Abstract
Calcineurin, a serine-threonine-specific, Ca2+-calmodulin-activated protein phosphatase, conserved from yeast to humans, plays a key role in regulating cardiac development, hypertrophy and pathological remodeling. Recent studies demonstrate that calcineurin regulates cardiomyocyte ion channels and receptors in a manner which often entails direct interaction with these target proteins. Here, we review the current state of knowledge of calcineurin-mediated regulation of ion channels in the myocardium with emphasis on the transient-outward potassium current (Ito) and L-type calcium current (ICa,L). We go on to discuss unanswered questions that surround these observations and provide perspective on future directions in this exciting field.
Introduction
Malignant ventricular arrhythmia accounts for a substantial component of the mortality associated with structural heart disease(Go, Mozaffarian, Roger et al. 2013). Disease-related electrical remodeling, a term intended to encompass alterations in multiple electrogenic transport processes within the heart, has emerged as an important pathophysiological mechanism. Whereas significant progress has been made in dissecting the molecular pathogenesis of cardiac hypertrophy and failure(Kehat, Molkentin 2010), our understanding of mechanisms underlying the numerous facets of electrical remodeling is limited. As a result, means of treating ventricular arrhythmias remain disappointingly ineffective.
Mechanisms underlying ventricular arrhythmia are multifactorial, but they derive, at least in part, from disordered electrical currents arising from prolongation of ventricular action potentials(Wang, Hill 2010). The resulting delay in the recovery of excitability, a consistent feature of ventricular hypertrophy and failure, predisposes to early- and late after-depolarizations. It further predisposes to abnormal impulse conduction and re-entry. Hypertrophic change is also associated with myocardial fibrosis, altered electrotonic coupling between cells, slowed conduction, and dispersion of refractoriness, all of which predispose to re-entrant arrhythmias. Together, these interlacing responses underlie the propensity to arrhythmia, syncope, and sudden death.
Prolongation of the ventricular action potential duration and spatial dispersion of this prolongation are each caused by a wide range of changes in myocyte ion channels and electrogenic transporters. Recent studies have uncovered an important role for calcineurin in the disease-associated electrical remodeling of cardiac hypertrophy and failure, which we discuss here.
Calcineurin
Calcineurin was first identified in neurons on the basis of its abundance in brain tissue(Klee, Crouch, Krinks 1979). Due to its high binding affinity for Ca2+ (Kd < 10−6 M), these authors originally proposed that this protein serves as an important regulator of intracellular Ca2+ in the nervous system(Klee, Crouch, Krinks 1979). Later studies of protein phosphatase-2 (PP2) revealed the presence of two distinct enzymatic activities that were further subclassified as PP2A and PP2B(Stewart, Ingebritsen, Manalan, Klee, Cohen 1982). Based on the subunit composition and co-purification with calcineurin from bovine brain, Stewart et al. suggested that PP2B and calcineurin are identical proteins(Stewart, Ingebritsen, Manalan, Klee, Cohen 1982).
Calcineurin is composed of two subunits, CnA and CnB. The B subunit of calcineurin is a Ca2+-binding protein. Like calmodulin (CaM), CnB possesses four EF hand motifs. This structural feature gives CnB the capability of binding four Ca2+ ions. CnB associates tightly with CnA even in the presence of Ca2+ chelators(Klee, Crouch, Krinks 1979; Aitken, Cohen, Santikarn et al. 1982). Despite the structural similarities of CnB and CaM, reconstitution of the holoenzyme from purified subunits demonstrated that CnB cannot substitute for the activity of CaM, nor can CaM functionally substitute CnB(Merat, Hu, Carter, Cheung 1985). While forming a complex with CnB and CaM, CnA exhibits phosphatase activity even in the absence of other subunits and, thus, has been recognized as the catalytic subunit(Merat, Hu, Carter, Cheung 1985; Gupta, Khandelwal, Sulakhe 1985). CnA possesses distinct and independent binding sites for CnB and CaM and can associate with either CnB or CaM alone(Merat, Hu, Carter, Cheung 1985). CnA harbors both regulatory and catalytic domains. The regulatory domain can itself be subdivided into a CaM binding site and an autoinhibitory domain. Upon Ca2+/CaM binding, the autoinhibitory domain is displaced, and the enzyme is activated(Hubbard, Klee 1989). At fixed concentrations of CaM, addition of Ca2+ triggers a steep rise in phosphatase activity with a 10- to 25-fold increase in Vmax with little change in Km(Stewart, Ingebritsen, Manalan, Klee, Cohen 1982; Tallant, Cheung 1984).
Shortly after the seminal characterization of calcineurin’s structure, subsequent studies revealed its importance in the immune system in addition to the brain(Feske, Okamura, Hogan, Rao 2003). Calcineurin was recognized as a key mediator for T-cell signaling and induction of cytokine gene expression via the transcription factor NFAT (nuclear factor of activated T-cells)(Clipstone, Crabtree 1992). These observations also helped uncover calcineurin as the target for two common, yet structurally unrelated, immunosuppressive drugs, cyclosporin A (CsA) and FK506 (tacrolimus); each binds to and inhibits calcineurin via mediation of the immunophilins cyclophilin A and FK506 binding protein 12 (FKBP12), respectively(Husi, Luyten, Zurini 1994). NFAT consists of at least two components, viz. a cytoplasmic and a nuclear component. Transcriptional activation requires association of these two components with translocation of the cytoplasmic component to the nucleus on transcriptional activation(Flanagan, Corthesy, Bram, Crabtree 1991). In some cell types, NFAT is constitutively localized to the nucleus(Shaw, Ho, Raghavan et al. 1995). However, phosphorylated NFAT binds to DNA with low affinity even in the presence of fos and jun. In heart, dephosphorylation of NFATs by CnA allows migration to the nucleus to promote gene expression. The best known example of this is the calcineurin-dependent pathway important in the pathogenesis of cardiac hypertrophy(Wilkins, Molkentin 2004).
Considerable progress has been made in defining the role of calcineurin in regulating cardiac development(Schulz, Yutzey 2004), muscle fiber phenotype transformation(Schiaffino, Serrano 2002), structural remodeling(Olson, Williams 2000), and apoptosis(Wang, Pathan, Ethell et al. 1999). Studies also indicate that the calcineurin pathway conveys a stabilizing function to counteract the myosin phenotype transformation that occurs with gravitational unloading (cardiac atrophy)(Shenkman, Nemirovskaya 2008).
The role of calcineurin in regulating ion channel expression and function has drawn particular attention. Although considerable progress has been achieved, our understanding in this area is still limited. Calcineurin has been involved in the regulation of several ion channels, including sodium channels(Chen, Law, Kondratyuk, Rossie 1995), potassium channels(Wilson, Jabr, Clapp 2000; Rossow, Dilly, Santana 2006), Ca2+-dependent chloride channels(Ledoux, Greenwood, Villeneuve, LeBlanc 2003; Go et al. 2013), N-type(Zhu, Yakel 1997) and L-type calcium channels(Tandan, Wang, Wang et al. 2009), ryanodine receptors(Ozawa 2008) and the sarcoplasmic reticulum ATPase (SERCA 2a)(Munch, Bolck, Karczewski, Schwinger 2002). Among these, the transient outward potassium channel (responsible for Ito) and L-type calcium channel (responsible for ICa,L) have been explored most extensively.
