Abstract
Study Design
Experimental correlation study design to quantify features of disc health, including signal intensity and distinction between the annulus fibrosus (AF) and nucleus pulposus (NP), with T2* magnetic resonance imaging (MRI) and correlate with the functional mechanics in corresponding motion segments.
Objective
Establish the relationship between disc health assessed by quantitative T2* MRI and functional lumbar mechanics.
Summary of Background Data
Degeneration leads to altered biochemistry in the disc, affecting the mechanical competence. Clinical routine MRI sequences are not adequate in detecting early changes in degeneration and fails to correlate with pain or improve patient stratification. Quantitative T2* relaxation time mapping probes biochemical features and may offer more sensitivity in assessing disc degeneration.
Methods
Cadaveric lumbar spines were imaged using quantitative T2* mapping, as well as conventional T2-weighted MRI sequences. Discs were graded by the Pfirrmann scale and features of disc health, including signal intensity (T2* Intensity Area) and distinction between the AF and NP (Transition Zone Slope), were quantified by T2*. Each motion segment was subjected to pure moment bending to determine range of motion (ROM), neutral zone (NZ), and bending stiffness.
Results
T2* Intensity Area and Transition Zone Slope were significantly correlated with flexion ROM (p=0.015; p=0.002), ratio of NZ/ROM (p=0.010; p=0.028), and stiffness (p=0.044; p=0.026), as well as lateral bending NZ/ROM (p=0.005; p=0.010) and stiffness (p=0.022; p=0.029). T2* Intensity Area was also correlated with LB ROM (p=0.023). Pfirrmann grade was only correlated with lateral bending NZ/ROM (p=0.001) and stiffness (p=0.007).
Conclusions
T2* mapping is a sensitive quantitative method capable of detecting changes associated with disc degeneration. Features of disc health quantified with T2* predicted altered functional mechanics of the lumbar spine better than traditional Pfirrmann grading. This new methodology and analysis technique may enhance the assessment of degeneration and enable greater patient stratification for therapeutic strategies.
Keywords: disc degeneration, quantitative magnetic resonance imaging, T2* (T2 star), biomechanics, in vitro, lumbar spine
Introduction
Intervertebral disc (IVD) degeneration is a primary cause for low back pain, one of the most prevalent musculoskeletal impairments in the U.S. affecting an estimated 70–85% of the population at some point in their life1. Currently, assessment of patients with low back pain often includes diagnostic imaging of the intervertebral discs to identify their morphological health. This data is complimented with a clinical evaluation, aimed at assessing the functional biomechanics of lumbar spine motion and the presentation of pain. Coupling these data provides a picture of an individual’s overall spinal health. Unfortunately, this often results in contradictory or inconclusive outcomes in spite of the phenomenological link between the intervertebral disc’s constituent makeup and mechanics. The ability to discern differences in biochemistry, structure, and mechanics, the likely nexus for this challenge, has become the focus for both imaging and biomechanics studies.
Conventional T2 weighted sagittal magnetic resonance imaging (MRI) sequences have been utilized to create a subjective grading scale for disc health based on morphological features2,3. The grading system is applied in the clinical decision making process and yet lacks specificity4,5. The Pfirrmann grading system with a score from 1–5 is based upon the MRI signal intensity, clarity of the transition zone between the annulus fibrosus (AF) and nucleus pulposus (NP), and disc height2. This subjective scoring system largely fails to correlate well with pain or provide clinically useful patient stratification, especially in detecting early signs of degeneration6–9. However, the underlying processes of the Pfirrmann grading system appear to be robust and quantification of these features may advance disc health assessment.
Recently, new quantitative MR imaging techniques have been developed including T1ρ, T2 mapping, chemical exchange saturation transfer (CEST), Magnetic Transfer Ratio, and T2* mapping which may offer promise in more accurately discriminating between patient discs of different health10–16. Many of these techniques utilize the physics of water molecule relaxation within a magnetic field to produce images, which highlight the presence of proteoglycans (T1rho), hydration level (T2), and structure of the macromolecule matrix along with hydration level (T2*). Quantitative T2* (T2 star) mapping is an emerging technique with the added benefit of a short acquisition time, high signal-to-noise ratio, and three-dimensionality over traditional T2 mapping11,17,18. T2* has been shown to probe biochemical properties of the tissues and has been beneficial in cartilage10,11,17–24. Specifically, T2* relaxation times provide information about spatial macromolecule architecture in conjunction with water molecule mobility11,18. The relationship between T2* value and histological grades of degeneration has been established in the hip joint, where a decrease in T2* relaxation time was significantly correlated with a higher degree of cartilage degeneration10.
To the authors knowledge, only two studies have been published which apply the T2* technique to the intervertebral disc17,25. Hoppe et al. proposed a technique for assessment of disc degeneration using axial T2* maps based on Watanabe et al. classification and found a significant correlation between T2* parameters and Pfirrmann grades25,26. Welsch et al. correlated quantitative T2 and T2* relaxation times with Pfirrmann Score in patients suffering from low back pain17. They reported both techniques correlated strongly with Pfirrmann Score regarding the nucleus pulposus, but T2* alone had the ability to identify changes in the posterior annulus fibrosus. They also performed a parametric analysis between T2* relaxation times at different regions of interest (ROI) and reported the highest spatial variation from AF to NP in Pfirrmann grade 1, and a decline at higher grades.
These T2* seminal works provide the basis for our investigation which aims to utilize the quantitative nature of the imaging technique and distinguish critical features of the Pfirrmann grading system. The features of the Pfirrmann grading system include disc signal intensity and clarity of the transition zone between the AF and NP. The interrogation of these features using the T2* images will enable the quantitative grading of disc degeneration without subjectivity or bias but with clinically recognized features of distinction. These efforts utilized ROI data alone to quantify disc health despite knowing that specific features of classification systems enable those systems to predict morphological changes better than others.
