Abstract
Data are available such that choice of Helicobacter pylori therapy for an individual patient can be reliably predicted. Here treatment success is defined at a cure rate of 90% or greater. Treatment outcome in a population or a patient can be calculated based on the effectiveness of a regimen for infections with susceptible and with resistant strains coupled with knowledge of the prevalence of resistance (i.e., based on formal measurement, clinical experience, or both). We provide the formula for predicting outcome and we illustrate the calculations. Because clarithromycin-containing triple therapy and 10 day sequential therapy are now only effective in special populations they are considered obsolete; neither should continue to be used as empiric therapies (i.e., 7 and 14 day triple therapies fail when clarithromycin resistance exceeds 5% and 15%, respectively and 10-day sequential therapy fails when metronidazole resistance exceeds 20%). Therapy should be individualized base on prior history and whether the patient is in a high risk group for resistance. The preferred choices for Western countries are 14 day concomitant therapy, 14 day bismuth quadruple therapy, and 14 day hybrid sequential-concomitant therapy. We also provide details regarding the successful use of fluoroquinolone-rifabutin-, and furazolidone-containing therapies. Finally, we give recommendations for efficient development (i.e., identification and optimization) of new regimens as well as how to prevent or minimized failures. The trial and error approach for identifying and testing regimens frequently resulted in poor treatment success. The approach described allows outcome to be predicted and should simplify treatment and drug development.
Keywords: Helicobacter pylori, treatment, quadruple therapy, review, treatment success, concomitant therapy, sequential therapy, bismuth, clarithromycin, tetracycline, metronidazole, amoxicillin, proton pump inhibitors, evidence based
Introduction
Like other infectious diseases the factors responsible for effective antimicrobial therapy of a Helicobacter pylori infection as well as those responsible treatment failure are both straight forward and easily discoverable. Poorly designed or executed regimens rarely produce good results. Treatment success depends on the details of the regimen including choice of drugs, doses, formulations, duration of therapy, administration in relation to meals, number of administrations/day, the use of adjuvants such as antisecretory drugs or mucolytics, etc 1. Results can be defined in terms of treatment success 2, 3. For exploratory studies the primary outcome is generally expressed per protocol (PP) which controls for compliance and other variables and thus provides an indication of the potential maximum success of regimen in clinical practice 1. For the information to be useful and to be used to predict success in other groups, regions, and populations, the results should also be provided as the outcomes with both susceptible and resistant strains (see below). In addition,, the data should also be expressed as both modified intention to treat (MTT) (which is the outcome of all who received a dose and for whom an outcome measure is available) and as intention to treat (ITT) in which those lost to follow-up are typically scored as treatment failures. ITT and MITT provide estimates of a regimen's actual success in clinical practice. PP and MITT are the most useful for development of new regimens whereas for large multicenter randomized comparisons most prefer ITT 4.
Considering that H. pylori is a common infectious disease and 100% success is obtainable, outcome (e.g., PP or ITT) is also scored in terms of efficacy (i.e., as excellent, good, borderline acceptable, or unacceptable) because efficacy is the most important measure for patient care. For evaluating new therapies we score success (PP with susceptible strains) as excellent (≥95% success), good (≥90 success), borderline acceptable (85-89% success), or unacceptable (<85% success). The most common causes for reliably good or excellent regimens to fail are the presence of organisms resistant to one or more of the antimicrobials used, poor compliance with therapy, or both. A number of studies have suggested a variety of miscellaneous factors that might be important including age, presentation (e.g., non-ulcer dyspepsia vs. duodenal ulcer, CagA status) 5-7. However, these candidates have typically been discovered in data dredging studies in which resistance was not assessed and most lack biologic plausibility. Although some of these factors (e.g., NUD vs. DU) have proven to be surrogates for differences in the prevalence of resistant strains 8, 9; none of the clinical correlates other than resistance and compliance have proven to be important in studies where compliance and resistance have been assessed.
Choice of therapy
As with other infectious diseases, treatment results are best when reliably excellent regimens are used to treat patients with organisms susceptible to the antimicrobials chosen. Pretreatment susceptibility testing, either by culture of the organism or indirectly by molecular testing of stools of infected patients or fluorescent in-situ hybridization (FISH) using parafin-embedded gastric biopsies, allows one to select regimen tailored by antimicrobial susceptibility (i.e., tailored therapy) 3. However, in many instances, one must choose therapy empirically and in this instance, the best approach is to use regimens that have proven to be reliably excellent locally 2. That choice should take advantage of knowledge of resistance patterns obtained from local or regional antimicrobial surveillance programs and/or based on local clinical experience with regard to which regimens are effective locally. Finally, the history of the patient's prior antibiotic use and any prior therapies will help identify which antibiotics are likely to be successful and those where resistance is probable.
All other things being equal, data from any area or region regarding the effects of resistance on outcome can reliably be used to predict outcome in any other area. Thus, strains with similar patterns of resistance in Italy, US, Iran, China, etc. should be expected to respond alike such that, if one knows the results with susceptible and with resistant strains in one place, one can reasonably predict the outcome of therapy anywhere.
Using available data to predict treatment success
An optimized regimen is defined as one that reliably achieves 95% or greater cures in patients with susceptible organisms. Although the effectiveness of any regimen can be undermined by antimicrobial resistance, the effect of resistance is not random and the effect of any particular level of resistance can be estimated based on studies with that combination elsewhere. For an example, the use the optimized regimen, 14 day concomitant therapy, consisting of a PPI, clarithromycin, metronidazole and amoxicillin given twice a day for 14 days 10. The regime contains 4 drugs but for the purpose of understanding the effects of resistance can best be considered as the simultaneous administration of two triple therapies plus a dual therapy (e.g. a PPI – amoxicillin - clarithromycin plus a PPI – amoxicillin - metronidazole plus a PPI -amoxicillin dual therapy). Both triple regimens individually will reliable achieve 95% or greater success PP with susceptible strains whereas the dual component will achieve approximately 50% success with clarithromycin and metronidazole resistant strains (i.e., the strains are only susceptible to amoxicillin). If resistance to clarithromycin or metronidazole was not present, there would be no indication to use the 4 drug regimen. However, when resistance results in unacceptably low treatment success when either are used empirically, the 4 drug combination might be considered.
