Abstract
OBJECTIVES
To determine the cumulative opioid doses administered to patients with Down syndrome (DS) after cardiac surgery and compare them to patients without DS.
DESIGN
Retrospective, observational, comparative study.
SETTING
PICU in a university-affiliated, freestanding pediatric teaching hospital.
PATIENTS
Infants and children who presented to our institution for heart surgery after July 1, 2008, and met the following criteria: 1) no opioid medications for 48 hours prior to surgery, 2) sternotomy approach with primary closure, 3) no additional operative procedures in the 5 days after surgery. All patients with DS were included, and patients without DS with similar age, type of cardiac lesion, and length of surgical procedure were selected in a ~2:1 ratio, blinded to opioid exposure.
INTERVENTIONS
None.
MEASUREMENTS AND MAIN RESULTS
Clinical and demographic data were extracted from electronic medical record data. Univariate analyses and mulitvariate linear regression modeling were performed to determine the influence of DS, patient characteristics, and clinical covariates on weight-adjusted opioid dose. The differences in median cumulative opioid doses between those with DS (n=44) and those without (n=77) were not significant in the first 24 hours [+0.39 mg/kg (95% CI −0.45 to +1.39 mg/kg)] or 96 hours [+0.54 mg/kg (−0.59 to +2.07 mg/kg)] after surgery. Age, cardiac bypass time, benzodiazepines, and neuromuscular blocking agents were significantly correlated with opioid dose, but DS, gender, pain score, creatinine, acetaminophen, NSAIDs, and steroid medications were not. Patients with DS had longer hospital stays; in multivariate analysis, higher opioid exposures in the first 96 hours after surgery and higher peak serum creatinine values correlated with longer hospitalization.
CONCLUSIONS
This cohort did not provide evidence for opioid resistance in patients with DS. Younger age, longer cardiac bypass time, exposure to benzodiazepines, and neuromuscular blockade did correlate with increased opioid doses after cardiac surgery.
Keywords: Opioid, Narcotic Analgesics, Pain, Postoperative, Analgesia, Thoracic Surgical Procedures, Down Syndrome
Introduction
Down Syndrome (DS) is the most common chromosomal abnormality in humans, affecting 1 in 691 live births.(1) Among patients with DS, approximately half are diagnosed with congenital heart disease, including atrioventricular septal defects (AVSD), ventricular septal defects, atrial septal defects, patent ductus arteriosus, Tetralogy of Fallot, and other complex configurations.(2) Many of these patients undergo surgical correction or palliation during infancy or childhood, with subsequent care in the critical care setting.
Patients with DS require specialized management during and after procedures due to higher risk for comorbidities such as pulmonary hypertension and atlantoaxial instability.(3) In addition, a general impression exists among some clinicians that achieving adequate postoperative analgesia among patients with DS is a greater challenge relative to patients without DS. In support of this impression, one Canadian study showed higher morphine dosing and more use of sedatives and muscle relaxants in 16 patients with DS after cardiac surgery in comparison to 16 matched controls without DS.(4) However, a more recent study showed comparable analgesic doses and pain scores in 15 patients with DS compared to 30 non-DS controls in the Netherlands.(5)
Given the limited and conflicting literature regarding opioid needs in patients with DS, we tested the hypothesis that pediatric patients with DS have higher cumulative opioid exposure after cardiac surgery than patients without DS. To that end, we performed retrospective chart reviews in a large cohort and compared cumulative weight-adjusted opioid exposure 24 hours and 96 hours after cardiac surgery in pediatric patients with and without DS.
Materials and Methods
The study was a nested retrospective study, with both this study and the larger prospective cohort study approved by the Vanderbilt Institutional Review Board. Parents provided written consent for their child’s participation. When appropriate, informed assent was also obtained. Eligibility for the larger cohort study, “Genetic Basis of Arrhythmias in Pediatrics and Congenital Heart Disease,” was defined as patients with congenital heart disease scheduled to undergo a corrective or palliative operative procedure at our institution.(6,7) Enrollment for the larger study began in September, 2007 and is ongoing. Of 1426 potential participants approached for consent, 254 (18%) declined enrollment, and 1 has withdrawn from the study. Analgesic and sedative selection and dosing were determined by the primary clinical team and were not impacted by study enrollment. Clinical teams were not blinded to study enrollment; however, analgesic medication use is not a primary outcome for the larger study.
Study subjects
The nested cohort for this retrospective study was the subset of participants in the larger study who met the following criteria: 1) no opioid medication exposure in the 48 hours prior to surgery, 2) sternotomy approach with primary closure for surgical procedure, 3) no additional operative procedures in the five days after surgery, and 4) surgery after July, 2008, the start date for electronic documentation of drug administration data in Vanderbilt electronic medical records (EMR). For patients with multiple surgical procedures, the first surgery for which medication exposure data were available was used for analysis. All pediatric patients, from neonates to 18 years of age, were considered for inclusion. The participants with DS were identified by stated diagnosis in the EMR, which was confirmed at the time of study enrollment. For each patient with DS, we attempted to select two study participants without DS with similar age, operative time, and type of cardiac lesion to serve as controls. This selection was conducted blinded to all medication exposures.