The ability of T2* mapping to detect changes related to the intrinsic biochemical properties has a potentially profound impact on diagnostic imaging of the intervertebral disc. Throughout the degeneration process there is a loss of proteoglycans and a change in the anabolic/catabolic balance of collagen homeostasis and its architecture. The alterations of the biochemical content and its structural integrity change the mechanical properties of the degenerating disc impacting the global mechanics of the spine27–29,30–36. Biomechanically, these changes lead to altered spinal stability and potentially cord occlusion, neural compressive lesions or nerve root pinching, causing pain.
Therefore, the purpose of this study was to create T2* maps of IVDs throughout the degenerative spectrum, quantify parameters of the Pfirrmann grading scale, and correlate these imaging parameters with mechanics outcomes of functional spinal units in an attempt to probe the relationship between structural constituent properties and functional mechanical parameters.
Methods
Eighteen osteoligamentous lumbar spines (L3-Sacrum), acquired from our University Bequest Program, (age: 53.2±15.5 years; range: 21–71 years), were examined using conventional and quantitative magnetic resonance imaging protocols and biomechanically exercised in flexion/extension, lateral bending, and axial rotation. A correlation study design was utilized to examine the relationship between the intervertebral disc morphology, based on MR imaging, and functional biomechanics of the lumbar spine.
Classic Disc Grading
MR imaging was performed on a Siemens 3T scanner (Magnetom Trio; Siemens Healthcare) on each IVD (L3-L4, L4-L5, L5-S1), 54 in total. Conventional T2-weighted sagittal images were acquired for Pfirrmann disc grading (Figure 1A). Each disc was graded via the Pfirrmann scale independently by seven spine surgeons (DWP) and three experienced spine researchers (AME, DJN, HM). These scores were then averaged and rounded to the nearest integer.
Figure 1. Magnetic Resonance Images of Lumbar Spine and Intervertebral Disc Using T2 and Quantitative T2* Techniques.
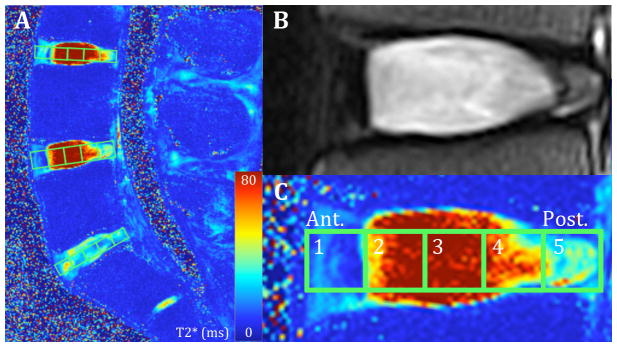
Left: T2* Map of Lumbar Spine. Top Right: Healthy Lumbar Intervertebral disc imaged with classical T2 MRI. Bottom Right: Same disc using T2* map showcasing the five regions of interest from anterior to posterior (1 to 5).
T2* Disc Health Quantification
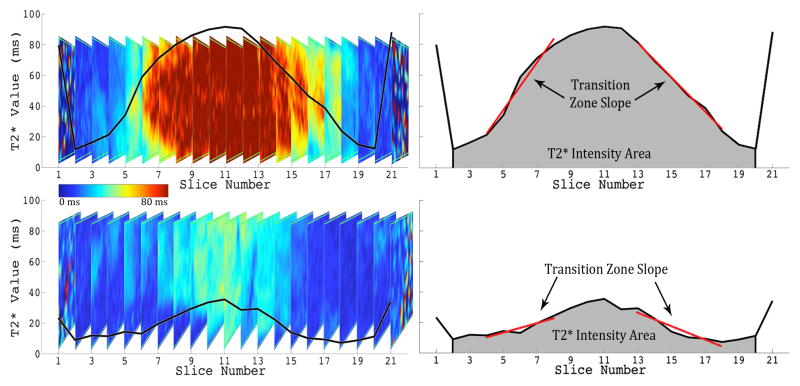
T2* relaxation maps were obtained [TR(ms): 500; TE(ms): 4.18, 11.32, 18.46, 25.60, 32.74, 39.88; Voxel Size(mm): 0.5×0.5×3.0, Slices: 33]. Quantitative T2* maps were calculated (MapIt, Siemens Healthcare) and mean T2* values were recorded using the open-source Osirix Imaging Software across five mid-sagittal plane regions of interest (ROI) from anterior (ROI 1) to posterior (ROI 5) (Figure 1C). Further analysis utilizing MATLAB (Mathworks Inc. Natick, MA), evaluated the central ROI (ROI 3) mean relaxation value in all sagittal slices across the coronal plane as the intensity changed from lateral to medial to lateral. Using a least squares method, the slope of the transition zone (ms/mm) between the annulus fibrosus and nucleus pulposus on each side was linearly regressed and then averaged. This metric quantified the distinction between the NP and AF. To quantify the signal intensity of the disc, the area underneath the curve was calculated by the trapezoidal integration method and normalized to the width of the IVD, measured by MRI (Figure 2).
Figure 2. Quantification of Imaging Parameters, Transition Zone Slope and T2* Intensity Area, from T2* Relaxation Profile in the Coronal Plane of ROI 3.
Top Left: Coronal Profile of Healthy Disc with Plot of Average T2* Relaxation Time (ms) overlaid. Top Right: Corresponding quantification of Transition Zone Slope and T2* Intensity Area of the healthy disc. Bottom Left: Coronal Profile of Degenerated Disc with Plot of Average T2* Relaxation Time (ms) overlaid. Bottom Right: Corresponding quantification of Transition Zone Slope and T2* Intensity Area of degenerated disc.
