Abstract
Objective
To determine incidence rates and predictors of epilepsy after childhood stroke and compare these to published estimates of 3–5% cumulative epilepsy incidence by five years post-stroke in adults.
Methods
In a retrospective population-based study of children with stroke (29 days−19 years) in an integrated health care system (1993–2007), post-stroke seizures were identified through electronic searches and confirmed by chart review. Stroke and seizure characteristics were abstracted from medical records. Survival analysis was used to determine rates and predictors of remote seizures and active epilepsy (anti-convulsant treatment for remote seizure within prior 6 months) at last follow-up.
Results
From a population of 2.5 million children, we identified 305 stroke cases. Over a median follow-up of 4.1 years (interquartile range 1.8–6.8), 49 children had a first unprovoked remote seizure. The average annual incidence rate of first remote seizure was 4.4% (95% confidence interval [CI] 3.3, 5.8) with a cumulative risk of 16% (CI 12%, 21%) at 5 years and 33% (CI 23%, 46%) at 10 years post-stroke. The cumulative risk of active epilepsy was 13% (CI 9%, 18%) at five years and 30% (CI 20%, 44%) at 10 years. Acute seizures at the time of stroke predicted development of active epilepsy (hazard ratio [HR] 4.2, CI 2.2, 8.1). At last follow-up, one-third of the children with active epilepsy had a recent breakthrough seizure despite anti-convulsant usage.
Interpretation
Unlike adults, children are uniquely vulnerable to epilepsy after stroke. Children with acute seizures at the time of stroke are at particularly high risk.
Introduction
Stroke occurs in an estimated 3.8 per 100,000 children annually, 1–3 and is an important cause of childhood brain injury and epilepsy. Estimates of seizure incidence after childhood stroke vary widely, in part due to variation in study referral populations and limitations of scope, sample size and length of follow-up. 4–9 In adults, remote seizures after stroke are relatively infrequent, and studies have suggested that stroke type and location influence their likelihood.10–12 Despite a growing body of literature on childhood stroke, epidemiologic data regarding the frequency, severity and predictors of remote seizures and epilepsy due to stroke in childhood are limited.
Currently, the frequency of remote seizures and epilepsy after stroke in children may be underestimated by neurologists because of extrapolation from stroke studies that demonstrate few adults develop post-stroke epilepsy. Accurate estimates of post-stroke seizure incidence and measurements of epilepsy severity in children are needed to offer accurate prognoses to families, assess the magnitude of the problems posed by post-stroke seizures to society, and design effective health care and service programs for children afflicted by stroke.13 Further, characterizing the children at greatest risk is important so that potentially vulnerable children can be targeted for closer follow-up and future studies of epileptogenesis and epilepsy prevention.
We hypothesized that children frequently develop remote seizures and epilepsy after a stroke, and that clinical factors such as age, stroke type, stroke location and acute seizures at the time of stroke affect their risk. We examined a large, population-based childhood stroke cohort to measure the incidence rate for first remote seizure post-stroke and determined clinical predictors. We also examined children with active epilepsy at last follow-up and described the severity of epilepsy in those children.
Methods
Study design, setting and population
We conducted a retrospective study of remote seizures and epilepsy within a population-based cohort of children with stroke enrolled in Kaiser Permanente Northern California (KPNC). All study procedures were approved by the Institutional Review Boards at KPNC and the University of California, San Francisco. KPNC is an integrated health care system that cares for about one third of the population in Northern California. KPNC electronic medical records include all outpatient and inpatient visits; encounters at outside facilities are also captured through the billing process. Electronic medical record coding is performed by the treating physician (for outpatient visits) or by professional coders reviewing admission records. All medications prescribed and filled are recorded in the KPNC electronic pharmacy database.
The study population included all children through 19 years of age enrolled in KPNC, January 1993– December 2007. From this population base, a cohort of children diagnosed with symptomatic stroke was identified in the Kaiser Pediatric Stroke Study (KPSS). Methods of case identification and characteristics of the cohort, including stroke etiology, have been previously described.14–17 The criteria for stroke were: (1) documented clinical presentation consistent with stroke, such as a sudden onset focal neurological deficit, headache, or seizure; and (2) computed tomography or magnetic resonance imaging showing a focal ischemic infarct or hemorrhage in a location and of a maturity consistent with the neurological signs and symptoms. KPSS excluded cases of sub-dural and epidural hematomas and strokes that occurred outside of the study period. For the current seizure study, neonatal strokes (strokes that occurred before 29 days of life) and children who died during their stroke hospitalization were excluded.
Data abstraction
A single pediatric nurse professional medical record analyst abstracted demographic and clinical data from electronic and traditional medical records. For children with a confirmed remote seizure, the same analyst abstracted additional data pertaining to seizures. All abstracted data were reviewed for accuracy by a pediatric neurologist.
