Abstract
This report describes a mutant of Listeria monocytogenes strain 10403S (serotype 1/2a) with a defective response to conditions of high osmolarity, an environment that L. monocytogenes encounters in some ready-to-eat foods. A library of L. monocytogenes clones mutagenized with Tn917 was generated and scored for sensitivity to 4% NaCl in order to identify genes responsible for growth or survival in elevated-NaCl environments. One of the L. monocytogenes Tn917 mutants, designated strain OSM1, was selected, and the gene interrupted by the transposon was sequenced. A BLAST search with the putative translated amino acid sequence indicated that the interrupted gene product was a homolog of htrA (degP), a gene coding for a serine protease identified as a stress response protein in several gram-positive and gram-negative bacteria. An htrA deletion strain, strain LDW1, was constructed, and the salt-sensitive phenotype of this strain was complemented by introduction of a plasmid carrying the wild-type htrA gene, demonstrating that htrA is necessary for optimal growth under conditions of osmotic stress. Additionally, strain LDW1 was tested for its response to temperature and H2O2 stresses. The results of these growth assays indicated that strain LDW1 grew at a lower rate than the wild-type strain at 44°C but at a rate similar to that of the wild-type strain when incubated at 4°C. In addition, strain LDW1 was significantly more sensitive to a 52°C heat shock than the wild-type strain. Strain LDW1 was also defective in its response to H2O2 challenge at 37°C, since 100 or 150 μg of H2O2 was more inhibitory for the growth of strain LDW1 than for that of the parent strain. The stress response phenotype observed for strain LDW1 is similar to that observed for other HtrA− organisms, which suggests that L. monocytogenes HtrA may play a role in degrading misfolded proteins that accumulate under stress conditions.
Listeria monocytogenes is a gram-positive bacterium associated with sporadic and outbreak cases of food-related illness. The consumption of L. monocytogenes-contaminated food can lead to listeriosis, a condition primarily affecting pregnant women, their neonates, and immunocompromised individuals. A mortality rate of 20 to 25% has been reported in these high-risk populations (25). While L. monocytogenes has been isolated from a variety of food sources (12, 14, 24), outbreaks of listeriosis have been strongly associated with ready-to-eat meat products, causing L. monocytogenes contamination of these products to remain a major public health concern. Because of the serious health risk this organism poses, the U.S. Department of Agriculture Food Safety Inspection Service currently stipulates a zero tolerance policy for L. monocytogenes in ready-to-eat foods such as delicatessen meats, sausages, and frankfurters. These products come in contact with L. monocytogenes primarily in the processing plant, after the cooking step but prior to packaging. If present, L. monocytogenes may not be eliminated from ready-to-eat foods before consumption, since these products are often consumed unheated or inadequately reheated.
L. monocytogenes has a number of characteristics that can allow it to remain a successful pathogen in contaminated foods. This organism is able to survive, and frequently grow, under adverse conditions such as low pH, refrigeration temperatures, and high osmolarity, often present in ready-to-eat meat products (6, 7, 15). Therefore, specific hurdles used in ready-to-eat foods to limit the growth of many food-borne pathogens may not adequately control L. monocytogenes growth. Furthermore, the ability of pathogens to overcome stressful conditions is of particular food safety interest, because exposure to hostile environments frequently provides cross-protection against additional stresses (2, 6, 26, 29, 30). In Salmonella enterica serovar Typhimurium, for example, acid adaptation provides resistance to salt and heat stress (20). In addition, starvation can induce a general stress response in Enterococcus faecalis, allowing enhanced survival under conditions of heat, ethanol, osmotic, and oxidative stress (30). A primary concern is whether the food environments that stimulate a stress response in pathogens also increase the infectivity of those organisms, either by inducing virulence genes or by aiding survival in the gastrointestinal tract or during invasion of eukaryotic cells. Indeed, several studies with L. monocytogenes imply a link between stress response and virulence (40, 44).
Stressful environments may result in deleterious protein effects such as misfolding and damage in stressed bacteria. One way in which Listeria adapts to stresses such as salt and cold is by the uptake and accumulation of small molecules called compatible solutes (1). These protective solutes relieve the damaging effects of stress by maintaining turgor, increasing the free water content of cells, and perhaps additionally increasing the hydration of molecule surfaces to allow for proper protein conformation (16). Two compatible solutes, carnitine and glycine betaine, are commonly present in food and may provide L. monocytogenes with some protection in that environment (39). However, under some stress conditions, compatible solutes alone are insufficient to alleviate the negative effects of stress, and therefore cells must utilize additional mechanisms to survive or adapt.
An understanding of how L. monocytogenes is able to survive high salt concetrations and low temperatures might point to strategies for controlling this organism in foods. The present study characterizes a L. monocytogenes mutant unable to grow at elevated salt concentrations. This mutant also has a reduced capacity to grow at high temperatures and a reduced tolerance to heat shock and H2O2 challenge.
MATERIALS AND METHODS
Strains, plasmids, and growth conditions.
Escherichia coli DH5α was obtained from GIBCO BRL (Rockville, Md.). L. monocytogenes strains 10403S (serotype 1/2a) and DP-L910 (10403S carrying the pLTV3 plasmid) (5) were gifts from Daniel Portnoy (University of California, Berkeley). Other strains constructed during the course of this study are described below. Plasmid pLTV3 (5) harbors the Tn917 transposon, the ColE1 origin of replication, the temperature-sensitive pE194ts gram-positive origin of replication, and genes encoding resistance to tetracycline, erythromycin and lincomycin, kanamycin, and chloramphenicol. The Tn917 transposon present on pLTV3 shows a high degree of random transposition in the L. monocytogenes chromosome, although there may be preferred “hot spots” (5). Plasmid pCON-1 is a shuttle vector (3), kindly provided by Nancy Freitag (Seattle Biomedical Research Institute, Seattle, Wash.). Plasmid pLW1 was created by PCR amplification of the chromosomal htrA gene using primers htrA2 (5′-CGCGGATCCGACCGA ATGAAAGGTCATATT-3′) and htrA3 (5′-GCGGGATCCACCCTCTTTTTCAAGAGAATG-3′). The PCR product included the htrA gene as well as 444 nucleotides upstream of the predicted translational start codon and 19 nucleotides downstream of the translational stop codon. BamHI sites introduced by the primers (underlined in the primer sequences) were used to clone the PCR product into the unique BamHI site of pCON-1, creating pLW1. Sequencing confirmed that the htrA gene sequence on pLW1 was identical to the sequence of the strain 10403S chromosomal htrA gene.