Biomechanical Testing
After imaging, the specimens were embedded in polymethylmethacrylate and tested in a six-axis Spine Kinetic Simulator (8821 Biopuls, Instron, Norwood, MA)37. Pure moments of 7 Nm with no axial preload (0 N) were applied with the spine unconstrained to displace inferiorly; this methodology is utilized in numerous studies and was also the initial basis for the ASTM F2423 standard, although it now includes an axial preload37–40. Input moments and forces were controlled by a 6 DOF load cell (AMTI M4380, Watertown, MA) in three cardinal planes. Inferiorly, movements were unconstrained using a passive X-Y table (resistance <0.1N) to minimize shear forces.
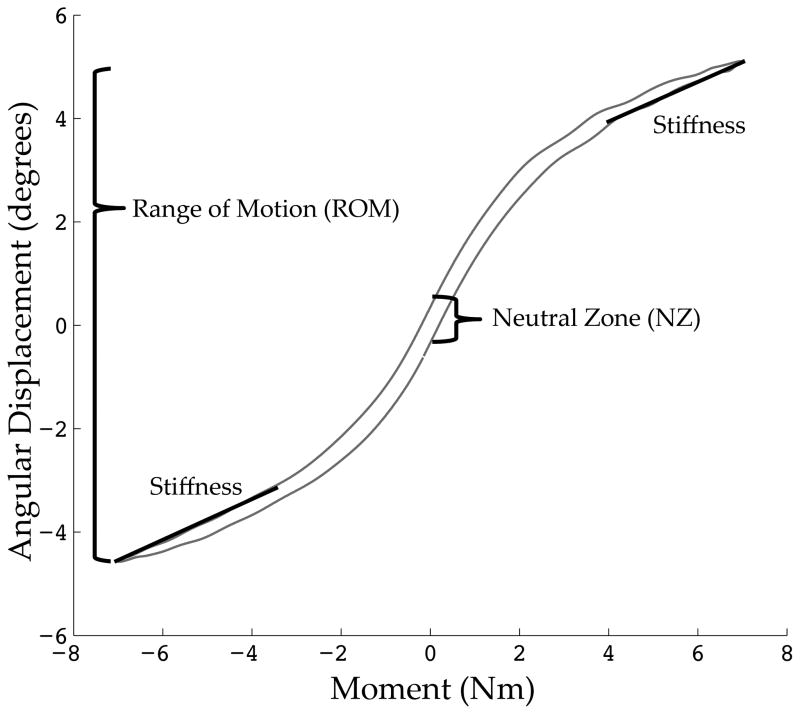
Three cycles of was performed sinusoidally (0.015 Hz) and the final cycle was used for analysis. Load and moment data were collected on two six-axis load cells (AMTI M4380, Watertown, MA) at 100 Hz on a data acquisition board connected to personal computer. Intersegmental angular displacements were recorded at 100 Hz using a 3D visual motion analysis 5-camera system (Vicon MX-F40NIR, Vicon Motion Systems, Centennial, CO) capturing a four ball infrared reflecting marker set attached to each vertebral body. Range of motion (ROM), neutral zone (NZ), ratio of NZ/ROM, and bending stiffness were quantified for each functional spinal units in all bending directions39. Figure 3 displays these metrics over top a representative motion profile of a healthy functional unit.
Figure 3. Representative Motion Profile.
A healthy functional unit motion profile with biomechanical metrics graphically defined. These include range of motion (ROM), neutral zone (NZ), and bending stiffness.
Statistical Treatment
The mechanical outcomes were compared with both T2* measurements and Pfirrmann grade using Pearson’s Correlation Tests (α=0.05). Furthermore, each T2* measurement was also correlated with respect to Pfirrmann grade (α=0.05). One-way single measure intra-class correlation (ICC) was calculated to determine the intra-observer variability in assessing Pfirrmann grades to the discs.
Results
All specimens were imaged and received the full battery of biomechanical testing to reveal moment-angular displacement curves for flexion/extension (FE), lateral bending (LB), and axial rotation (AR). One Pfirrmann grade 5 disc was unable to be quantified using the T2* technique due to the cadaveric nature of the tissues and this disc’s complete collapse.
The graders of disc health had an intra-class correlation coefficient value of 0.686. Pfirrmann grade was significantly correlated with ROI’s 2–5 from T2* maps, as well as the transition zone slope and T2* intensity area (Table 1).
Table 1.
Correlations between Pfirrmann Grade and T2* imaging parameters
| Pfirrmann Grade | ||
|---|---|---|
| r | p | |
| Transition Zone Slope | −0.604 | <0.001* |
| T2* Intensity Area | −0.774 | <0.001* |
| ROI 1 | 0.001 | 0.996 |
| ROI 2 | −0.698 | <0.001* |
| ROI 3 | −0.821 | <0.001* |
| ROI 4 | −0.737 | <0.001* |
| ROI 5 | −0.381 | <0.001* |
(r: Pearson’s correlation coefficient; p: p-value * represents significance at p=0.05.).