Remote seizure ascertainment
To determine the primary outcome of remote seizure, we first electronically searched for International Classification of Diseases, Ninth Revision (ICD-9) codes related to seizure and epilepsy in inpatient or outpatient databases, and searched the pharmacy database for prescriptions of anti-convulsant medications filled > one month after stroke. Two child neurologists then independently reviewed all potential cases to confirm the remote seizure. A third neurologist adjudicated in case of reviewer disagreement.
Definitions
Remote seizure was defined as at least one documented unprovoked seizure occurring > 30 days after stroke. In two cases of acute ischemic strokes, the event date was not clear from the medical record, so the date of evaluation was used for the date of stroke onset. Unprovoked seizures were seizures that were not in close temporal association or attributable to an acute systemic, metabolic or toxic insult (such as fever, hypoglycemia or other electrolyte disturbances) or an acute central nervous system insult (such as a recurrent stroke) after chart review by a neurologist. We defined active epilepsy as at least one unprovoked remote seizure and ongoing anti-convulsant treatment or less than 6 months seizure free off of anti-convulsants at the time of the last follow-up. This is consistent with the International League Against Epilepsy definition of active epilepsy as “a person who is either currently being treated for epilepsy or whose most recent seizure has occurred within a time interval usually defined as the past 2 or 5 years… but the time should be specified.”13
Predictors
Age (in years) was analyzed as a continuous variable. Acute seizure was defined as the presence of a clinical seizure documented at the time of stroke presentation. Neurologic deficit at hospital discharge was defined as any neurologic deficit documented at the time of the patient’s discharge from the acute stroke hospitalization. Location was defined by documentation in radiology reports. These categories were not mutually exclusive; a single stroke case could have multiple locations. Stroke type indicated stroke classification after chart review by a vascular neurologist (HJF) into mutually exclusive categories: any intraparenchymal hemorrhage, subarachnoid hemorrhage/intraventricular hemorrhage (SAH/IVH), arterial ischemic stroke or venous sinus thrombosis. Laterality classified side of stroke (left, right, bilateral or none) documented in radiology reports. Pure SAH/IVH and some venous sinus thromboses were listed as “none” if no lateralizing components of the stroke were identified.
Analysis
We used summary statistics to describe characteristics of the stroke cohort and compare groups stratified by onset of remote seizures, with non-parametric tests for age and length of follow-up. We used survival analysis to determine incidence rates and cumulative risk of remote seizure and active epilepsy, with time at risk beginning thirty days after stroke ictus. The first remote seizure was the failure event; children were also censored from the analysis at death or the last clinical follow-up in the KPNC system. We also used the first remote seizure as the failure event in analyses of active epilepsy. Children with a history of seizure prior to stroke were excluded from the survival analyses. Cox proportional hazards models were used to determine univariate predictors of children with remote seizures and active epilepsy due to stroke, using the log-rank test for statistical significance. Our multivariable model included univariate predictors of remote seizure with P<0.2, as well as predetermined demographic factors (sex and race). An interaction variable of “acute seizure” and “age at stroke” was used to examine independence of these clinical predictors of remote seizure and active epilepsy, but was not included in the final multivariable models because the interaction term did not reach significance (defined as P<0.2).
Results
Description of stroke cohort
From a study base of 2.5 million children (11.5 million person-years at risk), we identified 322 children with a non-neonatal stroke (2.9 strokes per 100,000 person-years). Of these, 17 died during the acute hospitalization, leaving 305 for inclusion in our final study cohort: 140 ischemic and 165 hemorrhagic strokes. Median age at the time of the stroke was 13.1 years (interquartile range [IQR] 6, 17.1), and the cohort was 41% Caucasian and 57% male. At stroke presentation, 27% (80/293, 12 missing) of the cases had an acute seizure and 60% (183/303, 2 missing) had a neurologic deficit documented at hospital discharge. Children who had a seizure at the time of the stroke were younger, with a median age of 8.4 (IQR 1.3, 16.2) years compared to 13.6 (IQR 7.8, 17.3) years among those who did not have an acute seizure (P<0.003). Median length of post-stroke follow-up was 4.1 years (IQR 1.8–6.8) with a total of 1111 person-years at risk for remote seizures.