Brain heart infusion (BHI) (Difco Laboratories, Detroit, Mich.) and Luria-Bertani (LB) broth (35) were used as complex media for growth of L. monocytogenes and E. coli, respectively, and Pine's defined medium with 0.5% added glucose as a carbon source (31) was used for several L. monocytogenes phenotypic studies. Where indicated, supplements were added to growth media to achieve the following final concentrations: 1 μg of erythromycin ml−1, 25 μg of lincomycin ml−1, 12.5 μg of tetracycline ml−1, 10 μg of chloramphenicol ml−1, 20 μg of kanamycin ml−1, and 1 mM glycine betaine.
Mutagenesis of strain 10403S.
Mutagenesis of 10403S with pLTV3 was performed essentially according to the method of Camilli et al. (5). Specifically, a single colony of strain DP-L910 was grown in BHI plus erythromycin, lincomycin, and tetracycline to stationary phase at 30°C (permissive temperature); then it was diluted into fresh BHI plus erythromycin and lincomycin and grown at 40°C (nonpermissive temperature) to stationary phase. Cultures were plated onto BHI-plus-erythromycin-and-lincomycin agar plates and incubated at 37°C. Colonies resistant to the antibiotics were patched onto a second BHI-plus-erythromycin-and-lincomycin agar plate and incubated at 37°C. This plate served as the master for replica plating onto the following media: Pine's medium plus 4% NaCl, erythromycin, and lincomycin (37°C); Pine's medium plus 4% NaCl, glycine betaine, erythromycin, and lincomycin (37°C); and BHI plus erythromycin, lincomycin, and tetracycline (30°C). Once a salt-sensitive mutant was selected, chromosomal DNA was isolated, and Southern blot analysis using a 32P-labeled transposon probe confirmed a single Tn917 insertion in the chromosome.
Sequencing of the gene interrupted by Tn917.
The DNA flanking the transposon insertion was sequenced according to the method of Camilli et al. (5). The unique XbaI site in pLTV3 allows excision of part of the plasmid along with a segment of the flanking genomic DNA. To this end, genomic DNA was isolated from strain OSM1 by the method of Flamm et al. (9), digested with XbaI, and self-ligated to form plasmid pOSM1. The ligated DNA was chemically transformed into the E. coli cloning strain DH5α according to the manufacturer's instructions, and kanamycin-resistant (Kmr) transformants were selected. Plasmid DNA was isolated from a transformant and sequenced.
Two PCR strategies were employed, using OSM1 genomic DNA as the template, in order to sequence the entire gene interrupted by Tn917. The first protocol (33) used a primer that annealed to a known sequence of the gene (obtained from pOSM1) to amplify single-stranded products of various lengths, which were subsequently C-tailed with terminal transferase (Roche Molecular Biochemicals, Mannheim, Germany). The C-tailed products were used as templates in a second PCR with a nested primer that annealed to the 3′ end of the gene (obtained from pOSM1) and a poly(G) primer for annealing to the C-tailed end. In the second PCR protocol, a single primer that annealed to a known region of the gene was used with a low annealing temperature to allow specific priming as well as nonspecific priming at other locations on the genome. For both procedures, the PCR products of various lengths were isolated from agarose gels and cloned into the pGEM-T Easy vector (Promega Corporation, Madison, Wis.), and the inserts were sequenced with an ABI Prism 3700 DNA analyzer (Perkin-Elmer Applied Biosystems, Foster City, Calif.). The combination of these two strategies allowed complete sequencing of the gene interrupted by Tn917. Homolog searches were performed using the BLAST programs provided by the National Center for Biotechnology Information. Protein homologs were aligned with the amino acid sequence predicted from the L. monocytogenes open reading frame (ORF) by using the ClustalW alignment program (43). The locations of the htrA genes in the sequenced genomes of L. monocytogenes strains EGD-e (11) and F2365 (The Institute for Genomic Research, Unfinished Microbial Genomes; www.tigr.org/tdb/ufmg/) and Listeria innocua strain CLIP 11262 (11) were analyzed by using the bioinformatics tools Artemis (34) and ACT (The Sanger Institute; http://www.sanger.ac.uk/Software/ACT).
Creation of the htrA deletion strain.
To create strain LDW1, the htrA gene from pLW1 was first cloned into the unique BamHI site of pUC19, resulting in plasmid pLW2. This plasmid was then sequentially digested with the restriction enzymes SwaI and PacI, removing 1,019 nucleotides of the htrA gene that corresponded to amino acids 8 to 348 of the translated protein. After digestion, the ends were filled in by using T4 polymerase (Invitrogen, Carlsbad, Calif.) and dephosphorylated by using shrimp alkaline phosphatase (U.S. Biochemicals, Cleveland, Ohio). Ten-base-pair PmeI linkers (New England Biolabs, Beverly, Mass.) were ligated into the blunt ends, creating an htrA gene with a PmeI site replacing approximately 1 kb of the interior region of the gene. The resulting plasmid, pLW3, was digested with BamHI, and the deleted htrA gene was cloned back into the pCON-1 shuttle vector. This plasmid, pLW4, was introduced into the wild-type strain 10403S. An allelic-exchange protocol (10) was utilized to replace the wild-type htrA gene on the chromosome with the deleted htrA gene. The resulting strain LDW1 was unable to grow on BHI-plus-5% NaCl agar plates. In addition, PCR confirmed that the wild-type htrA gene was no longer present on the chromosome of strain LDW1 but had been replaced by the deleted htrA allele.
