Abstract
The cyclooxygenase/prostaglandin (COX/PG) signaling pathway is of central importance in inflammation and neoplasia. COX inhibitors are widely used for analgesia and also have demonstrated activity for cancer prophylaxis. However, cardiovascular toxicity associated with this drug class diminishes their clinical utility and motivates the development of safer approaches both for pain relief and cancer prevention. The terminal synthase microsomal PGE synthase-1 (mPGES-1) has attracted considerable attention as a potential target. Overexpression of mPGES-1 has been observed in both colorectal and breast cancers, and gene knockout and overexpression approaches have established a role for mPGES-1 in gastrointestinal carcinogenesis. Here we evaluate the contribution of mPGES-1 to mammary tumorigenesis using a gene knockout approach. Mice deficient in mPGES-1 were crossed with a strain in which breast cancer is driven by overexpression of human epidermal growth factor receptor 2 (HER2/neu). Loss of mPGES-1 was associated with a substantial reduction in intramammary PGE2 levels, aromatase activity, and angiogenesis in mammary glands from HER2/neu transgenic mice. Consistent with these findings, we observed a significant reduction in multiplicity of tumors ≥1mm in diameter, suggesting that mPGES-1 contributes to mammary tumor growth. Our data identify mPGES-1 as a potential anti-breast cancer target.
Keywords: Mouse, mPGES-1, breast cancer, aromatase, angiogenesis, PGE2
1. Introduction
A wealth of evidence supports the inducible prostaglandin (PG) synthase prostaglandin-endoperoxide synthase 2, more commonly called cyclooxygenase-2 (COX-2), as a target for prevention of both breast and other cancers [1, 2]. COX-2 is overexpressed in numerous human cancers, including a significant proportion of breast neoplasias and the majority of colorectal cancers (CRC). Animal studies demonstrate convincing protective effects of both pharmacological inhibition and genetic ablation of COX-2 in numerous cancer models, most notably CRC models. We and others have shown that inhibiting or knocking out COX-2 suppresses mammary tumor formation in rodents [2–4]. Conversely, transgenic COX-2 over-expression induces mammary tumor formation [5]. Protective effects of COX inhibition are supported by epidemiological observations of correlations between use of COX-inhibiting non-steroidal antiinflammatory drugs (NSAIDs) and reduced incidence of breast and colon cancers [1, 2]. Furthermore, clinical trials have established that selective COX-2 inhibitors, or COXibs, suppress formation and induce regression of colorectal adenomas [6–9]. Nevertheless, COXibs are not considered clinically useful for cancer prophylaxis in the general population due to associated cardiovascular toxicity, ironically identified in some of the same trials which demonstrated chemopreventive efficacy [10–12].
The cardiovascular toxicity of COX-2 inhibitors may be partially attributable to perturbation of the thromboxane:prostacyclin (TXA2:PGI2) ratio, since COX-2 inhibition causes selective depression of endothelial, COX-2-derived PGI2 without reducing levels of COX-1-derived TXA2 released from platelets [13]. Prothrombotic effects of COX-2 suppression have now been demonstrated in multiple animal models, and are phenocopied by deletion of prostacyclin receptors [14–17].
The adverse prothrombotic effects of COX-2 inhibition provide substantial impetus for developing alternative strategies for suppressing inflammation and neoplasia which leverage the efficacy of COX-2 inhibition while sidestepping the associated toxicity. Potential targets include the terminal synthases responsible for conversion of COX-2-generated PGH2 to PGE2, because PGE2 is the prostanoid most strongly implicated in pain, inflammation and neoplasia. Three such enzymes have been identified with in vitro PGE2 synthetic capacity: microsomal PGE2 synthases (mPGES) 1 and 2, and a cytosolic PGES (cPGES/p23) [18, 19]. Gene knockout studies implicate mPGES-1 as playing a pivotal role in PGE2 synthesis in vivo under several conditions [20–24]. Notably, peritoneal macrophages from mPGES-1-null mice are unable to produce PGE2 in response to inflammatory challenge [22–24]. Similarly to COX-2, mPGES-1 is constitutively expressed in a limited number of organs, is upregulated in response to various proinflammatory stimuli, and expression is suppressed by glucocorticoids [25, 26]. Strikingly, mPGES-1 upregulation has been identified in numerous cancers, including those of the lung, head and neck, gastrointestinal tract, and breast [27–32]. Furthermore, genetic manipulation studies (overexpression, knockout and knockdown approaches) suggest that mPGES-1 may be a significant contributor to carcinogenesis [33–39], and thus potentially a viable alternative to COX-2 as an anti-neoplastic target.
In this study, we have used a genetic approach to evaluate the role of mPGES-1 in breast cancer, by crossing mPGES-1-deficient mice with a strain in which breast cancer is driven by HER2/neu overexpression. Loss of mPGES-1 was associated with a substantial reduction in intramammary PGE2 levels, aromatase activity, and angiogenesis in mammary glands from HER2/neu transgenic mice. Consistent with these findings, we observed a significant reduction in multiplicity of tumors greater than 1mm in diameter, suggesting that mPGES-1 contributes to mammary tumor growth.
2. Materials and methods
2.1. Materials
Enzyme-linked immunoassay (ELISA) kits for PGE2 analysis were purchased from Cayman Chemicals. Lowry protein assay kits were obtained from Sigma. 1β-[3H]-androstenedione was from Perkin-Elmer Life Science. RNeasy mini kits were purchased from Qiagen. MuLV reverse transcriptase, RNase inhibitor, oligo (dT)16, and SYBR green PCR master mix were obtained from Applied Biosystems. Real-time PCR primers were synthesized by Sigma-Aldrich. All other chemicals were obtained from Fisher Scientific or Sigma-Aldrich.
2.2. Mouse Experimental Procedure
All mice were used in accordance with protocols approved by the Institutional Animal Care and Use Committee of the Rockefeller University or the New York Blood Center. Both facilities are accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, and operate in accordance with Federal (PHS Policy on the Human Care and Use of Animals, Guide for the Use and Care of Laboratory Animals, Animal Welfare Act), State and local laws and regulations. Mice were provided food and water ad libitum. The previously reported mPGES-1 −/− strain [23] was obtained from Pfizer on a hybrid DBA/1:lacJ strain background, and crossed with FVB/J wildtype mice at The Jackson Laboratory using targeted speed congenics to introduce the targeted mPGES-1 allele onto an FVB background. Previous characterization of this strain confirmed the lack of mPGES-1 protein in mPGES-1-null mice [23]. MMTV/NDL mice express a mutationally activated HER2/neu allele (NDL, Neu Deletion mutant) that induces mammary hyperplasia and tumorigenesis [40]. MMTV/NDL and FVB-mPGES-1 +/− mice were interbred to generate MMTV/NDL, mPGES-1 +/− progeny, which were backcrossed with FVB-mPGES-1 +/− mice to generate females of the required test genotypes: MMTV/NDL, mPGES-1 +/+; MMTV/NDL, mPGES-1 +/−; and MMTV/NDL, mPGES-1 −/−. Genotypes were determined by PCR analysis of tail-tip-derived genomic DNA as previously described [4, 23]. Virgin test females were sacrificed at 20 weeks of age, and tissues were harvested as described below. All test animals were overtly healthy at sacrifice, and animal weights were not different between the three groups. Weights at sacrifice (mean±SD) of test animals were: MMTV/NDL, mPGES-1 +/+, 25.0±2.4g; MMTV/NDL, mPGES-1 +/−, 24.9±2.1g; MMTV/NDL, mPGES-1 −/−, 24.4±1.9g.