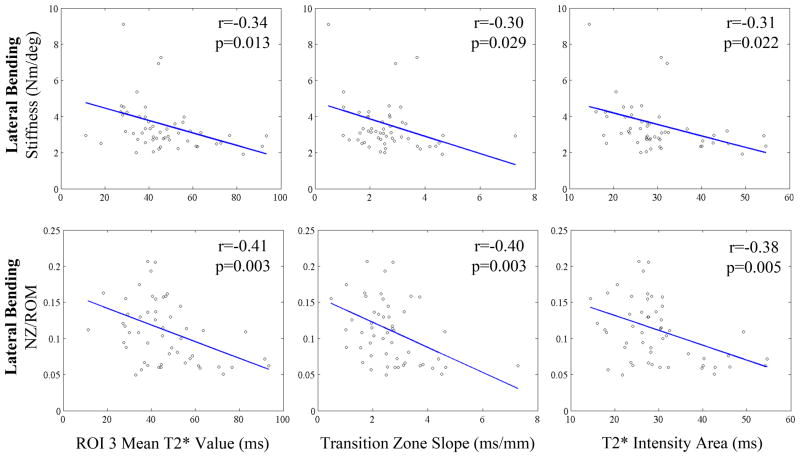
Range of motion measurements and stiffness correlations with imaging parameters are displayed in Tables 2 and 3, respectively. Pfirrmann grade was significantly correlated with only LB NZ/ROM (p=0.001) and stiffness (p=0.007). T2* Intensity Area and the Transition Zone Slope were also significantly correlated with LB NZ/ROM (p=0.005; p=0.010) and stiffness (p=0.022; p=0.029). T2* Intensity Area was also correlated with LB ROM (p=0.023). Select correlational plots for lateral bending are displayed in Figure 4. In addition, T2* Intensity Area and the Transition Zone Slope was significantly correlated with Flexion ROM (p=0.015; p=0.002), NZ/ROM (p=0.010; p=0.028), and stiffness (p=0.044; p=0.026). Several regions of interest also correlated with these variables as well as AR ROM (p=0.034) and AR stiffness (p=0.006).
Table 2.
Correlations between T2* parameters and range of motion and ratio neutral zone to range of motion
| Transition Zone Slope | T2* Intensity Area | ROI 1 | ROI 2 | ROI 3 | ROI 4 | ROI 5 | Pfirrmann Grade | |||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Flexion | ROM | r | 0.414 | 0.334 | 0.118 | 0.322 | 0.311 | 0.353 | 0.090 | −0.220 |
| p | 0.002* | 0.015* | 0.398 | 0.019* | 0.024* | 0.01* | 0.521 | 0.109 | ||
|
| ||||||||||
| NZ/ROM | r | −0.352 | −0.305 | .084 | −0.187 | −0.323 | −0.293 | −0.114 | 0.136 | |
| p | 0.010* | 0.028* | .554 | 0.185 | 0.019* | 0.035* | 0.422 | 0.332 | ||
|
| ||||||||||
| Lateral Bending | ROM | r | 0.179 | 0.312 | 0.164 | 0.206 | 0.262 | 0.188 | 0.335 | −0.223 |
| p | 0.199 | 0.023* | 0.241 | 0.139 | 0.058 | 0.177 | 0.014* | 0.105 | ||
|
| ||||||||||
| NZ/ROM | r | −0.402 | −0.378 | −0.346 | −0.363 | −0.406 | −0.399 | −0.258 | 0.468 | |
| p | 0.003* | 0.005* | 0.011* | 0.007* | 0.003* | 0.003* | 0.062 | 0.001* | ||
|
| ||||||||||
| Axial Rotation | ROM | r | −0.143 | −0.148 | −0.061 | −0.292 | −0.130 | −0.191 | 0.027 | 0.193 |
| p | 0.307 | 0.290 | 0.667 | 0.034* | 0.355 | 0.171 | 0.848 | 0.162 | ||
|
| ||||||||||
| NZ/ROM | r | 0.136 | 0.036 | 0.128 | 0.151 | 0.055 | 0.099 | −0.063 | 0.071 | |
| p | 0.332 | 0.799 | 0.361 | 0.280 | 0.696 | 0.481 | 0.653 | 0.608 | ||
(r: Pearson’s correlation coefficient; p: p-value * represents significance at p=0.05.)
Table 3.
Correlations between T2* parameters and stiffness measurements
| Transition Zone Slope | T2* Intensity Area | ROI 1 | ROI 2 | ROI 3 | ROI 4 | ROI 5 | Pfirrmann Grade | |||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Stiffness (deg/Nm) | Flexion | r | −0.306 | −0.278 | 0.105 | −0.079 | −0.308 | −0.163 | −0.228 | 0.230 |
| p | 0.026* | 0.044* | 0.454 | 0.574 | 0.025* | 0.244 | 0.101 | 0.094 | ||
|
| ||||||||||
| Extension | r | 0.002 | 0.191 | 0.106 | 0.249 | 0.126 | 0.172 | −0.021 | −0.031 | |
| p | 0.988 | 0.170 | 0.451 | 0.072 | 0.368 | 0.217 | 0.879 | 0.822 | ||
|
| ||||||||||
| Lateral Bending | r | −0.300 | −0.314 | −0.269 | −0.292 | −0.341 | −0.197 | −0.186 | 0.362 | |
| p | 0.029* | 0.022* | 0.052 | 0.034* | 0.013* | 0.157 | 0.182 | 0.007* | ||
|
| ||||||||||
| Axial Rotation | r | 0.276 | 0.241 | 0.186 | 0.270 | 0.233 | 0.371 | 0.229 | −0.208 | |
| p | 0.043* | 0.082 | 0.182 | 0.051 | 0.093 | 0.006* | 0.099 | 0.132 | ||
(r: Pearson’s correlation coefficient; p: p-value * represents significance at p=0.05.)
Figure 4. Representative Correlational Plots.
Top: Lateral Bending Stiffness and Bottom: Lateral Bending NZ/ROM correlated with ROI 3 Average T2* Value (ms), Transition Zone Slope (ms/mm), and T2* Intensity Area (ms). r: Pearson’s correlational coefficient and p: p-value are displayed on corresponding plot.
