Abstract
Objectives
To study the role of junctophilin 2 (JPH2) in atrial fibrillation (AF).
Background
JPH2 is believed to have an important role in sarcoplasmic reticulum (SR) Ca2+ handling and modulation of ryanodine receptor Ca2+ channels (RyR2). Whereas defective RyR2-mediated Ca2+ release contributes to the pathogenesis of AF, nothing is known about the potential role of JPH2 in atrial arrhythmias.
Methods
Screening 203 unrelated hypertrophic cardiomyopathy patients uncovered a novel JPH2 missense mutation (E169K) in 2 patients with juvenile-onset paroxysmal AF (pAF). Pseudo-knockin (PKI) mouse models were generated to determine the molecular defects underlying the development of AF caused by this JPH2 mutation.
Results
PKI mice expressing E169K mutant JPH2 exhibited a higher incidence of inducible AF compared with wildtype (WT)-PKI mice, while A399S-PKI mice expressing a HCM-linked JPH2 mutation not associated with atrial arrhythmias were not significantly different from WT-PKI. E169K-PKI but not A399A-PKI atrial cardiomyocytes showed an increased incidence of abnormal SR Ca2+ release events. These changes were attributed to reduced binding of E169KJPH2 to RyR2. Atrial JPH2 levels in WT-JPH2 transgenic, nontransgenic, and JPH2 knockdown mice correlated negatively with the incidence of pacing-induced AF. Ca2+ spark frequency in atrial myocytes and the open probability of single RyR2 channels from JPH2 knockdown mice was significantly reduced by a small JPH2-mimicking oligopeptide. Moreover, patients with pAF had reduced atrial JPH2 levels per RyR2 channel compared to sinus rhythm patients, and an increased frequency of spontaneous Ca2+ release events.
Conclusions
Our data suggest a novel mechanism by which reduced JPH2-mediated stabilization of RyR2 due to loss-of-function mutation or reduced JPH2:RyR2 ratios can promote SR Ca2+ leak and atrial arrhythmias, representing a potential novel therapeutic target for AF.
Keywords: Atrial fibrillation, Calcium, Junctophilin, Ryanodine receptor, Sarcoplasmic reticulum
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia causing significant morbidity and mortality (1). Although the mechanisms behind AF pathogenesis are complex, it is believed that AF is induced and maintained by a combination of reentry and triggered activity that includes early and delayed afterdepolarizations (EADs and DADs, respectively) (2,3). Spontaneous diastolic Ca2+ release from the sarcoplasmic reticulum (SR) generates depolarizing Na+/Ca2+-exchanger (NCX) current, which may induce DADs and triggered activity (4). The major SR Ca2+ release channel responsible for arrhythmogenic Ca2+ releases is the type-2 ryanodine receptor channel (RyR2) located within junctional membrane complexes (JMC) (5–7). Defective regulation and activity of RyR2 has been linked to AF in humans and various animal models (4,8,9). In addition, structural changes in the JMC may precipitate destabilization of RyR2 and diastolic Ca2+ leak (10). However, the molecular mechanisms underlying abnormal RyR2 Ca2+ leak from JMCs remain poorly understood, especially in the atrial myocardium.
Junctophilin 2 (JPH2) plays a critical structural role within JMCs (10,11). In mouse studies, knockdown of JPH2 was associated with loss of JMC numbers, reduced Ca2+-induced Ca2+ release, and development of acute heart failure (10). Several mutations in JPH2 were previously identified in patients with hypertrophic cardiomyopathy (HCM) (12,13). Here, we report two novel JPH2 mutations, one of which - E169K - was identified in a proband with the unusual clinical presentation of juvenile-onset paroxysmal AF (pAF). The proband's father, carrying the same mutation, also exhibited supraventricular tachycardias in addition to HCM. The patient with the other HCM-associated JPH2 mutation, A405S, did not exhibit atrial arrhythmias, similar to all other previously reported HCM patients with JPH2 mutations (12,13).
To determine how the E169K mutation in JPH2 might cause atrial arrhythmias, we generated genetically-modified mouse lines carrying the E169K or A399S (=A405S in humans) mutations in JPH2, respectively. Subsequently, `pseudo-knockin' (PKI) mice with total cardiac JPH2 levels similar to non-transgenic (NTg) mice were generated by crossing JPH2 transgenic (Tg) mice with inducible, cardiac-specific JPH2 knockdown mice. The E169K-PKI but not A399S-PKI mice exhibited an enhanced susceptibility to pacing-induced AF. Atrial myocytes from E169K-PKI but not A399S-PKI mice showed an increased frequency of spontaneous SR Ca2+ release events. Interestingly, JPH2 mutation E169K but not mutation A399S decreased the binding affinity of JPH2 to RyR2. To further examine the importance of JPH2 loss-of-function in AF, atrial JPH2 levels in mice were shown to have an inverse correlation with AF inducibility.
The addition of a small peptide containing the E169 region of JPH2 was able to ameliorate the Ca2+ spark frequency in atrial cardiomyocytes isolated from JPH2 knockdown mice, and to normalize the open probability of RyR2 channels from JPH2 knockdown mice. Finally, to assess the importance of JPH2 in clinical AF, JPH2 protein levels were assessed in atrial samples from non-HCM patients with pAF. JPH2 levels were reduced significantly in pAF patients compared to those in sinus rhythm, and isolated atrial cardiomyocytes showed an increased frequency of potential proarhythmic SR Ca2+ release events consistent with enhanced RyR2 activity.
Together, these data suggest that reduced JPH2-mediated stabilization of RyR2 (either due to the unique inherited loss-of-function mutation E169K in JPH2, or due to reduced JPH2 expression levels per RyR2 channel) can promote abnormal RyR2-mediated SR Ca2+ release events associated with AF. These findings may open new avenues for the development of anti-AF drugs that target either JPH2 or RyR2 abnormalities to reduce spontaneous proarrhythmic diastolic SR Ca2+-release events.
METHODS
Expanded methods can be found in the online only Supplement Materials.
Genetic Analysis
Comprehensive coding-region genetic analysis of JPH2 was accomplished by PCR, DHPLC, and direct DNA sequencing as described previously (12).
Generation of Animals
All animal studies were performed according to protocols approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine conforming to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85-23, revised 1996). Generation of Tg mice including WT-Tg, E169K and A399S JPH2 mutant Tg, and shRNA knockdown are described in detail in the online supplement. Pseudo-knockin (PKI) mice were generated by crossing the JPH2 Tg mice (WT, E169K, and A399S) with an inducible cardiac-specific JPH2 knockdown mouse line. The offspring were dosed with tamoxifen to induce shRNA-mediated knockdown of total JPH2 levels to protein levels similar to those in NTg mouse hearts. Experiments were performed between 2 and 3 weeks post-knockdown when JPH2 levels were stable.