Phenotypic characterization assays.
Growth assays were conducted as follows. A single colony was used to inoculate 2 to 5 ml of either Pine's or BHI medium plus the appropriate antibiotics and was grown at either 30 or 37°C overnight with shaking (150 rpm). The cultures were diluted 1:100 into fresh medium prepared with the indicated concentrations of NaCl and antibiotics where appropriate. Cultures were shaken continuously (150 rpm) at the indicated temperature, and optical density readings at 600 nm were taken to measure the growth of the cultures. Where appropriate, growth rates (expressed as doublings per hour) were calculated for exponentially growing cells by using the linear portion of the plotted growth curves. When comparisons of growth rates are discussed, the growth rates were calculated from the same time points for the cultures in question.
To test the response to heat shock conditions, overnight cultures were grown in BHI medium at 30°C. The cultures were then diluted 1:100 into 10 ml of BHI and shaken continuously at 30°C. Growth was measured by optical density at 600 nm, and once the cultures had reached approximately mid-log phase (optical density, 0.4 to 0.6), samples were removed and plated on BHI agar for determining CFU per milliliter. The cultures were then immediately shifted to incubators preequilibrated to 52°C. Samples were removed from the cultures for the next 4 h and plated on BHI agar. After incubation of the plates at 30°C for 24 to 48 h, colonies were enumerated to calculate CFU per milliliter.
To test for H2O2 sensitivity, overnight stationary-phase cultures of strains 10403S and LDW1 were diluted 1:100, and 150 μl of each dilution was spread onto BHI agar plates to generate a lawn. A 5-μl portion of sterile water containing either 100 or 150 μg of H2O2 (Sigma Chemical Co., St. Louis, Mo.) was added to filter disks placed on the spread plate. After overnight incubation at 30 or 37°C, the diameters of the zones of inhibition were measured in millimeters.
Nucleotide sequence accession number.
The 1,629-bp ORF sequenced in this study was submitted to GenBank and assigned accession number AYO49084.
RESULTS
Isolation of an L. monocytogenes mutant with increased sensitivity to NaCl.
Plasmid pLTV3 carrying Tn917 was used to mutagenize the chromosome of L. monocytogenes strain 10403S. The resulting Tn917 mutants were scored for the inability to grow on Pine's solid medium plus 4% NaCl with or without 1 mM glycine betaine. Since glycine betaine can serve as an osmoprotectant for L. monocytogenes (1, 16), allowing the organism to withstand conditions of high osmolarity, it was included in the screening medium to detect mutations that could not be suppressed by this compatible solute. Of the 1,000 colonies screened, three mutants showed an increased sensitivity to 4% NaCl. However, two of these mutants could grow on the 4% NaCl plate when glycine betaine was included in the medium, indicating that glycine betaine could rescue the salt-sensitive phenotype in these strains. One mutant, called OSM1, was unable to grow with 4% NaCl in the presence or absence of glycine betaine and was selected for further characterization. Southern blot analysis confirmed a single copy of the transposon on the chromosome of OSM1 (data not shown).
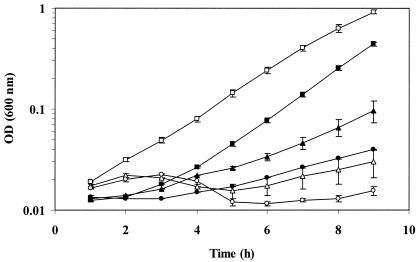
To confirm the salt-sensitive phenotype of the OSM1 mutant, both OSM1 and the wild-type 10403S strain were grown at 37°C in Pine's defined broth medium with three different concentrations of NaCl (Fig. 1). The two strains grew at comparable rates in Pine's broth with 1.8% NaCl. When 3% or 4% NaCl was added to the medium, the growth rate of the wild-type strain was 18 or 6%, respectively, of the wild-type growth rate observed with 1.8% added NaCl. In contrast, strain OSM1 grew slowly for the first 3 h in the presence of 3 or 4% added NaCl and then showed no growth for the next 5 h. The inability of strain OSM1 to sustain growth suggested that strain OSM1 was more sensitive than the wild-type strain to increased concentrations of NaCl.
FIG. 1.
Effects of NaCl on growth of L. monocytogenes strains 10403S and OSM1. Strains 10403S and OSM1 were grown at 37°C in Pine's defined broth with varying concentrations of NaCl. Growth was measured by optical density at 600 nm. Growth curves represent the average optical density from two or more individual growth assays. Error bars, standard deviations among the growth assays. Solid symbols, 10403S; open symbols, OSM1. Squares, 1.8% NaCl; triangles, 3% NaCl; circles, 4% NaCl.
Although glycine betaine is a powerful osmoprotectant for listeriae, it did not alleviate the OSM1 growth defect on solid medium with added NaCl. To test whether glycine betaine could rescue the salt-sensitive phenotype of the mutant in a liquid medium, 1 mM glycine betaine was added to Pine's medium, and the growth of strains 10403S and OSM1 were compared in the presence of 4% NaCl (Fig. 2). As a control, it was first established that glycine betaine had no effect on the growth of strains 10403S and OSM1 in Pine's medium when no NaCl was added, indicating that glycine betaine had little or no stimulatory growth effect in the absence of salt stress (data not shown). When 4% NaCl was included in the growth medium, the addition of 1 mM glycine betaine resulted in a 10.8-fold increase in the growth rate of 10403S over that in medium without glycine betaine, thus demonstrating the ability of this osmoprotectant to overcome conditions of high osmolarity (Fig. 2). The mutant strain OSM1 was unable to grow in Pine's medium when 4% NaCl was included in the growth medium, but the addition of glycine betaine stimulated a slow rate of growth for strain OSM1. These data suggested that glycine betaine could partially alleviate the salt-sensitive phenotype of strain OSM1 in liquid medium. However, the growth rate of strain OSM1 in the presence of 4% added NaCl and 1 mM glycine betaine was only 7% that of the 10403S parent strain under the same conditions. Additionally, the mutant strain had a much lower optical density than strain 10403S after 24 h of incubation. These results indicated that the addition of glycine betaine was not able to completely suppress the salt-sensitive phenotype of OSM1, since the growth rate of the mutant was not restored to wild-type levels.