Unless there is an interaction between the antibiotics, the treatment population can be visualized as 4 separate subgroups: one with organisms susceptible to all antibiotics, one with only clarithromycin resistant organisms, another with only metronidazole resistant organisms, and the final with organisms resistant to both (here we assume absence of resistance to amoxicillin). The subgroups without resistance and those resistant to a single drug will each receive an optimized triple therapy for their infection and most will be cured and the overall success will thus depend entirely on the success of the PPI-amoxicillin therapy for those with dual clarithromycin-metronidazole resistance.
In this example, both triple therapies achieve 97% treatment success and the dual therapy achieves 50% success (Table 1). One can calculate that treatment success will remain at or above 90% until dual resistance exceeds 15%. That calculation is based on the formula (% success with all-susceptible strains)(proportion with all-susceptible infections) plus (% success with clari-susceptible strains)(proportion with clari-susceptible infections) plus (% success with met-susceptible strains)(proportion with met-susceptible infections) plus (% success with dual resistant strains)(proportion with dual resistant strain) = 90%, Because the success with organisms susceptible to all antibiotics and single drug resistances is the same the two triple therapies can be combined to simplify the calculation (e.g., where X = proportion with dual resistance the formulas is .97(1−X) + .5X = ∼.90 and thus X = 14.9%). Table 1 lists approximate success rates with a number of common therapies.
Table 1. Approximate treatment success per protocol with susceptible strains (Western results)1.
| Therapy | Days | Success |
|---|---|---|
| Clarithromycin triple therapy | 7 | 94% |
| Clarithromycin triple therapy | 14 | 97% |
| Sequential therapy | 10 | 94% |
| Sequential therapy | 14 | 97% |
| Hybrid therapy | 14 | 97% |
| Fluoroquinolone triple | 7 | <80% |
| Fluoroquinolone triple | 10 | <90% |
| Fluoroquinolone triple | 14 | 96% |
| PPI + amoxicillin1 | 5 | 10% |
| PPI + amoxicillin1 | 7 | 15% |
| PPI + amoxicillin | 10 | 20% |
| PPI + amoxicillin1 | 14 | 50% |
| PPI metronidazole triple | 7 | 94% |
| PPI metronidazole triple | 14 | 97% |
| PPI- bismuth tetracycline, metronidazole | 14 | >95% |
=Equals triple therapies but with clari, met, or fluoroquinolone resistance infections
Effects of resistance
Triple therapies containing a PPI and amoxicillin plus clarithromycin, metronidazole, a fluoroquinolone, or rifabutin are all extremely sensitive to resistance to the third drug. Resistance to clarithromycin, fluoroquinolones and rifabutin can not be overcome by increasing the dose or duration. Using the formula above one can calculate that 7 day clarithromycin-containing triple therapy will fall below 90% success when clarithromycin resistance exceeds 5% (or 15% when the regimen is given for 14 days).
The 4 drug non-bismuth clarithromycin-containing sequential and concomitant therapies are extremely sensitive to dual clarithromycin-metronidazole resistance which reduces the regimens to contain only the PPI-amoxicillin component. Because the prevalence of dual resistance has such a great effect, it is important to consider how dual resistance might be acquired and what clinical factors might help predict its prevalence. Probably the most important variable is whether dual resistance is acquired from one encounter with both drugs or from separate encounters. For example, the prevalence of metronidazole resistance in many developing countries is >40% and often 80% or greater. In these countries both drugs are rarely given together and the prevalence of dual resistance will depend primarily on the prevalence of clarithromycin resistance such that the proportion with dual resistance will be approximately the same as the prevalence of clarithromycin resistance. In Nicaragua the prevalence of metronidazole resistance is at least 80% and thus dual resistance would exceed 15% whenever clarithromycin resistance exceeded 19% (i.e., 80% of 19 = 15.2%) 11. In Southern Europe metronidazole resistance is approximately 30% 12 and if acquisition of resistance to each drug were truly independent, clarithromycin resistance would need to exceed 50% to undermine 14 day concomitant therapy. However, even in low metronidazole resistance prevalence countries pockets of high prevalence metronidazole resistance often exist in which dual resistance may exceed 15% (e.g., in women where metronidazole is used for trichomonas infections, immigrants from developing countries, and patients who previously failed sequential or PPI-clarithromycin-metronidazole triple therapy). For such high risk groups, empiric concomitant or sequential therapies would likely be poor choices.
Recommended current regimens (Table 2)
Table 2. H. pylori therapies recommended for empiric therapy in Western countries1.
| For general use |
| 14 day concomitant therapy |
| 14 day bismuth quadruple therapy |
| 14 day hybrid sequential-concomitant therapy |
| Where clarithromycin-metronidazole dual resistance < 5% |
| 14 day sequential therapy |
| With fluoroquinolone resistance |
| 14 day fluoroquinolone triple therapy <13% |
| 14 day fluoroquinolone bismuth therapy <25% |
| 5 day fluoroquinolone concomitant therapy <20% |
| Salvage therapies (after 2 or more failures with different drug combinations) |
| Dependent on background rates of resistance and prior drug use by subject |
| One of the prior mentioned regimens |
| 14 day furazolidone bismuth quadruple therapy |
| One of the rifabutin regimens preferably for 14 days |
| Obsolete regimens for use only in special low resistance populations |
| 14 day clarithromycin-containing triple therapy |
| 14 day metronidazole-containing triple therapy |
| 10 day sequential therapy |
=These are recommendations for populations. See text for details of therapies and for modifications when considering an individual patient
Caveat
It should be recognized that the data pool from which the outcomes of various therapies with susceptible and resistant organisms are available is not large making the numbers we have used in our calculations imprecise and calculations only approximate. Sadly, this lack of data is related to the fact that resistance is not collected in most trials. Nonetheless, the results shown provide reasonable estimates of what can be expected and the appendix to the recent paper by Liou et al. 13 provides additional details, comparisons, and sensitivity analyses as well as a useful online calculator (https://hp-therapy.biomed.org.tw/) based on data from their comparison of 10 and 14 day sequential therapy and 14 day triple therapy in Taiwan.