Post-stroke incidence of remote seizure and active epilepsy
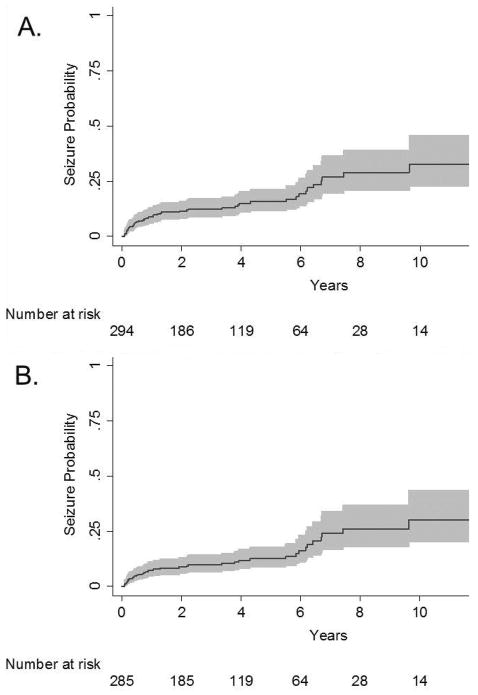
Seven children with remote seizures had a history of seizures prior to stroke and were excluded from survival analyses. We identified remote seizures in 49 children with no history of seizure prior to stroke. Of these, 40 children had active epilepsy at the time of last follow-up. The average annual incidence rate of a first remote seizure post-stroke was 4.4% (95% confidence interval [CI] 3.3, 5.8) with a 5-year cumulative risk of 16% (95% CI 12%, 21%) and 10 year cumulative risk of 33% (95% CI 23%, 46%) (Figure 1A). The average annual incidence rate of first remote seizure among those with active epilepsy at last follow-up was 3.6% (95% CI 2.6, 4.9%), with a 5 year cumulative risk of 12% (95% CI 9%, 18%) and a 10 year cumulative risk of 30% (95% CI 20%, 44%) (Figure 1B).
Figure 1.
Among children with stroke enrolled in Kaiser Permanente Northern California, 1993–2007, Kaplan-Meier plot demonstrating failure function (solid line) and 95% confidence intervals (gray shading) for (A) first remote seizure, and (B) first remote seizure among those with active epilepsy at last follow-up. The x-axis is time from 30 days post-stroke.
Predictors of remote seizure and active epilepsy
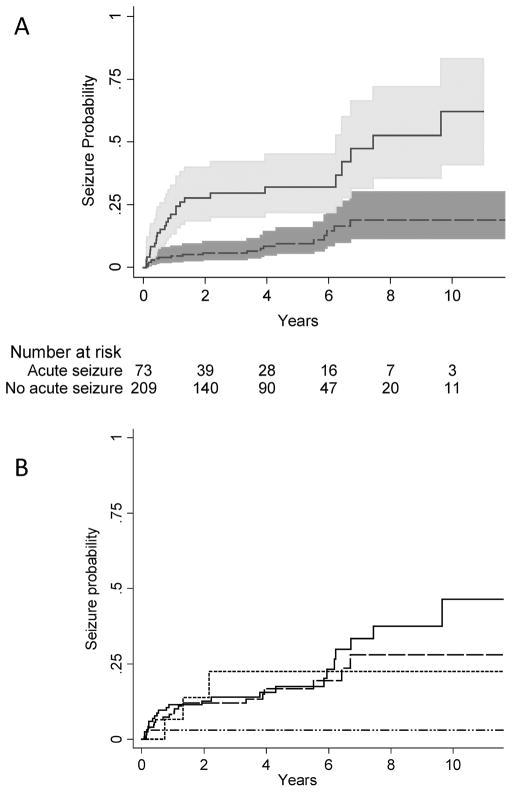
Children who had an acute sseizure at the time of stroke were four times as likely to have a remote seizure compared to children who did not have a seizure at the time of their stroke (hazard ratio [HR] 4.1, 95% CI 2.3, 7.3) (Figure 2A, Table 1). The average annual incidence rate of remote seizure among children who had an acute seizure was 10.6% compared to 2.5% for those without acute seizure, for an incidence rate difference of 8.1% (95% CI 3.8%, 12.2%). On univariate analysis, younger age also predicted remote seizures: for each 1 year increase in age at the time of stroke onset, the hazard ratio decreased by 4.3% (HR 0.96, 95% CI 0.92, 0.99). In our multivariate model, the hazard ratio for acute seizure was slightly attenuated (HR 3.5, 95% CI 1.9, 6.6) but remained a strong independent predictor of remote seizure, while age did not (Table 2). The cumulative incidence of first remote seizure was lowest among children with SAH/IVH (Figure 2B), although estimates stratified by stroke type had wide confidence intervals and the difference in estimates did not reach statistical significance. Excluding children with SAH/IVH, the cumulative incidence of first remote seizure was 4.9% (95% CI 4%, 7%) at 1 year, 17% (95% CI 13%, 24%) at 5 years and 37% (95% CI 26%, 51%) at 10 years. Younger age (HR 0.95, 95% CI 0.9, 1.0) and acute seizures (HR 4.1, 95% CI 2.1, 7.8) were also univariate predictors of active epilepsy, but only acute seizures predicted active epilepsy (HR 3.5, 95% CI 1.7, 7.1) in our multivariable model. Among children with acute seizures, the cumulative risk of active epilepsy was 25% (95% CI 16%, 38%) by five years.