Data extraction and storage
Demographic data and medical history were documented at the time of study enrollment. Surgical data, time of extubation, peak daily pain scores, creatinine lab values and length of hospitalization were extracted from the EMR. At our institution, pain is assessed and documented in non-verbal patients using the FLACC score, a tool that is reliable and validated in typical children, children with cognitive impairment, and critically ill children.(8–10) Self-report using Faces(11) or numeric scales is used for verbal patients. Medication exposures, including scheduled and as-needed intermittent dosing as well as continuous infusions after the postoperative admission to the intensive care unit were determined from the EMR and the Vanderbilt Enterprise Data Warehouse. The Enterprise Data Warehouse contains an electronic copy of both nurse administration and pharmacy operational data, enabling the computation of administered drug amounts over specific time periods.
For opioid medications, total administered doses of each medication were determined for 0 to 24 hours and 0 to 96 hours after surgery, using time windows relevant to previous studies.(4,5) These total doses were converted to the equivalent intravenous morphine dose as determined after consultation of the literature,(12) drug references,(13,14) and a pediatric pharmacist (Supplemental Table 1). Opioid exposures were divided by preoperative weight to determine per-kilogram dosing. Administration of acetaminophen, neuromuscular blockers, NSAIDS or steroids, and benzodiazepines was recorded as a binary factor for each category and time interval, and as cumulative doses per-kilogram within drug class using the conversions in Supplemental Table 1 for 24 and 96 hours. Analgesics and sedatives administered during operative procedures were determined from anesthetic care records and compared between groups in a separate analysis. Given their long duration of action, steroid medications administered intraoperatively were included with steroids given in the first 24 hours postoperatively. Because renal elimination is required for several opioid and non-opioid analgesics, peak postoperative creatinine was included as a covariate. Study data were collected and managed using REDCap electronic data capture tools, a secure, web-based application hosted at Vanderbilt University.(15)
Analysis
Statistical analyses were performed using Stata version 11.2. Continuous variables were compared by the Wilcoxon Rank Sum test. Dichotomous and ordinal outcomes were compared by Pearson’s Chi-square test or Fisher’s exact test. For the adjusted analysis, multivariate linear regression modeling of the outcome [log-transformed cumulative opioid dose/weight, ln(mg/kg)] was performed with the covariates of DS diagnosis, age, gender, cardiac bypass time, pain score, creatinine, and non-opioid medication exposures. Due to a significant portion of the cohort having no exposure to the non-opioid analgesics, sedatives, and/or neuromuscular blockers, the primary analysis used dichotomous variables to describe exposure to acetaminophen, benzodiazepines, neuromuscular blockade, and dexmedetomidine; all patients were exposed to NSAIDs or steroids in the first 24 hours after surgery, so this factor was eliminated. A secondary multivariate analysis was performed with each medication class as well as NSAID and steroid doses expressed as a continuous variable (mg/kg cumulative equivalent dose after 24 or 96 hours). For all linear regression analyses with log-transformed outcomes, coefficients were exponentiated to determine the ratio of geometric means of covariates to cumulative opioid dose after 24 and 96 hours. Time to extubation and length of hospitalization were analyzed using covariates of cumulative 96 hour opioid exposure, age, gender, DS status, bypass time, pain scores, and concomitant medication exposures. P-values < 0.05 were considered significant in the primary univariate analysis and regression model.
Post hoc subgroup analyses were performed to determine if there were differences in cumulative opioid dose at 24 and 96 hours after surgery between those with DS and without among infants (under 1 year of age) and children (over 1 year of age). Because of the smaller number of individuals in these subgroups, covariates used for the regression analysis were restricted to age and bypass time. Age was included due to different age distributions among children with and without DS (Supplemental Table 2), and bypass time was included as it was the most significant covariate in the primary analysis.
Post hoc power calculations were performed in PS version 3.0.43 assuming normal distributions, observed standard deviations, and alpha = 0.05.(16) This study had 80% power to detect a difference of 2.2 mg/kg for the first 24 hours, and 5.7 mg/kg for the first 96 hours between the patients with and without DS.