Programmed electrical stimulation
Atrial and ventricular intracardiac ECG were recorded using a 1.1F octapolar electrode catheter (EPR-800, Millar Instruments, Houston, Texas) inserted into the right ventricle via the right jugular vein, as described (14). AF inducibility was determined using rapid atrial pacing. AF was considered positive only if it lasted for ≥1 second.
Mouse myocyte isolation and confocal Ca2+ imaging
Mouse atrial myocytes were isolated and loaded with Ca2+ sensitive dye Fluo-4 AM as described previously (9). Once steady state Ca2+ transients were observed, pacing was paused for 30 seconds to record spontaneous Ca2+ release events (Ca2+ waves and Ca2+ sparks). Steady state SR Ca2+ was estimated by rapid application of 10 mM caffeine after pacing.
Simultaneous intracellular [Ca2+] and membrane current measurements in human atrial myocytes
Right atrial biopsies were dissected from appendages of patients with paroxysmal AF (pAF) or in sinus rhythm (SR), who underwent coronary artery bypass or valve replacement surgery. Protocols were approved by the medical ethics committees of the Dresden University of Technology and the Medical Faculty Mannheim, University of Heidelberg, Germany.
Statistical analyses
Results are expressed as mean ± SEM. Normality was examined using Shapiro Wilk test. Continuous variables with normal distribution were compared using ANOVA with post-hoc Tukey for equal variance and Dunnett's T3 for unequal variance, and paired samples were analyzed using paired T-test. Continuous variables that failed test of normality were compared using Kruskal-Wallis and pairs of data were analyzed using Mann-Whitney test. Ordinal variables were compared using Kendall's tau B. Categorical variables were compared using Pearson Chi-Square test and pairs of data were examined with Fisher's Exact Test. P<0.05 was considered statistically significant.
RESULTS
Identification of JPH2 mutation E169K in patients with HCM and AF
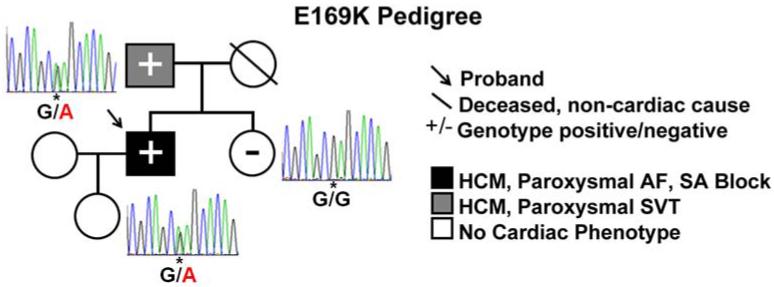
Previous studies have demonstrated the presence of rare, functionally disruptive JPH2 mutations in patients with HCM (12,13). JPH2 mutational analysis of an independent cohort of 203 unrelated patients with HCM revealed a new missense mutation annotated as E169K in a male index case with unusually early-onset of paroxysmal AF in the context of HCM (Figure 1). This JPH2 genotype positive patient was negative for mutations in the nine canonical sarcomeric HCM-associated genes. Diagnosed as having HCM at five months of age, patient went on to develop profound septal hypertrophy (32 mm) with marked diastolic dysfunction and labile left ventricular outflow obstruction (Online Figure S1). The onset of pAF was documented at the young age of 22 prior to significant atrial remodeling. At age 24, he required pacemaker implantation due to paroxysmal sinoatrial (SA) blocks and pre-syncope. Clinical and genetic evaluation of family members revealed the E169K mutation in the proband's father, who had HCM with 15 mm maximal left ventricular thickness and paroxysmal supraventricular tachycardia during clinical evaluation blinded to genotype (Figure 1). In contrast, the proband's sister was mutation negative and had a normal cardiac work-up. Both the proband and his father had prolonged QTc intervals, whereas the sister's QTc was normal (Online Figure S1). No other family members were available for genetic testing. In the same cohort, another JPH2 variant (A405S) was identified in a Caucasian male diagnosed with HCM at age 16. This proband presented with dyspnea, chest pain, and dizziness. He later developed basal septal morphology hypertrophy (23mm) and was diagnosed with HCM. He has no family history of HCM or SCD, both parents are genotype negative, and never developed QTc prolongation or supraventricular arrhythmias.
Figure 1. E169K-JPH2 is associated with HCM and paroxysmal atrial fibrillation (pAF).
Pedigree showing presence of HCM and supraventricular arrhythmias [pAF, sino-atrial (SA) block, supraventricular tachycardia (SVT)] in proband (arrow) and his father (grey square), both mutation positive (heterozygous G>A), and his sister who was genotype negative (white circle) as indicated by the asterisk.
E169K-PKI mice are more susceptible to AF induction
The unusual association of atrial arrhythmias with HCM in the 2 patients with the E169K mutation in JPH2 prompted further mechanistic analysis in animal models. In order to create a model resembling the heterozygous disease state of patients, pseudo-knockin mice were generated that express HA-tagged E169K mutant JPH2 (E169K-PKI), wild-type JPH2 (WT-PKI), or A399S (=A405S in humans) mutant JPH2. The total (i.e., combined endogenous and transgenic) cardiac JPH2 levels in these WT and mutant PKI mouse lines were similar to those in non-transgenic (NTg) littermates (Online Fig S2), whereas about half of the JPH2 in PKI mice is mutant/transgenic and the other half endogenous JPH2.