Figure 2.
Among children with stroke enrolled in Kaiser Permanente Northern California, 1993–2007, Kaplan-Meier plots demonstrating (A) a four-fold (HR 4.1, 95% CI 2.3, 7.3) increased risk of remote seizure among children with acute seizures (solid line, 95% confidence interval in light gray shading) compared to children without acute seizures (dashed line, 95% confidence interval in dark gray shading); and (B) the cumulative incidence of remote seizure stratified by stroke type: arterial ischemic stroke (solid line), intraparenchymal hemorrhage (large dashes), venous sinus thrombosis (small dashes) SAH/IVH (dash and dots). The x-axis is time from 30 days post-stroke.
Table 1.
Unadjusted hazard ratios for remote seizure after a stroke among children enrolled in Kaiser Permanente Northern California, 1993–2007. Location categories were not mutually exclusive.
| No remote seizure n/N (%) | Remote seizure n/N % | Unadjusted HR (95% CI) | Log-Rank P | |
|---|---|---|---|---|
| Age at stroke, median (IQR) | 13.4 (7.0, 17) | 9.8 (1.0, 17) | 0.96 (0.92, 0.99) | <0.0001 |
| Male sex | 141/249 (57) | 32/49 (65) | 1.4 (0.8, 2.6) | 0.2 |
| Caucasian race | 99/249 (40) | 20/49 (41) | 0.9 (0.5, 1.6) | 0.8 |
| Acute seizure | 48/239 (20) | 26/47 (55) | 4.1 (2.3, 7.3) | <0.0001 |
| Neurologic deficit at discharge | 144/247 (58) | 36/49 (73) | 1.7 (0.9, 3.2) | 0.1 |
| Location | ||||
| frontal | 84/249 (34) | 17/49 (35) | 1.1 (0.6, 1.9) | 0.8 |
| parietal | 70/249 (28) | 20/49 (41) | 1.7 (1.0, 3.0) | 0.1 |
| temporal | 54/249 (22) | 13/49 (27) | 1.4 (0.7, 2.7) | 0.3 |
| occipital | 33/249 (13) | 10/49 20) | 1.6 (0.8, 3.3) | 0.2 |
| cerebellum | 20/249 (8) | 2/49 (4) | 0.6 (0.1, 2.5) | 0.5 |
| Stroke type | 1.1 (0.9, 1.3) | 0.1 | ||
| Intraparenchymal hemorrhage | 108/249 (43) | 20/49 (41) | ||
| Arterial ischemic stroke | 96/249 (39) | 24/49 (49) | ||
| SAH/IVH | 32/249 (13) | 1/49 (2) | ||
| Venous sinus thrombosis | 13/249 (5) | 4/49 (8) | ||
| Laterality | 1.2 (0.8, 1.6) | 0.4 | ||
| left | 91/245 (37) | 21/49 (43) | ||
| right0 | 76/245 (31) | 18/49 (37) | ||
| bilateral | 40/245 (16) | 5/49 (10) | ||
| none | 38/245 (16) | 5/49 (10) | ||
Table 2.
Adjusted hazard ratios for remote seizure after a stroke among children enrolled in Kaiser Permanente Northern California, 1993–2007. The adjusted model included univariate predictors of remote seizure with P<0.2 and predetermined demographic factors (sex and race).
| HR (95% CI) | P | |
|---|---|---|
| Age at stroke (Years) | 1.0 (0.9, 1.0) | 0.5 |
| Male sex | 1.6 (0.9, 2.9) | 0.1 |
| Caucasian race | 1.1 (0.6, 2.1) | 0.7 |
| Acute seizure | 3.5 (1.9, 6.6) | < 0.001 |
| Neurologic deficit at discharge | 1.5 (0.8, 2.9) | 0.2 |
| Parietal location | 1.5 (0.8, 2.7) | 0.2 |
| Stroke type | 1.0 (0.8, 1.2) | 0.9 |
Measures of epilepsy severity
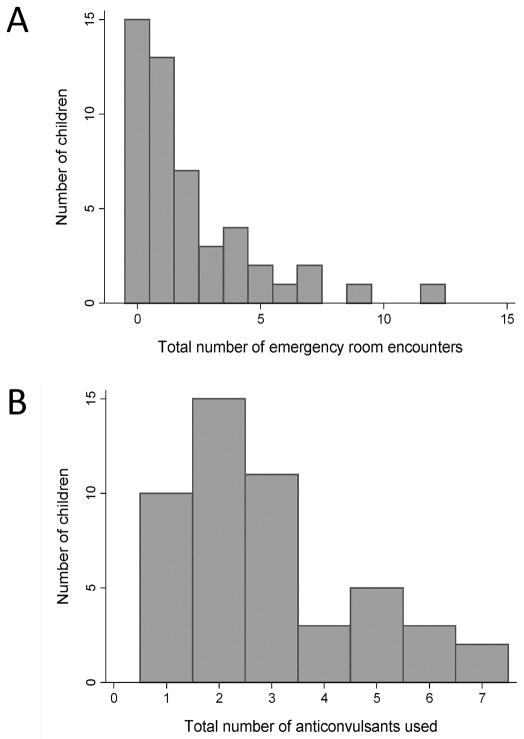
Among the 49 children with a remote seizure, 34 were seen in an emergency department at least once and 21 had multiple emergency encounters for seizure (Figure 3A). Fifteen children had been admitted to a hospital for a seizure, and 4 had been intubated and admitted to an ICU because of an episode of status epilepticus. All children with remote seizures after stroke were ultimately treated with an anti-convulsant medication. Most had been treated with at least two different anti-convulsants (range 1–7) during the follow-up period (Figure 3B). At last follow-up 9 children were on polytherapy with more than one concurrent anti-convulsant. Despite anti-convulsant usage, 13 (33%) of the children with active epilepsy had at least one breakthrough seizure in the month prior to the last follow-up and 8 were having multiple seizures per month.
