Abstract
To understand the drivers and consequences of malaria in epidemic-prone regions, it is important to know whether epidemics emerge independently in different areas as a consequence of local contingencies, or whether they are synchronized across larger regions as a result of climatic fluctuations and other broad-scale drivers. To address this question, we collected historical malaria surveillance data for the Amhara region of Ethiopia and analyzed them to assess the consistency of various indicators of malaria risk and determine the dominant spatial and temporal patterns of malaria within the region. We collected data from a total of 49 districts over years from 1999–2010. Data availability was higher for more recent years and more data was available for clinically-diagnosed outpatient malaria cases than confirmed malaria cases. Temporal patterns of outpatient malaria case counts were correlated with the proportion of outpatients diagnosed with malaria and confirmed malaria case counts. The proportion of outpatients diagnosed with malaria was spatially clustered, and these cluster locations were generally consistent from year to year. Outpatient malaria cases exhibited spatial synchrony at distances up to 300 km, supporting the hypothesis that regional climatic variability is an important driver of epidemics. Our results suggest that decomposing malaria risk into separate spatial and temporal components may be an effective strategy for modeling and forecasting malaria risk across large areas. They also emphasize both the value and limitations of working with historical surveillance datasets and highlight the importance of enhancing existing surveillance efforts.
Introduction
Malaria is one of the most common infectious diseases in the world and a major public health problem throughout sub-Saharan Africa. Within this region, malaria epidemics occur most frequently in highland and semi-arid zones and are often associated with interannual fluctuations in rainfall and temperature (Abeku, 2007). These epidemics can be particularly devastating because they occur in areas where large portions of the population lack immunity to malaria. Better information about the timing and locations of malaria epidemics would allow for more accurate targeting of resources for malaria prevention, control, and treatment. Therefore, there is general agreement about the importance of malaria surveillance for early detection of epidemics and the potential value of malaria early-warning systems based on environmental monitoring and seasonal climate forecasting (DaSilva et al., 2004). To develop effective early-detection and early-warning systems, it is essential to first understand the underlying pattern and scale of malaria occurrence in both time and space. In particular, it is important to know whether epidemics emerge independently in different areas as a consequence of local contingencies, or whether they are synchronized across larger regions as a result of climatic fluctuations and other broad-scale drivers.
As with other diseases, malaria is spatially autocorrelated over a range of scales from local to global (Ernst et al., 2006, Brooker et al., 2004, Hay et al., 2009). The geographic distribution of malaria is strongly influenced by climate, and is restricted to areas where there is enough rainfall to create mosquito breeding habitats, sufficient humidity for high activity and survival of vector mosquitoes, and high temperatures that support rapid gonotropic and sporogonic cycles (Stresman, 2010). In mountainous regions, there is typically an elevation limit above which stable malaria transmission is limited by low temperatures (Abeku et al., 2003). Malaria epidemics occur in areas of unstable transmission, which are often located in marginal climates where environmental conditions conducive to high rates of malaria transmission do not occur every year and human populations have low immunity. At more localized scales, topography and land cover influence the pooling of water to form breeding sites and the microenvironments in which mosquito larvae develop (Cohen et al., 2008, Munga et al., 2009). Other human impacts, such as dam construction and irrigation management, also affect local hydrology, development of mosquito breeding sites, and the resulting spatial patterns of malaria risk (Lautze et al., 2007).
Malaria often exhibits strong seasonality, reflecting seasonal patterns of precipitation, temperature, and land use (Mabaso et al., 2007). In the highlands of East Africa, seasonal peaks in malaria cases generally follow the major periods of monsoon rainfall. Interannual variability in malaria incidence arises as result of endogenous dynamics of susceptible, infected, and immune individuals as well as climatic variability (Alonso et al., 2011, Laneri et al., 2010). Numerous studies have shown that malaria case numbers exhibit lagged responses to patterns of temperature and precipitation, although the strength of these relationships, the relative importance of different weather variables, and the time lag of the climatic effects all vary with geographic locale (Zhou et al., 2004, Olson et al., 2009, Teklehaimanot et al., 2004). In general, it is expected that precipitation will be the major environmental driver of malaria outbreaks in semi-arid regions; whereas, the effects of temperature are greater in cooler highland areas (Abeku, 2007). There is considerable anecdotal information suggesting that large malaria epidemics in the Ethiopian highlands tend to be synchronized across broad regions, with climatic variability often implicated as a putative driver (Abeku et al., 2003, Fontaine et al., 1961). However, lack of historical data has limited our ability to quantify spatio-temporal patterns of malaria risk and identify the scales of spatial synchrony.
Epidemic malaria is a major public health issue in the Amhara region of Ethiopia, which covers nearly 16 million hectares and has a population of more than 17 million. Previous research in this region has included cross-sectional surveys of malaria prevalence and assessments of the effectiveness of large-scale malaria prevention campaigns (Graves et al., 2009, Jima et al., 2010, Otten et al., 2009, Baume et al., 2009). Other studies have examined the influences of temperature and precipitation on temporal patterns of malaria cases at selected sites within the region (Abeku et al., 2004, Teklehaimanot et al., 2004). Although the presence of geographic, seasonal, and interannual variability in malaria risk is widely recognized, to date there have been no spatially extensive, long-term data available to facilitate regional analysis of these spatial and temporal patterns. To remedy this knowledge gap, we collected and analyzed historical malaria surveillance data for the Amhara region of Ethiopia. Our specific objectives were to (1) assess the consistency of various indicators of temporal variability in malaria risk, (2) test for the presence of spatial and seasonal patterns of malaria cases, and (3) determine whether there is spatial synchrony in the interannual variability of malaria cases.
