Abstract
Atopic dermatitis is a chronic pruritic inflammatory skin disease. We recently described an animal model in which repeated epicutaneous applications of a house dust mite extract and staphylococcal enterotoxin B induced eczematous skin lesions. In this study we showed that global gene expression patterns are very similar between human atopic dermatitis skin and allergen/staphylococcal enterotoxin B-induced mouse skin lesions, particularly in expression of genes related to epidermal growth/differentiation, skin-barrier, lipid/energy metabolism, immune response, or extracellular matrix. In this model, mast cells and T cells, but not B cells or eosinophils, were shown to be required for the full expression of dermatitis, as revealed by reduced skin inflammation and reduced serum IgE levels in mice lacking mast cells or T cells (TCRβ−/− or Rag1−/−). The clinical severity of dermatitis correlated with the numbers of mast cells, but not eosinophils. Consistent with the idea that Th2 cells play a predominant role in allergic diseases, the receptor for the Th2-promoting cytokine thymic stromal lymphopoietin and the high-affinity IgE receptor, FcεRI, were required to attain maximal clinical scores. Therefore, this clinically relevant model provides mechanistic insights into the pathogenic mechanism of human atopic dermatitis.
Keywords: atopic dermatitis, T cell, mast cell, house dust mite, staphylococcal enterotoxin B, FcεRI, TSLP, GM-CSF
Introduction
Atopic dermatitis (AD), or eczema, is a chronic or chronically relapsing, pruritic inflammatory skin disease. The etiology of this disease is incompletely understood, but it is multifactorial and the disease is manifested by complex interactions between genetic and environmental factors (Bieber, 2008; Boguniewicz and Leung, 2011). Pathological examination reveals hyperkeratosis, spongiosis, and parakeratosis in acute lesions and marked epidermal hyperplasia and perivascular accumulation of lymphocytes and mast cells in chronic lesions. Immunological abnormalities of AD are characterized by sensitization with various allergens (e.g., foods, aeroallergens, microbes, and autoallergens), high serum IgE levels, and skin lesions with apoptotic keratinocytes and infiltration with immune cells such as CD4+ T cells, eosinophils, and mast cells. These T cells express IL-4, IL-5, and IL-13 (Grewe et al., 1998), and numerous studies suggest an association between AD development and T helper 2 (Th2) cell skewed immune responses. However, there are also data suggesting that AD development is independent of IgE, but correlates with an increase in interferon (IFN)-γ-producing Th1 cells (Thepen et al., 1996; Tsicopoulos et al., 1994; Werfel et al., 1996). Thus, as Irvine et al have stated, AD was considered for many years to be primarily immunologically driven disease with secondary barrier defect (the so-called insideoutside hypothesis). By contrast, some investigators had hypothesized that the primary defect is in the skin barrier (the outside-inside hypothesis) (Irvine et al., 2011). Various loss-of-function mutations in the FLG gene encoding filaggrin, a key protein for formation of the skin barrier, were recently found in a substantial proportion of AD patients (Palmer et al., 2006; Sandilands et al., 2007) and flaky tail mutant mice (Fallon et al., 2009). Furthermore, tight junction proteins claudin-1 and claudin-23 are reduced in AD patients. Silencing of claudin-1, whose expression is inversely correlated with Th2 biomarkers, in human keratinocytes diminishes tight junction function (De Benedetto et al, 2011). Thus, strong association of FLG mutations with AD and other studies have validated the outside-inside hypothesis (De Benedetto et al, 2012; Irvine et al, 2011). However, FLG mutations predispose subjects to allergen sensitization but these mutations are not sufficient for causing AD, as other genetic and environmental influences likely promote the Th2 immune response in susceptible individuals.
A number of mouse AD models have been developed over the last fifteen years, and have provided mechanistic insights into the pathogenesis of human AD (Gutermuth et al., 2004; Jin et al, 2009; Kawakami et al, 2009a). For example, a mouse model induced by epicutaneous (EC) sensitization with ovalbumin (OVA) mimicked skin lesions of human AD characterized by epidermal and dermal thickening, infiltration of CD4+ T cells and eosinophils, and local expression of mRNAs for IL-4, IL-5 and IL-13 (Spergel et al, 1998). Dermatitis in this model required αβ T cells, but not B cells or mast cells (Alenius et al, 2002; Woodward et al, 2001). Different roles of IL-4, IL-5, IL-10, IL-17, IFN-γ, chemokine receptors, complement components and complement receptors in this model were demonstrated using gene-manipulated mice (Jin et al, 2009). We also developed a highly reproducible mouse model that mimicked human AD, in which skin inflammation was induced by repeated treatments of Dermatophagoides farinae extract (Der f) and staphylococcal enterotoxin B (SEB) (Kawakami et al, 2007). Thus, AD patients often suffer from skin infections and more than 90% of AD patients are colonized with Staphylococcus aureus, as compared to about 5% of healthy subjects. S. aureus infection is thought to be critical in the pathogenesis and/or worsening of skin lesions (Jappe, 2000; Strange et al, 1996). Moreover, there is a strong association of human AD with house dust mite allergens (Fuiano and Incorvaia, 2012; Kimura et al, 1998; Scalabrin et al, 1999). In this study, we demonstrated the clinical relevance of this model to human AD by global gene expression analysis, and then investigated the cellular and molecular players involved in skin inflammation in this model. We focused particularly on the role of mast cells.
Results
Gene expression profiles in lesional skin of Der f/SEB-induced dermatitis are similar to those in human AD skin
Our previous study showed that AD-like skin lesions can be induced by epicutaneous applications of Der f and SEB in NC/Nga and C57BL/6 (B6) mice (Kawakami et al., 2007). Higher clinical scores were observed with dermatitis-prone NC/Nga mice than with B6 mice. Global gene expression analysis of skin RNAs showed r=0.956 (Spearman’s correlation coefficient) between normal and eczematous skin in NC/Nga mice, while r=0.976 between normal and eczematous skin in B6 mice. Thus, the lower values of Spearman’s correlation coefficient might reflect higher clinical scores in NC/Nga mice. Comparison between B6 and NC/Nga mice yielded r=0.962 when healthy skin was compared, and r=0.970 when eczematous skin was compared. Clustering analysis also showed higher similarity between eczematous B6 and eczematous NC/Nga mice than other comparisons (Fig. S1A). These data imply that the same pathogenic mechanisms may underlie the development of AD-like skin lesions in both strains of mice. By contrast, comparison between different tissues gave lower values, e.g., r=0.855 between normal skin and normal spleen of B6 mice; r=0.857 between eczematous skin and spleen of eczematous B6 mice.
