Abstract
Background
Major Depressive Disorder (MDD) is highly prevalent, is recurrent, and impairs people’s work, relationships, and leisure. Acute-phase treatments improve psychosocial impairment associated with MDD, but how these improvements occur is unclear. In this study, we tested the hypotheses that reductions in depressive symptoms exceed, precede, and predict improvements in psychosocial functioning.
Method
Patients with recurrent MDD (N = 523; 68% women, 81% Caucasian; M = 42 years old) received acute-phase Cognitive Therapy (CT; Beck, Rush, Shaw & Emery, 1979). We measured functioning and symptom severity with the Social Adjustment Scale—Self-Report (Weissman & Bothwell, 1976), Range of Impaired Functioning Tool (Leon et al., 1999), Beck Depression Inventory (Beck, Ward, Mendelson, Mock, & Erbaugh, 1961), Hamilton Rating Scale for Depression (Hamilton, 1960) and Inventory for Depressive Symptomatology—Self-Report (Rush et al., 1996). We tested cross-lagged correlations between functioning and symptoms measured at baseline and the beginning, middle and end of acute phase CT.
Results
Pre- to post- treatment improvement in psychosocial functioning and depressive symptoms was large and inter-correlated. Depressive symptoms improved more and sooner than did psychosocial functioning. But among four assessments across the course of treatment, improvements in functioning more strongly predicted later improvement in symptoms than vice versa.
Conclusions
Improvements in psychosocial functioning and depressive symptoms correlate substantially during acute-phase CT, and improvements in functioning may play a role in subsequent symptom reduction during acute-phase CT.
Introduction
During their lifetime, 16% of Americans will suffer from Major Depressive Disorder (MDD), a costly, chronic, and disabling disorder (Kessler, Berglund, Demler, Jin, & Walters, 2005). To be diagnosed with DSM-IV MDD, persons must evidence both depressive symptoms and impairment in psychosocial functioning (American Psychiatric Association, APA, 2000). Although impairment in psychosocial functioning associated with MDD rivals that of chronic nonpsychiatric diseases (e.g., Cassano & Fava, 2002; Hays, Wells, Sherbourne, Rogers, & Spritzer, 1995) and accounts for 62% of depression’s economic burden (over $50 billion annually in the US; Greenberg et al., 2003), few have investigated how psychosocial impairment changes during treatment. Instead, researchers investigating the treatment of MDD have focused primarily on changes in depressive symptoms.
Referencing a person’s performance in and satisfaction with occupational, interpersonal, and recreational roles (e.g., Dunn & Jarrett, 2009; Ro & Clark, 2009), impairment in psychosocial functioning is linked with the onset and persistence of depressive symptoms (e.g., Moos & Cronkite, 1999), poor response to treatment (e.g., Hirschfeld et al., 1998), and more frequent relapse and recurrence (e.g., Vittengl, Clark, & Jarrett, 2009a). Consequently, some researchers have suggested that psychosocial and pharmacological acute-phase treatments for MDD, lasting approximately 12 weeks (Rush et al., 2006), not only should reduce depressive symptom severity, but also should normalize functioning, or at least return individuals to premorbid functioning levels (e.g., Keller, 2003; Thase, 2003; ).
Research suggests that psychosocial and pharmacological acute-phase treatments improve psychosocial functioning in depressed patients (e.g., Gorenstein, Andrade, Moreno, & Artes, 2002; Hollon et al., 1992; Vittengl et al., 2004). Compared to depressive-symptom improvement, however, improvements in psychosocial functioning during acute-phase treatments are smaller, such that a significant number of depressed patients do not return to pre-morbid or normal levels of occupational (Mintz, Mintz, Arruda, & Hwang, 1992), interpersonal (Bothwell & Weissman, 1977), or recreational functioning (de Lisio et al., 1986), even when they experience symptom remission (Miller et al., 1998; Vittengl et al., 2004).
Researchers investigating how psychosocial functioning improves during treatment for MDD have identified depressive symptom reduction as a potential mediator (Finkelstein et al., 1996; Hirschfeld et al., 2002; Lenderking et al., 1999; Vittengl et al., 2004). In these studies, researchers compared pre- to post-treatment changes in psychosocial functioning and depressive symptom severity during acute-phase trials of psychotherapy (Vittengl et al.), anti-depressant medication (Finkelstein et al.; Lenderking et al.), and their combination (Hirschfeld et al.). Psychosocial functioning was measured with self-report instruments such as the Social Adjustment Scale--Self-Report (SAS-SR; Weissman & Bothwell, 1976) and Dyadic Adjustment Scale (DYS; Spanier, 1976); depressive symptom severity was measured with instruments including the self-report Beck Depression Inventory (BDI; Beck, Ward, Mendelson, Mock, & Erbaugh, 1961) and clinician-rated Hamilton Rating Scale for Depression (HRSD; Hamilton, 1960), and both the self-report and clinician-rated Inventory for Depressive Symptomatology (IDS-SR, IDS-CR; Rush et al., 1996). Using linear regression, these studies found reductions in depressive symptom severity explained much, if not all, of concurrent improvements in psychosocial functioning. As a result, Vittengl et al. (2009a) hypothesized that during acute-phase treatment “rapid decreases in depressive symptoms may facilitate slower improvements in psychosocial functioning as the social environment begins to notice and ‘trust’ (i.e., perceive as lasting) improvements in the patient’s functioning” (p. 141).
However, additional research is needed to test Vittengl et al.’s (2009a) hypothesis more directly. Before a mediating variable can be tested, researchers must first: a) determine temporal precedence of change (i.e., establish that the mediating variable changes before the outcome variable) and b) show that the mediating variable predicts or influences the outcome variable (Wilson, Fairburn, Agras, Walsh, & Kraemer, 2002). Once these steps are accomplished, , mediating variables can be tested and mechanisms of change established, allowing researchers to tailor interventions to optimize outcome and cost-effectiveness.