Results
Study cohort
We analyzed data from 121 individuals (age 5 days through 17 years), including 44 patients with DS and 77 without DS. The primary cardiac diagnoses among those with DS were different than those without DS, consistent with relatively high incidence of AVSD in patients with DS (Table 1). A total of 15 patients had prior cardiac surgeries (5 with DS and 10 without, p=0.87). For most individuals, pain scores were documented based on FLACC criteria (n=113/121, 93%), with the remainder based on self-report numeric (6/121, 5%, all over 10 years of age and none with DS) or Faces scales (2/121, 2%, 5 and 13 years old, neither with DS). Those with DS had lower weights and lower mean peak pain scores in the first 96 hours after surgery than those without DS, but there was no statistically significant difference between groups in age, gender, cardiac bypass time, creatinine, pain scores in the first 24 hours, or time to extubation (Table 1). With respect to concomitant medications, the two cohorts did not differ in their exposure to acetaminophen, NSAIDs/steroids, benzodiazepines, neuromuscular blockers or dexmedetomidine when treated as dichotomous traits (Table 2). Analysis of cumulative doses of these medications as continuous variables revealed no statistically significant differences between those with and without DS for any medications except dexmedetomidine. Patients with DS received less dexmedetomidine than those without DS at 24 hours (mean±SD: 1.6±2.8 mg/kg vs. 3.6±4.9 mg/kg, p=0.03) and 96 hours (2.6±5.0 mg/kg vs. 4.6±6.5 mg/kg, p=0.04).
Table 1.
Cohort demographics
| Cohort (N=121) | No Down Syndrome (N=77) | Down Syndrome (N=44) | P-value | |
|---|---|---|---|---|
| Age (months)a | 28 ± 48 (0.2 – 207) | 35 ± 55 (0.4 – 207) | 17 ± 27 (0.2 – 103) | 0.11 |
| Maleb | 64 (53%) | 43 (56%) | 21 (48%) | 0.39 |
| Weight (kg)a | 12.2 ± 16 (2.2 – 96.9) | 15 ± 19 (3.1 – 96.9) | 7.4 ± 4.8 (2.2 – 21.7) | 0.003 |
| Primary Diagnosisc | <0.001 | |||
| AVSD | 41 (34%) | 12 (16%) | 29 (66%) | |
| Tetralogy of Fallot | 37 (31%) | 35 (45%) | 2 (5%) | |
| Ventricular Septal Defect | 24 (20%) | 17 (22%) | 7 (16%) | |
| Atrial Septal Defect | 11 (9%) | 8 (10%) | 3 (7%) | |
| Otherd | 8 (7%) | 5 (6%) | 3 (7%) | |
| Bypass Time (min)a | 99 ± 39 (0 – 215) | 99 ± 36 (0 – 215) | 101 ± 44 (0 – 212) | 0.34 |
| Creatinine (mg/dL)a | 0.6 ± 0.2 (0.3 – 1.8) | 0.6 ± 0.2 (0.3 – 1.8) | 0.6 ± 0.1 (0.4 – 0.9) | 0.09 |
| Peak Pain Score in first 24 hoursa | 4 ± 3 (0 – 10) | 4 ± 3 (0 – 10) | 4 ± 2 (0 – 10) | 0.27 |
| Mean Peak Pain Score in first 96 hoursa | 5 ± 2 (0 – 10) | 5 ± 2 (1 – 10) | 4 ± 2 (0 – 8) | 0.02 |
| Time to Extubation (days)a | 1.2 ± 2.0 (0 – 14) | 1.0 ± 1.5 (0 – 6) | 1.6 ± 2.6 (0 – 14) | 0.08 |
| Hospital Length of Stay (days) a | 8.6 ± 9.6 (2 – 85) | 5.5 ± 9.5 (2 – 85) | 7 ± 9.6 (3 – 49) | 0.02 |
Mean ± SD (Min - Max), P-value from Wilcoxon Rank Sum Test
N (Column %), P-value from Pearson’s Chi-square Test
N (Column %), P-value from Fisher’s Exact Test
Other includes Mitral Valve Stenosis, Vascular Rings, and Aortic Arch Hypoplasia
Table 2.