Figure 3.
Histogram plots demonstrating measures of epilepsy severity among 49 children with remote seizure after stroke. Children were stratified by: (A) the number of emergency room encounters for seizure during the follow-up period, and (B) the number of separate anti-convulsants prescribed during the follow-up period.
Discussion
In this population-based childhood stroke cohort, one out of 6 children suffering from stroke had a remote seizure by 5 years, and this increased to one out of three children by 10 years post-stroke. Prior pediatric stroke studies have reported proportions of children with remote seizures or epilepsy ranging from 7% to 29%, although these studies were limited by variable duration of follow-up or losses to follow-up, and were not population-based. 5–9, 18, 19 A recent study of perinatal and childhood intracerebral hemorrhage found 13% of children developed epilepsy by two years. Several large population-based studies of post-stroke seizures in adults have used survival analysis techniques; in spite of varying definitions of late seizures and post-stroke epilepsy, the range of 3–5% cumulative incidence by five years20,10,11,21,22, 23 is relatively consistent (Table 3). While different definitions across studies might influence incidence rates, all of the comparable definitions result in lower estimates in the adult studies, suggesting that the developing brain may be more susceptible than the mature brain to remote seizures and epilepsy after an injury.
Table 3.
Comparison of incidence rate estimates of remote seizure after stroke from the current and previously published population-based studies. More than one seizure outcome was reported in some studies. Age is in years. AED = anti-convulsant.
| Authors | Seizure outcome | Estimated incidence rate | Mean age (range) | Stroke study, N |
|---|---|---|---|---|
| Kammersgaard, et al10 | recurrent seizures after stroke + AED | 1% by 3 years | 73 (All ages) | Copenhagen Stroke Study, N = 1195 |
| Chen, et al20 | 2 ICD-9 diagnoses in ambulatory care claims | 3% by 5 years | 63 (20–80) | Taiwan Health Insurance Database, N = 4126 |
| Lossius, et al21 | 1 seizure > 1 week after stroke recurrent seizures > 1 week after stroke |
3% by 1 year 3% by 7 years |
76 (>60) | Askershus Stroke Study, N = 484 |
| Burn, et al11 | 1 seizure > 1 month after stroke recurrent seizures > 24 hours after stroke |
4% by 5 years 4% by 2 years |
72 (All ages) | Oxfordshire Community Stroke Project, N = 675 |
| So, et al22 | 1 seizure > 1 week after stroke recurrent seizures > 1 week after stroke |
5% by 5 years 4% by 5 years |
72 (All ages) | Rochester, Minnesota, N = 535 |
| Viitanen, et al23 | recurrent seizures > 1 month after stroke + AED | 5% at 5 years | 72 (All ages) | Umea, Sweden, N = 409 |
| Fox, et al | 1 seizure > 1 month after stroke 1 seizure > 1 month after stroke + AED |
16% by 5 years 13% by 5 years |
11 (0.1–19) | Kaiser Pediatric Stroke Study, N = 305 |
We found that children with acute seizures at the time of stroke represent a particularly vulnerable subgroup with four times the risk of remote seizures; one-quarter of these children develop active epilepsy within five years. The greatest risk for a first remote seizure was within the first year after stroke, which may suggest a time period that warrants close clinical follow-up for seizure. Our findings are consistent with two adult stroke studies10, 11 and a recent childhood stroke study, which defined “epilepsy” as a seizure after discharge and treatment with an anticonvulsant: at six months after stroke, epilepsy was found in 5 of 21 children who presented with acute seizures and none of the 44 children without acute seizures.4
Studies that have examined age at the time of stroke in adults as a predictor of post-stroke epilepsy report conflicting results. Younger age was associated with post-stroke epilepsy in the Copenhagen Stroke Study, but the Askershus Stroke Study found no association.10, 21 We found that younger age was associated with remote seizures after childhood stroke on univariate analysis, but was no longer significant in multivariable analysis. This is likely due to the fact that younger age was highly correlated with risk of acute seizure at time of stroke in our cohort; therefore, adjustment for acute seizure in multivariable analysis removed the age effect.