Probably the most variable results are those regarding the expected outcomes of PPI-amoxicillin dual therapies. However, this group is generally represents only a small proportion of cases. The data used here is primarily derived from western studies which shows that 14 day dual therapy yields approximately 50% success and results above 50% are uncommon when using the doses and durations typically used with common therapies and success falls as the duration decreases. The actual results will depend in part on the effectiveness of the PPI in raising the intragastric pH to high levels (e.g., pH 6). PPI effectiveness depends in part on the PPI used, its dose and frequency of administration, the effects of CYP2C19 on the metabolism of the PPI 14 (and potentially some antibiotics) as well as the ability of the stomach to produce acid. The results reported here probably err slightly on the optimistic side but are consistent with the use of the formulas with data from clinical trials.
Concomitant therapy: (PPI-amoxicillin 1 g, clarithromycin 500 mg, metronidazole/tinidazole 500 mg, all b.i.d. for 14 days)
Meta-analyses have shown that the outcome is duration dependent 15, 16 and confirmed in a recent head-to-head comparison of 5 and 10 day concomitant therapies in Thailand where 5 day therapy proved unsatisfactory 17 and by failure of 5 day concomitant therapy in Central and South America (i.e., regions with known high levels of metronidazole resistance) 18. The Achilles heel of concomitant therapy is dual metronidazole-clarithromycin resistance. Fourteen day concomitant is a preferred initial empiric therapy for areas and patient groups where dual resistance is unlikely but is not recommended as a first line empiric regimen where metronidazole resistance is likely greater than 60% such as China, Iran, India, central and South America and populations at high risk of dual resistance (ie, following clarithromycin or metronidazole treatment failures).
Hybrid therapy: (PPI, amoxicillin 1 g for 14 days with amoxicillin 1 g, clarithromycin 500 mg, metronidazole/tinidazole 500 mg being given for the final 7 day, all b.i.d.)
Hybrid therapy combines sequential and concomitant therapy as all 4 drugs are given together. This is a new regimen with only a few studies 10, 19, 20. In a head to head comparison with 14 day concomitant therapy they appeared to be equivalent albeit hybrid therapy was more complicated. Further studies are needed to identify if there are important differences in relation to success in the face of different patterns of resistance. It could be considered in the same populations where concomitant therapy is recommended; 14 day hybrid therapy is expected to fall below 90% when clarithromycin-metronidazole resistance exceeds 9%.
Bismuth quadruple therapy: (PPI b.i.d., bismuth q.i.d., tetracycline HCl 500 mg q.i.d., metronidazole 500 mg t.i.d. for 14 days)
This is the oldest effective therapy and still one for which we do not know the optimal doses. With attention to detail regarding the doses and duration the primary Achilles heel is compliance. Tetracycline resistance is rare but currently many countries are experiencing a general unavailability of tetracycline. Generally doxycycline is not an adequate substitute.
Using this regimen at full doses and for 14 days one can expect 95% or greater treatment success irrespective the level of metronidazole resistance 21, 22. Therapy for 7 and likely 10 days is very susceptible to metronidazole resistance however the prevalence of resistance which results in a fall in outcome to below 90% is probably approximately 30% 23.
This regimen is also the one with the most unanswered questions regarding what are the optimal doses and frequencies of drug administration. For example, in Italy dosing only with the mid-day and evening meals was effective despite a dose reduction to one-half of recommend 24, 25. Treatment with resistant strains was less effective when administered at breakfast and the evening meal 26. Recent studies from China in a population with essentially universal metronidazole resistance also used twice a day bismuth and full q.i.d. doses and dosing intervals for the antibiotics with excellent results 22.
Because of the relative high rate of side effects, optimization is needed in terms of formulations, forms of bismuth, doses and dosing intervals as well as effectiveness in relation to the minimal inhibitory concentrations of metronidazole. Two caveats: the Etest overestimates the prevalence of metronidazole resistance such that resistance should always be confirmed (e.g., by agar dilution) for an accurate estimation of effectiveness in the presence of resistance 27, 28.
Therapies generally recommended only for low prevalence of resistance locations Clarithromycin-containing triple therapy: (PPI, amoxicillin 1 g, clarithromycin 500 mg, all b.i.d. for 14 days)
Despite the Maastricht IV recommendations, this is an obsolete therapy whether given for 7, 10, or 14 days 29. The Achilles heel is clarithromycin resistance with success depending on clarithromycin resistance and the duration of therapy (Tables 1 & 3). With 14 day therapy the combination remains effective until clarithromycin resistance exceeds approximately 15% whereas 7 day therapy is compromised by clarithromycin resistance exceeding 5%. Currently, there are few regions in the world where clarithromycin resistance is below 15% (i.e., 14-day regimen is still useful in such areas as Northern Europe and Thailand). Clarithromycin triple therapy has been superceded by 14 day concomitant therapy whose only Achilles heel is dual clarithromycin-metronidazole resistance.
Table 3. Achilles heels of individual common regimens1.
| Optimized therapies | Achilles heel1 |
|---|---|
| 14 day clarithromycin triple therapy | Clar®>15% |
| 14 day metronidazole triple therapy | Met® >15% |
| 14 day concomitant therapy | Clari®-Met® dual® >15% |
| 14 day sequential therapy | Clari®-Met® dual® >5% |
| 14 day hybrid therapy | Clari®-Met® dual® >9% |
| 14 day fluoroquinolone triple therapy | Levo® >13% |
| 14 day fluoroquinolone bismuth quadruple therapy | Levo® >25%2 |
| 14 day bismuth quadruple therapy | Tetracycline resistance (rare), compliance |
| 14 day bismuth-furazolidone therapy | Furazolidone resistance (rare), compliance |
| 5 day fluoroquinolone sequential therapy | Levo® ∼20%2 |
=The resistance level at which treatment success falls below 90%
=The number of subjects receiving these regimens is low such that the estimate is only approximate
Metronidazole-containing triple therapy: (PPI, amoxicillin 1 g, metronidazole/tinidazole 500 mg, all b.i.d. for 14 days)
The Achilles heel is metronidazole resistance and metronidazole-containing triple therapy is now rarely used except as a tailored therapy or in Japan where the general use of metronidazole has been strongly discouraged by the government because of possible genotoxicity 30, 31. Overall success parallels the experience with clarithromycin-containing triple therapy in relation to duration and to the presence of resistance.
