Abstract
Age-related macular degeneration (AMD) is a disease of the outer retina, characterized most significantly by atrophy of photoreceptors and retinal pigment epithelium accompanied with or without choroidal neovascularization. Development of AMD has been recognized as contingent on environmental and genetic risk factors, the strongest being advanced age. In this review, we highlight pathogenic changes that destabilize ocular homeostasis and promote AMD development. With normal aging, photoreceptors are steadily lost, Bruch's membrane thickens, the choroid thins, and hard drusen may form in the periphery. In AMD, many of these changes are exacerbated in addition to the development of disease-specific factors such as soft macular drusen. Para-inflammation, which can be thought of as an intermediate between basal and robust levels of inflammation, develops within the retina in an attempt to maintain ocular homeostasis, reflected by increased expression of the anti-inflammatory cytokine IL-10 coupled with shifts in macrophage plasticity from the pro-inflammatory M1 to the anti-inflammatory M2 polarization. In AMD, imbalances in the M1 and M2 populations together with activation of retinal microglia are observed and potentially contribute to tissue degeneration. Nonetheless, the retina persists in a state of chronic inflammation and increased expression of certain cytokines and inflammasomes is observed. Since not everyone develops AMD, the vital question to ask is how the body establishes a balance between normal age-related changes and the pathological phenotypes in AMD.
Keywords: age-related macular degeneration, ageing, homeostasis, para-inflammation, oxidative stress, retina
1. Introduction: Aging, Homeostasis, and Age-related Macular Degeneration
Age-related macular degeneration (AMD) is a degenerative disease of the photoreceptors and retinal pigment epithelium (RPE) in the human macula. Early stages of the disease feature deposition of extracellular debris, known as drusen, from the basal side of the RPE into Bruch's membrane. From this point on, the disease may either progress to one of two forms, known as geographic atrophy (GA) and neovascular AMD (nAMD). Patients with GA AMD exhibit areolar loss of the photoreceptors and RPE in the macula, whereas patients with choroidal neovascularization (CNV) experience breakthrough of choroidal neovascular vessels across Bruch's membrane, RPE, the neuroretinal layers in some cases, deposition of exudates, and hemorrhaging (Coleman et al., 2008).
Late-stage AMD is a leading cause of central blindness in industrialized nations (Congdon et al., 2004; Pascolini et al., 2004) and as of 2010 is responsible for approximately 5% of all blindness globally (Pascolini and Mariotti, 2011). Additionally, it is projected that AMD will afflict 3 million Americans over the age of 50 by the year 2020 (Friedman et al., 2004). It is important to note that AMD is a multifactorial disease with no one single cause. Known risks factors for development of AMD are many (AREDS, 2000): age, smoking status (Cackett et al., 2011; Kabasawa et al., 2011; Seddon et al., 1996), obesity and dietary fat consumption (Seddon et al., 2003a; (Seddon et al., 2003b; Seddon et al., 2006), and genetic polymorphisms, particularly the genes CFH, C2, C3, and ARMS2/HRTA1 (Chen, 2010). Additionally, much remains to be learned regarding the factors that lead to development of GA versus neovascularization. Only 10-15% of patients experience CNV and yet this form of the disease accounts for approximately 80% of all severe visual loss and blindness in AMD cases (Jager, 2008). As far as treatment strategies go, there has been success with intravitreal injection of anti-vascular endothelial growth factor (VEGF) to deter CNV, but there are no robust methods to treat patients suffering from GA AMD. In the latter cases, patients are encouraged to adopt lifestyle changes, including daily administration of the AREDS2 formulation (AREDS2, 2013).
The association of AMD with aging begs the same line of questioning geared towards all age-related disease. What is it about aging that makes individuals susceptible to diseases they never faced in their youth? Perhaps more poignantly, why is it that not all aging individuals experience age-related pathologies? In the case of AMD, what differentiates the anatomical, physiological, and biochemical changes common to every member of the elderly population from changes that cause disease and changes that do not? To these questions, we suggest that AMD be viewed as a disease very much to do with dysregulation and dysfunction of homeostatic processes that change as a function of age. These processes include imbalances that arise within the photoreceptor/RPE/choriocapillaris/choroid system (Bhutto and Lutty, 2012; Curcio et al., 2009) in addition to immunological and inflammatory imbalances resulting from the age-related increase in prevalence of the para-inflammatory state (Xu et al., 2009). Furthermore, we intend to distinguish some of the ways in which AMD can be viewed as a disease in which the aging process goes a few steps further than it should. The penultimate section frames these issues from a clinical/translational standpoint, exploring some of the ways in which this knowledge and the gaps in knowledge may be leveraged in order to treat patients suffering from AMD.
2. The Aging Paradigm
On the street, it is relatively simple to categorize individuals into one of a few subsets based on perceived biological age, including childhood, adulthood, and old age. Childhood, from infancy through adolescence, is very much a continuation of the developmental processes that begin during fertilization and terminate only when the body has reached maturity. It is from this point on that we call someone an adult, though there is no gold standard for distinguishing a 20-year-old from a 30-year-old, nor a 30-year-old from a 40-year-old. At least this does not hold true for everyone: there are the 30-year-olds who look 50 and 60-year-olds who look 40. However, we believe most people would agree that there is a high degree of variability when it comes to correlating biological age with chronological age using a purely observational method.
Further investigation into the philosophical nature of aging and how biological and chronological age are related led us to the work of noted Gerontologist Aubrey Nicholas Jasper De Grey. De Grey posits that biological aging stems from the body's need to undergo metabolism. Metabolism involves interactions between reactive chemical species, which lead to production of damaging toxins such as reactive oxygen species (ROS). The body is unable to protect itself fully from these damaging agents, and over time, accumulation of the toxin-induced damage leads to age-related pathology or diseases (Zealley and de Grey, 2012).
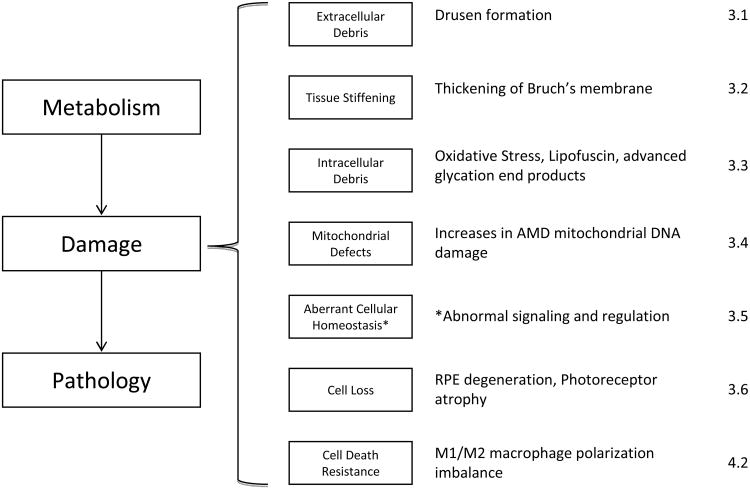
De Grey notes seven damaging events and has conceptualized how to deal with them in his goal of eliminating the phenomenon of age-related pathology. These include accumulation of extracellular debris, tissue stiffening, accumulation of intracellular debris, cell death resistance (loss of homeostatic balance of cell populations, a key finding in T lymphocyte populations observed in age-related immunosenescence), mitochondrial defects, cell overproliferation (as seen in cancer), and cell loss (mediated by apoptosis, autophagy, or necrosis) (Zealley and de Grey, 2012). Throughout the next sections focusing on the anatomical, physiological, and immunological changes observed in AMD, we draw on these facets to provide a framework with which to separate the many age-related and pathological changes seen in AMD (Figure 1).
Figure 1.
The aging paradigm in AMD. The metabolism-damage-pathology rubric follows from the work of Zealley and de Grey (2012). The seven damaging events are indicated in the second column and the correlates observed in AMD in the third column. The final column indicates the sections that discuss these events in AMD and aging. The asterisk (*) is meant to distinguish that de Grey's model is slightly modified when applied to AMD since no cell proliferation is observed in AMD.
3. The Aging Paradigm in AMD
3.1. Extracellular Drusen Formation
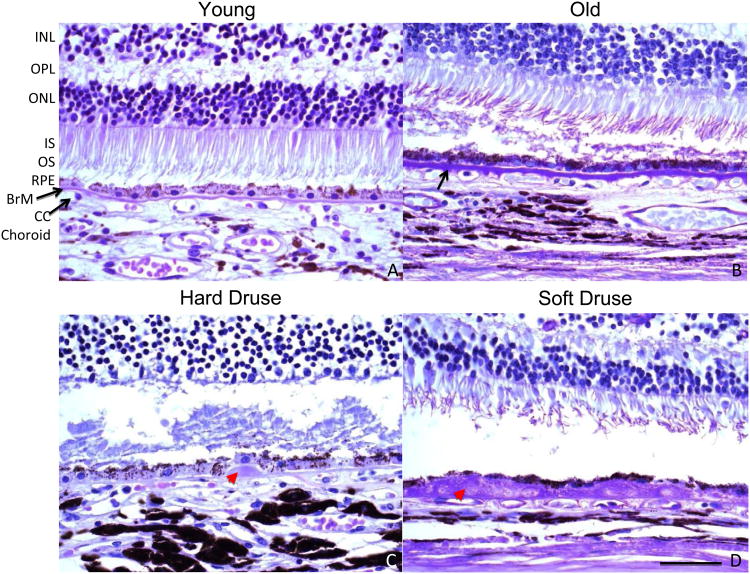
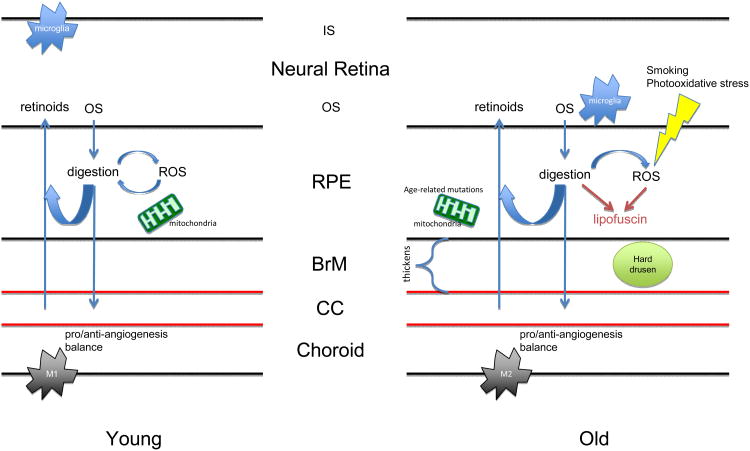
Drusen typically appear with age and fall into several categories depending on their appearance (Figure 2). Under the ophthalmoscope, hard drusen appear to be yellow dots, usually smaller than 63 μm in diameter, and have sharp borders. Soft drusen, which are not considered a part of normal aging, are larger, typically greater than 125 μm in diameter, and may have either sharply defined borders or fuzzy, indistinct borders. Basal linear deposits are composed of primarily membranous material in the inner collagenous layer of Bruch's membrane that is a critical component of soft drusen. Basal laminar deposits are comprised of fibrous long-spacing collagen and amorphous, basal linear-like material located between RPE and its basal lamina (Green, 1999). Of these different types, only hard drusen are considered to be a consequence of normal aging (Figure 3).
Figure 2.
High magnifications of the outer retina, RPE, Batch's membrane, and choroid. The only visibly significant difference between young (A, 23 years old) and old (B, 65 years old) is the thickening of Batch's membrane with age. In retinas with hard drusen (C, 49 years old), deposits form underneath the RPE monolayer within the inner collagenous layer of Batch's membrane, and decreasing intact CC. In retinas with soft drusen (D, 73 years old), significant displacement of the flattened RPE from the CC is observed, likely contributing to loss of homeostasis in the eye. The appearance of the photoreceptors in C and D is a post-mortem artifact unrelated to the finding of drusen. INL: inner nuclear layer, OPL: outer plexiform layer, ONL: outer nuclear layer, IS/OS: inner/outer segments of photoreceptors, RPE: retinal pigment epithelium, BrM: Batch's membrane. The black arrow indicates Batch's membrane. The red arrowhead indicates drusen. Panels depict PAS staining. Scale bar, 50 μm. Paraffin sections cut at 4-6 μm.
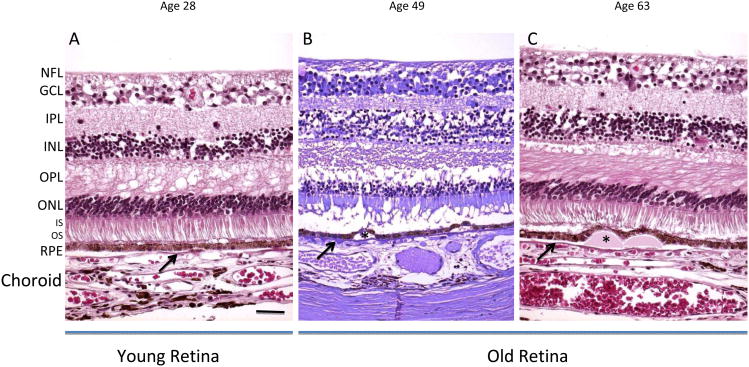
Figure 3.
Histological depiction of young and old. In the young image of a 28-year-old retina, the retinal layers are normal (A). The old retinas (49 and 63 years old) show thinning of the ONL and some RPE damage (B, C). In the drusen-containing retina of old, but healthy individuals, retinal layers appear histologically unaffected, except for the observed displacement of the RPE due to the physical presence of the drusen (asterisks). The arrows indicate Bruch's membrane. NFL: nerve fiber layer, GCL: ganglion cell layer, IPL: inner plexiform layer, INL: inner nuclear layer, OPL: outer plexiform layer, ONL: outer nuclear layer, IS/OS: inner/outer segments of photoreceptors, RPE: retinal pigment epithelium. Sections are paraffin at 4-6 μm. Left and Right panels: H&E; Middle panel: Periodic acid-Schiff (PAS). Scale bar, 50 μm. Paraffin sections cut at 4-6 μm.
Hard drusen are observed in both aging retina and AMD retina. the Copenhagen City Eye Study (2005) provided evidence that hard drusen do not significantly increase the risk of developing AMD, as only 2.9% of patients over a 14-year period ever developed GA or CNV (Buch et al., 2005). This same study indicated much higher rates of AMD progression in individuals with baseline soft distinct drusen (26.7%), and higher rates of GA (34.4%) and CNV (53.1%) in patients with soft drusen. Furthermore, soft drusen were not found outside the macula, whereas hard drusen were present in both the macula and periphery of the retina (Rudolf et al., 2008). Rudolf et al. (2008) identified no significant differences in RPE coverage, a measure of RPE health, between macular and peripheral areas with hard drusen, though they did find the presence of basal laminar deposits exclusively within the macula. Clinical grading systems take into account only the presence of macular hard drusen despite their occurrence in both the macula and periphery. Differential localization of soft and hard drusen, specifically with respect to the macula, underscores the dichotomous nature of the druse. It does not, unfortunately, indicate whether drusen and their compositional differences are causes or symptoms of AMD. Soft drusen are homogenous lesions that tend to cover large areas of the macula when present (Figure 2). Hard drusen may be present in both the macula and the periphery.
On the molecular level, drusen can be considered ocular landfills. However, the chemical composition of hard drusen depends on their location in the retina (Rudolf et al., 2008). Major druse constituents include cellular debris, lipids and lipoproteins (Curcio et al., 2005; Li et al., 2005; Wang et al., 2010a), aggregates of age-related amyloid deposits (Isas et al., 2010; Luibl et al., 2006), and at least 129 proteins including vitronectin, serum albumin, apolipoprotein E, and TIMP3 (Crabb et al., 2002; Wang et al., 2010a). In contrast to normal drusen, AMD drusen show more intense immunoreactivity against TIMP3 protein (Kamei and Hollyfield, 1999) and significantly higher immunoreactivity towards carboxyethylpyrrole (CEP) protein adducts, biomarkers of oxidative stress that are also noted in photoreceptor layers (Gu, 2003). The prevalence of amyloid assemblies and calcification is higher among macular hard drusen than peripheral drusen (Rudolf et al., 2008). Thus, there are two major differences in drusen due to normal aging in comparison to AMD, namely that soft drusen are found exclusively within the macula and that hard and soft drusen have different molecular components.
3.1.1. Reticular pseudo-drusen (RPD)
RPD are a type of drusenoid deposit found subretinally (Querques et al., 2011; Schmitz-Valckenberg et al., 2010; Spaide and Curcio, 2010), and are apparent internal to the RPE by optical coherence tomography (Zweifel et al., 2010b). Prospective studies have reported that RPD is a high risk factor for AMD (Klein et al., 2008; Knudtson et al., 2004; Wang et al., 2003; Zweifel et al., 2010a). However, further studies on RPD are required to confirm their pathogenic role in AMD.
3.2. Bruch's Membrane Stiffening
Bruch's membrane is a heterogeneous structure comprised of extracellular matrix that links the retina and the choroid (Figure 2). Its five layers include the inner basal lamina of the RPE basement membrane, the outer collagenous zone, the elastic zone, the inner collagenous zone, and the outer basal lamina of the choriocapillary basement membrane (CC). It is across these five layers that nutrients including glucose, amino acids, lipids, and oxygen diffuse in order to sustain the retina's high metabolic activity. Bruch's membrane diffusional capacity is likely a function of anatomical thickness and biochemical composition. This capacity is instrumental in keeping a stable balance between influx of nutrients and efflux of waste materials to maintain retinal health. These properties are likely intimately related to the turnover of extracellular matrix (ECM) within Bruch's membrane.
3.2.1. Age-related physiology
Age-related changes that are independent of AMD-induced changes include: increasing thickness of Bruch's membrane (Figure 2) (Ramrattan et al., 1994), lipid accumulation (Pauleikhoff et al., 1990), decreased amino acid diffusion (Hussain et al., 2002), decreased elasticity in response to hydrostatic pressure (Moore et al., 1995; Ugarte et al., 2006), and diminished diffusional capacity of various sized dextran macromolecules (Hussain et al., 2010b) and, in bovine Bruch's membrane, low density lipoprotein (Cankova et al., 2011). The decreases in diffusional capacity are significantly higher in the macula than in the periphery, showing a >95% reduction in the dextran diffusion rate between a 90-year old and a 9-year old macular Bruch's membrane but only a 56% decrease in the periphery (Hussain et al., 2010b).
3.2.2. ECM Turnover
3.2.2.1. TIMP3
One of the key observations made in Bruch's membrane is increased expression of the TIMP3 protein with advancing age (Kamei and Hollyfield, 1999). TIMP3 is found within the extracellular membrane (ECM) of epithelial cells in eyes, lungs, and kidneys (Macgregor et al., 2009). In AMD, it is highly expressed in Bruch's membrane, drusen, and some retinal ganglion cells (Fariss et al., 1997; Macgregor et al., 2009). TIMP3 has several regulatory functions: ECM remodeling by inhibiting matrix metalloproteinases (Brew and Nagase, 2010); inhibiting angiogenesis via VEGFR-2 (Qi et al., 2003); promoting apoptosis via TNF receptor 1, Fas, and TNF-related apoptosis inducing ligand receptor 1 (TRAIL-R1) in cancer cell lines and in vivo (Ahonen et al., 2003; Brew and Nagase, 2010). Most importantly for AMD, TIMP3 is more highly expressed in Bruch's membrane of AMD patients than in that of age-matched controls, though TIMP3 is undetectable in areas that have undergone RPE atrophy (Kamei and Hollyfield, 1999). Excess TIMP3 expression could boost its anti-angiogenic function and deter CNV. On the other, enhanced expression might prevent ECM remodeling and contribute to the thickening of Bruch's membrane, though this hypothesis may be speculative due to a lack of evidence for differences in Bruch's membrane thickness between AMD and age-matched healthy eyes (Spraul et al., 1996). Several studies have found an association with the TIMP3 single-nucleotide polymorphism (SNP) rs9621532 and development of AMD (Chen et al., 2010b; Yu et al., 2011) and our own study of TIMP3 suggests that the moderate protection from CNV offered by rs9625132 could be due to lower TIMP3 transcription in individuals with the minor allele (Ardeljan et al., 2013a). An additional layer of complexity exists regarding the generally accepted phenomenon that ECM in basal lamina has the capacity to sequester growth factors which may be released during ECM remodeling. It is not clear what factors reside within the ECM.
3.2.2.2. Gelatinase
Gelatinases are matrix metalloproteinases that are responsible for degradation of gelatin components of ECM, and are thus one of the big players in Bruch's membrane turnover. The gelatinases found in Bruch's membrane are matrix metalloproteinase (MMP) 9 and MMP2. MMP9 and MMP2 are zymogens, which require cleaving to be activated. As the eye ages, MMP9 and MMP2 are increasingly expressed in aging maculae, but only in their inactive forms (Guo et al., 1999). AMD tissues displayed significantly elevated levels of inactive MMP9, significantly reduced levels of inactive MMP2, and activated MMP9 and MMP2 (Hussain et al., 2011). This could potentially be due to sequestration of MMP9 and MMP2 into high molecular weight aggregates, which were shown to exhibit reduced gelatinase activity and likely contribute to age-related thickening of Bruch's membrane (Hussain et al., 2010a). It has been previously suggested that ECM turnover in Bruch's membrane is intimately dependent on the ratio of MMP to TIMP activity (Fariss et al., 1997; Kamei and Hollyfield, 1999). Considering lower levels of active MMP9 and MMP2 in AMD retinas and overexpression of TIMP3, it is certainly possible that the interplay of these factors contribute to disease insult.
3.2.3. Lipid Homeostasis
In addition to the transport of multi-molecular complexes required for retinal nutrition, previous studies have analyzed the importance for smooth fluid transport across Bruch's membrane in order to maintain retinal health (Starita et al., 1995; Starita et al., 1996; Starita et al., 1997). Disruption to this flow of nutrients, fluids, and debris is very likely a driving agent in the pathology of AMD. As the retina ages, lipid accumulation in Bruch's membrane is observed but there are differences between the composition of macular and peripheral lipids (Gulcan et al., 1993). For instance, significantly higher accumulation of esterified cholesterol occurs in the macular Bruch's membrane than in peripheral Bruch's membrane (Curcio et al., 2001), making this a possible discrepancy for why AMD affects the macula and not the periphery. Accumulation of lipoproteins results in formation of a lipid wall on the inner surface of Bruch's membrane (Curcio et al., 2011). Clearly, not all eyes accumulate lipid to the same extent. Otherwise, it would be seemingly impossible to determine why, if all eyes accumulate lipids in increasing quantities as a function of age, AMD is not observed in most individuals.