In this article, we attempt to improve understanding of how depression is treated acutely, by disentangling reciprocal relations between changes in psychosocial functioning and depressive symptom severity. We analyzed a large sample (N = 523) of outpatients who received acute-phase cognitive therapy (CT; Beck et al., 1979) for recurrent MDD. We estimated changes in levels (with ANOVA and regression analyses) and cross-lagged correlations (with structural equation modeling [SEM]) of psychosocial functioning and depressive symptom data obtained from both clinicians and patients at multiple assessment points. We tested hypotheses that: a) depressive symptom severity improves earlier, and to a greater extent, than psychosocial functioning and b) depressive symptom severity predicts subsequent improvement in psychosocial functioning measured at the beginning, middle, and end of the acute-phase, after controlling for previous levels of psychosocial impairment. Given the exploratory nature of this study, we also estimated psychosocial functioning’s potential influence on subsequent depressive symptom severity, controlling for previous levels of depressive symptom severity.
Method
The current analyses utilized data from an ongoing, two-site clinical trial comparing acute phase CT responders randomized to continuation phase CT, fluoxetine, or pill placebo (Jarrett & Thase, 2010). Below we summarize relevant methods from the acute phase of this trial and refer readers to Jarrett and Thase (2010) for additional detail, including continuation and follow-up phases not described further here. Patients were withdrawn from psychotropic medications before entering the study and were not prescribed medications in the acute phase CT protocol.
Participants
Participants consented to enter acute-phase CT as part of a randomized clinical trial approved by the Institutional Review Boards at The University of Texas Southwestern Medical Center at Dallas (UT Southwestern) and Western Psychiatric Institute and Clinic at Pittsburgh (WPIC). Potential participants were self or practitioner referred and/or informed of the study through newspaper, bulletin board, or Internet announcements. Clinic staff screened potential participants over the telephone or in-person, and scheduled them for initial and follow-up diagnostic evaluations to determine study eligibility. Included participants met DSM-IV criteria for recurrent MDD (APA, 2000), by protocol scored ≥ 14 on the 17-item HRSD at both diagnostic interviews (but two patients with HRSD = 13 were enrolled erroneously), and provided informed consent. Excluded participants: a) had severe or poorly controlled concurrent medical disorders that could cause depression, b) had any psychotic or organic mental disorder, bipolar disorder, active alcohol or drug dependence, primary obsessive-compulsive disorder, or primary eating disorders (primary refers to the disorder associated with the most impairment or distress), c) could not complete questionnaires in English, d) represented an active suicide risk, e) were outside 18–70 years of age, f) failed to respond to a previous trial of ≥ 8 weeks of CT or 6 weeks on 40 mg of fluoxetine, or g) were pregnant or planned to become pregnant during the first 11 months after intake. Diagnoses and lifetime history of psychiatric disorders were made with the Structured Clinical Interview for DSM-IV (First, Spitzer, Gibbon, & Williams, 1996) applied to the patient’s current and past symptoms, functioning, and previous treatment.
Participants consented to an initial diagnostic evaluation (UT Southwestern n = 1053, WPIC n = 306). Of these, 523 participants (UT Southwestern n = 276; WPIC n = 247) met study criteria at both the initial assessment and diagnostic follow-up visit and consented to enter acute-phase CT; 836 participants (UT Southwestern n = 777; WPIC n = 59) were excluded and referred to appropriate treatment. Participants were excluded most often because they did not meet criteria for recurrent MDD, scored below 14 on the HRSD, or had exclusionary disorders.
Consenting patients were mostly middle aged (M = 42.4, SD = 12.1, years), female (67.5%), single (58.1%), White (80.9%), and employed either full or part time (55.6%). Patients’ mean age of MDD onset was 21.2 years (SD = 10.8), with an average length of illness of 20.7 years (SD = 11.8). Patients reported that their current major depressive episode averaged 25.0 months (SD = 45.1), and they endorsed a median of 4 major depressive episodes during their lifetime.
Procedure
Patients who entered acute-phase CT received 16 or 20 sessions spread over 12–14 weeks. By protocol, patients received two sessions a week for 4 weeks, after which they were categorized as early (≥ 40% reduction in HRSD score compared to diagnostic follow-up) or late responders (< 40% reduction). Early responders then received one weekly session for the final 8 weeks of the acute phase, whereas late responders continued receiving two sessions weekly until the last 4 weeks of the acute-phase, when they also received one weekly session. Among 523 consenting, 410 patients completed the acute-phase protocol by attending ≥ 14 (early responders) or ≥ 18 (late responders) CT sessions.
Therapist competence
Fifteen therapists provided acute-phase CT and demonstrated competence by achieving and maintaining Cognitive Therapy Scale (CTS; Young & Beck, 1980) scores ≥ 40. Therapists attended weekly group supervision. Group supervisors and other therapists observed and rated videotaped sessions on the CTS, providing therapists with feedback on strengths and weaknesses.
Measures
Beck Depression Inventory
Using the 21-item BDI, patients rated their depressive symptom severity at the initial diagnostic evaluation, week one and seven of acute-phase CT, and the post-acute-phase CT evaluation, which occurred within 1 week after completion or premature termination of acute-phase CT. Total scores categorized depression as minimal (0–10), mild to moderate (10–18), moderate to severe (19–29), or severe (> 29; Beck et al., 1961). In the current data, the median internal consistency reliability was .89 (range = .83 to .92); median convergent validity r = .72 (range = .46 – .82) with the HRSD and r = .85 (range = .78 to .92) with the IDS-SR.