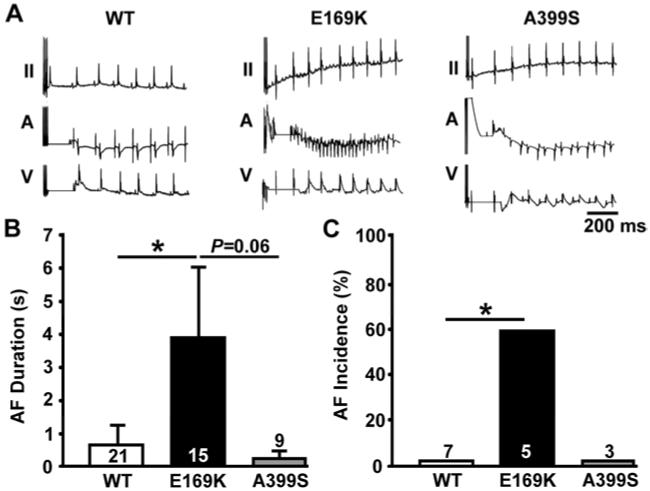
To determine whether mutation E169K in JPH2 increases susceptibility to AF, we subjected E169K-PKI mice to programmed electrical stimulation at an age prior to the onset of cardiac hypertrophy or dysfunction (Online Fig S3) (8). Following a rapid atrial burst pacing sequence, E169K-PKI mice exhibited a higher incidence of AF compared with WT-PKI and A399S-PKI mice (Figure 2A). The average duration of AF episodes in E169K-PKI mice was 3.9 ± 2.1 sec, whereas the duration of (non-reproducible) AF episodes in WT-PKI mice was only 0.6±0.6 sec (P<0.05 vs. E169K-PKI) and 0.2+/−0.2 sec for A399S-PKI (P=0.06 vs. E169K-PKI) (Figure 2B). The longest episode of pacing-induced AF recorded in an E169K-PKI mouse was 29 seconds. The incidence of reproducible AF (defined as ≥ 2 episodes of AF out of 3 attempts) was 60% in E169K-PKI mice, whereas it was 0% in WT-PKI mice (P<0.05 vs. E169K-PKI) and 0% in A399S-PKI mice (P=0.19 vs. E169K-PKI) (Figure 2C).
Figure 2. Higher susceptibility to pacing-induced AF in E169K-PKI but not A399S mice.
A, Representative ECG tracings after rapid atrial burst pacing in E169K-PKI mouse revealing AF, compared with sinus rhythm in WT-PKI mouse and A399S-PKI mouse. B, Bar graph showing average duration of AF episodes following rapid atrial pacing bursts. Number of incidences inside bars. C, Bar graph showing increased incidence of pacing-induced AF (reproducible, and lasting > 1 s) in E169K-PKI mice compared with WT-PKI and A399S-PKI mice. Number of mice per group inside bars. *P<0.05.
Mutation E169K in JPH2 increases spontaneous SR Ca2+ leak in atrial myocytes
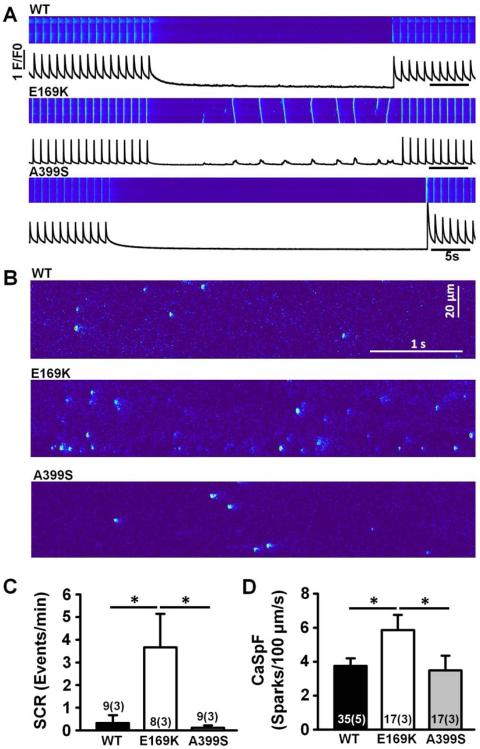
To determine whether the E169K mutation in JPH2 increases the susceptibility to AF by altering intracellular Ca2+ handling, we assessed the incidence of spontaneous SR Ca2+ release events in isolated atrial myocytes. Atrial myocytes isolated from E169K-PKI mice exhibited a significantly increased incidence of spontaneous Ca2+ waves following a 1-Hz pacing train (3.67 ± 1.47 /min) compared with myocytes from WT-PKI and A399S-PKI mice (0.33 ± 0.33 /min and 0.11 ± 0.11 /min, respectively; P<0.05; Figure 3A,C). These cell-wide spontaneous Ca2+ waves can directly activate NCX, trigger delayed afterdepolarizations (DADs) and cause triggered activity associated with AF (4).
Figure 3. Enhanced SR Ca2+ leak in E169K-PKI but not A399S-PKI mouse myocytes.
A, Representative confocal line-scan images and profiles showing spontaneous cell-wide Ca2+ waves in atrial myocytes from E169K-PKI mice following 1 Hz field stimulation. B, Representative confocal line-scan images showing increased incidence of Ca2+ sparks in atrial myocytes from E169K-PKI mice following 1-Hz field stimulation. C–D, Bar graphs showing quantification of Ca2+ wave (SCR) and Ca2+ spark frequencies (CaSpF). *P<0.05.
To gain more insights in the mechanisms of altered SR Ca2+ release, the incidence of spontaneous subcellular Ca2+ sparks were quantified (Figure 3B). The Ca2+ spark frequency was significantly increased in atrial myocytes from E169K-PKI mice (5.86 ± 0.88 /min) compared to those from WT-PKI mice and A399S-PKI (3.74 ± 0.45 and 3.48 ± 0.87 respectively; P<0.05; Figure 3B,D). This abnormal diastolic Ca2+ leak produced reduced SR Ca2+ load in E169K-PKI mice compared with WT-PKI mice (Online Fig S4). Thus, our data show that mutation E169K in JPH2 leads to abnormal SR Ca2+ release via RyR2, suggesting that the Ca2+ release channel is less stable in mice with the E169K but not the A399S JPH2 mutant variant.
Mutant E169K-JPH2 reduces JPH2-RyR2 binding
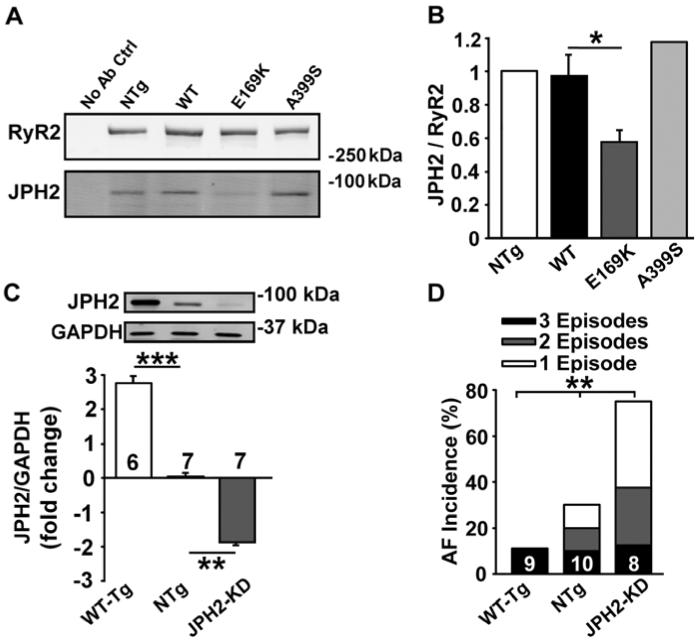
To gain more insights into the mechanisms by which E169K-JPH2 potentially affects RyR2 activity, we first assessed the amount of JPH2 binding to RyR2. Immunoprecipitation using anti-RyR2 antibody was performed using cardiac lysates from NTg, WT-PKI, E169K-PKI, and A399S-PKI mice (Figure 4A). Subsequent Western blotting for RyR2 and JPH2 revealed significantly reduced co-immunoprecipitation of E169K-mutant JPH2 with RyR2 compared with WT-JPH2 and A399S-mutant JPH2 (Figure 4B). These results suggest that the E169K mutation in JPH2 specifically disrupts its binding domain with RyR2.