Several of the children were treated with multiple anti-convulsant medications, which we speculate was likely related to either poor seizure control or unacceptable medication side effects. A third of the children who were on treatment with an anti-convulsant at last follow-up had a seizure within the month prior to last follow-up, suggesting treatment-refractory epilepsy. The multiple emergency room visits and hospitalizations for post-stroke epilepsy among the children in our cohort are further evidence of the heavy burden of disease that post-stroke epilepsy places on both individual families and society.
The cumulative risk and timing of remote seizures in our cohort are comparable to the patterns of remote seizures found after traumatic brain injury in children24, 25 and adults. 26, 27 Studies of traumatic brain injury in children suggest that in this group, early seizures and younger age may also predict remote epilepsy24, 28, 29 Post-traumatic epilepsy has recently received increasing attention because of its high incidence and its delayed but possibly predictable onset, suggesting a potential window for intervention and prevention.30 Post-stroke epilepsy in children appears to similarly manifest a delayed but predictable onset, presenting an opportunity for intervention if an agent to prevent epilepsy after acquired brain injury can be identified. The potential for intervention is important because even without a stroke, children with epilepsy are at an increased risk for comorbid physical, developmental and behavioral problems.31 Further, current literature suggests that children who develop epilepsy after a stroke are more likely to have poor neurodevelopmental outcomes compared with children who do not develop post-stroke epilepsy,32–37 even after accounting for stroke location.37 We do not know yet whether epilepsy is a marker for more severe initial brain injury or whether stroke recovery is impaired by seizures, interictal epileptiform discharges, or anti-convulsant treatment.
Our study had limitations. First, we were limited in assessing recurrent seizures because many of the children were treated with an anti-convulsant after the first remote seizure. We addressed this limitation by measuring “active epilepsy” – an outcome which should provide a more conservative estimate of epilepsy risk by limiting the outcome to those who had ongoing clinical treatment with an anticonvulsant. Secondly, because our initial screen for post-stroke seizure outcomes used electronic data, it is possible that some outcomes may have been missed if no seizure or epilepsy diagnostic codes were given and anti-convulsant medications were not filled through the integrated health care pharmacy. Missed cases would result in underestimating the incidence rate of remote seizures. Finally, the socio-demographic and health characteristics of the adult Kaiser population base are comparable to the Northern California adult population, and data obtained from KPNC is considered generalizable to wider populations.38 While it is likely that this is also true for children enrolled in Kaiser, it is possible that the Kaiser pediatric population may not be similarly generalizable.
Despite these limitations, our study provides strong evidence that remote seizures and epilepsy are common after stroke in children, particularly among children with acute seizures. The frequency of remote seizures after pediatric stroke has important implications for both public health and for individual families. These data highlight the urgency to understand the neurocognitive implications of seizures and anti-convulsant medication usage after childhood stroke, and to search for neuroprotective agents or other interventions to decrease epilepsy risk. Children with acute symptomatic seizures who are at higher risk may benefit from counseling and close monitoring for seizures in the outpatient setting, particularly during the first year post-stroke.
Acknowledgments
Research was supported by the National Institute of Neurological Disorders And Stroke under K12NS001692, K23NS066137 and K02 NS053883; and a UCSF grant through the Dubois Fund. The UCSF Neonatal Brain Research Institute supports Dr. Glass. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Barbara Rowe, RN, who performed data abstraction and Mike Sorel, MPH, who performed electronic searches of the Kaiser Permanente Northern California databases.