Hamilton Rating Scale for Depression
Clinicians (during acute phase CT) and evaluators (at intake and post-acute phase CT assessments) rated depressive symptom severity with the HRSD at both diagnostic evaluations, week one and seven of acute-phase CT, and the post-acute-phase CT evaluation. Total scores indicated very severe (> 24), 19–23 severe, 14–18 moderate, 6–13 mild, or no (< 6) depression. In the current study, the HRSD demonstrated interrater reliability of ICC = .91, concurrent validity with the IDS-SR (median r = .76, range = .52 – .86), and median internal consistency reliability of α = .68 (range = .52 – .83).
Inventory for Depressive Symptomatology – Self-Report
Patients also self-reported their depressive symptom severity with the IDS-SR at the initial diagnostic evaluation, week one and seven of acute-phase CT, and the post-acute-phase CT evaluation. Total scores represented very severe (> 49), severe (39–48), moderate (26–38), mild (14–25), or no (< 13) depression. In this study, the IDS-SR showed median internal consistency reliability of α = .86 (range = .80 to .91).
Range of Impaired Functioning Tool
At the post-acute-phase CT evaluation, clinicians and evaluators rated psychosocial functioning retrospectively with the Longitudinal Interval Follow-up Evaluation—Psychosocial Interview (Keller et al., 1987). From this interview, we scored the four-item RIFT (Leon et al., 1999) for periods coinciding with the diagnostic phase and the first, second, and third month of acute-phase CT. Higher scores indicate greater impairment. Leon et al. reported mean RIFT scores of 14 and 9 for depressed and non-depressed populations, respectively. In the current analysis, the RIFT showed convergence with the SAS-SR (median r = .46, range = .34 – .68) and median internal consistency reliability of α = .68 (range = .59 to .76).
Social Adjustment Scale-Self Report
Patients self-reported their psychosocial functioning on the 56-item SAS-SR at the first diagnostic evaluation, week one and seven of acute-phase CT, and the post-acute-phase CT evaluation. Higher scores indicate greater impairment. Weissman et al. (2001) reported the total score averaged 2.5 and 1.7 for depressed and non-depressed samples, respectively. In the current study, the SAS-SR total score showed median internal consistency reliability of α = .76 (range = .73 to .78).
Standardization of Scores
To compare levels of change among measures, we converted each to T-score units (M = 50, SD = 10) based on the measures’ distributions at intake. In addition, we averaged the depressive symptom-severity measures (BDI, HRSD, IDS-SR) to form a robust index, because past research shows that these measures mark the same construct concurrently and longitudinally during acute phase CT (Vittengl et al., 2004). Internal consistency reliability for the three-measure symptom composite for this study’s observations was high (median α = .91; range = .81–.95). Similarly, we standardized and averaged the psychosocial functioning measures (SAS-SR, RIFT) to form a composite index with moderate reliability (median α = .62; range = .51–.81). Previous research supports the convergence of the RIFT and SAS-SR concurrently and longitudinally for CT patients (Vittengl, Clark, & Jarrett, 2009a).
Statistical Analyses
We implemented a multiple-imputation procedure to utilize all available data, maximize statistical power of hypothesis tests, and increase the generalizability of results. Among the 523 patients, 5 measures, and 4 assessment periods, 16.8% of observations were missing (see Table 1). We generated 10 data sets with missing values imputed via the Markov chain Monte Carlo method in PROC MI, computed standard analyses (e.g., ANOVA, regression, SEM) on each dataset, and pooled the results via PROC MIANALYZE to test hypotheses (using SAS version 9.1; SAS Institute, Inc., Cary, NC). This procedure follows published guidelines for missing data (e.g., Schafer & Graham, 2002).
Table 1.
Descriptive Statistics for Depressive Symptom and Psychosocial Functioning Measures
| Measure | Diagnostic Evaluation | A-CT Week 1 | A-CT Week 7 | Post-A-CT Evaluation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | N | M | SD | N | M | SD | N | M | SD | N | |
| HRSD | 21.22 | 4.19 | 523 | 20.29 | 4.80 | 523 | 10.82 | 6.21 | 431 | 9.60 | 6.41 | 412 |
| BDI | 26.62 | 8.41 | 494 | 24.28 | 8.77 | 487 | 12.90 | 8.55 | 425 | 9.30 | 8.70 | 394 |
| IDS-SR | 39.33 | 10.26 | 494 | 36.40 | 10.49 | 480 | 20.20 | 12.00 | 422 | 14.96 | 11.56 | 396 |
| SAS-SR | 2.59 | 0.44 | 479 | 2.54 | 0.44 | 467 | 2.16 | 0.47 | 389 | 1.97 | 0.43 | 358 |
| RIFT | 14.33 | 2.72 | 401 | 12.53 | 2.86 | 401 | 10.85 | 2.99 | 399 | 10.05 | 3.08 | 332 |
Note. A-CT = acute-phase cognitive therapy. HRSD = 17-item Hamilton Rating Scale for Depression. BDI = 21-item Beck Depression Inventory. IDS-SR = Inventory of Depressive Symptomatology. SAS-SR = Social Adjustment Scale-Self-Report. RIFT = R
In our SEM, scores on the indices of psychosocial functioning and depressive symptom severity were cross-lagged over repeated measurements (see Figure 1). In addition, paths were added to the SEM to control for: a) covariation between measures of depressive symptoms and psychosocial functioning and b) independent changes in depressive symptom severity and psychosocial functioning. As a result, the SEM provided information regarding the extent to which change in variance unique to measures of depressive symptom severity predicted change in variance unique to measures of psychosocial functioning, and vice versa. We evaluated model fit using several common metrics: goodness of fit index (GFI), comparative fit index (CFI), non-normed fit index (NNFI), and root mean square error of approximation (RMSEA). Scores ≥ .90 on the GFI, CFI, and NNFI and ≤ .08 on the RMSEA indicate acceptable model fit (Kline, 2005).
Figure 1. Structural Equation Model Showing Potential Mediating Relationships between Psychosocial Functioning and Depressive Symptoms.