Sequential therapy
(PPI – amoxicillin 1 g for 5 or 7 days followed by a PPI – clarithromycin 500 mg – metronidazole/tinidazole 500 mg all b.i.d., for 5 or 7 days). While 14 day sequential therapy provides better results than 10 day therapy, both have the same Achilles heels (i.e., dual resistance and metronidazole resistance) 13 (Table 3). Metronidazole resistance undermines 10 day sequential therapy when it reaches 20% and 14 day sequential therapy at approximately 30% (Table 4, Figure 3). The regimens are complicated successful use is restricted to regions where clarithromycin resistance is high and metronidazole resistance is low.
Table 4. Effect of metronidazole resistance on 10 and 14 day sequential and 14 day triple therapies.
| Treatment | 10 d sequential | 14 d sequential | 14 d triple | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scenario | Metronidazole resistance >20%; clarithromycin resistance=0 | Metronidazole resistance=20%; clarithromycin resistance=18% | Metronidazole resistance=30%; Clarithromycin resistance=6% | Metronidazole resistance = N/A Clarithromycin resistance=15% | ||||||||
| Pattern1 | Succ2 | %3 | #4 | Succ | % | # | Succ | % | # | Succ | % | # |
| Cs-Ms | 95% | 80 | 76 | 99% | 65.6 | 65 | 99% | 65.8 | 65 | 97% | 85 | 82.5 |
| Cr-Ms | 80% | 0 | 0 | 88% | 14.4 | 12.6 | 88% | 4.2 | 3.7 | 50% | 15 | 7.5 |
| Cs-Mr | 75% | 20 | 15 | 75% | 16.4 | 12.3 | 75% | 28.2 | 21 | n/a | - | --- |
| Cr-Mr | 10% | 0 | 0 | 15% | 3.6 | 0.5 | 15% | 1.8 | 0.3 | n/a | - | --- |
| Overall success | 91 | 90.4 | 90 | 90 | ||||||||
= Resistance pattern in population ranging from clarithromycin susceptible (Cs) and metronidazole susceptible (Ms) to dual resistance (Cr-Mr)
= Predicted treatment success for the pattern of resistance
= Percent of the study population with that pattern of resistance
= Success rate percent of the resistant pattern group with successful therapy
Table 4 shows that at 20% metronidazole resistance success with 10 day sequential therapy is approximately 90% PP and any level of clarithromycin resistance would cause it to fall further. In contrast, despite 20% metronidazole resistance, success with 14 day sequential therapy remains above 90% until clarithromycin resistance exceeds 18%. There are instances when 14 day triple therapy will be superior to sequential therapy as it is not affected by metronidazole resistance and can withstand up to 15% clarithromycin resistance before falling below 90% success. The primary Achilles heel for sequential therapy is metronidazole resistance (i.e., the level of metronidazole resistance determines the level of clarithromycin resistance required for success to fall below 90%). If metronidazole resistance is absent or low, sequential therapy for 10 or 14 days is very resistant to clarithromycin resistance (e.g., ∼30% for 10 day and ∼80% for 14 day) but in that instance 14 day metronidazole triple therapy or concomitant therapy would likely be better choices. Because 10 day sequential therapy fails when metronidazole resistance exceeds 20% or clarithromycin-metronidazole dual resistance is >5% sequential therapy has had a poor showing in Asia and South and Central America 13, 32 (e.g., in Taiwan 10 day sequential therapy achieved 78.9% success despite no clarithromycin resistance 13.
Fluoroquinolone-containing triple therapy: (PPI b.i.d., amoxicillin 1 g b.i.d., a fluoroquinolone once a day such as levofloxacin, moxifloxacin, or sitofloxacin, for 14 days)
Only 14 day therapy is successful as fluoroquinolone triple therapy and success is restricted to areas with low fluoroquinolone resistance. Fluoroquinolone resistance can not be overcome by increasing the dose or duration of triple therapy which becomes ineffective when resistance reaches 13%. Fluoroquinolone therapy is not recommended for patients who have received any fluoroquinolone in the past where fluoroquinolone resistance exceeds 10%. Possibly better fluoroquinolones-containing regimes include: fluoroquinolone-bismuth therapy and fluoroquinolone concomitant therapy. Neither has been optimized or tested widely and generally they should be used as tailored therapies (see below).
Fluoroquinolone bismuth therapy: (PPI b.i.d., amoxicillin 1g b.i.d., bismuth b.i.d., levofloxacin 500 mg once daily for 14 days)
This is basically the addition of bismuth to fluoroquinolone triple therapy. The addition of bismuth is estimated to maintain effectiveness with fluoroquinolone resistance as high as 25%. This regimen has also not been optimized or tested except in China but likely would be a better empirc choice than 14 fluorquinolone triple therapy in most regions.
Fluoroquinolone concomitant therapy: [PPI (esomeprazole 40 mg or equivalent), amoxicillin 1 g, levofloxacin 500 mg, tinidazole/metronidazole 500; all b.i.d. for 5 days]
This regimen has been calculated to remain effective with fluoroquinolone resistance below 20%–25%, or metronidazole resistance below 50% but would be ineffective if dual resistance exceeded approximately 10% 33. The regimen has only been reported in one study 34 and has not been optimized in terms of doses (likely 500 mg of levofloxacin would be sufficient) or duration.
Salvage therapies (after at least two treatment failures with different regimens) Furazolidone bismuth quadruple therapy
There a number of different formulations but most successful ones are based on bismuth quadruple therapy. One substitutes furazolidone (100 mg t.i.d.) for metronidazole in 14 day bismuth quadruple therapy. Another substitutes amoxicillin (1 g t.i.d.) for tetracycline. Both have proven highly effective in China 22 and may prove especially useful in areas where furazolidone is available and tetracycline is difficult to obtain.
Furazolidone is only available in a limited number of countries but it is a highly effective antimicrobial and resistance is generally low. Furazolidone is a monoamine oxidase inhibitor and interacts with numerous other drugs and foods such that an “avoidance sheet” should always be given to the patient to reduce the rate of unnecessary side effects 35. Where it is available it is an excellent salvage regimen but one where side effects are to be expected.