Analgesic and sedative medication exposures
| Medication Name or Class | Cohort (N=121) | No Down Syndrome (N=77) | Down Syndrome (N=44) | P-value |
|---|---|---|---|---|
| Acetaminophen | ||||
| First 24 hours | 84 (69%) | 55 (71%) | 29 (66%) | 0.53 |
| First 96 hours | 110 (91%) | 70 (91%) | 40 (91%) | 1.0 |
| Benzodiazepine | ||||
| First 24 hours | 74 (61%) | 46 (60%) | 28 (64%) | 0.67 |
| First 96 hours | 81 (67%) | 47 (61%) | 34 (77%) | 0.07 |
| Neuromuscular Block | ||||
| First 24 hours | 20 (17%) | 14 (18%) | 6 (14%) | 0.52 |
| First 96 hours | 20 (17%) | 14 (18%) | 6 (14%) | 0.52 |
| Dexmedetomidine | ||||
| First 24 hours | 51 (42%) | 28 (36%) | 23 (52%) | 0.09 |
| First 96 hours | 56 (46%) | 31 (40%) | 25 (57%) | 0.08 |
N (Column %), for any exposure to medication in class, P-value from Pearson’s Chi-square Test
Opioid exposure
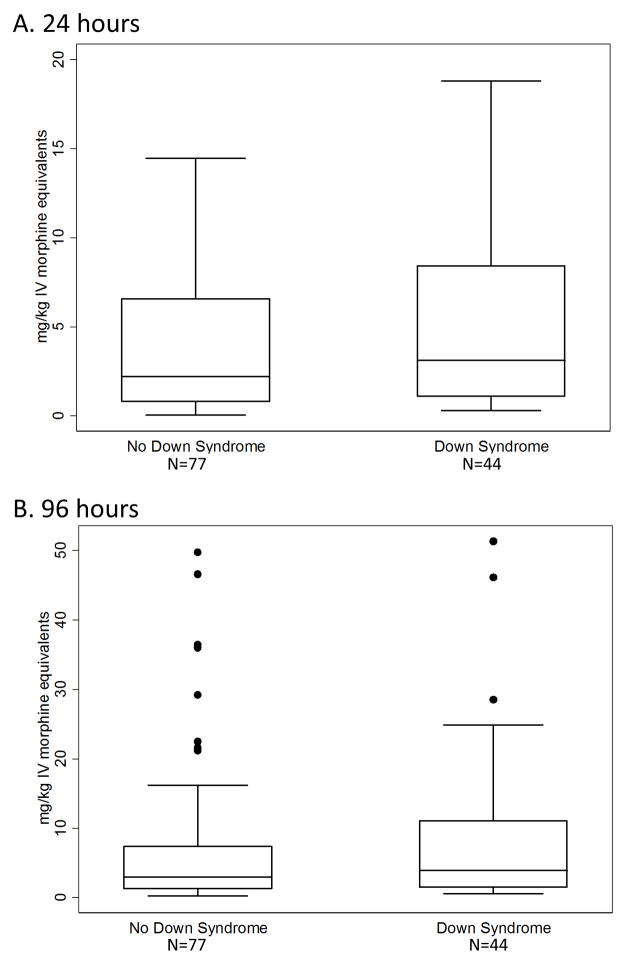
There was not a standardized anesthetic regimen in the timeframe of this study. All patients received fentanyl during surgery, and 82 (68%) received midazolam; intraoperative exposures to analgesics and sedatives were not different between groups. Postoperatively, six different opioid agents were used for analgesia within the first 96 hours (Supplemental Table 3). The most commonly used agent was morphine (111 patients, 92%), followed by fentanyl (109, 90%) and oxycodone (85, 70%). A small group of patients received hydromorphone (n=6), nalbuphine (n=1), or codeine (n=4). Nearly half of the cohort received opioid medication via infusion in the first 24 hours after surgery (n=58, 48%), nearly all of whom also received intermittent opioid dosing for breakthrough pain (52/58, 90%). Cumulative weight-adjusted opioid exposure was variable within the full cohort for the first 24 hours (median, interquartile range (IQR): 2.3, 0.9 – 6.6 mg/kg; range: 0.1 – 18.8 mg/kg) and 96 hours after surgery (median, IQR: 3.2, 1.3 – 9.5 mg/kg; range: 0.2 – 51.3 mg/kg). Averaging over time, these exposures correspond to median hourly doses of 0.096 mg/kg/hr for the first 24 hours and 0.033 mg/kg/hr for the initial 96 hours. When comparing patients with DS to those without DS, unadjusted analysis showed no difference in cumulative opioid exposure at 24 or 96 hours (Table 3, Figure 1). The differences in medians of opioid doses for patients with DS compared to those without was +0.39 mg/kg (95% CI −0.45 to +1.39 mg/kg) after 24 hours and +0.54 mg/kg (95% CI −0.59 to +2.07 mg/kg) after 96 hours, corresponding to average hourly rate differences of 0.016 and 0.006 mg/kg/hour.
Table 3.
Effect of Down syndrome on post-operative opioid dose
| 24 hours | 96 hours | |||
|---|---|---|---|---|
|
|
||||
| No Down Syndrome (N=77) | Down Syndrome (N=44) | No Down Syndrome (N=77) | Down Syndrome (N=44) | |
| Cumulative opioid (mg/kg) | 2.2 (0.8 – 6.6) | 3.1 (1.1 – 8.4) | 2.9 (1.2 – 7.4) | 3.9 (1.5 – 11.0) |
| Median (IQR) | ||||
| Differences in Medians (95% CI) | +0.39 (−0.45 to +1.39) | +0.54 (−0.59 to +2.07) | ||
| Unadjusted P-valuea | 0.33 | 0.33 | ||
| Adjusted P-valueb | 0.44 | 0.83 | ||
Wilcoxon Rank-Sum test
For Down syndrome in linear regression modeling
Figure 1.