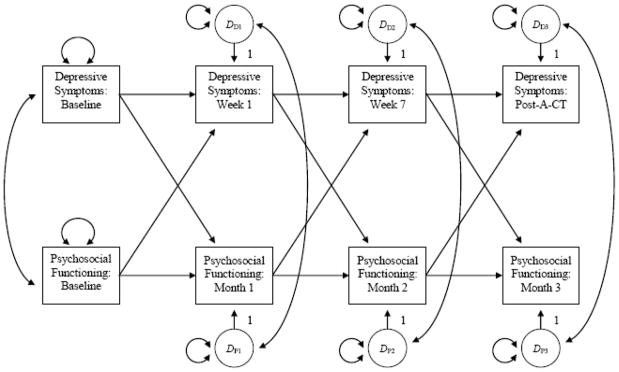
Note. Rectangles represent the indices of psychosocial functioning and depressive symptom severity. Circles with an uppercase D are called disturbances, which represent the effect of unexplained variation on the indices. One-sided arrows signify direct effects, and double-sided arrows represent covariances, which control for covariation between variables.
Results
How much do Psychosocial Functioning and Depressive Symptom Severity Change during Acute-Phase CT?
The depressive symptom and functioning composite measures’ standardized means are shown in Figure 2 (see Table 1 for raw scores). We analyzed changes in means with repeated-measures multilevel models including random subject effects, fixed effects of assessment time, and unstructured error patterns. Consistent with visual inspection of Figure 2, the main effect of assessment time was significant in predicting the symptom composite, F(3,1014) = 603.35, and psychosocial functioning composite, F(3,338) = 202.63, ps < .01. From pre- to post-acute-phase CT, decreases in depressive symptoms (d = 1.83) and psychosocial functioning (d = 1.24) were large. By week 7 of acute-phase CT, depressive symptoms (M = 26.28) were lower than psychosocial functioning (M = 39.10), t(223) = 25.90, p < .01. Similarly, at the post-acute-phase CT follow-up, depressive symptom scores (M = 22.23) were lower than psychosocial functioning (M = 35.63), t(185) = 30.94, p < .01. These results show that depressive symptoms changed sooner and more overall than did psychosocial functioning, replicating Vittengl et al. (2004).
Figure 2. Standardized Depressive Symptom and Psychosocial Functioning Scores across Acute-Phase Cognitive Therapy for Depression (CT).
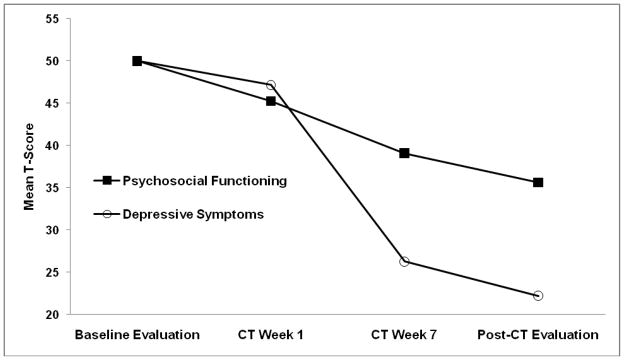
Note. Depressive symptoms are a composite of the Hamilton Rating Scale for Depression, Beck Depression Inventory, and the Inventory of Depressive Symptomatology-Self-Report. Psychosocial functioning is a composite of the Social Adjustment Scale—Self-Report and the Range of Impaired Functioning Tool.
Pre- to post-acute-phase CT decreases in depressive symptoms (Mdecrease = 27.77, SE = 0.71, p < .01) correlated moderately highly (r = .65, p < .01; 95% CI .58–.71) with decreases in psychosocial functioning (Mdecrease = 14.37, SE = 0.54, p < .01). Based on intercept tests in regressions predicting pre- to post-acute phase CT depressive symptom change from psychosocial functioning change and vice versa, the amount of change in depressive symptoms controlling change in psychosocial functioning (Mdecrease = 15.60, SE = 0.86, p < .01) was roughly one-third smaller than the unadjusted change, but remained substantial and statistically significant. In contrast, change in psychosocial functioning (Mdecrease = 0.50, SE = .92, p = .59) was very small and no longer significant when controlling change in depressive symptoms. This pattern of results also replicates Vittengl et al. (2004) in suggesting that pre- to post-acute phase CT change in depressive symptoms accounts for change in psychosocial functioning. However, these analyses do not address the extent to which change in depressive symptoms drives subsequent changes in psychosocial functioning and vice versa at four monthly time points during acute-phase CT.
Do Changes in Depressive Symptom Severity Drive Changes in Psychosocial Functioning or Vice Versa?
Cross-lagged correlations estimated via SEM appear in Figure 3 The model for depressive symptoms and psychosocial functioning fit acceptably by three indices (GFI = .96; CFI = .98; NNFI = .95) but not RMSEA = .11. Because the majority of fit indices were acceptable, and the model was specified a priori for hypothesis testing, we interpreted the model.
Figure 3. Correlations (95% Confidence Intervals) among Depressive Symptoms and Psychosocial Functioning during Acute Phase Cognitive Therapy (CT) for Depression.
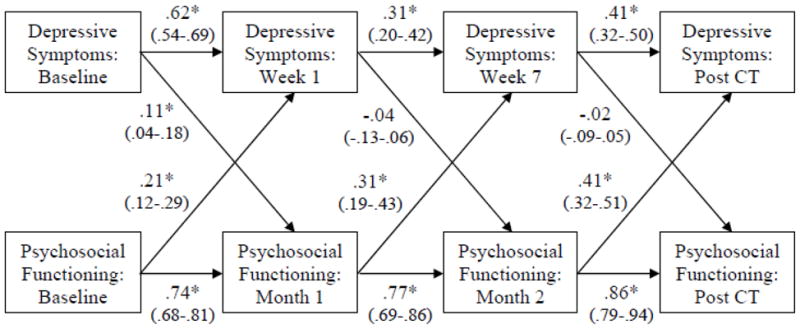
Note. Depressive symptoms are a composite of the Hamilton Rating Scale for Depression, Beck Depression Inventory, and the Inventory of Depressive Symptomatology-Self-Report. Psychosocial functioning is a composite of the Social Adjustment Scale—Self-Report and the Range of Impaired Functioning Tool.