FIG. 2.
Effects of glycine betaine and NaCl on growth of L. monocytogenes strains 10403S and OSM1. Strains were grown at 37°C in Pine's defined broth with 4% NaCl in either the presence or the absence of 1 mM glycine betaine. Growth was measured by optical density at 600 nm. Growth curves represent the average optical density from two or more individual growth assays. Error bars, standard deviations among the growth assays. Symbols: ▪, 10403S; □, OSM1. Dashed lines represent cultures where 1 mM glycine betaine was added to the growth medium.
The gene disrupted by Tn917 in strain OSM1 encodes a homolog of HtrA.
The gene interrupted by the Tn917 integration was sequenced. A BLAST search revealed that the interrupted gene encoded a protein homologous to HtrA, a serine protease identified in both gram-negative and gram-positive bacteria. The genomic sequences of L. monocytogenes strains EGD (serotype 1/2 a) and F2365 (serotype 4b) were examined and compared with the nucleotide sequence of the htrA gene from strain 10403S. Alignment of these three htrA gene sequences revealed that the genes were approximately 98% identical at the nucleotide level. The htrA sequence from the completed genome sequence of L. innocua strain CLIP 11262 was also examined. The L. innocua htrA sequence shared approximately 88% nucleotide identity with the htrA sequences from L. monocytogenes strains EGD, F2365, and 10403S. In addition, the annotation of the genomes of the three sequenced Listeria strains suggested that the translational start site was likely to be the second Met codon of the htrA ORF and was preceded by a ribosomal binding site. The annotated genomic data also predicted a transcriptional terminator for the htrA gene, suggesting that htrA is not transcribed as part of a multicistronic operon (Fig. 3).
FIG. 3.
Location and arrangement of the htrA gene on the chromosomes of L. monocytogenes strains EGD-e and F2365 and L. innocua strain CLIP 11262. lmo0289, lmo0290, lmo0291, and lmo0293 correspond to the ORF names obtained from the annotated genome sequence of strain EGD-e. Horizontal arrows show the 5′-to-3′ orientation of each ORF. Solid rectangles, predicted ribosomal binding sites. The vertical arrow indicates the position of the predicted transcriptional terminator sequence for the htrA gene. The ranges of nucleotide distances between the htrA gene and the adjacent ORFs represent the distances obtained from the completed genome sequences of L. monocytogenes strains EGD-e and F2365 and L. innocua strain CLIP 11262.
After tentative identification of the gene interrupted by Tn917 as the L. monocytogenes htrA gene, the translated amino acid sequence was aligned with homologous HtrA sequences from other organisms (Fig. 4). The proteins selected for comparison were limited to homologs that have been functionally shown to play a role in stress response (17, 27, 32, 38, 46). The L. monocytogenes HtrA amino acid sequence is 34 to 37% identical to the HtrA homologs from the gram-positive organisms Bacillus subtilis (27), Lactococcus lactis (32), and Lactobacillus helveticus (38). When compared to the HtrA proteins from the gram-negative organisms E. coli (21) and Yersinia enterocolitica (46), the L. monocytogenes HtrA protein was found to share approximately 17 to 22% identity at the amino acid level. The area of highest similarity (38 to 54% identity) occurs in the region surrounding the catalytic H-D-S domain and the C-terminal PDZ protein-protein interaction motif (28).
FIG. 4.
Alignment of the L. monocytogenes (Lm) HtrA protein with HtrA homologs from B. subtilis (Bs), L. lactis (Ll), L. helveticus (Lh), Y. enterocolitica (Ye), and E. coli (Ec). Symbols below residues represent amino acid identity (*) and strong similarity (:). Arrows indicate the H-D-S catalytic residues. Boxed region, putative PDZ domain.
Creation of the htrA deletion strain LDW1 and complementation with htrA on a plasmid.
Our analysis of available Listeria genome sequence data indicated that the transposon insertion in strain OSM1 was unlikely to have polar effects on genes downstream of the interrupted htrA gene. To confirm this analysis, we sought to demonstrate that the salt-sensitive phenotype of strain OSM1 could be complemented by a plasmid bearing the wild-type htrA gene. Complementation experiments can be difficult with strains carrying the Tn917 transposon due to the large number of antibiotic resistances coded for by the transposon; therefore, the use of an htrA deletion mutant could simplify complementation experiments. To this end, an htrA deletion strain, designated LDW1, was created by deleting about 1 kb from the central region of the chromosomal htrA gene of strain 10403S. The nucleotides deleted from htrA corresponded to amino acids 8 through 348 of the translated HtrA protein. Strain LDW1 was then tested for sensitivity to NaCl (Fig. 5a). Growth of the wild-type strain 10403S and growth of strain LDW1 were similar at 30°C in BHI broth with no added salt. However, when the strains were grown at 30°C in BHI plus 5% NaCl, the growth rate of strain LDW1 was approximately 4% of the growth rate observed for strain 10403S, demonstrating that strain LDW1 is sensitive to high levels of NaCl, as was observed with strain OSM1.
FIG. 5.