Methods
Study Area
The Amhara region encompasses 157,000 km2 with elevations ranging from 506 m at the bottom of the Blue Nile Gorge to 4533 m at Ras Dashen, the highest mountain in Ethiopia. The region is characterized by a monsoon climate, with a dry season extending from December through March, and a rainy season that begins in April with rainfall increasing to a peak from June through August. Temperature generally follows an inverse relationship. In Bahir Dar, the largest city in the region, mean daily temperatures range from 32.5 C at the end of the dry season to 14.6 C at the peak of the rainy season. The region is densely populated by more than 17 million inhabitants, and approximately 87.4% of the population lives in rural areas and practices subsistence agriculture. As a result, the landscape is heavily deforested and is dominated by croplands and pasture across most of the region.
Data Collection
Historical malaria surveillance data were collected from multiple woreda (district) health offices and the Federal Ministry of Health, Public Health Emergency Management office by the Health, Development, and Anti-Malaria Association, an Ethiopian NGO. In consultation with the Amhara Regional Health Bureau, sixty epidemic-prone districts were initially selected to encompass the ranges of environmental and sociocultural variability. Of these districts, fifty-six were actually visited because of logistical constraints. Forty-nine districts, encompassing 41% of the total area of the Amara region, were able to provide at least some historical data.
Most of the data that were collected were obtained from the Integrated Disease Surveillance and Response (IDSR) summary forms that are routinely used for surveillance of malaria and other infectious diseases in the Amhara region. All data were in the form of aggregated monthly, district-level statistics and did not include any information that could be used to identify individual patients. Hard copies of these surveillance forms were digitized and combined with other digital datasets into a unified database. The specific variables that were collected included (1) total outpatient visits, which was the number of outpatients attending the clinic for any reason, including malaria, (2) outpatient malaria cases, which was the total number of suspected malaria cases based on clinical diagnosis, and (3) total numbers of tested and of confirmed malaria cases. Confirmed cases were diagnosed via microscopy or multi-species rapid diagnostic test (RDT) and were aggregated by malaria species (Plasmodium falciparum, Plasmodium vivax, and mixed infection).
Data Analysis
To assess the concordance of temporal patterns of clinically-diagnosed outpatient malaria cases with other indicators of malaria risk, we analyzed a subset of eight districts that had a complete monthly record of outpatient malaria cases for at least eight years along with records of total outpatient visits and confirmed cases for the majority of these years. We computed Spearman’s rank correlation coefficients for each of these districts to quantify the associations of monthly outpatient malaria cases with (1) the proportion of outpatients with malaria, computed as the number of outpatient malaria cases divided by the total outpatient visits, (2) confirmed malaria cases, and (3) the proportions of confirmed cases that were identified as Plasmodium falciparum.
To analyze temporal patterns of malaria risk, we used a subset of 12 districts that had complete monthly outpatient malaria case data for 2001–2009. Mixed-effects models were used to estimate variance components for district, year, and month. The natural logarithm of outpatient malaria cases was the dependent variable, and district, year, and month were all modeled as random effects. Likelihood ratio tests were used to detect statistically significant seasonal (month) and interannual (year) effects in the dataset. Random effects and 95% confidence intervals were plotted to determine which years and months exhibited the largest deviations from the global mean.
Mapping and spatial analysis were carried out by computing the proportion of outpatients with malaria for the main epidemic season (September-December) for all district/year combinations that had available data on both outpatient malaria cases and total outpatient visits. The proportion of outpatients with malaria was used to adjust for spatial variability in total outpatient visits across different districts. The Moran’s I statistic was computed for every year from 2001–2009 to quantify spatial autocorrelation using a spatial weights matrix based on inverse distances. Statistical testing was carried out using a permutation test with 9999 replications.
Spatial synchrony of malaria cases was analyzed using outpatient malaria cases for September-December for all district/year combinations that had available data. We used a spline correlogram to quantify spatially lagged correlations between log-transformed time series of outpatient malaria cases over a range of spatial scales (Bjornstad and Falck, 2001, Bjornstad et al., 1999). High positive values of this correlation statistic indicate that districts separated by a given distance exhibit synchronous patterns of interannual variability; whereas, values close to zero indicate that temporal variability is independent. The spline correlogram avoids the limitation of arbitrarily chosen distance classes by modeling temporal correlation as a non-parametric function of distance in space. We used 9999 bootstrap resamples to generate 95% confidence intervals for the covariance function. All districts and years with outpatient case data were used to fit the nonparametric correlation function, and missing data was handled via pairwise deletion of missing values for each pair of time series.
All analyses were carried out in the R statistical analysis environment (R Development Core Team, 2011). The lme4 library (Bates et al., 2011) was used for random effects modeling, the spdep (Bivand, 2012) library was used for spatial autocorrelation analysis, and the ncf library (Bjornstad, 2009) was used to generate the spline correlogram.