A recent study suggested that contribution to deposits could come from circulating low-density lipoprotein in a rat model (Yin et al., 2012), but much evidence from human tissue samples concludes that Bruch's membrane lipids come from RPE (Curcio et al., 2011; Ebrahimi and Handa, 2011). RPE contribution to Bruch's membrane lipid deposits is thought to be highly similar to the formation of atherosclerotic plaques in cardiovascular disease (Curcio et al., 2009, 2010). One mouse model relying on ApoB expression develops early AMD-like features including lipid deposition in Bruch's membrane (Fujihara et al., 2009) whereas another featuring knockout of LDL receptor resulted in AMD-like disease marked by accumulation of Bruch's membrane lipid deposits and VEGF expression, both of which did not occur in wild-type mice (Rudolf et al., 2005).
An oxidized derivative of cholesterol (oxysterol) known as 7-ketocholesterol (7KCh) has been reported in lipoprotein deposits in Bruch's membrane, CC, and RPE cells (Moreira et al., 2009). 7KCh had been previously identified in atherosclerotic plaque formation in cardiovascular disease (Rodriguez and Larrayoz, 2010). With aging, the deposition and oxidation of 7KCh exists at an equilibrium mediated by macrophage phagocytosis (Tabas, 2010). However, equilibrium disruption in macrophage populations (see 4.2) results in 7KCh accumulation in Bruch's membrane and CC, potentially contributing to AMD development (Rodriguez and Larrayoz, 2010). Sterculic acid, a 7KCh inhibitor, can effectively suppress laser-induced CNV in the rat (Huang et al., 2012).
3.3. Intracellular Debris
3.3.1. Oxidative Stress
Oxidative stress is a principle pathogenic element in AMD. It is widely accepted that environmental factors such as smoking significantly increase the risk of developing AMD (Seddon et al., 1996) and incorporating antioxidants and zinc into the diet reduces this risk (AREDS, 2001). Extracellular accumulation of peroxidized lipids has been observed in Bruch's membrane of aged donors (Spaide et al., 1999). In vitreous specimens obtained post-mortem from 38 donors free of AMD, antioxidant capacity was inversely correlated with age, thickness of Bruch's membrane, photoreceptor density, and fundus autofluorescence (Berra et al., 2002). Serum markers of oxidative stress, including malondialdehyde (MDA), protein carbonyl (PC), and 8-Hydroxy-29-deoxyguanosine (8-OHdG), as well as measures of total oxidative stress (TOS) and total antioxidant capacity (TAC), were assessed in recent clinical studies; oxidative stress markers as well as TOS are significantly elevated in AMD patient serum with respect to age-matched health controls (Totan et al., 2009; Venza et al., 2012). Furthermore, TOS in the serum increased with age whereas TAC decreased, albeit to a greater magnitude in AMD patients with respect to controls (Venza et al., 2012). Young and old RPE isolated from rats share only approximately 50% of their proteomic profiles, and much of qualitative and quantitative differences concern proteins involved in the oxidative stress pathways, including but not limited to catalase, glutathione peroxidase 1, superoxide dismutase (Gu et al., 2012).
These data indicate an exacerbated response occurs in AMD versus normal aging. Treatment with antioxidants and omega-3 fatty acids has been reported to preserve RPE health in vitro as well as prevent retinal degeneration in animal models (Cao et al., 2010; Tuo et al., 2012; Tuo et al., 2009). Tissue damage from oxidative stress results from an imbalance of pro- and anti-oxidants. Oxidative stress is highly intertwined with inflammation, as the latter is capable of generating reactive oxygen species and the former may activate NF-κB-regulated inflammatory genes (Han et al., 2001; Li et al., 2012).
3.3.2. Lipofuscin
Lipofuscin granules are byproducts of photoreceptor outer segment turnover and are seen within both healthy and pathological RPE (Sparrow et al., 2012). Lipofuscin in RPE accumulates throughout life (Feeney, 1978), and is readily detectable after age 40 but not necessarily earlier (Feeney-Burns et al., 1980). Lipofuscin pigments are generated from random non-enzymatic reactions of retinaldehyde in photoreceptors throughout life and enter RPE via phagocytosis, from which point pigment-containing phagosomes fuse with lysosomes. However, the random nature of lipofuscin pigment generation results in compounds that evade lysosomal digestion and thus accumulate within the RPE. In a study of 145 normal individuals ranging from 15-80 years, lipofuscin fluorescence was found to increase linearly through age 70, at which point it declined, potentially due to age-related atrophy of the tissue (Delori et al., 2001). Delori et al. (2001) noted that lipofuscin fluorescence matched the spatial distribution of rods and therefore reflected the pattern of age-related loss of photoreceptors, but could not predict areas of photoreceptor degeneration, suggesting a correlation but unable to prove causation. Lipofuscin levels, though increasing with age, were statistically indistinguishable between AMD and healthy RPE from age-matched eyes (Feher et al., 2006), a result that may be affected by the fact that cells in AMD eyes are dying and so lipofuscin is lost or formation is slowed. One pilot study has provided evidence that increasing fluorescence from lipofuscin may be due to overlap of RPE cells and thus only appear to be due to increased sequestration of lipofuscin within RPE (Rudolf et al., 2013).
One of the principle lipofuscin fluorophores, A2E, has been experimentally shown to mediate apoptosis in blue-light exposed RPE cell culture (Sparrow et al., 2000) as well as induce activation of the complement system in vitro (Sparrow, 2010; Zhou et al., 2006; Zhou et al., 2009), providing a link to AMD pathogenesis. This link likely hinges more significantly on genetic predisposition as well as on other pathogenic changes than it does on any specific accumulation of lipofuscin in AMD versus normal aging.
3.3.3. Advanced Glycation End Products (AGEs)
AGEs are oxidative protein modifications resulting from the Maillard nonenzymatic glycation reaction that have been associated with aging and age-related diseases (Baynes, 2001). AGE accumulation has been observed in age-associated inclusions in the human brain (Kimura et al., 1998), aged neurons in the hippocampus (Jono et al., 2002), intervertebral disc tissue (Pillin et al., 2007), inversely correlated with age-related loss in bone density (Odetti et al., 2005). AGEs have been associated with pathogenesis of a variety of age-related diseases including rheumatoid arthritis and osteoarthritis (DeGroot et al., 2001) as well as osteoporosis (Hein et al., 2003). One study reported increases in AGE immunoreactivity co-localized with lipofuscin in the brain of both patients with Alzheimer's disease as well as age-matched controls but not in young, healthy brain tissue (Horie et al., 1997). Within the eye, AGE accumulation— i.e. carboxymethyllysine—has been detected in Bruch's membrane, drusen, RPE, and choroidal extracellular matrix in healthy donor eyes (Crabb et al., 2002; Farboud et al., 1999) and in aging vitreous samples from both men and women after age 50 (van Deemter et al., 2009). AGEs have also been detected in AMD tissues (Hammes et al., 1999; Handa et al., 1999; Howes et al., 2004). Serum levels of the AGEs pentosidine and N-epsilon-carboxymethyllysine (CML) have been identified in significant quantities in AMD patients (Ni, 2009). CML and pentosidine both increase with age in Bruch's membrane (Glenn et al., 2007; Handa et al., 1999). Recently, it was suggested that AGEs may be generated from bisretinoids within RPE (Yoon et al., 2012). Accumulation of AGEs within Bruch's membrane has been shown to alter the RPE proteome, potentially reducing the monolayer's degradative capacity (Glenn et al., 2012).
AGEs communicate to cells via the aptly named receptors, receptor for advanced glycation end products (RAGE), AGE Receptor 1 (R1), AGE R2, and AGE R3. RAGE is a member of the immunoglobulin superfamily activated by multiple ligands including AGEs, amyloid-b, S100B/calgranulin, Mac-1, and others (Ma et al., 2007; Xu et al., 2009). Expression of the AGE receptors is elevated in RPE adjacent to basal laminar deposits with respect to those adjacent to normal Bruch's membrane (Howes et al., 2004; Yamada et al., 2006). RAGE activation by AGEs has been shown to activate NF-kB (Ma et al., 2007; Schmidt et al., 1995), vascular endothelial cell adhesion markers, and pro-inflammatory factors such as IL-6 (Miyata et al., 1996; Schmidt et al., 1994; Schmidt et al., 1995). Amyloid-β has been shown to stimulate microglia chemotaxis and TNF-α expression (Miyata et al., 1996; Yan et al., 1999).
3.4. Mitochondrial Defects
Age-related damage to mitochondrial DNA (mtDNA) (Barron et al., 2001) has been associated with development of AMD in patients (Karunadharma et al., 2010; Kenney et al., 2010). Animal and in vitro studies have provided the basis for mechanistic insight into this phenomenon. Human RPE treated with H2O2 show preferential mtDNA damage over nuclear DNA damage as measured by real-time PCR (Ballinger et al., 1999). Age-related increases in an oxidative DNA damage marker and down-regulated expression of DNA repair enzymes such as 8-oxoguanine-DNA glycosylase 1 (OGG1), mutY homolog (MYH), and thymine DNA glycosylase were observed in rodent RPE and choroid (Wang et al., 2008). Ultrastructural changes occurring in the RPE as a function of both age and AMD include a decrease in the number and area of mitochondria, a loss of cristae and matrix density, proliferation of peroxisomes and lipofuscin granules, though only peroxisomal proliferation was significantly enhanced in AMD (Feher et al., 2006). Feher et al. (2006) hypothesized that alterations in mitochondrial membranes, evidenced by findings of membrane destruction, was a part of aging in the eye and that damage to mitochondria, though present in both aged and AMD retinas, was more severe in AMD retinas. Further evidence is required in order to confirm or refute this hypothesis.
Genome-wide association studies have associated an insertion-deletion polymorphism in what was believed to be a mitochondrial-associated protein age-related maculopathy susceptibility 2 (ARMS2), with development of AMD (Fritsche et al., 2008). However, ARMS2 was later shown distributed throughout the cytosol and not in any specific association with mitochondria (Wang et al., 2009b). AMD RPE was shown to have diminished DNA repair systems, increases in mtDNA damage, and an increased prevalence of mitochondrial heteroplasmic mutations (Lin et al., 2011). Polymorphisms in DNA repair enzymes have been suggested as candidate contributors to AMD susceptibility but these data are largely inference-based with little to no experimental support (Blasiak et al., 2012; Blasiak and Szaflik, 2011).
Several studies have analyzed mitochondrial haplotypes and associated haplotype J with risk of developing AMD in both Austrian and Californian case-control cohorts (Kenney et al., 2013b; Mueller et al., 2012). Functional studies in vitro featuring introduction of mitochondria with either haplotype J or H into ARPE-19 cells without mitochondrial DNA resulted in significantly lower levels of ATP and higher levels of lactate in J-receiving lines, illustrating clear metabolic differences resulting from the haplotype (Kenney et al., 2013a). Such severe metabolic changes occurring within the RPE could be pertinent drivers of pathology. For instance, RPE with reduced levels of ATP, a model of aged RPE in vitro, exhibit reduced autophagy as well as reduced outer segment phagocytic capacity and in increase in oxidative load (Schutt et al., 2012). Loss of the RPE's key role as homeostatic agent in the retina throws the entire system out of balance. Of note, patients with the MELAS A to G 3243 mtDNA point mutation have pigmentary changes in the macula similar to those seen in AMD (Sue et al., 1997), though very few AMD cases feature this mutation (Jones et al., 2004).
3.5. Abnormal Signaling and Regulation
Classical regulators of cell proliferation and differentiation include the MAPK and PI3K/Akt signaling pathways (Chang and Karin, 2001; Manning and Cantley, 2007). MAPK and Akt have been previously implicated in regulating RPE cell viability and proliferation in vitro, but more convincing in vivo studies have surfaced within the last year. It was shown that extracellular signal-regulated kinase-1/2 (ERK1/2) is overly active in GA AMD ocular tissue sections and that ERK1/2 mediates RPE damage in a GA mouse model driven by intracellular accumulation of Alu RNA in murine RPE (Dridi et al., 2012; Kaneko et al., 2011; Tarallo et al., 2012). In the Ccl2−/−/Cx3cr1−/−/Crb1rd8 we have observed that a MAPK-dependent pathway drives tissue pathology in that anti-inflammatory treatment is able to reduce ameliorate retinal degeneration which is mirrored by reduced MAPK activity (Ardeljan et al., 2013b). Thus, while involvement of these intracellular signaling pathways are not proliferative per se, they are indicative of a stress response in the cells mediated by factors classically identified in the cell growth pathways.
3.5.1. Epigenetic Change
Epigenetic modifications are instrumental in regulating gene expression and are one of the principle mechanisms by which germ layers and tissues develop distinct phenotypes along the developmental time course. One study monitored risk factors for stage and severity of AMD in monozygotic twins with discordant disease and found that in such pairs, the twin who smoked more cigarettes or had lower dietary vitamin D also had more severe or advanced AMD phenotype (Seddon et al., 2011). Hypomethylation of the IL-17RC promoter exists in monozygotic twins with discordant disease corresponding to increased mRNA and protein expression in AMD macular lesions (Park et al., 2012; Wei et al., 2012). Hypermethylation of the AMD-associated glutathione S-transferase isoform mu1 (GSTM1) and mu5 (GSTM5) corresponding with reduced expression (Guven et al., 2011; Hunter et al., 2012) has also been reported in AMD patients. In mouse RPE, microsomal glutathione S-transferase (MGST1) has been shown to decrease in expression as a function of age to almost undetectable levels by 18 months (Maeda et al., 2005). These changes result in priming of the environment such that cells are less able to handle oxidative stress and more susceptible to pro-inflammatory signals. Though data in this field is limited, developing methods to alter the gene regulation of all involved inflammatory factors would be certain to aid in the battle against AMD and most other human disease.
3.6. Cell Loss/Tissue Degeneration
3.6.1. Retinal Pigment Epithelium (RPE)
The RPE is a main homeostatic agent in the retina. RPE cells are polarized (Figure 2); the apical side of the RPE is in contact with the photoreceptors and is responsible for constitutive phagocytosis and recycling of shedding outer segments, recycling of all-trans-retinol to 11-cis retinal for use in the visual cycle, as well as providing nutrients to the retina, whereas the basal side is responsible for transporting nutrients and waste materials between the retina and the choroidal blood flow (Kinnunen et al., 2012). RPE pigmentation is essential for focusing clear images on the retina.
Much of the present article includes intimate discussion of RPE-related functions due to the primary role RPE plays in retinal health. The RPE has been implicated as the direct source of drusen lipids due to evidence of bidirectional ApoE and/or B-mediated trafficking of neutral lipids from both the apical RPE to and from the photoreceptors as well as from the basal RPE to the choroidal circulation (Curcio et al., 2011). Furthermore, a recent report revealed that RPE grown in a monolayer deposit basal debris that react with complement (Johnson et al., 2011). According to this experimental system, one would expect that deposition of these lipid-dense materials is a normal physiological process. Absent other cell types, the debris accumulates over time, allowing for the possibility that in AMD the typical debris clearance processes deteriorate over time and at an accelerated rate from what occurs with normal aging. In this light, disease pathology is more so the result of faulty debris removal systems rather than of degeneration of RPE. Prime candidates include immune cells such as macrophages and microglia (see sections 4.2 and 4.3), which, once attracted to the site for clearance purposes, could then become perpetrators of the disease. Perhaps the biggest caveat with most data concerning the RPE is that the majority of in vitro studies use ARPE-19 cells, which lack important properties of primary RPE including but not limited to hexagonal shape, high trans-epithelial resistance, and apical-basolateral polarity (Ablonczy et al., 2011; Kannan et al., 2011). Ablonczy et al. (2011) suggest, however, that ARPE19 cells may mimic aged RPE in their properties.
3.6.2. Neural Retina
Normal aging of the human eye results in remodeling of rod and On-cone bipolar cells as well as horizontal cells of the human retina. The dendritic fibers are significantly elongated and denser, changes that are most pronounced in the peripheral retina and not in the central retina (Eliasieh et al., 2007). The authors speculate that remodeling occurs in response to age-related decline in neuronal synapses, representing the systems' ability to maintain visual capability despite cell death with age. Similar outgrowth of rod bipolar cell and horizontal cell dendrites is observed in mice beginning at 12 months of age and continuing through old age (Terzibasi et al., 2009). These changes have been induced in mouse models of retinal detachment, likely as the systems' attempt to maintain retinal circuitry in response to injury (Lewis et al., 1998). Pathogenic changes in AMD-affected retinas include retraction of photoreceptor dendrites into the outer nuclear layer, an event which spurs dendritic outgrowth from post-synaptic bipolar cells in the periphery (Sullivan et al., 2007), suggesting that similar processes occur in both aging and AMD to the peripheral neurosensory layers.
As rod photoreceptors age in the mouse, their transcriptional profiles change, with significant differences emerging in expression of genes related to angiogenesis, transcriptional regulation, and apoptosis (Parapuram et al., 2010). As the human retina ages, it tends to lose peripheral photoreceptors and RPE at about equal rates (Gao and Hollyfield, 1992), but specifically within the macula, loss of photoreceptors is not seen in cones but is seen in rods, which feature a 30% reduction in density (Curcio et al., 1993). An immunohistochemical study of young, old, and AMD-afflicted retinas suggested that signs of nonfoveal macular cone degeneration are present as early as age 22 and are similar to anomalies found in regions of AMD retinas adjacent to areas of overt degeneration (Shelley et al., 2009). Cones tend to surround AMD disciform scars, whereas rods are absent from these locations (Shelley et al., 2009), corroborating the observation that rods are preferentially lost in AMD (Curcio et al., 1996), but also suggesting that cones exhibit enhanced capacity to survive following age-related damage. No doubt many of these age-related changes in the human and mouse are due to conserved changes in genetic regulation as a function of age. It is within this framework of changing regulation that one must tease out not which age-related pathways are responsible for disease, but rather which are permissive of disease.
3.6.3. Retinal cell apoptosis
3.6.3.1. Endoplasmic reticulum (ER) stress
ER stress may result from misfolded/unfolded proteins, viral infection, energy/nutrient deprivation, or imbalance of redox status of a cell. Misfolded/unfolded proteins activate the unfolded protein response (UPR), a pathway that promotes suppression of translation, upregulates ER-associated chaperones, and activates ER-associated protein degradation systems to remove debris. When this system fails, such as in the case of prolonged ER stress, apoptosis is activated through the functions of C/EBP homologous protein (CHOP), the interplay between mitochondria and the Bcl-2 family, cytosolic Caspase-12, and JNK signaling (Jing et al., 2012).
Though direct evidence of protein misfolding in AMD does not exist, findings of amyloid-β in drusen arouse suspicion (Crabb et al., 2002). Consequently, ER stress in AMD could very well result based on the variety of risk factors present in the aging retina, particularly the combination of high metabolic activity, intense light exposure, lipofuscin accumulation in the RPE, age-related declines in antioxidant capacity, impaired choroidal hemodynamics, and sub-RPE drusen deposits (Sauer et al., 2008). Groups have proposed that ER stress could result in chronic oxidative stress that leads to complement dysregulation and AMD (Libby and Gould, 2010), and furthermore that ER stress spurs angiogenesis in nAMD (Salminen et al., 2010). ER stress has been shown to affect genes that regulate cell fate, including anti- and pro-apoptotic factors including Bax and Bcl-2 (Galehdar et al., 2010; McCullough et al., 2001). Nonetheless, the majority of publications addressing ER stress in the pathogenesis of AMD are largely speculative reviews or in vitro studies of RPE. We need in vivo data confirming the ER's relevance to AMD.
3.6.3.2. Autophagic stress
Autophagy, one of the cell lysosomal systems, is activated by several AMD-related pathological conditions, including hypoxia, oxidative stress, UPR, or inflammation (Kaarniranta et al., 2010). Autophagic markers have been identified in both large drusen in AMD human eyes as well as in small drusen and sub-RPE spaces in healthy, age-matched tissue (Wang et al., 2009a). Furthermore, Wang et al. (2009a) provided evidence that observed increases in damage to mitochondrial DNA that result from age are capable of reducing the lysosomal capacity of cultured ARPE-19 cells and lead to enhanced exocytosis of incompletely digested intracellular material, the components of which were reactive with complement cascade factors and identified in AMD donor drusen. In vitro studies of ARPE-19 cells also revealed that lysosomal inhibition by chloroquine caused vacuolation of cells and accumulation of dense intracellular debris (Chen et al., 2011b), implicating the RPE's debris removal systems as vital to cell viability. Human RPE treated with lysosomal inhibitor aluminum chloride or peroxidized lipids accumulate lipofuscin and showed marked decreases in autophagic activity (Krohne et al., 2010). Dysfunction of autophagic mechanisms may lead to increases in protein-misfolding and oxidative stress, contributing to the overall para-inflammatory state and driving the system towards a pathological equilibrium. We have also shown enhanced apoptosis in cultured RPE cells under inflammatory stimuli and oxidative stress (Wang et al., 2012).
3.6.4. Choriocapillaris/Choroid
The CC is a microvascular fenestrated capillary bed within the inner choroid. The basement membrane of the choriocapillaris is the outer basal lamina of Bruch's membrane. A principle function of Bruch's membrane is to exchange nutrients, oxygen, and waste with the outer retina (Figure 2). The choroid is a macrovascular tissue with overlapping function to the CC and which supplies blood to the CC. Choroidal thickness as well as CC density and diameter decrease as a function of age in healthy human eyes, but are significantly reduced in AMD eyes near regions with basal laminar deposits, GA, or disciform scarring (Ramrattan et al., 1994). Submacular choroidal vessels were fewer in AMD eyes and submacular CC density was greater in AMD versus age-matched non-AMD tissues, whereas in the periphery, choroidal vascular diameters were greater in AMD versus non-AMD eyes whereas CC density was greater in AMD versus non-AMD (Spraul et al., 1996; Spraul et al., 1999). A previous study indicated that choroidal thickness was reduced in GA AMD and increased in nAMD with respect to healthy choroids (Sarks, 1976). However, contemporary studies using optical coherence tomography (OCT) have verified the decrease in choroidal thickness resulting from age (Margolis and Spaide, 2009) but have uncovered no significant changes in choroidal thickness due to nAMD (Wood et al., 2011). Furthermore, reductions in CC blood flow are observed as a function of age and are exacerbated in AMD patients (Boltz et al., 2010; Lutty et al., 1999; Xu et al., 2010). Overall, it is not clear how the differences in CC and choroid are related to one another temporally and mechanistically, nor is it clear whether one specific change is more relevant to the development of AMD than another. Further attention to the differences in vascular development and pathology are required to delineate the contribution of vascular disease to AMD. It is worth highlighting some key studies in this area, with a particular emphasis on the differences observed in GA versus nAMD.
