Abstract
Inflammation-associated pigmentation changes are extremely common, but the etiology behind this clinical observation remains elusive. Particularly, it is unclear how the myriad of cytokines known to be involved in inflammatory skin processes affect epidermal melanocytes. We sought to determine how IL-17 and TNF influence normal human melanocytes, as these two cytokines have been implicated in various skin diseases. IL-17 and TNF jointly stimulated broad inductions of cytokines , including melanoma mitogens CXCL1 and IL-8. Moreover, IL-17 and TNF synergistically inhibited pigmentation-related signaling and melanin production, and induced keratinocytes production of β-defensin 3, an antagonist for melanocortin-receptor 1. When analyzing psoriasis lesions that are known to over express IL-17 and TNF, we observed an increase in melanocyte number and a simultaneous decrease in pigmentation signaling. Furthermore, therapeutic neutralization of TNF and IL-17 with mAbs results in a rapid recovery of pigment gene expression in psoriasis lesions. These results demonstrate that IL-17 and TNF can impact both the growth and pigment production of melanocytes, which may contribute to the pigmentation changes associated with psoriasis. These findings may allow the development of novel therapeutics for pigmentary disorders and bring new insights into the immune milieu surrounding melanocytes and related neoplasms.
Introduction
Psoriasis is an inflammatory skin disease characterized by keratinocytes hyperplasia, epidermal thickness and infiltrations of dermal T-cells and leukocytes. A myriad of inflammatory mediators are overexpressed in psoriasis skin which may contribute to psoriatic skin inflammation, including cytokines such as TNF, IFNγ, IL-17, IL-1α, TGFβ1, IL-22 and IL-6. Among these cytokines, the roles of TNF and IL-17 in psoriasis pathogenesis are best understood (Di Cesare et al., 2009; Krueger et al., 2012). In recent clinical trials, major disease reversal for moderate-to-severe psoriasis was attained using IL-17 inhibitors or IL-17 receptor A blockers (Leonardi et al., 2012; Papp et al., 2012). TNF also plays an important role in the pathogenesis of psoriasis, and TNF inhibitors (e.g. etanercept) have been proven to be highly effective treatments (Gottlieb et al., 2005). The mechanism of action of TNF is thought to involve the inhibition of the Th17 axis (Zaba et al., 2007), suggesting an intricate relationship between these two pathways.
Interestingly, IL-17 and TNF are also known to interplay and drive common molecular pathways. Their synergy has been described in multiple cells types (Iyoda et al., 2010; Koenders et al., 2011; Nonaka et al., 2009; Shen et al., 2006). Although IL-17 can induce pro-inflammatory cytokines by itself, its effects are vastly increased when cooperating with TNF (Liang et al., 2006). Our lab and other groups have previously reported the synergy between IL-17 and TNF in cultured keratinocytes. The two cytokines synergistically enhanced the production of signature molecules for psoriasis disease onset, such as β-defensin 4, S100A7, LCN2 (Chiricozzi et al., 2011; Guilloteau et al., 2010).
In this study, we sought to examine the effects of IL-17 and TNF on epidermal melanocytes, either alone or in combination. Our data revealed a dichotomous effect of IL-17 and TNF on melanocytes, which not only elicit potentially mitogenic cytokines but also suppress melanogenesis by down-regulating genes of the pigmentation pathway. We reasoned that this direct effect of IL-17 and TNF on melanocytes may contribute to the pigmentary disorders frequently associated with skin inflammation, and delineating this process may allow the development of new targeted therapies for pigmentary disorders. Moreover, our understanding of how IL-17 influences melanocytes is limited (Kotobuki et al., 2012), our investigation into the effect of Th17-mediated inflammation on normal melanocytes brings new insights into the complex layers of immune interactions surrounding epidermal melanocytes.
Results
Primary human melanocytes form clusters in culture in response to IL-17 and TNF
To test the effect of IL-17 and TNF, normal human epidermal melanocytes (NHEM) in serum-free media were stimulated with either TNF alone, IL-17 alone, or in combination. At 48 h, treated cells formed clusters, and the most striking clustering effects were observed in cells stimulated with both cytokines (Figure S1 a-d). This clustering effect was not observed in keratinocytes or fibroblasts (data not shown). Melanocyte clustering can be completely reversed 3 days following the removal of TNF and IL-17 in the media (Figure S2). Apoptosis and cell viability assays did not reveal a cytotoxic effect in melanocytes after cytokine treatment.
Microarray profiling of gene expressions confirms IL-17 and TNF responses in melanocytes
cDNA microarray analysis was performed to capture broad transcriptional changes in melanocytes treated with IL-17 and TNF. In a two-dimensional principal component analysis (PCA) plot, the addition of TNF incited major changes in the gene expression profiles of melanocytes (Figure S3). In addition, cells treated with a high dose of IL-17 produced transcriptional profiles (PCA) that were distinct from those treated with a low dose, indicating that the melanocyte response to IL-17 was dose-dependent.
Induction of mitogenic cytokines and growth factors genes
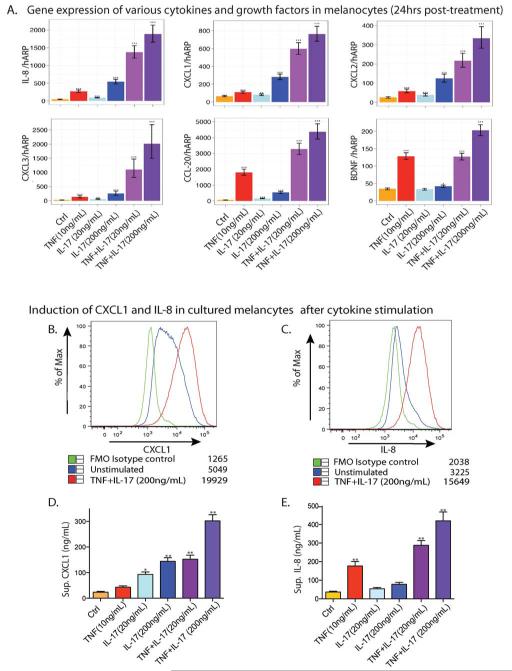
Gene set enrichment analysis (GSEA) of microarray data showed strong positive enrichment of cytokines and growth factors genes that may promote an increase cell number in melanocytes treated with TNF and IL-17 for 24 hours (Figure S4 a-b). Hierarchical clustering of the expression profiles of cytokines and growth factors genes showed most enhanced expressions in cells co-stimulated with TNF and IL-17 (Figure S 4 c-d). Among the induced genes, several are known for their growth promoting effects on melanoma cells and melanocytes (e.g. CXCL1, 2, 3, IL-6, IL-8). Microarray data was validated with qRT-PCR (Figure 1a), demonstrating strong inductions of CXCL1, 2,3, IL-8, BDNF, and CCL20, a Th17 marker gene. Marked synergism was observed in the induction of CXCL1, CXCL2. CXCL3, BDNF and IL-8 after co-stimulation with TNF and IL-17 (Figure S6). Notably, the expression of several cytokine receptors were not seen to be affected, including IL-17RA, IL-17RC, IL22R1, IL-1R1, IL-1R2 etc (data not shown).
Figure 1. IL-17 and TNF synergistically induced cytokines and growth factors in cultured melanocytes.