Opioid exposure in patients with and without DS. A) First 24 postoperative hours. B) First 96 postoperative hours. Bars represent the median, boxes the 25th to 75th percentile, whiskers the 5th to 95th percentile, and solid circles any data outside the whiskers.
Adjusted analyses using multivariate linear regression and dichotomous non-opioid medication exposure covariates showed no significant association between DS and cumulative opioid dosing for either time interval (24 hour model: R2=0.54, p<0.001 for model, p=0.44 for DS; 96 hour model R2=0.59, p<0.001 for model, p=0.83 for DS). Younger age, longer bypass time, exposure to benzodiazepines, and exposure to neuromuscular blockade significantly correlated with increased opioid exposure after 24 and 96 hours (Supplemental Table 4). Additionally, exposure to dexmedetomidine was associated at 96 hours. Gender, peak pain score, creatinine, and acetaminophen exposure did not have a statistically significant correlation with opioid dose in the model at 24 or 96 hours (Supplemental Table 4). Using total doses of acetaminophen, NSAIDs, steroids, benzodiazepines, neuromuscular blockers, and dexmedetomidine as continuous variables rather than dichotomous did not affect results with respect to DS diagnosis, but eliminated the statistically significant association of opioid dose with neuromuscular blockers at 24 and 96 hours, and dexmedetomidine at 96 hours. Exclusion of patients without cardiac bypass (n=6) did not affect results.
The post hoc univariate analysis stratified by age [infant (< 1 year of age) and child (age > 1 year)] revealed no differences between those with and without DS in opioid exposure after 24 or 96 hours among infants or children (Supplemental Table 5). There was a trend towards increased opioid use among children over 1 year of age with DS at 24 hours, but this association is not statistically significant (p=0.07). Correction for age and bypass time in linear regression modeling further reduced the statistical significance of this association (Supplemental Table 5).
Time to extubation and length of hospitalization
There was no difference in time to extubation between patients with and without DS (Table 1). Regression analysis revealed that of the covariates included, cumulative opioid dose after 96 hours was the only factor significantly correlated to this outcome (p<0.001, beta 0.1, 95% CI 0.07 – 0.14). Patients with DS did have significantly longer hospital stays (Table 1). Regression analysis of this outcome identified cumulative 96 hour opioid dose (p=0.03, beta 0.24, 95% CI 0.02 – 0.45) and peak creatinine (p=0.04, beta 13.0, 95% CI 0.82 – 25.2) as significant correlates to length of stay. In the adjusted analysis, DS status was not significantly associated with length of hospitalization.
Discussion
The goal of this study was to compare total opioid doses administered after cardiac surgery to pediatric patients with and without DS. In this large cohort of postoperative pediatric patients, opioid administration was not different among those with DS when compared to those without DS. In both univariate analyses and linear regression adjusting for age, gender, cardiac bypass time, creatinine, pain scores, and concomitant medication exposure, DS was not associated with increased opioid doses used after surgery. Among patients over 1 year of age, a trend towards increased opioid dosing in patients with DS was found when comparing opioid doses in the first 24 postoperative hours. However, the confidence in this finding is limited as it was a post hoc analysis among a small subset of patients. In all, our findings are consistent with Valkenburg et al.(5) indicating no difference, and do not support the hypothesis that patients with DS are opioid resistant. Instead, these findings indicate that patients with DS in this cohort were managed with similar doses of opioids as patients without DS.
For the purposes of comparison, non-DS patients were selected in a ~2:1 ratio to DS patients, with the goal of matching on age and surgical complexity. While this matching strategy resulted in similar ages, surgical bypass times, time to extubation, and medication exposures for the two groups, the DS cohort individuals had lower weights than those in the non-DS cohort. This may be attributable to the DS cohort being slightly younger, though the difference in age was not statistically significant, coupled with expected lower birth weights and growth velocities of patients with DS.(17–19) Drug doses were weight adjusted for analysis, and therefore weight was not included as a covariate in the regression models. Furthermore, time to extubation was not included as a covariate, as prolonged intubation may be a consequence of opioid sensitivity or administration of large doses.
Perhaps more importantly, the primary cardiac diagnoses in patients with DS were different than in the patients without DS. Due to the relatively high incidence of AVSD in patients with DS, insufficient AVSD cases without DS were available to allow for 2:1 matching for this specific cardiac diagnosis. Patients with Tetralogy of Fallot were included in non-DS group, as surgical repair was performed at a similar age as AVSD repair with similar cross-clamp and bypass times in this cohort; furthermore, both surgical procedures are associated with similar postoperative risk.(20) Due to the total sample size and number of covariates to be included, the effects of the specific cardiac diagnoses on opioid use could not be assessed in this cohort without overfitting the linear regression model. A larger cohort may enable exploration of the association of specific diagnoses or procedures to analgesic drug exposures.