3.6.4.1. Geographic atrophy (GA) versus neovascular AMD
Differences do exist in the CC and choroid with respect to GA AMD and nAMD. In GA, reduction in CC density and diameter undoubtedly results in decreased blood flow to the capillary bed and by extension to the outer retina. Nonetheless, atrophy of the CC seems to be secondary to damage to RPE in AMD eyes, based on the dual observations that loss of RPE occurs proportionately to loss of CC and areas of damaged RPE having underlying, not-yet-lost CC (Bhutto and Lutty, 2012; Korte et al., 1984; McLeod et al., 2009). These areas devoid of RPE but with intact CC contained significantly constricted capillaries with small diameter, indicative of poor tissue health (Bhutto and Lutty, 2012; McLeod et al., 2009). Constrictions in the capillaries are possibly accounted for by a reduction in endothelial nitric oxide synthases (eNOS) and neuronal NOS (nNOS) signaling in AMD RPE and choroid (Bhutto et al., 2010). CC vascular density has also been inversely correlated with drusen density (Mullins et al., 2011). In eyes with nAMD, RPE consistently persists in areas surrounding CNV, suggesting a link between RPE and CNV, whereas in GA, RPE are lost before the CC (Bhutto and Lutty, 2012; McLeod et al., 2009; McLeod et al., 2002). It is unclear how the decision for CNV is made, and thus why some eyes develop GA whereas others develop nAMD, or why nAMD may develop at the borders of GA lesions. The answer may indeed lie in a deeper understanding of the factors influencing CNV.
4. Para-inflammation and Immunity
The physiologic changes described in Section 3 detail the many aspects of aging and AMD seen in the retina. However, none of these changes occur in isolation and none alone have been shown to be sufficient to cause AMD either in a model system or in the AMD patient. The immune system is a major player in AMD, as it is this homeostatic agent in the body which continually and throughout the life of the organism interacts with and potentially influences the many age-related and pathological changes seen in AMD.
Para-inflammation is a phenomenon whereby the immune system teeters somewhere between the active and inactive states, the purpose thereof being the restoration of tissue functionality and homeostasis (Medzhitov, 2008; Xu et al., 2009). If a mature inflammatory response is typically recognized as the response to infection or tissue injury, then para-inflammation should be thought of as the tissue response to noxious stress induced by various stressors such as oxidative stress, hyperglycemia, or hypercholesterolemia (Xu et al., 2009). Common diseases resulting from dysregulation of para-inflammation include type 2 diabetes, atherosclerosis, and neurodegenerative diseases (Medzhitov, 2008).
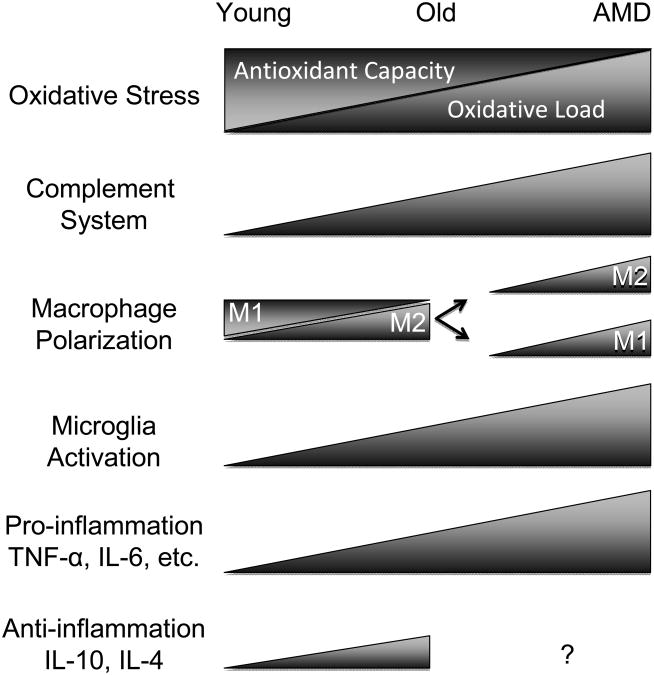
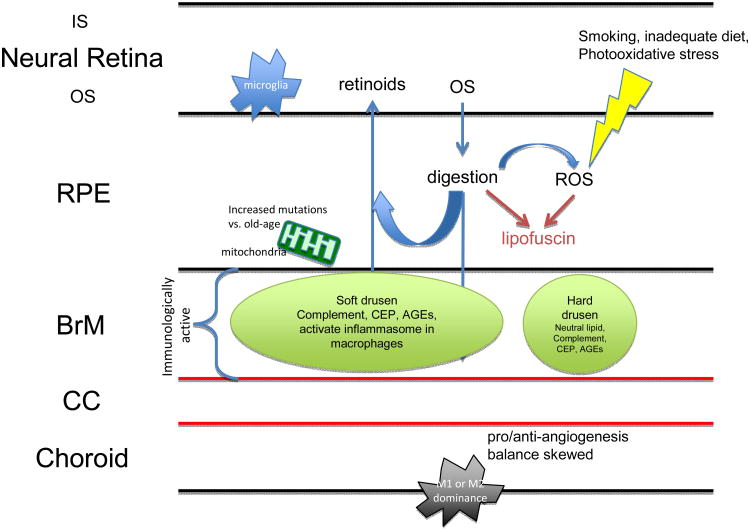
Slowly, this chronic low-grade inflammation intensifies with age. Triggers for para-inflammatory activation in the aging retina include the increasing prevalence of retinal cell death, the loss of ocular immune privilege, the burden of increasing oxidative stress from reactive oxygen and nitrogen species, and accumulation of AGEs (Xu et al., 2009). Dysregulation or dysfunction of para-inflammation can result in several age-related diseases, including AMD. AMD inflammatory processes include complement activation, microglia activation, and macrophage infiltration (Patel and Chan, 2008). As will soon become evident, a trend is seen whereby each of these systems becomes increasingly active in the aging retina and even more so in the AMD retina (Figure 4).
Figure 4.
Immune changes in aging vs. AMD. The aging eye sees shows increased oxidative load and diminished antioxidant capacity, increased immunoreactivity towards complement factors, shifts in macrophage polarization, general increased microglia activity, and upregulation of pro-and anti-inflammatory factors. In AMD, each of these balances is disturbed, evidenced by excessive increases in each of these categories that synergize to launch the system into a disease state. There is a lack of data regarding IL-10 and IL-4 levels in AMD patients with respect to age-matched controls (indicated by “?”).
4.1. Complement System
The complement system is part of the innate immune response and can be subdivided into three distinct pathways: classical, lectin, and alternative. Classical activation results from immune complexes, i.e. antibody bound to antigen; mannose-binding lectin activation occurs from binding of mannose and N-acetyl glucosamine residues, which are abundant on bacterial cell surfaces; the alternative pathway activates from a variety of substances including microbial surfaces and polysaccharides. Overall, activation of these pathways results in a proinflammatory response including generation of membrane attack complexes (MAC) which mediate cell lysis, release of chemokines to attract inflammatory cells to the site of damage, and enhancement of capillary permeability to promote extravasation of infiltrating leukocytes (Kindt et al., 2007).
Genetic association studies have linked complement factor H (CFH) (Edwards et al., 2005; Haines et al., 2005; Klein et al., 2005), C2/complement factor B (CFB) (Gold et al., 2006; Spencer et al., 2007), CFHR1/CFHR3 (Fritsche et al., 2010; Hughes et al., 2006), C3 (Yates et al., 2007), and C5 (Baas et al., 2010) to AMD. Complement proteins including C5 and MAC components C5b-9 have been identified in significant quantity in subretinal drusen and complement dysregulation has been proposed to be a central inflammatory driver in AMD (Anderson et al., 2010; Crabb et al., 2002; Mullins et al., 2000). Complement component C5a has been shown to reduce RPE cell viability in vitro (Hu et al., 2011) as well as promote production of pro-inflammatory cytokines such as IL-17 and IL-22 (Liu et al., 2011).
Basal complement activation at the level of the RPE/Bruch's membrane increases steadily with age in cultured RPE cells (Chen et al., 2008b). Cultured RPE can synthesize endogenous CFH (Chen et al., 2007; Kim et al., 2009), CFB increasingly with age (Chen et al., 2008b), and C3 (Wang et al., 2004), and serum levels of complement are higher in AMD patients (Scholl et al., 2008). In mice, upregulation of 9 complement cascade proteins was observed with age in RPE/choroid, and tissue localization of C3 was different in young and old animals, pointing to evidence of possible disturbances in the integrity of Bruch's membrane as a result of age (Chen et al., 2008a). CFH functions as a complement inhibitor, preventing activation of the alternative pathway via recognition and binding of heparin on cell surfaces as well as blocking alternative activation via C-reactive protein (CRP) in response to tissue injury (Alsenz et al., 1985; Ferreira et al., 2010; Prosser et al., 2007). In mice, increased CFH expression has been shown to occur with age, presumably to combat the proinflammatory changes that accumulate over the lifetime of the organism (Mandal et al., 2006). For instance, reports of significant imbalances in the localization of CRP and CFH showed that in AMD versus age-matched control eyes, immunoreactivity against CRP is significantly higher in the RPE/Bruch's membrane/choroid complex, whereas that against CFH is significantly lower (Bhutto et al., 2011). C1INH, an inhibitor of the classical and lectin complement pathways encoded by the gene SERPING1, has been inconsistently genetically associated with development of AMD (Ennis et al., 2008; Park et al., 2009). Nonetheless, an immunohistochemical study reported higher immunoreactivity against C1INH in AMD CC tissue than in age-matched healthy donor tissue, irrespective of genotype at the disputed genetic locus (Mullins et al., 2009). C1INH serum protein was found to be significantly higher in AMD patients than in age-matched controls, a result that was significant when statistically adjusting for gender and smoking status but not for age (Gibson et al., 2012). Up-regulation of complement inhibitors is indicative of the body's attempt at restoring balance to the immune response. Together, these data point to a delicate balance in the activation status of the complement cascade that, once tipped in favor of activation has the potential to throw the body into a disease state.
4.2. Macrophages
Macrophage activation is highly relevant to AMD pathology, as macrophages have been identified in regions of RPE atrophy, breakdown of Bruch's membrane, and choroidal neovascularization (Coleman et al., 2008; Dastgheib and Green, 1994; Penfold et al., 1985). A recent study showed that while macrophages localize to the choroid in normal tissue, beginning with the appearance of drusen and basal laminar deposits in early AMD, macrophages of a pro-angiogenic phenotype are recruited to Bruch's membrane (Cherepanoff et al., 2010). In truth, macrophages are innate immune cells of a highly dichotomous nature, capable of eliciting responses related to pro/anti-inflammation, immune activation/suppression, and tissue maintenance/destruction (Ferguson and Apte, 2008; Skeie and Mullins, 2009). Indeed, macrophages play a particularly complicated role in the regulation of angiogenesis, as experimental models of CNV have been used to generate contradictory reports on the role of macrophages in regulating the angiogenic response to tissue injury (Apte et al., 2006; Espinosa-Heidmann et al., 2003). Aging macrophages express less MHC class II (Herrero et al., 2002), suggesting diminished capacity to clear pathogens. At the same time, with age macrophages are increasingly prevalent in the choroid and express complement receptor CRIg (Xu et al., 2009), which is involved in complement-mediated phagocytosis (Gorgani et al., 2008; Helmy et al., 2006). The finding that ARPE-19 cells are better-able to phagocytose apoptotic cells than are macrophages in vitro (Petrovski et al., 2011) allows for the possibility that age-related alterations in macrophages together with reduced viability of RPE lends to chronic para-inflammation in the retinal tissues.
The dual nature of macrophage plasticity can be attributed to the two subgroups referred to as M1 and M2 macrophages (Mantovani et al., 2004). M1 macrophages are identified immunohistochemically as CD86+ positive cells and by flow cytometry as IL-12hi, IL-23hi, IL-10lo populations (Mantovani et al., 2005). Classical M1 macrophages are immune effector cells that are aggressive against microbes and can engulf and digest infected/damaged cells. M1 macrophages can be activated by IFN-γ or LPS and produce an array of factors that result in pro-inflammatory/anti-angiogenic environments, including generation of reactive oxygen and nitrogen species (Grossniklaus et al., 2002; Mantovani et al., 2004). M2 macrophages are marked immunohistochemically as CD163+ cells and by flow cytometry as IL-12lo, IL-23lo, IL-10hi populations (Mantovani et al., 2005). Alternative M2 macrophages are activated by cytokines such as IL-4 and IL-10, are described as anti-inflammatory/pro-angiogenic, and promote immunoregulation, tissue remodeling/wound healing, and have been implicated in tumor growth (Ambati et al., 2003; Apte et al., 2006; Mantovani et al., 2004). As age advances, IL-10 transcripts in the posterior eye increase, pushing macrophages towards an M2 polarization, which is anti-inflammatory and pro-angiogenic, and thus potentiating CNV (Apte et al., 2006; Ferguson and Apte, 2008; Kelly et al., 2007). As the ratio of M1 to M2 macrophages destabilizes, pathological AMD features begin to manifest. In the normal aging process, young individuals tend to have more M1 and fewer M2 macrophages whereas older people have the opposite (Cao et al., 2011). AMD patients show a reversion to the younger phenotype, however, with an M1:M2 ratio similar to that in young individuals (Cao et al., 2011). Increased M2 polarization likely has protective effects in the aged eye as it attempts to combat the cumulative effects of life-long oxidative stress. The reversion to M1 phenotype in macrophages recruited to the eye has the potential to further damage retinal tissues due to the pro-inflammatory environment. Furthermore, the fact that no disease is evident in M1-dominated young eyes suggests a loss of ocular immune privilege with age (Ferguson and Apte, 2008), which has profound consequences for the aging eye. However, macrophages seem to display a diverse range of phenotypes that can change over time (Mosser and Edwards, 2008). Additionally, they are highly plastic depending on the tissue microenvironment (Mantovani et al., 2009). In fact, both M1 and M2 predominant phenotypes were reported in AMD-associated CNV membranes (Cao et al., 2011), as either pro- or anti-angiogenic M2 macrophages are found in areas of angiogenesis (Wu et al., 2010). A more recent review describes the newer Mox and M4 macrophage subsets found in the atherosclerotic aorta (Butcher and Galkina, 2012), further raising the possibility that the balance of macrophage plasticity and the complexity of macrophage heterogeneity are of utmost importance in tissue homeostasis.
4.3. Microglia
Microglia are resident macrophages of the central nervous system that monitor and maintain the health of surrounding tissues (Karlstetter et al., 2010). Microglia are highly plastic, enabling rapid migration of their processes in response to tissue injury or stress (Gehrmann et al., 1995). Aging causes poorly understood alterations in microglia behavior and polarization. In the human brain, increased accumulation of IL-1-overexpressing microglia has been reported (Mrak and Griffin, 2005; Sheng et al., 1998) and postulated to contribute to risk of developing age-related cognitive disorders such as Alzheimer's. One study found increased expression of the pro-inflammatory cytokine IL-6 in aged brain microglia from BALB/c mice (Ye and Johnson, 1999).
The retina is a neurological tissue susceptible to similar changes in microglia populations, which may contribute to AMD etiology. Microglia populations expand with age in normal rat retinas (Chan-Ling et al., 2007). Aged microglia tend to show signs of activation, including increased expression of activation markers as well as morphological changes such as large cell bodies and shortened dendrites (Xu et al., 2009; Xu et al., 2008). Whereas in young eyes microglia are localized to the inner retina, with age they increasingly migrate to subretinal spaces and begin to uptake lipofuscin (Xu et al., 2008; Xu et al., 2007). These processes occur merely as a function of age, suggesting a protective role for the differential tissue localization of these cells. However, aged microglia show increased signs of dystrophic morphologies, such as reduced size of dendritic arbors, and also become slower to both migrate to sites of tissue stress as well as disaggregate from those sites thereafter (Damani et al., 2011; Lopes et al., 2008; Streit et al., 2004). Subretinal microglia come in close contact with RPE, which has been shown to subsequently induce secretion of pro-inflammatory and angiogenic factors relevant to AMD pathogenesis (Ma et al., 2009; Xu et al., 2008). An in vitro study showed induction of cell death in photoreceptors co-cultured with retinal microglia (Roque et al., 1999). Furthermore, in a mouse model of retinal degeneration contingent on Ccl2 or Ccr2 deletion, photoreceptor and RPE death is associated with sites of microglial aggregation (Chen et al., 2011a). Knockouts of Cx3cr1, a G-protein coupled receptor involved in microglia activation and chemotaxis, result in retinal degeneration coincident with subretinal accumulation of microglia (Combadiere et al., 2007). The process of subretinal microglial accumulation has been suggested to be caused by the accumulation of the lipofuscin constituent A2E within microglia, which was shown to reduce microglial migratory capabilities, increase deposition of CFB, and reduce expression of CFH (Ma et al., 2013). Moreover, in Ccl2−/−/Cx3cr1−/−/Crb1rd8 mice, which undergo progressive focal retinal degeneration, treatment with the microglial inhibitor naloxene significantly ameliorated retinal lesion formation (Shen et al., 2011). Furthermore, adult Sprague-Dawley rats treated with short interfering RNA targeting Ccl2 showed reductions in apoptotic photoreceptor populations (Rutar et al., 2012). Thus, the microglia, which normally maintain homeostasis of the retina, become pathogenic with age. Chronic activation of these cells is likely due to accumulation of debris not found in the young eye, and found to lesser extent in the aged healthy eye.
A recent study identified hyrperreflective dots (HRD) in outer retinal layers of patients undergoing treatment for nAMD using spectral domain OCT (SD-OCT) (Coscas et al., 2012). Coscas et al. (2012) described that the HRD regressed following treatments and suggested that they could indicate an accumulation of microglia, paralleling the findings described in the preceding paragraphs regarding microglial accumulation in animal models.
4.4. Macroglia
Macroglia in the human retina consist of Müller cells and astrocytes. Müller glia are the predominant glial cell type found in the vertebrate retina and whose primary functionality is to provide support for neuronal cell health. They maintain homeostasis of the microenvironments in several ways, namely by providing nutrients to retinal neurons, regulating neurotransmitter recycling, keeping K+ balances, regulating water concentrations, and by secreting glutathione to curb oxidative stress (Bringmann et al., 2006). Retinal astrocytes reside in the neurofiber layer and are vital for proper vasculogenesis during development (Jiang et al., 1995; Jiang et al., 1994).
Age-related changes in Müller glia include steady decreases in K+ conductance (Bringmann et al., 2003). Müller cells have been shown to accumulate markers of oxidative stress with age, specifically as seen by increasing immunoreactivity towards 4-hydroxy-2-enal, a peroxidized lipid in aged tissue (Nag et al., 2011). One group observed in aging rat retinas that light-induced loss of RPE and photoreceptors resulted in remodeling of Müller cells in order to generate scaffolding to enable migration of remaining retinal neurons to allow for the maintenance of retinal circuits, which was also observed in AMD retinas (Sullivan et al., 2003). Retinal stress is often assessed by analyzing expression of glial fibrillary acidic protein (GFAP). GFAP was seen to increase in expression as a result of age alone in Müller cells (Madigan et al., 1994). Additionally, it was reported that GFAP expression was significantly higher in Müller cells in eyes affected with GA (Wu et al., 2003). In regions of RPE atrophy, Müller processes exhibited gliosis (Guidry et al., 2002; Wu et al., 2003), potentially in an attempt to maintain ocular immune privilege. Gliosis, also termed reactive gliosis, is a process in which Müller cells hypertrophy and proliferate. During reactive gliosis, Müller glia may secrete chemokines that attract phagocytic macrophages and microglia to the affected area and in turn promote an inflammatory response that results in photoreceptor apoptosis (Bringmann and Wiedemann, 2012). Alternatively, gliosis may protect photoreceptors and neurons from cell death via neurotrophic and growth factors such as fibroblast growth factor-2 (FGF-2) or glial cell-derived neurotrophic factor (GDNF) (Bringmann and Wiedemann, 2012).
Aging astrocytes undergo morphological changes featuring increased density of intermediate filaments and hypertrophy in addition to increasing GFAP immunoreactivity (Madigan et al., 1994; Ramirez et al., 2001). Furthermore, with aging, astrocytes decrease in number but show an increase in the number of cellular organelles, indicative of higher activity to compensate for the loss of cells (Ramirez et al., 2001). GFAP is significantly upregulated in astrocytes from AMD retinas with respect to age-matched controls and associated with the presence of drusen (Ramirez et al., 2001; Wu et al., 2003).
4.5. Carboxyethylpyrrole (CEP)
CEP is a byproduct generated from the oxidation of the omega-3 fatty acid docosahexaenoic (DHA) acid in the retina (Crabb et al., 2002). Anti-CEP antibodies have been identified in the serum of AMD patients, and when used along side genomic risk markers, provide approximately 80% accuracy for determining one's predisposition to developing AMD (Gu, 2009; Gu, 2003). CEP appears to have a functional involvement in AMD, though its role is not yet clear and the oxidative mechanisms governing its formation has not been demonstrated. In one experimental system, CEP-immunized mice developed features of atrophic retinal degeneration, (Hollyfield, 2008,Hollyfield 2010), whereas in another CEP was shown to induce neovascularization (Ebrahem, 2006), likely via TLR2 (West et al., 2010). Furthermore, autoantibody generation towards CEP potentially contributes to chronic inflammation and propagates the disease phenotype (Gu et al., 2003). DHA is typically a precursor for neuroprotective factors that inhibit apoptosis and inflammation (Bazan, 2005; Mukherjee, 2007) but oxidative damage in the retina is capable of diverting DHA for use in these protective functions towards generation of pathogenic intermediates (Ardeljan et al., 2011). Omega-3 fatty acid supplements have been shown to be beneficial in animal models of age-related pathologies including retinal degeneration (Hodge et al., 2007; Seddon et al., 2006; Tuo et al., 2009) but not in humans (AREDS2, 2013).
4.6. Gene Expression
Aging has been associated with increased expression of the pro-inflammatory cytokine milieu, in particular TNF-α, IL-1, IL-6, IL-17, IFN-γ, and TGF-β (Hobbs et al., 1993; Kubo and Cinader, 1990; Miller, 1991; Riancho et al., 1994). These changes are associated with changes in CD4+ T-cells from a predominantly Th1 to Th2 population as organisms age, a paradigm shift thought to contribute to the increasing prevalence of autoimmune disease in the elderly and which also parallels the polarization from M1 to M2 macrophages with age (Goronzy and Weyand, 2012; Prelog, 2006). Together these observations constitute the inflammatory hypothesis of aging (Chung et al., 2001). Evidence also exists of upregulation of antiinflammatory cytokines such as IL-4 and IL-10 in aging (Plackett et al., 2003), though it is unclear how the balance of pro-to-anti-inflammatory cytokines plays out in generating a disease phenotype. Imbalances in cytokines such as IL-17 and IL-22 seem to play a role in AMD, as it was recently reported that although IL-17 was undetectable in the majority of human subjects in one study (Njemini et al., 2007), it was found to be significantly elevated along with serum proinflammatory cytokine IL-22 in AMD patients with respect to age-matched controls (Liu et al., 2011). One study used microarray technology to probe the differences in gene expression between young and aged neural retina and RPE/choroid extracted from mice (Chen et al., 2008a), in which 10 upregulated pathways were identified in RPE/choroid (notably leukocyte extravasation and complement factors) related to enhanced immunological activation as well as regulation (such as IL-10) and 9 upregulated pathways in neural retina related to stress responses and apoptosis. Another group reported upregulation of immune response genes such as CC/CXC chemokines and complement components, markers of microglia activation, as well as calcitonin receptor (Chen et al., 2010a). These differences provide further evidence for the transformation of the eye into an immunologically active tissue as the organism ages.