(a) Cytokines and growth factors inductions in melanocytes treated with IL-17 and/or TNF for 24 h. (b-c) Intracellular cytokine staining shows induction of CXCL1 and IL-8 in melanocytes after treatment with TNF and IL-17 for 24 h. Median fluorescent intensity is indicated next to each condition. (d) CXCL1 secretion was assessed by ELISA after 24 h in culture. (e) IL-8 secretion was assessed by ECL assay after 24 h in culture. (*P<0.05, **P<0.01, vs. Ctrl). Data represent results from three independent cultures using melanocytes from three different skin donors.
Enhanced production of CXCL1 and IL-8
We assessed the level of CXCL1 and IL-8 by FACS-based intracellular cytokine staining and ELISA-based assays. In cells treated with both IL-17 and TNF, we observed over 5-fold increases in the MFI of CXCL1 and IL-8 vs. control (Figure 1b-c). Melanocytes treated with both IL-17 (200ng/mL) and TNF secreted nearly 10 times the amount of CXCL1 and IL-8 into the culture supernatant compared to controls at 24 h (Figure 1d-e). Our data showed that IL-17 is more potent in stimulating CXCL1 production, whereas TNF is more effective in inducing IL-8 production.
Inhibition of pigmentation signaling pathway and melanin production
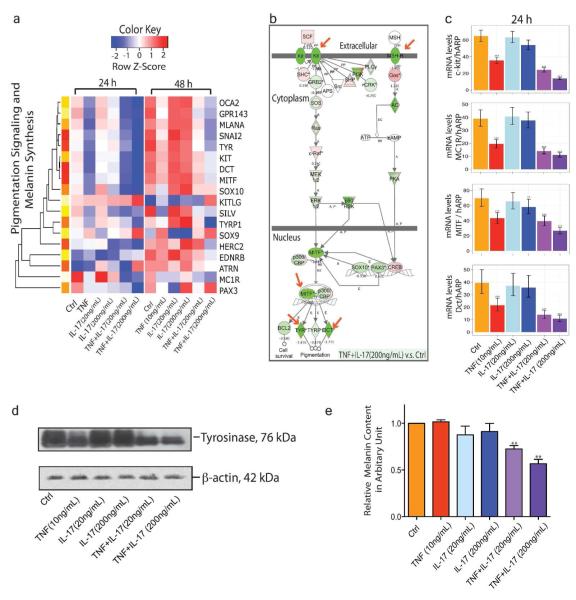
GSEA also showed significant negative enrichment of melanogenesis genes in the expression profiles of melanocytes treated with TNF and IL-17 at 24 h (Figure S5 a&c). Based on recent reviews, a gene set for human skin pigmentation signaling and melanin synthesis was curated (Baxter et al., 2009; Rees and Harding, 2012; Sturm, 2009). Hierarchical clustering of this gene set reveals decreased expression in cells co-stimulated with TNF and IL-17 (Figure 2a). Notable among them are lineage specific melanocyte transcriptional factors such as Mitf, SOX10 and Mitf-regulated genes that code for the rate limiting enzyme Tyr, Tyrp1, and OCA2 which are required for efficient maturation of tyrosinase, and Dct, another catalytic enzyme in melanogenesis. Hierarchical clustering of genes in the category of Melanosome Transport and Uptake also shows decreased expressions in cells co-stimulated with TNF and IL-17 (Figure S5 b&d). In Ingenuity Pathway Analysis (IPA) system, Melanocyte Development and Pigmentation Signaling Pathway was enriched with significant repression for nearly all members of this pathway after treatment with IL-17 and TNF for 24 h (Figure 2b).
Figure 2. Suppression of pigmentation signaling and melanin synthesis after combined IL-17 and TNF treatment in melanocytes.
(a) Hierarchical clusters of genes involved in human skin pigmentation signaling and melanin synthesis. (b) Ingenuity Pathway Analysis reveals global inhibition of Melanocyte Development and Pigmentation Signaling. Arrow indicates genes where the expression was validated by qRT-PCR or western blot. (c) mRNA expression of c-Kit, MC1R, Mitf and Dct in melanocytes treated with both IL-17 and TNF at 24h. (d) Tyrosinase levels in melanocytes after 48 h exposure to IL-17 and TNF (e) Cellular melanin content in melanocytes treated with IL-17 and TNF for 48h. Data show results from three independent cultures using melanocytes derived from different donors (*P < 0.05; **P < 0.01; ***P<0.001, vs. Ctrl)
qRT-PCR for c-kit, MC1R, Mitf and Dct confirmed microarray data (Figure 2c). When melanocytes were treated with IL-17 alone, minimal changes were detected in c-kit, Dct, Mitf expressions. However, 24 h after treatment with IL-17 and TNF, the expression of Dct dropped to less than a quarter of its level in control samples. Marked synergism was observed in the inhibition c-Kit, MC1R, Mitf and Dct after combined treatment of TNF and IL-17 (Figure S6).
Tyrosinase is a melanosome membrane glycoprotein that catalyzes the rate-limiting steps of melanogenesis. A significant decrease in tyrosinase levels as well as in cellular melanin content was detected in melanocytes after 48h exposure to IL-17 and TNF (Figure 2 d&e).
Down-regulation of pigmentation signaling pathway in lesional psoriatic skin
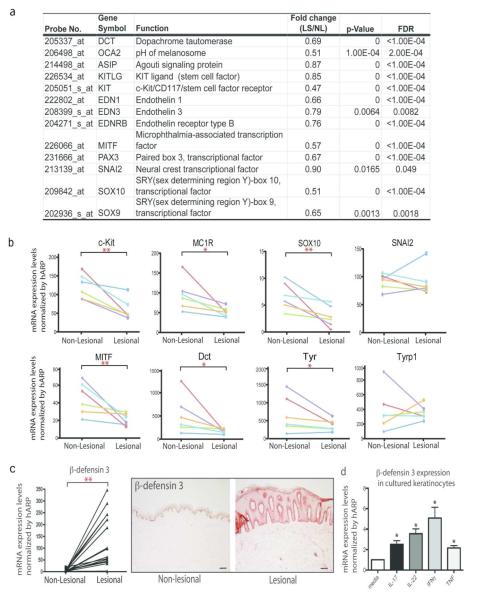
Psoriasis lesions contain high levels of IL-17 and TNF, providing us with a relevant in vivo system to study how skin inflammation can influence melanocyte biology. We accessed a meta-analysis-derived psoriasis transcriptome established by our group (MAD-3), which combined paired sets of lesional vs. non-lesional skin of over 190 patients (Tian et al., 2012). A broad inhibition of pigmentation genes was found in this transcriptome, including cell surface receptors and their ligands, (e.g. Kit Ligand/Stem cell factor, c-Kit, ET-3, ET-1 and EDNR-B), lineage specific melanocyte transcriptional factor (e.g. Mitf, Sox10 and Snai2), as well as catalytic enzymes and melanosome proteins involved in melanin synthesis (e.g. Dct, OCA2) (Figure 3a). This inhibition was confirmed by qRT-PCR using paired lesional and non-lesional skin biopsies (n=6). There was significantly decreased expression of c-kit, MC1R, Mitf, Sox10 (p<0.01), Dct and Tyr (p<0.05) (Figure 3b).
Figure 3. A broad inhibition of pigmentation genes in lesional psoriasis skin.