1) Regulation of the transient outward potassium channel (Ito)
The importance of the transient outward potassium channel (responsible for Ito) in action potential duration is well established, and prolongation of the action potential duration (APD) has consistently been observed in experimental models of cardiac hypertrophy and failure partially attributed to a reduction of a hyperpolarizing current provided by Ito. In fact, over-expression of Kv4.2 and Kv4.3 (responsible for Ito) both in vitro and in vivo has been associated with an attenuation of pathophysiological remodeling in a variety of hypertrophic models(Zobel, Kassiri, Nguyen, Meng, Backx 2002; Lebeche, Kaprielian, del Monte et al. 2004). One interesting finding is that calcineurin down-regulates functional expression of Ito(Perrier, Perrier, Richard, Benitah 2004), and this effect is suggested to contribute to the transmural gradient of Ito current in heart(Rossow, Dilly, Santana 2006). Consistent with this, Rossow et al. presented evidence that calcineurin is likely a key mediator of myocardial infarction-induced Ito down-regulation(Rossow, Dilly, Santana 2006). Conversely, there was also evidence in cultured neonatal rat ventricular myocytes that over-expression of constitutively calcineurin up-regulates Kv4.2 expression, a major subunit underlying Ito, via the NFAT transcriptional pathway, without affecting Kv4.3 or KChIP2(Gong, Bodi, Zobel, Schwartz, Molkentin, Backx 2006). Thus, the precise role of calcineurin in the regulation of Ito remains unclear. This inconsistency may derive from differences in disease models investigated (hypertrophy vs. myocardial infarction), crosstalk between the direct effect of calcineurin on the channel and indirect actions on the channel in the setting of calcineurin-induced hypertrophy. In a calcineurin-transgenic model of hypertrophy, Ito peak density is increased with no change in Kv4.2 protein expression compared to non-transgenic controls. In addition, Kv1.5 (underlying IKur) and Kv2.1 (encoding for IK, slow) expression levels were reduced significantly in calcineurin-over-expressors vs non-transgenics. Intriguingly, studies performed at a later age in these same transgenic mice who went on to develop heart failure, Kv4.2 α-subunit protein expression also decreased, as was Ito current density in heart failure in the transgenic model which was reversed by CsA administration(Petrashevskaya, Bodi, Rubio, Molkentin, Schwartz 2002; Dong, Duan, Guo et al. 2003). Importantly, these studies were conducted in rodents in which Kv4.2 is the predominant isoform underlying Ito(Niwa, Wang, Sha, Marionneau, Nerbonne 2008). In humans, on the other hand, Kv4.3 is the predominant isoform, and along with KChIP2 subunits is predominantly responsible for Ito. Therefore, additional studies are required to define the role of calcineurin in the regulation of Ito in non-rodents.
More recently, using a rapid-rate sustained canine model of ventricular tachycardia, Kv4.3 mRNA and protein expression were each found to be decreased with no change in KChIP2 expression. The concomitant down-regulation of Ito was abolished with inhibition of Ca2+-sensing mechanisms including CaMKII and calcineurin. In fact, specific inhibition of CaMKII prevented calcineurin activation and NFAT nuclear translocation, suggesting that CaMKII was upstream of calcineurin-mediated regulation of Kv4.3 and Ito in canine ventricular myocytes(Xiao, Coutu, Villeneuve et al. 2008).
In addition to its effects on Ito, calcineurin participates in the regulation of other potassium channels as mentioned above, including the ATP-sensitive K+ channel (IKATP) and the delayed-rectifier K+ channel(Wilson, Jabr, Clapp 2000).
2) Calcineurin-mediated regulation of L-type calcium channel (LTCC)
2a) Cardiac LTCC structure
Voltage-dependent calcium channels (VDCCs) were first identified by Fatt and Katz in 1953(FATT, Katz 1953). Based on their pharmacological and biophysical properties, VDCCs were subdivided into functional subtypes denoted T-type, L-type, N-type, P/Q-type, and R-type Ca2+channels(De Waard, Gurnett, Campbell 1996). Cardiac L-type Ca2+ channels (LTCCs) are composed of four polypeptide subunits (α1, β, α2/δ) that form a hetero-tetrameric complex with a molecular mass of about 400 kDa. The α1 subunit forms the channel pore for Ca2+ ion movement, and it is also responsible for voltage-dependent Ca2+ channel opening and channel selectivity for Ca2+ ions. More recently, the discovery of at least 10 different α1 subunit genes led to a revised numerical nomenclature of LTCCs(Catterall, Perez-Reyes, Snutch, Striessnig 2005), but only the α11.2 (Cav1.2/α1C) isoform is expressed at high levels in cardiac muscle(Bodi, Mikala, Koch, Akhter, Schwartz 2005). Similarly, there are four different β subunit isoforms (β1-β4) that have been discovered, with β2 the predominant cardiac isoform(Bodi, Mikala, Koch, Akhter, Schwartz 2005). The β subunit plays an important role in the trafficking of α1 subunits from the endoplasmic/sarcoplasmic reticulum to the plasma membrane, channel activation kinetics, Ca2+ channel facilitation, and β-adrenergic regulation(Bodi, Mikala, Koch, Akhter, Schwartz 2005). In addition, β subunits harbor sites of channel phosphorylation by cyclic GMP dependent protein kinase (PKG), cyclic ATP dependent protein kinase (PKA), and protein kinase C (PKC)(Keef, Hume, Zhong 2001). The α2/δ subunits, in contrast, have less influence on the action of LTCC than the β subunits in terms of current amplitude and channel activation and inactivation kinetics. The α2/δ subunits participate in regulating channel density and probably foster trafficking of the α11.2 subunit to the plasma membrane(Bodi, Mikala, Koch, Akhter, Schwartz 2005).