Rifabutin-containing regimens
Rifabutin is primarily used as an anti-tuberculosis drug. Resistance among H. pylori is rare. The initial trials particularly as a 7 day triple therapy proved disappointing 36 but several regimens are promising and it is expected that an optimized rifabutin will soon be identified for use especially as a salvage therapy. The original successful trial (i.e., 96.6%) was consisted of rifabutin 150 mg daily, amoxicillin 1.5 g t.i.d., pantoprazole 80 mg (or an equivalent PPI) t.i.d. for 12 days 37. We have used this regimen with success as a salvage therapy when given for 14 days. More recent studies have tested lower doses of amoxicillin and PPI (rifabutin 150 mg once daily, amoxicillin 1 g b.i.d., and esomeprazole 40 mg b.i.d. for 12 days with lower results 88.6% 38. Clearly additional studies are needed to optimize the regimen in terms of doses and duration. Finally, a recent study from Western Australia evaluated the combination of a PPI, bismuth, rifabutin and ciprofloxacin and reported an eradication rate of 95.2% in susceptible strains 39. The recent increase in fluoroquinolone resistance makes it unlikely that the combination will prove useful as other than a tailored regimen but it brings up the intriguing question regarding what improvement if any would be obtained by the addition of bismuth to rifabutin triple therapy 40.
Second or subsequent treatments for treatment failures
Generally one should have a two preferred “first line” regimens known to be effective locally with the choice between them being based on the patients history of prior drug use and exposure (Figure 2). The one with the highest predicted outcome should always be used first 41. Treatment success should always be confirmed generally using a non-invasive test for active infection such as the stool antigen or urea breath test 42. Confirmation of cure also provides the clinician with an early warning of the development of increasing resistance in the community.
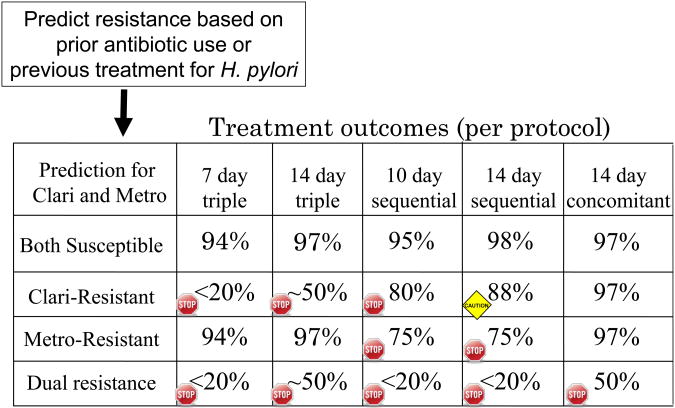
Figure 2.
Example of choices of clarithromycin-containing regimens for an individual patient based on predicted resistance to clarithomycin and metronidazole.
H. pylori is naturally resistant to many antimicrobials and rapidly has become resistant to others. The use of agents to which the organism is resistant to either as natural or acquired resistance has no effect on the outcome of therapy with agents to which the strain is susceptible. Prior use of an antibiotic for another often results in the H. pylori becoming resistant (a bystander effect) and clarithromycin and other macrolides, fluoroquinolones, and rifabutin should not be used again. Generally, amoxicillin and tetracycline can be reused as resistance rarely develops. The key outcome variable is whether the infecting strain is susceptible. In our experience the same high success rates are obtained with the first and the “nth-line” regimen provided the organisms are susceptible and good compliance is obtained. To the infection, all attempts with agents to which it is susceptible are first attempts. We do not know whether some patients may be more difficult to cure than others but, all things being equal, effectiveness of first line, first line alternative agents or even salvage therapies is similar if the organisms are susceptible. Repeated failures should prompt assessment of compliance and rare events such as the development of amoxicillin resistance.
Compliance/Adherence
Poor compliance with a regimen and antimicrobial resistance are the primary reasons for failure of what is otherwise a reliably excellent regimen. Large multicenter clinical trials have shown that although side effects related to the antibiotics used are common, in the majority of trials the drop out rates because of side effects are low (e.g., in the range of 5%). While there is considerable literature regarding compliance with medication use, treatment of H. pylori has not been a popular area of such research. The fact that H. pylori therapy often involves multiple drugs and multiple dosing intervals makes patient education extremely important. Emphasizing the importance of taking all the drugs as is generally done in large multicenter has repeatedly shown that this is associated a high degree of compliance despite the complexity of some regimens. When tested in a randomized trial, patient counseling and follow-up have been shown to improve outcome and compliance of H. pylori therapy and it is recommended 43. It is worthwhile to consider direct counseling regarding the regimen and the need to be compliant as well as to give handouts regarding the objectives and the details of the regimen. While it is important to try to keep patients on therapy despite side effects, it is also important to test for cure even if patients were unable to complete the regimen because even short course of therapy will result in the cure of a proportion of patients.
Recommendations regarding developing new regimens
The trial and error approach to the development of H. pylori therapies has proven to be inefficient and to provide misleading results. The history of sequential therapy is a good example Originally 10 day sequential therapy was devised in response to failure of triple therapy in Italy 44, 45 and it proved to be successful and superior to triple therapy 46. Unfortunately, it was presumed to be optimal and no further attempts were made to optimize it or to systematically examine its limitations. Rather, sequential therapy was repeatedly in the same population to “prove” its superiority to triple therapy. These multiple samplings were then combined in meta-analyses to confirm that at least, in that population, sequential therapy was superior to triple therapy 47-49. Importantly, the detrimental effect of clarithromycin resistance was noted, but the critical effect of metronidazole resistance on outcome remained unrecognized 32. When sequential therapy was tested in Southern Italy and other populations with higher metronidazole resistance it generally failed to achieve it prior success 32, 50, 51. The process took approximately 10 years during which thousands of subjects were randomized to triple therapy which had been repeatedly proven to provide unacceptable results 1, 52; many meta-analyses were done but the severe limitations of the regimen remained unrecognized. It was finally optimized in 2013 13. Additional details are available in the supplemental material.