2.3. Mammary Tissue Harvesting and Analysis
Abdominal (#4) mammary glands (MGs) were either snap-frozen in liquid nitrogen and stored at −80°C, or stained with carmine alum and mammary wholemounts prepared as previously described [4]. Tumor multiplicity was scored in carmine alum-stained mammary wholemounts in a blinded manner by two independent investigators using an eyepiece graticule on a dissecting microscope, and a threshold diameter of 0.25mm. To evaluate the effect of mPGES-1 deficiency on tumor size, tumor multiplicity was rescored using 1.0mm as the threshold diameter. Axillary (#3) MGs were fixed in formalin and embedded in paraffin for histopathological analysis.
Anti-CD31 immunohistochemistry (IHC) was performed using monoclonal rat anti-mouse CD31 antibody (Pharmingen) on 5μm sections of formalin-fixed, paraffin-embedded MGs, and sections were counterstained with methyl green as previously described [4]. One slide was examined from each animal, and several microscopic fields were examined on each slide. The number of CD31-positive blood vessels associated with a ductal or lobular unit was scored in each field, and a mean value was generated for each animal.
PGE2 in snap-frozen MGs was assayed using an ELISA kit as previously described [41]. Aromatase activity in microsomes prepared from snap-frozen MGs was assayed by measuring tritiated water release from 1β-[3H]-androstenedione, as previously described [42].
Vascular endothelial growth factor A (VEGF-A) expression was assayed by quantitative reverse transcriptase-coupled PCR (RT-PCR) of cDNA prepared from snap-frozen MGs. Primers used were: forward, 5′-GAA AGG GAA AGG GTC AAA AA-3′; reverse, 5′-CAC ATC TGC AAG TAC GTT CG-3′. VEGF-A transcript levels were normalized to GAPDH transcript level for each sample. Relative fold induction was determined using the ddCT (relative quantification) analysis protocol.
2.4. Statistical Analysis
Tumor multiplicity data
The mean tumor counts from the two investigators among the three groups (MMTV/NDL, mPGES-1 +/+; MMTV/NDL, mPGES-1 +/−; and MMTV/NDL, mPGES-1 −/−) were compared using ANOVA followed by a t-test for pair-wise comparisons. P values from the pair-wise comparisons were adjusted for multiple comparisons using Tukey’s method.
PGE2 levels, aromatase activity, and gene expression
The non-parametric Wilcoxon rank-sum test was used to compare PGE2 levels, aromatase activity, and VEGF expression levels, between MGs from MMTV/NDL, mPGES-1 +/+ and MMTV/NDL, mPGES-1 −/− mice.
Microvessel density
Average CD31-positive blood vessel counts in multiple microscopic fields per mouse MG sample were compared between MMTV/NDL, mPGES-1 +/+ and MMTV/NDL, mPGES-1 −/− mice using a Student t-test.
3. Results
The goal of this study was to evaluate the contribution of the terminal PGE2 synthase mPGES-1 to mammary tumorigenesis. Despite the identification of multiple proteins with in vitro PGE2 synthase activity, mPGES-1 has emerged as a key determinant of PGE2 synthesis under numerous conditions, such as in response to inflammatory stimuli (e.g. lipopolysaccharide) as well as in lactating murine mammary gland [22–24, 43]. Furthermore, numerous studies using overexpression and genetic ablation approaches have implicated mPGES-1 in experimental tumorigenesis, particularly in gastrointestinal cancer models [33–35, 37, 39]. Here we used a genetic approach to evaluate the potential contribution of mPGES-1 to breast cancer. Specifically, we determined the consequences of knocking out mPGES-1 in MMTV/NDL mice, a model of HER2/neu-overexpressing breast cancer that we previously employed to demonstrate that Cox-2 contributes to HER2/neu-induced mammary tumorigenesis [4]. The MMTV/NDL strain expresses a mammary-targeted, mutationally activated HER2/neu transgene, and exhibits mammary hyperplasia which progresses through mammary intraepithelial neoplastic (MIN) lesions resembling human ductal carcinoma in situ (DCIS) to invasive breast cancers, which ultimately metastasize to the lung [40]. Multiple MIN lesions develop in each mammary gland in virgin females by 20 weeks of age [4]. Thus, we used the MMTV/NDL strain as a useful quantitative model of intraepithelial neoplasia analogous to mutant Apc strains frequently used to study intestinal neoplasia.
3.1. Effect of mPGES-1 deletion on HER2/neu-induced mammary tumorigenesis
Mice with targeted deletion of mPGES-1 were obtained on a DBA/1lacJ background, and the mutant mPGES-1 allele was introgressed onto an FVB background to negate potential confounding effects due to mixed strain backgrounds. Subsequently, MMTV/NDL mice were interbred with mPGES-1-deficient mice to generate test females of three genotypes in which to compare mammary tumor multiplicity: MMTV/NDL, mPGES-1 +/+; MMTV/NDL, mPGES-1 +/−; and MMTV/NDL, mPGES-1 −/−. Tumor multiplicity was analyzed in carmine alum-stained #4 abdominal mammary gland wholemounts harvested from 20-week old virgin females of all three genotypes, using two size thresholds (0.25mm and 1.0mm diameter). Tumor multiplicity was similar in all three cohorts when we scored all tumors of ≥ 0.25mm diameter (Table 1). Interestingly however, mPGES-1 deficiency was associated with a reduction in the number of tumors of ≥ 1.0mm in diameter: tumor number was significantly reduced in both mPGES-1 heterozygous and null animals relative to those carrying two wildtype mPGES-1 alleles (Table 1; P=0.008 and 0.025, respectively). These data suggest that mPGES-1 contributes to mammary tumor growth. Given the similar findings in MMTV/NDL mice that were mPGES-1 heterozygous and nullizygous, we focused exclusively on comparisons of mPGES-1 wildtype and null tissues for subsequent mechanistic analyses.
Table 1.