2b) Kinase-dependent regulation of cardiac LTCC
Several studies over the years have shed light on LTCC phosphorylation and regulation by a variety of kinases. The β-adrenergic receptor (β-AR)/PKA pathway is the main and best-investigated pathway for LTCC regulation. PKA phosphorylates the α11.2 subunit at Ser-1928(De Jongh, Murphy, Colvin, Hell, Takahashi, Catterall 1996) as well as the auxiliary β subunit at sites Ser-459, Ser-478, and Ser-479(Gerhardstein, Puri, Chien, Hosey M.M. 1999). The phosphorylation of α11.2 by PKA is critically dependent on close localization of these two proteins (PKA and α11.2), a process facilitated by A-kinase anchoring proteins (AKAPs) which serve as scaffolds to compartmentalize cAMP- and Ca2+-responsive enzymes close to their substrates. In particular, it is suggested that PKA and α11.2 are associated with AKAP79/150 or AKAP15/18 in cardiomyocytes(Hulme, Lin, Westenbroek, Scheuer, Catterall 2003; Scott, Santana 2010). The latter, which through lipid modifications localizes to the plasma membrane, has been shown to associate with the carboxy-terminus of LTCCs(Hulme, Lin, Westenbroek, Scheuer, Catterall 2003). The role of AKAP79/150-mediated co-localization of PKA and α11.2 has been demonstrated extensively in neuronal cells and recombinant studies. However, expression of AKAP79/150 in heart remains a topic of debate. Furthermore, the role of Ser1928 as the site of PKA-mediated ICa,L regulation has come under scrutiny. Recent evidence suggests that much of PKA-dependent activation of ICa,L in ventricular myocytes may not involve Ser1928(Lemke, Welling, Christel et al. 2008). Regardless of the precise site of PKA-mediated regulation of LTCC, or the means of co-localization, most studies agree upon the downstream effects of PKA-mediated phosphorylation and potentiation of LTCC function.
The mechanism for PKC-dependent regulation of cardiac LTCCs is not well documented. It is suggested that both α11.2 and β2 subunits can be phosphorylated by PKC in vitro(Kamp, Hell 2000). PKC phosphorylates the amino-terminus of the α11.2 subunit with the effect of either stimulating(Weiss, Doan, Bernstein, Dascal 2004) or inhibiting(McHugh, Sharp, Scheuer, Catterall 2000) LTCC function. A source of additional complication is that the effect of PKC on LTCC activity seems to be PKC isoform dependent. For instance, it has been shown that the βPKC isoform stimulates ICa,L (Alden, Goldspink, Ruch, Buttrick, Garcia 2002) while εPKC suppresses ICa,L (Yue, Qu, Boutjdir 2004). Disparity in these findings, compounded by the existence of multiple PKC isoforms, has made it difficult to pinpoint accurately both the effects of PKC-mediated regulation LTCC function and underlying mechanisms.
The effect of cGMP/PKG signaling pathway on the regulation of cardiac LTCCs is less clear, and reported observations are quite diverse. A recent report mapped PKG-mediated inhibition of heterologously expressed LTCC activity to Ser496 on the β2a subunit(Yang, Liu, Zakharov, Bellinger, Mongillo, Marx 2007). There are several possible pathways involved in cGMP/PKG-dependent regulation of LTCC, including direct phosphorylation of the Ca2+ channel itself by PKG, activation of phosphatases by PKG, or cGMP-dependent stimulation of a phosphodiesterase (PDE2) that hydrolyzes cAMP(Schroder, Klein, Fiedler et al. 2003; Fischmeister, Castro, Abi-Gerges, Rochais, Vandecasteele 2005). Due to the multifactoral effects of cGMP/PKA on LTCCs, the net effect on channel function can be either suppressive(Schroder et al. 2003) or stimulating(Wang, Wagner, Joyner, Kumar 2000).
Recent work has uncovered a binding site specific to Ca2+-calmodulin-dependent protein kinase II (CaMKII) on the LTCC β subunit that allows for targeting of a neighboring amino acid for phosphorylation(Grueter, Abiria, Dzhura et al. 2006). CaMKII-dependent phosphorylation of threonine 498 on the β2 subunit is required for facilitation(Grueter et al. 2006). Other studies have demonstrated that CaMKII binds directly to the pore-forming α subunit of the channel(Hudmon, Schulman, Kim, Maltez, Tsien, Pitt 2005), with a requirement for phosphorylation of Ser1512 and Ser1570 on the α subunit for ICa,L facilitation.
3) Phosphatases, calcineurin, and LTCC
Regulation of cardiac LTCC function includes channel phosphorylation by protein kinases and dephosphorylation by phosphatases. The main protein phosphatases (PP) found in heart are PP1, PP2A, and PP2B (calcineurin)(Herzig, Neumann 2000). LTCC is regulated by all these phosphatases(Herzig, Neumann 2000), but the relative role in LTCC regulation for these phosphatases may be different in normal and diseased heart. For instance, the expression and activity of PP1 and calcineurin were increased in human heart failure(Neumann, Eschenhagen, Jones et al. 1997; Carr, Schmidt, Suzuki et al. 2002) while expression and activity of PP2A in failing human myocardium was reduced(Chen, Piacentino, III, Furukawa, Goldman, Margulies, Houser 2002). On the other hand, in animal models of heart failure, there is evidence of an increase in PP2A(Ai, Pogwizd 2005) localization at the cellular gap junction. Furthermore, PP2A has also been shown to co-localize with LTCC α11.2 and antagonize PKA-mediated phosphorylation of α11.2(Tandan et al. 2009).
Reports of the effects of calcineurin or its inhibitors on LTCC are quite disparate. While stimulation of LTCC by CsA has been described by some groups(Schuhmann, Romanin, Baumgartner, Groschner 1997; Santana, Chase, Votaw, Nelson, Greven 2002), others found no effect(Yatani, Honda, Tymitz, Lalli, Molkentin 2001) or even a decrease in ICa,L (Norris, Blalock, Chen, Porter, Landfield 2002). Similar to findings with PP2A as above, some studies implicate the scaffolding protein AKAP79/150 localizing calcineurin to the LTCC in the PKA-dependent antagonism of LTCC function in hippocampal neurons and heterologous expression systems35. A recent study showed that CIB1 (calmyrin) interacts with the LTCC and enables calcineurin to translocate to the sarcolemma, facilitating calcineurin-LTCC interaction(Heineke, Auger-Messier, Correll et al. 2010). It is possible that localization of calcineurin to this region of the cell facilitates sensing of local Ca2+ concentrations which might differ from bulk intracellular Ca2+ involved in calcineurin activation. Indeed, in primary rat myocytes, in which the LTCC was inhibited, CIB1-dependent down-regulation of calcineurin-NFAT activation was abolished(Heineke et al. 2010).
We have reported that in the murine heart calcineurin binds to both the N and C termini of LTCC α11.2 and that this interaction is direct and does not require an intermediary protein(Tandan et al. 2009). We have also mapped residues 1943 to 1971 on the LTCC α11.2 carboxy-terminus as the minimal region required for specific calcineurin binding(Tandan et al. 2009). In vitro assays demonstrated that calcineurin can dephosphorylate α11.2. However, channel function was increased in voltage-clamp recordings of ICa,L from cultured cardiomyocytes expressing constitutively active calcineurin, while acute suppression of calcineurin by calcineurin inhibitors or with specific peptides decreased ICa,L (Tandan et al. 2009). These data reveal direct physical interaction between α11.2 and calcineurin in heart and a stimulatory role of calcineurin in regulating cardiac LTCC(Tandan et al. 2009). However, specific mechanisms underlying the functional regulation of ICa,L by calcineurin remain to be elucidated. Intriguingly, we have localized calcineurin binding to a region of α11.2 close to a site where PP2A binds(Hall, Feekes, Arachchige Don et al. 2006); therefore one possible mechanism by which calcineurin regulates LTCC function may involve interference with PP2A binding and consequent antagonism of PP2A-dependent α11.2 dephosphorylation. Studies are currently underway to test this hypothesis.