Results
Historical malaria surveillance data were collected from a total of 49 districts within the Amhara region for years between 1999 and 2010. Data availability generally increased over this period. The percent of districts with outpatient malaria case data increased from 29% in 1999 to 98% in 2009. The percent of districts with data on total outpatient visits was slightly lower, increasing from 22% in 1999 to 84% in 2009. Data availability was lowest for confirmed malaria cases, which were obtained for only 14% of the districts in 1999 increasing to 59% in 2009. Within a given year, many districts also had missing data for one or more months. The percent of months with missing data ranged from 16% for outpatient malaria cases to 35% for total outpatient visits to 52% for confirmed malaria cases. Overall, there were 20 districts that contained eight or more years of complete data on malaria outpatient cases, compared to only 13 districts with comparable data on total outpatient visits and 8 districts with comparable data on confirmed malaria cases.
There were strong and statistically significant positive correlations between monthly outpatient malaria cases and proportions of outpatients with malaria (0.72–0.91) in all eight of the districts examined (Table 2). Correlations between monthly outpatient cases and slide-confirmed cases were also statistically significant for all eight districts. These correlations were relatively strong for four districts (0.70 – 0.84), and low-to-moderate for the other four districts (0.32 – 0.58). There were relatively weak but statistically significant positive correlations between monthly outpatient malaria cases and the proportion of P. falciparum confirmed cases for five of the eight districts examined.
Table 2.
Associations of clinically-diagnosed outpatient malaria cases (OMC) with proportion of outpatients with clinically-diagnosed malaria (POM), confirmed malaria cases (CMC) and the proportion of confirmed cases identified as Plasomodium falciparum (PPF) for eight districts in the Amhara region of Ethiopia.
| District | Mean Monthly Values | Spearman Rank Correlations with OMC | ||||||
|---|---|---|---|---|---|---|---|---|
| N† | OMC | POM | CMC | PPF | POM | CMC | PPF | |
| Bati | 108 | 765 | 0.30 | 148 | 0.53 | 0.74* | 0.84* | 0.24* |
| Dawa Chefa | 130 | 1720 | 0.35 | 543 | 0.77 | 0.85* | 0.70* | 0.01 |
| Guba Lafto | 118 | 385 | 0.16 | 41 | 0.50 | 0.90* | 0.70* | −0.08 |
| Mecha | 102 | 3134 | 0.42 | 290 | 0.65 | 0.86* | 0.58* | 0.31* |
| Meket | 128 | 359 | 0.12 | 7 | 0.36 | 0.91* | 0.40* | 0.26* |
| Minjar Shenkora | 120 | 75 | 0.13 | 22 | 0.29 | 0.79* | 0.38* | 0.01 |
| South Achefer | 107 | 1681 | 0.36 | 241 | 0.53 | 0.90* | 0.84* | 0.50* |