(a) Decreased expressions of pigmentation genes (paired lesional vs. non-lesional skin) in a meta-analysis derived of transcriptome of over 190 psoriasis patients (P<0.05, FDR<0.05) (b) qRT-PCR analysis confirms suppression of pigmentation genes in paired psoriasis lesional vs. non-lesional skin (n=6). (*P<0.05, *P<0.01). Gene expression changes for each patient were represented by a line with a different color. (c) Increased expression of β-defensin 3 in lesional psoriasis skin, compared to non-lesional skin (n=10). Bar=100μm (d) IL-17 and TNF induces the expression of β-defensin 3, an antagonist for melanocortin-1 receptor, in keratinocytes after 24 h treatment with individual cytokines: IL-17 (200ng/mL), IL-22 (200ng/mL), IFNγ (20ng/mL), and TNF (10ng/mL) (*P < 0.05; **P < 0.01; ***P<0.001, vs. Ctrl).
Induction of β-defensin 3 in cultured keratinocytes after IL-17 or TNF treatment
β-defensin 3 is an anti-microbial peptide that was identified as a novel antagonist for MC1R (melanocortin 1-receptor) (Beaumont et al., 2012; Swope et al., 2012). It is capable of inhibiting α-melanocyte-stimulating hormone (a-MSH)–induced increase in the activities of adenylate cyclase and tyrosinase. Consistent with previous reports (Harder et al., 2001; Harder et al., 2010; Hollox et al., 2008; Peric et al., 2009), we detected over-expression of β-defensin 3 in psoriasis lesional skin (> 150-fold increase in mRNA), and a strong tissue staining (Figure 3c). Keratinocytes are the only source of β-defensin 3 in human skin (Nomura et al., 2003). We observed over two-fold induction of β-defensin 3 mRNA after 24 h treatment with IL-17 or TNF in cultured keratinocytes (Figure 3d). Meanwhile, we did not detect significant changes in the expression levels of POMC (MSH) and ASIP(Agouti), the ligand and the other antagonist of M1CR, respectively.
Increased numbers of melanocytes in psoriasis lesional skin
In a published study of patients with moderate-to-severe psoriasis who were treated with etanercept (anti-TNF) for 12 weeks (Zaba et al., 2007; Zaba et al., 2009), a majority of the patients developed hyper-pigmentation during treatment (Figure 4a). Melan-A staining and cell counting was performed on paired biopsies of 10 patients who had favorable responses to etanercept (non-lesional vs. lesional vs. 12 wks recovery). Lesional psoriasis skin contained almost twice the number of Melan-A+ cells per field compared to non-lesional skin (p <0.001). In some patients, we observed a high density of Melan+ cells embedded at resolved lesions (Figure 4b). Melan-A staining on another set of biopsies showed that psoriatic lesions (n=11) contained nearly three-fold Melan-A+ cells per field (p<0.001) than normal skin from healthy volunteers (n=6) (Figure 4c). An “activated” morphology of melanocytes in psoriasis lesions was observed as the cells appeared dilated, more dendritic and their processes elongated.
Figure 4. Lesional psoriasis skin contained increased number of melanocytes.
(a) A patient treated with etanercept developed post-inflammatory hyper-pigmentation. (b) Melan-A staining on paired psoriasis skin biopsies. Bar chart shows Melan-A+ cell counts. (c) Melan-A staining and cell counts of psoriasis lesional skin and normal skin from healthy volunteers. Right panel: the morphology of melan-A+ cells at psoriasis lesions. Melan-A+ cell counts of psoriasis lesional skin and normal skin from healthy volunteers (***P<0.001). Bar=100μm. (d) Melanocytes containing Ki67+ nuclei were found in lesional psoriasis skin by confocal microscopy. Arrow designates a Ki67+ (green) and MART-1+ (red) melansomes, which can be found either on melanocyte surface or in the cytoplasm. Boxes show images from x-z and y-z axis of selected melanocyte containing a Ki67+ nucleus. Bar=10μm.
Double immunofluorescence staining of Ki67, a cell proliferation marker that stains the nuclei, and MART-1 (alternative symbol: Melan-A), a melanosome surface protein, was performed (n=3). In confocal microscopy, Ki67+ /MART-1+ cells were found in psoriasis lesional skin (Figure 4d), but not in non-lesional skin, nor in resolved lesions after 12 weeks of etanercept treatment.
Recovery of pigmentation signaling in psoriatic lesions after therapeutic neutralization of IL-17 and TNF
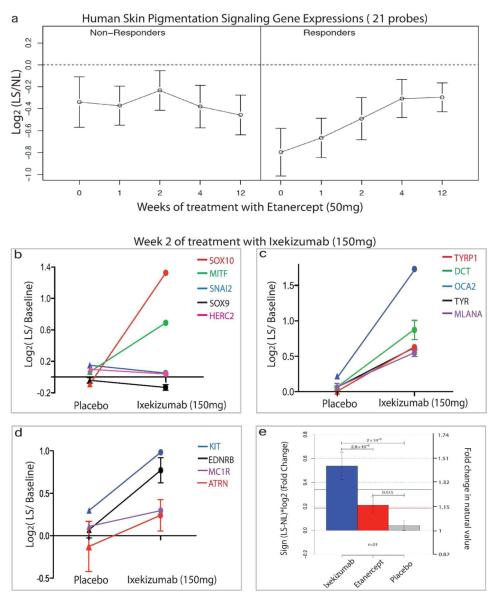
Our group has evaluated the changes in transcriptional profiles of psoriasis lesional skin after treatment with either etanercept (anti-TNF) or ixekinumab (anti-IL-17) (Krueger et al., 2012; Zaba et al., 2009) This data was mined to assess pigmentation gene responses of psoriasis patients during therapy. In the 12-week etanercept study, a group of 15 patients were selected based on sufficient RNA quantity and quality for performing microarrays, which included 11 responders, and 4 non-responders (Zaba et al., 2009). Progressive recovery of pigmentation genes was detected in 11 patients who responded to treatment and showed histological improvement of psoriasis (Figure 5a, Table S1). This recovery was not observed in the lesions of 4 non-responders. The expression levels of pigmentation genes from psoriasis skin lesions in patients treated with ixekizumab (anti-IL-17) were also examined. All patients (n=8) in this trial had successful responses by week 6. Overall, significant increases in the pigmentation genes were observed after 2-week ixekizumab treatment, but not in the placebo group (Figure 5b-d, Table S2). In comparison with etanercept at the same time-point in treatment (week 2), recovery of pigmentation signaling following anti-IL-17 treatment appeared to be more rapid (Figure 5e), which is consistent with a short time course for effective clinical responses for ixekizumab (6 weeks).
Figure 5. Rapid recovery of pigmentation genes in psoriasis lesions after therapeutic neutralization of TNF and IL-17.
(a) Pigmentation gene expressions (21 probes) in lesional skin at weeks 0, 1, 2, 4, and 12 were normalized to non-lesional skin and are shown as average cluster gene expression ±SEM. Progressive recovery of pigmentation genes was observed in patients with good clinical response to etanercept (n=11), but not in non-responders (n=4). (b-d) IL-17 neutralization by ixekizumab results in increase of pigmentation gene expressions in psoriasis lesions compared to placebo. Fold changes in log2, vs. baseline. SEMs are included for genes with multiple cDNA probes. (e) Recovery of pigmentation signaling in psoriasis lesions at week 2 of treatment with either ixekizumab or etanercept (* P<0.05 vs. placebo).