Figure 4. JPH2 mutant E169K but not A399S reduces JPH2-RyR2 binding and JPH2 levels inversely correlate with AF inducibility.
A, Western blots showing reduced JPH2 co-immunoprecipitation following RyR2 pull-down from cardiac lysates of E169K-PKI mouse heart compared with WT-PKI, A399S-PKI and NTg mice. B, Bar graph showing quantification of JPH2 co-immunoprecipitated with RyR2 pull-down. C, Western blots showing total JPH2 levels compared with GAPDH in atria from MCM-shJPH2-WT-Tg mice that overexpressed higher cardiac JPH2 levels (WT-Tg), control non-Tg MCM mice with normal JPH2 levels (NTg), or MCM-shJPH2 knockdown mice with reduced cardiac JPH2 levels (JPH2-KD). Bar graphs showing JPH2 levels normalized to GAPDH in these 3 groups, on a log scale. D, Stacked bar graph showing AF incidence after atrial burst pacing in the 3 groups of mice. Number of mice per group inside bars. *P<0.05, **P<0.01, ***P<0.001.
JPH2 levels inversely correlate with atrial arrhythmogenesis in mice
To determine whether there is an association between reduced atrial JPH2 protein levels and AF susceptibility, we studied three genetically modified mouse models expressing different levels of JPH2 in the heart. Tg mice overexpressing WT JPH2 (WT-Tg) had ~2.9 fold higher JPH2 protein levels than NTg mice (Figure 4C). Compared to NTg mice, cardiac-specific knockdown mice (JPH2-KD) (10) exhibited ~1.9 fold lower JPH2 levels in the heart. When subjected to rapid atrial burst pacing, WT-Tg mice were less susceptible to pacing-induced AF compared to NTg mice, whereas JPH2-KD mice were more susceptible (P=0.04 across the groups; Figure 4D). Thus, these experiments demonstrated that the susceptibility to AF in mouse models is inversely correlated with atrial JPH2 protein levels. These data suggest that reduced JPH2 levels might be a culprit mechanism for RyR2 dysfunction and the increased AF susceptibility identified in E169K-PKI mice.
JPH2-derived peptide stabilizes RyR2-mediated SR Ca2+ release
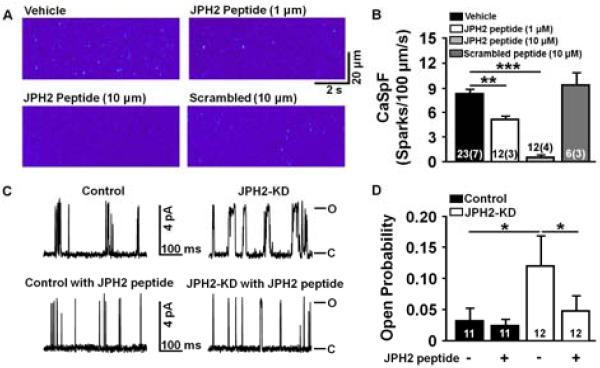
To assess whether JPH2 binding to RyR2 modulates SR Ca2+ release, we synthesized a peptide corresponding to the 25 amino acids of the JPH2 region that flanks residue E169. Next, the JPH2 peptide was added to permeabilized atrial myocytes from JPH2-KD mice, in which JPH2 expression was reduced. Baseline Ca2+ spark frequency in vehicle-treated JPH2 knockdown was relatively high (8.2 ± 0.6 sparks/100μm/s; Figure 5A,B). Addition of the JPH2-derived peptide significantly decreased the Ca2+ spark frequency in a dose-dependent manner (1 μM peptide: 5.2 ± 0.4; P<0.01, and 10 μM peptide: 0.48 ± 0.27; P <0.001 vs. vehicle). A control peptide (10 μM) in which the same 25 amino acids were scrambled did not affect the Ca2+ spark frequency (9.4 ± 1.5; P=N.S. vs. vehicle).
Figure 5. JPH2-derived small peptide stabilizes RyR2-mediated SR Ca2+ leak in atrial myocytes from JPH2 knockdown mice.
A, Representative confocal line scan images showing spontaneous Ca2+ sparks recorded from permeabilized atrial myocytes treated with vehicle only (upper left), JPH2 peptide at 1 μM (upper right), JPH2 peptide at 10 μM (bottom left), and scrambled control peptide (10 μM, bottom right). B, Bar graph showing Ca2+ spark frequency (CaSpF), which was significantly decreased by JPH2-derived peptide but not by scrambled control peptides. **P<0.01, ***P<0.001. Numbers indicate number of cells (number of mice). C, Representative planar lipid bilayer RyR2 single channel tracings from Mer-Cre-Mer (Control) and JPH2 knockdown (JPH2-KD) mice at baseline (top) and with the addition of JPH2 peptide (bottom). D, Bar graph showing significant increase in RyR2 open probability (Po) in JPH2-KD vs control and significant reduction in RyR2 Po with the addition of JPH2 peptide in JPH2-KD mice. P <0.05
Furthermore, single channel measurements in planar lipid bilayers revealed a high average open probability (Po) of RyR2 channels isolated from JPH2-KD mice (0.12±0.048), which was significantly reduced after the addition of JPH2 peptide (0.048±0.024; p<0.05; Figure 5C,D). In contrast, the addition of JPH2 peptide did not change the average Po of RyR2 isolated from αMHC-mER-Cre-mER (MCM) control mice (0.032 ± 0.02 vs 0.024 ± 0.01,P=NS). The specificity of the peptide effects was confirmed by the demonstration that the scrambled peptide did not have a significant effect on the open probability of RyR2 isolated from both MCM control mice or JPH2-KD mice (Online Figure S5). Taken together, these data strongly suggest that JPH2, in particular the domain containing the E169 residue, are critical for stabilization of RyR2 channel activity.