Significant contributions to the microenvironment's inflammatory milieu are made by RPE. Through formation of tight junctions, RPE constitutes a physical barrier that assists in establishing the immune privilege of the eye. RPE secrete immunosuppressive factors including TGF-β, somatostatin, thrombospondin, and pigment epithelial-derived factor (PEDF) (Zamiri et al., 2007). RPE have been shown to suppress B and T cells and can induce T regulatory cell differentiation (Kawazoe et al., 2012; Sugita et al., 2010). Additionally, RPE expresses a variety of toll-like receptors (Kumar et al., 2004) and is capable of producing TNF-α, IL-1, and IL-8 (Cao et al., 2010; Ebihara et al., 2007; Hu et al., 2011; Wang et al., 2010b). Significantly, evidence suggests that RPE gene expression is modulated by the health of surrounding tissues. Analysis of differentially expressed genes in ARPE-19 grown on either old or young donor Bruch's membrane revealed significant changes in expression with respect to apoptotic genes, as well as downregulation of immune related genes such as the complement inhibitor vitronectin (Cai and Del Priore, 2006). Absent proper regulation, this para-inflammatory state contributes to tissue damage.
5. Returning the System to Homeostasis
5.1. Suppress excessive inflammation
The purpose of the tissue inflammatory response is to restore homeostasis in the face of injury or stress. In the case of AMD, however, this attempt to restore homeostasis fails and instead propagates the disease, ultimately resulting in blindness of the patient. Blocking the deleterious inflammatory response can be achieved from a variety of angles by targeting either the many contributors to the para-inflammatory state of the retina (e.g., complement, macrophages, or microglia) or those factors that tip the balance towards pathology (drusen). Changes in the eye that occur as a result of normal aging include heightened upregulation of the para-inflammatory state, so treatments targeted towards eliminating this environment may in fact prove harmful to the patient. Perhaps the most attractive therapeutic strategy would be to attempt to remove soft drusen as they are the earliest clinical manifestation of disease and a principle activator of the associated inflammatory response. One potential model system for exploring such a method is that developed by Johnson et al. (2011). Using this culture system, it would be possible to analyze the kinetics of basal debris deposition and develop experimental methods to either slow this process or promote extracellular degradation of the debris. Following the hypothesis that macrophages and microglia are recruited by immune recognition of soft drusen, a more sophisticated system could be developed to analyze ways of promoting either immune cell clearance of soft drusen and subsequent deactivation, or immunosuppressive techniques geared towards rendering soft drusen harmless to the surrounding tissues.
Treatment strategies may be pursued that seek to instead quell inflammation. Macrophages and microglia are likely drawn to the subretinal and sub-RPE spaces due to the presence of immunoreactive drusen. In the case of macrophages, whereas in normal aged eyes M2 macrophages would be attracted, in AMD eyes it is both M1 and M2 macrophages found at the site of insult with relatively higher M1 activity in GA AMD and higher M2 activity in nAMD (Cao et al., 2011). The presence of these macrophages could contribute to pro-inflammatory and angiogenic factors that in turn negatively affect RPE cells. Discovering how to manipulate the macrophage plasticity in vivo or potentially restore the immune privilege of the eye is necessary to deter pathogenic events downstream of M1, M2, or other subtype macrophage recruitment. As for microglia, we must further analyze the differences between microglia from AMD tissue and from age-matched counterparts. Microglial inhibition shows promise in a mouse model of subretinal hemorrhage and could potentially translate to the treatment of human AMD (Zhao et al., 2011).
Inhibition of complement-related proteins has also been seen as a possibility. One novel agent, an inhibitor of the alternative pathway with fusion proteins of the inhibitory CFH domain and a fragment targeting complement receptor 2, was shown to prevent angiogenesis in vivo and mitigate the oxidative damage done to challenge RPE in vitro (Rohrer et al., 2012; Rohrer et al., 2010). Inhibition of complement C3 by intravitreal injection of a small peptide inhibitor called compstatin resulted in clearance of drusen within 6 months in a non-human primate model of macular degeneration (Chi et al., 2010) and was shown in ARPE-19 to deter alternative pathway complement activation that normally occurs in response to lipofuscin (Zhou et al., 2009). Further avenues regarding the RPE's ability to upregulate the complement inhibitor vitronectin in response to complement exposure (Wasmuth et al., 2009) may be pursued, the goal being to boost the body's ability to restore balance to the system.
We have recently reported beneficial effects resulting from treatment in vivo of an anti-inflammatory protein, tumor necrosis factor-inducible gene 6 (TSG-6) (Tuo et al., 2012). Tuo et al. (2012) showed that treatment with TSG-6 prevented retinal pathology in a mouse model of focal retinal degeneration, mediated by reductions in pro-inflammatory cytokines such as IL-17. High serum IL-17 as well as high expression of IL-17 and its receptor IL-17RC in the macula has been associated with AMD patients (Liu et al., 2011; Park et al., 2012). Unpublished data from our lab indicate that knockdown of IL-17 in the retina of a mouse model of focal retinal degeneration significantly ameliorates photoreceptor degeneration and reduces A2E/lipofuscin accumulation in the RPE of these mice.
Recently, several studies have reported on the involvement of IL-18 and the inflammasome in the pathogenesis of AMD. AMD patients show higher activity of the NLRP3 inflammasome, a pathway that responds to exogenous as well as endogenous danger signals, whose activation contributes to degenerative events in RPE (Kaneko et al., 2011; Tarallo et al., 2012). Activation of this immune response system has been described to occur in macrophages in a CEP-induced animal model and to inhibit CNV in laser-induced mouse models (Doyle et al., 2012). Interestingly, activation of the inflammasome produces abundant IL-1β and promotes Th17 cell differentiation and IL-17 secretion (Eyerich et al., 2010). Identification of additional inflammatory mediators, which are distinctly pathogenic, and not age-related in nature, will lead to more potential therapeutic targets. Modulation of targets downstream of IL-18 might prove beneficial in treating nAMD or GA AMD (Rosenbaum, 2012).
The best strategy remains to be determined. First, the mechanism for drusen accumulation must be uncovered. This is likely a complex interplay between genetics, the immune system, and environmental factors that influence Bruch's membrane, the RPE, and the choroid. A method to prevent or reverse drusen accumulation would likely be the most beneficial to patients, who above all want to maintain their vision.
5.2. Downgrade oxidative stress
Many studies have illustrated the beneficial effects of anti-oxidant administration towards reducing the oxidative burden on retinal cells both in vitro as well as in vivo. AREDS guidelines have approved anti-oxidant treatments such as beta-carotene and zinc for prevention of AMD (AREDS, 2001 , 2002; Prasad, 2009). Quercetin was shown to be effective in against oxidative stress in human RPE and limited amelioration of focal photoreceptor degeneration in Ccl2−/−/Cx3cr1−/−/Crb1rd8 mice (Cao et al., 2010). A study in primates concluded that macular pigments lutein and zeaxanthin protected fovea from blue light-induced damage as measured by lesion size after light exposure, and that omega-3 fatty acids protected the parafoveal region (Barker et al., 2011). Blockage of pro-inflammatory mediators such as NF-κB have been implicated in these protective effects (Kaarniranta and Salminen, 2009).
Uncovering methods for removing stressors such as lipofuscin will likely prove to be essential. A recent study reported successful removal of lipofuscin from RPE of cynomolgus monkeys by oral administration of a reversible H+/K+ ATPase inhibitor known as Remofuscin, which belongs to the tetrahydropyridoether family of compounds; lipofuscin granules were exocytosed by RPE and cleared by macrophages recruited to the area (Julien and Schraermeyer, 2012). Drugs such as these hold the promise to reverse the deleterious effects secondary to lipofuscin accumulation. They also hold the power to determine whether lipofuscin accumulation in RPE is related to drusen formation, and could be used in animal models to determine causality or correlation. Thampi and colleagues (2012) recently reported on the protective effects of 8-hydroxy-2-(di-n-proprylamino)tetralin (8-OH DPAT), an agonist of the serotonin-responsive 5-hydroxytryptamine receptor 1A (HTR1A), a G protein-coupled receptor. 8-OH DPAT treatment of cultured RPE significantly reduced lipofuscin levels derived from phagocytosis of outer segments, reduced intracellular levels of superoxide anion and increased the ratio of reduced to oxidized glutathione, indicating an overall increase in the oxidative capacity of cultured cells (Thampi et al., 2012). In vivo, Sod2−/− mice, which have increased oxidative burdens due to a lack of superoxide dismutase, treatment with 8-OH DPAT resulted in 60% less lipofuscin accumulation compared to untreated controls in the RPE (Thampi et al., 2012). In another recent proof-of-principle finding, intracellular A2E, the principle lipofuscin fluorophore, was degraded using exogenous enzyme in both cell-free and cell-based assays (Wu et al., 2011). Despite a lack of evidence for any increased accumulation of lipofuscin in AMD with respect to aged eyes, its ability to activate complement (Zhou et al., 2009) suggests that its removal may be beneficial to prevent pathology.
5.3. Reverse epigenetic changes
The epigenetic features of AMD are not well explored. Of the many animal systems used to model AMD, it would be informative to identify epigenetic contributions that could potentially play major roles in development of the disease. Wei et al. (2012) identified hypomethylation of the IL-17RC promoter in AMD using CD14+ peripheral blood monocytes (PBMCs). It is possible that these monocytes, potentiated to respond to IL-17RC, may be associated with damage to the retina in AMD. A recent study of lung tumor biology suggested that IL-17 production by the tumor was sufficient to draw macrophages to the site in an IL-17RA- and IL-17RC-dependent manner, after which macrophages predominantly polarized into M2 phenotypes and facilitated tumor growth (Liu et al., 2012).
5.4. Neuroprotection (anti-apoptosis)
Atrophy of the neural retina is likely secondary to RPE degeneration (Curcio et al., 1996). Despite this fact, photoreceptor atrophy in AMD results in preferential loss of rods over cones (Curcio et al., 1996), suggesting the presence of neuroprotective factors within cones that aid in their survival. Use of neuroprotective factors to prevent apoptosis in photoreceptors could significantly reduce the rate of vision loss in patients afflicted with the disease. One mechanism for neuronal loss in the retina is apoptosis triggered by oxidative or inflammatory stress.
The neuroprotective, anti-apoptotic effects of the omega-3 fatty acid DHA have been illustrated in rat models of hypoxia-ischemia (Belayev et al., 2009; Berman et al., 2009; Mayurasakorn et al., 2011) and in rat models of spinal cord injury (Ward et al., 2010). These effects are mediated via DHA conversion into neuroprotectin D1 (Bazan, 2009). Neuroprotectin D1 also inhibits cytokine-mediated cyclooxygenase-2 (COX-2) expression and leukocyte infiltration. Omega-3 supplements are beneficial in humans (Hodge et al., 2006) and in a mouse model of focal retinal degeneration (Tuo et al., 2009). Furthermore, it has been hypothesized that omega-3 supplements work because increasing concentrations of DHA promotes conversion into its neuroprotective intermediates and away from its oxidative conversion into CEP at least in part due to Le Chatlier's principle (Ardeljan et al., 2011).
5.4.1. PEDF
PEDF is one potential factor that could be administered to treat AMD. PEDF overexpression in transgenic mice resulted in inhibition of retinal inflammation and prevention of neovascularization (Park et al., 2011). PEDF naturally secreted by Müller cells has also been neuroprotective towards retinal ganglion cells (Unterlauft et al., 2012) and lentiviral-mediated gene therapy using PEDF has resulted in neuroprotection in animal models (Miyazaki et al., 2011). Porcine retinal pericytes were protected from AGE-induced cytotoxicity (Sheikpranbabu et al., 2011), suggesting that PEDF could also be beneficial to retinal cells in AMD. PEDF was shown to mitigate apoptosis in photoreceptors by inhibiting nuclear translocation of apoptosis-inducing factor (AIF) in a mouse model of retinitis pigmentosa (Murakami et al., 2008). An in vitro study of ARPE-19 determined that PEDF promotes the conversion of the omega-3 fatty acid DHA into neuroprotectin D1, suggesting that PEDF may work in conjunction with DHA to promote RPE cell survival (Reinoso et al., 2008). In rat brain, neuroprotection seems to be mediated via PEDF-dependent ERK1/2 activity and induction of the anti-apoptotic Bcl-2 protein (Sanchez et al., 2012).
5.4.2. CTNF
After a beneficial effect was observed in a pilot study using ciliary neurotrophic factor (CNTF) in patients with retinitis pigmentosa (Sieving et al., 2006), intraocular CNTF implant was evaluated in a phase 2 clinical trial of 51 patients with GA AMD. At 12 months, patients who received the low- and high-dose implants presented a significant increase in retinal thickness that may reflect increased photoreceptor metabolic activity or an increased number of photoreceptors. CNTF seemed to preserve vision; however, it did not stop the progression of GA (Zhang et al., 2011). Further work into the identification of neuroprotective treatments is needed to reduce to the rate of vision loss in AMD patients.
6. Summary
Broadly defined, homeostasis is a process whereby a dynamic system maintains a stable equilibrium. AMD can be thought of as a loss of retinal homeostasis, whereby a series of age-related changes are permissive for age-related pathology, and it is with the combination of environmental and genetic risk factors that disease manifests. This becomes apparent when comparing the changes that occur as a result of aging with those that occur during the AMD disease process (Table 1).
Table 1.
Comparing young, old, and AMD retinas.
| Young | Old | AMD | |||
|---|---|---|---|---|---|
| Drusen | Hard | absent | present - peripheral | present - peripheral and macula | |
| Soft | absent | absent | present - macula | ||
| Basal Linear Deposits | absent | absent | present - macula | ||
| Bruch's Membrane | baseline | thickening, lipid accumulation, decreased elasticity, decreased diffusion, increased TIMP3 | With respect to old retina, diminished capacity for ECM turnover (TIMP3 and MMP ratios skewed) | ||
| Photoreceptors | baseline | Periphery: photoreceptors and RPE atrophy at the same rate; macula: cones are preserved but rods are lost over time | Rods prefenentially lost to cones, cones degenerate as well | ||
| CC/Choroid | baseline | choroidal thinning and CC density decreases, CC blood flow decreases | choroid thinning and CC density decreases even lower, CC blood flow decreases | ||
| Mitochondria | baseline | mtDNA damage increases with age | mtDNA damage increased with respect to old age, some evidence for mitochondrial membrane damage in AMD | ||
| Oxidative | baseline | increases with age | exacerbated antioxidant deficiency | ||
| Lipofuscin | baseline | increases with age | complement activation in AMD | ||
| AGEs | minimal | increase with age | AGE receptor expression higher in RPE adjacent to drusen deposits | ||
| Epigenetics | baseline | unclear, but natural tendency is more methylation | hypomethylation of IL-17RC, hypermethylation of GSTM1, GSTM5 | ||
| Para- inflammation | Overall | baseline | enhanced | not controlled | |
| Complement System | baseline | increase with age of balancing factors such as CRP and CFH | activating factors increased, inhibitory factors decreased | ||
| Macrophages | M1 dominant | M2 dominant | reversion to M1 dominance; also possible that exacerbation of M2 dominance may occur | ||
| Microglia | quiescent, in inner retina | active, in outer retina | Active and accumulated in outer retina, likely drawn by drusen | ||
| Macroglia | Müller | quiescent | markers of oxidative stress, increased GFAP | exacerbated GFAP increase, gliosis | |
| Astrocytes | quiescent | hypertrophy, increased GFAP, decreased number, higher activity | exacerbated GFAP increase, may be associated with drusen | ||
| CEP | absent | absent/limited | dectable serum (autoantibodies), drusen (adducts) | ||
| Gene Expression | Pro-inflammatory | baseline | increase with age | increase vs. old age | |
| Anti-inflammatory | baseline | increase with age | unknown vs. old age, potentially decrease | ||
| Special Case -IL-17, IL-18, IL-22, etc. | baseline | might increase | increase vs. baseline/old age |
The various factors at play in ocular homeostasis include the interplay between age-related changes occurring to tissues with age and the ever-increasing burden of para-inflammation on the retina. There exists a delicate balance of the cytokine profile, influenced by resident immune cells of the retina, RPE, and choroid. As we age, our eyes become an immunologically active tissue, featuring steady increases in complement activation, general increases in the expression of pro-inflammatory cytokines and chemokines. Microglia are activated and contribute to this system. In an attempt to combat these changes, a shift in the macrophage polarization from a predominantly M1 state to an M2 state occurs, accompanied by upregulation of the anti-inflammatory cytokine IL-10 (Figure 4).
In the young eye, retinoids are cycled between the shedding outer segments (OS) and RPE (Figure 5). This process involves generation of ROS, which are carefully balanced by anti-oxidant mechanisms within the RPE. Debris is trafficked through Bruch's membrane and into the CC, from which it enters the systemic circulation and is disposed of. Resident microglia reside within the inner neural retina, responding to stressors when necessary but generally remaining docile. Resident M1 macrophages reside within the choroid, from which they maintain health of the surrounding tissue. Basal secretion of VEGF from the RPE to the CC maintains CC health and angiogenesis is suppressed with expression of anti-angiogenic factors such as PEDF, thrombospondin 1, and endostatin. In the aging eye (Figure 5), accumulation of oxidative stressors may lead to build-up of lipofuscin, generated physiologically from visual cycle flux of bisretinoids (Sparrow, 2010) within RPE. Hard drusen may accumulate within BrM, but mostly in the periphery and not in the macula. Mitochondrial DNA damage accumulates with normal aging and is not specifically pathologic. Expression of both VEGF and PEDF/anti-angiogenic factors increases. Choroidal blood flow decreases, which places additional stress on the outer retina and RPE, and visual acuity ultimately worsens with age. Nonetheless, neither choroidal neovascularization nor GA occurs. The authors state no conflicts of interest.
Figure 5.
Homeostasis of the healthy eye. Spatial organization of ocular homeostatic processes. IS: inner segments; OS: outer segments; RPE: retinal pigment epithelium; ROS: reactive oxygen species; BrM: Bruch's membrane; CC: choriocapillaris; Ml: Ml macrophages; VEGF: vascular endothelial growth factor. Hard drusen are primarily composed of neutral lipid and phospholipid (>40%).
Given the proper genetic (e.g., CFH, ARMS2/HTRA1) and environmental (e.g., smoking, stress) risk factors, the initiation of a low-grade immune response results in complement-laden drusen and accumulation of AGEs and lipofuscin, all of which work together to tip the balance and contribute to a chronic immune response seen in AMD. AMD can be thought of as exacerbated aging-related changes mediated by immune activation (Figure 6). Accumulation of soft drusen within Bruch's membrane leads to blockages in debris removal from RPE. Choroidal macrophages and retinal microglia respond to cues (chemokines, cytokines, CFH, Amyloid-β, CEP, AGEs), activating them and tipping the para-inflammatory state into one of chronic inflammation. In the case of nAMD, diminished expression of anti-angiogenic factors with increasing VEGF results in CNV, allowing for nutrient supply and gas diffusion to RPE and photoreceptors. In GA AMD, oxygen transport is slowed, lipofuscin-laden RPE atrophy in a process that ultimately results in photoreceptor and CC degeneration and atrophy. Activated microglia are associated with RPE and photoreceptor cell death, macrophage polarization to a destructive imbalance of either M1 or M2 phenotypes, and increased expression of inflammatory cytokines such as inflammasome regulators, IL-17 and IL-22 is observed.
Figure 6.
Homeostasis of AMD eyes. Spatial organization of AMD-related processes. IS: inner segments; OS: outer segments; RPE: retinal pigment epithelium; ROS: reactive oxygen species; BrM: Bruch's membrane; CC: choriocapillaris; M1: M1 macrophages; M2: M2 macrophages; VEGF: vascular endothelial growth factor; CEP: carboxyethylpyrrole; AGEs: advanced glycation end products.
The salient question to ask is not how to prevent age-related changes, but rather how to distinguish age-related changes from pathological ones, since not every aged individual develops AMD. The key is in developing a significant understanding of the interplay between aging physiology and age-related immune activation/pathology (Figure 7). Uncovering the differences in how the body finds a dynamic balance between the pro-/anti-inflammatory and the age/disease-associated changes is vital in order to discover a cure for AMD. Aging sets the stage for disease to set in, but the factors that tip the balance in favor of disease should be our targets for therapy.
Figure 7.
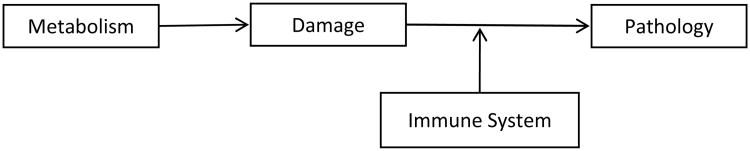
An AMD disease model. The Zealley and de Grey (2012) model of aging as applied to AMD, where a key aspect features activation of the immune system.