Lesser use of adjunct analgesics would be expected to increase opioid use. However, despite receiving lower weight-adjusted doses of dexmedetomidine, patients with DS did not have significantly higher opioid exposures. Administration of other non-opioid medications such as NSAIDs and sedatives was not different in patients with DS compared to those without, indicating that non-opioid medication management was similar across the two groups with the stated exception of dexmedetomidine.
Pain scores are an important covariate to consider in the evaluation of analgesics. Patients with DS had similar pain scores to those without DS in the first 24 hours, but lower mean pain scores for the first 96 hours after surgery. Given that the pain scales used have been validated in typically developing and developmentally delayed pediatric populations,(8,10,11) this finding of lower scores after surgery indicates that patients with DS achieved the same or better level of analgesia than the non-DS patients, provided pain scores were accurately assessed in both groups. It is possible that patients with DS were assigned lower pain scores due to evaluator bias or different manifestations of pain in these patients. This bias could not be assessed in this retrospective study.
This retrospective observational study did not include a protocol driving medication choice or dosing decisions made by the clinical care team. Hence this retrospective approach enables conclusions to be drawn based on the actual medications given in the patient care setting, without restrictions or bias introduced by a research protocol. However, because information regarding clinical decision making behind opioid administration or changes in dose are not available, it is not possible to ascertain to what degree biases such as presumed pain due to the different surgical procedures influenced opioid doses. In comparison to previously reported studies investigating opioid exposures in children with DS and/or cardiac surgery, the cumulative opioid exposures in this cohort are higher.(4,5,21–23) One reason for this may be the methodology for calculation, including extraction and conversion of all opioid exposures, and use of the pre-surgical “dry” weight. In addition, the specific cardiac surgeries done, patient age, and the common use of fentanyl in this cohort may influence the results. Pain assessment was documented in this cohort initially at an hourly basis, more frequently than reported by Valkenburg et al. and Lynn et al;(5,23) it is possible that this frequent assessment may have contributed to increased exposures. Detailed assessment of total exposures requires further study through both retrospective and prospective designs in order to determine optimal ways to balance adequate analgesia while minimizing exposures.
The larger cohort study enrolled nearly all patients presenting to our institution for cardiac surgery since late-2007, but only 44 individuals with DS were eligible for these analyses. A larger sample size may reveal a difference in opioid doses between patients with and without DS. The upper bounds of the confidence intervals indicate that the median difference in iv morphine dose for patients with DS vs. those without is at most +1.39 mg/kg over the first 24 hours after surgery (0.06 mg/kg/hr), and +2.07 mg/kg over the first 96 hours after surgery (0.02 mg/kg/hr); if true, a median difference of this degree may appear to be clinically relevant, as it approximates initial infusion rates for opioid medications. However, the marked variability of opioid dosing within each group (Figure 1) demonstrates that factors other than DS contribute to opioid exposure, a topic for further study.
Although this study did not demonstrate clear differences in opioid administration between patients with DS to those without, observations of the cohort as a whole will guide future studies. First, some patients were exposed to large cumulative doses of opioid drugs. One-quarter of the cohort received ≥6.6 mg/kg morphine equivalents in the first 24 hours after surgery (>0.25mg/kg/hour). Manual review of these individuals confirmed accurate extraction of this data from the EMR, but did not provide indications for the high doses delivered. In contrast, the 25th percentile of 24 hours cumulative dose (0.9mg/kg), nearly 10-fold lower, highlights the extreme variability in dosing received. Linear regression analysis did reveal that younger age, longer bypass time, and exposure to benzodiazepines were consistently associated with increased cumulative opioid exposures at 24 and 96 hours after surgery. Each of these factors may be associated with more complex surgical procedures, often completed at a young age, which require longer operative times and more postoperative sedation and analgesia. Of note, gender, peak pain score, and creatinine were not associated with opioid exposure, indicating that in this cohort these variables did not predict opioid use. Study of a larger cohort to determine robust predictors of opioid doses is currently underway.
The regression modeling indicated that ~60% of the variability in cumulative opioid exposure could be accounted for using demographic, surgical, and medical information, leaving significant additional variability unaccounted for by these factors. Potential influences on postoperative opioid dose not assessed by this study include surgical factors such as the specific procedure completed, environmental factors such as opioid exposures more than 48 hours before the procedure or non-pharmaceutical comfort measures, and genetic factors influencing drug disposition and response. Further retrospective studies of a variety of populations as well as prospective studies of effective doses of analgesics in children are warranted to determine predictors of differences in this patient population.