Discussion
Inflammation-associated pigmentary changes are extremely common. They can be triggered by psoriasis, atopic dermatitis, acne vulgaris. Typically, hypo-pigmentation of the lesions accompanies active inflammation, but upon resolution of the inflammatory process, patients are at a high risk of developing hyper-pigmentation (Taylor et al., 2009; Vachiramon and Thadanipon, 2011). One existing hypothesis of hypo-pigmentation associated with inflammation is that edema, or the rapid turnover of keratinocytes during epidermal hyperplasia will interfere with melanosome transfer to keratinocytes (Brenner and Hearing, 2008; Burge et al., 1986), but that does not explain the hyper-pigmentation that frequently occurs with disease resolution. Beyond the case of vitiligo, where an immune response is thought to be responsible for the destruction of normal melanocytes; or mastocytosis (Urticaria pigmentosa) where histamine, leukotrienes and prostaglandins released by mast cells are thought to accelerate melanin transfer and increase pigmentation (Tomita et al., 1989), the etiology behind inflammation-associated pigment disorders remains elusive.
Here, we revealed the synergistic action of IL-17 and TNF in regulating melanin production. We previously reported the presence of Th17 cells in the depigmenting skin of vitiligo (Wang et al., 2011). Additionally, it has been shown that treatment of melanocytes with agents that induce vitiligo in the occupational setting causes increased expression of IL-17 and TNF with a concomitant increase in the expression of cytokines CXCL1, 3, IL-6, IL-8, and CCL20 (Toosi et al., 2012). Kotobuki T. et al reported that a combination of 4 cytokines (i.e. TNF, IL-1β, IL-6 and IL-17) could inhibit melanin production (Kotobuki et al., 2012). However, to our knowledge, the synergy between IL-17 and TNF in regulating pigment production has not been reported. IL-17 can act in concert with a number of cytokines (e.g. IL-1β, TNF, CXCL1) (Shen and Gaffen, 2008). This synergy can be achieved either by activating common transcriptional factors, or by stabilizing the mRNA of other cytokines at a post-transcriptional level (Hartupee et al., 2007; Shen et al., 2006). Our data showed that IL-17 by itself does not inhibit pigmentation signaling, however, IL-17 can dramatically amplify the inhibitory effect of TNF on melanogenesis.
Psoriasis lesions contained high levels of both TNF and IL-17, thus epidermal melanocytes in psoriatic skin are under the influence of at least these two cytokines. Therapeutic blockade of IL-17 in psoriatic skin may therefore alleviate the synergistic inhibition of IL-17 and TNF on melanogenesis, which we observed during ixekizumab (anti-IL-17) treatment. In the etanercept trial, although psoriasis does not improve significantly in “non-responders”, all patients have rapid inhibition of direct TNF-related genes, such as IL-6 and IL-8 (Zaba et al., 2010). For non-responders, TNF was most likely not a primary disease driver, and may not be highly expressed in the psoriatic lesions of these patients. Hence, TNF blockade had very little effect on melanocytes in the skin lesions of non-responders. In contrast, for the 11 responders, blockade of TNF resulted in a quick restoration of pigmentation genes expressions in psoriasis lesions. The rapid reversal of pigmentation signaling indicates the therapeutic potential of anti-IL-17 or anti-TNF for treating pigmentary disorders. However, further prospective studies to monitor changes in skin pigmentation after localized anti-IL-17 or anti-TNF treatment are warranted to investigate this possibility.
Although this study focuses only on TNF and IL-17, pigmentary changes in psoriasis may be modulated by a myriad of inflammatory mediators that are overexpressed in psoriasis lesions, including IFNγ, IL-1α, TGFβ1, IL-22 and IL6. Among these cytokines, several are known to have hypo-pigmenting effects (TNF, IL-6, IL-1α, TGFβ1), and can independently modulate the expression of tyrosinase and related enzymes (e.g. TYRP1 and DCT) (Brenner and Hearing, 2008; Levy et al., 2006; Martinez-Esparza et al., 2001; Martinez-Esparza et al., 1999; Swope et al., 1991; Yang et al., 2008). Our study also focused on the direct effect of IL-17 and TNF on melanocytes. However, during psoriatic inflammation, melanocytes are also affected by keratinocytes-derived secretary products. For instance, IL-17 can induce several hypo-pigmenting cytokines in keratinocytes (e.g. TNF, IL-6, IL-1β etc), as well as endothelin-1, CXCL1, IL-8, which have growth stimulating properties. How keratinocytes-derived inflammatory products influence melanocyte is an important aspect of the biology, which we would like to focus on in future studies.
The observation of increased melanocytes numbers in psoriasis lesions was unexpected, since the prevailing dogma suggests that epidermal melanocytes in adult skin are quiescent cells and their numbers stay unchanged despite alterations in skin color. Hyper-pigmented skin lesions at sites of inflammation are thought to have no increase in melanocyte number, though the cells may produce higher amounts of eumelanin (Brenner and Hearing, 2008). We observed increased density of melanocytes at the basal layer of the epidermis during psoriasis resolution, which highly resembled the pathology of Lentigo (Figure 4b). Increased number of melanocytes, combined with a rapid recovery of pigmentation function during psoriasis resolution, may lead to an abundant production of melanin, which will persist in keratinocytes during treatment until the pigmented keratinocytes are shed. Hence, a period of post-inflammatory hyper-pigmentation may persist for weeks to months, even as melanocyte number returns to baseline as lesions resolve. This was observed in the majority of patients treated with etanercept. Our data showed IL-17 and TNF induced an array of melanoma and melanocyte mitogens, including CXCL1, IL-8, IL-6, CXCL2, CXCL3, BDNF (Crawford et al., 2008; Haghnegahdar et al., 2000; Mockenhaupt et al., 2003; Norgauer et al., 2003; Schadendorf et al., 1993; Scheibenbogen et al., 1995; Singh et al., 1994; Singh and Varney, 2000; Wang et al., 2000), which may contribute to a proliferative milieu that supports melanocyte growth (Figure 6). Neighboring keratinocytes are known to produce growth stimulators (e.g. Endothelin-1, CXCL1, IL-6 and IL-8) upon IL-17 and TNF stimulation (Nograles et al., 2008; Venner et al., 1995).
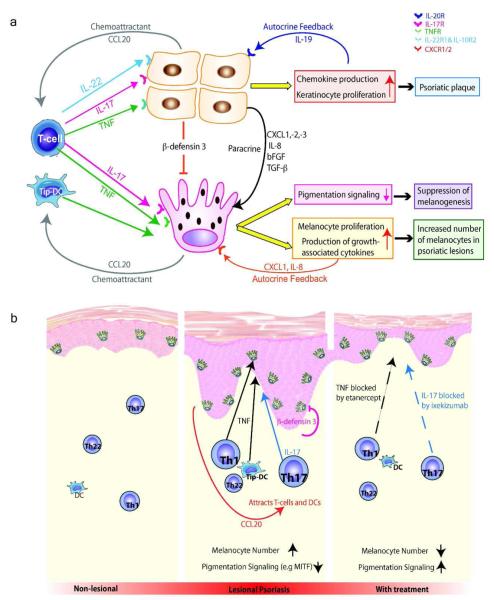
Figure 6. Proposed model of IL-17 and TNF mediated interactions between T-cells, dendritic cell, keratinocytes and melanocytes during skin inflammation.