| Tacharmacho | 130 | 1172 | 0.38 | 259 | 0.66 | 0.72* | 0.32* | 0.20* |
Months of data for which all variables were available
Statistically significant at α=0.05 level
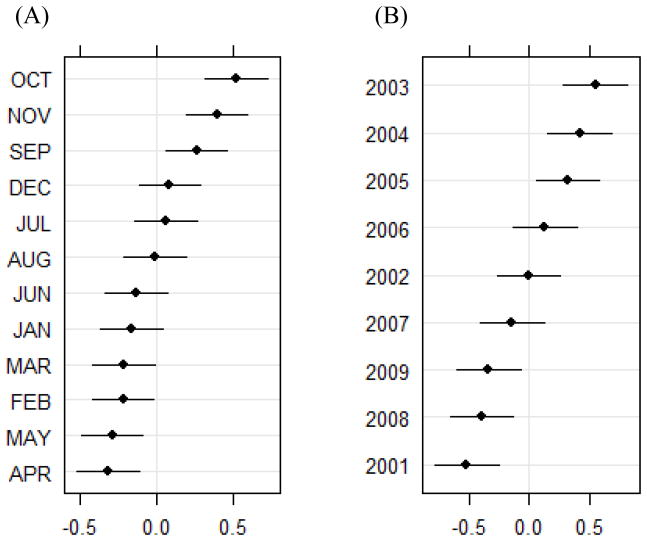
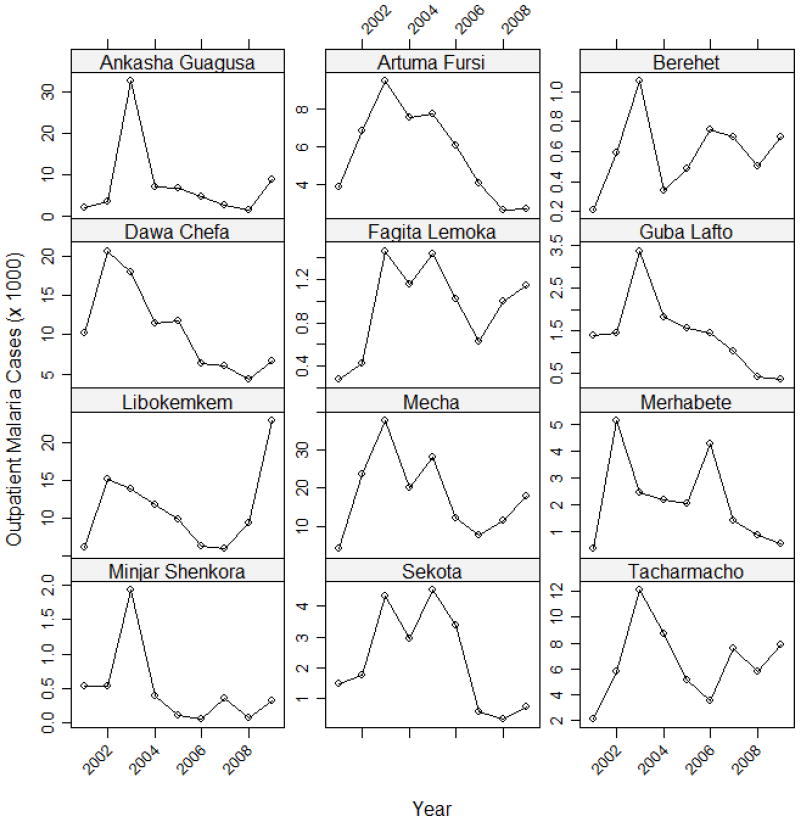
The variance components analysis revealed statistically significant seasonal (X2 = 139.0, df = 1, p < 0.0001) and interannual (X2 = 257.7, df = 1, p < 0.0001) variability in the twelve districts that were examined. October was the month with the largest positive random effect, followed by November, September, and December. The random effects for October, November, and September were all greater than 0 at a 95% confidence interval (Figure 2). The years with the largest positive random effects were 2003, 2004, and 2005 and these values were all greater than 0 at a 95% confidence interval. These interannual patterns were also apparent in the line graphs of total outpatient malaria cases during the September-December peak season plotted for individual districts (Figure 3). Most of the districts exhibited a peak in 2002 or 2003, but the subsequent temporal patterns were more variable across the different districts. Many of the districts also showed an increase in outpatient malaria cases after 2008.
Figure 2.
Random effects and 95% confidence intervals (plotted on the x-axis) for (A) month, and (B) year from a random effects model with the natural logarithm of outpatient malaria cases as the dependent variable and month, year, and district as independent variables.
Figure 3.
Interannual variability in clinically-diagnosed outpatient malaria cases during the peak malaria season (September-December) for 12 districts with complete data.
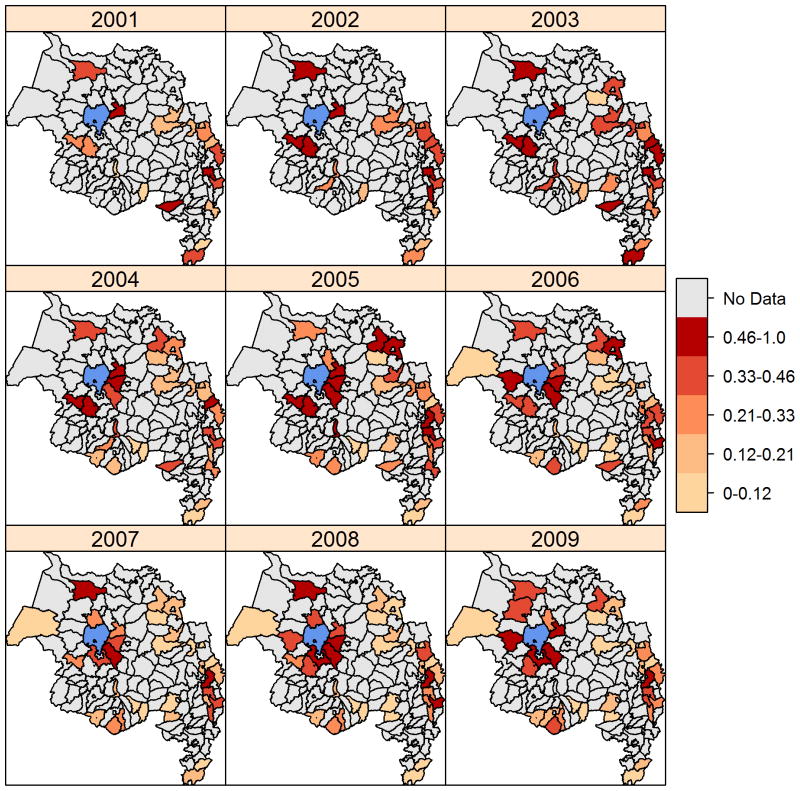
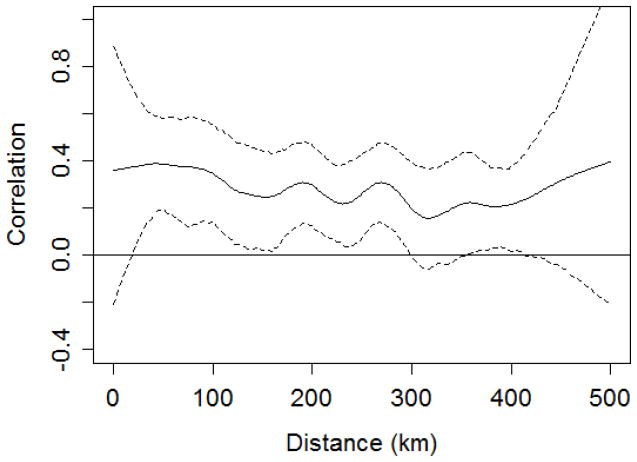
There was statistically significant positive spatial autocorrelation of the proportion of outpatients with malaria in all years except 2001 and 2006 (Table 3). Maps showed that, in most years, there were clusters of districts with high values in the low elevation areas around Lake Tana (e.g., Libokemkem, Mecha, and South Achefer) and along the eastern edge of the Amhara region (e.g., Artuma Fursi, Bati, and Dawa Chefa) (Figure 4). In contrast, districts with lower proportions of outpatients with malaria were concentrated in the center of the region and along the southern edge. The spline correlogram indicated statistically significant spatial synchrony in outpatient malaria cases at distances from approximately 25 km to 300 km (Figure 5). The correlation coefficient was highest from 25 km and 75 km and decreased slightly at longer distances. Confidence intervals overlapping zero at distances from 0 to 25 km reflected the threshold minimum distance between the centroids of adjacent districts.