Decreased JPH2:RyR2 ratio in patients with paroxysmal AF correlate with enhanced SR Ca2+ leak
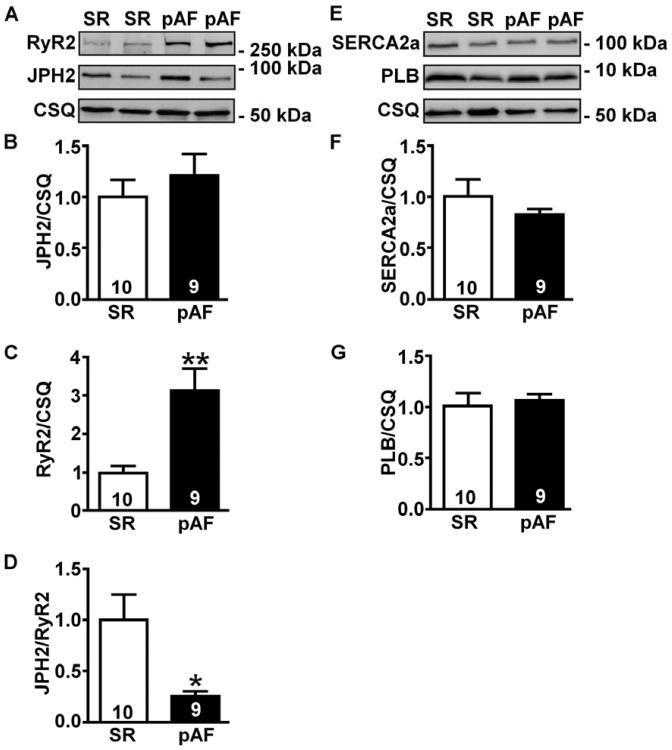
In view of the inverse correlation between atrial JPH2 levels and AF susceptibility in Tg mice, we set out to determine whether changes in JPH2 and RyR2 expression levels might occur in more common AF patients without either JPH2 mutations or HCM. Overall, the pAF and sinus rhythm patient cohorts included mostly elderly patients with significant co-morbidities including ischemic and valvular heart disease, diabetes mellitus, hypertension, and hyperlipidemia (Online Table 1). There were no significant differences between patient characteristics, except for a slightly higher average age in the pAF group. However, there was no significant correlation between age and atrial JPH2 protein levels (Online Fig S6). Western blotting revealed increased RyR2 but unaltered JPH2 levels in atrial tissues from patients with pAF compared with those in SR, resulting in a significantly lower JPH2/RyR2 ratio (Figure 6A–D). In contrast, the protein levels of SERCA2a and phospholamban (PLN), Ca2+ cycling proteins involved in Ca2+ resequestration into the SR, were unchanged (Figure 6E–G).
Figure 6. Reduced JPH2:RyR2 ratios in patients with paroxysmal AF.
Representative Western blots (A) and bar graphs (B–C) showing RyR2 and JPH2 protein levels normalized to calsequestrin (CSQ) in atria from patients in sinus rhythm (SR) and paroxysmal AF (pAF). (D) Ratio of JPH2-to-RyR2 levels in human atria. Representative Western blots (E) and bar graphs (F–G) showing SERCA2a and PLB levels normalized to CSQ.
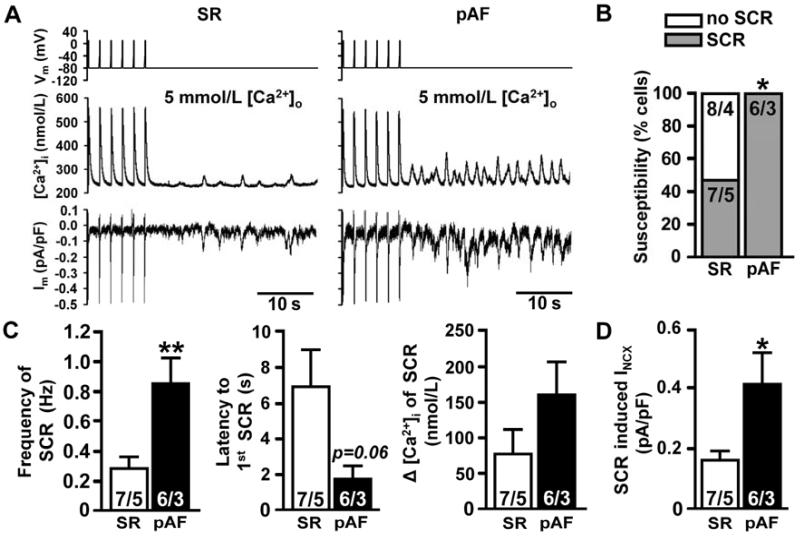
Whereas several recent studies have documented profound changes in SR Ca2+ handling in atrial myocytes from patients in chronic AF, much less is known about Ca2+ handling in paroxysmal AF. Therefore, atrial myocytes were isolated from right atrial samples from pAF patients and control individuals in sinus rhythm. In the presence of high intracellular Ca2+ concentrations, atrial myocytes from pAF patients were significantly more likely to exhibit spontaneous SR Ca2+ release events (SCR) compared to myocytes from sinus rhythm patients (Figure 7A–B). In addition, the frequency of SCR was increased in atrial myocytes from patients in pAF, and there was a trend towards a shorter interval between the final regularly triggered Ca2+ transient and first SCR (Figure 7C). There was also a non-significant increase in the amplitude of the SCR in patients with pAF compared to sinus rhythm patients. Finally, the amplitude of the NCX current (INCX) induced by SCR was approximately 2-fold higher in atrial myocytes from patients with pAF compared to those in SR (Figure 7D). Thus, reduced atrial JPH2 levels per RyR2 chnanel correlated with an increased incidence of spontaneous SR Ca2+ release events and enhanced pro-arrhythmogenic NCX currents in atrial myocytes from patients with pAF.
Figure 7. Enhanced pro-arrhythmic Ca2+ release events in patients with paroxysmal AF.
A, Representative Ca2+ transient and membrane current (Im) tracings from isolated atrial myocytes from SR and pAF patients following 1 min of pacing at 0.5-Hz. B, Bar graph showing incidence of spontaneous Ca2+ release events (SCR). C, Frequency (left), latency (middle), amplitude (right) of SCR. D, Amplitude of SCR-mediated NCX current. *P<0.05, **P<0.01. Numbers represent number of cells/number of patients.