(a) IL-17 secreted by skin Th17 cells, together with TNF secreted by T cells, dendritic cells, can jointly induce mitogenic cytokines in both keratinocytes (KC) and melanocytes (MC), creating a milieu that supports cell proliferation. This leads to an increase in melanocytes numbers at psoriasis lesions. This is accompanied by a synergistic suppression of pigmentation signaling and melanin synthesis. (b) Therapeutic neutralization by monoclonal antibodies can lead to a rapid restoration of pigment function within 2-4 weeks. An elevated number of melanocytes in lesional skin will be producing abundant melanin, which persists in keratinocytes during early phases of clinical improvement, leading to post-inflammatory hyper-pigmentation.
In conclusion, we demonstrate that TNF and IL-17 contribute to reduction of melanogenesis in psoriasis lesions. Our data further suggests that these two cytokines modulate signaling that impacts melanocytes proliferation. Our investigation into the effect of Th17-mediated inflammation on normal human epidermal melanocytes may thus help unravel the complex layers of immune interactions surrounding melanocytes or melanoma, especially since the pro-tumor or anti-tumor role of IL-17 is still debated, and opposing data have been reported in both mice and human studies (Kryczek et al., 2009; Martin-Orozco et al., 2009; Muranski et al., 2008). The presence of Ki67+ melanocytes in psoriasis lesions suggests that immune infiltration surrounding nevi or melanoma might be a double-edged sword, where cytokines such as TNF and IL-17 may contribute to excess melanocyte proliferation, while associated T-cells also provide immune surveillance for melanoma.
Materials and Methods
Subjects and Skin Samples
Skin punch biopsies (6 mm diameter) were obtained from normal volunteers and psoriasis patients under a Rockefeller University Institutional Review Board approved protocol. Written informed consent was obtained for all studies, and performed in adherence with the Declaration of Helsinki Principles. See Supplementary Methods for details.
Reagents
Table S3 and S4 in Supplementary Material lists the sources of primers, antibodies and their concentrations for each experiment.
Immunohistochemistry
Primary antibodies for immunohistochemistry are listed in Table S4. Standard procedures were employed as previously described (Fuentes-Duculan et al., 2010).
Immunofluorescence
Primary antibodies used for staining were listed in Table S4. See Supplementary Methods for details.
Primary Cell Culture
Cryopreserved normal human epidermal melanocytes (NHEM) isolated from juvenile foreskin are purchased from PromoCell (Catalog #. C-12402, Heidelberg, Germany). Primary pooled human keratinocytes were obtained from Yale Skin Diseases Research Center core facility. See Supplementary Methods for details.
Microarray Analysis
Total RNA samples from human melanocytes were hybridized to Affymetrix Gene Chip Human Genome U133Plus2. (Affymetrix, Santa Clara, CA). Raw data have been deposited in the NCBI’s Gene Expression Omnibus repository and are accessible through accession number GSE40413. See Supplementary Methods for details.
Real-time reverse-transcription PCR (qRT-PCR)
qRT-PCR was performed using Taqman gene expression assay, as previously published (Chamian et al., 2005). Target gene expressions were normalized against the house keeping gene hARP. Primers are listed in Table S3.
FACS analysis
Cells were collected 24 h after cytokine stimulation, and were incubated with Brefeldin-A (10μg/mL) for 3 hours prior to harvest, and stained with the antibodies listed in Table S4. See Supplementary Methods for details.
ELISA and Electrochemiluminescent Assay
ELISA: Quantikine® human CXCL1/GROα kit was used to quantify CXCL1 level in melanocyte culture supernatant (Catalog # DGR00, R&D systems). ELISA-based ECL assay: Human IL-8 Ultra-Sensitive Kit was used to quantify IL-8 in melanocyte culture supernatant (Catalog # K151ANC-1, Meso Scale Diagnostics, Gaithersburg MD).
Western Blot
Standard procedures were used as previously described (Toosi et al., 2012).
Melanin Content Assay
Total cellular melanin content was determined as previously described (Manga and Orlow, 2001).
Statistical Analysis
Data presented as mean±SD and assessed using repeated measures ANOVA. See Supplementary Method for details.
Supplementary Material
Figure S1: IL-17 and TNF induced dramatic morphological changes in cultured normal human melanocytes.
IL-17 and TNF induced dramatic morphological changes in cultured normal human melanocytes. Primary human melanocytes aggregated and formed large clusters over 48 hours in response to cytokine treatment (a) Ctrl; (b) TNF alone (10ng/mL); (c) IL-17 alone (200ng/mL); (d) IL-17 (200ng/mL)+ TNF (10ng/mL). The formation of cell aggregates is most pronounced when melanocytes were stimulated with both cytokines. Bar=100μm.
Figure S2: The clustering of melanocytes disappears after removal of TNF and IL-17 in culture media.
The clustering of melanocytes disappears after removal of TNF and IL-17 in culture media. (a) Primary human melanocytes formed large clusters after 48 h in exposure to IL-17 (200ng/mL) and TNF (10ng/mL). (b-d) Melanocyte clusters gradually disappeared after switching to regular M2 media (Promocell, Germany) without IL-17 or TNF. 72 h after removal of cytokines in media, no clustering was observed in cells previous treated with IL-17 and/or TNF. (e) Melanocytes continued to proliferate in subcultures without forming clusters.Bar=100μm.
Figure S3: Principal Component Plot of Melanocytes Stimulated with IL-17 and TNF
Principal Component Plot of Melanocytes Stimulated with IL-17 and TNF (a) Two-dimensional principal component analysis (PCA) of cDNA microarray data of melanocytes treated in 6 different conditions for 24 hours and 48 hours (n=1). Over 70% of the variance in gene expression data sets were accounted for by the first two components: PC1 (52.2%) and PC2 (18.7%). The addition of TNF (10ng/mL) incited major shift in transcriptional profile of primary human melanocytes on the PC1 axis. Melanocytes treated with a high dose of IL-17 (200ng/mL) also produced distinct dataset from low dose IL-17 treatment (20ng/mL).
Figure S4: Cytokine and growth factor inductions in cultured melanocytes after TNF and IL-17 treatment
Cytokine and growth factor inductions in cultured melanocytes after TNF and IL-17 treatment. (a-b) GSEA plots of melanocytes treated with IL-17 (200ng/mL) and TNF (10ng/mL) for 24 hours revealed strong positive enrichment of cytokine and growth factor genes. (c-d) Hierarchical clustering of cytokine and growth factor genes show enhanced expression levels in melanocytes treated with both IL-17 and TNF. Gene set belongs to gene ontology/molecular function.
Figure S5: Suppression of melanogenesis in cultured melanocytes after TNF and IL-17 treatment.
Suppression of melanogenesis in cultured melanocytes after TNF and IL-17 treatment. (a) GSEA plot of the expression profiles of melanocytes treated with IL-17 (200ng/mL) and TNF (10ng/mL) for 24 h shows negative enrichment of melanogenesis genes (KEGG) and (b) genes related to melanosome transport and uptake (curated based on recent reviews). In hierarchical clusters of genes involved in melanogenesis (c) or melanosome transport and uptake (d), The most marked decreases in gene expression was observed in cells treated with both IL-17 and TNF. Gene symbols were shown on the right.
Figure S6: Genes that are synergistically induced by IL-17 and TNF treatment.