Although we have shown that calcineurin is capable of recognizing α11.2 as a substrate in vitro, the specific enzymatic substrate remains unknown. In addition, if calcineurin does regulate channel function by enzymatic action, then the cognate kinase that calcineurin antagonizes also remains to be determined. Alternatively, actions mediated indirectly by downstream targets of calcineurin may also exist, for example activation of CaMKII signaling cascades and LTCC facilitation or another, as yet, unidentified mediator (Figure 1).
Figure 1. Possible mechanisms of calcineurin-dependent regulation of cardiac LTCC.
(A) Calcineurin dephosphorylates the LTCC. The cognate kinase and specific enzymatic site(s) are currently undetermined. (B) Calcineurin mechanically alters LTCC function by direct protein-protein binding and conformational change. (C) Calcineurin interferes with PP2A interaction with LTCC. (D) Calcineurin alters LTCC function by activation of an intermediary protein(s). The identity of the downstream signaling target is currently unresolved. Key: α11.2 = LTCC pore-forming α subunit, CnA = calcineurin A catalytic subunit, P = phosphate, PP2A = protein phosphatase 2A, XYZ = unknown downstream target of calcineurin.
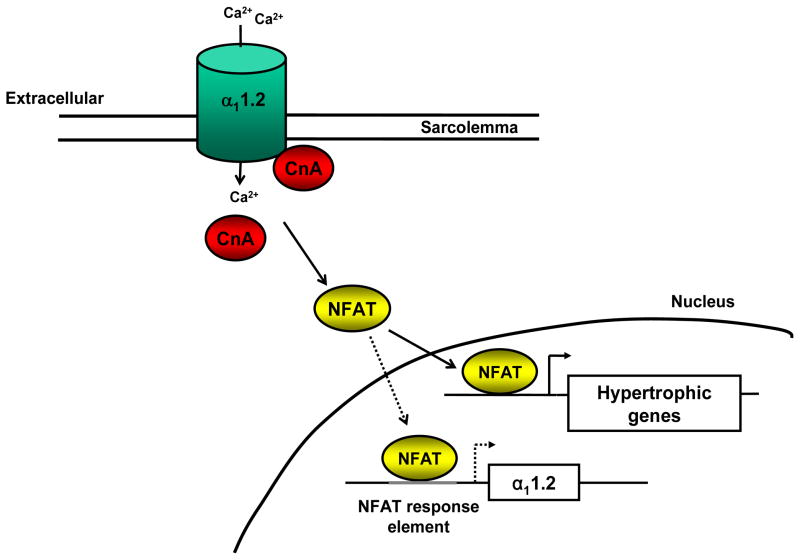
While the majority of studies focus on calcineurin-mediated regulation of LTCC phosphorylation state and channel function, it is unclear whether calcineurin also alters LTCC via effects on gene transcription, transcript translational, or channel subunit trafficking. Previous studies have demonstrated the presence of NFAT binding response elements in the human and rat cardiac α11.2 promoter regions, raising the prospect that α11.2 is a downstream target of the calcineurin-NFAT signaling pathway11. In addition to NFAT, we have identified GATA 4 and MEF 2 transcription factor binding sequences within 2 Kb of the murine cardiac α11.2 promoter (Tandan S and Hill JA, unpublished observations) (Figure 2). Studies are currently underway to assess the role of these response elements in LTCC channel expression in both normal and diseased heart.
Figure 2. Putative calcineurin-mediated regulation of LTCC transcription.
The calcineurin-NFAT signaling pathway is an established inducer of the fetal gene program in cardiac hypertrophy (solid arrows). We posit that the cardiac LTCC α11.2 subunit is one such downstream target of calcineurin-NFAT transcriptional activity in myocytes (dotted arrows).
4) Calcineurin-dependent and disease-related LTCC regulation
In structural heart disease, alterations in LTCC function are generally accompanied by an increase in intracellular calcium ([Ca2+]i) and calcineurin activity. Activation of calcineurin is triggered by [Ca2+]i and the [Ca2+]i itself also directly affects LTCC function. On the other hand, calcineurin alters [Ca2+]i via its modulating influences on LTCC function, provoking changes in Ca2+ influx. Furthermore, changes in [Ca2+]i also modulate activation of other intracellular calcium signaling pathways which may interface with calcineurin, e.g. CaMKII(MacDonnell, Weisser-Thomas, Kubo et al. 2009) and PKA(Santana, Chase, Votaw, Nelson, Greven 2002; Oliveria, Dell’Acqua, Sather 2007). Activation of CaMKII and PKA can, in turn, modify LTCC function and [Ca2+]i and, as a consequence, calcineurin activity. On the other hand, both calcineurin and CaMKII are activated by the same mechanism, Ca2+ and CaM. Whereas calcineurin has higher affinity for Ca2+/CaM, the relative activities of calcineurin and CaMKII are different in the dyadic cleft and cytosol due to differences in Ca2+ oscillation and CaM concentration(Saucerman, Bers 2012). Also, the activity of these emzymes manifests different patterns in response to changes in beating cycle length(Saucerman, Bers 2008). This spatial signaling and frequency modulation provide a prototype of complex behaviors. Clearly, intricate interplay among these calcium signaling pathways makes it difficult to dissect the specific role of calcineurin in regulating LTCC in myocytes and other cell types, including recombinant cell lines.