Table 3.
Spatial autocorrelation of the proportion of outpatients with clinically-diagnosed malaria (POM) for nine years in the Amhara region of Ethiopia.
| Year | I | N† | P-value |
|---|---|---|---|
| 2001 | −0.12 | 18 | 0.691 |
| 2002 | 0.30 | 19 | 0.017 |
| 2003 | 0.18 | 24 | 0.041 |
| 2004 | 0.43 | 28 | < 0.001 |
| 2005 | 0.21 | 31 | 0.015 |
| 2006 | 0.12 | 32 | 0.070 |
| 2007 | 0.17 | 32 | 0.031 |
| 2008 | 0.26 | 35 | 0.003 |
| 2009 | 0.20 | 33 | 0.019 |
Number of districts with available POM data for September-December in the given year
Figure 4.
Maps of the proportion of outpatients clinically diagnosed with malaria during the peak malaria season (September-December) from 2001–2009, including all districts in each year that had data on both outpatient malaria cases and total outpatient visits. Water bodies are displayed in blue.
Figure 5.
Spline correlogram illustrating the nonparametric spatial covariance function and 95% confidence interval for the natural logarithm of clinically-diagnosed outpatient malaria cases during the peak malaria season (September-December).
Discussion
Data availability and data quality are two major limiting factors in long-term analyses of malaria risk. As in many other parts of Africa, counts of clinically diagnosed malaria cases were the most widely available indicator of historical malaria risk in the Amhara region. The proportion of outpatients with malaria is recommended as an indicator of malaria epidemics because it corrects for temporal variability in attendance at health facilities (Guintran et al., 2006), but data on total outpatient visits were only available for a subset of the districts that had outpatient malaria case counts. However, our analyses found strong correlations between counts and proportions of outpatient malaria cases in the eight districts we examined, indicating that the counts of outpatient malaria cases captured the temporal variability of the outpatient malaria burden on the health system within a given district.
Data on confirmed malaria cases are preferred as indicators of malaria risk because the specificity of clinical diagnosis is limited by the overlap between symptoms of malaria and those of other tropical diseases (Wongsrichanalai et al., 2007). However, in the Amhara region, routine microscopy is only conducted at health centers and hospitals and not at the more peripheral health posts. Since 2005, multi-species RDTs have been increasingly used at the health post level to confirm malaria diagnosis, as reflected in the increased availability of confirmed malaria case data over time. The effectiveness of malaria diagnosis through either microscopy or RDTs is also influenced by fluctuating levels of parasitemia in the individuals tested, inconsistency in the quality of microscopy, and variability in the accuracy of RDTs (Guintran et al., 2006, O’Meara et al., 2007, Endeshaw et al., 2012). The correlations between outpatient case counts and slide-confirmed case counts showed that the major temporal fluctuations in these two data sources were generally concordant and increased our confidence that malaria indicators based on clinically-diagnosed outpatient malaria cases captured major seasonal and interannual patterns of malaria occurrence. The correlations between outpatient case numbers and the proportion of slide-confirmed cases that were P. falciparum further suggested that in many areas, but not all, malaria outbreaks were driven by increases in the more severe P. falciparum malaria.
Results of the spatial autocorrelation analysis provided evidence of spatial clustering in most years, and visualization of these patterns across multiple years suggested the presence of at least two foci that have persisted in both epidemic and non-epidemic years. This finding is in agreement with previous research suggesting that spatial clusters of high malaria risk tend to remain in the same places over time (Ernst et al., 2006). Because of variability in data availability across years, it was not possible to carry out a rigorous statistical test of the temporal stability of these potential “hot spots”. However, future research efforts can develop models based on spatial and temporal autocorrelation and relationships with environmental covariates to created interpolated malaria risk surfaces covering the districts not sampled (Gething et al., 2006, Wimberly et al., 2008).
The seasonal pattern of outpatient malaria cases supports the generally-accepted notion that the main malaria season extends from September-December in the Amhara region, but also emphasizes that seasonal peaks are highest in October and November. The variance components analysis indicated a consistent pattern of higher outpatient malaria cases in 2003–2005 across the 12 districts with nine years of complete data, and lower case numbers in 2001, 2008, and 2009 across the same 12 districts. The spline correlogram provided further evidence of broad-scale spatial synchrony at distances up to 300 km, indicating that a significant portion of the temporal variability in malaria risk is synchronized over large portions of the Amhara region. The correlation coefficient remained above zero at distances great than 300 km, suggesting that synchrony might be detectable at even larger distances if a more extensive dataset was available.