To examine the clinical relevance of our Der f/SEB induction model, we compared skin gene expression data derived with B6 and NC/Nga mice with human AD skin data deposited in the NCBI Gene Expression Omnibus (GEO) database. The changes in expression of genes in human AD versus healthy skin from AD patients or healthy subjects were compared with those of orthologous genes in mouse eczematous versus healthy skin, using OrderedList algorithm (Lottaz et al., 2006; Yang et al., 2006). This analysis detected significant similarity in gene expression in the skin between human AD and mouse AD-like dermatitis (Table 1 and Fig. S1B). The top genes contributing to 95% of the similarity score were similar when our B6 and NC/Nga results were compared with different human AD data, and were related to epidermal growth/differentiation (e.g., several keratin genes, cornified cell envelope-related genes [Sprr1b, Sprr2k, Tgm3, Lce1m]), skin barrier function (e.g., several kallikrein-related peptidases, serine protease inhibitors [Serpinb3d, 3a, 13a]), immune responses (e.g., several cytokines, chemokines, and their receptors, S100A8, S100A9), and lipid/energy metabolism (e.g., Slc27a2, Pck1), extracellular matrix/adhesion (e.g., several matrix metalloproteinases, their inhibitor Timp4, Tnc) Among these genes, Il7r, Il21r, CD8a, Ltb, Ccl5, Cxcl9, Cxcl10, Dlg2, Zap70, Pik3r1, and Fos are involved in the development and/or various aspects of functions of T cells, and Fcer1a, Hck, Ccl2, Pik3r1, and Fos are involved in the development and/or functions of mast cells (see more detail in Supplementary Description of Microarray Data and Table S1). Consistent with the altered expression of skin barrier-related genes, Der f/SEB-induced mice had impaired skin barrier, as revealed by high levels of TEWL (Fig. S2). Expression of select genes among the top similarity contributors was confirmed by RT-qPCR (Fig. S1C).
Table 1.
Similarity analysis of human and mouse microarray data.
| GEO accession |
Orthologs common in the lists |
Expression fold-change between | B6AD-like lesional / B6 Normal Skin |
NC/NgaAD-like lesional / NC/Nga Normal Skin |
||||
|---|---|---|---|---|---|---|---|---|
| Score | p-value | Genes | Score | p-value | Genes | |||
| GSE6012 | 10873 | AD lesional / Normal Skin | 1198.9 | <0.001 | 94 | 1327.5 | <0.001 | 131 |
| GSE5667 | 14325 | AD lesional / Normal Skin | 972.3 | <0.001 | 75 | 976.0 | <0.001 | 97 |
| AD lesional / Non-lesional Skin | 1029.0 | <0.001 | 61 | 976.6 | <0.001 | 81 | ||
| AD non-lesional / Normal Skin | 277.8 | 0.845 | 69 | 366.0 | 0.502 | 65 | ||
| GSE32924 | 14878 | AD lesional / Normal Skin | 768.2 | <0.001 | 56 | 553.7 | 0.014 | 75 |
| AD lesional / Non-lesional Skin | 1283.8 | <0.001 | 126 | 1015.4 | <0.001 | 143 | ||
| AD non-lesional / Normal Skin | 552.4 | 0.022 | 29 | 485.2 | 0.068 | 41 | ||
| GSE16161 | 10873 | AD lesional / Normal Skin | 994.1 | <0.001 | 97 | 753.1 | 0.004 | 116 |
| GSE27887 | 14878 | AD lesional / Non-lesional Skin | 1156.9 | <0.001 | 117 | 910.6 | <0.001 | 134 |
| GSE26952 | 14889 | AD non-lesional / Normal Epidermis | 888.8 | <0.001 | 46 | 849.5 | <0.001 | 50 |
| PS non-lesional / Normal Epidermis | 577.9 | 0.006 | 29 | 484.5 | 0.069 | 34 | ||
Similarity of expression changes in human AD or psoriasis (PS) and mouse induction models were compared using OrderedList algorithm as described in the Materials and Methods. Human data series obtained from NCBI Gene Expression Omnibus (GEO) are shown by GEO accession. Since each dataset uses a different microarray platform, first, orthologs were matched between human and mouse data according to NCBI HomoloGene Build 65. Numbers of the matched orthologs used in the comparisons are shown. OrderedList algorithm calculates similarity score according to the ranks of the genes listed in human and mouse data. We used expression fold changes for ranking. When the same genes were listed in the top (upregulated) or the bottom (downregulated) end of both human and mouse expression data, it gives a high similarity score. The similarity scores were compared with the random distribution of similarity scores (Fig. S1B), and p-values for the significance of similarity were obtained. Numbers of top genes that contributed to a total of 95% of the similarity score are also shown. A full gene list is available in Table S1.
The above expression data, together with previous results showing high serum IgE levels in both the majority of AD patients and allergen-induced eczematous mice (Jin et al., 2009; Kawakami et al., 2009a), showed high similarity between human AD and Der f/SEB-induced skin inflammation, supporting the clinical relevance of our model. Thus, these results set the stage for detailed mechanistic investigations.