Effect of mPGES-1 deficiency on mammary tumor multiplicity
| Genotype (n) | Tumors ≥0.25mm (Mean +/− sd) |
P vs control ‡ | Tumors ≥1.0mm (Mean +/− sd) |
P vs control |
|---|---|---|---|---|
| MMTV/NDL, mPGES-1 +/+ (22) | 14.34 +/− 9.29 | - | 1.05 +/− 1.13 | - |
| MMTV/NDL, mPGES-1 +/− (20) | 11.2 +/− 4.58 | 0.336 | 0.25 +/− 0.5 | 0.008 |
| MMTV/NDL, mPGES-1 −/− (30) | 12.8 +/− 6.76 | 0.724 | 0.42 +/− 0.76 | 0.025 |
Comparisons effected with MMTV/NDL, mPGES-1 +/+
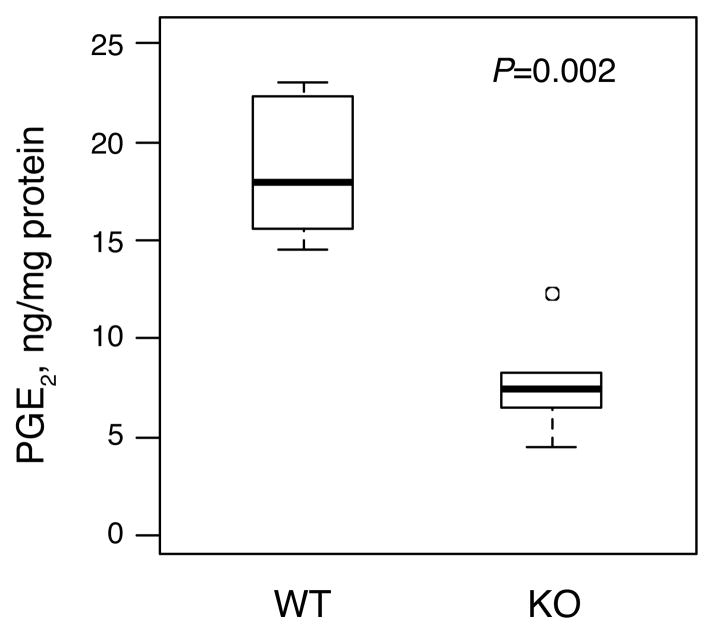
As anticipated, mammary PGE2 levels were substantially reduced by genetic ablation of mPGES-1. Median PGE2 levels of 18.0 ng/mg protein were detected in MGs from MMTV/NDL, mPGES-1 +/+ mice (Figure 1). Deletion of mPGES-1 caused an approximately 60% reduction in intramammary PGE2 levels (MMTV/NDL, mPGES-1 −/−, median=7.4 ng/mg protein; P=0.002).
Fig. 1.
Mammary PGE2 levels are markedly decreased in mPGES-1 knockout MMTV/NDL mice. MGs were harvested from 20 week old virgin female mice that were MMTV/NDL, mPGES-1 +/+ (WT) or MMTV/NDL, mPGES-1 −/− (KO), and PGE2 levels were assayed by ELISA. Mammary PGE2 levels were reduced from 18.0 [14.5, 23.0] ng/mg protein (median [range], n=6) in MMTV/NDL, mPGES-1 +/+ samples to 7.4 [4.5, 12.3] ng/mg protein in MMTV/NDL, mPGES-1 −/− samples (P=0.002; Wilcoxon rank-sum test).
3.2. Microvessel density and VEGF expression are reduced by genetic ablation of mPGES-1
Our subsequent studies focused on identifying potential mechanisms by which mPGES-1 might regulate HER2/neu-dependent mammary tumor growth. COX enzymes and COX-derived PGE2 are strongly implicated in angiogenesis [44, 45]. Notably, we previously reported a profound reduction in vascularization both of MIN lesions and normal-appearing mammary gland in MMTV/NDL mice lacking functional Cox-2 [4]. Furthermore, transgenic COX-2 overexpression in mouse MG drives extensive vascular development [46]. Importantly, angiogenesis is considered to be an obligate step in tumor growth. Based on the observed reduction in intramammary PGE2 levels and corresponding suppression of tumor growth in MMTV/NDL mice lacking functional mPGES-1, we therefore explored the possibility that mammary gland vascularization was defective in mPGES-1-null animals.
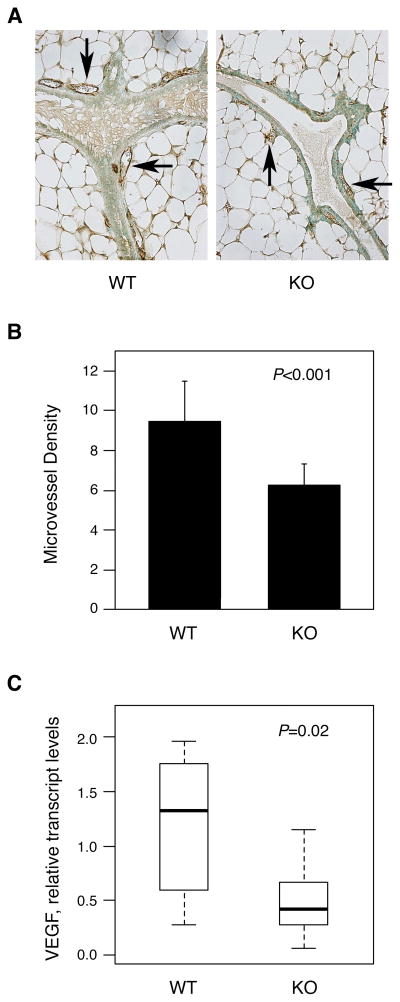
Microvessel density was scored in MGs from MMTV/NDL, mPGES-1 +/+ and MMTV/NDL, mPGES-1 −/− mice by quantitating the number of CD31-positive blood vessels observed in association with ductal or lobular units in each microscopic field (Figure 2A). The mean CD31-positive blood vessel count was significantly reduced in mPGES-1-null mammary tissues (Figure 2B; P<0.001), implicating mPGES-1 in mammary vascularization. Consistent with this observation, expression of VEGF-A, a key driver of angiogenesis, was significantly reduced in MGs lacking mPGES-1 (Figure 2C; P=0.02). The median VEGF-A transcript level in MGs from MMTV/NDL, mPGES-1 −/− mice was only 32% of that in glands with wildtype mPGES-1. Our data suggest that mPGES-1 may regulate tumor growth at least in part via controlling vascular development.
Fig. 2.