Genes that are synergistically induced by IL-17 and TNF treatment. (a) Average fold changes in the mRNA expression levels of key pigmentation genes and cytokines/growth factors. Fold changes (FCH) are presented in log2 values. (b) Comparisons of the sum of single cytokine effects vs. combined cytokine effects. The induction of a gene is defined as synergistic when the log2-FCH of the combined stimulus is greater than the sum of the log2-FCH of each stimuli individually. (* indicates synergistic induction of gene expressions)
Table S1: Fold changes (log2) of pigmentation gene expression in lesional skin of psoriasis patients treated with etanercept vs. paired non-lesional skin (week 0 to week 12)
Table S2: Fold changes of pigmentation genes expressions in lesional skin of psoriasis patients treated with ixekinumab at week 2 vs. baseline.
Table S3. Primers for qRT-PCR (Applied Biosystems)
Table S4. Antibodies and their working condition
ACKNOWLEGEMENTS
The Milstein Medical Program provided the major support for this study. Research was also supported by a Clinical and Translational Science Award grant UL1RR024143 (JGK); NIH/NIAMS grant AR41880 (SJO); grant AR060222 (MAL) and a pilot project grant from New York University School of Medicine (SJO&PM). We thank Mr. Shivaprasad Bhuvanendran and Dr. Alison North from the RU Bio-Imaging Resource Center for technical advice, and Dr. Kristine E. Nograles for technical assistance.
Abbreviations
- GSEA
Gene set enrichment analysis
- IL-17
Interleukin-17A
- TNF
Tumor Necrosis Factor α
- Th17
T helper 17 cells
Footnotes
CONFLICT OF INTEREST The authors do not have any conflict of interest.
REFERENCES
- Baxter LL, Loftus SK, Pavan WJ. Networks and pathways in pigmentation, health, and disease. Wiley Interdiscip Rev Syst Biol Med. 2009;1:359–371. doi: 10.1002/wsbm.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont KA, Smit DJ, Liu YY, Chai E, Patel MP, Millhauser GL, et al. Melanocortin-1 receptor-mediated signalling pathways activated by NDP-MSH and HBD3 ligands. Pigment Cell Melanoma Res. 2012;25:370–374. doi: 10.1111/j.1755-148X.2012.00990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner M, Hearing VJ. Modifying skin pigmentation - approaches through intrinsic biochemistry and exogenous agents. Drug Discov Today Dis Mech. 2008;5:e189–e199. doi: 10.1016/j.ddmec.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge SM, Bristol M, Millard PR, Dawber RP. Pigment changes in human skin after cryotherapy. Cryobiology. 1986;23:422–432. doi: 10.1016/0011-2240(86)90027-1. [DOI] [PubMed] [Google Scholar]
- Chamian F, Lowes MA, Lin SL, Lee E, Kikuchi T, Gilleaudeau P, et al. Alefacept reduces infiltrating T cells, activated dendritic cells, and inflammatory genes in psoriasis vulgaris. Proc Natl Acad Sci U S A. 2005;102:2075–2080. doi: 10.1073/pnas.0409569102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiricozzi A, Guttman-Yassky E, Suarez-Farinas M, Nograles KE, Tian S, Cardinale I, et al. Integrative responses to IL-17 and TNF-α in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J Invest Dermatol. 2011;131:677–687. doi: 10.1038/jid.2010.340. [DOI] [PubMed] [Google Scholar]
- Crawford S, Belajic D, Wei J, Riley JP, Dunford PJ, Bembenek S, et al. A novel B-RAF inhibitor blocks interleukin-8 (IL-8) synthesis in human melanoma xenografts, revealing IL-8 as a potential pharmacodynamic biomarker. Mol Cancer Ther. 2008;7:492–499. doi: 10.1158/1535-7163.MCT-07-0307. [DOI] [PubMed] [Google Scholar]
- Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol. 2009;129:1339–1350. doi: 10.1038/jid.2009.59. [DOI] [PubMed] [Google Scholar]
- Fuentes-Duculan J, Suarez-Farinas M, Zaba LC, Nograles KE, Pierson KC, Mitsui H, et al. A subpopulation of CD163-positive macrophages is classically activated in psoriasis. J Invest Dermatol. 2010;130:2412–2422. doi: 10.1038/jid.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb AB, Chamian F, Masud S, Cardinale I, Abello MV, Lowes MA, et al. TNF inhibition rapidly down-regulates multiple proinflammatory pathways in psoriasis plaques. J Immunol. 2005;175:2721–2729. doi: 10.4049/jimmunol.175.4.2721. [DOI] [PubMed] [Google Scholar]
- Guilloteau K, Paris I, Pedretti N, Boniface K, Juchaux F, Huguier V, et al. Skin Inflammation Induced by the Synergistic Action of IL-17A, IL-22, Oncostatin M, IL-1 α, and TNFα Recapitulates Some Features of Psoriasis. J Immunol. 2010;184:5263–5270. doi: 10.4049/jimmunol.0902464. [DOI] [PubMed] [Google Scholar]
- Haghnegahdar H, Du J, Wang D, Strieter RM, Burdick MD, Nanney LB, et al. The tumorigenic and angiogenic effects of MGSA/GRO proteins in melanoma. J Leukoc Biol. 2000;67:53–62. doi: 10.1002/jlb.67.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder J, Bartels J, Christophers E, Schroder JM. Isolation and characterization of human beta -defensin-3, a novel human inducible peptide antibiotic. J Biol Chem. 2001;276:5707–5713. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- Harder J, Dressel S, Wittersheim M, Cordes J, Meyer-Hoffert U, Mrowietz U, et al. Enhanced expression and secretion of antimicrobial peptides in atopic dermatitis and after superficial skin injury. J Invest Dermatol. 2010;130:1355–1364. doi: 10.1038/jid.2009.432. [DOI] [PubMed] [Google Scholar]
- Hartupee J, Liu C, Novotny M, Li X, Hamilton T. IL-17 enhances chemokine gene expression through mRNA stabilization. J Immunol. 2007;179:4135–4141. doi: 10.4049/jimmunol.179.6.4135. [DOI] [PubMed] [Google Scholar]
- Hollox EJ, Huffmeier U, Zeeuwen PL, Palla R, Lascorz J, Rodijk-Olthuis D, et al. Psoriasis is associated with increased beta-defensin genomic copy number. Nat Genet. 2008;40:23–25. doi: 10.1038/ng.2007.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyoda M, Shibata T, Kawaguchi M, Hizawa N, Yamaoka T, Kokubu F, et al. IL-17A and IL-17F stimulate chemokines via MAPK pathways (ERK1/2 and p38 but not JNK) in mouse cultured mesangial cells: synergy with TNF-alpha and IL-1beta. Am J Physiol Renal Physiol. 2010;298:F779–787. doi: 10.1152/ajprenal.00198.2009. [DOI] [PubMed] [Google Scholar]
- Koenders MI, Marijnissen RJ, Devesa I, Lubberts E, Joosten LA, Roth J, et al. Tumor necrosis factor-interleukin-17 interplay induces S100A8, interleukin-1beta, and matrix metalloproteinases, and drives irreversible cartilage destruction in murine arthritis: rationale for combination treatment during arthritis. Arthritis Rheum. 2011;63:2329–2339. doi: 10.1002/art.30418. [DOI] [PubMed] [Google Scholar]
- Kotobuki Y, Tanemura A, Yang L, Itoi S, Wataya-Kaneda M, Murota H, et al. Dysregulation of melanocyte function by Th17-related cytokines: significance of Th17 cell infiltration in autoimmune vitiligo vulgaris. Pigment Cell Melanoma Res. 2012;25:219–230. doi: 10.1111/j.1755-148X.2011.00945.x. [DOI] [PubMed] [Google Scholar]