T cells, but not B cells, are required for maximal skin inflammation
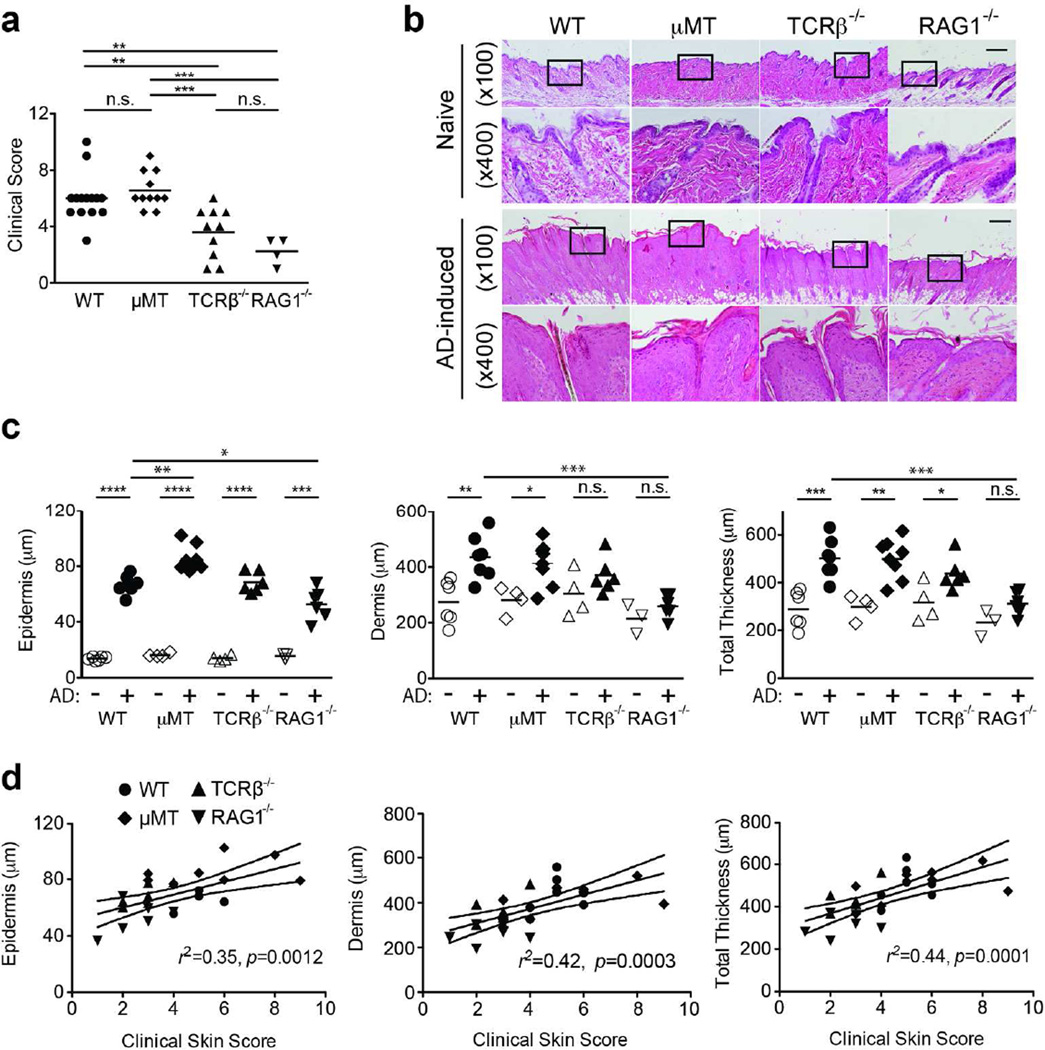
CD4 T cells, particularly Th2 cells, play a predominant role in allergic diseases including AD (Boguniewicz and Leung, 2011; Novak and Leung, 2011). To begin to analyze the cellular requirement for allergen-induced dermatitis, we performed the Der f/SEB experiments on T celldeficient TCRβ−/− and T cell/B cell-deficient Rag1−/− mice. For comparison, B cell-deficient µMT and WT mice were also tested. Both TCRβ−/− and Rag1−/− mice exhibited substantially lower clinical scores than WT mice (Fig. 1A). By contrast, the clinical scores of µMT mice were similar to those of WT mice. These observations were reflected in the thicknesses of skin (Fig. 1B–D). While the epidermis was thickened in all AD-induced mice, the dermis in TCRβ−/− or Rag1−/− mice was not thickened following Der f/SEB treatment (Fig. 1C). The clinical scores correlated with the thicknesses of epidermis and dermis (Fig. 1D). In comparison to the non-AD WT sample, the increased thickness of the epidermis in AD-induced samples could be attributed to expansion of differentiated layers, such as the stratum spinosum denoted by keratin 1 (K1) and the stratum granulosum marked by loricrin (Fig. S3). Consistent with the perturbation of epidermal homeostasis, there was an increase in keratin 6 expression. Despite a defect in tight junction formation, E-cadherin (which nucleates adherens junctions) expression appeared normal. Consistent with our previous data (Kawakami et al., 2007), serum IgE levels (3076 ± 839 ng/ml, n=7) were high in eczematous WT mice. By contrast, without T cell help, IgE levels (700 ± 279 ng/ml, n=6) were lower in TCRβ−/− mice. As expected from the lack of antibodyproducing B cells, µMT and Rag1−/− mice did not have detectable levels of serum IgE (<15.6 ng/ml, the detection limit, n=8 or 6), indicating that IgE is not essential for skin inflammation. However, this does not exclude the possibility that IgE might contribute to some aspects of skin inflammation (see below). These results demonstrate that T cells, but not B cells, are required for the full expression of dermatitis in this model, similar to the EC OVA model.
Figure 1. T cells, but not B cells, are indispensable for maximal skin inflammation.
Dermatitis induction by epicutaneous applications of Der f and SEB was performed as described in the Materials and Methods. Each symbol represents one mouse. (A) Clinical skin scores. (B) H&E staining of naïve (upper rows) and lesional (lower rows) skin tissues. Bar, 200 µm. (C) Thicknesses of epidermis, dermis, and total skin (epidermis + dermis) layers, as measured in H&E-stained tissues. (D) Relationships between clinical scores and skin layer thicknesses. Linear regression lines are shown. *, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001; n.s., not significant.
Mast cells, but not eosinophils, are required for maximal skin inflammation
Histological analysis showed increased numbers of eosinophils in lesional skin in TCRβ−/−, Rag1− /−, µMT, and WT mice (Fig. 2A). However, no correlation was found between the numbers of eosinophils in skin lesions and clinical scores observed in these mice (Fig. 2B). Furthermore, the clinical scores in Der f/SEB-treated eosinophil-deficient PHIL or ΔdblGATA mice were not different from those in WT control (Fig. 2C–D), indicating that eosinophils are dispensable for allergen-induced skin inflammation. By contrast, the numbers of mast cells correlated positively with clinical scores (Fig. S4). Clinical scores were significantly lower in Der f/SEB-treated mast cell-deficient KitW-sh/W-sh mice than in the corresponding WT mice (Fig. 3A). Consistent with these observations, the thicknesses of the lesional epidermis and dermis were significantly reduced in KitW-sh/W-sh mice (Fig. 3B–C). To further confirm the role of mast cells, KitW-sh/W-sh mice were engrafted with BMMCs generated from WT mice. These mice exhibited clinical scores similar to WT mice (Fig. 3A). The numbers of engrafted mast cells were at near-normal levels (1131 ± 98/mm2 in engrafted mice versus 1770 ± 49 /mm2 in WT mice). In the absence of mast cells, the decreased thickening of AD-induced skin was consistent with a lower expression of K1 in AD-induced KitW-sh/W-sh mice versus AD-induced WT mice (Fig. 3D). Concerned about the possible role of abnormalities other than the mast cell deficiency in KitW-sh/W-sh mice (Reber et al, 2012; Rodewald and Feyerabend, 2012), we performed Der f/SEB experiments using the recently engineered mast cell-deficient mouse strain Cpa3-Cre;Mcl-1fl/f/ (Lilla et al, 2011). These mice also exhibited significantly blunted skin inflammation (Fig. S5). Interestingly, eosinophil infiltration was increased in mast cell-deficient mice (Fig. 3E), while neutrophils were decreased (Fig. 3F). Moreover, the numbers of neutrophils were significantly correlated with the clinical scores (Fig. S6). However, the role of neutrophils in our model remains to be determined. These results strongly indicate that mast cells are required for maximal skin inflammation.
Figure 2. Eosinophils are dispensable for allergen-induced skin inflammation.