Broad-scale spatial synchrony of malaria risk is consistent with the hypothesis that climatic variability is a key driver of malaria epidemics in the Amhara region and other parts of East Africa (Zhou et al., 2004). There is considerable spatial variability in climate across this region that arises from the interaction of heterogeneous topography with multiple major air streams and convergence zones. However, interannual deviations from these climatic normals are correlated across much larger areas, reflecting atmospheric teleconnections with large-scale climate modes (Nicholson, 1996). In Ethiopia, for example, temporal patterns of rainfall are correlated across large watersheds (Cheung et al., 2008). Other factors besides climatic variability, including large-scale population movements and public health campaigns at regional to national levels, may also contribute to broad-scale synchrony in malaria cases. In the Amhara region, the mass distribution of LLIN in 2006 and 2007 likely accounts for at least some of the observed temporal variability in malaria cases (Otten et al., 2009), although synchronous patterns of malaria cases were also observed prior to 2006.
An important public health implication of these spatial and temporal patterns is that the problem of modeling and forecasting malaria risk can potentially be decomposed into separate spatial and temporal components. Spatial clusters of high potential malaria risk can be identified based on land use, topography, hydrology, and other aspects of the landscape that vary at relatively slow rates. These clusters are expected to remain relatively static, although rapid land use changes, building of dams and irrigation projects, or population movements can trigger localized outbreaks. Despite these local effects, a significant component of the interannual variability in malaria risk is likely to be synchronized across larger areas as a result of climatic fluctuations. This synchrony suggests a potential for extrapolating malaria early detection results and early warning forecasts from sentinel sites or modeling temporal fluctuations in malaria risk using data aggregated across multiple districts.
In summary, our results document temporally consistent geographic patterns of malaria risk and broad-scale spatial synchrony in malaria outbreaks in the Amhara region of Ethiopia, a malaria epidemic-prone region. These patterns provide indirect evidence that malaria epidemics are at least partially driven by regional climatic variability. This hypothesis is further supported by recent studies that have found lagged associations between remotely-sensed climatic anomalies and temporal patterns of malaria cases in the Amhara region (Midekisa et al., 2012, Wimberly et al., 2012). Further research is still needed to more clearly elucidate the types of climatic anomalies that trigger malaria outbreaks, and the need for greater availability of climate data to support this type of work has recently been emphasized (Thomson et al., 2011). Our research also underscores the importance of long-term epidemiological datasets for conducting these types of assessments and demonstrates the potential insights that can be gained despite the limitations of historical surveillance data. There is need both to acquire spatially explicit, long-term historical data where they are currently available and to expand and enhance current malaria surveillance efforts to ensure that data availability and quality will continue to improve in the future.
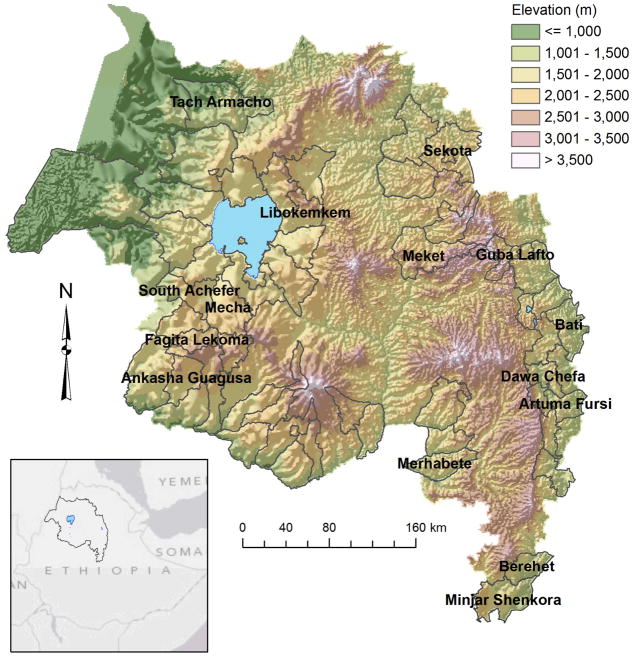
Figure 1.
Map of the Amhara region with outlines of the 49 surveyed districts. The 15 districts referenced by name in the paper are labeled.
Table 1.
Summary of data availability from a database of historical malaria surveillance variables collected for the Amhara region.