* p < .05
Not surprisingly, the depressive symptom and psychosocial functioning measures’ retest correlations were moderate to high and stronger than cross-correlations between measures (see Figure 3). Psychosocial functioning at baseline predicted depressive symptoms at week 1 of acute-phase CT, and depressive symptoms at baseline predicted psychosocial functioning at week 1. Thus before acute-phase CT, analyses suggested reciprocal causality between improvements in symptoms and functioning. However, from weeks 1 to 7 of acute-phase CT, and from week 7 to post-acute-phase CT, depressive symptom reduction did not predict subsequent improvements in psychosocial functioning, whereas improvements in psychosocial functioning predicted subsequent symptom reduction. In sum, we found limited support for the hypothesis that changes in depressive symptoms drive improvements in psychosocial functioning. But the evidence was somewhat stronger that changes in psychosocial functioning drive changes in depressive symptoms.
Discussion
In this study, we tested the hypotheses that change in depressive symptom severity would exceed, precede, and predict change in psychosocial functioning during acute-phase CT. In replication of Vittengl et al. (2004), we analyzed mean changes and intercorrelations of pre-post-acute-phase CT improvements in depressive symptoms and psychosocial functioning. We then extended analyses with SEM to control covariation between constructs and estimate interdependent changes across four assessment points. Results supported our hypothesis that depressive symptom severity showed greater improvement sooner than psychosocial functioning. Further, pre-post changes in symptoms and functioning were moderately highly correlated, and improvement in psychosocial functioning was expected only when depressive symptoms decreased, replicating Vittengl et al.’s (2004) findings in a smaller dataset. Counter to our additional hypotheses, however, shorter-interval changes in depressive symptom severity did not consistently predict subsequent improvement in psychosocial functioning. Instead, improvement in psychosocial functioning more strongly predicted subsequent depressive symptom reduction across lagged assessments points.
The current findings may differ from expectations based on previous research for several reasons (Finkelstein et al., 1996; Hirschfeld et al., 2002; Lenderking et al., 1999; Vittengl et al., 2004). First, when investigating the extent to which change in depressive symptom severity accounted for change in psychosocial functioning, or vice versa, the previous studies did not consider precedence of change (Finkelstein et al., 1996; Hirschfeld et al., 2002; Lenderking et al., 1999; Vittengl et al., 2004). Instead, these studies compared global pre- to post-treatment changes in each construct. As a result, even if changes in depressive symptom severity accounted for changes in psychosocial functioning, the mediational relation might also exist when constructs were reversed (Kraemer, Stice, Kazdin, Offord, & Kupfer, 2001). By dividing the acute phase into shorter time intervals and establishing temporal precedence, this study possibly was more sensitive to changes in reciprocal relations between psychosocial functioning and depressive symptom severity.
Second, whereas previous studies relied on linear regression (Finkelstein et al., 1996; Hirschfeld et al., 2002; Lenderking et al., 1999; Vittengl et al., 2004), this study used SEM to investigate mediational relations between psychosocial functioning and depressive symptom severity. Kline (2005) suggests that the inability to enter variables as both predictor and criterion in the same analysis, and to control covariation between variables across data sets, limits linear regression analyses. As a result, this study may have revealed a different mediational relationship between psychosocial functioning and depressive symptom severity because SEM did not have these limitations.
Finally, a yet-to-be-identified change process may have been at work such that early improvements in psychosocial functioning influenced depressive symptom severity later in the acute phase. For example, relations between the two sets of variables may be different in the early and late phases of CT and/or early phase change may be necessary for late phase change. So, despite changing more slowly (see Figure 2), early improvements in psychosocial functioning, or perhaps the initial mobilization of resources to improve psychosocial functioning (e.g., behavioral activation), might serve important roles in the alleviation of depressive symptoms. Moreover, cognitive therapists often focus on improving psychosocial functioning in addition to reducing depressive symptoms, particularly when functioning is impaired and selected as a target for treatment. A detailed analysis of individual patients’ session content (e.g., from videotapes) could be used in future research to determine whether observed relations between psychosocial functioning and depressive symptoms vary with individual patients’ CT goals.
Implications for Treatment of Psychosocial Impairment Associated with Depression
According to results, changes in a depressed patient’s psychosocial functioning played a role in reducing subsequent depressive symptom severity during acute-phase CT. As such, it could be said that these findings substantiated cognitive and behavioral theorists’ idea that behavior change early in the acute phase makes the shift from negative to euthymic mood possible by increasing access to social reinforcement, reducing exposure to social punishment, and activating constructive schemas (e.g., Beck et al., 1979; Follette & Greenberg, 2005). Development of behavioral activation as a free-standing treatment for depression separate from cognitive interventions (e.g., Dimidjian et al., 2006) can be viewed as an extension of behavioral techniques in beginning sessions of CT. Behavioral activation emphasizes reduction of avoidance and reengagement in psychosocial activities (e.g., in fulfilling social role obligations) with potential for long-term reinforcement and reduction of depressive symptoms.
The current findings also are consistent theoretically with interventions that focus on improving patients’ interpersonal and social functioning to reduce depressive symptomatology (e.g., Interpersonal Psychotherapy; Weissman, Markowitz, & Klerman, 2000). For example, Interpersonal Psychotherapists suggest that “change and improvement in depressive symptoms occur through working on mastery and competence in the social sphere” (p. 44; Crowe & Luty, 2005). Therefore, the current study’s conceptualization of change in psychosocial functioning in relation to depressive symptom severity appears robust and applicable to psychosocial interventions other than CT.