Acknowledgments
The authors state no conflicts of interest. We appreciate Dr. Janet Sparrow's critical review of the manuscript. This work was supported by the NEI intramural fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ablonczy Z, Dahrouj M, Tang PH, Liu Y, Sambamurti K, Marmorstein AD, Crosson CE. Human retinal pigment epithelium cells as functional models for the RPE in vivo. Invest Ophthalmol Vis Sci. 2011;52:8614–8620. doi: 10.1167/iovs.11-8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahonen M, Poukkula M, Baker AH, Kashiwagi M, Nagase H, Eriksson JE, Kahari VM. Tissue inhibitor of metalloproteinases-3 induces apoptosis in melanoma cells by stabilization of death receptors. Oncogene. 2003;22:2121–2134. doi: 10.1038/sj.onc.1206292. [DOI] [PubMed] [Google Scholar]
- Alsenz J, Schulz TF, Lambris JD, Sim RB, Dierich MP. Structural and functional analysis of the complement component factor H with the use of different enzymes and monoclonal antibodies to factor H. Biochem J. 1985;232:841–850. doi: 10.1042/bj2320841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambati J, Anand A, Fernandez S, Sakurai E, Lynn BC, Kuziel WA, Rollins BJ, Ambati BK. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat Med. 2003;9:1390–1397. doi: 10.1038/nm950. [DOI] [PubMed] [Google Scholar]
- Anderson DH, Radeke MJ, Gallo NB, Chapin EA, Johnson PT, Curletti CR, Hancox LS, Hu J, Ebright JN, Malek G, Hauser MA, Rickman CB, Bok D, Hageman GS, Johnson LV. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog Retin Eye Res. 2010;29:95–112. doi: 10.1016/j.preteyeres.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte RS, Richter J, Herndon J, Ferguson TA. Macrophages Inhibit Neovascularization in a Murine Model of Age-Related Macular Degeneration. PLoS Med. 2006;3 doi: 10.1371/journal.pmed.0030310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardeljan D, Meyerle CB, Agron E, Jin Wang J, Mitchell P, Chew EY, Zhao J, Maminishkis A, Chan CC, Tuo J. Influence of TIMP3/SYN3 polymorphisms on the phenotypic presentation of age-related macular degeneration. European journal of human genetics: EJHG. 2013a doi: 10.1038/ejhg.2013.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardeljan D, Tuo J, Chan CC. Carboxyethylpyrrole plasma biomarkers in age-related macular degeneration. Drugs Future. 2011;36:713–718. doi: 10.1358/dof.2011.036.09.1678338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardeljan D, Wang Y, Abu-Asab M, Yu CR, Tuo J, Shen D, White G, Wadsworth S, Scaria A, Chan CC. Association for Research in Vision and Ophthalmology (ARVO) Seattle, WA: 2013b. Interleukin-17 neutralization ameliorates retinal degeneration in Cx3cr1−/−/Ccl2−/−/Crb1rd8 mice. [Google Scholar]
- AREDS2 Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309:2005–2015. doi: 10.1001/jama.2013.4997. [DOI] [PubMed] [Google Scholar]
- AREDS. Risk factors associated with age-related macular degeneration. A case-control study in the age-related eye disease study: age-related eye disease study report number 3. Ophthalmology. 2000;107:2224–2232. doi: 10.1016/s0161-6420(00)00409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AREDS. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AREDS. The effect of five-year zinc supplementation on serum zinc, serum cholesterol and hematocrit in persons randomly assigned to treatment group in the age-related eye disease study: AREDS Report No. 7. J Nutr. 2002;132:697–702. doi: 10.1093/jn/132.4.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas DC, Ho L, Ennis S, Merriam JE, Tanck MW, Uitterlinden AG, de Jong PT, Cree AJ, Griffiths HL, Rivadeneira F, Hofman A, van Duijn C, Smith RT, Barile GR, Gorgels TG, Vingerling JR, Klaver CC, Lotery AJ, Allikmets R, Bergen AA. The complement component 5 gene and age-related macular degeneration. Ophthalmology. 2010;117:500–511. doi: 10.1016/j.ophtha.2009.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballinger SW, Van Houten B, Jin GF, Conklin CA, Godley BF. Hydrogen peroxide causes significant mitochondrial DNA damage in human RPE cells. Exp Eye Res. 1999;68:765–772. doi: 10.1006/exer.1998.0661. [DOI] [PubMed] [Google Scholar]
- Barker FM, 2nd, Snodderly DM, Johnson EJ, Schalch W, Koepcke W, Gerss J, Neuringer M. Nutritional manipulation of primate retinas, V: effects of lutein, zeaxanthin, and n-3 fatty acids on retinal sensitivity to blue-light-induced damage. Invest Ophthalmol Vis Sci. 2011;52:3934–3942. doi: 10.1167/iovs.10-5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron MJ, Johnson MA, Andrews RM, Clarke MP, Griffiths PG, Bristow E, He LP, Durham S, Turnbull DM. Mitochondrial abnormalities in ageing macular photoreceptors. Invest Ophthalmol Vis Sci. 2001;42:3016–3022. [PubMed] [Google Scholar]
- Baynes JW. The role of AGEs in aging: causation or correlation. Exp Gerontol. 2001;36:1527–1537. doi: 10.1016/s0531-5565(01)00138-3. [DOI] [PubMed] [Google Scholar]
- Bazan NG. Neuroprotecting D1 (NPD1): a DHA-derived mediator that protects brain and retina against cell injury-induced oxidative stress. Brain Pathol. 2005;15:159–166. doi: 10.1111/j.1750-3639.2005.tb00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan NG. Neuroprotectin D1-mediated anti-inflammatory and survival signaling in stroke, retinal degenerations, and Alzheimer's disease. Journal of lipid research. 2009;50 Suppl:S400–405. doi: 10.1194/jlr.R800068-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belayev L, Khoutorova L, Atkins KD, Bazan NG. Robust docosahexaenoic acid-mediated neuroprotection in a rat model of transient, focal cerebral ischemia. Stroke. 2009;40:3121–3126. doi: 10.1161/STROKEAHA.109.555979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman DR, Mozurkewich E, Liu Y, Barks J. Docosahexaenoic acid pretreatment confers neuroprotection in a rat model of perinatal cerebral hypoxia-ischemia. American journal of obstetrics and gynecology. 2009;200:305, e301–306. doi: 10.1016/j.ajog.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berra A, Ferreira S, Stanga P, Llesuy S. Age-related antioxidant capacity of the vitreous and its possible relationship with simultaneous changes in photoreceptors, retinal pigment epithelium and Bruchs' membrane in human donors' eyes. Archives of gerontology and geriatrics. 2002;34:371–377. doi: 10.1016/s0167-4943(02)00013-4. [DOI] [PubMed] [Google Scholar]
- Bhutto I, Lutty G. Understanding age-related macular degeneration (AMD): Relationships between the photoreceptor/retinal pigment epithelium/Bruch's membrane/choriocapillaris complex. Mol Aspects Med. 2012;33:295–317. doi: 10.1016/j.mam.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutto IA, Baba T, Merges C, Juriasinghani V, McLeod DS, Lutty GA. C-reactive protein and complement factor H in aged human eyes and eyes with age-related macular degeneration. Br J Ophthalmol. 2011;95:1323–1330. doi: 10.1136/bjo.2010.199216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutto IA, Baba T, Merges C, McLeod DS, Lutty GA. Low nitric oxide synthases (NOSs) in eyes with age-related macular degeneration (AMD) Exp Eye Res. 2010;90:155–167. doi: 10.1016/j.exer.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasiak J, Synowiec E, Salminen A, Kaarniranta K. Genetic variability in DNA repair proteins in age-related macular degeneration. International journal of molecular sciences. 2012;13:13378–13397. doi: 10.3390/ijms131013378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasiak J, Szaflik JP. DNA damage and repair in age-related macular degeneration. Frontiers in bioscience: a journal and virtual library. 2011;16:1291–1301. doi: 10.2741/3789. [DOI] [PubMed] [Google Scholar]
- Boltz A, Luksch A, Wimpissinger B, Maar N, Weigert G, Frantal S, Brannath W, Garhofer G, Ergun E, Stur M, Schmetterer L. Choroidal blood flow and progression of age-related macular degeneration in the fellow eye in patients with unilateral choroidal neovascularization. Invest Ophthalmol Vis Sci. 2010;51:4220–4225. doi: 10.1167/iovs.09-4968. [DOI] [PubMed] [Google Scholar]
- Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2010;1803:55–71. doi: 10.1016/j.bbamcr.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringmann A, Kohen L, Wolf S, Wiedemann P, Reichenbach A. Age-related decrease of potassium currents in glial (Muller) cells of the human retina. Canadian journal of ophthalmology. Journal canadien d'ophtalmologie. 2003;38:464–468. doi: 10.1016/s0008-4182(03)80024-8. [DOI] [PubMed] [Google Scholar]
- Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, Osborne NN, Reichenbach A. Muller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006;25:397–424. doi: 10.1016/j.preteyeres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Bringmann A, Wiedemann P. Muller glial cells in retinal disease. Ophthalmologica. 2012;227:1–19. doi: 10.1159/000328979. [DOI] [PubMed] [Google Scholar]
- Buch H, Nielsen NV, Vinding T, Jensen GB, Prause JU, la Cour M. 14-year incidence, progression, and visual morbidity of age-related maculopathy: the Copenhagen City Eye Study. Ophthalmology. 2005;112:787–798. doi: 10.1016/j.ophtha.2004.11.040. [DOI] [PubMed] [Google Scholar]
- Butcher MJ, Galkina EV. Phenotypic and functional heterogeneity of macrophages and dendritic cell subsets in the healthy and atherosclerosis-prone aorta. Frontiers in physiology. 2012;3:44. doi: 10.3389/fphys.2012.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cackett P, Yeo I, Cheung CMG, Vithana EN, Wong D, Tay WT, Tai ES, Aung T, Wong TY. Relationship of Smoking and Cardiovascular Risk Factors with Polypoidal Choroidal Vasculopathy and Age-related Macular Degeneration in Chinese Persons. Ophthalmology. 2011;118:846–852. doi: 10.1016/j.ophtha.2010.09.026. [DOI] [PubMed] [Google Scholar]
- Cai H, Del Priore LV. Bruch membrane aging alters the gene expression profile of human retinal pigment epithelium. Curr Eye Res. 2006;31:181–189. doi: 10.1080/02713680500514628. [DOI] [PubMed] [Google Scholar]
- Cankova Z, Huang JD, Kruth HS, Johnson M. Passage of low-density lipoproteins through Bruch's membrane and choroid. Exp Eye Res. 2011;93:947–955. doi: 10.1016/j.exer.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Liu M, Tuo J, Shen D, Chan CC. The effects of quercetin in cultured human RPE cells under oxidative stress and in Ccl2/Cx3cr1 double deficient mice. Exp Eye Res. 2010;91:15–25. doi: 10.1016/j.exer.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Shen D, Patel MM, Tuo J, Johnson TM, Olsen TW, Chan CC. Macrophage polarization in the maculae of age-related macular degeneration: a pilot study. Pathol Int. 2011;61:528–535. doi: 10.1111/j.1440-1827.2011.02695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan-Ling T, Hughes S, Baxter L, Rosinova E, McGregor I, Morcos Y, van Nieuwenhuyzen P, Hu P. Inflammation and breakdown of the blood-retinal barrier during “physiological aging” in the rat retina: a model for CNS aging. Microcirculation. 2007;14:63–76. doi: 10.1080/10739680601073451. [DOI] [PubMed] [Google Scholar]
- Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- Chen H, Liu B, Lukas TJ, Neufeld AH. The aged retinal pigment epithelium/choroid: a potential substratum for the pathogenesis of age-related macular degeneration. PLoS One. 2008a;3:e2339. doi: 10.1371/journal.pone.0002339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Forrester JV, Xu H. Synthesis of complement factor H by retinal pigment epithelial cells is down-regulated by oxidized photoreceptor outer segments. Exp Eye Res. 2007;84:635–645. doi: 10.1016/j.exer.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Chen M, Forrester JV, Xu H. Dysregulation in retinal para-inflammation and age-related retinal degeneration in CCL2 or CCR2 deficient mice. PLoS One. 2011a;6:e22818. doi: 10.1371/journal.pone.0022818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Muckersie E, Forrester JV, Xu H. Immune activation in retinal aging: a gene expression study. Invest Ophthalmol Vis Sci. 2010a;51:5888–5896. doi: 10.1167/iovs.09-5103. [DOI] [PubMed] [Google Scholar]
- Chen M, Muckersie E, Robertson M, Forrester JV, Xu H. Up-regulation of complement factor B in retinal pigment epithelial cells is accompanied by complement activation in the aged retina. Exp Eye Res. 2008b;87:543–550. doi: 10.1016/j.exer.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Chen PM, Gombart ZJ, Chen JW. Chloroquine treatment of ARPE-19 cells leads to lysosome dilation and intracellular lipid accumulation: possible implications of lysosomal dysfunction in macular degeneration. Cell & bioscience. 2011b;1:10. doi: 10.1186/2045-3701-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Stambolian D, Edwards AO, Branham KE, Othman M, Jakobsdottir J, Tosakulwong N, Pericak-Vance MA, Campochiaro PA, Klein ML, Tan PL, Conley YP, Kanda A, Kopplin L, Li Y, Augustaitis KJ, Karoukis AJ, Scott WK, Agarwal A, Kovach JL, Schwartz SG, Postel EA, Brooks M, Baratz KH, Brown WL, Brucker AJ, Orlin A, Brown G, Ho A, Regillo C, Donoso L, Tian L, Kaderli B, Hadley D, Hagstrom SA, Peachey NS, Klein R, Klein BE, Gotoh N, Yamashiro K, Ferris Iii F, Fagerness JA, Reynolds R, Farrer LA, Kim IK, Miller JW, Corton M, Carracedo A, Sanchez-Salorio M, Pugh EW, Doheny KF, Brion M, Deangelis MM, Weeks DE, Zack DJ, Chew EY, Heckenlively JR, Yoshimura N, Iyengar SK, Francis PJ, Katsanis N, Seddon JM, Haines JL, Gorin MB, Abecasis GR, Swaroop A. Genetic variants near TIMP3 and high-density lipoprotein-associated loci influence susceptibility to age-related macular degeneration. Proc Natl Acad Sci U S A. 2010b;107:7401–7406. doi: 10.1073/pnas.0912702107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Bedell M, Zhang K. Age-related Macular Degeneration: Genetic and Environmental Factors of Disease. Mol Interv. 2010;10:271–281. doi: 10.1124/mi.10.5.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherepanoff S, McMenamin P, Gillies MC, Kettle E, Sarks SH. Bruch's membrane and choroidal macrophages in early and advanced age-related macular degeneration. Br J Ophthalmol. 2010;94:918–925. doi: 10.1136/bjo.2009.165563. [DOI] [PubMed] [Google Scholar]
- Chi ZL, Yoshida T, Lambris JD, Iwata T. Suppression of drusen formation by compstatin, a peptide inhibitor of complement C3 activation, on cynomolgus monkey with early-onset macular degeneration. Adv Exp Med Biol. 2010;703:127–135. doi: 10.1007/978-1-4419-5635-4_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HY, Kim HJ, Kim JW, Yu BP. The inflammation hypothesis of aging: molecular modulation by calorie restriction. Ann N Y Acad Sci. 2001;928:327–335. [PubMed] [Google Scholar]
- Coleman HR, Chan CC, Ferris FL, III, Chew EY. Age-related macular degeneration. Lancet. 2008;372:1835–1845. doi: 10.1016/S0140-6736(08)61759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combadiere C, Feumi C, Raoul W, Keller N, Rodero M, Pezard A, Lavalette S, Houssier M, Jonet L, Picard E, Debre P, Sirinyan M, Deterre P, Ferroukhi T, Cohen SY, Chauvaud D, Jeanny JC, Chemtob S, Behar-Cohen F, Sennlaub F. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J Clin Invest. 2007;117:2920–2928. doi: 10.1172/JCI31692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon N, O'Colmain B, Klaver CC, Klein R, Munoz B, Friedman DS, Kempen J, Taylor HR, Mitchell P. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- Coscas G, De Benedetto U, Coscas F, Li Calzi CI, Vismara S, Roudot-Thoraval F, Bandello F, Souied E. Hyperreflective Dots: A New Spectral-Domain Optical Coherence Tomography Entity for Follow-Up and Prognosis in Exudative Age-Related Macular Degeneration. Ophthalmologica. 2012 doi: 10.1159/000342159. [DOI] [PubMed] [Google Scholar]
- Crabb JW, Miyagi M, Gu X, Shadrach K, West KA, Sakaguchi H, Kamei M, Hasan A, Yan L, Rayborn ME, Salomon RG, Hollyfield JG. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci U S A. 2002;99:14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio CA, Johnson M, Huang JD, Rudolf M. Aging, age-related macular degeneration, and the response-to-retention of apolipoprotein B-containing lipoproteins. Prog Retin Eye Res. 2009;28:393–422. doi: 10.1016/j.preteyeres.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio CA, Johnson M, Huang JD, Rudolf M. Apolipoprotein B-containing lipoproteins in retinal aging and age-related macular degeneration. Journal of lipid research. 2010;51:451–467. doi: 10.1194/jlr.R002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio CA, Johnson M, Rudolf M, Huang JD. The oil spill in ageing Bruch membrane. British Journal of Ophthalmology. 2011;95:1638–1645. doi: 10.1136/bjophthalmol-2011-300344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio CA, Medeiros NE, Millican CL. Photoreceptor loss in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1996;37:1236–1249. [PubMed] [Google Scholar]
- Curcio CA, Millican CL, Allen KA, Kalina RE. Aging of the human photoreceptor mosaic: evidence for selective vulnerability of rods in central retina. Invest Ophthalmol Vis Sci. 1993;34:3278–3296. [PubMed] [Google Scholar]
- Curcio CA, Millican CL, Bailey T, Kruth HS. Accumulation of cholesterol with age in human Bruch's membrane. Invest Ophthalmol Vis Sci. 2001;42:265–274. [PubMed] [Google Scholar]
- Curcio CA, Presley JB, Malek G, Medeiros NE, Avery DV, Kruth HS. Esterified and unesterified cholesterol in drusen and basal deposits of eyes with age-related maculopathy. Exp Eye Res. 2005;81:731–741. doi: 10.1016/j.exer.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Damani MR, Zhao L, Fontainhas AM, Amaral J, Fariss RN, Wong WT. Age-related alterations in the dynamic behavior of microglia. Aging Cell. 2011;10:263–276. doi: 10.1111/j.1474-9726.2010.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dastgheib K, Green WR. Granulomatous reaction to Bruch's membrane in age-related macular degeneration. Arch Ophthalmol. 1994;112:813–818. doi: 10.1001/archopht.1994.01090180111045. [DOI] [PubMed] [Google Scholar]
- DeGroot J, Verzijl N, Wenting-Van Wijk MJ, Bank RA, Lafeber FP, Bijlsma JW, TeKoppele JM. Age-related decrease in susceptibility of human articular cartilage to matrix metalloproteinase-mediated degradation: the role of advanced glycation end products. Arthritis and rheumatism. 2001;44:2562–2571. doi: 10.1002/1529-0131(200111)44:11<2562::aid-art437>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Delori FC, Goger DG, Dorey CK. Age-related accumulation and spatial distribution of lipofuscin in RPE of normal subjects. Invest Ophthalmol Vis Sci. 2001;42:1855–1866. [PubMed] [Google Scholar]
- Doyle SL, Campbell M, Ozaki E, Salomon RG, Mori A, Kenna PF, Farrar GJ, Kiang AS, Humphries MM, Lavelle EC, O'Neill LA, Hollyfield JG, Humphries P. NLRP3 has a protective role in age-related macular degeneration through the induction of IL-18 by drusen components. Nat Med. 2012;18:791–798. doi: 10.1038/nm.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dridi S, Hirano Y, Tarallo V, Kim Y, Fowler BJ, Ambati BK, Bogdanovich S, Chiodo VA, Hauswirth WW, Kugel JF, Goodrich JA, Ponicsan SL, Hinton DR, Kleinman ME, Baffi JZ, Gelfand BD, Ambati J. ERK1/2 activation is a therapeutic target in age-related macular degeneration. Proc Natl Acad Sci U S A. 2012;109:13781–13786. doi: 10.1073/pnas.1206494109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara N, Chen L, Tokura T, Ushio H, Iwatsu M, Murakami A. Distinct functions between toll-like receptors 3 and 9 in retinal pigment epithelial cells. Ophthalmic Res. 2007;39:155–163. doi: 10.1159/000103235. [DOI] [PubMed] [Google Scholar]
- Ebrahem Q, Renganathan K, Sears J, Vasanji A, Gu X, Lu L, Salomon RG, Crabb JW, Anand-Apte B. Carboxyethylpyrrole oxidative protein modifications stimulate neovascularization: Implications for age-related macular degeneration. Proc Natl Acad Sci U S A. 2006;103:13480–13484. doi: 10.1073/pnas.0601552103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi KB, Handa JT. Lipids, lipoproteins, and age-related macular degeneration. Journal of lipids. 2011;2011:802059. doi: 10.1155/2011/802059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AO, Ritter IR, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement Factor H Polymorphism and Age-Related Macular Degeneration. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- Eliasieh K, Liets LC, Chalupa LM. Cellular reorganization in the human retina during normal aging. Invest Ophthalmol Vis Sci. 2007;48:2824–2830. doi: 10.1167/iovs.06-1228. [DOI] [PubMed] [Google Scholar]
- Ennis S, Jomary C, Mullins R, Cree A, Chen X, Macleod A, Jones S, Collins A, Stone E, Lotery A. Association between the SERPING1 gene and age-related macular degeneration: a two-stage case-control study. Lancet. 2008;372:1828–1834. doi: 10.1016/S0140-6736(08)61348-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa-Heidmann DG, Suner IJ, Hernandez EP, Monroy D, Csaky KG, Cousins SW. Macrophage depletion diminishes lesion size and severity in experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3586–3592. doi: 10.1167/iovs.03-0038. [DOI] [PubMed] [Google Scholar]