(A,B) Eosinophils stained with Congo red were enumerated in the skin sections derived from experiments shown in Fig. 1. Each symbol represents one mouse. There was no significant correlation between eosinophil numbers and clinical scores. (C,D) Dermatitis induction by epicutaneous applications of Der f and SEB was performed on eosinophil-deficient PHIL (C) and ΔdblGATA (D) mice. Clinical scores are shown.
Figure 3. Mast cells are indispensable for maximal skin inflammation.
(A) Mast cell-deficient KitW-sh/W-sh mice exhibited lower clinical scores than WT mice. The scores similar to WT mice were restored by engraftment of BMMC (W-sh + BMMC). (B) H&E staining of naïve and lesional skin tissues. Enlarged images of the areas indicated by rectangles are shown below. Bar, 200 µm. (C) Thicknesses of epidermis, dermis, and total skin layers. (D) Immunofluorescence microscopy was performed on naïve and lesional skin tissues. Numbers of eosinophil (E) and neutrophil (F) before and after AD induction. *, p<0.05; **, p<0.01; ***, p<0.001; n.s., not significant.
FcεRI contributes to skin inflammation
High clinical scores in µMT mice (Fig. 1A) do not necessarily indicate that immunoglobulins are not involved in AD pathogenesis, because there are both activating and inhibitory Fc receptors (Nimmerjahn and Ravetch, 2006) and IgE binding to FcεRI has positive effects on mast cell survival and activation (Asai et al, 2001; Kalesnikoff et al., 2001; Kitaura et al, 2003). Elevated IgE levels are found in up to 80 percent of AD patients (Leung and Bieber, 2003) and anti-IgE therapy is efficacious to treat severe AD patients (Amrol, 2010; Vigo et al., 2006). Therefore, we tested whether the IgE-FcεRI axis is involved in skin inflammation. As shown in Fig. 4A, the clinical scores were significantly lower in FcεRIα−/− mice than in WT mice. Although H&E staining showed that epidermal/dermal thicknesses were not altered compared with WT mice (Fig. 4B–C), immunofluorescence microscopy analysis indicated that the increase in differentiating cell populations is slightly higher in AD-induced FcεRIα−/− mice compared with AD-induced WT counterparts (Fig. 4D). The same trend of expression changes could be seen for K6 and E-cadherin. However, consistent with the lower clinical scores, lesional skin had less infiltration of neutrophils in FcεRIα−/− mice (Fig. 4E). One of the major cytokines acutely secreted from FcεRI-activated mast cells is TNF-α, which is important for late-phase allergic reactions and neutrophil accumulation (Wershil et al, 1991). However, mice lacking TNF-a failed to show a reduction in clinical scores (Fig. 4F), suggesting that a factor(s) other than TNF-α may be critical for the mast cell contribution to skin inflammation in this model of AD.
Figure 4. FcεRI, but not TNF-α, is required for maximal skin inflammation.
Der f/SEB induction experiments were performed on FcεRIα−/− (A–E) and TNF-α−/− (F) mice. (A, F) Clinical skin scores. Thicknesses of epidermis, dermis, and total skin layers (B, C, D), and inflammatory cell infiltration (E) for these mice are also shown. (B) H&E staining and (D) immunofluorescence microscopy were performed on naïve and lesional skin tissues in FcεRIα−/− mice. *, p<0.05; n.s., not significant.
TSLP contributes to skin inflammation
TSLP activates dendritic cells (DCs) and TSLP-activated DCs prime naive T cells to produce several cytokines such as IL-4, IL-5, IL-13 and TNF-α (Liu, 2006). TSLP is highly expressed by keratinocytes from AD patients (Soumelis et al., 2002), and transgenic mice overexpressing TSLP in keratinocytes develop AD-like eczematous lesions (Li et al., 2005; Yoo et al., 2005). As shown in Fig. 5A, TSLP protein was highly expressed by keratinocytes in lesional skin of B6 mice. Skin sections in which the primary antibody was omitted suggested that the fluorescence in hair follicles might be non-specific. TSLP mRNA levels were also increased in lesional skin (data not shown). Next, we tested whether TSLP is involved in skin inflammation in the Der f/SEB model. Importantly, mice lacking TSLPR exhibited substantial reduction in clinical scores (Fig. 5B). Although the thicknesses of epidermis and dermis were not significantly different between WT and TSLPR−/− mice (Fig. 5C–D), the numbers of neutrophils and eosinophils, but not mast cells, were drastically reduced in TSLPR−/− mice (Fig. 5E). Consistent with the histological analysis of AD-induced samples, expression of markers of epidermal differentiation was not significantly different between WT and TSLPR−/− mice (Fig. 5F). Interestingly, K6 expression was higher in AD-induced skin from TSLPR−/− mice versus WT mice. However, serum IgE levels were not lower in TSLPR−/− mice (10.1 ± 3.8 µg/ml, n=4 versus WT 4.4 ± 1.1 µg/ml, n=7). These results collectively indicate that the TSLP-TSLPR axis is critically involved in certain aspects of this AD model.
Figure 5. The Th2-promoting cytokine TSLP contributes to skin inflammation.
(A) Expression of TSLP (red) before (Upper) and after (Lower) dermatitis induction with Der f/SEB in WT mice was revealed by immunofluorescence microscopy. Also shown are enlarged images of the areas indicated by rectangles as well as negative control without primary antibody. Bar, 100 µm. (B–F) Der f/SEB induction experiments were performed on TSLPR−/− mice. (B) Clinical skin scores, (C,D) thicknesses of epidermis, dermis, and total skin layers, and (E) inflammatory cell infiltration are shown. (C) H&E staining and (F) immunofluorescence microscopy were performed on naïve and lesional skin tissues in TSLPR−/− mice. **, p<0.01; ***, p<0.001 by Student’s t -test.
Discussion
This and previous (Kawakami et al, 2007) studies strongly support the clinical relevance of our Der f/SEB-induced AD model for the following reasons. First, eczematous mice thus induced exhibited similarity to human AD skin in gross and microscopic morphology and pruritus. Second, eczematous mice showed Th2 predominant skin inflammation and elevated serum IgE levels. Third, global gene expression in eczematous skin was similar to human AD skin, confirming altered epidermal differentiation (leading to impaired barrier function) and immune dysregulation in both human and mouse diseases. Fourth, consistent with the efficacy of anti-IgE therapy in treating severe AD patients (Belloni et al, 2008; Liu et al, 2011), the IgE-FcεRI axis was involved in Der f/SEB-induced dermatitis. Finally, the requirement of TSLPR for Der f/SEB-induced dermatitis was also consistent with Th2 inflammation in human AD.