Mammary gland vascularization is reduced in mPGES-1-null MMTV/NDL mice. (A, B) Microvessel density is reduced in mPGES-1-null tissue. MG tissue sections from 20 week old virgin MMTV/NDL females that were mPGES-1 wildtype (WT) or mPGES-1 null (KO) were immunohistochemically stained with anti-CD31 antibody. Several microscopic fields were evaluated for each animal. The number of CD31-positive blood vessels associated with a ductal or lobular unit was scored in each microscopic field, and a mean value was calculated for each mouse. The mean CD31-positive blood vessel count in WT mice was significantly greater than that in KO mice: 9.45+/−2.05 (mean+/−SD, n=10) vs 6.26+/−1.07 (mean+/−SD, n=8); P<0.001 (Student t-test). Panel A shows representative images for mPGES-1 wildtype (WT) and mPGES-1 null (KO) mammary glands. Examples of CD31-positive blood vessels are indicated by arrows. Panel B shows the data obtained from numerical evaluation of anti-CD31-stained tissue sections. (C) VEGF levels are strikingly reduced in mPGES-1-null tissue. Transcript levels of VEGF-A were assayed in MGs harvested from 20 week old virgin female mice that were MMTV/NDL, mPGES-1 +/+ (WT) or MMTV/NDL, mPGES-1 −/− (KO). Relative VEGF-A transcript levels (normalized to GAPDH) were reduced from 1.33 [0.28, 1.96] (median [range], n=10) in MMTV/NDL, mPGES-1 +/+ samples to 0.42 [0.06, 1.15] in MMTV/NDL, mPGES-1 −/− samples (P=0.02; Wilcoxon rank-sum test).
3.3. Mammary aromatase activity is substantially reduced in mPGES-1-deficient MGs
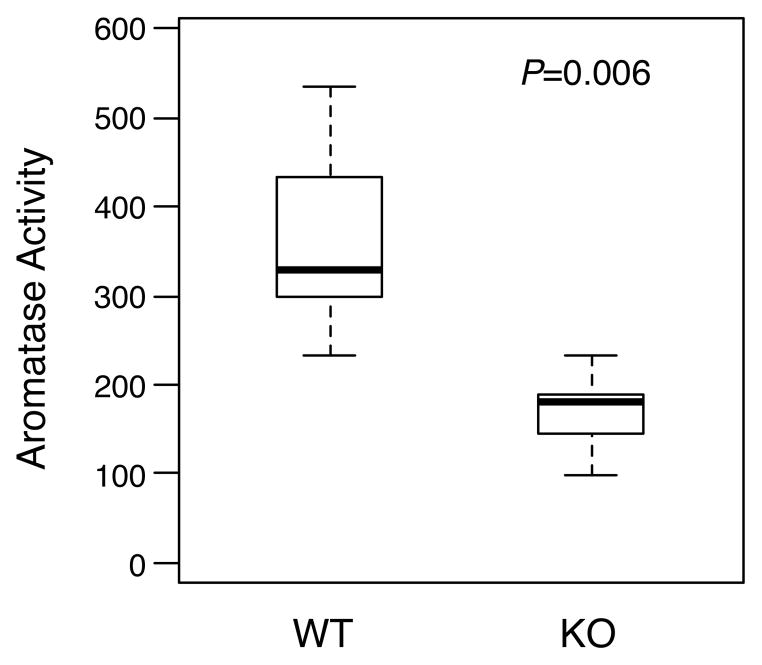
Extensive data support the estrogen synthase aromatase as a potentially key effector of PGE2 signaling in mammary neoplasia [47]. Expression of the CYP19 gene encoding aromatase is increased by PGE2 signaling via a cascade involving cAMP and cyclic AMP response element-binding protein (CREB), resulting in promoter switching and upregulated aromatase expression and activity [48–53]. Activation of this axis has been identified both in breast neoplasia and in normal mammary tissues in the context of obesity [41, 42, 54–56], and is presumed to be an important determinant of NSAID-mediated suppression of postmenopausal breast cancer. Thus it was logical to test the effect of mPGES-1 deficiency on mammary aromatase activity in our study. We observed an approximately 45% reduction in aromatase activity in MGs from MMTV/NDL mice lacking mPGES-1 (Figure 3; P=0.006). These data support the notion that mPGES-1-derived PGE2 drives aromatase expression in MMTV/NDL mammary tissues.
Fig. 3.
Aromatase activity is substantially reduced in MGs from mPGES-1-deficient MMTV/NDL mice. Aromatase activity was assayed in microsomes prepared from MGs harvested from 20 week old virgin female mice that were MMTV/NDL, mPGES-1 +/+ (WT) or MMTV/NDL, mPGES-1 −/− (KO). Aromatase activity was assayed by measuring tritiated water release from 1β-[3H]-androstenedione. Mammary aromatase activity was reduced from 328 [234, 534] fmoles/μg protein/hr (median [range], n=6) in MMTV/NDL, mPGES-1 +/+ samples to 182 [98, 234] fmoles/μg protein/hr in MMTV/NDL, mPGES-1 −/− samples (P=0.006; Wilcoxon rank-sum test).
4. Discussion
In this study we tested the role of mPGES-1 in mammary tumorigenesis by crossing mPGES-1-deficient mice with the MMTV/NDL breast cancer model, and quantitating the multiplicity of MIN tumors. Based on our previous study in which we knocked out COX-2 in the MMTV/NDL strain [4], we anticipated that we would see a global reduction in tumor number. Unexpectedly however, only tumors above the 1.0mm threshold were affected by loss of mPGES-1 (Table 1). Our data are strikingly similar to findings from the Rosenberg group, who have studied the role of mPGES-1 in intestinal tumorigenesis. They found that small intestinal polyp multiplicity in ApcΔ14/+ mice was substantially reduced by mPGES-1 deletion [35]. However, only tumors > 1.0mm diameter were decreased, whereas the multiplicity of polyps ≤ 1.0mm in diameter was significantly increased. Together the parallel findings in our study and in the ApcΔ14/+ model suggest that mPGES-1, and by extension PGE2, may contribute to tumor growth rather than to initial tumor formation in some experimental systems. This finding does not hold true for all models: mPGES-1 deficiency impacts carcinogen-induced neoplasia at all stages including decreasing aberrant crypt focus formation [35, 37], and unexpectedly, Elander and colleagues observed increased tumor formation in ApcMin/+ mice in the context of mPGES-1 deficiency [57].
Interestingly, direct comparison of the consequences of knocking out COX-2 and mPGES-1 in MMTV/NDL mice suggests that other COX-derived eicosanoids could contribute to early tumor formation in this strain, since both knockouts result in comparable magnitudes of reduction of intramammary PGE2 levels (COX-2 ko, 50% reduction [4]; mPGES-1 ko, 59% reduction, Figure 1), but only COX-2 nulls exhibit a reduction in tumors <1mm in diameter. Knocking out mPGES-1 was previously shown to cause a greater than 90% reduction in PGE2 levels in lactating mammary gland [43], consistent with data from other systems where mPGES-1 has been identified as the predominant source of PGE2 [20–24]. Residual PGE2 levels in MGs from MMTV/NDL, mPGES-1-null mice in our experiment may reflect the activity of other PGE synthases or potentially non-enzymatic isomerization. It is conceivable that selective suppression of PGE2 synthesis leads to a synthetic “shunt”, resulting in increased conversion of the PGH2 precursor to other protumorigenic eicosanoids in the mPGES-1 nulls relative to the COX-2 ko mice. This could provide a rational basis for the decreased magnitude of tumor protection afforded by mPGES-1 ablation.