Discussion
Quantitative T2* MRI has been shown to be beneficial in cartilage imaging by probing the biochemical content of the tissue. Specifically, T2* relaxation times provide information about spatial macromolecule architecture and its interaction with water molecule mobility10, 11. T2* relaxation is a combination of inherent “true” T2 relaxation and additional relaxation due to magnetic inhomogeneities, on both a microscopic and macroscopic scale (1/T2* = 1/T2 + 1/T2’, where T2’ is the relaxation due to magnetic field inhomogeneities). While T2* is affected by bulk inhomogeneities in the magnetic field, which are typically not of interest, it is also affected by the differences in tissue composition at microscopic level, such as the change from cartilage to bone, annulus to nucleus pulposus or susceptibility-induced changes related to para- or diamagnetic depositions within the disc41. These principals extrapolate to other tissues, such as the intervertebral disc. This study highlights the quantitative nature of the T2* imaging to distinguish features utilized in the Pfirrmann grading system, the current gold standard for assessing disc health, and correlate them with intersegmental spinal functional mechanics. The features of the Pfirrmann grading system quantified by T2*, including disc signal intensity and clarity of the transition zone between the AF and NP, were found to be more sensitive in detecting change in the global mechanics of the lumbar spine.
Disc health was correlated with the bending stiffness of the lumbar spine in flexion, but not in extension. Flexion bending stiffness increased with degeneration severity, resulting in a decrease of flexion ROM. Furthermore, the NZ/ROM ratio, a relative measure of joint laxity, was shown to increase with worsening degeneration, indicating a decrease in the stability of the segment. There is more laxity in the joint until the end range of motion, where the tissue is much stiffer, allowing for little motion outside of the neutral zone. These findings were significantly correlated with the Transition Zone Slope, T2* Intensity Area, and specific ROI’s, however no correlation was determined with Pfirrmann grade. Similarly to flexion, lateral bending stiffness was found to increase with degeneration, while ROM decreased. Pfirrmann grade was only significantly correlated with LB NZ/ROM and stiffness, while parameters of T2* were correlated with flexion, lateral bending, and axial rotation range of motion and stiffness measurements.
Previous studies have also reported a decrease in range of motion for both flexion and lateral bending, while axial rotation increased with degeneration31,34–36. Mimura et al. reported an increase in lateral bending NZ/ROM ratio with disc degeneration similar to the results herein34. The literature also suggests an increase in the bending stiffness of both lateral bending and flexion with degeneration32. Haughton et al. observed a decrease in axial rotation bending stiffness with degeneration33. Thus, the mechanical results of the present study align with the prevailing literature and exhibit identifiable changes across the disc degeneration spectrum.
The observed changes in global mechanics are supported by the changes locally in the AF and NP through degeneration. The mechanical properties of the AF in tension are deteriorated, as there is a shift of collagen production from Type I to Type II42–48. This results in an increase in AR ROM while stiffness decreases49. Conversely, flexion and lateral bending places the ipsilateral AF in compression and previous literature has shown that the compressive modulus of the AF increases with degeneration28,29,50, causing the bending stiffness of the functional spinal unit to increase. This phenomenon is exacerbated when the NP loses hydration due to the loss of proteoglycans, associated with degeneration. The NP no longer acts like a pressure vessel and the disc height decreases while a larger compressive load is placed on the outer AF.
It is important to note that the correlation coefficients obtained in this present study are weak to moderate, but was determined to be significant because of the large sample size. In order to expand the clinical utility of these imaging metrics, a post-hoc multiple linear regression analysis was performed to predict the biomechanical outcome measures by using combinations of all the T2* MR imaging parameters (ROI’s 1–5, Transition Zone Slope, and T2* Intensity Area). The correlation coefficients are displayed in Table 4. Also included in the table is the highest correlation coefficient achieved from the univariate linear regression analysis and the percent improvement in prediction. The improvement in agreement between the two analysis techniques illustrates the ability of various T2* parameters to identify independent characteristics of disc health that relate to the functional mechanics of the lumbar spine.
Table 4.
Correlation Coefficient Comparison between Multiple Linear Regression Analysis and Univariate
| Flexion | Lateral Bending | Axial Rotation | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ROM | NZ / ROM | Stiffness | ROM | NZ / ROM | Stiffness | ROM | NZ / ROM | Stiffness | |
| Univariate (r) | 0.414 | 0.352 | 0.308 | 0.335 | 0.406 | 0.341 | 0.292 | 0.151 | 0.371 |
| Multivariate (r) | 0.536 | 0.434 | 0.496 | 0.484 | 0.575 | 0.611 | 0.486 | 0.344 | 0.486 |
| Improvement (%) | 29.5 | 23.3 | 61.0 | 44.5 | 41.6 | 79.2 | 66.4 | 127.8 | 31.0 |
(r: Correlation Coefficient). Multivariate coefficient includes all T2* Imaging Parameters (ROI’s 1–5, Transition Zone Slope, and T2* Intensity Area). The Univariate value represents the highest correlation coefficient achieved between the all imaging metrics and the corresponding biomechanical output.
As with any cadaveric study, the limitations to this work should be considered to contextualize the results. Biomechanical tests were conducted on cadaveric tissue without musculature at a low loading rate commensurate with flexing fully forward over a 12 second duration. Although pure moments were applied to the spine and coupled motions could have occurred, only the primary direction of bending was analyzed for each rotation sequence. Although three levels were all compared against each other, there was no statistical difference as a function of disc level within a single Pfirrmann grade (p>0.10). Also, imaging was free of any motion artifacts, and therefore idealized, due to stationary specimens, which were at room temperature.