Limitations and Directions for Future Research
The current study’s assessment strategy and design limit its generalizability. First, our depressive symptom composite reduced variability and enhanced validity across instruments, but it may limit the degree to which findings could be replicated in clinical settings where providers are overburdened and unable to use multimodal, multi-measure assessment. Similarly, we cannot rule out differences between clinicians’ and evaluators’ use of the HRSD (e.g., biases), although any such differences appear small because convergence with self-reported symptom measures was strong at all assessments. Also, because we used the RIFT retrospectively, it relied heavily on patients’ ability to recall past events when they were often more depressed, thereby potentially introducing mood-congruent memory biases in the index of psychosocial functioning (Barry et al., 2004). Future research might improve this study’s assessment strategy by administering the RIFT at the same time as symptom measures.
Second, the current study’s design did not include random assignment or a control group. As a result, we could not control for the impact of extraneous factors on changes in psychosocial functioning and depressive symptom severity. Given that past randomized controlled trials (e.g., Dimidjian et al., 2006; Elkin et al., 1989; Evans 1992; Hollon et al., 2005) reported similar changes in psychosocial functioning and depressive symptom severity, it could be inferred that the observed changes in this study were due to exposure to acute-phase CT. However, replication of this study’s hypotheses within a randomized trial comparing treatment vs. control conditions is necessary before drawing firm conclusions regarding causation.
Finally, the current findings may have limited generalizability due to sample demographics and treatment specificity. Despite targeting ethnic/racial minorities with specific recruitment strategies, ethnic minorities did not participate in proportion with the United States population. Also, because all patients underwent acute-phase CT for MDD, whether results generalize to populations with other diagnoses or treatments is unclear. Future research could clarify the extent to which our findings generalize to more ethnically diverse patient populations and treatment modalities.
Summary
Acute-phase CT, like other psychotherapeutic interventions, is a complex process with multiple potential mechanisms of change (e.g., environmental, biological, cognitive, etc.; Garratt et al., 2007; Whisman, 1993). Although efforts have been made to understand how acute-phase treatments reduce the psychosocial impairment associated with depression (e.g., Hirschfeld et al., 2002; Vittengl et al., 2004), this area of research is nascent. The current study advances the field by disentangling, to some degree, the sequence and process of change in psychosocial functioning and depressive symptom severity during one acute-phase treatment. In short, we found that change in depressive symptom severity had less impact on subsequent change in psychosocial functioning than vice versa across four acute-phase CT assessments. Depressed patient’s psychosocial functioning at treatment baseline and the beginning and middle of acute-phase CT predicted subsequent depressive symptom severity at each lag-one assessment point. Although researchers need to clarify how psychosocial functioning changes during acute-phase treatment, the current study suggests that early efforts by CT therapists to change depressed patients’ behavior are well warranted.
Acknowledgments
We are indebted to our research teams and our colleagues at The University of Texas Southwestern Medical Center at Dallas, the University of Pittsburgh (where Dr. Thase was located during patient accrual), and the University of Pennsylvania (Dr. Thase’s current affiliation). We appreciate the assistance of Julie Kangas, B.A., Lauren Dunlap, B.A., Joanne Sanders, M.S., and Abu Minhajuddin, Ph.D. in preparing this manuscript.
This report was supported by Grants Number K24 MH001571, R01 MH58397, R01 MH69619 (to Robin B. Jarrett, Ph.D.) and R01 MH58356 and R01 MH69618 (to Michael E. Thase, M.D.) from the National Institute of Mental Health (NIMH). We appreciate the support of our NIMH Program Officer, Jane Pearson, Ph.D. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH or the National Institutes of Health. We also appreciate the careful review by members of the trial’s Data Safety and Monitoring Board.
Footnotes
Declaration of Interest:
During the past two years Dr. Thase has consulted with, served on advisory boards for, or received honoraria for talks from: AstraZeneca, Bristol-Myers Squibb Company, Eli Lilly and Company, Forest Laboratories, GlaxoSmithKline, Janssen Pharmaceutica, Lundbeck, MedAvante, Inc., Neuronetics, Inc., Novartis, Otsuka, Pamlab, Pfizer Pharmaceuticals, Schering-Plough, Shionogi, Shire US Inc., Supernus Pharmaceuticals, Takeda, Transcept Pharmaceuticals, and Wyeth Pharmaceuticals and he has received grant support from Eli Lilly and Company, Forest Laboratories, GlaxoSmithKline, Otsuka, and Sepracor, Inc., in addition to funding from the NIMH. He has equity holdings for MedAvante, Inc. and has received royalties from American Psychiatric Publishing, Inc. (APPI), Guilford Publications, Herald House, and W.W. Norton & Company, Inc. Two books currently promoted by the APPI specifically pertain to cognitive therapy. Dr. Thase also discloses that his spouse is an employee of Embryon, Inc. (formerly Cardinal Health and Advogent), which does business with several pharmaceutical companies that market medications used to treat depression.
Dr. Jarrett’s medical center receives the fees from the cognitive therapy she provides to patients. Dr. Jarrett is a paid consultant to the NIMH.
Thomas Carmody has been a consultant for Cyberonics, Inc.
Contributor Information
Todd W. Dunn, The University of Texas Southwestern Medical Center at Dallas
Jeffrey R. Vittengl, Truman State University.
Lee Anna Clark, University of Iowa.
Thomas Carmody, The University of Texas Southwestern Medical Center at Dallas.