Subsequent analyses in our study focused on identifying potential mechanistic explanations for the observed reduction in tumor growth in mPGES-1-deficient animals. Based on the known link between PGE2 and angiogenesis [1, 44, 45], we first explored the impact of mPGES-1 ablation on mammary vascularization. Consistent with the observed reduction in PGE2 levels, both microvessel density and VEGF-A expression were significantly decreased in mPGES-1 null MGs (Figure 2). These data are consistent with our previous reports of decreased vasculature in COX-2 null MGs, and increased vascular development in COX-2 transgenic glands [4, 46]. Previous reports similarly implicate mPGES-1 in angiogenesis [38, 58, 59], with a clear role for stromal mPGES-1 identified by transplant studies. Consistent with a role for mPGES-1 in angiogenesis, correlations have been observed between levels of mPGES-1 and proangiogenic factors in some human cancers [60]. Given the well-established requirement for neovascularization for tumor growth, reduced angiogenesis provides a plausible explanation for the observed reduction in mammary tumor growth in mPGES-1 knockout mice.
Also of interest was to determine the impact of mPGES-1 ablation on the activity of the estrogen synthetase aromatase. PGE2 is an established regulator of the CYP19 gene encoding aromatase, acting via a clearly defined pathway involving cAMP and CREB, and ultimately resulting in increased transcription from cAMP-sensitive promoters [48–53]. MMTV/NDL MGs lacking mPGES-1 exhibited similar magnitudes of reduction in aromatase activity and PGE2 levels (Figures 1 & 3), consistent with our previous data from COX-2 knockout mice [52]. These data suggest that local estrogen production in mammary tissues is impaired in the absence of mPGES-1.
The role of estrogen receptor (ER) signaling in HER2/neu-driven breast cancer is complex. HER2-overexpressing human breast carcinomas tend to lack ER expression, as do invasive cancers in HER2/neu transgenic strains. Nevertheless, treatment of post-pubertal MMTV/neu mice with a selective estrogen receptor modulator (SERM) delays mammary tumor formation, implicating estrogen signaling in HER2/neu-induced tumor development [61]. In this context, estrogen may regulate mammary tumor formation through direct effects on epithelial cells prior to loss of ER expression during development of invasive lesions. Alternatively, the role of estrogen in HER2/neu-driven tumor formation may be primarily regulation of angiogenesis through interaction with ER-expressing stromal cells [62–64]. The ability of estrogen to regulate angiogenesis is well established [65], but the capacity of estrogen to promote ER-negative breast tumor growth via modulation of stromal cells in the tumor microenvironment is a comparatively recent discovery [62–64]. These findings suggest the possibility that the decreased vascularization observed in mPGES-1-null mammary glands could be a consequence not only of attenuation of PGE2-driven synthesis of proangiogenic factors, but also of decreased PGE2-dependent estrogen synthesis impacting stromal angiogenic responses.
In summary, using a genetic approach we have established a role for mPGES-1 in mammary tumor growth and angiogenesis. Multiplicity of HER2/neu-induced MIN lesions ≥1mm in diameter is reduced by mPGES-1 nullizygosity, with corresponding reductions in mammary PGE2, aromatase activity and angiogenesis. These data suggest mPGES-1 as a potential anti-breast cancer target, based on the reported upregulation of mPGES-1 in tumor epithelium in almost four-fifths of human breast cancers, as well as in DCIS [31]. Relative safety of this approach compared with COX-2 inhibition is suggested by the lack of prothrombotic phenotype associated with knocking out mPGES-1 [14]. Importantly, we have recently identified that breast adipose inflammation with consequent upregulation of the PG-aromatase-estrogen signaling axis is associated with overweight and obesity [41, 42, 56], which may provide at least a partial explanation for the increased risk of breast cancer associated with obesity in post-menopausal women. Findings of the present study suggest mPGES-1 as a potential target for intervention to reduce the increased breast cancer risk associated with obesity in the post-menopausal setting.
Pharmacological mPGES-1 inhibitors for antiinflammatory and antineoplastic applications are currently under development. Evaluation of numerous compounds has identified several promising candidates [66–71], including some with in vivo analgesic/antiinflammatory properties, although no clinical studies have thus far been reported. Preclinical evaluation of these molecules in animal cancer models has been hampered by structural dissimilarities between human and rodent mPGES-1 enzymes at the active site, which render mouse and rat orthologs insensitive to multiple compounds that have activity towards human mPGES-1. Nevertheless, mPGES-1 inhibitor-mediated suppression of tumor xenograft growth has recently been reported [59], providing important proof-of-principle for the validity of mPGES-1 inhibition as an anticancer approach.
Highlights.
Knocking out mPGES-1 suppresses mouse mammary tumor growth
Angiogenesis is reduced by genetic ablation of mPGES-1
Aromatase activity is substantially reduced in mPGES-1-deficient mammary glands
Acknowledgments
Funding
This work was supported by National Institutes of Health (CA154481 to A.J.D.); Breast Cancer Research Foundation (to A.J.D., C.A.H.); and the Botwinick-Wolfensohn Foundation (in memory of Mr. and Mrs. Benjamin Botwinick). The sponsors played no role in study design; collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Sara Khalil and Grace Tan are gratefully acknowledged for their excellent technical assistance.