Previous studies have investigated the relationship between T2* relaxation times and Pfirrmann grade. Welsch et al. investigated the relationship between T2* relaxation times, grouped into 5 ROI’s from anterior to posterior, and Pfirrmann grades of subjects with low back pain17. Only including grades 2–5 they found a significant correlation between Pfirrmann grade and all but the anterior most ROI. Hoppe et al. performed a similar study using 7 ROIs13. They reported a significant correlation with Pfirrmann grade for all ROIs except for the anterior and posterior most regions. Their study did not include severely degenerated discs. The present study incorporated 54 discs from the entire degeneration spectrum, which followed a normal distribution (7 grade 1, 12 grade 2, 17 grade 3, 11 grade 4, 7 grade 5). Similarly to Welsch et al. there was a significant correlation between Pfirrmann grade and ROI’s 2–5 as well as the newly defined variables, T2* Intensity Area and Transition Zone Slope.
Quantitative T2* assessment of disc health has the potential to be more sensitive in detecting the local changes associated with disc degeneration, particularly due to its intrinsic nature to detect water mobility, or the interaction of water within the macromolecular network11,18. T2* also predicted altered kinematics better than traditional Pfirrmann grading. Therefore it is postulated that the T2* relaxation times may be correlated to the concentration of proteoglycan or collagen, which are related to its biomechanical competence. This technique allows for the quantification of key characteristics of disc health, without subjectivity or bias. These include the T2* Intensity Area and more uniquely the Transition Zone Slope, which defines the distinction between the NP and AF. T2* also benefits from a short acquisition time, high signal-to-noise ratio, three-dimensionality and is currently available on clinical scanners.
T2* MRIs produce a sensitive measure capable of detecting alterations in the lumbar intervertebral disc morphology which are correlated with their mechanical performance. These morphologic changes are likely due to altered biochemical content during degeneration. Affecting the local mechanical properties of the tissue, and ultimately leads to affected kinematics and spinal function. Further investigation is needed to establish the relationship between T2* relaxation times and precise biochemical content, such as water, proteoglycans and collagen. Nonetheless, this work highlights a clinically available MR imaging sequence (T2*) and disc health quantification methods that are correlated with the functional mechanics of the spinal segment. This new methodology and analysis technique may enhance quantitative data on the degenerative cascade, and enable greater patient stratification for therapeutic strategies with a currently available technology.
Key Points.
Key features of the Pfirrmann grading scale were quantified using T2* magnetic resonance imaging.
These metrics, T2* relaxation times, T2* Intensity Area, and Transition Zone Slope, predicted altered kinematics better than traditional Pfirrmann grading.
This new methodology and analysis technique is a potential diagnostic tool for the assessment of disc degeneration.
Acknowledgments
The authors would like to thank Peter Kollasch from the Center of Magnetic Resonance Research at the University of Minnesota for help acquiring MR images. Also, the authors would like to acknowledge Jonathan Sembrano MD, Edward Santos MD, Charles Ledonio MD, Matthew Hunt MD, Ann Parr MD PhD, and Robert Morgan MD for grading the discs.
NIH/NIAMS T32 AR050938 Musculoskeletal Training grant funds were received in support of this work. Relevant financial activities outside the submitted work: patents.
Footnotes
The manuscript submitted does not contain information about medical device(s)/drug(s).
References
- 1.Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999 Aug 14;354(9178):581–585. doi: 10.1016/S0140-6736(99)01312-4. [DOI] [PubMed] [Google Scholar]
- 2.Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 2001 Sep 1;26(17):1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 3.Thompson JP, Pearce RH, Schechter MT, Adams ME, Tsang IK, Bishop PB. Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine (Phila Pa 1976) 1990 May;15(5):411–415. doi: 10.1097/00007632-199005000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Arana E, Royuela A, Kovacs FM, et al. Lumbar spine: agreement in the interpretation of 1.5-T MR images by using the Nordic Modic Consensus Group classification form. Radiology. 2010 Mar;254(3):809–817. doi: 10.1148/radiol.09090706. [DOI] [PubMed] [Google Scholar]
- 5.Raininko R, Manninen H, Battie MC, Gibbons LE, Gill K, Fisher LD. Observer variability in the assessment of disc degeneration on magnetic resonance images of the lumbar and thoracic spine. Spine (Phila Pa 1976) 1995 May 1;20(9):1029–1035. doi: 10.1097/00007632-199505000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990 Mar;72(3):403–408. [PubMed] [Google Scholar]
- 7.Savage RA, Whitehouse GH, Roberts N. The relationship between the magnetic resonance imaging appearance of the lumbar spine and low back pain, age and occupation in males. Eur Spine J. 1997;6(2):106–114. doi: 10.1007/BF01358742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensen MC, Brant-Zawadzki MN, Obuchowski N, Modic MT, Malkasian D, Ross JS. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994 Jul 14;331(2):69–73. doi: 10.1056/NEJM199407143310201. [DOI] [PubMed] [Google Scholar]