Growth of strain LDW1 in BHI with 5% added NaCl and complementation of the salt-sensitive phenotype with plasmid pLW1. Growth curves represent the average optical density from two or more individual growth assays. Error bars, standard deviations among the growth assays. (a) Growth of strains 10403S and LDW1 at 30°C in BHI broth with or without 5% added NaCl. Symbols: ▪, 10403S in BHI; □, LDW1 in BHI; ▴, 10403S in BHI plus 5% added NaCl; ▵, LDW1 in BHI plus 5% added NaCl. (b) Complementation of the NaCl-sensitive phenotype with a plasmid carrying the wild-type htrA gene. All strains were grown at 30°C in BHI broth plus chloramphenicol and 5% added NaCl. Symbols: ▪, LDW2 (10403S plus pCON-1); □, LDW4 (LDW1 plus pCON-1); ▴, LDW3 (10403S plus pLW1); ▵, LDW5 (LDW1 plus pLW1).
The salt-sensitive phenotype was complemented by introducing plasmid pLW1, bearing a copy of the htrA gene, into strain LDW1 to create strain LDW5. To serve as controls, strains LDW2 (10403S plus pCON-1), LDW3 (10403S plus pLW1), and LDW4 (LDW1 plus pCON-1) were also created. The growth of these four strains at 30°C in BHI plus chloramphenicol plus 5% NaCl was compared. The results (Fig. 5b) demonstrated that very little growth occurred for strain LDW4, revealing the sensitivity of strain LDW4 to increased levels of NaCl. In addition, after 24 h of growth, strain LDW4 was unable to reach the optical density observed for strains LDW2 and LDW3. In contrast, the complemented strain LDW5 grew at a rate similar to that of the control strain LDW3 and reached a similar optical density. These results indicated that the htrA gene in trans could rescue the salt-sensitive phenotype caused by the deletion of the htrA gene on the chromosome. Interestingly, the growth rate of the control strain LDW3 was approximately 30% of the growth rate observed with strain LDW2, suggesting that the htrA gene in trans may have some deleterious effects on the growth of the wild-type strain.
Stress response phenotype of strain LDW1.
L. monocytogenes strain LDW1 was tested for its response to elevated temperatures, reduced temperatures, and oxidative stress in order to determine the effect that htrA has in allowing L. monocytogenes to overcome these stressful conditions. To test for a temperature-sensitive phenotype, strains LDW1 and 10403S were comparatively characterized for growth at 30, 37, 42, and 44°C in BHI. When incubated at 30, 37, or 42°C, the wild-type and mutant strains grew to similar levels (data not shown). However, when strains were incubated at 44°C (Fig. 6), the growth rate of strain LDW1 was 33% of the growth rate observed for strain 10403S, indicating that deletion of the htrA gene resulted in sensitivity to high temperatures. To test for the ability of strain LDW1 to withstand a heat shock stress, strains LDW1 and 10403S were grown at 30°C to approximately mid-log phase and then shifted to the heat shock temperature of 52°C. The survival of the cultures following the temperature shift was measured by plating on BHI agar. After 3 h at 52°C, strain LDW1 suffered an approximately 2.8-log-unit decrease in CFU per milliliter compared to a 0.4-log-unit decrease for the wild-type strain 10403S (Fig. 7). By 4 h post-heat shock, strain LDW1 had suffered a 2.8-log-unit decrease in CFU per milliliter compared to a 1.5-log-unit decrease for strain 10403S. These results indicate that the L. monocytogenes htrA gene is involved in the heat shock response in this organism.
FIG. 6.
Effect of elevated temperature on growth of L. monocytogenes strains 10403S and LDW1. Cultures were grown in BHI with aeration at 44°C, and optical densities at 600 nm were measured hourly. Growth curves represent the average optical density from two or more individual growth assays. Error bars, standard deviations among the growth assays. Symbols: ▪, 10403S; □, LDW1.
FIG. 7.
Effect of heat shock on survival of L. monocytogenes strains 10403S and LDW1. Cultures were grown at 30°C in BHI to mid-log phase and then shifted to 52°C. Samples were taken during the 4 h of heat shock and plated on BHI agar to enumerate survivors. Inactivation data were obtained from four individual cultures for each strain, and each experiment was run in duplicate. Error bars, standard deviations. Symbols: ▪, 10403S; □, LDW1.
Since L. monocytogenes is well known for its ability to adapt and grow at refrigeration temperatures, the htrA strain was also tested for its ability to grow at 4°C. Strains LDW1 and 10403S were incubated in BHI broth at 4°C for 6 days, and the growth of the cultures was recorded. The results of this experiment indicated that strain LDW1 was able to grow at a rate similar to that of the wild-type strain (data not shown), suggesting that the htrA gene in L. monocytogenes is not involved in adaptation to cold temperatures.
To test the oxidative stress response, the sensitivities of strains LDW1 and 10403S to H2O2 were compared. The strains were each cultured as a lawn of growth on BHI agar plates, and H2O2-saturated disks were placed over the lawns. Plates were incubated at 30 and 37°C, and zones of inhibition surrounding the H2O2-saturated disks were measured. When the plates were incubated at 30°C, the parent strain 10403S and strain LDW1 displayed similar zones of inhibition, suggesting that there was no significant difference in their responses to H2O2-induced stress (data not shown). However, when the plates were incubated at 37°C, the zones of inhibition for strain LDW1 were significantly larger than those for strain 10403S (diameters of zones of inhibition [means ± standard deviations], 14.8 ± 0.52 versus 16.4 ± 0.49 mm for 10403S versus LDW1, respectively, at 150 μg of H2O2 and 11.9 ± 0.58 versus 13.5 ± 1.14 mm, respectively, at 100 μg of H2O2; P < 0.02). These results suggest that a minor temperature stress renders strain LDW1 more sensitive to oxidative stress than the wild-type strain.
DISCUSSION
L. monocytogenes is a continuing problem for the food industry because the organism has the potential to survive and grow in high-osmolarity and low-temperature environments, conditions commonly found in ready-to-eat foods and relied on to control food-borne pathogens. It is important to understand the mechanisms of L. monocytogenes survival in stressful environments, because this pathogen continues to be a significant public health concern. In the present study, a L. monocytogenes mutant with increased sensitivity to NaCl was obtained by random Tn917 mutagenesis of a serotype 1/2a strain. This mutant strain, called OSM1, demonstrated a lower growth rate than the parent strain when cells were grown in the presence of NaCl.