To the best of our knowledge, this study represents a previously unreported comparison in gene expression at the genomic level between human AD and a mouse model of AD. The genes that contribute to similarity in human AD and our mouse model are related to epidermal growth and differentiation, skin barrier, lipid and energy metabolism, immune response, and extracellular matrix. Many of these genes have been implicated in the pathophysiology of human AD (Barnes, 2010).
This study showed that mast cells and αβ T cells, but not B cells or eosinophils, are required for the full expression of AD-like skin lesions in B6 mice. This report also demonstrates the requirement for mast cells in an AD model (Kawakami et al, 2009a) by using a strict set of approaches including mast cell knock-in (Nakano et al., 1985). According to the widely accepted notion for AD development (Bieber, 2008; Boguniewicz and Leung, 2011), the impaired skin barrier function allows easy access of allergens to the inside of epidermis and dermis; allergens are taken up by Langerhans cells and/or dermal dendritic cells, and these cells migrate and mature to present allergens to naïve helper T cells in lymph nodes; activated and differentiated Th2 cells migrate back to skin sites re-exposed to allergens; these effector Th2 cells recruit eosinophils, mast cells, and other granulocytes to cause tissue damage. Our results support this scenario, particularly the roles of αβ T cells and mast cells. The dispensability of eosinophils shown in this study, as well as the dispensability of CCR3 (the chemokine receptor essential for eosinophil recruitment) in another AD model (Ma et al, 2002), probably indicates that tissuedamaging functions of eosinophils are redundant with those of other cells. Despite the apparent involvement of the IgE-FcεRI axis in certain features of Der f/SEB-induced dermatitis, including clinical score and numbers of neutrophils, B cells were not required for the full expression of the dermatitis. This could be interpreted as reflecting a balance between positive and negative regulatory functions of immunoglobulins in allergic inflammation. IgG receptors such as FcγRI, FcγRIIIA, and FcγRIV are activating receptors, whereas FcγRIIB is an inhibitory receptor (Nimmerjahn and Ravetch, 2006). FcγRIIB inhibits FcεRI-mediated activation as well (Kraft and Novak, 2006). The cellular requirements for dermatitis development in our model were not identical to those of the EC OVA model, as dermatitis in the latter model required αβ T cells, but not B or mast cells (Alenius et al, 2002; Woodward et al, 2001). By contrast, skin inflammation induced by EC sensitization with cedar pollen antigens was abolished in mast cell-deficient mice (Oiwa et al, 2008). The mast cell contribution to dermatitis development in that model and our model, but not in the EC OVA model, might be due to the use of complex allergens containing component(s) that trigger mast cell activation. Similar to our model, FcεRI was shown to be involved in dermatitis in an EC OVA sensitization model (Abboud et al., 2009). While reduced NK cell activity was shown in our model and it led to severe erosive skin lesions upon vaccinia virus infection (Kawakami et al., 2009b), NK cell activity seemed normal in the EC OVA model. Therefore, the two AD models might mimic different aspects of the AD phenotype. Alternatively, these different models reflect heterogeneity of human AD.
Several studies implicated TNF-α as an important factor in skin inflammation: TNF-α expression is high in AD and psoriatic lesional skin (Zimmermann et al., 2011); TNF-α and IFN-γ induce keratinocyte apoptosis (Konur et al., 2005); TNF-α inhibits barrier protein expression (filaggrin and loricrin) via a JNK-dependent pathway (Kim et al., 2011); TNF-α and TWEAK (TNF-like weak inducer of apoptosis) cooperate in the induction of apoptosis in primary keratinocytes and artificial skin equivalents. TWEAK upregulates TNF-α expression in keratinocytes. High TWEAK expression was observed in AD lesions, but not in healthy skin or psoriatic lesions (Zimmermann et al., 2011). Although TNF-α could be produced by T cells and mast cells, the two cell types required for the full expression of dermatitis, TNF-α was not required for Der f/SEB-induced dermatitis. Since anti-TNF-α therapy is effective in treating psoriasis (Kircik and Del Rosso, 2009; Langley et al., 2010), but not AD (Belloni et al., 2008; Pua and Barnetson, 2006), this result also supports the relevance of our Der f/SEB-induced dermatitis as a model of human AD.
TSLP is considered a master regulator of allergic inflammation (Liu, 2006). TSLP activates DCs and TSLP-activated DCs prime naive T cells to produce Th2 cytokines (IL-4, IL-5, IL-13) and TNF-α. TSLPR is expressed on other immune cells as well, and TSLP is necessary and sufficient for allergic inflammation (Ziegler and Artis, 2010). Given that TSLP is highly expressed by keratinocytes from AD patients (Soumelis et al., 2002), and Der f/SEB-induced dermatitis and transgenic mice overexpressing TSLP in keratinocytes develop AD-like eczematous lesions (Li et al., 2005; Yoo et al., 2005), it was not surprising that TSLPR was required for Der f/SEB-induced dermatitis. Considering the requirement of T cells in maximal Der f/SEB-induced dermatitis and the dispensability of T cells for dermatitis in keratinocytespecific TSLP transgenic mice, it is tempting to speculate that T cells are required for the expression of TSLP in keratinocytes and they become dispensable after high-level expression of TSLP is attained. In this context, mast cells, which express TSLPR (Allakhverdi et al., 2007), might exert an effector role downstream of TSLP. Alternatively, mast cells, together with T cells, might also be required for TSLP production in keratinocytes, since mast cells stimulated via FcεRI produce TSLP (Okayama et al., 2009; Soumelis et al., 2002) and combinations of Th2 cytokines and inflammatory cytokines (IL-1α or TNF-α) can induce TSLP production in keratinocytes (Bogiatzi et al., 2007).
In summary, this study has strengthened the clinical relevance of Der f/SEB-induced model of AD. By establishing its cellular and molecular basis, this model should be a useful tool for further studying the pathogenesis of AD and developing novel therapeutic strategies to the treatment of human AD.