In structural heart disease, e.g. cardiac hypertrophy and heart failure, extensive alterations in LTCC density and metabolic modification of channel function occur in the heart(Aiba, Tomaselli 2010). For instance, some studies reported a reduction or no change in LTCC density (and ICa,L) in heart failure, while we and others reported an increase in LTCC density in hypertrophied and failing hearts(Bers, Despa 2006; Wang, Tandan, Cheng et al. 2008). However, molecular mechanisms underlying these alterations in LTCC abundance have not been well documented. It has been shown that CaMKII phosphorylates nuclear factor-kappaB (NFκB) component p65, provoking nuclear translocation and consequent suppression of LTCC channel expression in smooth muscle(Ishiguro, Green, Rapley et al. 2006). However, whether this applies to heart and how this mechanism may participate in the increased ICa,L density in the diseased heart remains unknown. Interestingly, recent studies have shown that metabolic inhibition suppresses LTCC function by ATP hydrolysis, but yet this occurs independently of reduced channel phosphorylation(Treinys, Jurevicius 2008). In addition to alterations in cardiac function and structure in the setting of heart failure, a wide array of more global changes in cardiac regulatory systems takes place. For instance, the level of cardiac Gs-mediated β1-ARs is reduced and Gi is increased(Lohse, Engelhardt, Eschenhagen 2003). These changes significantly impact PKA signaling and its downstream targets, calcineurin, and CaMKII-mediated LTCC regulation. Furthermore, due to the complex of interplay among these enzymes, the net effect on LTCC function and expression varies with disease etiology and stage of progression. We have reported a role of calcineurin in modulating LTCC current and accordingly the action potential duration in hypertrophic heart(Wang, Kutschke, Richardson, Karimi, Hill 2001) -In general, ICa,L is increased or unchanged in mild-to-moderate hypertrophy and decreased in severe hypertrophy and heart failure(Akar, Tomaselli 2005)
5) T-type calcium channels
While the L-type Ca2+ channel is the main source of Ca2+ influx for cardiac E-C coupling and pacemaker activity, the functional role of cardiac T-type Ca2+ channels is diverse, varying by species, heart region, age, and the presence of various cardiac diseases(Ono, Iijima 2010). T-type Ca2+ channels were initially considered to play only a minor functional role, because of their small amplitude and transient nature. It is now well established that the T-type Ca2+ channel contributes significantly to cardiac automaticity, development, and E-C coupling in normal cardiac myocytes. Furthermore, its functional role becomes more significant during the progression of pathological cardiac hypertrophy and heart failure(Perez-Reyes 2003). Recent studies have demonstrated that the T-type Ca2+ channel Cav3.2 is required for pressure overload–induced cardiac hypertrophy(Chiang, Huang, Chieng et al. 2009) and serum-induced hypertrophy in cultured neonatal mouse ventricular myocytes(Horiba, Muto, Ueda et al. 2008). However, whether calcineurin also plays a role in regulating T-type calcium channels is unknown.
Perspective and directions for future studies
In most cases, ion channel phosphorylation leads to an increase in channel activity, while dephosphorylation inhibits channel activity. In heart, however, our results(Tandan et al. 2009) and some results from others(Norris, Blalock, Chen, Porter, Landfield 2002; Fauconnier, Lacampagne, Rauzier et al. 2005) revealed an increase in LTCC function triggered by the phosphatase calcineurin. Meanwhile, there are also results that show no effect(McCall, Li, Satoh, Shannon, Blatter, Bers 1996; Yatani, Honda, Tymitz, Lalli, Molkentin 2001) or inhibitory effects of calcineurin on ICa,L (Schuhmann, Romanin, Baumgartner, Groschner 1997; Santana, Chase, Votaw, Nelson, Greven 2002). The underlying molecular mechanisms for either stimulatory or suppressive effects are not understood. However, reasons underlying these inconsistencies may be multifactorial. First, calcineurin activity could be different from one species to another. Second, the activity of calcineurin-associated kinases, e.g. CaMKII and PKA may vary with species and experimental conditions, which will affect the net results of calcineurin action on LTCC function due to the interplay between calcineurin and the Ca2+ regulatory kinases. For example, calcineurin can only modify Ca2+ transients when PKA activity is high(Santana, Chase, Votaw, Nelson, Greven 2002). Third, the effect of calcineurin on LTCC may also be affected by the expression and activity of other phosphatases, e.g. PP1 and PP2A. In fact, our recent results suggest a direct coupling of calcineurin to cardiac LTCC and α11.2 dephosphorylation(Tandan et al. 2009). However, we have not yet determined whether the effects of calcineurin on LTCC function occurs via direct calcineurin-dependent dephosphorylation of a channel subunit or an indirect effect via PP2A or other mediator and downstream targets. Fourth, calcineurin-mediated and disease-related LTCC regulation co-exist, adding an additional layer of complexity to this biology. And the fact that calcineurin, along with the major LTCC phosphorylation kinases, CaMKII and PKA, all couple with LTCCs(Santana, Chase, Votaw, Nelson, Greven 2002; Hudmon, Schulman, Kim, Maltez, Tsien, Pitt 2005; Oliveria, Dell’Acqua, Sather 2007; Tandan et al. 2009), bespeaks the importance of carefully titrated LTCC activity in health and disease. This fact also raises the question whether the effect of calcineurin on LTCC activity requires the co-regulation of other kinases as a functional unit or whether these multi-protein complexes govern LTCC channel function mechanically. The limited understanding of the molecular mechanisms and the complexity of kinase interplay make it difficult to dissect the actual role of calcineurin in disease-related electrical remodeling. Additional experimentation is required to elucidate these questions, but the ensuing answers may yield insights of importance in our understanding of heart failure and malignant ventricular arrhythmia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Ai X, Pogwizd SM. Connexin 43 downregulation and dephosphorylation in nonischemic heart failure is associated with enhanced colocalized protein phosphatase type 2A. Circ Res. 2005;96:54–63. doi: 10.1161/01.RES.0000152325.07495.5a. [DOI] [PubMed] [Google Scholar]