- 9.Powell MC, Wilson M, Szypryt P, Symonds EM, Worthington BS. Prevalence of lumbar disc degeneration observed by magnetic resonance in symptomless women. Lancet. 1986 Dec 13;2(8520):1366–1367. doi: 10.1016/s0140-6736(86)92008-8. [DOI] [PubMed] [Google Scholar]
- 10.Bittersohl B, Miese FR, Hosalkar HS, et al. T2 * mapping of hip joint cartilage in various histological grades of degeneration. Osteoarthritis Cartilage. 2012 Mar 30; doi: 10.1016/j.joca.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Krause FG, Klammer G, Benneker LM, Werlen S, Mamisch TC, Weber M. Biochemical T2* MR quantification of ankle arthrosis in pes cavovarus. J Orthop Res. 2010 Dec;28(12):1562–1568. doi: 10.1002/jor.21192. [DOI] [PubMed] [Google Scholar]
- 12.Carballido-Gamio J, Stahl R, Blumenkrantz G, Romero A, Majumdar S, Link TM. Spatial analysis of magnetic resonance T1rho and T2 relaxation times improves classification between subjects with and without osteoarthritis. Med Phys. 2009 Sep;36(9):4059–4067. doi: 10.1118/1.3187228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmitt B, Zbyn S, Stelzeneder D, et al. Cartilage quality assessment by using glycosaminoglycan chemical exchange saturation transfer and (23)Na MR imaging at 7 T. Radiology. 2011 Jul;260(1):257–264. doi: 10.1148/radiol.11101841. [DOI] [PubMed] [Google Scholar]
- 14.Singh A, Haris M, Cai K, et al. Chemical exchange saturation transfer magnetic resonance imaging of human knee cartilage at 3 T and 7 T. Magn Reson Med. 2012 Aug;68(2):588–594. doi: 10.1002/mrm.23250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buchbender C, Scherer A, Kropil P, et al. Cartilage quality in rheumatoid arthritis: comparison of T2* mapping, native T1 mapping, dGEMRIC, DeltaR1 and value of pre-contrast imaging. Skeletal Radiol. 2012 Jun;41(6):685–692. doi: 10.1007/s00256-011-1276-2. [DOI] [PubMed] [Google Scholar]
- 16.Welsch GH, Trattnig S, Hughes T, et al. T2 and T2* mapping in patients after matrix-associated autologous chondrocyte transplantation: initial results on clinical use with 3.0-Tesla MRI. European Radiology. 2009;20(6):1515–1523. doi: 10.1007/s00330-009-1669-y. [DOI] [PubMed] [Google Scholar]
- 17.Welsch GH, Trattnig S, Paternostro-Sluga T, et al. Parametric T2 and T2* mapping techniques to visualize intervertebral disc degeneration in patients with low back pain: initial results on the clinical use of 3.0 Tesla MRI. Skeletal Radiol. 2011 May;40(5):543–551. doi: 10.1007/s00256-010-1036-8. [DOI] [PubMed] [Google Scholar]
- 18.Mamisch TC, Hughes T, Mosher TJ, et al. T2 star relaxation times for assessment of articular cartilage at 3 T: a feasibility study. Skeletal Radiol. 2011 Apr 19; doi: 10.1007/s00256-011-1171-x. [DOI] [PubMed] [Google Scholar]
- 19.Welsch GH, Trattnig S, Hughes T, et al. T2 and T2* mapping in patients after matrix-associated autologous chondrocyte transplantation: initial results on clinical use with 3.0-Tesla MRI. Eur Radiol. 2010 Jun;20(6):1515–1523. doi: 10.1007/s00330-009-1669-y. [DOI] [PubMed] [Google Scholar]
- 20.Williams A, Qian Y, Bear D, Chu CR. Assessing degeneration of human articular cartilage with ultra-short echo time (UTE) T2* mapping. Osteoarthritis and Cartilage. 2010;18(4):539–546. doi: 10.1016/j.joca.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marik W, Apprich S, Welsch GH, Mamisch TC, Trattnig S. Biochemical evaluation of articular cartilage in patients with osteochondrosis dissecans by means of quantitative T2- and T2-mapping at 3T MRI: a feasibility study. Eur J Radiol. 2012 May;81(5):923–927. doi: 10.1016/j.ejrad.2011.01.124. [DOI] [PubMed] [Google Scholar]
- 22.Miese FR, Zilkens C, Holstein A, et al. Assessment of early cartilage degeneration after slipped capital femoral epiphysis using T2 and T2* mapping. Acta Radiol. 2011 Feb 1;52(1):106–110. doi: 10.3109/02841851.2010.516015. [DOI] [PubMed] [Google Scholar]
- 23.Williams A, Qian Y, Chu CR. UTE-T2 * mapping of human articular cartilage in vivo: a repeatability assessment. Osteoarthritis Cartilage. 2011 Jan;19(1):84–88. doi: 10.1016/j.joca.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qian Y, Williams AA, Chu CR, Boada FE. Multicomponent T2* mapping of knee cartilage: technical feasibility ex vivo. Magn Reson Med. 2010 Nov;64(5):1426–1431. doi: 10.1002/mrm.22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoppe S, Quirbach S, Mamisch TC, Krause FG, Werlen S, Benneker LM. Axial T2* mapping in intervertebral discs: a new technique for assessment of intervertebral disc degeneration. Eur Radiol. 2012 Apr 29; doi: 10.1007/s00330-012-2448-8. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe A, Benneker LM, Boesch C, Watanabe T, Obata T, Anderson SE. Classification of intervertebral disk degeneration with axial T2 mapping. AJR Am J Roentgenol. 2007 Oct;189(4):936–942. doi: 10.2214/AJR.07.2142. [DOI] [PubMed] [Google Scholar]
- 27.Ellingson AM, Nuckley DJ. Intervertebral disc viscoelastic parameters and residual mechanics spatially quantified using a hybrid confined/in situ indentation method. J Biomech. 2012 Feb 2;45(3):491–496. doi: 10.1016/j.jbiomech.2011.11.050. [DOI] [PubMed] [Google Scholar]
- 28.Iatridis JC, Setton LA, Foster RJ, Rawlins BA, Weidenbaum M, Mow VC. Degeneration affects the anisotropic and nonlinear behaviors of human anulus fibrosus in compression. J Biomech. 1998 Jun;31(6):535–544. doi: 10.1016/s0021-9290(98)00046-3. [DOI] [PubMed] [Google Scholar]