- Eyerich S, Eyerich K, Cavani A, Schmidt-Weber C. IL-17 and IL-22: siblings, not twins. Trends in immunology. 2010;31:354–361. doi: 10.1016/j.it.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Farboud B, Aotaki-Keen A, Miyata T, Hjelmeland LM, Handa JT. Development of a polyclonal antibody with broad epitope specificity for advanced glycation endproducts and localization of these epitopes in Bruch's membrane of the aging eye. Mol Vis. 1999;5:11. [PubMed] [Google Scholar]
- Fariss RN, Apte SS, Olsen BR, Iwata K, Milam AH. Tissue inhibitor of metalloproteinases-3 is a component of Bruch's membrane of the eye. Am J Pathol. 1997;150:323–328. [PMC free article] [PubMed] [Google Scholar]
- Feeney L. Lipofuscin and melanin of human retinal pigment epithelium. Fluorescence, enzyme cytochemical, and ultrastructural studies. Invest Ophthalmol Vis Sci. 1978;17:583–600. [PubMed] [Google Scholar]
- Feeney-Burns L, Berman ER, Rothman H. Lipofuscin of human retinal pigment epithelium. Am J Ophthalmol. 1980;90:783–791. doi: 10.1016/s0002-9394(14)75193-1. [DOI] [PubMed] [Google Scholar]
- Feher J, Kovacs I, Artico M, Cavallotti C, Papale A, Balacco Gabrieli C. Mitochondrial alterations of retinal pigment epithelium in age-related macular degeneration. Neurobiol Aging. 2006;27:983–993. doi: 10.1016/j.neurobiolaging.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Ferguson TA, Apte RS. Angiogenesis in eye disease: immunity gained or immunity lost? Semin Immunopathol. 2008;30:111–119. doi: 10.1007/s00281-008-0113-8. [DOI] [PubMed] [Google Scholar]
- Ferreira VP, Pangburn MK, Cortes C. Complement control protein factor H: the good, the bad, and the inadequate. Mol Immunol. 2010;47:2187–2197. doi: 10.1016/j.molimm.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman DS, O'Colmain BJ, Munoz B, Tomany SC, McCarty C, de Jong PT, Nemesure B, Mitchell P, Kempen J. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- Fritsche LG, Lauer N, Hartmann A, Stippa S, Keilhauer CN, Oppermann M, Pandey MK, Kohl J, Zipfel PF, Weber BH, Skerka C. An imbalance of human complement regulatory proteins CFHR1, CFHR3 and factor H influences risk for age-related macular degeneration (AMD) Hum Mol Genet. 2010;19:4694–4704. doi: 10.1093/hmg/ddq399. [DOI] [PubMed] [Google Scholar]
- Fritsche LG, Loenhardt T, Janssen A, Fisher SA, Rivera A, Keilhauer CN, Weber BH. Age-related macular degeneration is associated with an unstable ARMS2 (LOC387715) mRNA. Nat. Genet. 2008;40:892–896. doi: 10.1038/ng.170. [DOI] [PubMed] [Google Scholar]
- Fujihara M, Bartels E, Nielsen LB, Handa JT. A human apoB100 transgenic mouse expresses human apoB100 in the RPE and develops features of early AMD. Exp Eye Res. 2009;88:1115–1123. doi: 10.1016/j.exer.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galehdar Z, Swan P, Fuerth B, Callaghan SM, Park DS, Cregan SP. Neuronal apoptosis induced by endoplasmic reticulum stress is regulated by ATF4-CHOP-mediated induction of the Bcl-2 homology 3-only member PUMA. J Neurosci. 2010;30:16938–16948. doi: 10.1523/JNEUROSCI.1598-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Hollyfield JG. Aging of the human retina. Differential loss of neurons and retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1992;33:1–17. [PubMed] [Google Scholar]
- Gehrmann J, Matsumoto Y, Kreutzberg GW. Microglia: intrinsic immuneffector cell of the brain. Brain research. Brain research reviews. 1995;20:269–287. doi: 10.1016/0165-0173(94)00015-h. [DOI] [PubMed] [Google Scholar]
- Gibson J, Hakobyan S, Cree AJ, Collins A, Harris CL, Ennis S, Morgan BP, Lotery AJ. Variation in complement component C1 inhibitor in age-related macular degeneration. Immunobiology. 2012;217:251–255. doi: 10.1016/j.imbio.2011.07.015. [DOI] [PubMed] [Google Scholar]
- Glenn JV, Beattie JR, Barrett L, Frizzell N, Thorpe SR, Boulton ME, McGarvey JJ, Stitt AW. Confocal Raman microscopy can quantify advanced glycation end product (AGE) modifications in Bruch's membrane leading to accurate, nondestructive prediction of ocular aging. FASEB J. 2007;21:3542–3552. doi: 10.1096/fj.06-7896com. [DOI] [PubMed] [Google Scholar]
- Glenn JV, Mahaffy H, Dasari S, Oliver M, Chen M, Boulton ME, Xu H, Curry WJ, Stitt AW. Proteomic profiling of human retinal pigment epithelium exposed to an advanced glycation-modified substrate. Graefes Arch Clin Exp Ophthalmol. 2012;250:349–359. doi: 10.1007/s00417-011-1856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold B, Merriam JE, Zernant J, Hancox LS, Taiber AJ, Gehrs K, Cramer K, Neel J, Bergeron J, Barile GR, Smith RT, Hageman GS, Dean M, Allikmets R. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat Genet. 2006;38:458–462. doi: 10.1038/ng1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgani NN, He JQ, Katschke KJ, Jr, Helmy KY, Xi H, Steffek M, Hass PE, van Lookeren Campagne M. Complement receptor of the Ig superfamily enhances complement-mediated phagocytosis in a subpopulation of tissue resident macrophages. J Immunol. 2008;181:7902–7908. doi: 10.4049/jimmunol.181.11.7902. [DOI] [PubMed] [Google Scholar]
- Goronzy JJ, Weyand CM. Immune aging and autoimmunity. Cell Mol Life Sci. 2012;69:1615–1623. doi: 10.1007/s00018-012-0970-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green WR. Histopathology of age-related macular degeneration. Mol Vis. 1999;5:27–36. [PubMed] [Google Scholar]
- Grossniklaus HE, Ling JX, Wallace TM, Dithmar S, Lawson DH, Cohen C, Elner VM, Elner SG, Sternberg P., Jr Macrophage and retinal pigment epithelium expression of angiogenic cytokines in choroidal neovascularization. Mol Vis. 2002;8:119–126. [PubMed] [Google Scholar]
- Gu J, Pauer GJ, Yue X, Narendra U, Sturgill GM, Bena J, Gu X, Peachey NS, Salomon RG, Hagstrom SA, Crabb JW the Clinical Genomic Proteomic AMD Study Group. Assessing susceptibility to age-related macular degeneration with proteomic and genomic biomarkers. Mol Cell Proteomics. 2009;8:1338–1349. doi: 10.1074/mcp.M800453-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, M SG, Miyagi M, Rayborn ME, Hollyfield JG, Crabb JW, Salomon RG. Carboxyethylpyrrole protein adducts and autoantibodies, biomarkers for age-related macular degeneration. J Biol Chem. 2003;278:42027–42035. doi: 10.1074/jbc.M305460200. [DOI] [PubMed] [Google Scholar]
- Gu X, Meer SG, Miyagi M, Rayborn ME, Hollyfield JG, Crabb JW, Salomon RG. Carboxyethylpyrrole protein adducts and autoantibodies, biomarkers for age-related macular degeneration. J Biol Chem. 2003;278:42027–42035. doi: 10.1074/jbc.M305460200. [DOI] [PubMed] [Google Scholar]
- Gu X, Neric NJ, Crabb JS, Crabb JW, Bhattacharya SK, Rayborn ME, Hollyfield JG, Bonilha VL. Age-related changes in the retinal pigment epithelium (RPE) PLoS One. 2012;7:e38673. doi: 10.1371/journal.pone.0038673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidry C, Medeiros NE, Curcio CA. Phenotypic variation of retinal pigment epithelium in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2002;43:267–273. [PubMed] [Google Scholar]
- Gulcan HG, Alvarez RA, Maude MB, Anderson RE. Lipids of human retina, retinal pigment epithelium, and Bruch's membrane/choroid: comparison of macular and peripheral regions. Invest Ophthalmol Vis Sci. 1993;34:3187–3193. [PubMed] [Google Scholar]
- Guo L, Hussain AA, Limb GA, Marshall J. Age-dependent variation in metalloproteinase activity of isolated human Bruch's membrane and choroid. Invest Ophthalmol Vis Sci. 1999;40:2676–2682. [PubMed] [Google Scholar]
- Guven M, Gorgun E, Unal M, Yenerel M, Batar B, Kucumen B, Dinc UA, Guven GS, Ulus T, Yuksel A. Glutathione S-transferase M1, GSTT1 and GSTP1 genetic polymorphisms and the risk of age-related macular degeneration. Ophthalmic Res. 2011;46:31–37. doi: 10.1159/000321940. [DOI] [PubMed] [Google Scholar]
- Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, Spencer KL, Kwan SY, Noureddine M, Gilbert JR, Schnetz-Boutaud N, Agarwal A, Postel EA, Pericak-Vance MA. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- Hammes HP, Hoerauf H, Alt A, Schleicher E, Clausen JT, Bretzel RG, Laqua H. N(epsilon)(carboxymethyl)lysin and the AGE receptor RAGE colocalize in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1999;40:1855–1859. [PubMed] [Google Scholar]
- Han YJ, Kwon YG, Chung HT, Lee SK, Simmons RL, Billiar TR, Kim YM. Antioxidant enzymes suppress nitric oxide production through the inhibition of NF-kappa B activation: role of H(2)O(2) and nitric oxide in inducible nitric oxide synthase expression in macrophages. Nitric Oxide. 2001;5:504–513. doi: 10.1006/niox.2001.0367. [DOI] [PubMed] [Google Scholar]
- Handa JT, Verzijl N, Matsunaga H, Aotaki-Keen A, Lutty GA, te Koppele JM, Miyata T, Hjelmeland LM. Increase in the advanced glycation end product pentosidine in Bruch's membrane with age. Invest Ophthalmol Vis Sci. 1999;40:775–779. [PubMed] [Google Scholar]
- Hein G, Wiegand R, Lehmann G, Stein G, Franke S. Advanced glycation end-products pentosidine and N epsilon-carboxymethyllysine are elevated in serum of patients with osteoporosis. Rheumatology (Oxford) 2003;42:1242–1246. doi: 10.1093/rheumatology/keg324. [DOI] [PubMed] [Google Scholar]
- Helmy KY, Katschke KJ, Jr, Gorgani NN, Kljavin NM, Elliott JM, Diehl L, Scales SJ, Ghilardi N, van Lookeren Campagne M. CRIg: a macrophage complement receptor required for phagocytosis of circulating pathogens. Cell. 2006;124:915–927. doi: 10.1016/j.cell.2005.12.039. [DOI] [PubMed] [Google Scholar]
- Herrero C, Sebastian C, Marques L, Comalada M, Xaus J, Valledor AF, Lloberas J, Celada A. Immunosenescence of macrophages: reduced MHC class II gene expression. Exp Gerontol. 2002;37:389–394. doi: 10.1016/s0531-5565(01)00205-4. [DOI] [PubMed] [Google Scholar]
- Hobbs MV, Weigle WO, Noonan DJ, Torbett BE, McEvilly RJ, Koch RJ, Cardenas GJ, Ernst DN. Patterns of cytokine gene expression by CD4+ T cells from young and old mice. J Immunol. 1993;150:3602–3614. [PubMed] [Google Scholar]
- Hodge WG, Barnes D, Schachter HM, Pan YI, Lowcock EC, Zhang L, Sampson M, Morrison A, Tran K, Miguelez M, Lewin G. Evidence for the effect of omega-3 fatty acids on progression of age-related macular degeneration: a systematic review. Retina. 2007;27:216–221. doi: 10.1097/01.iae.0000233322.83713.2d. [DOI] [PubMed] [Google Scholar]
- Hodge WG, Schachter HM, Barnes D, Pan Y, Lowcock EC, Zhang L, Sampson M, Morrison A, Tran K, Miguelez M, Lewin G. Efficacy of omega-3 fatty acids in preventing age-related macular degeneration: a systematic review. Ophthalmology. 2006;113:1165–1172. doi: 10.1016/j.ophtha.2006.02.043. [DOI] [PubMed] [Google Scholar]
- Hollyfield JG, Bonilha VL, Rayborn ME, Yang X, Shadrach KG, Lu L, Ufret RL, Salomon RG, Perez VL. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat Med. 2008;14:194–198. doi: 10.1038/nm1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollyfield JG, Perez VL, Salomon RG. A hapten generated from an oxidation fragment of docosahexaenoic acid is sufficient to initiate age-related macular degeneration. Mol Neurobiol. 2010;41:290–298. doi: 10.1007/s12035-010-8110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie K, Miyata T, Yasuda T, Takeda A, Yasuda Y, Maeda K, Sobue G, Kurokawa K. Immunohistochemical localization of advanced glycation end products, pentosidine, and carboxymethyllysine in lipofuscin pigments of Alzheimer's disease and aged neurons. Biochem Biophys Res Commun. 1997;236:327–332. doi: 10.1006/bbrc.1997.6944. [DOI] [PubMed] [Google Scholar]
- Howes KA, Liu Y, Dunaief JL, Milam A, Frederick JM, Marks A, Baehr W. Receptor for advanced glycation end products and age-related macular degeneration. Invest Ophthalmol Vis Sci. 2004;45:3713–3720. doi: 10.1167/iovs.04-0404. [DOI] [PubMed] [Google Scholar]
- Hu M, Liu B, Jawad S, Ling D, Casady M, Wei L, Nussenblatt RB. C5a contributes to intraocular inflammation by affecting retinal pigment epithelial cells and immune cells. Br J Ophthalmol. 2011;95:1738–1744. doi: 10.1136/bjophthalmol-2011-300235. [DOI] [PubMed] [Google Scholar]
- Huang JD, Amaral J, Lee JW, Larrayoz IM, Rodriguez IR. Sterculic acid antagonizes 7-ketocholesterol-mediated inflammation and inhibits choroidal neovascularization. Biochimica et biophysica acta. 2012;1821:637–646. doi: 10.1016/j.bbalip.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AE, Orr N, Esfandiary H, az-Torres M, Goodship T, Chakravarthy U. A common CFH haplotype, with deletion of CFHR1 and CFHR3, is associated with lower risk of age-related macular degeneration. Nat Genet. 2006;38:1173–1177. doi: 10.1038/ng1890. [DOI] [PubMed] [Google Scholar]
- Hunter A, Spechler PA, Cwanger A, Song Y, Zhang Z, Ying GS, Hunter AK, Dezoeten E, Dunaief JL. DNA methylation is associated with altered gene expression in AMD. Invest Ophthalmol Vis Sci. 2012;53:2089–2105. doi: 10.1167/iovs.11-8449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain AA, Lee Y, Marshall J. High molecular-weight gelatinase species of human Bruch's membrane: compositional analyses and age-related changes. Invest Ophthalmol Vis Sci. 2010a;51:2363–2371. doi: 10.1167/iovs.09-4259. [DOI] [PubMed] [Google Scholar]
- Hussain AA, Lee Y, Zhang JJ, Marshall J. Disturbed matrix metalloproteinase activity of Bruch's membrane in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:4459–4466. doi: 10.1167/iovs.10-6678. [DOI] [PubMed] [Google Scholar]
- Hussain AA, Rowe L, Marshall J. Age-related alterations in the diffusional transport of amino acids across the human Bruch's-choroid complex. J Opt Soc Am A Opt Image Sci Vis. 2002;19:166–172. doi: 10.1364/josaa.19.000166. [DOI] [PubMed] [Google Scholar]
- Hussain AA, Starita C, Hodgetts A, Marshall J. Macromolecular diffusion characteristics of ageing human Bruch's membrane: implications for age-related macular degeneration (AMD) Exp Eye Res. 2010b;90:703–710. doi: 10.1016/j.exer.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Isas JM, Luibl V, Johnson LV, Kayed R, Wetzel R, Glabe CG, Langen R, Chen J. Soluble and mature amyloid fibrils in drusen deposits. Invest Ophthalmol Vis Sci. 2010;51:1304–1310. doi: 10.1167/iovs.09-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager RD, Mieler WF, Miller JW. Age-related Macular Degeneration. N Engl J Med. 2008;358:2606–2617. doi: 10.1056/NEJMra0801537. [DOI] [PubMed] [Google Scholar]
- Jiang B, Bezhadian MA, Caldwell RB. Astrocytes modulate retinal vasculogenesis: effects on endothelial cell differentiation. Glia. 1995;15:1–10. doi: 10.1002/glia.440150102. [DOI] [PubMed] [Google Scholar]
- Jiang B, Liou GI, Behzadian MA, Caldwell RB. Astrocytes modulate retinal vasculogenesis: effects on fibronectin expression. J Cell Sci. 1994;107(Pt 9):2499–2508. doi: 10.1242/jcs.107.9.2499. [DOI] [PubMed] [Google Scholar]
- Jing G, Wang JJ, Zhang SX. ER stress and apoptosis: a new mechanism for retinal cell death. Experimental diabetes research. 2012;2012:589589. doi: 10.1155/2012/589589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LV, Forest DL, Banna CD, Radeke CM, Maloney MA, Hu J, Spencer CN, Walker AM, Tsie MS, Bok D, Radeke MJ, Anderson DH. Cell culture model that mimics drusen formation and triggers complement activation associated with age-related macular degeneration. Proc Natl Acad Sci U S A. 2011;108:18277–18282. doi: 10.1073/pnas.1109703108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M, Mitchell P, Wang JJ, Sue C. MELAS A3243G mitochondrial DNA mutation and age related maculopathy. Am J Ophthalmol. 2004;138:1051–1053. doi: 10.1016/j.ajo.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Jono T, Kimura T, Takamatsu J, Nagai R, Miyazaki K, Yuzuriha T, Kitamura T, Horiuchi S. Accumulation of imidazolone, pentosidine and N(epsilon)-(carboxymethyl)lysine in hippocampal CA4 pyramidal neurons of aged human brain. Pathol Int. 2002;52:563–571. doi: 10.1046/j.1320-5463.2002.01390.x. [DOI] [PubMed] [Google Scholar]
- Julien S, Schraermeyer U. Lipofuscin can be eliminated from the retinal pigment epithelium of monkeys. Neurobiol Aging. 2012;33:2390–2397. doi: 10.1016/j.neurobiolaging.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Kaarniranta K, Hyttinen J, Ryhanen T, Viiri J, Paimela T, Toropainen E, Sorri I, Salminen A. Mechanisms of protein aggregation in the retinal pigment epithelial cells. Front Biosci (Elite Ed) 2010;2:1374–1384. doi: 10.2741/e198. [DOI] [PubMed] [Google Scholar]
- Kaarniranta K, Salminen A. NF-kappaB signaling as a putative target for omega-3 metabolites in the prevention of age-related macular degeneration (AMD) Exp Gerontol. 2009;44:685–688. doi: 10.1016/j.exger.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Kabasawa S, Mori K, Horie-Inoue K, Gehlbach PL, Inoue S, Awata T, Katayama S, Yoneya S. Associations of Cigarette Smoking But Not Serum Fatty Acids with Age-related Macular Degeneration in a Japanese Population. Ophthalmology. 2011;118:1082–1088. doi: 10.1016/j.ophtha.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Kamei M, Hollyfield JG. TIMP-3 in Bruch's membrane: changes during aging and in age-related macular degeneration. Investigative Ophthalmology & Visual Science. 1999;40:2367–2375. [PubMed] [Google Scholar]
- Kaneko H, Dridi S, Tarallo V, Gelfand BD, Fowler BJ, Cho WG, Kleinman ME, Ponicsan SL, Hauswirth WW, Chiodo VA, Kariko K, Yoo JW, Lee Dk, Hadziahmetovic M, Song Y, Misra S, Chaudhuri G, Buaas FW, Braun RE, Hinton DR, Zhang Q, Grossniklaus HE, Provis JM, Madigan MC, Milam AH, Justice NL, Albuquerque RJC, Blandford AD, Bogdanovich S, Hirano Y, Witta J, Fuchs E, Littman DR, Ambati BK, Rudin CM, Chong MMW, Provost P, Kugel JF, Goodrich JA, Dunaief JL, Baffi JZ, Ambati J. DICER1 deficit induces Alu RNA toxicity in age-related macular degeneration. Nature. 2011;471:325–330. doi: 10.1038/nature09830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan R, Sreekumar PG, Hinton DR. VEGF and PEDF secretion in ARPE-19 and fhRPE cells. Invest Ophthalmol Vis Sci. 2011;52:9047. doi: 10.1167/iovs.11-8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlstetter M, Ebert S, Langmann T. Microglia in the healthy and degenerating retina: insights from novel mouse models. Immunobiology. 2010;215:685–691. doi: 10.1016/j.imbio.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Karunadharma PP, Nordgaard CL, Olsen TW, Ferrington DA. Mitochondrial DNA damage as a potential mechanism for age-related macular degeneration. Invest Ophthalmol Vis Sci. 2010;51:5470–5479. doi: 10.1167/iovs.10-5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawazoe Y, Sugita S, Keino H, Yamada Y, Imai A, Horie S, Mochizuki M. Retinoic acid from retinal pigment epithelium induces T regulatory cells. Exp Eye Res. 2012;94:32–40. doi: 10.1016/j.exer.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Kelly J, Ali KA, Yin J, Ferguson TA, Apte RS. Senescence regulates macrophage activation and angiogenic fate at sites of tissue injury in mice. J Clin Invest. 2007;117:3421–3426. doi: 10.1172/JCI32430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney MC, Atilano SR, Boyer D, Chwa M, Chak G, Chinichian S, Coskun P, Wallace DC, Nesburn AB, Udar NS. Characterization of retinal and blood mitochondrial DNA from age-related macular degeneration patients. Invest Ophthalmol Vis Sci. 2010;51:4289–4297. doi: 10.1167/iovs.09-4778. [DOI] [PubMed] [Google Scholar]
- Kenney MC, Chwa M, Atilano SR, Pavlis JM, Falatoonzadeh P, Ramirez C, Malik D, Hsu T, Woo G, Soe K, Nesburn AB, Boyer DS, Kuppermann BD, Jazwinski SM, Miceli MV, Wallace DC, Udar N. Mitochondrial DNA variants mediate energy production and expression levels for CFH, C3 and EFEMP1 genes: implications for age-related macular degeneration. PLoS One. 2013a;8:e54339. doi: 10.1371/journal.pone.0054339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney MC, Hertzog D, Chak G, Atilano SR, Khatibi N, Soe K, Nobe A, Yang E, Chwa M, Zhu F, Memarzadeh M, King J, Langberg J, Small K, Nesburn AB, Boyer DS, Udar N. Mitochondrial DNA haplogroups confer differences in risk for age-related macular degeneration: a case control study. BMC medical genetics. 2013b;14:4. doi: 10.1186/1471-2350-14-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, He S, Kase S, Kitamura M, Ryan SJ, Hinton DR. Regulated secretion of complement factor H by RPE and its role in RPE migration. Graefes Arch Clin Exp Ophthalmol. 2009;247:651–659. doi: 10.1007/s00417-009-1049-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Takamatsu J, Miyata T, Miyakawa T, Horiuchi S. Localization of identified advanced glycation end-product structures, N epsilon(carboxymethyl)lysine and pentosidine, in age-related inclusions in human brains. Pathol Int. 1998;48:575–579. doi: 10.1111/j.1440-1827.1998.tb03953.x. [DOI] [PubMed] [Google Scholar]
- Kindt TJ, Goldsby RA, Osborne BA, Kuby J. Kuby Immunology. 6th. W. H. Freeman; New York: 2007. [Google Scholar]
- Kinnunen K, Petrovski G, Moe MC, Berta A, Kaarniranta K. Molecular mechanisms of retinal pigment epithelium damage and development of age-related macular degeneration. Acta Ophthalmol. 2012;90:299–309. doi: 10.1111/j.1755-3768.2011.02179.x. [DOI] [PubMed] [Google Scholar]