- Aiba T, Tomaselli GF. Electrical remodeling in the failing heart. Curr Opin Cardiol. 2010;25:29–36. doi: 10.1097/HCO.0b013e328333d3d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken A, Cohen P, Santikarn S, et al. Identification of the NH2-terminal blocking group of calcineurin B as myristic acid. FEBS Lett. 1982;150:314–18. doi: 10.1016/0014-5793(82)80759-x. [DOI] [PubMed] [Google Scholar]
- Akar FG, Tomaselli GF. Ion channels as novel therapeutic targets in heart failure. Ann Med. 2005;37:44–54. doi: 10.1080/07853890510007214. [DOI] [PubMed] [Google Scholar]
- Alden KJ, Goldspink PH, Ruch SW, Buttrick PM, Garcia J. Enhancement of L-type Ca(2+) current from neonatal mouse ventricular myocytes by constitutively active PKC-betaII. Am J Physiol Cell Physiol. 2002;282:C768–C774. doi: 10.1152/ajpcell.00494.2001. [DOI] [PubMed] [Google Scholar]
- Bers DM, Despa S. Cardiac myocytes Ca2+ and Na+ regulation in normal and failing hearts. J Pharmacol Sci. 2006;100:315–22. doi: 10.1254/jphs.cpj06001x. [DOI] [PubMed] [Google Scholar]
- Bodi I, Mikala G, Koch SE, Akhter SA, Schwartz A. The L-type calcium channel in the heart: the beat goes on. J Clin Invest. 2005;115:3306–17. doi: 10.1172/JCI27167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr AN, Schmidt AG, Suzuki Y, et al. Type 1 phosphatase, a negative regulator of cardiac function. Mol Cell Biol. 2002;22:4124–35. doi: 10.1128/MCB.22.12.4124-4135.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005;57:411–25. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- Chen TC, Law B, Kondratyuk T, Rossie S. Identification of soluble protein phosphatases that dephosphorylate voltage-sensitive sodium channels in rat brain. J Biol Chem. 1995;270:7750–7756. doi: 10.1074/jbc.270.13.7750. [DOI] [PubMed] [Google Scholar]
- Chen X, Piacentino V, III, Furukawa S, Goldman B, Margulies KB, Houser SR. L-type Ca2+ channel density and regulation are altered in failing human ventricular myocytes and recover after support with mechanical assist devices. Circ Res. 2002;91:517–24. doi: 10.1161/01.res.0000033988.13062.7c. [DOI] [PubMed] [Google Scholar]
- Chiang CS, Huang CH, Chieng H, et al. The Ca(v)3. 2 T-type Ca(2+) channel is required for pressure overload-induced cardiac hypertrophy in mice. Circ Res. 2009;104:522–30. doi: 10.1161/CIRCRESAHA.108.184051. [DOI] [PubMed] [Google Scholar]
- Clipstone NA, Crabtree GR. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992;357:695–97. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- De Jongh KS, Murphy BJ, Colvin AA, Hell JW, Takahashi M, Catterall WA. Specific phosphorylation of a site in the full-length form of the alpha 1 subunit of the cardiac L-type calcium channel by adenosine 3’,5’-cyclic monophosphate-dependent protein kinase. Biochemistry. 1996;35:10392–402. doi: 10.1021/bi953023c. [DOI] [PubMed] [Google Scholar]
- De Waard M, Gurnett CA, Campbell KP. Structural and functional diversity of voltage-activated calcium channels. Ion Channels. 1996;4:41–87. doi: 10.1007/978-1-4899-1775-1_2. [DOI] [PubMed] [Google Scholar]
- Dong D, Duan Y, Guo J, et al. Overexpression of calcineurin in mouse causes sudden cardiac death associated with decreased density of K+ channels. Cardiovasc Res. 2003;57:320–332. doi: 10.1016/s0008-6363(02)00661-2. [DOI] [PubMed] [Google Scholar]
- FATT P, Katz B. The electrical properties of crustacean muscle fibres. J Physiol. 1953;120:171–204. doi: 10.1113/jphysiol.1953.sp004884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauconnier J, Lacampagne A, Rauzier JM, et al. Frequency-dependent and proarrhythmogenic effects of FK-506 in rat ventricular cells. Am J Physiol Heart Circ Physiol. 2005;288:H778–H786. doi: 10.1152/ajpheart.00542.2004. [DOI] [PubMed] [Google Scholar]
- Feske S, Okamura H, Hogan PG, Rao A. Ca2+/calcineurin signalling in cells of the immune system. Biochem Biophys Res Commun. 2003;311:1117–32. doi: 10.1016/j.bbrc.2003.09.174. [DOI] [PubMed] [Google Scholar]
- Fischmeister R, Castro L, Abi-Gerges A, Rochais F, Vandecasteele G. Species- and tissue-dependent effects of NO and cyclic GMP on cardiac ion channels. Comp Biochem Physiol A Mol Integr Physiol. 2005;142:136–43. doi: 10.1016/j.cbpb.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Flanagan WM, Corthesy B, Bram RJ, Crabtree GR. Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporin A. Nature. 1991;352:803–7. doi: 10.1038/352803a0. [DOI] [PubMed] [Google Scholar]
- Gerhardstein BL, Puri TS, Chien AJ, Hosey MM. Identification of the sites phosphorylated by cyclic AMP-dependent protein kinase on the β2 subunit of L-type voltage dependent calcium channels. Biochemistry. 1999;38:10361–70. doi: 10.1021/bi990896o. [DOI] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong N, Bodi I, Zobel C, Schwartz A, Molkentin JD, Backx PH. Calcineurin increases cardiac transient outward K+ currents via transcriptional up-regulation of Kv4. 2 channel subunits. J Biol Chem. 2006;281:38498–506. doi: 10.1074/jbc.M607774200. [DOI] [PubMed] [Google Scholar]
- Grueter CE, Abiria SA, Dzhura I, et al. L-type Ca2+ channel facilitation mediated by phosphorylation of the beta subunit by CaMKII. Mol Cell. 2006;23:641–50. doi: 10.1016/j.molcel.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Gupta RC, Khandelwal RL, Sulakhe PV. Resolution of bovine brain calcineurin subunits: stimulatory effect of subunit B on subunit A phosphatase activity. FEBS Lett. 1985;190:104–8. doi: 10.1016/0014-5793(85)80437-3. [DOI] [PubMed] [Google Scholar]
- Hall DD, Feekes JA, Arachchige Don AS, et al. Binding of protein phosphatase 2A to the L-type calcium channel Cav1. 2 next to Ser1928, its main PKA site, is critical for Ser1928 dephosphorylation. Biochemistry. 2006;45:3448–59. doi: 10.1021/bi051593z. [DOI] [PubMed] [Google Scholar]
- Heineke J, Auger-Messier M, Correll RN, et al. CIB1 is a regulator of pathological cardiac hypertrophy. Nat Med. 2010;16:872–79. doi: 10.1038/nm.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig S, Neumann J. Effects of serine/threonine protein phosphatases on ion channels in excitable membranes. Physiol Rev. 2000;80:173–210. doi: 10.1152/physrev.2000.80.1.173. [DOI] [PubMed] [Google Scholar]
- Horiba M, Muto T, Ueda N, et al. T-type Ca2+ channel blockers prevent cardiac cell hypertrophy through an inhibition of calcineurin-NFAT3 activation as well as L-type Ca2+ channel blockers. Life Sci. 2008;82:554–60. doi: 10.1016/j.lfs.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Hubbard MJ, Klee CB. Functional domain structure of calcineurin A: mapping by limited proteolysis. Biochemistry. 1989;28:1868–74. doi: 10.1021/bi00430a066. [DOI] [PubMed] [Google Scholar]