Materials and Methods
Der f/SEB-induced dermatitis
Dermatitis was induced in NC/Nga, C57BL/6 (B6) mice or mutant mice with a C57BL/6 genetic background as previously described (Kawakami et al, 2007). NC/Nga mice were purchased from Charles River Japan (www.crj.co.jp). µMT (Kitamura et al, 1991), TCRβ−/− (Mombaerts et al, 1991), Rag1−/− (Mombaerts et al., 1992), KitW-sh/W-sh (Grimbaldeston et al., 2005), Cpa3-Cre;Mcl-1f/fl (Lilla et al., 2011), PHIL (Lee et al, 2004), ΔdblGATA (Yu et al, 2002), FcεRIα−/− (Dombrowicz et al, 1993), TNF-α−/− (Pasparakis et al, 1996), TSLPR−/− (Al-Shami et al, 2004), and GM-CSF−/− (Stanley et al, 1994) mice were previously described. Briefly, solutions of 500 ng of SEB (Sigma-Aldrich, St. Louis, MO) and 10 µg of Der f extract (100 µg/ml, Greer Laboratories, Lenoir, NC) were pipetted on a 1 cm × 1 cm square gauze pad placed on the shaved area. This portion of the back skin was occluded with a Tegaderm™ Transparent Dressing (3M HealthCare, St. Paul, MN) using bandages. Three days later, the dressings were replaced with a new one. After an additional 4 days had passed, the dressings were removed and the mice were kept without treatment for the next week. The one-week Der f/SEB treatment was repeated once more. Clinical severity was scored by an investigator who did not know the identities of mice 2 days after removing the dressings in the last cycle. Clinical scores were based on the severity (0, no symptoms; 1, mild; 2, intermediate; 3, severe) of four possible symptoms (redness, bleeding, eruption, and scaling). Der f/SEB experiments were performed using 3–6 mice per group and cumulative data from 2–5 experiments are presented. Animal experiments were approved by the Animal Care and Use Committee of the La Jolla Institute for Allergy and Immunology. Other experimental procedures, together with detailed description of microarray data, supplementary Table and Figures, can be found in the Supplementary Data.
Supplementary Material
Acknowledgments
The authors thank Drs. Glenn Dranoff and Steven Ziegler for providing mice; Dr. Michael R. Comeau (Amgen) for providing anti-TSLP antibody. This study was funded in part by the Atopic Dermatitis Research Network (Contract Number HHSN26620040033C) supported by the National Institute of Allergy and Infectious Diseases/National Institutes of Health (T.K.) and the National Institute of Biomedical Innovation (K. M). H.Y. was supported by a fellowship from Banyu Life Science Foundation International. This study is publication No. 1527 from the La Jolla Institute for Allergy and Immunology.
Abbreviations
- AD
atopic dermatitis
- B6
C57BL/6
- BMMC
bone marrow-derived mast cell
- CDSN
corneodesmosin
- DC
dendritic cell
- Der f
Dermatophagoides farinae extract
- EC
epicutaneous
- K
keratin
- KLK
kallikrein
- MMP
metalloproteinase
- OVA
ovalbumin
- SEB
staphylococcal enterotoxin B
- Th2
T helper 2
Footnotes
Conflict of Interest
The authors state no conflict of interest.
References
- Abboud G, Staumont-Salle D, Kanda A, Roumier T, Deruytter N, Lavogiez C, et al. Fc(epsilon)RI and FcgammaRIII/CD16 differentially regulate atopic dermatitis in mice. Journal of immunology. 2009;182:6517–6526. doi: 10.4049/jimmunol.0801055. [DOI] [PubMed] [Google Scholar]
- Al-Shami A, Spolski R, Kelly J, Fry T, Schwartzberg PL, Pandey A, et al. A role for thymic stromal lymphopoietin in CD4(+) T cell development. The Journal of experimental medicine. 2004;200:159–168. doi: 10.1084/jem.20031975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alenius H, Laouini D, Woodward A, Mizoguchi E, Bhan AK, Castigli E, et al. Mast cells regulate IFN-gamma expression in the skin and circulating IgE levels in allergen-induced skin inflammation. The Journal of allergy and clinical immunology. 2002;109:106–113. doi: 10.1067/mai.2002.120553. [DOI] [PubMed] [Google Scholar]
- Allakhverdi Z, Comeau MR, Jessup HK, Yoon BR, Brewer A, Chartier S, et al. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. The Journal of experimental medicine. 2007;204:253–258. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrol D. Anti-immunoglobulin e in the treatment of refractory atopic dermatitis. Southern medical journal. 2010;103:554–558. doi: 10.1097/SMJ.0b013e3181de0cf6. [DOI] [PubMed] [Google Scholar]
- Asai K, Kitaura J, Kawakami Y, Yamagata N, Tsai M, Carbone DP, et al. Regulation of mast cell survival by IgE. Immunity. 2001;14:791–800. doi: 10.1016/s1074-7613(01)00157-1. [DOI] [PubMed] [Google Scholar]
- Barnes KC. An update on the genetics of atopic dermatitis: scratching the surface in 2009. The Journal of allergy and clinical immunology. 2010;125:16–29. doi: 10.1016/j.jaci.2009.11.008. e1–11; quiz 30-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belloni B, Andres C, Ollert M, Ring J, Mempel M. Novel immunological approaches in the treatment of atopic eczema. Current opinion in allergy and clinical immunology. 2008;8:423–427. doi: 10.1097/ACI.0b013e32830fb8fd. [DOI] [PubMed] [Google Scholar]
- Bieber T. Atopic dermatitis. N Engl J Med. 2008;358:1483–1494. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- Bogiatzi SI, Fernandez I, Bichet JC, Marloie-Provost MA, Volpe E, Sastre X, et al. Cutting Edge: Proinflammatory and Th2 cytokines synergize to induce thymic stromal lymphopoietin production by human skin keratinocytes. Journal of immunology. 2007;178:3373–3377. doi: 10.4049/jimmunol.178.6.3373. [DOI] [PubMed] [Google Scholar]
- Boguniewicz M, Leung DY. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunological reviews. 2011;242:233–246. doi: 10.1111/j.1600-065X.2011.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? The Journal of investigative dermatology. 2012;132:949–963. doi: 10.1038/jid.2011.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Benedetto A, Rafaels NM, McGirt LY, Ivanov AI, Georas SN, Cheadle C, et al. Tight junction defects in patients with atopic dermatitis. The Journal of allergy and clinical immunology. 2011;127:773–786. doi: 10.1016/j.jaci.2010.10.018. e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrowicz D, Flamand V, Brigman KK, Koller BH, Kinet JP. Abolition of anaphylaxis by targeted disruption of the high affinity immunoglobulin E receptor alpha chain gene. Cell. 1993;75:969–976. doi: 10.1016/0092-8674(93)90540-7. [DOI] [PubMed] [Google Scholar]
- Fallon PG, Sasaki T, Sandilands A, Campbell LE, Saunders SP, Mangan NE, et al. A homozygous frameshift mutation in the mouse Flg gene facilitates enhanced percutaneous allergen priming. Nat Genet. 2009;41:602–608. doi: 10.1038/ng.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuiano N, Incorvaia C. Dissecting the causes of atopic dermatitis in children: less foods, more mites. Allergology international : official journal of the Japanese Society of Allergology. 2012;61:231–243. doi: 10.2332/allergolint.11-RA-0371. [DOI] [PubMed] [Google Scholar]