- Klein R, Meuer SM, Knudtson MD, Iyengar SK, Klein BE. The epidemiology of retinal reticular drusen. Am J Ophthalmol. 2008;145:317–326. doi: 10.1016/j.ajo.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, Bracken MB, Ferris FL, Ott J, Barnstable C, Hoh J. Complement Factor H Polymorphism in Age-Related Macular Degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudtson MD, Klein R, Klein BE, Lee KE, Meuer SM, Tomany SC. Location of lesions associated with age-related maculopathy over a 10-year period: the Beaver Dam Eye Study. Invest Ophthalmol Vis Sci. 2004;45:2135–2142. doi: 10.1167/iovs.03-1085. [DOI] [PubMed] [Google Scholar]
- Korte GE, Reppucci V, Henkind P. RPE destruction causes choriocapillary atrophy. Invest Ophthalmol Vis Sci. 1984;25:1135–1145. [PubMed] [Google Scholar]
- Krohne TU, Stratmann NK, Kopitz J, Holz FG. Effects of lipid peroxidation products on lipofuscinogenesis and autophagy in human retinal pigment epithelial cells. Exp Eye Res. 2010;90:465–471. doi: 10.1016/j.exer.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Kubo M, Cinader B. Polymorphism of age-related changes in interleukin (IL) production: differential changes of T helper subpopulations, synthesizing IL 2, IL 3 and IL 4. Eur J Immunol. 1990;20:1289–1296. doi: 10.1002/eji.1830200614. [DOI] [PubMed] [Google Scholar]
- Kumar MV, Nagineni CN, Chin MS, Hooks JJ, Detrick B. Innate immunity in the retina: Toll-like receptor (TLR) signaling in human retinal pigment epithelial cells. J Neuroimmunol. 2004;153:7–15. doi: 10.1016/j.jneuroim.2004.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis GP, Linberg KA, Fisher SK. Neurite outgrowth from bipolar and horizontal cells after experimental retinal detachment. Invest Ophthalmol Vis Sci. 1998;39:424–434. [PubMed] [Google Scholar]
- Li CM, Chung BH, Presley JB, Malek G, Zhang X, Dashti N, Li L, Chen J, Bradley K, Kruth HS, Curcio CA. Lipoprotein-like particles and cholesteryl esters in human Bruch's membrane: initial characterization. Invest Ophthalmol Vis Sci. 2005;46:2576–2586. doi: 10.1167/iovs.05-0034. [DOI] [PubMed] [Google Scholar]
- Li DY, Xue MY, Geng ZR, Chen PY. The suppressive effects of Bursopentine (BP5) on oxidative stress and NF-kB activation in lipopolysaccharide-activated murine peritoneal macrophages. Cell Physiol Biochem. 2012;29:9–20. doi: 10.1159/000337581. [DOI] [PubMed] [Google Scholar]
- Libby RT, Gould DB. Endoplasmic reticulum stress as a primary pathogenic mechanism leading to age-related macular degeneration. Adv Exp Med Biol. 2010;664:403–409. doi: 10.1007/978-1-4419-1399-9_46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Xu H, Liang FQ, Liang H, Gupta P, Havey AN, Boulton ME, Godley BF. Mitochondrial DNA damage and repair in RPE associated with aging and age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:3521–3529. doi: 10.1167/iovs.10-6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Wei L, Meyerle C, Tuo J, Sen HN, Li Z, Chakrabarty S, Agron E, Chan CC, Klein ML, Chew E, Ferris F, Nussenblatt RB. Complement Component C5a Promotes Expression of IL-22 and IL-17 from Human T cells and its Implication in Age-related Macular Degeneration. J Transl Med. 2011;9:111. doi: 10.1186/1479-5876-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Ge D, Ma L, Mei J, Liu S, Zhang Q, Ren F, Liao H, Pu Q, Wang T, You Z. Interleukin-17 and prostaglandin E2 are involved in formation of an M2 macrophage-dominant microenvironment in lung cancer. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2012;7:1091–1100. doi: 10.1097/JTO.0b013e3182542752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes KO, Sparks DL, Streit WJ. Microglial dystrophy in the aged and Alzheimer's disease brain is associated with ferritin immunoreactivity. Glia. 2008;56:1048–1060. doi: 10.1002/glia.20678. [DOI] [PubMed] [Google Scholar]
- Luibl V, Isas JM, Kayed R, Glabe CG, Langen R, Chen J. Drusen deposits associated with aging and age-related macular degeneration contain nonfibrillar amyloid oligomers. J Clin Invest. 2006;116:378–385. doi: 10.1172/JCI25843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutty G, Grunwald J, Majji AB, Uyama M, Yoneya S. Changes in choriocapillaris and retinal pigment epithelium in age-related macular degeneration. Mol Vis. 1999;5:35. [PubMed] [Google Scholar]
- Ma W, Coon S, Zhao L, Fariss RN, Wong WT. A2E accumulation influences retinal microglial activation and complement regulation. Neurobiol Aging. 2013;34:943–960. doi: 10.1016/j.neurobiolaging.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Lee SE, Guo J, Qu W, Hudson BI, Schmidt AM, Barile GR. RAGE ligand upregulation of VEGF secretion in ARPE-19 cells. Invest Ophthalmol Vis Sci. 2007;48:1355–1361. doi: 10.1167/iovs.06-0738. [DOI] [PubMed] [Google Scholar]
- Ma W, Zhao L, Fontainhas AM, Fariss RN, Wong WT. Microglia in the mouse retina alter the structure and function of retinal pigmented epithelial cells: a potential cellular interaction relevant to AMD. PLoS One. 2009;4:e7945. doi: 10.1371/journal.pone.0007945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macgregor AM, Eberhart CG, Fraig M, Lu J, Halushka MK. Tissue inhibitor of matrix metalloproteinase-3 levels in the extracellular matrix of lung, kidney, and eye increase with age. J Histochem Cytochem. 2009;57:207–213. doi: 10.1369/jhc.2008.952531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madigan MC, Penfold PL, Provis JM, Balind TK, Billson FA. Intermediate filament expression in human retinal macroglia. Histopathologic changes associated with age-related macular degeneration. Retina. 1994;14:65–74. [PubMed] [Google Scholar]
- Maeda A, Crabb JW, Palczewski K. Microsomal glutathione S-transferase 1 in the retinal pigment epithelium: protection against oxidative stress and a potential role in aging. Biochemistry. 2005;44:480–489. doi: 10.1021/bi048016f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal MN, Vasireddy V, Reddy GB, Wang X, Moroi SE, Pattnaik BR, Hughes BA, Heckenlively JR, Hitchcock PF, Jablonski MM, Ayyagari R. CTRP5 Is a Membrane-Associated and Secretory Protein in the RPE and Ciliary Body and the S163R Mutation of CTRP5 Impairs Its Secretion. Invest Ophthalmol Vis Sci. 2006;47:5505–5513. doi: 10.1167/iovs.06-0312. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Garlanda C, Locati M. Macrophage diversity and polarization in atherosclerosis: a question of balance. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:1419–1423. doi: 10.1161/ATVBAHA.108.180497. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23:344–346. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends in immunology. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009;147:811–815. doi: 10.1016/j.ajo.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Mayurasakorn K, Williams JJ, Ten VS, Deckelbaum RJ. Docosahexaenoic acid: brain accretion and roles in neuroprotection after brain hypoxia and ischemia. Current opinion in clinical nutrition and metabolic care. 2011;14:158–167. doi: 10.1097/MCO.0b013e328342cba5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod DS, Grebe R, Bhutto I, Merges C, Baba T, Lutty GA. Relationship between RPE and choriocapillaris in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50:4982–4991. doi: 10.1167/iovs.09-3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod DS, Taomoto M, Otsuji T, Green WR, Sunness JS, Lutty GA. Quantifying changes in RPE and choroidal vasculature in eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2002;43:1986–1993. [PubMed] [Google Scholar]
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Miller RA. Aging and immune function. Int Rev Cytol. 1991;124:187–215. doi: 10.1016/s0074-7696(08)61527-2. [DOI] [PubMed] [Google Scholar]
- Miyata T, Hori O, Zhang J, Yan SD, Ferran L, Iida Y, Schmidt AM. The receptor for advanced glycation end products (RAGE) is a central mediator of the interaction of AGE-beta2microglobulin with human mononuclear phagocytes via an oxidant-sensitive pathway. Implications for the pathogenesis of dialysis-related amyloidosis. J Clin Invest. 1996;98:1088–1094. doi: 10.1172/JCI118889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki M, Ikeda Y, Yonemitsu Y, Goto Y, Murakami Y, Yoshida N, Tabata T, Hasegawa M, Tobimatsu S, Sueishi K, Ishibashi T. Pigment epithelium-derived factor gene therapy targeting retinal ganglion cell injuries: neuroprotection against loss of function in two animal models. Human gene therapy. 2011;22:559–565. doi: 10.1089/hum.2010.132. [DOI] [PubMed] [Google Scholar]
- Moore DJ, Hussain AA, Marshall J. Age-related variation in the hydraulic conductivity of Bruch's membrane. Invest Ophthalmol Vis Sci. 1995;36:1290–1297. [PubMed] [Google Scholar]
- Moreira EF, Larrayoz IM, Lee JW, Rodriguez IR. 7-Ketocholesterol is present in lipid deposits in the primate retina: potential implication in the induction of VEGF and CNV formation. Invest Ophthalmol Vis Sci. 2009;50:523–532. doi: 10.1167/iovs.08-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrak RE, Griffin WS. Glia and their cytokines in progression of neurodegeneration. Neurobiol Aging. 2005;26:349–354. doi: 10.1016/j.neurobiolaging.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Mueller EE, Schaier E, Brunner SM, Eder W, Mayr JA, Egger SF, Nischler C, Oberkofler H, Reitsamer HA, Patsch W, Sperl W, Kofler B. Mitochondrial haplogroups and control region polymorphisms in age-related macular degeneration: a case-control study. PLoS One. 2012;7:e30874. doi: 10.1371/journal.pone.0030874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee P, Marcheselli V, de Rivero Vacari J, Gordon W, Jackson F, Bazan N. Photoreceptor outer segment phagocytosis attenuates oxidative-stress induced apoptosis with concomitant neuroprotecting D1 synthesis. Proc Natl Acad Sci U S A. 2007;104:13158–13163. doi: 10.1073/pnas.0705963104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins RF, Faidley EA, Daggett HT, Jomary C, Lotery AJ, Stone EM. Localization of complement 1 inhibitor (C1INH/SERPING1) in human eyes with age-related macular degeneration. Exp Eye Res. 2009;89:767–773. doi: 10.1016/j.exer.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins RF, Johnson MN, Faidley EA, Skeie JM, Huang J. Choriocapillaris vascular dropout related to density of drusen in human eyes with early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:1606–1612. doi: 10.1167/iovs.10-6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins RF, Russell SR, Anderson DH, Hageman GS. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000;14:835–846. [PubMed] [Google Scholar]
- Murakami Y, Ikeda Y, Yonemitsu Y, Onimaru M, Nakagawa K, Kohno R, Miyazaki M, Hisatomi T, Nakamura M, Yabe T, Hasegawa M, Ishibashi T, Sueishi K. Inhibition of nuclear translocation of apoptosis-inducing factor is an essential mechanism of the neuroprotective activity of pigment epithelium-derived factor in a rat model of retinal degeneration. Am J Pathol. 2008;173:1326–1338. doi: 10.2353/ajpath.2008.080466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag TC, Wadhwa S, Alladi PA, Sanyal T. Localization of 4-hydroxy 2-nonenal immunoreactivity in aging human retinal Muller cells. Annals of anatomy = Anatomischer Anzeiger: official organ of the Anatomische Gesellschaft. 2011;193:205–210. doi: 10.1016/j.aanat.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Ni J, Yuan X, Gu J, Yue X, Gu X, Nagaraj RH, Crabb JW. Plasma Protein Pentosidine and Carboxymethyllysine, Biomarkers for Age-related Macular Degeneration. Mol Cell Proteomics. 2009;8:1921–1933. doi: 10.1074/mcp.M900127-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njemini R, Bautmans I, Lambert M, Demanet C, Mets T. Heat shock proteins and chemokine/cytokine secretion profile in ageing and inflammation. Mech Ageing Dev. 2007;128:450–454. doi: 10.1016/j.mad.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Odetti P, Rossi S, Monacelli F, Poggi A, Cirnigliaro M, Federici M, Federici A. Advanced glycation end products and bone loss during aging. Ann N Y Acad Sci. 2005;1043:710–717. doi: 10.1196/annals.1333.082. [DOI] [PubMed] [Google Scholar]
- Parapuram SK, Cojocaru RI, Chang JR, Khanna R, Brooks M, Othman M, Zareparsi S, Khan NW, Gotoh N, Cogliati T, Swaroop A. Distinct Signature of Altered Homeostasis in Aging Rod Photoreceptors: Implications for Retinal Diseases. PLoS One. 2010;5:e13885. doi: 10.1371/journal.pone.0013885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K, Jin J, Hu Y, Zhou K, Ma JX. Overexpression of pigment epithelium-derived factor inhibits retinal inflammation and neovascularization. Am J Pathol. 2011;178:688–698. doi: 10.1016/j.ajpath.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KH, Ryu E, Tosakulwong N, Wu Y, Edwards AO. Common variation in the SERPING1 gene is not associated with age-related macular degeneration in two independent groups of subjects. Mol Vis. 2009;15:200–207. [PMC free article] [PubMed] [Google Scholar]
- Park S, Shen D, Wei L, Wang Y, Eberhart CG, Nussenblatt RB, Chan CC. IL-17A and IL-17RC expression in the macula of the human eye with Age-related Macular Degeneration, ARVO #1667, Ft. Lauderdale: 2012. [Google Scholar]
- Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. British Journal of Ophthalmology. 2011 doi: 10.1136/bjophthalmol-2011-300539. [DOI] [PubMed] [Google Scholar]
- Pascolini D, Mariotti SP, Pokharel GP, Pararajasegaram R, Etya'ale D, Negrel AD, Resnikoff S. 2002 global update of available data on visual impairment: a compilation of population-based prevalence studies. Ophthalmic Epidemiol. 2004;11:67–115. doi: 10.1076/opep.11.2.67.28158. [DOI] [PubMed] [Google Scholar]
- Patel M, Chan CC. Immunopathological aspects of age-related macular degeneration. Semin Immunopathol. 2008;30:97–110. doi: 10.1007/s00281-008-0112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauleikhoff D, Harper CA, Marshall J, Bird AC. Aging changes in Bruch's membrane. A histochemical and morphologic study. Ophthalmology. 1990;97:171–178. [PubMed] [Google Scholar]
- Penfold PL, Killingsworth MC, Sarks SH. Senile macular degeneration: the involvement of immunocompetent cells. Graefes Arch Clin Exp Ophthalmol. 1985;223:69–76. doi: 10.1007/BF02150948. [DOI] [PubMed] [Google Scholar]
- Petrovski G, Berenyi E, Moe MC, Vajas A, Fesus L, Berta A, Facsko A. Clearance of dying ARPE-19 cells by professional and nonprofessional phagocytes in vitro- implications for age-related macular degeneration (AMD) Acta Ophthalmol. 2011;89:e30–34. doi: 10.1111/j.1755-3768.2010.02047.x. [DOI] [PubMed] [Google Scholar]
- Pillin A, Pudil F, Bencko V, Bezdickova D. Contents of pentosidine in the tissue of the intervertebral disc as an indicator of the human age. Soudni lekarstvi/casopis Sekce soudniho lekarstvi Cs. lekarske spolecnosti. J Ev Purkyne. 2007;52:60–64. [PubMed] [Google Scholar]
- Plackett TP, Schilling EM, Faunce DE, Choudhry MA, Witte PL, Kovacs EJ. Aging enhances lymphocyte cytokine defects after injury. FASEB J. 2003;17:688–689. doi: 10.1096/fj.02-0452fje. [DOI] [PubMed] [Google Scholar]
- Prasad AS. Zinc: role in immunity, oxidative stress and chronic inflammation. Current opinion in clinical nutrition and metabolic care. 2009;12:646–652. doi: 10.1097/MCO.0b013e3283312956. [DOI] [PubMed] [Google Scholar]
- Prelog M. Aging of the immune system: a risk factor for autoimmunity? Autoimmun Rev. 2006;5:136–139. doi: 10.1016/j.autrev.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Prosser BE, Johnson S, Roversi P, Herbert AP, Blaum BS, Tyrrell J, Jowitt TA, Clark SJ, Tarelli E, Uhrin D, Barlow PN, Sim RB, Day AJ, Lea SM. Structural basis for complement factor H linked age-related macular degeneration. J Exp Med. 2007;204:2277–2283. doi: 10.1084/jem.20071069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi JH, Ebrahem Q, Moore N, Murphy G, Claesson-Welsh L, Bond M, Baker A, Anand-Apte B. A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nat Med. 2003;9:407–415. doi: 10.1038/nm846. [DOI] [PubMed] [Google Scholar]
- Querques G, Querques L, Martinelli D, Massamba N, Coscas G, Soubrane G, Souied EH. Pathologic insights from integrated imaging of reticular pseudodrusen in age-related macular degeneration. Retina. 2011;31:518–526. doi: 10.1097/IAE.0b013e3181f04974. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Ramirez AI, Salazar JJ, de Hoz R, Trivino A. Changes of astrocytes in retinal ageing and age-related macular degeneration. Exp Eye Res. 2001;73:601–615. doi: 10.1006/exer.2001.1061. [DOI] [PubMed] [Google Scholar]
- Ramrattan RS, van der Schaft TL, Mooy CM, de Bruijn WC, Mulder PG, de Jong PT. Morphometric analysis of Bruch's membrane, the choriocapillaris, and the choroid in aging. Invest Ophthalmol Vis Sci. 1994;35:2857–2864. [PubMed] [Google Scholar]
- Reinoso MA, Mukherjee P, Marcheselli V, Bergsma D, Hesse R, Bazan N. PEDF Promotes Biosynthesis of a Novel Anti-inflammatory and Anti-apoptotic Mediator NPD1 in Retinal Pigment Epithelial Cells. The Ochsner journal. 2008;8:39–43. [PMC free article] [PubMed] [Google Scholar]
- Riancho JA, Zarrabeitia MT, Amado JA, Olmos JM, Gonzalez-Macias J. Age-related differences in cytokine secretion. Gerontology. 1994;40:8–12. doi: 10.1159/000213568. [DOI] [PubMed] [Google Scholar]
- Rodriguez IR, Larrayoz IM. Cholesterol oxidation in the retina: implications of 7KCh formation in chronic inflammation and age-related macular degeneration. Journal of lipid research. 2010;51:2847–2862. doi: 10.1194/jlr.R004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer B, Coughlin B, Bandyopadhyay M, Holers VM. Systemic Human CR2-Targeted Complement Alternative Pathway Inhibitor Ameliorates Mouse Laser-Induced Choroidal Neovascularization. Journal of ocular pharmacology and therapeutics: the official journal of the Association for Ocular Pharmacology and Therapeutics. 2012;28:402–409. doi: 10.1089/jop.2011.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer B, Long Q, Coughlin B, Renner B, Huang Y, Kunchithapautham K, Ferreira VP, Pangburn MK, Gilkeson GS, Thurman JM, Tomlinson S, Holers VM. A targeted inhibitor of the complement alternative pathway reduces RPE injury and angiogenesis in models of age-related macular degeneration. Adv Exp Med Biol. 2010;703:137–149. doi: 10.1007/978-1-4419-5635-4_10. [DOI] [PubMed] [Google Scholar]
- Roque RS, Rosales AA, Jingjing L, Agarwal N, Al-Ubaidi MR. Retina-derived microglial cells induce photoreceptor cell death in vitro. Brain Res. 1999;836:110–119. doi: 10.1016/s0006-8993(99)01625-x. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JT. Eyeing macular degeneration--few inflammatory remarks. N Engl J Med. 2012;367:768–770. doi: 10.1056/NEJMcibr1204973. [DOI] [PubMed] [Google Scholar]
- Rudolf M, Clark ME, Chimento MF, Li CM, Medeiros NE, Curcio CA. Prevalence and morphology of druse types in the macula and periphery of eyes with age-related maculopathy. Invest Ophthalmol Vis Sci. 2008;49:1200–1209. doi: 10.1167/iovs.07-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolf M, Vogt SD, Curcio CA, Huisingh C, McGwin G, Jr, Wagner A, Grisanti S, Read RW. Histologic Basis of Variations in Retinal Pigment Epithelium Autofluorescence in Eyes with Geographic Atrophy. Ophthalmology. 2013;120:821–828. doi: 10.1016/j.ophtha.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolf M, Winkler B, Aherrahou Z, Doehring LC, Kaczmarek P, Schmidt-Erfurth U. Increased expression of vascular endothelial growth factor associated with accumulation of lipids in Bruch's membrane of LDL receptor knockout mice. Br J Ophthalmol. 2005;89:1627–1630. doi: 10.1136/bjo.2005.071183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutar MV, Natoli RC, Provis JM. Small interfering RNA-mediated suppression of Ccl2 in Muller cells attenuates microglial recruitment and photoreceptor death following retinal degeneration. J Neuroinflammation. 2012;9:221. doi: 10.1186/1742-2094-9-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Kauppinen A, Hyttinen JM, Toropainen E, Kaarniranta K. Endoplasmic reticulum stress in age-related macular degeneration: trigger for neovascularization. Mol Med. 2010;16:535–542. doi: 10.2119/molmed.2010.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez A, Tripathy D, Yin X, Luo J, Martinez J, Grammas P. Pigment epithelium-derived factor (PEDF) protects cortical neurons in vitro from oxidant injury by activation of extracellular signal-regulated kinase (ERK) 1/2 and induction of Bcl-2. Neuroscience research. 2012;72:1–8. doi: 10.1016/j.neures.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarks SH. Ageing and degeneration in the macular region: a clinico-pathological study. Br J Ophthalmol. 1976;60:324–341. doi: 10.1136/bjo.60.5.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer T, Patel M, Chan CC, Tuo J. Unfolding the therapeutic potential of chemical chaperones for age-related macular degeneration. Expert Review of Ophthalmology. 2008;3:29–42. doi: 10.1586/17469899.3.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt AM, Hasu M, Popov D, Zhang JH, Chen J, Yan SD, Brett J, Cao R, Kuwabara K, Costache G, et al. Receptor for advanced glycation end products (AGEs) has a central role in vessel wall interactions and gene activation in response to circulating AGE proteins. Proc Natl Acad Sci U S A. 1994;91:8807–8811. doi: 10.1073/pnas.91.19.8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt AM, Hori O, Chen JX, Li JF, Crandall J, Zhang J, Cao R, Yan SD, Brett J, Stern D. Advanced glycation endproducts interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and in mice. A potential mechanism for the accelerated vasculopathy of diabetes. J Clin Invest. 1995;96:1395–1403. doi: 10.1172/JCI118175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz-Valckenberg S, Steinberg JS, Fleckenstein M, Visvalingam S, Brinkmann CK, Holz FG. Combined confocal scanning laser ophthalmoscopy and spectral-domain optical coherence tomography imaging of reticular drusen associated with age-related macular degeneration. Ophthalmology. 2010;117:1169–1176. doi: 10.1016/j.ophtha.2009.10.044. [DOI] [PubMed] [Google Scholar]