- Krueger JG, Fretzin S, Suarez-Farinas M, Haslett PA, Phipps KM, Cameron GS, et al. IL-17A is essential for cell activation and inflammatory gene circuits in subjects with psoriasis. J Allergy Clin Immunol. 2012;130:145–154. e149. doi: 10.1016/j.jaci.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczek I, Wei S, Szeliga W, Vatan L, Zou W. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood. 2009;114:357–359. doi: 10.1182/blood-2008-09-177360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi C, Matheson R, Zachariae C, Cameron G, Li L, Edson-Heredia E, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190–1199. doi: 10.1056/NEJMoa1109997. [DOI] [PubMed] [Google Scholar]
- Levy C, Khaled M, Fisher DE. MITF: master regulator of melanocyte development and melanoma oncogene. Trends Mol Med. 2006;12:406–414. doi: 10.1016/j.molmed.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manga P, Orlow SJ. Inverse correlation between pink-eyed dilution protein expression and induction of melanogenesis by bafilomycin A1. Pigment Cell Res. 2001;14:362–367. doi: 10.1034/j.1600-0749.2001.140508.x. [DOI] [PubMed] [Google Scholar]
- Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31:787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Esparza M, Ferrer C, Castells MT, Garcia-Borron JC, Zuasti A. Transforming growth factor β 1 mediates hypopigmentation of B16 mouse melanoma cells by inhibition of melanin formation and melanosome maturation. Int J Biochem Cell Biol. 2001;33:971–983. doi: 10.1016/s1357-2725(01)00068-1. [DOI] [PubMed] [Google Scholar]
- Martinez-Esparza M, Solano F, Garcia-Borron JC. Independent regulation of tyrosinase by the hypopigmenting cytokines TGF β 1 and TNF α and the melanogenic hormone αMSH in B16 mouse melanocytes. Cell Mol Biol (Noisy-le-grand) 1999;45:991–1000. [PubMed] [Google Scholar]
- Mockenhaupt M, Peters F, Schwenk-Davoine I, Herouy Y, Schraufstatter I, Elsner P, et al. Evidence of involvement of CXC-chemokines in proliferation of cultivated human melanocytes. Int J Mol Med. 2003;12:597–601. [PubMed] [Google Scholar]
- Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nograles KE, Zaba LC, Guttman-Yassky E, Fuentes-Duculan J, Suarez-Farinas M, Cardinale I, et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol. 2008;159:1092–1102. doi: 10.1111/j.1365-2133.2008.08769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura I, Goleva E, Howell MD, Hamid QA, Ong PY, Hall CF, et al. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol. 2003;171:3262–3269. doi: 10.4049/jimmunol.171.6.3262. [DOI] [PubMed] [Google Scholar]
- Nonaka M, Ogihara N, Fukumoto A, Sakanushi A, Kusama K, Pawankar R, et al. Synergistic Induction of Macrophage Inflammatory Protein-3alpha;/CCL20 Production by Interleukin-17A and Tumor Necrosis Factor-alpha; in Nasal Polyp Fibroblasts. World Allergy Organ J. 2009;2:218–223. doi: 10.1097/WOX.0b013e3181bdd219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgauer J, Dichmann S, Peters F, Mockenhaupt M, Schraufst tter I, Herouy Y. Tumor necrosis factor alpha induces upregulation of CXC-chemokine receptor type II expression and magnifies the proliferative activity of CXC-chemokines in human melanocytes. Eur J Dermatol. 2003;13:124–129. [PubMed] [Google Scholar]
- Papp KA, Leonardi C, Menter A, Ortonne JP, Krueger JG, Kricorian G, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366:1181–1189. doi: 10.1056/NEJMoa1109017. [DOI] [PubMed] [Google Scholar]
- Peric M, Koglin S, Dombrowski Y, Gross K, Bradac E, Buchau A, et al. Vitamin D analogs differentially control antimicrobial peptide/“alarmin” expression in psoriasis. PLoS One. 2009;4:e6340. doi: 10.1371/journal.pone.0006340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees JL, Harding RM. Understanding the evolution of human pigmentation: recent contributions from population genetics. J Invest Dermatol. 2012;132:846–853. doi: 10.1038/jid.2011.358. [DOI] [PubMed] [Google Scholar]
- Schadendorf D, Moller A, Algermissen B, Worm M, Sticherling M, Czarnetzki BM. IL-8 produced by human malignant melanoma cells in vitro is an essential autocrine growth factor. J Immunol. 1993;151:2667–2675. [PubMed] [Google Scholar]
- Scheibenbogen C, Mohler T, Haefele J, Hunstein W, Keilholz U. Serum interleukin-8 (IL-8) is elevated in patients with metastatic melanoma and correlates with tumour load. Melanoma Res. 1995;5:179–181. doi: 10.1097/00008390-199506000-00006. [DOI] [PubMed] [Google Scholar]
- Shen F, Gaffen SL. Structure-function relationships in the IL-17 receptor: implications for signal transduction and therapy. Cytokine. 2008;41:92–104. doi: 10.1016/j.cyto.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen F, Hu Z, Goswami J, Gaffen SL. Identification of common transcriptional regulatory elements in interleukin-17 target genes. J Biol Chem. 2006;281:24138–24148. doi: 10.1074/jbc.M604597200. [DOI] [PubMed] [Google Scholar]
- Singh RK, Gutman M, Radinsky R, Bucana CD, Fidler IJ. Expression of interleukin 8 correlates with the metastatic potential of human melanoma cells in nude mice. Cancer Res. 1994;54:3242–3247. [PubMed] [Google Scholar]
- Singh RK, Varney ML. IL-8 expression in malignant melanoma: implications in growth and metastasis. Histol Histopathol. 2000;15:843–849. doi: 10.14670/HH-15.843. [DOI] [PubMed] [Google Scholar]
- Sturm RA. Molecular genetics of human pigmentation diversity. Hum Mol Genet. 2009;18:R9–17. doi: 10.1093/hmg/ddp003. [DOI] [PubMed] [Google Scholar]
- Swope VB, Abdel-Malek Z, Kassem LM, Nordlund JJ. Interleukins 1 alpha and 6 and tumor necrosis factor-alpha are paracrine inhibitors of human melanocyte proliferation and melanogenesis. J Invest Dermatol. 1991;96:180–185. doi: 10.1111/1523-1747.ep12460991. [DOI] [PubMed] [Google Scholar]
- Swope VB, Jameson JA, McFarland KL, Supp DM, Miller WE, McGraw DW, et al. Defining MC1R Regulation in Human Melanocytes by Its Agonist α Melanocortin and Antagonists Agouti Signaling Protein and β-Defensin 3. J Invest Dermatol. 2012;132:2255–2262. doi: 10.1038/jid.2012.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S, Grimes P, Lim J, Im S, Lui H. Postinflammatory hyperpigmentation. J Cutan Med Surg. 2009;13:183–191. doi: 10.2310/7750.2009.08077. [DOI] [PubMed] [Google Scholar]
- Tian S, Krueger JG, Li K, Jabbari A, Brodmerkel C, Lowes MA, et al. Meta-Analysis derived (MAD) transcriptome of psoriasis defines the “core” pathogenesis of disease. PLoS One. 2012;7(9):e44274. doi: 10.1371/journal.pone.0044274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita Y, Maeda K, Tagami H. Mechanisms for hyperpigmentation in postinflammatory pigmentation, urticaria pigmentosa and sunburn. Dermatologica. 1989;179(Suppl 1):49–53. doi: 10.1159/000248449. [DOI] [PubMed] [Google Scholar]