- Grewe M, Bruijnzeel-Koomen CA, Schopf E, Thepen T, Langeveld-Wildschut AG, Ruzicka T, et al. A role for Th1 and Th2 cells in the immunopathogenesis of atopic dermatitis. Immunol Today. 1998;19:359–361. doi: 10.1016/s0167-5699(98)01285-7. [DOI] [PubMed] [Google Scholar]
- Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. Mast celldeficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005;167:835–848. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutermuth J, Ollert M, Ring J, Behrendt H, Jakob T. Mouse models of atopic eczema critically evaluated. International archives of allergy and immunology. 2004;135:262–276. doi: 10.1159/000082099. [DOI] [PubMed] [Google Scholar]
- Irvine AD, McLean WH, Leung DY. Filaggrin mutations associated with skin and allergic diseases. The New England journal of medicine. 2011;365:1315–1327. doi: 10.1056/NEJMra1011040. [DOI] [PubMed] [Google Scholar]
- Jappe U. Superantigens and their association with dermatological inflammatory diseases: facts and hypotheses. Acta Derm Venereol. 2000;80:321–328. doi: 10.1080/000155500459231. [DOI] [PubMed] [Google Scholar]
- Jin H, He R, Oyoshi M, Geha RS. Animal models of atopic dermatitis. The Journal of investigative dermatology. 2009;129:31–40. doi: 10.1038/jid.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalesnikoff J, Huber M, Lam V, Damen JE, Zhang J, Siraganian RP, et al. Monomeric IgE stimulates signaling pathways in mast cells that lead to cytokine production and cell survival. Immunity. 2001;14:801–811. doi: 10.1016/s1074-7613(01)00159-5. [DOI] [PubMed] [Google Scholar]
- Kawakami T, Ando T, Kimura M, Wilson BS, Kawakami Y. Mast cells in atopic dermatitis. Curr Opin Immunol. 2009a;21:666–678. doi: 10.1016/j.coi.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y, Tomimori Y, Yumoto K, Hasegawa S, Ando T, Tagaya Y, et al. Inhibition of NK cell activity by IL-17 allows vaccinia virus to induce severe skin lesions in a mouse model of eczema vaccinatum. The Journal of experimental medicine. 2009b;206:1219–1225. doi: 10.1084/jem.20082835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y, Yumoto K, Kawakami T. An improved mouse model of atopic dermatitis and suppression of skin lesions by an inhibitor of tec family kinases. Allergol Int. 2007;56:403–409. doi: 10.2332/allergolint.O-07-486. [DOI] [PubMed] [Google Scholar]
- Kim BE, Howell MD, Guttman-Yassky E, Gilleaudeau PM, Cardinale IR, Boguniewicz M, et al. TNF-alpha downregulates filaggrin and loricrin through c-Jun N-terminal kinase: role for TNF-alpha antagonists to improve skin barrier. The Journal of investigative dermatology. 2011;131:1272–1279. doi: 10.1038/jid.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M, Tsuruta S, Yoshida T. Correlation of house dust mite-specific lymphocyte proliferation with IL-5 production, eosinophilia, and the severity of symptoms in infants with atopic dermatitis. The Journal of allergy and clinical immunology. 1998;101:84–89. doi: 10.1016/S0091-6749(98)70197-6. [DOI] [PubMed] [Google Scholar]
- Kircik LH, Del Rosso JQ. Anti-TNF agents for the treatment of psoriasis. Journal of drugs in dermatology : JDD. 2009;8:546–559. [PubMed] [Google Scholar]
- Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- Kitaura J, Song J, Tsai M, Asai K, Maeda-Yamamoto M, Mocsai A, et al. Evidence that IgE molecules mediate a spectrum of effects on mast cell survival and activation via aggregation of the FcepsilonRI. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:12911–12916. doi: 10.1073/pnas.1735525100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konur A, Schulz U, Eissner G, Andreesen R, Holler E. Interferon (IFN)-gamma is a main mediator of keratinocyte (HaCaT) apoptosis and contributes to autocrine IFN-gamma and tumour necrosis factor-alpha production. The British journal of dermatology. 2005;152:1134–1142. doi: 10.1111/j.1365-2133.2005.06508.x. [DOI] [PubMed] [Google Scholar]
- Kraft S, Novak N. Fc receptors as determinants of allergic reactions. Trends in immunology. 2006;27:88–95. doi: 10.1016/j.it.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Langley RG, Strober BE, Gu Y, Rozzo SJ, Okun MM. Benefit-risk assessment of tumour necrosis factor antagonists in the treatment of psoriasis. The British journal of dermatology. 2010;162:1349–1358. doi: 10.1111/j.1365-2133.2010.09707.x. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O'Neill KR, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305:1773–1776. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- Leung DY, Bieber T. Atopic dermatitis. Lancet. 2003;361:151–160. doi: 10.1016/S0140-6736(03)12193-9. [DOI] [PubMed] [Google Scholar]
- Li M, Messaddeq N, Teletin M, Pasquali JL, Metzger D, Chambon P. Retinoid X receptor ablation in adult mouse keratinocytes generates an atopic dermatitis triggered by thymic stromal lymphopoietin. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:14795–14800. doi: 10.1073/pnas.0507385102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilla JN, Chen CC, Mukai K, BenBarak MJ, Franco CB, Kalesnikoff J, et al. Reduced mast cell and basophil numbers and function in Cpa3-Cre; Mcl-1fl/fl mice. Blood. 2011;118:6930–6938. doi: 10.1182/blood-2011-03-343962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu FT, Goodarzi H, Chen HY. IgE, mast cells, and eosinophils in atopic dermatitis. Clinical reviews in allergy & immunology. 2011;41:298–310. doi: 10.1007/s12016-011-8252-4. [DOI] [PubMed] [Google Scholar]
- Liu YJ. Thymic stromal lymphopoietin: master switch for allergic inflammation. The Journal of experimental medicine. 2006;203:269–273. doi: 10.1084/jem.20051745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lottaz C, Yang X, Scheid S, Spang R. OrderedList--a bioconductor package for detecting similarity in ordered gene lists. Bioinformatics. 2006;22:2315–2316. doi: 10.1093/bioinformatics/btl385. [DOI] [PubMed] [Google Scholar]