- 29.Périé D, Iatridis JC, Demers CN, et al. Assessment of compressive modulus, hydraulic permeability and matrix content of trypsin-treated nucleus pulposus using quantitative MRI. Journal of Biomechanics. 2006;39(8):1392–1400. doi: 10.1016/j.jbiomech.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 30.Fujiwara A, Lim TH, An HS, et al. The effect of disc degeneration and facet joint osteoarthritis on the segmental flexibility of the lumbar spine. Spine (Phila Pa 1976) 2000 Dec 1;25(23):3036–3044. doi: 10.1097/00007632-200012010-00011. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka N, An HS, Lim TH, Fujiwara A, Jeon CH, Haughton VM. The relationship between disc degeneration and flexibility of the lumbar spine. Spine J. 2001 Jan-Feb;1(1):47–56. doi: 10.1016/s1529-9430(01)00006-7. [DOI] [PubMed] [Google Scholar]
- 32.Brown MD, Holmes DC, Heiner AD. Measurement of cadaver lumbar spine motion segment stiffness. Spine (Phila Pa 1976) 2002 May 1;27(9):918–922. doi: 10.1097/00007632-200205010-00006. [DOI] [PubMed] [Google Scholar]
- 33.Haughton VM, Lim TH, An H. Intervertebral disk appearance correlated with stiffness of lumbar spinal motion segments. AJNR Am J Neuroradiol. 1999 Jun-Jul;20(6):1161–1165. [PMC free article] [PubMed] [Google Scholar]
- 34.Mimura M, Panjabi MM, Oxland TR, Crisco JJ, Yamamoto I, Vasavada A. Disc degeneration affects the multidirectional flexibility of the lumbar spine. Spine (Phila Pa 1976) 1994 Jun 15;19(12):1371–1380. doi: 10.1097/00007632-199406000-00011. [DOI] [PubMed] [Google Scholar]
- 35.Kettler A, Rohlmann F, Ring C, Mack C, Wilke HJ. Do early stages of lumbar intervertebral disc degeneration really cause instability? Evaluation of an in vitro database. European Spine Journal. 2010;20(4):578–584. doi: 10.1007/s00586-010-1635-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niosi CA, Oxland TR. Degenerative mechanics of the lumbar spine. Spine J. 2004 Nov-Dec;4(6 Suppl):202S–208S. doi: 10.1016/j.spinee.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 37.Wheeler DJ, Freeman AL, Ellingson AM, et al. Inter-laboratory variability in in vitro spinal segment flexibility testing. Journal of Biomechanics. 2011;44(13):2383–2387. doi: 10.1016/j.jbiomech.2011.06.034. [DOI] [PubMed] [Google Scholar]
- 38.Panjabi M, Henderson G, Abjornson C, Yue J. Multidirectional testing of one- and two-level ProDisc-L versus simulated fusions. Spine (Phila Pa 1976) 2007 May 20;32(12):1311–1319. doi: 10.1097/BRS.0b013e318059af6f. [DOI] [PubMed] [Google Scholar]
- 39.Wilke HJ, Wenger K, Claes L. Testing criteria for spinal implants: recommendations for the standardization of in vitro stability testing of spinal implants. Eur Spine J. 1998;7(2):148–154. doi: 10.1007/s005860050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goel VK, Panjabi MM, Patwardhan AG, Dooris AP, Serhan H. Test protocols for evaluation of spinal implants. J Bone Joint Surg Am. 2006 Apr;88( Suppl 2):103–109. doi: 10.2106/JBJS.E.01363. [DOI] [PubMed] [Google Scholar]
- 41.Chavhan GB, Babyn PS, Thomas B, Shroff MM, Haacke EM. Principles, techniques, and applications of T2*-based MR imaging and its special applications. Radiographics. 2009 Sep-Oct;29(5):1433–1449. doi: 10.1148/rg.295095034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fujita Y, Duncan NA, Lotz JC. Radial tensile properties of the lumbar annulus fibrosus are site and degeneration dependent. J Orthop Res. 1997 Nov;15(6):814–819. doi: 10.1002/jor.1100150605. [DOI] [PubMed] [Google Scholar]
- 43.Acaroglu ER, Iatridis JC, Setton LA, Foster RJ, Mow VC, Weidenbaum M. Degeneration and aging affect the tensile behavior of human lumbar anulus fibrosus. Spine (Phila Pa 1976) 1995 Dec 15;20(24):2690–2701. doi: 10.1097/00007632-199512150-00010. [DOI] [PubMed] [Google Scholar]
- 44.Urban JP, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5(3):120–130. doi: 10.1186/ar629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skaggs DL, Weidenbaum M, Iatridis JC, Ratcliffe A, Mow VC. Regional variation in tensile properties and biochemical composition of the human lumbar anulus fibrosus. Spine (Phila Pa 1976) 1994 Jun 15;19(12):1310–1319. doi: 10.1097/00007632-199406000-00002. [DOI] [PubMed] [Google Scholar]
- 46.Hollander AP, Heathfield TF, Liu JJ, et al. Enhanced denaturation of the alpha (II) chains of type-II collagen in normal adult human intervertebral discs compared with femoral articular cartilage. J Orthop Res. 1996 Jan;14(1):61–66. doi: 10.1002/jor.1100140111. [DOI] [PubMed] [Google Scholar]
- 47.Antoniou J, Steffen T, Nelson F, et al. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996 Aug 15;98(4):996–1003. doi: 10.1172/JCI118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roughley PJ. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine (Phila Pa 1976) 2004 Dec 1;29(23):2691–2699. doi: 10.1097/01.brs.0000146101.53784.b1. [DOI] [PubMed] [Google Scholar]
- 49.Jensen GM. Biomechanics of the lumbar intervertebral disk: a review. Phys Ther. 1980 Jun;60(6):765–773. doi: 10.1093/ptj/60.6.765. [DOI] [PubMed] [Google Scholar]
- 50.Umehara S, Tadano S, Abumi K, Katagiri K, Kaneda K, Ukai T. Effects of degeneration on the elastic modulus distribution in the lumbar intervertebral disc. Spine. 1996 Apr 1;21(7):811–819. doi: 10.1097/00007632-199604010-00007. discussion 820. [DOI] [PubMed] [Google Scholar]