- Scholl HP, Charbel Issa P, Walier M, Janzer S, Pollok-Kopp B, Borncke F, Fritsche LG, Chong NV, Fimmers R, Wienker T, Holz FG, Weber BH, Oppermann M. Systemic complement activation in age-related macular degeneration. PLoS One. 2008;3:e2593. doi: 10.1371/journal.pone.0002593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutt F, Aretz S, Auffarth GU, Kopitz J. Moderately reduced ATP levels promote oxidative stress and debilitate autophagic and phagocytic capacities in human RPE cells. Invest Ophthalmol Vis Sci. 2012;53:5354–5361. doi: 10.1167/iovs.12-9845. [DOI] [PubMed] [Google Scholar]
- Seddon JM, Cote J, Davis N, Rosner B. Progression of age-related macular degeneration: association with body mass index, waist circumference, and waist-hip ratio 34. Arch Ophthalmol. 2003a;121:785–792. doi: 10.1001/archopht.121.6.785. [DOI] [PubMed] [Google Scholar]
- Seddon JM, Cote J, Rosner B. Progression of age-related macular degeneration: association with dietary fat, transunsaturated fat, nuts, and fish intake. Arch Ophthalmol. 2003b;121:1728–1737. doi: 10.1001/archopht.121.12.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon JM, George S, Rosner B. Cigarette smoking, fish consumption, omega-3 fatty acid intake, and associations with age-related macular degeneration: the US Twin Study of Age-Related Macular Degeneration. Arch Ophthalmol. 2006;124:995–1001. doi: 10.1001/archopht.124.7.995. [DOI] [PubMed] [Google Scholar]
- Seddon JM, Reynolds R, Shah HR, Rosner B. Smoking, dietary betaine, methionine, and vitamin D in monozygotic twins with discordant macular degeneration: epigenetic implications. Ophthalmology. 2011;118:1386–1394. doi: 10.1016/j.ophtha.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon JM, Willett WC, Speizer FE, Hankinson SE. A prospective study of cigarette smoking and age-related macular degeneration in women. JAMA. 1996;276:1141–1146. [PubMed] [Google Scholar]
- Sheikpranbabu S, Haribalaganesh R, Gurunathan S. Pigment epithelium-derived factor inhibits advanced glycation end-products-induced cytotoxicity in retinal pericytes. Diabetes & metabolism. 2011;37:505–511. doi: 10.1016/j.diabet.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Shelley EJ, Madigan MC, Natoli R, Penfold PL, Provis JM. Cone degeneration in aging and age-related macular degeneration. Arch Ophthalmol. 2009;127:483–492. doi: 10.1001/archophthalmol.2008.622. [DOI] [PubMed] [Google Scholar]
- Shen D, Cao X, Zhao L, Tuo J, Wong WT, Chan CC. Naloxone ameliorates retinal lesions in Ccl2/Cx3cr1 double-deficient mice via modulation of microglia. Invest Ophthalmol Vis Sci. 2011;52:2897–2904. doi: 10.1167/iovs.10-6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng JG, Mrak RE, Griffin WS. Enlarged and phagocytic, but not primed, interleukin-1 alpha-immunoreactive microglia increase with age in normal human brain. Acta neuropathologica. 1998;95:229–234. doi: 10.1007/s004010050792. [DOI] [PubMed] [Google Scholar]
- Sieving PA, Caruso RC, Tao W, Coleman HR, Thompson DJ, Fullmer KR, Bush RA. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc Natl Acad Sci U S A. 2006;103:3896–3901. doi: 10.1073/pnas.0600236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeie JM, Mullins RF. Macrophages in neovascular age-related macular degeneration: friends or foes? Eye (Lond) 2009;23:747–755. doi: 10.1038/eye.2008.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaide RF, Curcio CA. Drusen characterization with multimodal imaging. Retina. 2010;30:1441–1454. doi: 10.1097/IAE.0b013e3181ee5ce8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaide RF, Ho-Spaide WC, Browne RW, Armstrong D. Characterization of peroxidized lipids in Bruch's membrane. Retina. 1999;19:141–147. doi: 10.1097/00006982-199902000-00010. [DOI] [PubMed] [Google Scholar]
- Sparrow JR. Bisretinoids of RPE lipofuscin: trigger for complement activation in age-related macular degeneration. Adv Exp Med Biol. 2010;703:63–74. doi: 10.1007/978-1-4419-5635-4_5. [DOI] [PubMed] [Google Scholar]
- Sparrow JR, Gregory-Roberts E, Yamamoto K, Blonska A, Ghosh SK, Ueda K, Zhou J. The bisretinoids of retinal pigment epithelium. Prog Retin Eye Res. 2012;31:121–135. doi: 10.1016/j.preteyeres.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow JR, Nakanishi K, Parish CA. The lipofuscin fluorophore A2E mediates blue light-induced damage to retinal pigmented epithelial cells. Invest Ophthalmol Vis Sci. 2000;41:1981–1989. [PubMed] [Google Scholar]
- Spencer KL, Hauser MA, Olson LM, Schmidt S, Scott WK, Gallins P, Agarwal A, Postel EA, Pericak-Vance MA, Haines JL. Protective effect of complement factor B and complement component 2 variants in age-related macular degeneration. Hum Mol Genet. 2007;16:1986–1992. [Google Scholar]
- Spraul CW, Lang GE, Grossniklaus HE. Morphometric analysis of the choroid, Bruch's membrane, and retinal pigment epithelium in eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 1996;37:2724–2735. [PubMed] [Google Scholar]
- Spraul CW, Lang GE, Grossniklaus HE, Lang GK. Histologic and morphometric analysis of the choroid, Bruch's membrane, and retinal pigment epithelium in postmortem eyes with age-related macular degeneration and histologic examination of surgically excised choroidal neovascular membranes. Surv Ophthalmol. 1999;441(1):S10–32. doi: 10.1016/s0039-6257(99)00086-7. [DOI] [PubMed] [Google Scholar]
- Starita C, Hussain AA, Marshall J. Decreasing hydraulic conductivity of Bruch's membrane: relevance to photoreceptor survival and lipofuscinoses. Am J Med Genet. 1995;57:235–237. doi: 10.1002/ajmg.1320570224. [DOI] [PubMed] [Google Scholar]
- Starita C, Hussain AA, Pagliarini S, Marshall J. Hydrodynamics of ageing Bruch's membrane: implications for macular disease. Exp Eye Res. 1996;62:565–572. doi: 10.1006/exer.1996.0066. [DOI] [PubMed] [Google Scholar]
- Starita C, Hussain AA, Patmore A, Marshall J. Localization of the site of major resistance to fluid transport in Bruch's membrane. Invest Ophthalmol Vis Sci. 1997;38:762–767. [PubMed] [Google Scholar]
- Streit WJ, Sammons NW, Kuhns AJ, Sparks DL. Dystrophic microglia in the aging human brain. Glia. 2004;45:208–212. doi: 10.1002/glia.10319. [DOI] [PubMed] [Google Scholar]
- Sue CM, Mitchell P, Crimmins DS, Moshegov C, Byrne E, Morris JG. Pigmentary retinopathy associated with the mitochondrial DNA 3243 point mutation. Neurology. 1997;49:1013–1017. doi: 10.1212/wnl.49.4.1013. [DOI] [PubMed] [Google Scholar]
- Sugita S, Horie S, Yamada Y, Mochizuki M. Inhibition of B-cell activation by retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2010;51:5783–5788. doi: 10.1167/iovs.09-5098. [DOI] [PubMed] [Google Scholar]
- Sullivan R, Penfold P, Pow DV. Neuronal migration and glial remodeling in degenerating retinas of aged rats and in nonneovascular AMD. Invest Ophthalmol Vis Sci. 2003;44:856–865. doi: 10.1167/iovs.02-0416. [DOI] [PubMed] [Google Scholar]
- Sullivan RK, Woldemussie E, Pow DV. Dendritic and synaptic plasticity of neurons in the human age-related macular degeneration retina. Invest Ophthalmol Vis Sci. 2007;48:2782–2791. doi: 10.1167/iovs.06-1283. [DOI] [PubMed] [Google Scholar]
- Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. 2010;10:36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarallo V, Hirano Y, Gelfand BD, Dridi S, Kerur N, Kim Y, Cho WG, Kaneko H, Fowler BJ, Bogdanovich S, Albuquerque RJ, Hauswirth WW, Chiodo VA, Kugel JF, Goodrich JA, Ponicsan SL, Chaudhuri G, Murphy MP, Dunaief JL, Ambati BK, Ogura Y, Yoo JW, Lee DK, Provost P, Hinton DR, Nunez G, Baffi JZ, Kleinman ME, Ambati J. DICER1 loss and Alu RNA induce age-related macular degeneration via the NLRP3 inflammasome and MyD88. Cell. 2012;149:847–859. doi: 10.1016/j.cell.2012.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzibasi E, Calamusa M, Novelli E, Domenici L, Strettoi E, Cellerino A. Age-dependent remodelling of retinal circuitry. Neurobiol Aging. 2009;30:819–828. doi: 10.1016/j.neurobiolaging.2007.08.017. [DOI] [PubMed] [Google Scholar]
- Thampi P, Rao HV, Mitter SK, Cai J, Mao H, Li H, Seo S, Qi X, Lewin AS, Romano C, Boulton ME. The 5HT1a receptor agonist 8-Oh DPAT induces protection from lipofuscin accumulation and oxidative stress in the retinal pigment epithelium. PLoS One. 2012;7:e34468. doi: 10.1371/journal.pone.0034468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totan Y, Yagci R, Bardak Y, Ozyurt H, Kendir F, Yilmaz G, Sahin S, Sahin Tig U. Oxidative macromolecular damage in age-related macular degeneration. Curr Eye Res. 2009;34:1089–1093. doi: 10.3109/02713680903353772. [DOI] [PubMed] [Google Scholar]
- Tuo J, Ardeljan D, Meyerle CB, Wang JJ, Mitchell P, Chew EY, Chan CC. Influence of TIMP3/SYN1 variation on the phenotypic presentation of age-related macular degeneration, ARVO Abstract #3314, Ft. Lauderdale, FL: 2012. [Google Scholar]
- Tuo J, Ross RJ, Herzlich AA, Shen D, Ding X, Zhou M, Coon SL, Hussein N, Salem N, Jr, Chan CC. A high omega-3 fatty acid diet reduces retinal lesions in a murine model of macular degeneration. Am J Pathol. 2009;175:799–807. doi: 10.2353/ajpath.2009.090089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugarte M, Hussain AA, Marshall J. An experimental study of the elastic properties of the human Bruch's membrane-choroid complex: relevance to ageing. Br J Ophthalmol. 2006;90:621–626. doi: 10.1136/bjo.2005.086579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterlauft JD, Eichler W, Kuhne K, Yang XM, Yafai Y, Wiedemann P, Reichenbach A, Claudepierre T. Pigment epithelium-derived factor released by Muller glial cells exerts neuroprotective effects on retinal ganglion cells. Neurochem Res. 2012;37:1524–1533. doi: 10.1007/s11064-012-0747-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deemter M, Ponsioen TL, Bank RA, Snabel JM, van der Worp RJ, Hooymans JM, Los LI. Pentosidine accumulates in the aging vitreous body: a gender effect. Exp Eye Res. 2009;88:1043–1050. doi: 10.1016/j.exer.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Venza I, Visalli M, Cucinotta M, Teti D, Venza M. Association between oxidative stress and macromolecular damage in elderly patients with age-related macular degeneration. Aging clinical and experimental research. 2012;24:21–27. doi: 10.3275/7659. [DOI] [PubMed] [Google Scholar]
- Wang AL, Lukas TJ, Yuan M, Du N, Tso MO, Neufeld AH. Autophagy and exosomes in the aged retinal pigment epithelium: possible relevance to drusen formation and age-related macular degeneration. PLoS One. 2009a;4:e4160. doi: 10.1371/journal.pone.0004160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AL, Lukas TJ, Yuan M, Neufeld AH. Increased mitochondrial DNA damage and down-regulation of DNA repair enzymes in aged rodent retinal pigment epithelium and choroid. Mol Vis. 2008;14:644–651. [PMC free article] [PubMed] [Google Scholar]
- Wang G, Spencer KL, Court BL, Olson LM, Scott WK, Haines JL, Pericak-Vance MA. Localization of age-related macular degeneration-associated ARMS2 in cytosol, not mitochondria. Invest Ophthalmol Vis Sci. 2009b;50:3084–3090. doi: 10.1167/iovs.08-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JJ, Foran S, Smith W, Mitchell P. Risk of age-related macular degeneration in eyes with macular drusen or hyperpigmentation: the Blue Mountains Eye Study cohort. Arch Ophthalmol. 2003;121:658–663. doi: 10.1001/archopht.121.5.658. [DOI] [PubMed] [Google Scholar]
- Wang L, Clark ME, Crossman DK, Kojima K, Messinger JD, Mobley JA, Curcio CA. Abundant lipid and protein components of drusen. PLoS One. 2010a;5:e10329. doi: 10.1371/journal.pone.0010329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XF, Cui JZ, Nie W, Prasad SS, Matsubara JA. Differential gene expression of early and late passage retinal pigment epithelial cells. Exp Eye Res. 2004;79:209–221. doi: 10.1016/j.exer.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Wang Y, Bian ZM, Yu WZ, Yan Z, Chen WC, Li XX. Induction of interleukin-8 gene expression and protein secretion by C-reactive protein in ARPE-19 cells. Exp Eye Res. 2010b;91:135–142. doi: 10.1016/j.exer.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Wang Y, Shen D, Wang VM, Yu CR, Wang RX, Tuo J, Chan CC. Enhanced apoptosis in retinal pigment epithelium under inflammatory stimuli and oxidative stress. Apoptosis. 2012;17:1144–1155. doi: 10.1007/s10495-012-0750-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RE, Huang W, Curran OE, Priestley JV, Michael-Titus AT. Docosahexaenoic acid prevents white matter damage after spinal cord injury. Journal of neurotrauma. 2010;27:1769–1780. doi: 10.1089/neu.2010.1348. [DOI] [PubMed] [Google Scholar]
- Wasmuth S, Lueck K, Baehler H, Lommatzsch A, Pauleikhoff D. Increased vitronectin production by complement-stimulated human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2009;50:5304–5309. doi: 10.1167/iovs.08-3326. [DOI] [PubMed] [Google Scholar]
- Wei L, Liu B, Tuo J, Shen D, Chen P, Li Z, Liu X, Ni J, Dagur P, Sen HN, Jawad S, Ling D, Park S, Chakrabarty S, Meyerle C, Agron E, Ferris FL, 3rd, Chew EY, McCoy JP, Blum E, Francis PJ, Klein ML, Guymer RH, Baird PN, Chan CC, Nussenblatt RB. Hypomethylation of the IL17RC Promoter Associates with Age-Related Macular Degeneration. Cell reports. 2012;2:1151–1158. doi: 10.1016/j.celrep.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West XZ, Malinin NL, Merkulova AA, Tischenko M, Kerr BA, Borden EC, Podrez EA, Salomon RG, Byzova TV. Oxidative stress induces angiogenesis by activating TLR2 with novel endogenous ligands. Nature. 2010;467:972–976. doi: 10.1038/nature09421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood A, Binns A, Margrain T, Drexler W, Povazay B, Esmaeelpour M, Sheen N. Retinal and choroidal thickness in early age-related macular degeneration. Am J Ophthalmol. 2011;152:1030, 1038–e1032. doi: 10.1016/j.ajo.2011.05.021. [DOI] [PubMed] [Google Scholar]
- Wu KH, Madigan MC, Billson FA, Penfold PL. Differential expression of GFAP in early v late AMD: a quantitative analysis. Br J Ophthalmol. 2003;87:1159–1166. doi: 10.1136/bjo.87.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WK, Llewellyn OP, Bates DO, Nicholson LB, Dick AD. IL-10 regulation of macrophage VEGF production is dependent on macrophage polarisation and hypoxia. Immunobiology. 2010;215:796–803. doi: 10.1016/j.imbio.2010.05.025. [DOI] [PubMed] [Google Scholar]
- Wu Y, Zhou J, Fishkin N, Rittmann BE, Sparrow JR. Enzymatic degradation of A2E, a retinal pigment epithelial lipofuscin bisretinoid. J Am Chem Soc. 2011;133:849–857. doi: 10.1021/ja107195u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Chen M, Forrester JV. Para-inflammation in the aging retina. Prog Retin Eye Res. 2009;28:348–368. doi: 10.1016/j.preteyeres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Xu H, Chen M, Manivannan A, Lois N, Forrester JV. Age-dependent accumulation of lipofuscin in perivascular and subretinal microglia in experimental mice. Aging Cell. 2008;7:58–68. doi: 10.1111/j.1474-9726.2007.00351.x. [DOI] [PubMed] [Google Scholar]
- Xu H, Chen M, Mayer EJ, Forrester JV, Dick AD. Turnover of resident retinal microglia in the normal adult mouse. Glia. 2007;55:1189–1198. doi: 10.1002/glia.20535. [DOI] [PubMed] [Google Scholar]
- Xu W, Grunwald JE, Metelitsina TI, DuPont JC, Ying GS, Martin ER, Dunaief JL, Brucker AJ. Association of risk factors for choroidal neovascularization in age-related macular degeneration with decreased foveolar choroidal circulation. Am J Ophthalmol. 2010;150:40–47. e42. doi: 10.1016/j.ajo.2010.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y, Ishibashi K, Ishibashi K, Bhutto IA, Tian J, Lutty GA, Handa JT. The expression of advanced glycation endproduct receptors in rpe cells associated with basal deposits in human maculas. Exp Eye Res. 2006;82:840–848. doi: 10.1016/j.exer.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan SD, Roher A, Schmidt AM, Stern DM. Cellular cofactors for amyloid beta-peptide-induced cell stress. Moving from cell culture to in vivo. Am J Pathol. 1999;155:1403–1411. doi: 10.1016/s0002-9440(10)65452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates JR, Sepp T, Matharu BK, Khan JC, Thurlby DA, Shahid H, Clayton DG, Hayward C, Morgan J, Wright AF, Armbrecht AM, Dhillon B, Deary IJ, Redmond E, Bird AC, Moore AT. Complement C3 variant and the risk of age-related macular degeneration. N Engl J Med. 2007;357:553–561. doi: 10.1056/NEJMoa072618. [DOI] [PubMed] [Google Scholar]
- Ye SM, Johnson RW. Increased interleukin-6 expression by microglia from brain of aged mice. J Neuroimmunol. 1999;93:139–148. doi: 10.1016/s0165-5728(98)00217-3. [DOI] [PubMed] [Google Scholar]
- Yin L, Shi Y, Liu X, Zhang H, Gong Y, Gu Q, Wu X, Xu X. A rat model for studying the biological effects of circulating LDL in the choriocapillaris-BrM-RPE complex. Am J Pathol. 2012;180:541–549. doi: 10.1016/j.ajpath.2011.10.015. [DOI] [PubMed] [Google Scholar]
- Yoon KD, Yamamoto K, Ueda K, Zhou J, Sparrow JR. A novel source of methylglyoxal and glyoxal in retina: implications for age-related macular degeneration. PLoS One. 2012;7:e41309. doi: 10.1371/journal.pone.0041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Bhangale TR, Fagerness J, Ripke S, Thorleifsson G, Tan PL, Souied EH, Richardson AJ, Merriam JE, Buitendijk GH, Reynolds R, Raychaudhuri S, Chin KA, Sobrin L, Evangelou E, Lee PH, Lee AY, Leveziel N, Zack DJ, Campochiaro B, Campochiaro P, Smith RT, Barile GR, Guymer RH, Hogg R, Chakravarthy U, Robman LD, Gustafsson O, Sigurdsson H, Ortmann W, Behrens TW, Stefansson K, Uitterlinden AG, van Duijn CM, Vingerling JR, Klaver CC, Allikmets R, Brantley MA, Jr, Baird PN, Katsanis N, Thorsteinsdottir U, Ioannidis JP, Daly MJ, Graham RR, Seddon JM. Common Variants near FRK/COL10A1 and VEGFA are Associated with Advanced Age-related Macular Degeneration. Hum Mol Genet. 2011 doi: 10.1093/hmg/ddr270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamiri P, Sugita S, Streilein JW. Immunosuppressive properties of the pigmented epithelial cells and the subretinal space. Chemical immunology and allergy. 2007;92:86–93. doi: 10.1159/000099259. [DOI] [PubMed] [Google Scholar]
- Zealley B, de Grey AD. Strategies for Engineered Negligible Senescence. Gerontology. 2012 doi: 10.1159/000342197. [DOI] [PubMed] [Google Scholar]
- Zhang K, Hopkins JJ, Heier JS, Birch DG, Halperin LS, Albini TA, Brown DM, Jaffe GJ, Tao W, Williams GA. Ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for treatment of geographic atrophy in age-related macular degeneration. Proc Natl Acad Sci U S A. 2011;108:6241–6245. doi: 10.1073/pnas.1018987108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Ma W, Fariss RN, Wong WT. Minocycline attenuates photoreceptor degeneration in a mouse model of subretinal hemorrhage microglial: inhibition as a potential therapeutic strategy. Am J Pathol. 2011;179:1265–1277. doi: 10.1016/j.ajpath.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Jang YP, Kim SR, Sparrow JR. Complement activation by photooxidation products of A2E, a lipofuscin constituent of the retinal pigment epithelium. Proc Natl Acad Sci U S A. 2006;103:16182–16187. doi: 10.1073/pnas.0604255103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Kim SR, Westlund BS, Sparrow JR. Complement activation by bisretinoid constituents of RPE lipofuscin. Invest Ophthalmol Vis Sci. 2009;50:1392–1399. doi: 10.1167/iovs.08-2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel SA, Imamura Y, Spaide TC, Fujiwara T, Spaide RF. Prevalence and significance of subretinal drusenoid deposits (reticular pseudodrusen) in age-related macular degeneration. Ophthalmology. 2010a;117:1775–1781. doi: 10.1016/j.ophtha.2010.01.027. [DOI] [PubMed] [Google Scholar]
- Zweifel SA, Spaide RF, Curcio CA, Malek G, Imamura Y. Reticular pseudodrusen are subretinal drusenoid deposits. Ophthalmology. 2010b;117:303–312. e301. doi: 10.1016/j.ophtha.2009.07.014. [DOI] [PubMed] [Google Scholar]