Footnotes
Conflict of Interest Statement: None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brown JR, DuBois RN. COX-2: a molecular target for colorectal cancer prevention. J Clin Oncol. 2005;23:2840–55. doi: 10.1200/JCO.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 2.Howe LR. Inflammation and breast cancer. Cyclooxygenase/prostaglandin signaling and breast cancer. Breast Cancer Res. 2007;9:210. doi: 10.1186/bcr1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris RE, Alshafie GA, Abou-Issa H, Seibert K. Chemoprevention of breast cancer in rats by celecoxib, a cyclooxygenase 2 inhibitor. Cancer Res. 2000;60:2101–3. [PubMed] [Google Scholar]
- 4.Howe LR, Chang SH, Tolle KC, Dillon R, Young LJ, Cardiff RD, et al. HER2/neu-induced mammary tumorigenesis and angiogenesis are reduced in cyclooxygenase-2 knockout mice. Cancer Res. 2005;65:10113–9. doi: 10.1158/0008-5472.CAN-05-1524. [DOI] [PubMed] [Google Scholar]
- 5.Liu CH, Chang SH, Narko K, Trifan OC, Wu MT, Smith E, et al. Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem. 2001;276:18563–9. doi: 10.1074/jbc.M010787200. [DOI] [PubMed] [Google Scholar]
- 6.Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–52. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 7.Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Solomon SD, Kim K, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355:873–84. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 8.Arber N, Eagle CJ, Spicak J, Racz I, Dite P, Hajer J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355:885–95. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 9.Baron JA, Sandler RS, Bresalier RS, Quan H, Riddell R, Lanas A, et al. A randomized trial of rofecoxib for the chemoprevention of colorectal adenomas. Gastroenterology. 2006;131:1674–82. doi: 10.1053/j.gastro.2006.08.079. [DOI] [PubMed] [Google Scholar]
- 10.Solomon SD, Pfeffer MA, McMurray JJ, Fowler R, Finn P, Levin B, et al. Effect of celecoxib on cardiovascular events and blood pressure in two trials for the prevention of colorectal adenomas. Circulation. 2006;114:1028–35. doi: 10.1161/CIRCULATIONAHA.106.636746. [DOI] [PubMed] [Google Scholar]
- 11.Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, et al. Cardiovascular Events Associated with Rofecoxib in a Colorectal Adenoma Chemoprevention Trial. N Engl J Med. 2005 doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- 12.Rostom A, Dube C, Lewin G, Tsertsvadze A, Barrowman N, Code C, et al. Nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors for primary prevention of colorectal cancer: a systematic review prepared for the U.S. Preventive Services Task Force. Ann Intern Med. 2007;146:376–89. doi: 10.7326/0003-4819-146-5-200703060-00010. [DOI] [PubMed] [Google Scholar]
- 13.Funk CD, FitzGerald GA. COX-2 inhibitors and cardiovascular risk. J Cardiovasc Pharmacol. 2007;50:470–9. doi: 10.1097/FJC.0b013e318157f72d. [DOI] [PubMed] [Google Scholar]
- 14.Cheng Y, Wang M, Yu Y, Lawson J, Funk CD, Fitzgerald GA. Cyclooxygenases, microsomal prostaglandin E synthase-1, and cardiovascular function. J Clin Invest. 2006;116:1391–9. doi: 10.1172/JCI27540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hennan JK, Huang J, Barrett TD, Driscoll EM, Willens DE, Park AM, et al. Effects of selective cyclooxygenase-2 inhibition on vascular responses and thrombosis in canine coronary arteries. Circulation. 2001;104:820–5. doi: 10.1161/hc3301.092790. [DOI] [PubMed] [Google Scholar]
- 16.Murata T, Ushikubi F, Matsuoka T, Hirata M, Yamasaki A, Sugimoto Y, et al. Altered pain perception and inflammatory response in mice lacking prostacyclin receptor. Nature. 1997;388:678–82. doi: 10.1038/41780. [DOI] [PubMed] [Google Scholar]
- 17.Seta F, Chung AD, Turner PV, Mewburn JD, Yu Y, Funk CD. Renal and cardiovascular characterization of COX-2 knockdown mice. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1751–60. doi: 10.1152/ajpregu.90985.2008. [DOI] [PubMed] [Google Scholar]
- 18.Kudo I, Murakami M. Prostaglandin E synthase, a terminal enzyme for prostaglandin E2 biosynthesis. J Biochem Mol Biol. 2005;38:633–8. doi: 10.5483/bmbrep.2005.38.6.633. [DOI] [PubMed] [Google Scholar]
- 19.Smith WL, Urade Y, Jakobsson PJ. Enzymes of the cyclooxygenase pathways of prostanoid biosynthesis. Chem Rev. 2011;111:5821–65. doi: 10.1021/cr2002992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jania LA, Chandrasekharan S, Backlund MG, Foley NA, Snouwaert J, Wang IM, et al. Microsomal prostaglandin E synthase-2 is not essential for in vivo prostaglandin E2 biosynthesis. Prostaglandins Other Lipid Mediat. 2009;88:73–81. doi: 10.1016/j.prostaglandins.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lovgren AK, Kovarova M, Koller BH. cPGES/p23 is required for glucocorticoid receptor function and embryonic growth but not prostaglandin E2 synthesis. Mol Cell Biol. 2007;27:4416–30. doi: 10.1128/MCB.02314-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boulet L, Ouellet M, Bateman KP, Ethier D, Percival MD, Riendeau D, et al. Deletion of microsomal prostaglandin E2 (PGE2) synthase-1 reduces inducible and basal PGE2 production and alters the gastric prostanoid profile. J Biol Chem. 2004;279:23229–37. doi: 10.1074/jbc.M400443200. [DOI] [PubMed] [Google Scholar]
- 23.Trebino CE, Stock JL, Gibbons CP, Naiman BM, Wachtmann TS, Umland JP, et al. Impaired inflammatory and pain responses in mice lacking an inducible prostaglandin E synthase. Proc Natl Acad Sci U S A. 2003;100:9044–9. doi: 10.1073/pnas.1332766100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uematsu S, Matsumoto M, Takeda K, Akira S. Lipopolysaccharide-dependent prostaglandin E(2) production is regulated by the glutathione-dependent prostaglandin E(2) synthase gene induced by the Toll-like receptor 4/MyD88/NF-IL6 pathway. J Immunol. 2002;168:5811–6. doi: 10.4049/jimmunol.168.11.5811. [DOI] [PubMed] [Google Scholar]