DISCUSSION
This study describes the first example of a defect in a structural protein (i.e., junctophilin-2) causing atrial arrhythmias due to perturbed Ca2+ handling. Specifically, the inherited mutation E169K in JPH2 affects RyR2-mediated SR Ca2+ release from the sarcoplasmic reticulum, causing triggered activity and supraventricular arrhythmias in both patients and mice with E169K-mutant JPH2 in the heart. Biochemical studies revealed that the mutation E169K impairs binding of JPH2 to RyR2. This molecular defect was not observed for JPH2 mutation A405S identified in HCM patients without atrial arrhythmias. Our studies identified residue E169 as being part of a critical domain within JPH2 that inhibits RyR2 channel activity. Furthermore, reduced amounts of functionally intact (wild-type) JPH2 per RyR2 channel resulting in a loss of JPH2-mediated stabilization of RyR2 that may also contribute to arrhythmogenesis in patients with paroxysmal AF (15).
Recent studies have shown that genetic mutations in JPH2 are a rare cause of HCM (12,13,16). The first JPH2 variant specifically linked to cardiac arrhythmias, E169K, was identified in a HCM index patient with atypical disease features, most notably juvenile-onset of paroxysmal AF, supraventricular arrhythmias and paroxysmal sinoatrial blocks, which are rarely seen in patients with HCM in the absence of significant atrial remodeling (17). In contrast, AF is more commonly observed in HCM with increasing age and/or atrial dilatation (17). Thus, these clinical findings suggested that the E169K mutation in JPH2 promoted atrial arrhythmias independently of the mechanism related to HCM formation caused by other JPH2 variants, including A405S (A399S in mice). Indeed, our findings revealed that E169K-PKI mice were susceptible to AF without evidence of structural cardiomyopathy at the age of 2 months. Moreover, A399S-PKI mice did not develop a susceptibility to AF, similarly to WT-PKI mice. Therefore, it is likely that the proarrhythmogenic effects of the E169K mutation are due to the reduced binding of E169K-JPH2 to RyR2, whereas HCM development likely involves other cellular mechanisms that remain to be explored in future studies.
The E169K mutation in JPH2 is located in a flexible `joining domain' connecting two sets of membrane-binding domains that attach JPH2 to the sarcolemma (16). Because the RyR2 channel is large and predicted to occupy most of the dyadic space between the sarcolemma and SR, the joining domain is a prime candidate to mediate the physical interaction of JPH2 with RyR2 (18). The region around residue E169 is highly conserved in evolution, highlighting the functional importance of this domain (16). Indeed, our data using a small JPH2-derived peptide containing the E169 residue was able to alter RyR2 functional activity, indicating direct channel modulation. Furthermore, co-immunoprecipitation experiments revealed that mutation E169K greatly reduced JPH2 binding to RyR2, whereas another HCM-associated mutation A399S (in mice) did not affect RyR2 binding. The effects of the peptide centered around the E169 residue were very specific, as a scrambled peptide containing the same amino acids did not inhibit RyR2 in single channel experiments nor did it suppress Ca2+ sparks in atrial myocytes.
JPH2 was first identified as a structural protein within the Ca2+-release unit (CRU) that anchors the SR membrane to the plasma membrane (11). The current study uncovered an important additional cellular role of JPH2 in cardiomyocytes, namely regulation of RyR2-mediated SR Ca2+ release. Mutation E169K in JPH2, which interferes with RyR2 modulation, caused increased SR Ca2+ leak (Figure 3), which is similar to what we recently demonstrated with knockdown of JPH2 in ventricular myocytes (10). It is theoretically possible that the JPH2 mutation also affects regulation of other known JPH2-binding partners within the CRU such as the L-type Ca2+ channel (LTCC) and the transient-receptor potential channel, subfamily C, member 3 (TRPC3) (15,19,20). However, it is unlikely that the E169K mutation affects the LTCC function, since ICa was not altered in atrial myocytes isolated from E169K-PKI mice (data not shown). Interestingly, it has been shown that in skeletal muscle, mutation S165F in JPH2 can block a potential PKC phosphorylation site, which in turn might reduce binding of JPH2 with TRPC3 (15). However, at this time it is unknown whether the JPH2 mutation E169K affects gating of the TRPC3 Ca2+ channel. Our peptide data suggest that JPH2 strongly regulates RyR2-mediated Ca2+ release, and that the E169K mutation decreases binding to RyR2. Thus, it is very likely that the cellular mechanisms underlying arrhythmogenesis caused by the E169K mutation involve reduced JPH2 binding to RyR2, diastolic SR Ca2+ release, and arrhythmogenic afterdepolarizations leading to atrial arrhythmias.
A growing body of literature suggests that altered intracellular Ca2+ handling is a key contributor to AF, in particular ectopic activity associated with AF induction (21,22). Ectopic activity is caused by abnormal spontaneous action potentials that can result from DADs induced by diastolic SR Ca2+ release (4,9,22,23). However, the mechanisms by which RyR2 exhibit increased sensitivity to Ca2+-mediated activation have been only partially understood and likely involve hyperphosphorylation of RyR2 in patients with chronic AF (4,9). The present study extends these previous findings by showing that another cellular defect, namely reduced JPH2 levels per RyR2 channel could also contribute to RyR2 dysfunction, especially in patients with pAF.
Our current data suggest that a lower JPH2:RyR2 ratio may promote increased RyR2-mediated SR Ca2+ release events that are likely to cause proarrhythmic DADs in pAF patients. Similar findings have been reported for another accessory subunit that stabilizes RyR2, namely FK506-binding protein 12.6 (FKBP12.6). Patients in chronic AF exhibit a reduced FKBP12.6:RyR2 ratio (23). In mice, reduced FKBP12.6 levels predispose to both ventricular and atrial arrhythmias due to an increased sensitivity to Ca2+-mediated activation of RyR2 resulting in spontaneous Ca2+ release events and triggered activity (9,24,25).
Finally, studies in atrial cardiomyocytes from both mouse models and patients with chronic AF have established that aberrant RyR2-mediated SR Ca2+ release events activate NCX, which causes DADs providing the trigger for AF (4,8,9). Future studies using JPH2 peptides might prove whether loss of JPH2-mediated RyR2 stabilization is causally linked to arrhythmogenenic SR Ca2+ release events in patients with AF.