Michael E. Thase, University of Pennsylvania
Robin B. Jarrett, The University of Texas Southwestern Medical Center at Dallas.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR (Text Revision) American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Barry ES, Naus MJ, Rehm LP. Depression and implicit memory: Understanding mood congruent memory bias. Cognitive Therapy and Research. 2004;28:387–414. [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive Therapy of Depression. New York: Guilford; 1979. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock JE, Erbaugh JK. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bothwell S, Weissman MM. Social impairments four years after an acute depressive episode. American Journal of Orthopsychiatry. 1977;47:231–237. doi: 10.1111/j.1939-0025.1977.tb00978.x. [DOI] [PubMed] [Google Scholar]
- Cassano P, Fava M. Depression and public health: an overview. Journal of Psychosomatic Research. 2002;53:849–857. doi: 10.1016/s0022-3999(02)00304-5. [DOI] [PubMed] [Google Scholar]
- Crowe M, Luty S. The process of change in Interpersonal Psychotherapy (IPT) for depression: A case study for the new IPT therapist. Psychiatry: Interpersonal and Biological Processes. 2005;68:43–54. doi: 10.1521/psyc.68.1.43.64184. [DOI] [PubMed] [Google Scholar]
- De Lisio G, Maremmani I, Perugi G, Cassano GB, Deltito J, Akiskal HS. Impairment of work and leisure in depressed outpatients. A preliminary communication. Journal of Affective Disorders. 1986;10:79–84. doi: 10.1016/0165-0327(86)90029-7. [DOI] [PubMed] [Google Scholar]
- Dimidjian S, Hollon SD, Dobson KS, Schmaling KB, Kohlenberg RJ, Addis ME, et al. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. Journal of Consulting & Clinical Psychology. 2006;74:658–670. doi: 10.1037/0022-006X.74.4.658. [DOI] [PubMed] [Google Scholar]
- Dunn TW, Jarrett RB. Psychosocial functioning. In: Ingram R, editor. The International Encyclopedia of Depression. Springer; New York, NY: 2009. pp. 468–471. [Google Scholar]
- Elkin I, Shea TM, Watkins JT, Imber SD, Sotsky SM, Collins JF, et al. National Institute of Mental Health Treatment of Depression Collaborative Research Program: General effectiveness of treatments. Archives of General Psychiatry. 1989;46:971–982. doi: 10.1001/archpsyc.1989.01810110013002. [DOI] [PubMed] [Google Scholar]
- Evans MD, Hollon SD, DeRubeis RJ, Piasecki JM, Grove WM, Garvey MJ, Tuason VB. Differential relapse following cognitive therapy and pharmacotherapy for depression. Archives of General Psychiatry. 1992;49:802–808. doi: 10.1001/archpsyc.1992.01820100046009. [DOI] [PubMed] [Google Scholar]
- Finkelstein SN, Berndt ER, Greenberg PE, Parsley RA, Russell JM, Keller MB. Improvement in subjective work performance after treatment of chronic depression: Some preliminary results. Psychopharmacology Bulletin. 1996;32:33–40. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID-I/P, Version 2.0) New York State Psychiatric Institute, Biometrics Research Department; New York, NY: 1996. [Google Scholar]
- Follette WC, Greenberg LS. Technique factors in treating dysphoric disorders. In: Castonguay LG, Beutler LE, editors. Principles of Therapeutic Change that Work. Oxford University Press; New York: 2005. pp. 83–110. [Google Scholar]
- Garratt G, Ingram RE, Rand KL, Sawalani G. Cognitive processes in cognitive therapy: Evaluation of the mechanisms of change in the treatment of depression. Clinical Psychology: Science and Practice. 2007;14:224–239. [Google Scholar]
- Gorenstein C, Andrade L, Moreno RA, Artes R. Social adjustment in depressed patients treated with venlafaxine and amitriptyline. International Clinical Psychopharmacology. 2002;17:171–175. doi: 10.1097/00004850-200207000-00003. [DOI] [PubMed] [Google Scholar]
- Greenberg PE, Kessler RC, Birnbaum HG, Leong SA, Lowe SW, Berglund PA, et al. The economic burden of depression in the United States: How did it change between 1990 and 2000? Journal of Clinical Psychiatry. 2003;64:1465–1475. doi: 10.4088/jcp.v64n1211. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;12:52–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays RD, Wells KB, Sherbourne CD, Rogers W, Spritzer K. Functioning and well-being outcomes of patients with depression compared with chronic general medical illnesses. Archives of General Psychiatry. 1995;52:11–19. doi: 10.1001/archpsyc.1995.03950130011002. [DOI] [PubMed] [Google Scholar]
- Hirschfeld RMA, Dunner DL, Keitner G, Klein DN, Koran LM, Kornstein SG, et al. Does psychosocial functioning improve independent of depressive symptoms? A comparison of nefazodone, psychotherapy, and their combination. Biological Psychiatry. 2002;51:123–133. doi: 10.1016/s0006-3223(01)01291-4. [DOI] [PubMed] [Google Scholar]
- Hirschfeld RM, Russell JM, Delgado PL, Fawcett J, Friedman RA, Harrison WM, et al. Predictors of response to acute treatment of chronic and double depression with sertraline or imipramine. The Journal of Clinical Psychiatry. 1998;59:669–675. doi: 10.4088/jcp.v59n1205. [DOI] [PubMed] [Google Scholar]
- Hollon SD, DeRubeis RJ, Evans MD, Wiemer MJ, Garvey MJ, Grove WM, Tuason VB. Cognitive therapy and pharmacotherapy for depression: Singly and in combination. Archives of General Psychiatry. 1992;49:774–781. doi: 10.1001/archpsyc.1992.01820100018004. [DOI] [PubMed] [Google Scholar]
- Hollon SD, DeRubeis RJ, Shelton RC, Amsterdam JD, Salomon RM, O’Reardon JP, et al. Prevention of relapse following cognitive therapy vs. medications in moderate to severe depression. Archives of General Psychiatry. 2005;62:417–422. doi: 10.1001/archpsyc.62.4.417. [DOI] [PubMed] [Google Scholar]