Analysis of the outcomes of time to extubation and length of hospitalization revealed no difference between groups for the former, but patients with DS had longer hospitalizations. In multivariate analysis, length of stay was correlated to opioid exposure and peak creatinine after surgery, while age, bypass time, DS status, and other medication exposures were not significant. Acute kidney injury has previously been associated with longer hospitalization.(24,25) With respect to length of hospitalization, this retrospective study cannot assess whether drug exposures directly contributed to longer stays. The association of postoperative opioid exposure to length of stay may be due to drug effects (e.g. dependence and prolonged taper), or this single variable acting as a surrogate for many factors (e.g. age and/or complexity of surgical procedure). Determining the nature of this association merits further study.
Conclusions
Based upon our findings, patients with DS are not expected to need higher opioid doses than individuals without DS for adequate pain management after cardiac surgery. In a patient requiring high doses of opioid medications, alternate explanations should be sought, regardless of whether the patient has a DS diagnosis.
Supplementary Material
Acknowledgments
The authors wish to thank Jill Owen, R.N., for her contribution to this study.
This work was supported in part by the Vanderbilt Institute for Clinical and Translational Research grant (1 UL1 RR024975 from NCRR/NIH), NIH/NIEHS grant K12 ES015855, the NIH/NIGMS Clinical Pharmacology Training Program (5T32 GM007569-33), American Heart Association 12CRP10560001, and NIH/NCI grant R01CA141307.
Abbreviations
- AVSD
Atrioventricular Septal Defects
- CI
Confidence Interval
- DS
Down Syndrome
- EMR
Electronic Medical Record
- IQR
Interquartile Range
- NSAIDs
Non-Steroidal Anti-Inflammatory Drugs
Footnotes
Conflicts of interest and sources of funding
SLV, AS, MDM, HX, AHS, TLM, and PJK: Portions of this study were supported by NCRR/NIH grant UL1 RR024975, supporting the Vanderbilt Institute for Clinical and Translational Research (VICTR).
SLV has been supported by NIH/NIGMS Clinical Pharmacology Training Program 5T32 GM007569-33.
AS and HX are supported by NIH/NCI grant R01CA141307.
AHS is supported by American Heart Association Clinical Research Program Grant 12CRP10560001.
TLM is supported by NIH/NIEHS grant K12 ES015855.
References
- 1.Parker SE, Mai CT, Canfield MA, Rickard R, Wang Y, Meyer RE, et al. Updated national birth prevalence estimates for selected birth defects in the United States, 2004–2006. Birt Defects Res. 2010 Dec 1;88(12):1008–16. doi: 10.1002/bdra.20735. [DOI] [PubMed] [Google Scholar]
- 2.Vis JC, Duffels MGJ, Winter MM, Weijerman ME, Cobben JM, Huisman SA, et al. Down syndrome: a cardiovascular perspective. J Intellect Disabil Res. 2009 May 1;53(5):419–25. doi: 10.1111/j.1365-2788.2009.01158.x. [DOI] [PubMed] [Google Scholar]
- 3.Meitzner MC, Skurnowicz JA. Anesthetic considerations for patients with Down syndrome. AANA J. 2005 Apr;73(2):103–7. [PubMed] [Google Scholar]
- 4.Gakhal B, Scott CS, MacNab AJ. Comparison of morphine requirements for sedation in Down’s syndrome and non-Down’s patients following paediatric cardiac surgery. Paediatr Anaesth. 1998;8(3):229–33. doi: 10.1046/j.1460-9592.1998.00764.x. [DOI] [PubMed] [Google Scholar]
- 5.Valkenburg AJ, Van Dijk M, De Leeuw TG, Meeussen CJ, Knibbe CA, Tibboel D. Anaesthesia and Postoperative Analgesia in Surgical Neonates with or Without Down’s Syndrome: Is It Really Different? Br J Anaesth. 2012 Feb 1;108(2):295–301. doi: 10.1093/bja/aer421. [DOI] [PubMed] [Google Scholar]
- 6.Smith AH, Owen J, Borgman KY, Fish FA, Kannankeril PJ. Relation of milrinone after surgery for congenital heart disease to significant postoperative tachyarrhythmias. Am J Cardiol. 2011 Dec 1;108(11):1620–4. doi: 10.1016/j.amjcard.2011.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borgman KY, Smith AH, Owen JP, Fish FA, Kannankeril PJ. A genetic contribution to risk for postoperative junctional ectopic tachycardia in children undergoing surgery for congenital heart disease. Heart Rhythm. 2011 Dec;8(12):1900–4. doi: 10.1016/j.hrthm.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voepel-Lewis T, Merkel S, Tait AR, Trzcinka A, Malviya S. The reliability and validity of the Face, Legs, Activity, Cry, Consolability observational tool as a measure of pain in children with cognitive impairment. Anesth Analg. 2002 Nov;95(5):1224–1229. doi: 10.1097/00000539-200211000-00020. [DOI] [PubMed] [Google Scholar]