After sequencing of the gene interrupted by Tn917 in strain OSM1, a BLAST search revealed that the gene encodes a protein homologous to the HtrA protein in E. coli. This protein has been identified as a serine protease in E. coli, and its involvement in responses to salt, temperature, and oxidative stress has been well documented (17, 21, 22, 37). In addition, HtrA homologs have a demonstrated role in various stress responses in Yersinia spp. (45, 46), B. subtilis (27), L. lactis (32), and L. helveticus (38), indicating the widespread role of serine proteases in stress responses. The primary role of serine proteases is the degradation of improperly folded proteins that can accumulate under stressful conditions and interfere with normal cell functioning (28). In addition, the E. coli HtrA protein acts as a chaperone at nonstressful temperatures, assisting in the folding and transport of proteins (42). With further studies it may become evident that HtrA homologs in other organisms serve similar “housekeeping” roles under nonstressful conditions.
Elements of L. monocytogenes HtrA shared with other homologs are the catalytic H-D-S residues located in the central region of the proteins and the C-terminal PDZ motifs (28). The H-D-S domain has catalytic activity at sequences likely to be concealed in properly folded proteins, preferably following a valine or isoleucine residue (18). As indicated by studies with the E. coli HtrA protein (36, 41), the PDZ motifs are required for oligomerization into a hexameric ring structure and appear to be involved in substrate recognition. Since the E. coli HtrA protein is periplasmic and the amino acid sequences of the gram-positive homologs indicate membrane localizations (28), there may be significant differences in oligomerization and substrate recognition among the homologs. An additional indication of differences is the presence of two PDZ motifs in the gram-negative homologs, while the gram-positive HtrA proteins possess only one PDZ domain (28). The presence of the second PDZ domain in the gram-negative homologs accounts for some of the differences in amino acid sequence noted between the L. monocytogenes HtrA and its gram-negative counterparts.
Glycine betaine is an effective osmoprotectant for L. monocytogenes and has been suggested to increase the hydration of protein surfaces so as to prevent misfolding (16). The growth assays with strain OSM1 indicated that glycine betaine could not completely suppress the phenotype of the L. monocytogenes htrA mutant. Since HtrA has a proposed role in the degradation of misfolded proteins, glycine betaine might be expected to reduce or eliminate the need for HtrA function. Glycine betaine was able to reduce, but not completely ameliorate, the deleterious effects associated with NaCl stress in the mutant. This was likely due to the activity of glycine betaine in reducing the degree of protein misfolding. We hypothesize that in the mutant strain, glycine betaine is unable to adequately prevent protein misfolding and hence these proteins accumulate. At this point, a functional HtrA protein is required to degrade the abnormal proteins and maintain the growth rate originally observed in the absence of the stress. Another possibility is that products of HtrA degradation may serve as signals that induce stress adaptations. Under these conditions, glycine betaine may be unable to fully compensate for the loss of a global stress response in the htrA mutant. The cooperative roles of HtrA, glycine betaine, and other factors may become more evident as additional L. monocytogenes stress response genes and proteins are identified.
The demonstrated stress response role of the L. monocytogenes htrA gene has food safety implications. Due to its involvement in stress adaptation, HtrA may be important for L. monocytogenes survival in foods, particularly foods containing added salt such as ready-to-eat meat products, which have been associated with outbreaks of listeriosis. The concentrations of NaCl used in this study correlate with salt concentrations found in frankfurters (19), suggesting that an L. monocytogenes htrA mutant may have a reduced capability to survive in some food environments. The widespread role that the HtrA protein plays in stress responses may also point to the importance of this protein for L. monocytogenes survival under conditions found in the environment, in food-processing plants, and during distribution to the consumer. Since stressful conditions have been shown to induce htrA expression (4, 27, 38), it is noteworthy that several pathogenic bacteria have a demonstrated dependence on HtrA for virulence (8, 13, 45). For example, Y. enterocolitica GsrA (an HtrA homolog), which is essential for optimal growth under conditions of high temperature, high osmolarity, and oxidative stress, has been shown to be required for Y. enterocolitica survival in a macrophage-like cell line (46). Since L. monocytogenes and Y. enterocolitica have HtrA homologs that are involved in similar stress responses and since both organisms are intracellular pathogens, the L. monocytogenes HtrA protein may be a previously unrecognized virulence factor. Therefore, environments such as foods that induce L. monocytogenes htrA expression may contribute to increased virulence of this organism. Further experiments will be necessary to determine if the L. monocytogenes htrA gene is a virulence factor.
If L. monocytogenes HtrA is shown to be required for virulence or survivability in foods, it will be interesting to study the expression and proteolytic activity of HtrA in strains isolated at points along the food continuum. Perhaps the L. monocytogenes serotype 4b, 1/2a, and 1/2b strains, which account for the majority of listeriosis outbreaks, have an increased HtrA activity that contributes to the survival of these pathogens in foods. A second potential area of study is the use of HtrA inhibitors to reduce the survivability of L. monocytogenes in foods. Serine protease inhibitors, such as diisopropyl fluorophosphate and phenylmethylsulfonyl fluoride (22, 23), prevent proteolytic cleavage by binding to the catalytic site of the enzyme. Analysis of the chemical structures of known inhibitors may suggest additives currently approved for foods that may be useful for controlling L. monocytogenes.
Acknowledgments
We are grateful to D. Portnoy for the gift of strains 10403S and DP-L90, to N. Frietag for the gift of plasmid pCON-1, and to Connie Briggs for excellent sequencing efforts.
This work was supported in part by award 2002-35201-12781 from the National Research Initiative Competitive Grants Program of the U.S. Department of Agriculture to B.J.W.
Mention of brand or firm names is not an endorsement by the U.S. Department of Agriculture over others of a similar nature not mentioned.