References
- 1.Agrawal N, Johnston SC, Wu YW, Sidney S, Fullerton HJ. Imaging data reveal a higher pediatric stroke incidence than prior US estimates. Stroke. 2009;40:3415–3421. doi: 10.1161/STROKEAHA.109.564633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zahuranec DB, Brown DL, Lisabeth LD, Morgenstern LB. Is it time for a large, collaborative study of pediatric stroke? Stroke; a journal of cerebral circulation. 2005;36:1825–1829. doi: 10.1161/01.STR.0000177882.08802.3c. [DOI] [PubMed] [Google Scholar]
- 3.Broderick J, Talbot GT, Prenger E, Leach A, Brott T. Stroke in children within a major metropolitan area: the surprising importance of intracerebral hemorrhage. J Child Neurol. 1993;8:250–255. doi: 10.1177/088307389300800308. [DOI] [PubMed] [Google Scholar]
- 4.Singh RK, Zecavati N, Singh J, et al. Seizures in acute childhood stroke. J Pediatr. 160:291–296. doi: 10.1016/j.jpeds.2011.07.048. [DOI] [PubMed] [Google Scholar]
- 5.Aydinli N, Tatli B, Caliskan M, et al. Stroke in childhood: experience in Istanbul, Turkey. J Trop Pediatr. 2006;52:158–162. doi: 10.1093/tropej/fml016. [DOI] [PubMed] [Google Scholar]
- 6.Lee JC, Lin KL, Wang HS, et al. Seizures in childhood ischemic stroke in Taiwan. Brain Dev. 2008 doi: 10.1016/j.braindev.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 7.De Schryver EL, Kappelle LJ, Jennekens-Schinkel A, Boudewyn Peters AC. Prognosis of ischemic stroke in childhood: a long-term follow-up study. Dev Med Child Neurol. 2000;42:313–318. doi: 10.1017/s0012162200000554. [DOI] [PubMed] [Google Scholar]
- 8.Yang JS, Park YD, Hartlage PL. Seizures associated with stroke in childhood. Pediatr Neurol. 1995;12:136–138. doi: 10.1016/0887-8994(94)00152-r. [DOI] [PubMed] [Google Scholar]
- 9.Steinlin M, Roellin K, Schroth G. Long-term follow-up after stroke in childhood. Eur J Pediatr. 2004;163:245–250. doi: 10.1007/s00431-003-1357-x. [DOI] [PubMed] [Google Scholar]
- 10.Kammersgaard LP, Olsen TS. Poststroke epilepsy in the Copenhagen stroke study: incidence and predictors. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2005;14:210–214. doi: 10.1016/j.jstrokecerebrovasdis.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Burn J, Dennis M, Bamford J, Sandercock P, Wade D, Warlow C. Epileptic seizures after a first stroke: the Oxfordshire Community Stroke Project. BMJ. 1997;315:1582–1587. doi: 10.1136/bmj.315.7122.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leone MA, Tonini MC, Bogliun G, et al. Risk factors for a first epileptic seizure after stroke: a case control study. J Neurol Sci. 2009;277:138–142. doi: 10.1016/j.jns.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Thurman DJ, Beghi E, Begley CE, et al. Standards for epidemiologic studies and surveillance of epilepsy. Epilepsia. 2011;52 (Suppl 7):2–26. doi: 10.1111/j.1528-1167.2011.03121.x. [DOI] [PubMed] [Google Scholar]
- 14.Fullerton HJ, Wu YW, Sidney S, Johnston SC. Risk of recurrent childhood arterial ischemic stroke in a population-based cohort: the importance of cerebrovascular imaging. Pediatrics. 2007;119:495–501. doi: 10.1542/peds.2006-2791. [DOI] [PubMed] [Google Scholar]
- 15.Fullerton HJ, Wu YW, Sidney S, Johnston SC. Recurrent hemorrhagic stroke in children: a population-based cohort study. Stroke. 2007;38:2658–2662. doi: 10.1161/STROKEAHA.107.481895. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong-Wells J, Johnston SC, Wu YW, Sidney S, Fullerton HJ. Prevalence and predictors of perinatal hemorrhagic stroke: results from the kaiser pediatric stroke study. Pediatrics. 2009;123:823–828. doi: 10.1542/peds.2008-0874. [DOI] [PubMed] [Google Scholar]
- 17.Jordan LC, Johnston SC, Wu YW, Sidney S, Fullerton HJ. The importance of cerebral aneurysms in childhood hemorrhagic stroke: a population-based study. Stroke. 2009;40:400–405. doi: 10.1161/STROKEAHA.108.518761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.deVeber GA, MacGregor D, Curtis R, Mayank S. Neurologic outcome in survivors of childhood arterial ischemic stroke and sinovenous thrombosis. Journal of child neurology. 2000;15:316–324. doi: 10.1177/088307380001500508. [DOI] [PubMed] [Google Scholar]
- 19.Beslow LA, Abend NS, Gindville MC, et al. Pediatric Intracerebral Hemorrhage: Acute Symptomatic Seizures and Epilepsy. JAMA Neurol. 2013:1–7. doi: 10.1001/jamaneurol.2013.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen TC, Chen YY, Cheng PY, Lai CH. The incidence rate of post-stroke epilepsy: A 5-year follow-up study in Taiwan. Epilepsy Res. doi: 10.1016/j.eplepsyres.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Lossius MI, Ronning OM, Slapo GD, Mowinckel P, Gjerstad L. Poststroke epilepsy: occurrence and predictors--a long-term prospective controlled study (Akershus Stroke Study) Epilepsia. 2005;46:1246–1251. doi: 10.1111/j.1528-1167.2005.57904.x. [DOI] [PubMed] [Google Scholar]