- Toosi S, Orlow SJ, Manga P. Vitiligo Inducing Phenols Activate the Unfolded Protein Response in Melanocytes Resulting in Upregulation of IL6 and IL8. J Invest Dermatol. 2012 doi: 10.1038/jid.2012.181. In press. doi: 10.1038/jid.2012.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vachiramon V, Thadanipon K. Postinflammatory hypopigmentation. Clin Exp Dermatol. 2011;36:708–714. doi: 10.1111/j.1365-2230.2011.04088.x. [DOI] [PubMed] [Google Scholar]
- Venner TJ, Sauder DN, Feliciani C, McKenzie RC. Interleukin-8 and melanoma growth-stimulating activity (GRO) are induced by ultraviolet B radiation in human keratinocyte cell lines. Exp Dermatol. 1995;4:138–145. doi: 10.1111/j.1600-0625.1995.tb00237.x. [DOI] [PubMed] [Google Scholar]
- Wang CQ, Cruz-Inigo AE, Fuentes-Duculan J, Moussai D, Gulati N, Sullivan-Whalen M, et al. Th17 cells and activated dendritic cells are increased in vitiligo lesions. PLoS One. 2011;6:e18907. doi: 10.1371/journal.pone.0018907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Yang W, Du J, Devalaraja MN, Liang P, Matsumoto K, et al. MGSA/GRO-mediated melanocyte transformation involves induction of Ras expression. Oncogene. 2000;19:4647–4659. doi: 10.1038/sj.onc.1203820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Li Y, Nishimura EK, Xin H, Zhou A, Guo Y, et al. Inhibition of PAX3 by TGF-β modulates melanocyte viability. Mol Cell. 2008;32:554–563. doi: 10.1016/j.molcel.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Zaba LC, Cardinale I, Gilleaudeau P, Sullivan-Whalen M, Suarez-Farinas M, Fuentes-Duculan J, et al. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J Exp Med. 2007;204:3183–3194. doi: 10.1084/jem.20071094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaba LC, Fuentes-Duculan J, Eungdamrong NJ, Johnson-Huang LM, Nograles KE, White TR, et al. Identification of TNF-related apoptosis-inducing ligand and other molecules that distinguish inflammatory from resident dendritic cells in patients with psoriasis. J Allergy Clin Immunol. 2010;125:1261–1268. e1269. doi: 10.1016/j.jaci.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaba LC, Suarez-Farinas M, Fuentes-Duculan J, Nograles KE, Guttman-Yassky E, Cardinale I, et al. Effective treatment of psoriasis with etanercept is linked to suppression of IL-17 signaling, not immediate response TNF genes. J Allergy Clin Immunol. 2009;124:1022–1010. e1021–1395. doi: 10.1016/j.jaci.2009.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: IL-17 and TNF induced dramatic morphological changes in cultured normal human melanocytes.
IL-17 and TNF induced dramatic morphological changes in cultured normal human melanocytes. Primary human melanocytes aggregated and formed large clusters over 48 hours in response to cytokine treatment (a) Ctrl; (b) TNF alone (10ng/mL); (c) IL-17 alone (200ng/mL); (d) IL-17 (200ng/mL)+ TNF (10ng/mL). The formation of cell aggregates is most pronounced when melanocytes were stimulated with both cytokines. Bar=100μm.
Figure S2: The clustering of melanocytes disappears after removal of TNF and IL-17 in culture media.
The clustering of melanocytes disappears after removal of TNF and IL-17 in culture media. (a) Primary human melanocytes formed large clusters after 48 h in exposure to IL-17 (200ng/mL) and TNF (10ng/mL). (b-d) Melanocyte clusters gradually disappeared after switching to regular M2 media (Promocell, Germany) without IL-17 or TNF. 72 h after removal of cytokines in media, no clustering was observed in cells previous treated with IL-17 and/or TNF. (e) Melanocytes continued to proliferate in subcultures without forming clusters.Bar=100μm.
Figure S3: Principal Component Plot of Melanocytes Stimulated with IL-17 and TNF
Principal Component Plot of Melanocytes Stimulated with IL-17 and TNF (a) Two-dimensional principal component analysis (PCA) of cDNA microarray data of melanocytes treated in 6 different conditions for 24 hours and 48 hours (n=1). Over 70% of the variance in gene expression data sets were accounted for by the first two components: PC1 (52.2%) and PC2 (18.7%). The addition of TNF (10ng/mL) incited major shift in transcriptional profile of primary human melanocytes on the PC1 axis. Melanocytes treated with a high dose of IL-17 (200ng/mL) also produced distinct dataset from low dose IL-17 treatment (20ng/mL).
Figure S4: Cytokine and growth factor inductions in cultured melanocytes after TNF and IL-17 treatment
Cytokine and growth factor inductions in cultured melanocytes after TNF and IL-17 treatment. (a-b) GSEA plots of melanocytes treated with IL-17 (200ng/mL) and TNF (10ng/mL) for 24 hours revealed strong positive enrichment of cytokine and growth factor genes. (c-d) Hierarchical clustering of cytokine and growth factor genes show enhanced expression levels in melanocytes treated with both IL-17 and TNF. Gene set belongs to gene ontology/molecular function.
Figure S5: Suppression of melanogenesis in cultured melanocytes after TNF and IL-17 treatment.
Suppression of melanogenesis in cultured melanocytes after TNF and IL-17 treatment. (a) GSEA plot of the expression profiles of melanocytes treated with IL-17 (200ng/mL) and TNF (10ng/mL) for 24 h shows negative enrichment of melanogenesis genes (KEGG) and (b) genes related to melanosome transport and uptake (curated based on recent reviews). In hierarchical clusters of genes involved in melanogenesis (c) or melanosome transport and uptake (d), The most marked decreases in gene expression was observed in cells treated with both IL-17 and TNF. Gene symbols were shown on the right.
Figure S6: Genes that are synergistically induced by IL-17 and TNF treatment.
Genes that are synergistically induced by IL-17 and TNF treatment. (a) Average fold changes in the mRNA expression levels of key pigmentation genes and cytokines/growth factors. Fold changes (FCH) are presented in log2 values. (b) Comparisons of the sum of single cytokine effects vs. combined cytokine effects. The induction of a gene is defined as synergistic when the log2-FCH of the combined stimulus is greater than the sum of the log2-FCH of each stimuli individually. (* indicates synergistic induction of gene expressions)
Table S1: Fold changes (log2) of pigmentation gene expression in lesional skin of psoriasis patients treated with etanercept vs. paired non-lesional skin (week 0 to week 12)
Table S2: Fold changes of pigmentation genes expressions in lesional skin of psoriasis patients treated with ixekinumab at week 2 vs. baseline.
Table S3. Primers for qRT-PCR (Applied Biosystems)
Table S4. Antibodies and their working condition