| Year | Outpatient Malaria Cases | Total Outpatient Visits | Confirmed Malaria Cases | |||
|---|---|---|---|---|---|---|
| Districts† | % Incomplete‡ | Districts† | % Incomplete‡ | Districts† | % Incomplete‡ | |
| 1999 | 14 | 100.0 | 11 | 100.0 | 7 | 100.0 |
| 2000 | 23 | 43.5 | 17 | 41.2 | 11 | 36.4 |
| 2001 | 26 | 15.4 | 20 | 20.0 | 13 | 15.4 |
| 2002 | 29 | 17.2 | 21 | 19.0 | 14 | 35.7 |
| 2003 | 34 | 17.6 | 25 | 24.0 | 18 | 44.4 |
| 2004 | 40 | 22.5 | 30 | 23.3 | 21 | 38.1 |
| 2005 | 42 | 14.3 | 32 | 18.8 | 24 | 33.3 |
| 2006 | 47 | 23.4 | 38 | 39.5 | 29 | 48.3 |
| 2007 | 45 | 13.3 | 37 | 27.0 | 27 | 25.9 |
| 2008 | 47 | 19.1 | 38 | 23.7 | 29 | 34.5 |
| 2009 | 48 | 29.2 | 41 | 34.1 | 29 | 31.0 |
| 2010 | 43 | 100.0 | 36 | 100.0 | 26 | 100.0 |
Total number of districts with available data in the given year
Percent of districts missing data for at least one month in the given year
Acknowledgments
We thank Abere Mihretie of the Health, Development, and Anti-Malaria Association for his support in coordinating data collection activities and facilitating communication with the public health sector. We also acknowledge the cooperation and support of the Amhara Regional Health Bureau and the Federal Democratic Republic of Ethiopia Ministry of Health. This study was supported by National Institute of Health/National Institute of Allergy and Infectious Diseases Grant R01AI079411.
References
- Abeku TA. Response to malaria epidemics in Africa. Emerging Infectious Diseases. 2007;13:681–686. doi: 10.3201/eid1305.061333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeku TA, De Vlas SJ, Borsboom G, et al. Effects of meteorological factors on epidemic malaria in Ethiopia: a statistical modelling approach based on theoretical reasoning. Parasitology. 2004;128:585–593. doi: 10.1017/s0031182004005013. [DOI] [PubMed] [Google Scholar]
- Abeku TA, Van Oortmarssen GJ, Borsboom G, De Vlas SJ, Habbema JDF. Spatial and temporal variations of malaria epidemic risk in Ethiopia: factors involved and implications. Acta Tropica. 2003;87:331–340. doi: 10.1016/s0001-706x(03)00123-2. [DOI] [PubMed] [Google Scholar]
- Alonso D, Bouma MJ, Pascual M. Epidemic malaria and warmer temperatures in recent decades in an East African highland. Proceedings of the Royal Society B-Biological Sciences. 2011;278:1661–1669. doi: 10.1098/rspb.2010.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B. lme4: Linear mixed-effects models using S4 classes. R package version 0.999375–42 2011 [Google Scholar]
- Baume CA, Reithinger R, Woldehanna S. Factors associated with use and non-use of mosquito nets owned in Oromia and Amhara Regional States, Ethiopia. Malaria Journal. 2009;8:264. doi: 10.1186/1475-2875-8-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivand R. spdep: Spatial dependence: weighting schemes, statistics and models. R package version 0.5–45 2012 [Google Scholar]
- Bjørnstad ON. R package version 1.1–3. 2009. ncf: spatial nonparametric covariance functions. [Google Scholar]
- Bjørnstad ON, Falck W. Nonparametric spatial covariance functions: Estimation and testing. Environmental and Ecological Statistics. 2001;8:53–70. [Google Scholar]
- Bjørnstad ON, Ims RA, Lambin X. Spatial population dynamics: analyzing patterns and processes of population synchrony. Trends in Ecology & Evolution. 1999;14:427–432. doi: 10.1016/s0169-5347(99)01677-8. [DOI] [PubMed] [Google Scholar]
- Brooker S, Clarke S, Njagi JK, et al. Spatial clustering of malaria and associated risk factors during an epidemic in a highland area of western Kenya. Tropical Medicine & International Health. 2004;9:757–766. doi: 10.1111/j.1365-3156.2004.01272.x. [DOI] [PubMed] [Google Scholar]
- Cheung WH, Senay GB, Singh A. Trends and spatial distribution of annual and seasonal rainfall in Ethiopia. International Journal of Climatology. 2008;28:1723–1734. [Google Scholar]
- Cohen JM, Ernst KC, Lindblade KA, Vulule JM, John CC, Wilson ML. Topography-derived wetness indices are associated with household-level malaria risk in two communities in the western Kenyan highlands. Malaria Journal. 2008;7:40. doi: 10.1186/1475-2875-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasilva J, Garanganga B, Teveredzi V, Marx SM, Mason SJ, Connor SJ. Improving epidemic malaria planning, preparedness and response in Southern Africa. Malaria Journal. 2004;3:37. doi: 10.1186/1475-2875-3-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endeshaw T, Graves PM, Ayele B, et al. Performance of Local Light Microscopy and the ParaScreen Pan/Pf Rapid Diagnostic Test to Detect Malaria in Health Centers in Northwest Ethiopia. PLoS One. 2012;7:e33014. doi: 10.1371/journal.pone.0033014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst KC, Adoka SO, Kowuor DO, Wilson ML, John CC. Malaria hotspot areas in a highland Kenya site are consistent in epidemic and non-epidemic years and are associated with ecological factors. Malaria Journal. 2006;5:78. doi: 10.1186/1475-2875-5-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine RE, Najjar AE, Prince JS. The 1958 malaria epidemic in Ethiopia. American Journal of Tropical Medicine and Hygeine. 1961;10:795–803. doi: 10.4269/ajtmh.1961.10.795. [DOI] [PubMed] [Google Scholar]