- 22.So EL, Annegers JF, Hauser WA, O’Brien PC, Whisnant JP. Population-based study of seizure disorders after cerebral infarction. Neurology. 1996;46:350–355. doi: 10.1212/wnl.46.2.350. [DOI] [PubMed] [Google Scholar]
- 23.Viitanen M, Eriksson S, Asplund K. Risk of recurrent stroke, myocardial infarction and epilepsy during long-term follow-up after stroke. European neurology. 1988;28:227–231. doi: 10.1159/000116272. [DOI] [PubMed] [Google Scholar]
- 24.Emanuelson I, Uvebrant P. Occurrence of epilepsy during the first 10 years after traumatic brain injury acquired in childhood up to the age of 18 years in the south western Swedish population-based series. Brain injury : [BI] 2009;23:612–616. doi: 10.1080/02699050902973913. [DOI] [PubMed] [Google Scholar]
- 25.Annegers JF, Hauser WA, Coan SP, Rocca WA. A population-based study of seizures after traumatic brain injuries. The New England journal of medicine. 1998;338:20–24. doi: 10.1056/NEJM199801013380104. [DOI] [PubMed] [Google Scholar]
- 26.Ferguson PL, Smith GM, Wannamaker BB, Thurman DJ, Pickelsimer EE, Selassie AW. A population-based study of risk of epilepsy after hospitalization for traumatic brain injury. Epilepsia. 2010;51:891–898. doi: 10.1111/j.1528-1167.2009.02384.x. [DOI] [PubMed] [Google Scholar]
- 27.Temkin NR. Risk factors for posttraumatic seizures in adults. Epilepsia. 2003;44 (Suppl 10):18–20. doi: 10.1046/j.1528-1157.44.s10.6.x. [DOI] [PubMed] [Google Scholar]
- 28.Jennett B. Trauma as a cause of epilepsy in childhood. Developmental medicine and child neurology. 1973;15:56–62. doi: 10.1111/j.1469-8749.1973.tb04866.x. [DOI] [PubMed] [Google Scholar]
- 29.Asikainen I, Kaste M, Sarna S. Early and late posttraumatic seizures in traumatic brain injury rehabilitation patients: brain injury factors causing late seizures and influence of seizures on long-term outcome. Epilepsia. 1999;40:584–589. doi: 10.1111/j.1528-1157.1999.tb05560.x. [DOI] [PubMed] [Google Scholar]
- 30.Christensen J. Traumatic brain injury: Risks of epilepsy and implications for medicolegal assessment. Epilepsia. 2012;53 (Suppl 4):43–47. doi: 10.1111/j.1528-1167.2012.03612.x. [DOI] [PubMed] [Google Scholar]
- 31.Russ SA, Larson K, Halfon N. A national profile of childhood epilepsy and seizure disorder. Pediatrics. 2012;129:256–264. doi: 10.1542/peds.2010-1371. [DOI] [PubMed] [Google Scholar]
- 32.Kolk A, Ennok M, Laugesaar R, Kaldoja ML, Talvik T. Long-term cognitive outcomes after pediatric stroke. Pediatr Neurol. 44:101–109. doi: 10.1016/j.pediatrneurol.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 33.Koelfen W, Freund M, Konig S, Varnholt V, Rohr H, Schultze C. Results of parenchymal and angiographic magnetic resonance imaging and neuropsychological testing of children after stroke as neonates. Eur J Pediatr. 1993;152:1030–1035. doi: 10.1007/BF01957231. [DOI] [PubMed] [Google Scholar]
- 34.Ballantyne AO, Spilkin AM, Hesselink J, Trauner DA. Plasticity in the developing brain: intellectual, language and academic functions in children with ischaemic perinatal stroke. Brain. 2008;131:2975–2985. doi: 10.1093/brain/awn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harbert MJ, Jett M, Appelbaum M, Nass R, Trauner DA. Perinatal risk factors and later social, thought, and attention problems after perinatal stroke. Stroke research and treatment. 2012;2012:914546. doi: 10.1155/2012/914546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vargha-Khadem F, Isaacs E, van der Werf S, Robb S, Wilson J. Development of intelligence and memory in children with hemiplegic cerebral palsy. The deleterious consequences of early seizures. Brain : a journal of neurology. 1992;115(Pt 1):315–329. doi: 10.1093/brain/115.1.315. [DOI] [PubMed] [Google Scholar]
- 37.Muter V, Taylor S, Vargha-Khadem F. A longitudinal study of early intellectual development in hemiplegic children. Neuropsychologia. 1997;35:289–298. doi: 10.1016/s0028-3932(96)00079-6. [DOI] [PubMed] [Google Scholar]
- 38.Gordon N. Similarity of the Adult Kaiser Permanente Membership in Northern California to the Insured and General Population in Northern California: Statistics from the 2007 California Health Interview Survey. Kaiser Permanente Division of Research; Jan, 2012. [Google Scholar]