- Hudmon A, Schulman H, Kim J, Maltez JM, Tsien RW, Pitt GS. CaMKII tethers to L-type Ca2+ channels, establishing a local and dedicated integrator of Ca2+ signals for facilitation. J Cell Biol. 2005;171:537–47. doi: 10.1083/jcb.200505155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme JT, Lin TW, Westenbroek RE, Scheuer T, Catterall WA. Beta-adrenergic regulation requires direct anchoring of PKA to cardiac CaV1. 2 channels via a leucine zipper interaction with A kinase-anchoring protein 15. Proc Natl Acad Sci U S A. 2003;100:13093–98. doi: 10.1073/pnas.2135335100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husi H, Luyten MA, Zurini MG. Mapping of the immunophilin-immunosuppressant site of interaction on calcineurin. J Biol Chem. 1994;269:14199–204. [PubMed] [Google Scholar]
- Ishiguro K, Green T, Rapley J, et al. Ca2+/calmodulin-dependent protein kinase II is a modulator of CARMA1-mediated NF-kappaB activation. Mol Cell Biol. 2006;26:5497–508. doi: 10.1128/MCB.02469-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamp TJ, Hell JW. Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ Res. 2000;87:1095–102. doi: 10.1161/01.res.87.12.1095. [DOI] [PubMed] [Google Scholar]
- Keef KD, Hume JR, Zhong J. Regulation of cardiac and smooth muscle Ca(2+) channels (Ca(V)1. 2a,b) by protein kinases. Am J Physiol Cell Physiol. 2001;281:C1743–C1756. doi: 10.1152/ajpcell.2001.281.6.C1743. [DOI] [PubMed] [Google Scholar]
- Kehat I, Molkentin JD. Molecular pathways underlying cardiac remodeling during pathophysiological stimulation. Circulation. 2010;122:2727–35. doi: 10.1161/CIRCULATIONAHA.110.942268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee CB, Crouch TH, Krinks MH. Calcineurin: a calcium- and calmodulin-binding protein of the nervous system. Proc Natl Acad Sci U S A. 1979;76:6270–6273. doi: 10.1073/pnas.76.12.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeche D, Kaprielian R, del Monte F, et al. In vivo cardiac gene transfer of Kv4. 3 abrogates the hypertrophic response in rats after aortic stenosis. Circulation. 2004;110:3435–43. doi: 10.1161/01.CIR.0000148176.33730.3F. [DOI] [PubMed] [Google Scholar]
- Ledoux J, Greenwood I, Villeneuve LR, LeBlanc N. Modulation of Ca2+-dependent Cl-channels by calcineurin in rabbit coronary arterial myocytes. J Physiol. 2003;552:701–14. doi: 10.1113/jphysiol.2003.043836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke T, Welling A, Christel CJ, et al. Unchanged beta-adrenergic stimulation of cardiac L-type calcium channels in Ca v 1. 2 phosphorylation site S1928A mutant mice. J Biol Chem. 2008;283:34738–44. doi: 10.1074/jbc.M804981200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse MJ, Engelhardt S, Eschenhagen T. What is the role of beta-adrenergic signaling in heart failure? Circ Res. 2003;93:896–906. doi: 10.1161/01.RES.0000102042.83024.CA. [DOI] [PubMed] [Google Scholar]
- MacDonnell SM, Weisser-Thomas J, Kubo H, et al. CaMKII negatively regulates calcineurin-NFAT signaling in cardiac myocytes. Circ Res. 2009;105:316–25. doi: 10.1161/CIRCRESAHA.109.194035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall E, Li L, Satoh H, Shannon TR, Blatter LA, Bers DM. Effects of FK-506 on contraction and Ca2+ transients in rat cardiac myocytes. Circ Res. 1996;79:1110–1121. doi: 10.1161/01.res.79.6.1110. [DOI] [PubMed] [Google Scholar]
- McHugh D, Sharp EM, Scheuer T, Catterall WA. Inhibition of cardiac L-type calcium channels by protein kinase C phosphorylation of two sites in the N-terminal domain. Proc Natl Acad Sci U S A. 2000;97:12334–38. doi: 10.1073/pnas.210384297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merat DL, Hu ZY, Carter TE, Cheung WY. Bovine brain calmodulin-dependent protein phosphatase. Regulation of subunit A activity by calmodulin and subunit B. J Biol Chem. 1985;260:11053–59. [PubMed] [Google Scholar]
- Munch G, Bolck B, Karczewski P, Schwinger RH. Evidence for calcineurin-mediated regulation of SERCA 2a activity in human myocardium. J Mol Cell Cardiol. 2002;34:321–34. doi: 10.1006/jmcc.2001.1515. [DOI] [PubMed] [Google Scholar]
- Neumann J, Eschenhagen T, Jones LR, et al. Increased expression of cardiac phosphatases in patients with end-stage heart failure. J Mol Cell Cardiol. 1997;29:265–72. doi: 10.1006/jmcc.1996.0271. [DOI] [PubMed] [Google Scholar]
- Niwa N, Wang W, Sha Q, Marionneau C, Nerbonne JM. Kv4. 3 is not required for the generation of functional Ito, f channels in adult mouse ventricles. J Mol Cell Cardiol. 2008;44:95–104. doi: 10.1016/j.yjmcc.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris CM, Blalock EM, Chen KC, Porter NM, Landfield PW. Calcineurin enhances L-type Ca(2+) channel activity in hippocampal neurons: increased effect with age in culture. Neuroscience. 2002;110:213–25. doi: 10.1016/s0306-4522(01)00574-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveria SF, Dell’Acqua ML, Sather WA. AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling. Neuron. 2007;55:261–75. doi: 10.1016/j.neuron.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EN, Williams RS. Calcineurin signaling and muscle remodeling. Cell. 2000;101:689–92. doi: 10.1016/s0092-8674(00)80880-6. [DOI] [PubMed] [Google Scholar]
- Ono K, Iijima T. Cardiac T-type Ca(2+) channels in the heart. J Mol Cell Cardiol. 2010;48:65–70. doi: 10.1016/j.yjmcc.2009.08.021. [DOI] [PubMed] [Google Scholar]
- Ozawa T. Effects of FK506 on ca release channels (review) Perspect Medicin Chem. 2008;2:51–55. doi: 10.4137/pmc.s382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev. 2003;83:117–61. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- Perrier E, Perrier R, Richard S, Benitah JP. Ca2+ controls functional expression of the cardiac K+ transient outward current via the calcineurin pathway. J Biol Chem. 2004;279:40634–39. doi: 10.1074/jbc.M407470200. [DOI] [PubMed] [Google Scholar]
- Petrashevskaya NN, Bodi I, Rubio M, Molkentin JD, Schwartz A. Cardiac function and electrical remodeling of the calcineurin-overexpressed transgenic mouse. Cardiovasc Res. 2002;54:117–32. doi: 10.1016/s0008-6363(02)00241-9. [DOI] [PubMed] [Google Scholar]
- Rossow CF, Dilly KW, Santana LF. Differential calcineurin/NFATc3 activity contributes to the Ito transmural gradient in the mouse heart. Circ Res. 2006;98:1306–13. doi: 10.1161/01.RES.0000222028.92993.10. [DOI] [PubMed] [Google Scholar]