- 9.Voepel-Lewis T, Malviya S, Merkel S, Tait AR. Behavioral pain assessment and the Face, Legs, Activity, Cry and Consolability instrument. Expert Rev Pharmacoecon Outcomes Res. 2003 Jun;3(3):317–25. doi: 10.1586/14737167.3.3.317. [DOI] [PubMed] [Google Scholar]
- 10.Voepel-Lewis T, Zanotti J, Dammeyer JA, Merkel S. Reliability and validity of the face, legs, activity, cry, consolability behavioral tool in assessing acute pain in critically ill patients. Am J Crit Care. 2010 Jan;19(1):55–61. doi: 10.4037/ajcc2010624. quiz 62. [DOI] [PubMed] [Google Scholar]
- 11.Hicks CL, von Baeyer CL, Spafford PA, van Korlaar I, Goodenough B. The Faces Pain Scale-Revised: toward a common metric in pediatric pain measurement. Pain. 2001 Aug;93(2):173–83. doi: 10.1016/S0304-3959(01)00314-1. [DOI] [PubMed] [Google Scholar]
- 12.Pereira J, Lawlor P, Vigano A, Dorgan M, Bruera E. Equianalgesic Dose Ratios for Opioids: A Critical Review and Proposals for Long-Term Dosing. J Pain Symptom Manage. 2001 Aug;22(2):672–87. doi: 10.1016/s0885-3924(01)00294-9. [DOI] [PubMed] [Google Scholar]
- 13.Hudson, Ohio: Lexi-Comp, Inc. Lexicomp Online: Calculations [Internet] Pediatr Neonatal Lexi-Drugs Online. [cited 2012 May 1]. Available from: http://www.crlonline.com/lco/action/calc/calculator/70050#.
- 14.Johns Hopkins Hospital. The Harriet Lane handbook: a manual for pediatric house officers. Chicago: Children’s Medical and Surgical Center. [Google Scholar]
- 15.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009 Apr;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dupont WD, Plummer WD., Jr Power and sample size calculations. A review and computer program. Control Clin Trials. 1990 Apr;11(2):116–28. doi: 10.1016/0197-2456(90)90005-m. [DOI] [PubMed] [Google Scholar]
- 17.Cronk C, Crocker AC, Pueschel SM, Shea AM, Zackai E, Pickens G, et al. Growth charts for children with Down syndrome: 1 month to 18 years of age. Pediatrics. 1988 Jan;81(1):102–10. [PubMed] [Google Scholar]
- 18.Ratcliffe SG. The Effect of Chromosome Abnormalities on Human Growth. Br Med Bull. 1981 Sep 1;37(3):291–5. doi: 10.1093/oxfordjournals.bmb.a071717. [DOI] [PubMed] [Google Scholar]
- 19.Weijerman ME, van Furth AM, Vonk Noordegraaf A, van Wouwe JP, Broers CJM, Gemke RJBJ. Prevalence, Neonatal Characteristics, and First-Year Mortality of Down Syndrome: A National Study. J Pediatr. 2008 Jan;152(1):15–9. doi: 10.1016/j.jpeds.2007.09.045. [DOI] [PubMed] [Google Scholar]
- 20.Jenkins KJ, Gauvreau K, Newburger JW, Spray TL, Moller JH, Iezzoni LI. Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg. 2002 Jan 1;123(1):110–8. doi: 10.1067/mtc.2002.119064. [DOI] [PubMed] [Google Scholar]
- 21.Lynn AM, Nespeca MK, Opheim KE, Slattery JT. Respiratory Effects of Intravenous Morphine Infusions in Neonates, Infants, and Children After Cardiac Surgery. 1993 Oct 1;77(4):695–701. doi: 10.1213/00000539-199310000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Lynn A, Nespeca MK, Bratton SL, Strauss SG, Shen DD. Clearance of morphine in postoperative infants during intravenous infusion: the influence of age and surgery. Anesth Analg. 1998 May;86(5):958–63. doi: 10.1097/00000539-199805000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Lynn AM, Nespeca MK, Bratton SL, Shen DD. Intravenous morphine in postoperative infants: intermittent bolus dosing versus targeted continuous infusions. Pain. 2000 Oct;88(1):89–95. doi: 10.1016/S0304-3959(00)00313-4. [DOI] [PubMed] [Google Scholar]
- 24.Moffett BS, Goldstein SL. Acute kidney injury and increasing nephrotoxic-medication exposure in noncritically-ill children. Clin J Am Soc Nephrol. 2011 Apr;6(4):856–63. doi: 10.2215/CJN.08110910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005 Nov;16(11):3365–70. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.