- Ma W, Bryce PJ, Humbles AA, Laouini D, Yalcindag A, Alenius H, et al. CCR3 is essential for skin eosinophilia and airway hyperresponsiveness in a murine model of allergic skin inflammation. The Journal of clinical investigation. 2002;109:621–628. doi: 10.1172/JCI14097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P, Clarke AR, Hooper ML, Tonegawa S. Creation of a large genomic deletion at the T-cell antigen receptor beta-subunit locus in mouse embryonic stem cells by gene targeting. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:3084–3087. doi: 10.1073/pnas.88.8.3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- Nakano T, Sonoda T, Hayashi C, Yamatodani A, Kanayama Y, Yamamura T, et al. Fate of bone marrow-derived cultured mast cells after intracutaneous, intraperitoneal, and intravenous transfer into genetically mast cell-deficient W/Wv mice. Evidence that cultured mast cells can give rise to both connective tissue type and mucosal mast cells. The Journal of experimental medicine. 1985;162:1025–1043. doi: 10.1084/jem.162.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Novak N, Leung DY. Advances in atopic dermatitis. Current opinion in immunology. 2011;23:778–783. doi: 10.1016/j.coi.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oiwa M, Satoh T, Watanabe M, Niwa H, Hirai H, Nakamura M, et al. CRTH2-dependent, STAT6-independent induction of cedar pollen dermatitis. Clin Exp Allergy. 2008;38:1357–1366. doi: 10.1111/j.1365-2222.2008.03007.x. [DOI] [PubMed] [Google Scholar]
- Okayama Y, Okumura S, Sagara H, Yuki K, Sasaki T, Watanabe N, et al. FcepsilonRI-mediated thymic stromal lymphopoietin production by interleukin-4-primed human mast cells. Eur Respir J. 2009;34:425–435. doi: 10.1183/09031936.00121008. [DOI] [PubMed] [Google Scholar]
- Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38:441–446. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. The Journal of experimental medicine. 1996;184:1397–1411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pua VS, Barnetson RS. Recent developments in the treatment of adult atopic dermatitis. The Australasian journal of dermatology. 2006;47:84–89. doi: 10.1111/j.1440-0960.2006.00237.x. [DOI] [PubMed] [Google Scholar]
- Reber LL, Marichal T, Galli SJ. New models for analyzing mast cell functions in vivo. Trends in immunology. 2012;33:613–625. doi: 10.1016/j.it.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodewald HR, Feyerabend TB. Widespread immunological functions of mast cells: fact or fiction? Immunity. 2012;37:13–24. doi: 10.1016/j.immuni.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Sandilands A, Terron-Kwiatkowski A, Hull PR, O'Regan GM, Clayton TH, Watson RM, et al. Comprehensive analysis of the gene encoding filaggrin uncovers prevalent and rare mutations in ichthyosis vulgaris and atopic eczema. Nat Genet. 2007;39:650–654. doi: 10.1038/ng2020. [DOI] [PubMed] [Google Scholar]
- Scalabrin DM, Bavbek S, Perzanowski MS, Wilson BB, Platts-Mills TA, Wheatley LM. Use of specific IgE in assessing the relevance of fungal and dust mite allergens to atopic dermatitis: a comparison with asthmatic and nonasthmatic control subjects. The Journal of allergy and clinical immunology. 1999;104:1273–1279. doi: 10.1016/s0091-6749(99)70024-2. [DOI] [PubMed] [Google Scholar]
- Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nature immunology. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- Spergel JM, Mizoguchi E, Brewer JP, Martin TR, Bhan AK, Geha RS. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J Clin Invest. 1998;101:1614–1622. doi: 10.1172/JCI1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E, Lieschke GJ, Grail D, Metcalf D, Hodgson G, Gall JA, et al. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:5592–5596. doi: 10.1073/pnas.91.12.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange P, Skov L, Lisby S, Nielsen PL, Baadsgaard O. Staphylococcal enterotoxin B applied on intact normal and intact atopic skin induces dermatitis. Arch Dermatol. 1996;132:27–33. [PubMed] [Google Scholar]
- Thepen T, Langeveld-Wildschut EG, Bihari IC, van Wichen DF, van Reijsen FC, Mudde GC, et al. Biphasic response against aeroallergen in atopic dermatitis showing a switch from an initial TH2 response to a TH1 response in situ: an immunocytochemical study. The Journal of allergy and clinical immunology. 1996;97:828–837. doi: 10.1016/s0091-6749(96)80161-8. [DOI] [PubMed] [Google Scholar]
- Tsicopoulos A, Hamid Q, Haczku A, Jacobson MR, Durham SR, North J, et al. Kinetics of cell infiltration and cytokine messenger RNA expression after intradermal challenge with allergen and tuberculin in the same atopic individuals. The Journal of allergy and clinical immunology. 1994;94:764–772. doi: 10.1016/0091-6749(94)90185-6. [DOI] [PubMed] [Google Scholar]
- Vigo PG, Girgis KR, Pfuetze BL, Critchlow ME, Fisher J, Hussain I. Efficacy of anti-IgE therapy in patients with atopic dermatitis. Journal of the American Academy of Dermatology. 2006;55:168–170. doi: 10.1016/j.jaad.2005.12.045. [DOI] [PubMed] [Google Scholar]
- Werfel T, Morita A, Grewe M, Renz H, Wahn U, Krutmann J, et al. Allergen specificity of skin-infiltrating T cells is not restricted to a type-2 cytokine pattern in chronic skin lesions of atopic dermatitis. The Journal of investigative dermatology. 1996;107:871–876. doi: 10.1111/1523-1747.ep12331164. [DOI] [PubMed] [Google Scholar]
- Wershil BK, Wang ZS, Gordon JR, Galli SJ. Recruitment of neutrophils during IgEdependent cutaneous late phase reactions in the mouse is mast cell-dependent. Partial inhibition of the reaction with antiserum against tumor necrosis factor-alpha. J Clin Invest. 1991;87:446–453. doi: 10.1172/JCI115016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward AL, Spergel JM, Alenius H, Mizoguchi E, Bhan AK, Castigli E, et al. An obligate role for T-cell receptor alphabeta+ T cells but not T-cell receptor gammadelta+ T cells, B cells, or CD40/CD40L interactions in a mouse model of atopic dermatitis. The Journal of allergy and clinical immunology. 2001;107:359–366. doi: 10.1067/mai.2001.112695. [DOI] [PubMed] [Google Scholar]
- Yang X, Bentink S, Scheid S, Spang R. Similarities of ordered gene lists. Journal of bioinformatics and computational biology. 2006;4:693–708. doi: 10.1142/s0219720006002120. [DOI] [PubMed] [Google Scholar]
- Yoo J, Omori M, Gyarmati D, Zhou B, Aye T, Brewer A, et al. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. The Journal of experimental medicine. 2005;202:541–549. doi: 10.1084/jem.20041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Cantor AB, Yang H, Browne C, Wells RA, Fujiwara Y, et al. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. The Journal of experimental medicine. 2002;195:1387–1395. doi: 10.1084/jem.20020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler SF, Artis D. Sensing the outside world: TSLP regulates barrier immunity. Nature immunology. 2010;11:289–293. doi: 10.1038/ni.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M, Koreck A, Meyer N, Basinski T, Meiler F, Simone B, et al. TNF-like weak inducer of apoptosis (TWEAK) and TNF-alpha cooperate in the induction of keratinocyte apoptosis. The Journal of allergy and clinical immunology. 2011;127:200–207. doi: 10.1016/j.jaci.2010.11.005. 7 e1–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.