- Santana LF, Chase EG, Votaw VS, Nelson MT, Greven R. Functional coupling of calcineurin and protein kinase A in mouse ventricular myocytes. J Physiol. 2002;544:57–69. doi: 10.1113/jphysiol.2002.020552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saucerman JJ, Bers DM. Calmodulin mediates differential sensitivity of CaMKII and calcineurin to local Ca2+ in cardiac myocytes. Biophys J. 2008;95:4597–612. doi: 10.1529/biophysj.108.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saucerman JJ, Bers DM. Calmodulin binding proteins provide domains of local Ca2+ signaling in cardiac myocytes. J Mol Cell Cardiol. 2012;52:312–16. doi: 10.1016/j.yjmcc.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino S, Serrano A. Calcineurin signaling and neural control of skeletal muscle fiber type and size. Trends Pharmacol Sci. 2002;23:569–75. doi: 10.1016/s0165-6147(02)02111-9. [DOI] [PubMed] [Google Scholar]
- Schroder F, Klein G, Fiedler B, et al. Single L-type Ca(2+) channel regulation by cGMP-dependent protein kinase type I in adult cardiomyocytes from PKG I transgenic mice. Cardiovasc Res. 2003;60:268–77. doi: 10.1016/s0008-6363(03)00546-7. [DOI] [PubMed] [Google Scholar]
- Schuhmann K, Romanin C, Baumgartner W, Groschner K. Intracellular Ca2+ inhibits smooth muscle L-type Ca2+ channels by activation of protein phosphatase type 2B and by direct interaction with the channel. J Gen Physiol. 1997;110:503–13. doi: 10.1085/jgp.110.5.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz RA, Yutzey KE. Calcineurin signaling and NFAT activation in cardiovascular and skeletal muscle development. Dev Biol. 2004;266:1–16. doi: 10.1016/j.ydbio.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Scott JD, Santana LF. A-kinase anchoring proteins: getting to the heart of the matter. Circulation. 2010;121:1264–71. doi: 10.1161/CIRCULATIONAHA.109.896357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw KT, Ho AM, Raghavan A, et al. Immunosuppressive drugs prevent a rapid dephosphorylation of transcription factor NFAT1 in stimulated immune cells. Proc Natl Acad Sci U S A. 1995;92:11205–9. doi: 10.1073/pnas.92.24.11205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenkman BS, Nemirovskaya TL. Calcium-dependent signaling mechanisms and soleus fiber remodeling under gravitational unloading. J Muscle Res Cell Motil. 2008;29:221–30. doi: 10.1007/s10974-008-9164-7. [DOI] [PubMed] [Google Scholar]
- Stewart AA, Ingebritsen TS, Manalan A, Klee CB, Cohen P. Discovery of a Ca2+- and calmodulin-dependent protein phosphatase: probable identity with calcineurin (CaM-BP80) FEBS Lett. 1982;137:80–84. doi: 10.1016/0014-5793(82)80319-0. [DOI] [PubMed] [Google Scholar]
- Tallant EA, Cheung WY. Characterization of bovine brain calmodulin-dependent protein phosphatase. Arch Biochem Biophys. 1984;232:269–79. doi: 10.1016/0003-9861(84)90543-5. [DOI] [PubMed] [Google Scholar]
- Tandan S, Wang Y, Wang TT, et al. Physical and functional interaction between calcineurin and the cardiac L-type Ca2+ channel. Circ Res. 2009;105:51–60. doi: 10.1161/CIRCRESAHA.109.199828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treinys R, Jurevicius J. L-type Ca2+ channels in the heart: structure and regulation. Medicina (Kaunas) 2008;44:491–99. [PubMed] [Google Scholar]
- Wang HG, Pathan N, Ethell IM, et al. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science. 1999;284:339–43. doi: 10.1126/science.284.5412.339. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hill JA. Electrophysiological remodeling in heart failure. J Mol Cell Cardiol. 2010;48:619–32. doi: 10.1016/j.yjmcc.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Tandan S, Cheng J, et al. Ca2+/calmodulin-dependent protein kinase II-dependent remodeling of Ca2+ current in pressure overload heart failure. J Biol Chem. 2008;283:25524–32. doi: 10.1074/jbc.M803043200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YG, Wagner MB, Joyner RW, Kumar R. cGMP-dependent protein kinase mediates stimulation of L-type calcium current by cGMP in rabbit atrial cells. Cardiovasc Res. 2000;48:310–322. doi: 10.1016/s0008-6363(00)00178-4. [DOI] [PubMed] [Google Scholar]
- Wang Z, Kutschke W, Richardson KE, Karimi M, Hill JA. Electrical remodeling in pressure-overload cardiac hypertrophy: role of calcineurin. Circulation. 2001;104:1657–63. doi: 10.1161/hc3901.095766. [DOI] [PubMed] [Google Scholar]
- Weiss S, Doan T, Bernstein KE, Dascal N. Modulation of cardiac Ca2+ channel by Gq- activating neurotransmitters reconstituted in Xenopus oocytes. J Biol Chem. 2004;279:12503–10. doi: 10.1074/jbc.M310196200. [DOI] [PubMed] [Google Scholar]
- Wilkins BJ, Molkentin JD. Calcium-calcineurin signaling in the regulation of cardiac hypertrophy. Biochem Biophys Res Commun. 2004;322:1178–91. doi: 10.1016/j.bbrc.2004.07.121. [DOI] [PubMed] [Google Scholar]
- Wilson AJ, Jabr RI, Clapp LH. Calcium modulation of vascular smooth muscle ATP-sensitive K(+) channels: role of protein phosphatase-2B. Circ Res. 2000;87:1019–25. doi: 10.1161/01.res.87.11.1019. [DOI] [PubMed] [Google Scholar]
- Xiao L, Coutu P, Villeneuve LR, et al. Mechanisms underlying rate-dependent remodeling of transient outward potassium current in canine ventricular myocytes. Circ Res. 2008;103:733–42. doi: 10.1161/CIRCRESAHA.108.171157. [DOI] [PubMed] [Google Scholar]
- Yang L, Liu G, Zakharov SI, Bellinger AM, Mongillo M, Marx SO. Protein kinase G phosphorylates Cav1. 2 alpha1c and beta2 subunits. Circ Res. 2007;101:465–74. doi: 10.1161/CIRCRESAHA.107.156976. [DOI] [PubMed] [Google Scholar]
- Yatani A, Honda R, Tymitz KM, Lalli MJ, Molkentin JD. Enhanced Ca2+ channel currents in cardiac hypertrophy induced by activation of calcineurin-dependent pathway. J Mol Cell Cardiol. 2001;33:249–59. doi: 10.1006/jmcc.2000.1296. [DOI] [PubMed] [Google Scholar]
- Yue Y, Qu Y, Boutjdir M. Beta- and alpha-adrenergic cross-signaling for L-type Ca current is impaired in transgenic mice with constitutive activation of epsilonPKC. Biochem Biophys Res Commun. 2004;314:749–54. doi: 10.1016/j.bbrc.2003.12.155. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Yakel JL. Calcineurin modulates G protein-mediated inhibition of N-type calcium channels in rat sympathetic neurons. J Neurophysiol. 1997;78:1161–65. doi: 10.1152/jn.1997.78.2.1161. [DOI] [PubMed] [Google Scholar]
- Zobel C, Kassiri Z, Nguyen TT, Meng Y, Backx PH. Prevention of hypertrophy by overexpression of Kv4. 2 in cultured neonatal cardiomyocytes. Circulation. 2002;106:2385–91. doi: 10.1161/01.cir.0000033970.22130.93. [DOI] [PubMed] [Google Scholar]