Potential Limitations
The link between the E169K mutation in JPH2 and atrial arrhythmias is based on two patients with the unusual presentation of atrial arrhythmias and HCM. Unfortunately, there were no additional family members available for genotyping. On the other hand, the association of HCM and supraventricular arrhythmias at a young age and prior to significant atrial remodeling is rather unique and not seen in carriers of the A405S mutation or other previously published JPH2 mutations linked to HCM (12,13).
Although knock-in mice can be used to study the effects of point mutations on protein function, our repeated efforts to generate E169K knockin mice failed thusfar. However, we were able to establish E169K mutant mice with physiological JPH2 levels in the heart by crossing E169K-Tg mice with MCM-shJPH2 knockdown mice (10). Thus, our pseudo-knockin mouse models serve as an adequate model when more traditional knockin mice are not available or feasible.
The E169K-PKI did not develop spontaneous atrial arrhythmias, unlike the human carriers of the E169K mutation in JPH2. This is not surprising, since it is well established that mice are relatively resistant to developing AF unless there is gross atrial remodeling or dilatation. In other mouse models of genetic variants that cause spontaneous AF in humans, we did not observe spontaneous AF although such mutant mice were vulnerable to pacing-induced arrhythmias (8,26). Although it is well accepted that an enhanced susceptibility to AF in mice is an approximate validation of a genetic variant that causes spontaneous AF in humans, using mice as an experimental model is a clear general limitation.
Finally, we only used right atrial tissue samples collected from the right atrial appendage of humans with paroxysmal AF. Although it is well established that ectopic activity often arises from the pulmonary vein region, there is also evidence for highly frequent focal sources in the right atrium in up to 33% of patients (27). Our finding of reduced JPH2 levels per RyR2 channel might not apply to other atrial regions or the left atrium. On the other hand, analysis of right atrial tissue samples and myocytes has proven to be useful and confirmed the presence of increased Ca2+ sparks and remodeling of Ca2+ handling proteins in patients with paroxysmal and chronic AF (4,28,29).
Conclusions
We describe a novel mechanism by which JPH2 mutation E169K can cause atrial arrhythmias as a result of defective RyR2-mediated SR Ca2+ release events. The inherited mutation E169K in JPH2 disrupts the binding of mutant JPH2 to RyR2 and destabilizes RyR2, thereby increasing SR Ca2+ leak and the inducibility of AF in mice. These mechanisms are only observed in E169K mutant mice and not in another HCM-associated (A399S) mutant mouse, suggesting that the mechanisms underlying HCM-related ventricular remodeling are distinct and unrelated to RyR2 destabilization. Interestingly, patients with paroxysmal AF (non-genetic and not associated with HCM) exhibit decreased atrial JPH2 levels per RyR2 channel, suggesting that a similar mechanism of RyR2 destabilization due to a relative depletion of JPH2 could be involved in a much larger population of patients with paroxysmal AF. Our work suggests that future studies are warranted to explore whether JPH2-mediated stabilization of RyR2 might represent a novel therapeutic target for atrial arrhythmias. Specifically, with the emergence of cell-penetrating peptides and small peptide therapies, it may be possible to develop a targeted therapy to reduce atrial arrhythmias (30).
Supplementary Material
Acknowledgments
Disclosures: M.J.A. is a consultant for Biotronik, Boston Scientific, Medtronic, St. Jude Medical, Inc., and Transgenomic. Intellectual property derived from M.J.A.'s research program resulted in license agreements in 2004 between Mayo Clinic Health Solutions (formerly Mayo Medical Ventures) and PGxHealth (formerly Genaissance Pharmaceuticals, now Transgenomic).
Funding sources X.H.T.W. is a W.M. Keck Foundation Distinguished Young Scholar in Medical Research, Established Investigator of the American Heart Association (AHA), and is supported by the Muscular Dystrophy Association and NIH grants HL089598 and HL091947. D.B., S.A. and A.P.L are supported by AHA predoctoral fellowships. D.B. was supported by NIH grant T32HL007676-21A1, and S.A. was supported by an Alkek Foundation fellowship from Baylor College of Medicine. N.L. as supported by a postdoctoral fellowship and currently holds a beginning grant in-aid from the AHA. M.J.A. is an Established Investigator of the AHA and is supported by NIH grants HD42569 and HL94291, and the Mayo Clinic Windland Smith Rice Comprehensive Sudden Cardiac Death Program. This work was also supported in part by Fondation Leducq (`Alliance for CaMKII Signaling in Heart') to X.H.T.W. and M.J.A., and the `European North-American Atrial Fibrillation Research Alliance' to D.D.), and by the DZHK (German Center for Cardiovascular Research; to D.D.), and by the European Union Network for Translational Research in Atrial Fibrillation (EUTRAF, FP7-HEALTH-2010, large-scale integrating project, Proposal No. 261057) to D.D..