- 25.Jakobsson PJ, Thoren S, Morgenstern R, Samuelsson B. Identification of human prostaglandin E synthase: a microsomal, glutathione-dependent, inducible enzyme, constituting a potential novel drug target. Proc Natl Acad Sci U S A. 1999;96:7220–5. doi: 10.1073/pnas.96.13.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samuelsson B, Morgenstern R, Jakobsson PJ. Membrane prostaglandin E synthase-1: a novel therapeutic target. Pharmacol Rev. 2007;59:207–24. doi: 10.1124/pr.59.3.1. [DOI] [PubMed] [Google Scholar]
- 27.Yoshimatsu K, Altorki NK, Golijanin D, Zhang F, Jakobsson PJ, Dannenberg AJ, et al. Inducible prostaglandin E synthase is overexpressed in non-small cell lung cancer. Clin Cancer Res. 2001;7:2669–74. [PubMed] [Google Scholar]
- 28.Yoshimatsu K, Golijanin D, Paty PB, Soslow RA, Jakobsson PJ, DeLellis RA, et al. Inducible microsomal prostaglandin E synthase is overexpressed in colorectal adenomas and cancer. Clin Cancer Res. 2001;7:3971–6. [PubMed] [Google Scholar]
- 29.Cohen EG, Almahmeed T, Du B, Golijanin D, Boyle JO, Soslow RA, et al. Microsomal prostaglandin E synthase-1 is overexpressed in head and neck squamous cell carcinoma. Clin Cancer Res. 2003;9:3425–30. [PubMed] [Google Scholar]
- 30.van Rees BP, Sivula A, Thoren S, Yokozaki H, Jakobsson PJ, Offerhaus GJ, et al. Expression of microsomal prostaglandin E synthase-1 in intestinal type gastric adenocarcinoma and in gastric cancer cell lines. Int J Cancer. 2003;107:551–6. doi: 10.1002/ijc.11422. [DOI] [PubMed] [Google Scholar]
- 31.Mehrotra S, Morimiya A, Agarwal B, Konger R, Badve S. Microsomal prostaglandin E2 synthase-1 in breast cancer: a potential target for therapy. J Pathol. 2006;208:356–63. doi: 10.1002/path.1907. [DOI] [PubMed] [Google Scholar]
- 32.Nakanishi M, Gokhale V, Meuillet EJ, Rosenberg DW. mPGES-1 as a target for cancer suppression: A comprehensive invited review “Phospholipase A2 and lipid mediators”. Biochimie. 2010;92:660–4. doi: 10.1016/j.biochi.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamei D, Murakami M, Nakatani Y, Ishikawa Y, Ishii T, Kudo I. Potential role of microsomal prostaglandin E synthase-1 in tumorigenesis. J Biol Chem. 2003;278:19396–405. doi: 10.1074/jbc.M213290200. [DOI] [PubMed] [Google Scholar]
- 34.Oshima H, Oshima M, Inaba K, Taketo MM. Hyperplastic gastric tumors induced by activated macrophages in COX-2/mPGES-1 transgenic mice. EMBO J. 2004;23:1669–78. doi: 10.1038/sj.emboj.7600170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakanishi M, Montrose DC, Clark P, Nambiar PR, Belinsky GS, Claffey KP, et al. Genetic deletion of mPGES-1 suppresses intestinal tumorigenesis. Cancer Res. 2008;68:3251–9. doi: 10.1158/0008-5472.CAN-07-6100. [DOI] [PubMed] [Google Scholar]
- 36.Hanaka H, Pawelzik SC, Johnsen JI, Rakonjac M, Terawaki K, Rasmuson A, et al. Microsomal prostaglandin E synthase 1 determines tumor growth in vivo of prostate and lung cancer cells. Proc Natl Acad Sci U S A. 2009;106:18757–62. doi: 10.1073/pnas.0910218106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakanishi M, Menoret A, Tanaka T, Miyamoto S, Montrose DC, Vella AT, et al. Selective PGE(2) suppression inhibits colon carcinogenesis and modifies local mucosal immunity. Cancer Prev Res (Phila) 2011;4:1198–208. doi: 10.1158/1940-6207.CAPR-11-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamei D, Murakami M, Sasaki Y, Nakatani Y, Majima M, Ishikawa Y, et al. Microsomal prostaglandin E synthase-1 in both cancer cells and hosts contributes to tumour growth, invasion and metastasis. Biochem J. 2010;425:361–71. doi: 10.1042/BJ20090045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sasaki Y, Kamei D, Ishikawa Y, Ishii T, Uematsu S, Akira S, et al. Microsomal prostaglandin E synthase-1 is involved in multiple steps of colon carcinogenesis. Oncogene. 2012;31:2943–52. doi: 10.1038/onc.2011.472. [DOI] [PubMed] [Google Scholar]
- 40.Siegel PM, Ryan ED, Cardiff RD, Muller WJ. Elevated expression of activated forms of Neu/ErbB-2 and ErbB-3 are involved in the induction of mammary tumors in transgenic mice: implications for human breast cancer. Embo J. 1999;18:2149–64. doi: 10.1093/emboj/18.8.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Subbaramaiah K, Morris PG, Zhou XK, Morrow M, Du B, Giri D, et al. Increased levels of COX-2 and prostaglandin E2 contribute to elevated aromatase expression in inflamed breast tissue of obese women. Cancer Discov. 2012;2:356–65. doi: 10.1158/2159-8290.CD-11-0241. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Subbaramaiah K, Howe LR, Bhardwaj P, Du B, Gravaghi C, Yantiss RK, et al. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res (Phila) 2011;4:329–46. doi: 10.1158/1940-6207.CAPR-10-0381. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Chandrasekharan S, Foley NA, Jania L, Clark P, Audoly LP, Koller BH. Coupling of COX-1 to mPGES1 for prostaglandin E2 biosynthesis in the murine mammary gland. J Lipid Res. 2005;46:2636–48. doi: 10.1194/jlr.M500213-JLR200. [DOI] [PubMed] [Google Scholar]
- 44.Gately S, Li WW. Multiple roles of COX-2 in tumor angiogenesis: a target for antiangiogenic therapy. Semin Oncol. 2004;31:2–11. doi: 10.1053/j.seminoncol.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 45.Leahy KM, Koki AT, Masferrer JL. Role of cyclooxygenases in angiogenesis. Curr Med Chem. 2000;7:1163–70. doi: 10.2174/0929867003374336. [DOI] [PubMed] [Google Scholar]
- 46.Chang SH, Liu CH, Conway R, Han DK, Nithipatikom K, Trifan OC, et al. Role of prostaglandin E2-dependent angiogenic switch in cyclooxygenase 2-induced breast cancer progression. Proc Natl Acad Sci U S A. 2004;101:591–6. doi: 10.1073/pnas.2535911100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diaz-Cruz ES, Brueggemeier RW. Interrelationships between cyclooxygenases and aromatase: unraveling the relevance of cyclooxygenase inhibitors in breast cancer. Anticancer Agents Med Chem. 2006;6:221–32. doi: 10.2174/187152006776930873. [DOI] [PubMed] [Google Scholar]
- 48.Agarwal VR, Bulun SE, Leitch M, Rohrich R, Simpson ER. Use of alternative promoters to express the aromatase cytochrome P450 (CYP19) gene in breast adipose tissues of cancer-free and breast cancer patients. J Clin Endocrinol Metab. 1996;81:3843–9. doi: 10.1210/jcem.81.11.8923826. [DOI] [PubMed] [Google Scholar]