- Jarrett RB, Thase ME. Comparative efficacy and durability of continuation phase cognitive therapy for preventing recurrent depression: Design of a double-blinded fluoxetine- and pill placebo-controlled randomized trial with 2-year follow-up. Contemporary Clinical Trials. 2010;31:355–377. doi: 10.1016/j.cct.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller MB. Past, present, and future directions for defining optimal treatment outcome in depression: Remission and beyond. JAMA. 2003;289:3152–3160. doi: 10.1001/jama.289.23.3152. [DOI] [PubMed] [Google Scholar]
- Keller MB, Lavori PW, Friedman B, Nielsen E, Endicott J, McDonald-Scott P, et al. The longitudinal interval follow-up evaluation: A comprehensive method for assessing outcome in prospective longitudinal studies. Archives of General Psychiatry. 1987;44:540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kline RB. Principles and Practice of Structural Equation Modeling. Guilford Press; New York: 2005. [Google Scholar]
- Kraemer HC, Stice E, Kazdin A, Offord D, Kupfer D. How do risk factors work together? Mediators, moderators, independent, overlapping, and proxy risk factors. American Journal of Psychiatry. 2001;158:848–856. doi: 10.1176/appi.ajp.158.6.848. [DOI] [PubMed] [Google Scholar]
- Lenderking WR, Tennen H, Nackley JF, Hale MS, Turner RR, Testa MA. The effects of venlafaxine on social activity level in depressed outpatients. Journal of Clinical Psychiatry. 1999;60:157–163. doi: 10.4088/jcp.v60n0312. [DOI] [PubMed] [Google Scholar]
- Leon AC, Solomon DA, Mueller TI, Turvey CL, Endicott J, Keller MB. The Range of Impairment Functioning Tool (LIFE-RIFT): A brief measure of functional impairment. Psychological Medicine. 1999;29:869–878. doi: 10.1017/s0033291799008570. [DOI] [PubMed] [Google Scholar]
- Miller IW, Keitner GI, Schatzberg AF, Klein DN, Thase ME, Rush AJ, et al. The treatment of chronic depression, part 3: Psychosocial functioning before and after treatment with sertraline or imipramine. Journal of Clinical Psychiatry. 1998;59:608–619. doi: 10.4088/jcp.v59n1108. [DOI] [PubMed] [Google Scholar]
- Mintz J, Mintz LI, Arruda MJ, Hwang SS. Treatments of depression and the functional capacity to work. Archives of General Psychiatry. 1992;49:761–768. doi: 10.1001/archpsyc.1992.01820100005001. [DOI] [PubMed] [Google Scholar]
- Moos RH, Cronkite RC. Symptom-Based Predictors of a 10-Year Chronic Course of Treated Depression. Journal of Nervous & Mental Disease. 1999;187:360–368. doi: 10.1097/00005053-199906000-00005. [DOI] [PubMed] [Google Scholar]
- Ro E, Clark LA. Psychosocial functioning in the context of diagnosis: Assessment and theoretical issues. Psychological Assessment. 2009;21:313–324. doi: 10.1037/a0016611. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): Psychometric properties. Psychological Medicine. 1996;26:477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Kraemer HC, Sackeim HA, Fava M, Trivedi MH, Frank E, et al. Report by the ACNP task force on response and remission in major depressive disorder. Neuropsychopharmacology. 2006;31:1841–1853. doi: 10.1038/sj.npp.1301131. [DOI] [PubMed] [Google Scholar]
- Schafer J, Graham J. Missing data: Our view of the state of the art. Psychological Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- Spanier GB. Measuring dyadic adjustment: New scales for assessing the quality of marriage and similar dyads. Journal of Marriage & the Family. 1976;38:15–28. [Google Scholar]
- Thase ME. Evaluating antidepressant therapies: Remission as the optimal outcome. Journal of Clinical Psychiatry. 2003;64 (Supplement 13):18–25. [PubMed] [Google Scholar]
- Vittengl JR, Clark LA, Jarrett RB. Improvement in social-interpersonal functioning after cognitive therapy for recurrent depression. Psychological Medicine. 2004;34:643–658. doi: 10.1017/S0033291703001478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittengl JR, Clark LA, Jarrett RB. Deterioration in psychosocial functioning predicts relapse/recurrence after cognitive therapy for depression. Journal of Affective Disorders. 2009a;112:139–143. doi: 10.1016/j.jad.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittengl JR, Clark LA, Jarrett RB. Continuation phase cognitive therapy’s effects on remission and recovery from depression. Journal of Consulting and Clinical Psychology. 2009b;77:367–371. doi: 10.1037/a0015238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Bothwell S. Assessment of social adjustment by patient self-report. Archives of General Psychiatry. 1976;33:1111–1115. doi: 10.1001/archpsyc.1976.01770090101010. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Markowitz JC, Klerman GL. Comprehensive Guide to Interpersonal Psychotherapy. Basic Books; New York: 2000. [Google Scholar]
- Weissman MM, Olfson M, Gameroff MJ, Feder A, Fuentes M. A comparison of three scales for assessing social functioning in primary care. American Journal of Psychiatry. 2001;158:460–466. doi: 10.1176/appi.ajp.158.3.460. [DOI] [PubMed] [Google Scholar]
- Whisman MA. Mediators and moderators of change in cognitive therapy of depression. Psychological Bulletin. 1993;114:248–265. doi: 10.1037/0033-2909.114.2.248. [DOI] [PubMed] [Google Scholar]
- Wilson G, Fairburn CC, Agras W, Walsh B, Kraemer H. Cognitive-behavioral therapy for bulimia nervosa: Time course and mechanisms of change. Journal of Consulting and Clinical Psychology. 2002;70:267–274. [PubMed] [Google Scholar]
- Young J, Beck AT. Cognitive Therapy Scale: Rating Manual. Center for Cognitive Therapy; Philadelphia: 1980. [Google Scholar]