Summary recommendations
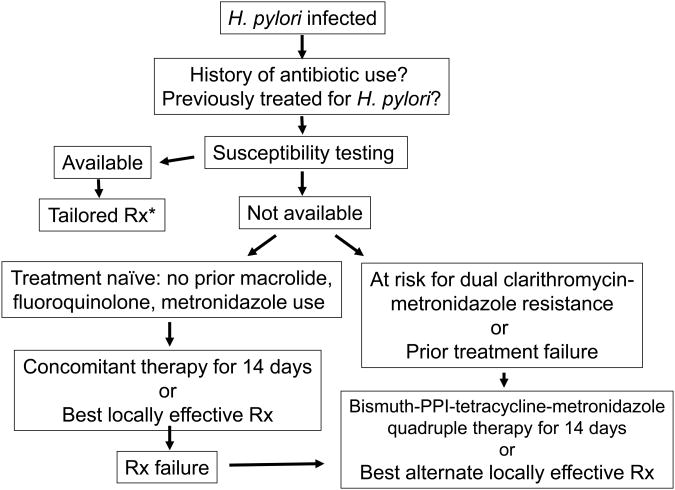
Sufficient data from treatment trials in which the outcomes in relation to susceptibility-resistance have been provided to allow an evidence-based approach to choosing anti-H. pylori therapies. We can now add to the admonition to use what works locally by being able to reliably identify which regimens have the greatest chance of working. Figure 1 outlines a general schema with therapy chosen based on pretreatment susceptibility testing, or if unavailable, based on a combination of local experience and information obtained from the patients and the patients' records. The data and discussions above generally focus on therapeutic choices for a population. Whether one considers an individual patient (i.e., population = n of 1) or a group the outcome variables are determined by resistance and compliance. Figure 3 shows how suspected resistance markedly influences choice for an individual subject (e.g., previous use of a macrolide or metronidazole would make triple or sequential therapy poor choices) such that what might be recommended for a population often differs when individualized to a single patient.
Figure 1. Recommended approach to treatment of H. pylori infections.
*Rx= treatment
Supplementary Material
Acknowledgments
Support: Dr. Graham is supported in part by the Office of Research and Development Medical Research Service Department of Veterans Affairs, Public Health Service grant DK56338 which funds the Texas Medical Center Digestive Diseases Center, DK067366 and CA116845. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the VA or NIH. Dr. Yi-Chia Lee and Dr. Ming-Shiang Wu received research grants from the National Science Council of Taiwan for research into the prevention of gastric cancer and the pathogenesis of Helicobacter pylori. Dr. Ming-Shiang Wu also received grants from the National Center of Excellence for Clinical Trial and Research in the National Taiwan University Hospital for the foundation of Taiwan Helicobacter Consortium.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Graham DY. Helicobacter pylori eradication therapy research: Ethical issues and description of results. Clin Gastroenterol Hepatol. 2010;8:1032–1036. doi: 10.1016/j.cgh.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Graham DY, Fischbach LA. Empiric therapies for Helicobacter pylori infections. CMAJ. 2011 doi: 10.1503/cmaj.101460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rimbara E, Fischbach LA, Graham DY. Optimal therapy for Helicobacter pylori infections. Nat Rev Gastroenterol Hepatol. 2011;8:79–88. doi: 10.1038/nrgastro.2010.210. [DOI] [PubMed] [Google Scholar]
- 4.Sheiner LB, Rubin DB. Intention-to-treat analysis and the goals of clinical trials. Clin Pharmacol Ther. 1995;57:6–15. doi: 10.1016/0009-9236(95)90260-0. [DOI] [PubMed] [Google Scholar]
- 5.Broutet N, Marais A, Lamouliatte H, de MA, Samoyeau R, Salamon R, Megraud F. cagA Status and eradication treatment outcome of anti-Helicobacter pylori triple therapies in patients with nonulcer dyspepsia. J Clin Microbiol. 2001;39:1319–1322. doi: 10.1128/JCM.39.4.1319-1322.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scaccianoce G, Hassan C, Panarese A, Piglionica D, Morini S, Zullo A. Helicobacter pylori eradication with either 7-day or 10-day triple therapies, and with a 10-day sequential regimen. Can J Gastroenterol. 2006;20:113–117. doi: 10.1155/2006/258768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki T, Matsuo K, Ito H, Sawaki A, Hirose K, Wakai K, Sato S, Nakamura T, Yamao K, Ueda R, Tajima K. Smoking increases the treatment failure for Helicobacter pylori eradication. Am J Med. 2006;119:217–224. doi: 10.1016/j.amjmed.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Taneike I, Nami A, O'connor A, Fitzgerald N, Murphy P, Qasim A, O'Connor H, O'Morain C. Analysis of drug resistance and virulence-factor genotype of Irish Helicobacter pylori strains: is there any relationship between resistance to metronidazole and cagA status? Aliment Pharmacol Ther. 2009;30:784–790. doi: 10.1111/j.1365-2036.2009.04095.x. [DOI] [PubMed] [Google Scholar]
- 9.Zullo A, Perna F, Hassan C, Ricci C, Saracino I, Morini S, Vaira D. Primary antibiotic resistance in Helicobacter pylori strains isolated in northern and central Italy. Aliment Pharmacol Ther. 2007;25:1429–1434. doi: 10.1111/j.1365-2036.2007.03331.x. [DOI] [PubMed] [Google Scholar]
- 10.Molina-Infante J, Romano M, Fernandez-Bermejo M, Federico A, Gravina AG, Pozzati L, Garcia-Abadia E, Vinagre-Rodriguez G, Martinez-Alcala C, Hernandez-Alonso M, Miranda A, Iovene MR, Pazos-Pacheco C, Gisbert JP. Optimized Non-Bismuth Quadruple Therapies Cure Most Patients with Helicobacter pylori Infection in Populations with High Rates of Antibiotic Resistance. Gastroenterology. 2013 Apr 3; doi: 10.1053/j.gastro.2013.03.050. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 11.Graham DY, Gonzalez C, Palacios C, Rodriguez J, Morales G, Lundin BS, Rabeneck L, Reddy R, Paszat L. Importance of determining the pattern of H.pylori resistance in countries with a high prevalence of gastric cancer such as Nicaragua. Helicobacter. 2011;11(Suppl 1):136. [Google Scholar]
- 12.Megraud F, Coenen S, Versporten A, Kist M, Lopez-Brea M, Hirschl AM, Andersen LP, Goossens H, Glupczynski Y. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut. 2013;62:34–42. doi: 10.1136/gutjnl-2012-302254. [DOI] [PubMed] [Google Scholar]