ABBREVIATION LIST
- AF
atrial fibrillation
- Ca2+
calcium
- DAD
delayed afterdepolarizations
- EAD
early afterdepolarizations
- HCM
hypertrophic cardiomyopathy
- JMC
junctional membrane complex
- JPH2
junctophilin 2
- NCX
Na+/Ca2+-exchanger
- NTg
non-transgenic
- PKI
pseudo-knockin
- RyR2
ryanodine receptor type 2
- SR
sarcoplasmic reticulum
- Tg
transgenic
- WT
wildtype
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–6. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 2.Burashnikov A, Antzelevitch C. Late-phase 3 EAD. A unique mechanism contributing to initiation of atrial fibrillation. Pacing and clinical electrophysiology : PACE. 2006;29:290–5. doi: 10.1111/j.1540-8159.2006.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeh YH, Wakili R, Qi XY, et al. Calcium-handling abnormalities underlying atrial arrhythmogenesis and contractile dysfunction in dogs with congestive heart failure. Circulation Arrhythmia and electrophysiology. 2008;1:93–102. doi: 10.1161/CIRCEP.107.754788. [DOI] [PubMed] [Google Scholar]
- 4.Voigt N, Li N, Wang Q, et al. Enhanced sarcoplasmic reticulum Ca2+ leak and increased na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation. 2012;125:2059–70. doi: 10.1161/CIRCULATIONAHA.111.067306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greiser M, Neuberger HR, Harks E, et al. Distinct contractile and molecular differences between two goat models of atrial dysfunction: AV block-induced atrial dilatation and atrial fibrillation. Journal of molecular and cellular cardiology. 2009;46:385–94. doi: 10.1016/j.yjmcc.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 6.Wehrens XH, Ather S, Dobrev D. Role of abnormal sarcoplasmic reticulum function in the management of atrial fibrillation. Therapy. 2010;7:147–158. [PMC free article] [PubMed] [Google Scholar]
- 7.Jayasinghe ID, Baddeley D, Kong CH, Wehrens XH, Cannell MB, Soeller C. Nanoscale organization of junctophilin-2 and ryanodine receptors within peripheral couplings of rat ventricular cardiomyocytes. Biophysical journal. 2012;102:L19–21. doi: 10.1016/j.bpj.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chelu MG, Sarma S, Sood S, et al. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. The Journal of clinical investigation. 2009;119:1940–51. doi: 10.1172/JCI37059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li N, Wang T, Wang W, et al. Inhibition of CaMKII phosphorylation of RyR2 prevents induction of atrial fibrillation in FKBP12.6 knockout mice. Circ Res. 2012;110:465–70. doi: 10.1161/CIRCRESAHA.111.253229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Oort RJ, Garbino A, Wang W, et al. Disrupted junctional membrane complexes and hyperactive ryanodine receptors after acute junctophilin knockdown in mice. Circulation. 2011;123:979–88. doi: 10.1161/CIRCULATIONAHA.110.006437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takeshima H, Komazaki S, Nishi M, Iino M, Kangawa K. Junctophilins: a novel family of junctional membrane complex proteins. Mol Cell. 2000;6:11–22. doi: 10.1016/s1097-2765(00)00003-4. [DOI] [PubMed] [Google Scholar]
- 12.Landstrom AP, Weisleder N, Batalden KB, et al. Mutations in JPH2-encoded junctophilin-2 associated with hypertrophic cardiomyopathy in humans. J Mol Cell Cardiol. 2007;42:1026–35. doi: 10.1016/j.yjmcc.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsushita Y, Furukawa T, Kasanuki H, et al. Mutation of junctophilin type 2 associated with hypertrophic cardiomyopathy. J Hum Genet. 2007;52:543–8. doi: 10.1007/s10038-007-0149-y. [DOI] [PubMed] [Google Scholar]
- 14.Li N, Wehrens XH. Programmed electrical stimulation in mice. J Vis Exp. 2010;39:1730. doi: 10.3791/1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woo JS, Hwang JH, Ko JK, et al. S165F mutation of junctophilin 2 affects Ca2+ signalling in skeletal muscle. Biochem J. 2010;427:125–34. doi: 10.1042/BJ20091225. [DOI] [PubMed] [Google Scholar]
- 16.Garbino A, van Oort RJ, Dixit SS, Landstrom AP, Ackerman MJ, Wehrens XH. Molecular evolution of the junctophilin gene family. Physiol Genomics. 2009;37:175–86. doi: 10.1152/physiolgenomics.00017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olivotto I, Cecchi F, Casey SA, Dolara A, Traverse JH, Maron BJ. Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation. 2001;104:2517–24. doi: 10.1161/hc4601.097997. [DOI] [PubMed] [Google Scholar]
- 18.Inui M, Saito A, Fleischer S. Isolation of the ryanodine receptor from cardiac sarcoplasmic reticulum and identity with the feet structures. J Biol Chem. 1987;262:15637–42. [PubMed] [Google Scholar]
- 19.Golini L, Chouabe C, Berthier C, et al. Junctophilin 1 and 2 Proteins Interact with the L-type Ca2+ Channel Dihydropyridine Receptors (DHPRs) in Skeletal Muscle. J Biol Chem. 2011;286:43717–25. doi: 10.1074/jbc.M111.292755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamazaki D, Yamazaki T, Takeshima H. New molecular components supporting ryanodine receptor-mediated Ca(2+) release: roles of junctophilin and TRIC channel in embryonic cardiomyocytes. Pharmacol Ther. 2009;121:265–72. doi: 10.1016/j.pharmthera.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Nattel S, Burstein B, Dobrev D. Molecular Remodeling and Chronic Atrial Fibrillation. In: Zipes DP, Jalife J, editors. Cardiac Electrophysiology: From Cell to Bedside. 5th ed. Saunders; Philadelphia: 2009. pp. 453–465. [Google Scholar]
- 22.Dobrev D, Voigt N, Wehrens XH. The ryanodine receptor channel as a molecular motif in atrial fibrillation: pathophysiological and therapeutic implications. Cardiovasc Res. 2011;89:734–43. doi: 10.1093/cvr/cvq324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vest JA, Wehrens XH, Reiken SR, et al. Defective cardiac ryanodine receptor regulation during atrial fibrillation. Circulation. 2005;111:2025–32. doi: 10.1161/01.CIR.0000162461.67140.4C. [DOI] [PubMed] [Google Scholar]
- 24.Wehrens XH, Lehnart SE, Reiken SR, et al. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science. 2004;304:292–6. doi: 10.1126/science.1094301. [DOI] [PubMed] [Google Scholar]
- 25.Wehrens XH, Lehnart SE, Huang F, et al. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell. 2003;113:829–40. doi: 10.1016/s0092-8674(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Klysik E, Sood S, Johnson RL, Wehrens XH, Martin JF. Pitx2 prevents susceptibility to atrial arrhythmias by inhibiting left-sided pacemaker specification. Proc Natl Acad Sci U S A. 2010;107:9753–8. doi: 10.1073/pnas.0912585107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narayan SM, Krummen DE, Clopton P, Shivkumar K, Miller JM. Direct Or Coincidental Elimination of Stable Rotors or Focal Sources May Explain Successful Atrial Fibrillation Ablation: On-Treatment Analysis of the CONFIRM (CONventional ablation for AF with or without Focal Impulse and Rotor Modulation) Trial. J Am Coll Cardiol. 2013 doi: 10.1016/j.jacc.2013.03.021. Apr 3 [ePub online] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hove-Madsen L, Llach A, Bayes-Genis A, et al. Atrial fibrillation is associated with increased spontaneous calcium release from the sarcoplasmic reticulum in human atrial myocytes. Circulation. 2004;110:1358–63. doi: 10.1161/01.CIR.0000141296.59876.87. [DOI] [PubMed] [Google Scholar]
- 29.Neef S, Dybkova N, Sossalla S, et al. CaMKII-dependent diastolic SR Ca2+ leak and elevated diastolic Ca2+ levels in right atrial myocardium of patients with atrial fibrillation. Circ Res. 2010;106:1134–44. doi: 10.1161/CIRCRESAHA.109.203836. [DOI] [PubMed] [Google Scholar]
- 30.Heitz F, Morris MC, Divita G. Twenty years of cell-penetrating peptides: from molecular mechanisms to therapeutics. Br J Pharmacol. 2009;157:195–206. doi: 10.1111/j.1476-5381.2009.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.