REFERENCES
- 1.Bayles, D. O., and B. J. Wilkinson. 2000. Osmoprotectants and cryoprotectants for Listeria monocytogenes. Lett. Appl. Microbiol. 30:23-27. [DOI] [PubMed] [Google Scholar]
- 2.Begley, M., C. Hill, and C. G. M. Gahan. 2003. Identification and disruption of btlA, a locus involved in bile tolerance and general stress resistance in Listeria monocytogenes. FEMS Microbiol. Lett. 218:31-38. [DOI] [PubMed] [Google Scholar]
- 3.Behari, J., and P. Youngman. 1998. Regulation of hly expression in Listeria monocytogenes by carbon sources and pH occurs through separate mechanisms mediated by PrfA. Infect. Immun. 66:3635-3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bianchi, A. A., and F. Baneyx. 1999. Hyperosmotic shock induces the σ32 and σE stress regulons of Escherichia coli. Mol. Microbiol. 34:1029-1038. [DOI] [PubMed] [Google Scholar]
- 5.Camilli, A., D. A. Portnoy, and P. Youngman. 1990. Insertional mutagenesis of Listeria monocytogenes with a novel Tn917 derivative that allows direct cloning of DNA flanking transposon insertions. J. Bacteriol. 172:3738-3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheroutre-Vialette, M., I. Lebert, M. Hebraud, J. C. Labadie, and A. Lebert. 1998. Effects of pH or aw stress on growth of Listeria monocytogenes. Int. J. Food Microbiol. 42:71-77. [DOI] [PubMed] [Google Scholar]
- 7.Conner, D. E., R. E. Brackett, and L. R. Beuchat. 1986. Effect of temperature, sodium chloride, and pH on growth of Listeria monocytogenes in cabbage juice. Appl. Environ. Microbiol. 52:59-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cortés, G., B. de Astorza, V. J. Benedí, and S. Albertí. 2002. Role of the htrA gene in Klebsiella pneumoniae virulence. Infect. Immun. 70:4772-4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flamm, R. K., D. J. Hinrichs, and M. F. Thomashow. 1984. Introduction of pAMβ1 into Listeria monocytogenes by conjugation and homology between native L. monocytogenes plasmids. Infect. Immun. 44:157-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freitag, N. E. 2000. Genetic tools for use with Listeria monocytogenes, p. 488-498. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. ASM Press, Washington, D.C.
- 11.Glaser, P., L. Frangeul, C. Buchrieser, C. Rusniok, A. Amend, F. Baquero, P. Berche, H. Bloecker, P. Brandt, T. Chakraborty, A. Charbit, F. Chetouani, E. Couvé, A. de Daruvar, P. Dehoux, E. Domann, G. Domínguez-Bernal, E. Duchaud, L. Durant, O. Dussurget, K.-D. Entian, H. Fsihi, F. G. Portillo, P. Garrido, L. Gautier, W. Goebel, N. Gómez-López, T. Hain, J. Hauf, D. Jackson, L.-M. Jones, U. Kaerst, J. Kreft, M. Kuhn, F. Kunst, G. Kurapkat, E. Madueño, A. Maitournam, J. M. Vicente, E. Ng, H. Nedjari, G. Nordsiek, S. Novella, B. de Pablos, J. C. Pérez-Diaz, R. Purcell, B. Remmel, M. Rose, T. Schlueter, N. Simoes, A. Tierrez, J. A. Vázquez-Boland, H. Voss, J. Wehland, and P. Cossart. 2001. Comparative genomics of Listeria species. Science 294:849-852. [DOI] [PubMed] [Google Scholar]
- 12.Heisick, J. E., D. E. Wagner, M. L. Nierman, and J. T. Peeler. 1989. Listeria spp. found on fresh market produce. Appl. Environ. Microbiol. 55:1925-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson, K., I. Charles, G. Dougan, D. Pickard, P. O'Gaora, G. Costa, T. Ali, I. Miller, and C. Hormaeche. 1991. The role of a stress-response protein in Salmonella typhimurium virulence. Mol. Microbiol. 5:401-407. [DOI] [PubMed] [Google Scholar]
- 14.Jørgensen, L. V., and H. H. Huss. 1998. Prevalence and growth of Listeria monocytogenes in naturally contaminated seafood. Int. J. Food Microbiol. 42:127-131. [DOI] [PubMed] [Google Scholar]
- 15.Juneja, V. K., T. A. Foglia, and B. S. Marmer. 1998. Heat resistance and fatty acid composition of Listeria monocytogenes: effect of pH, acidulant, and growth temperature. J. Food Prot. 61:683-687. [DOI] [PubMed] [Google Scholar]
- 16.Kempf, B., and E. Bremer. 1998. Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments. Arch. Microbiol. 170:319-330. [DOI] [PubMed] [Google Scholar]
- 17.Kim, K. I., S.-C. Park, S. H. Kang, G.-W. Cheong, and C. H. Chung. 1999. Selective degradation of unfolded proteins by the self-compartmentalizing HtrA protease, a periplasmic heat shock protein in Escherichia coli. J. Mol. Biol. 294:1363-1374. [DOI] [PubMed] [Google Scholar]
- 18.Kolmar, H., P. R. Waller, and R. T. Sauer. 1996. The DegP and DegQ periplasmic endoproteases of Escherichia coli: specificity for cleavage sites and substrate conformation. J. Bacteriol. 178:5925-5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kramlich, W. E. 1978. Sausage products, p. 484-512. In J. F. Price and B. S. Schweigert (ed.), The science of meat and meat products. Food and Nutrition Press, Inc., Westport, Conn.