- 13.Liou JM, Chen CC, Chen MJ, Chen CC, Chang CY, Fang YJ, Lee JY, Hsu SJ, Luo JC, Chang WH, Hsu YC, Tseng CH, Tseng PH, Wang HP, Yang UC, Shun CT, Lin JT, Lee YC, Wu MS. Sequential versus triple therapy for the first-line treatment of Helicobacter pylori: a multicentre, open-label, randomised trial. Lancet. 2013;381:205–213. doi: 10.1016/S0140-6736(12)61579-7. [DOI] [PubMed] [Google Scholar]
- 14.Furuta T, Graham DY. Pharmacologic aspects of eradication therapy for Helicobacter pylori Infection. Gastroenterol Clin North Am. 2010;39:465–480. doi: 10.1016/j.gtc.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Essa AS, Kramer JR, Graham DY, Treiber G. Meta-analysis: four-drug, three-antibiotic, non-bismuth-containing “concomitant therapy” versus triple therapy for Helicobacter pylori eradication. Helicobacter. 2009;14:109–118. doi: 10.1111/j.1523-5378.2009.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gisbert JP, Calvet X. Review article: non-bismuth quadruple (concomitant) therapy for eradication of Helicobacter pylori. Aliment Pharmacol Ther. 2011;34:604–617. doi: 10.1111/j.1365-2036.2011.04770.x. [DOI] [PubMed] [Google Scholar]
- 17.Kongchayanun C, Vilaichone RK, Pornthisarn B, Amornsawadwattana S, Mahachai V. Pilot studies to identify the optimum duration of concomitant Helicobacter pylori eradication therapy in Thailand. Helicobacter. 2012;17:282–285. doi: 10.1111/j.1523-5378.2012.00953.x. [DOI] [PubMed] [Google Scholar]
- 18.Greenberg ER, Anderson GL, Morgan DR, Torres J, Chey WD, Bravo LE, Dominguez RL, Ferreccio C, Herrero R, Lazcano-Ponce EC, Meza-Montenegro MM, Pena R, Pena EM, Salazar-Martinez E, Correa P, Martinez ME, Valdivieso M, Goodman GE, Crowley JJ, Baker LH. 14-day triple, 5-day concomitant, and 10-day sequential therapies for Helicobacter pylori infection in seven Latin American sites: a randomised trial. Lancet. 2011;378:507–514. doi: 10.1016/S0140-6736(11)60825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sardarian H, Fakheri H, Hosseini V, Taghvaei T, Maleki I, Mokhtare M. Comparison of Hybrid and Sequential Therapies for Helicobacter pylori Eradication in Iran: A Prospective Randomized Trial. Helicobacter. 2013;18:129–134. doi: 10.1111/hel.12017. [DOI] [PubMed] [Google Scholar]
- 20.Hsu PI, Wu DC, Wu JY, Graham DY. Modified sequential Helicobacter pylori therapy: proton pump inhibitor and amoxicillin for 14 days with clarithromycin and metronidazole added as a quadruple (Hybrid) therapy for the final 7 days. Helicobacter. 2011;16:139–145. doi: 10.1111/j.1523-5378.2011.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salazar CO, Cardenas VM, Reddy RK, Dominguez DC, Snyder LK, Graham DY. Greater than 95% success with 14-day bismuth quadruple anti-Helicobacter pylori therapy: A pilot study in US Hispanics. Helicobacter. 2012;17:382–389. doi: 10.1111/j.1523-5378.2012.00962.x. [DOI] [PubMed] [Google Scholar]
- 22.Liang X, Xu X, Zheng Q, Zhang W, Sun Q, Liu W, Xiao S, Lu H. Efficacy of bismuth-containing quadruple therapies for clarithromycin-, metronidazole-, and fluoroquinolone-resistant Helicobacter pylori infections in a prospective study. Clin Gastroenterol Hepatol. 2013 Jan 29; doi: 10.1016/j.cgh.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Fischbach L, Evans EL. Meta-analysis: the effect of antibiotic resistance status on the efficacy of triple and quadruple first-line therapies for Helicobacter pylori. Aliment Pharmacol Ther. 2007;26:343–357. doi: 10.1111/j.1365-2036.2007.03386.x. [DOI] [PubMed] [Google Scholar]
- 24.Dore MP, Farina V, Cuccu M, Mameli L, Massarelli G, Graham DY. Twice-a-day bismuth-containing quadruple therapy for Helicobacter pylori eradication: a randomized trial of 10 and 14 days. Helicobacter. 2011;16:295–300. doi: 10.1111/j.1523-5378.2011.00857.x. [DOI] [PubMed] [Google Scholar]
- 25.Dore MP, Maragkoudakis E, Pironti A, Tadeu V, Tedde R, Realdi G, Delitala G. Twice-a-day quadruple therapy for eradication of Helicobacter pylori in the elderly. Helicobacter. 2006;11:52–55. doi: 10.1111/j.0083-8703.2006.00370.x. [DOI] [PubMed] [Google Scholar]
- 26.Graham DY, Belson G, Abudayyeh S, Osato MS, Dore MP, El-Zimaity HM. Twice daily (mid-day and evening) quadruple therapy for H. pylori infection in the United States. Dig Liver Dis. 2004;36:384–387. doi: 10.1016/j.dld.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 27.Osato MS, Reddy R, Reddy SG, Penland RL, Graham DY. Comparison of the Etest and the NCCLS-approved agar dilution method to detect metronidazole and clarithromycin resistant Helicobacter pylori. Int J Antimicrob Agents. 2001;17:39–44. doi: 10.1016/s0924-8579(00)00320-4. [DOI] [PubMed] [Google Scholar]
- 28.Osato MS, Graham DY. Etest for metronidazole susceptibility in H. pylori: use of the wrong standard may have led to the wrong conclusion. Am J Gastroenterol. 2004;99:769. doi: 10.1111/j.1572-0241.2004.04146.x. [DOI] [PubMed] [Google Scholar]
- 29.Malfertheiner P, Megraud F, O'Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T, el-Omar EM, Kuipers EJ. Management of Helicobacter pylori infection--the Maastricht IV/Florence Consensus Report. Gut. 2012;61:646–664. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 30.Hori K, Miwa H, Matsumoto T. Efficacy of 2-week, second-line Helicobacter pylori eradication therapy using rabeprazole, amoxicillin, and metronidazole for the Japanese population. Helicobacter. 2011;16:234–240. doi: 10.1111/j.1523-5378.2011.00842.x. [DOI] [PubMed] [Google Scholar]
- 31.Graham DY, Rimbara E. Helicobacter pylori therapy in the west. Japanese J Helicobacter Res. 2012;13:4–9. [PMC free article] [PubMed] [Google Scholar]