- Gething PW, Noor AM, Gikandi PW, et al. Improving imperfect data from health management information systems in Africa using space-time geostatistics. Plos Medicine. 2006;3:825–831. doi: 10.1371/journal.pmed.0030271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves PM, Richards FO, Ngondi J, et al. Individual, household and environmental risk factors for malaria infection in Amhara, Oromia and SNNP regions of Ethiopia. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2009;103:1211–1220. doi: 10.1016/j.trstmh.2008.11.016. [DOI] [PubMed] [Google Scholar]
- Guintran J, Delacollette C, Trigg P. WHO/HTM/MAL/2006.1115. World Health Organization; Geneva: 2006. Systems for the early detection of malaria epidemics in Africa: An analysis of current practices and future priorities. [Google Scholar]
- Hay SI, Guerra CA, Gething PW, et al. A World Malaria Map: Plasmodium falciparum Endemicity in 2007. PLoS Medicine. 2009;6:286–302. doi: 10.1371/journal.pmed.1000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jima D, Getachew A, Bilak H, et al. Malaria indicator survey 2007, Ethiopia: coverage and use of major malaria prevention and control interventions. Malaria Journal. 2010;9:58. doi: 10.1186/1475-2875-9-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laneri K, Bhadra A, Ionides EL, et al. Forcing versus feedback: epidemic malaria and monsoon rains in northwest India. Plos Computational Biology. 2010;6:e1000898. doi: 10.1371/journal.pcbi.1000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautze J, McCartney M, Kirshen P, Olana D, Jayasinghe G, Spielman A. Effect of a large dam on malaria risk: the Koka reservoir in Ethiopia. Tropical Medicine & International Health. 2007;12:982–989. doi: 10.1111/j.1365-3156.2007.01878.x. [DOI] [PubMed] [Google Scholar]
- Mabaso MLH, Craig M, Ross A, Smith T. Environmental predictors of the seasonality of malaria transmission in Africa: The challenge. American Journal of Tropical Medicine and Hygiene. 2007;76:33–38. [PubMed] [Google Scholar]
- Midekisa A, Senay G, Henebry GM, Semuniguse P, Wimberly MC. Remote sensing-based time series models for malaria early warning in the highlands of Ethiopia. Malaria Journal. 2012;11:165. doi: 10.1186/1475-2875-11-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munga S, Yakob L, Mushinzimana E, et al. Land use and land cover changes and spatiotemporal dynamics of Anopheline larval habitats during a four-year period in a highland community of Africa. American Journal of Tropical Medicine and Hygiene. 2009;81:1079–1084. doi: 10.4269/ajtmh.2009.09-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson SE. A review of climate dynamics and climate variability in Eastern Africa. In: Johnson TC, editor. The Limnology, Climatology, and Paleoclimatology of East African Lakes. Gordon and Breach; Amsterdam: 1996. pp. 25–56. [Google Scholar]
- O’meara WP, Collins WE, McKenzie FE. Parasite prevalence: A static measure of dynamic infections. American Journal of Tropical Medicine and Hygiene. 2007;77:246–249. [PMC free article] [PubMed] [Google Scholar]
- Olson SH, Gangnon R, Elguero E, et al. Links between climate, malaria, and wetlands in the Amazon Basin. Emerging Infectious Diseases. 2009;15:659–662. doi: 10.3201/eid1504.080822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten M, Aregawi M, Were W, et al. Initial evidence of reduction of malaria cases and deaths in Rwanda and Ethiopia due to rapid scale-up of malaria prevention and treatment. Malaria Journal. 2009;8:14. doi: 10.1186/1475-2875-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. [Google Scholar]
- Stresman GH. Beyond temperature and precipitation Ecological risk factors that modify malaria transmission. Acta Tropica. 2010;116:167–172. doi: 10.1016/j.actatropica.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Teklehaimanot HD, Lipsitch M, Teklehaimanot A, Schwartz J. Weather-based prediction of Plasmodium falciparum malaria in epidemic-prone regions of Ethiopia I. Patterns of lagged weather effects reflect biological mechanisms. Malaria Journal. 2004;3:41. doi: 10.1186/1475-2875-3-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson MC, Connor SJ, Zebiak SE, Jancloes M, Mihretie A. Africa needs climate data to fight disease. Nature. 2011;471:440–442. doi: 10.1038/471440a. [DOI] [PubMed] [Google Scholar]
- Wimberly MC, Baer AB, Yabsley MJ. Enhanced spatial models for predicting the geographic distributions of tick-borne pathogens. International Journal of Health Geographics. 2008;7:15. doi: 10.1186/1476-072X-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimberly MC, Chuang T-W, Henebry GM, et al. A Computer System for Forecasting Malaria Epidemic Risk Using Remotely-Sensed Environmental Data. Proceedings of the Sixth Biennial Conference of the International Environmental Modelling and Software Society; Leipzig, Germany. 2012. [Google Scholar]
- Wongsrichanalai C, Barcus MJ, Muth S, Sutamihardja A, Wernsdorfer WH. A review of malaria diagnostic tools: Microscopy and rapid diagnostic test (RDT) American Journal of Tropical Medicine and Hygiene. 2007;77:119–127. [PubMed] [Google Scholar]
- Zhou G, Minakawa N, Githeko AK, Yan GY. Association between climate variability and malaria epidemics in the East African highlands. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:2375–2380. doi: 10.1073/pnas.0308714100. [DOI] [PMC free article] [PubMed] [Google Scholar]