- 20.Leyer, G. J., and E. A. Johnson. 1993. Acid adaptation induces cross-protection against environmental stresses in Salmonella typhimurium. Appl. Environ. Microbiol. 59:1842-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lipinska, B., O. Fayet, L. Baird, and C. Georgopoulos. 1989. Identification, characterization, and mapping of the Escherichia coli htrA gene, whose product is essential for bacterial growth only at elevated temperatures. J. Bacteriol. 171:1574-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lipinska, B., M. Zylicz, and C. Georgopoulos. 1990. The HtrA (DegP) protein, essential for Escherichia coli survival at high temperatures, is an endopeptidase. J. Bacteriol. 172:1791-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loosmore, S. M., Y. P. Yang, R. Oomen, J. M. Shortreed, D. C. Coleman, and M. H. Klein. 1998. The Haemophilus influenzae HtrA protein is a protective antigen. Infect. Immun. 66:899-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lovett, J., D. W. Francis, and J. M. Hunt. 1987. Listeria monocytogenes in raw milk: detection, incidence, and pathogenicity. J. Food Prot. 50:188-192. [DOI] [PubMed] [Google Scholar]
- 25.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy, C., C. Carroll, and K. N. Jordan. 2003. Induction of an adaptive tolerance response in the foodborne pathogen Campylobacter jejuni. FEMS Microbiol. Lett. 223:89-93. [DOI] [PubMed] [Google Scholar]
- 27.Noone, D., A. Howell, and K. M. Devine. 2000. Expression of ykdA, encoding a Bacillus subtilis homologue of HtrA, is heat shock inducible and negatively autoregulated. J. Bacteriol. 182:1592-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pallen, M. J., and B. W. Wren. 1997. The HtrA family of serine proteases. Mol. Microbiol. 26:209-221. [DOI] [PubMed] [Google Scholar]
- 29.Periago, P. M., W. van Schaik, T. Abee, and J. A. Wouters. 2002. Identification of proteins involved in the heat stress response of Bacillus cereus ATCC 14579. Appl. Environ. Microbiol. 68:3486-3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pichereau, V., A. Hartke, and Y. Auffray. 2000. Starvation and osmotic stress induced multiresistances. Influence of extracellular compounds. Int. J. Food Microbiol. 55:19-25. [DOI] [PubMed] [Google Scholar]
- 31.Pine, L., G. B. Malcolm, J. B. Brooks, and M. I. Daneshvar. 1989. Physiological studies on the growth and utilization of sugars by Listeria species. Can. J. Microbiol. 35:245-254. [DOI] [PubMed] [Google Scholar]
- 32.Poquet, I., V. Saint, E. Seznec, N. Simoes, A. Bolotin, and A. Gruss. 2000. HtrA is the unique surface housekeeping protease in Lactococcus lactis and is required for natural protein processing. Mol. Microbiol. 35:1042-1051. [DOI] [PubMed] [Google Scholar]
- 33.Rudi, K., T. Fossheim, and K. S. Jakobsen. 1999. Restriction cutting independent method for cloning genomic DNA segments outside the boundaries of known sequences. BioTechniques 27:1170-1177. [DOI] [PubMed] [Google Scholar]
- 34.Rutherford, K., J. Parkhill, J. Crook, T. Horsnell, P. Rice, M.-A. Rajandream, and B. Barrell. 2000. Artemis: sequence visualization and annotation. Bioinformatics 16:944-945. [DOI] [PubMed] [Google Scholar]
- 35.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 36.Sassoon, N., J. P. Arie, and J. M. Betton. 1999. PDZ domains determine the native oligomeric structure of the DegP (HtrA) protease. Mol. Microbiol. 33:583-589. [DOI] [PubMed] [Google Scholar]
- 37.Skorko-Glonek, J., D. Zurawa, E. Kuczwara, M. Wozniak, Z. Wypych, and B. Lipinska. 1999. The Escherichia coli heat shock protease HtrA participates in defense against oxidative stress. Mol. Gen. Genet. 262:342-350. [DOI] [PubMed] [Google Scholar]
- 38.Smeds, A., P. Varmanen, and A. Palva. 1998. Molecular characterization of a stress-inducible gene from Lactobacillus helveticus. J. Bacteriol. 180:6148-6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith, L. T. 1996. Role of osmolytes in adaptation of osmotically stressed and chill-stressed Listeria monocytogenes grown in liquid media and on processed meat surfaces. Appl. Environ. Microbiol. 62:3088-3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sokolovic, Z., J. Riedel, M. Wuenscher, and W. Goebel. 1993. Surface-associated, PrfA-regulated proteins of Listeria monocytogenes synthesized under stress conditions. Mol. Microbiol. 8:219-227. [DOI] [PubMed] [Google Scholar]
- 41.Spiers, A., H. K. Lamb, S. Cocklin, K. A. Wheeler, J. Budworth, A. L. Dodds, M. J. Pallen, D. J. Maskell, I. G. Charles, and A. R. Hawkins. 2002. PDZ domains facilitate binding of high temperature requirement protease A (HtrA) and tail-specific protease (Tsp) to heterologous substrates through recognition of the small stable RNA A (ssrA)-encoded peptide. J. Biol. Chem. 277:39443-39449. [DOI] [PubMed] [Google Scholar]
- 42.Spiess, C., A. Beil, and M. Ehrmann. 1999. A temperature-dependent switch from chaperone to protease in a widely conserved heat shock protein. Cell 97:339-347. [DOI] [PubMed] [Google Scholar]
- 43.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. ClustalW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiedmann, M., T. J. Arvik, R. J. Hurley, and K. J. Boor. 1998. General stress transcription factor σB and its role in acid tolerance and virulence of Listeria monocytogenes. J. Bacteriol. 180:3650-3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams, K., P. C. Oyston, N. Dorrell, S.-R. Li, R. W. Titball, and B. W. Wren. 2000. Investigation into the role of the serine protease HtrA in Yersinia pestis pathogenesis. FEMS Microbiol. Lett. 186:281-286. [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto, T., T. Hanawa, S. Ogata, and S. Kamiya. 1996. Identification and characterization of the Yersinia enterocolitica gsrA gene, which protectively responds to intracellular stress induced by macrophage phagocytosis and to extracellular environmental stress. Infect. Immun. 64:2980-2987. [DOI] [PMC free article] [PubMed] [Google Scholar]