- 32.Gisbert JP, Calvet X, O'connor A, Megraud F, O'Morain CA. Sequential therapy for Helicobacter pylori eradication: A critical review. J Clin Gastroenterol. 2010;44:313–325. doi: 10.1097/MCG.0b013e3181c8a1a3. [DOI] [PubMed] [Google Scholar]
- 33.Graham DY, Shiotani A. Which Therapy for Helicobacter pylori Infection? Gastroenterology. 2012;143:10–12. doi: 10.1053/j.gastro.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Federico A, Nardone G, Gravina AG, Iovene MR, Miranda A, Compare D, Pilloni PA, Rocco A, Ricciardiello L, Marmo R, Loguercio C, Romano M. Efficacy of 5-day levofloxacin-containing concomitant therapy in eradication of Helicobacter pylori infection. Gastroenterology. 2012;143:55–61. doi: 10.1053/j.gastro.2012.03.043. [DOI] [PubMed] [Google Scholar]
- 35.Graham DY, Lu H. Furazolidone in Helicobacter pylori therapy: Misunderstood and often unfairly maligned drug told in a story of French bread. Saudi J Gastroenterol. 2012;18:1–2. doi: 10.4103/1319-3767.91724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gisbert JP, Calvet X. Review article: rifabutin in the treatment of refractory Helicobacter pylori infection. Aliment Pharmacol Ther. 2012;35:209–221. doi: 10.1111/j.1365-2036.2011.04937.x. [DOI] [PubMed] [Google Scholar]
- 37.Borody TJ, Pang G, Wettstein AR, Clancy R, Herdman K, Surace R, Llorente R, Ng C. Efficacy and safety of rifabutin-containing ‘rescue therapy’ for resistant Helicobacter pylori infection. Aliment Pharmacol Ther. 2006;23:481–488. doi: 10.1111/j.1365-2036.2006.02793.x. [DOI] [PubMed] [Google Scholar]
- 38.Fiorini G, Vakil N, Zullo A, Saracino IM, Castelli V, Ricci C, Zaccaro C, Gatta L, Vaira D. Culture-based selection therapy for patients who did not respond to previous treatment for Helicobacter pylori infection. Clin Gastroenterol Hepatol. 2013;11:507–511. doi: 10.1016/j.cgh.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 39.Tay CY, Windsor HM, Thirriot F, Lu W, Conway C, Perkins TT, Marshall BJ. Helicobacter pylori eradication in Western Australia using novel quadruple therapy combinations. Aliment Pharmacol Ther. 2012;36:1076–1083. doi: 10.1111/apt.12089. [DOI] [PubMed] [Google Scholar]
- 40.Graham DY, Gisbert JP. Helicobacter pylori: tailored therapy with novel sequential quadruple therapies. Nat Rev Gastroenterol Hepatol. 2013;10:6–8. doi: 10.1038/nrgastro.2012.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Graham DY, Calvet X. Guide regarding choice of second-line therapy to obtain a high cumulative cure rate. Helicobacter. 2012;17:243–245. doi: 10.1111/j.1523-5378.2012.00952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Attumi TA, Graham DY. Follow-up testing after treatment of Helicobacter pylori infections: Cautions, caveats, and recommendations. Clin Gastroenterol Hepatol. 2011;9:373–375. doi: 10.1016/j.cgh.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 43.Al-Eidan FA, McElnay JC, Scott MG, McConnell JB. Management of Helicobacter pylori eradication--the influence of structured counselling and follow-up. Br J Clin Pharmacol. 2002;53:163–171. doi: 10.1046/j.0306-5251.2001.01531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buzas GM. Gastric tubes as vectors of Helicobacter pylori transmission. Med Hypotheses. 2010;75:47–49. doi: 10.1016/j.mehy.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 45.Laheij RJ, Rossum LG, Jansen JB, Straatman H, Verbeek AL. Evaluation of treatment regimens to cure Helicobacter pylori infection- a meta-analysis. Aliment Pharmacol Ther. 1999;13:857–864. doi: 10.1046/j.1365-2036.1999.00542.x. [DOI] [PubMed] [Google Scholar]
- 46.Zullo A, Rinaldi V, Winn S, Meddi P, Lionetti R, Hassan C, Ripani C, Tomaselli G, Attili AF. A new highly effective short-term therapy schedule for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2000;14:715–718. doi: 10.1046/j.1365-2036.2000.00766.x. [DOI] [PubMed] [Google Scholar]
- 47.Jafri NS, Hornung CA, Howden CW. Meta-analysis: sequential therapy appears superior to standard therapy for Helicobacter pylori infection in patients naive to treatment. Ann Intern Med. 2008;148:923–931. doi: 10.7326/0003-4819-148-12-200806170-00226. [DOI] [PubMed] [Google Scholar]
- 48.Gatta L, Vakil N, Leandro G, Di MF, Vaira D. Sequential therapy or triple therapy for Helicobacter pylori infection: systematic review and meta-analysis of randomized controlled trials in adults and children. Am J Gastroenterol. 2009;104:3069–3079. doi: 10.1038/ajg.2009.555. [DOI] [PubMed] [Google Scholar]
- 49.Zullo A, De F V, Hassan C, Morini S, Vaira D. The sequential therapy regimen for Helicobacter pylori eradication: a pooled-data analysis. Gut. 2007;56:1353–1357. doi: 10.1136/gut.2007.125658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iovene MR, Romano M, Pilloni AP, Giordano B, Montella F, Caliendo S, Tufano MA. Prevalence of Antimicrobial Resistance in Eighty Clinical Isolates of Helicobacter pylori. Chemotherapy. 1999;45:8–14. doi: 10.1159/000007159. [DOI] [PubMed] [Google Scholar]
- 51.Romano M, Cuomo A, Gravina AG, Miranda A, Iovene MR, Tiso A, Sica M, Rocco A, Salerno R, Marmo R, Federico A, Nardone G. Empirical levofloxacin-containing versus clarithromycin-containing sequential therapy for Helicobacter pylori eradication: a randomised trial. Gut. 2010;59:1465–1470. doi: 10.1136/gut.2010.215350. [DOI] [PubMed] [Google Scholar]
- 52.Pounder RE, Talley NJ. Letter: the ethics of using inferior regimens in H. pylori randomised trials - editors' reply. Aliment Pharmacol Ther. 2012;35:858. doi: 10.1111/j.1365-2036.2011.04944.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.