- 49.Chen S, Zhou D, Okubo T, Kao YC, Yang C. Breast tumor aromatase: functional role and transcriptional regulation. Endocr Relat Cancer. 1999;6:149–56. doi: 10.1677/erc.0.0060149. [DOI] [PubMed] [Google Scholar]
- 50.Diaz-Cruz ES, Shapiro CL, Brueggemeier RW. Cyclooxygenase inhibitors suppress aromatase expression and activity in breast cancer cells. J Clin Endocrinol Metab. 2005;90:2563–70. doi: 10.1210/jc.2004-2029. [DOI] [PubMed] [Google Scholar]
- 51.Prosperi JR, Robertson FM. Cyclooxygenase-2 directly regulates gene expression of P450 Cyp19 aromatase promoter regions pII, pI.3 and pI. 7 and estradiol production in human breast tumor cells. Prostaglandins Other Lipid Mediat. 2006;81:55–70. doi: 10.1016/j.prostaglandins.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 52.Subbaramaiah K, Howe LR, Port ER, Brogi E, Fishman J, Liu CH, et al. HER-2/neu status is a determinant of mammary aromatase activity in vivo: evidence for a cyclooxygenase-2-dependent mechanism. Cancer Res. 2006;66:5504–11. doi: 10.1158/0008-5472.CAN-05-4076. [DOI] [PubMed] [Google Scholar]
- 53.Zhao Y, Agarwal VR, Mendelson CR, Simpson ER. Estrogen biosynthesis proximal to a breast tumor is stimulated by PGE2 via cyclic AMP, leading to activation of promoter II of the CYP19 (aromatase) gene. Endocrinology. 1996;137:5739–42. doi: 10.1210/endo.137.12.8940410. [DOI] [PubMed] [Google Scholar]
- 54.Brodie AM, Lu Q, Long BJ, Fulton A, Chen T, Macpherson N, et al. Aromatase and COX-2 expression in human breast cancers. J Steroid Biochem Mol Biol. 2001;79:41–7. doi: 10.1016/s0960-0760(01)00131-5. [DOI] [PubMed] [Google Scholar]
- 55.Brueggemeier RW, Quinn AL, Parrett ML, Joarder FS, Harris RE, Robertson FM. Correlation of aromatase and cyclooxygenase gene expression in human breast cancer specimens. Cancer Lett. 1999;140:27–35. doi: 10.1016/s0304-3835(99)00050-6. [DOI] [PubMed] [Google Scholar]
- 56.Morris PG, Hudis CA, Giri D, Morrow M, Falcone DJ, Zhou XK, et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res (Phila) 2011;4:1021–9. doi: 10.1158/1940-6207.CAPR-11-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Elander N, Ungerback J, Olsson H, Uematsu S, Akira S, Soderkvist P. Genetic deletion of mPGES-1 accelerates intestinal tumorigenesis in APC(Min/+) mice. Biochem Biophys Res Commun. 2008;372:249–53. doi: 10.1016/j.bbrc.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 58.Kamata H, Hosono K, Suzuki T, Ogawa Y, Kubo H, Katoh H, et al. mPGES-1-expressing bone marrow-derived cells enhance tumor growth and angiogenesis in mice. Biomed Pharmacother. 2010;64:409–16. doi: 10.1016/j.biopha.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 59.Finetti F, Terzuoli E, Bocci E, Coletta I, Polenzani L, Mangano G, et al. Pharmacological inhibition of microsomal prostaglandin E synthase-1 suppresses epidermal growth factor receptor-mediated tumor growth and angiogenesis. PLoS One. 2012;7:e40576. doi: 10.1371/journal.pone.0040576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.von Rahden BH, Brucher BL, Langner C, Siewert JR, Stein HJ, Sarbia M. Expression of cyclo-oxygenase 1 and 2, prostaglandin E synthase and transforming growth factor beta1, and their relationship with vascular endothelial growth factors A and C, in primary adenocarcinoma of the small intestine. Br J Surg. 2006;93:1424–32. doi: 10.1002/bjs.5426. [DOI] [PubMed] [Google Scholar]
- 61.Liby K, Rendi M, Suh N, Royce DB, Risingsong R, Williams CR, et al. The combination of the rexinoid, LG100268, and a selective estrogen receptor modulator, either arzoxifene or acolbifene, synergizes in the prevention and treatment of mammary tumors in an estrogen receptor-negative model of breast cancer. Clin Cancer Res. 2006;12:5902–9. doi: 10.1158/1078-0432.CCR-06-1119. [DOI] [PubMed] [Google Scholar]
- 62.Gupta PB, Proia D, Cingoz O, Weremowicz J, Naber SP, Weinberg RA, et al. Systemic stromal effects of estrogen promote the growth of estrogen receptor-negative cancers. Cancer Res. 2007;67:2062–71. doi: 10.1158/0008-5472.CAN-06-3895. [DOI] [PubMed] [Google Scholar]
- 63.Iyer V, Klebba I, McCready J, Arendt LM, Betancur-Boissel M, Wu MF, et al. Estrogen Promotes ER-Negative Tumor Growth and Angiogenesis through Mobilization of Bone Marrow-Derived Monocytes. Cancer Res. 2012;72:2705–13. doi: 10.1158/0008-5472.CAN-11-3287. [DOI] [PubMed] [Google Scholar]
- 64.Pequeux C, Raymond-Letron I, Blacher S, Boudou F, Adlanmerini M, Fouque MJ, et al. Stromal Estrogen Receptor-alpha Promotes Tumor Growth by Normalizing an Increased Angiogenesis. Cancer Res. 2012;72:3010–9. doi: 10.1158/0008-5472.CAN-11-3768. [DOI] [PubMed] [Google Scholar]
- 65.Losordo DW, Isner JM. Estrogen and angiogenesis: A review. Arterioscler Thromb Vasc Biol. 2001;21:6–12. doi: 10.1161/01.atv.21.1.6. [DOI] [PubMed] [Google Scholar]
- 66.Chang HH, Meuillet EJ. Identification and development of mPGES-1 inhibitors: where we are at? Future Med Chem. 2011;3:1909–34. doi: 10.4155/fmc.11.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bruno A, Di Francesco L, Coletta I, Mangano G, Alisi MA, Polenzani L, et al. Effects of AF3442 [N-(9-ethyl-9H-carbazol-3-yl)-2-(trifluoromethyl)benzamide], a novel inhibitor of human microsomal prostaglandin E synthase-1, on prostanoid biosynthesis in human monocytes in vitro. Biochem Pharmacol. 2010;79:974–81. doi: 10.1016/j.bcp.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 68.Koeberle A, Rossi A, Zettl H, Pergola C, Dehm F, Bauer J, et al. The molecular pharmacology and in vivo activity of 2-(4-chloro-6-(2,3-dimethylphenylamino)pyrimidin-2-ylthio)octanoic acid (YS121), a dual inhibitor of microsomal prostaglandin E2 synthase-1 and 5-lipoxygenase. J Pharmacol Exp Ther. 2010;332:840–8. doi: 10.1124/jpet.109.160663. [DOI] [PubMed] [Google Scholar]
- 69.Mbalaviele G, Pauley AM, Shaffer AF, Zweifel BS, Mathialagan S, Mnich SJ, et al. Distinction of microsomal prostaglandin E synthase-1 (mPGES-1) inhibition from cyclooxygenase-2 inhibition in cells using a novel, selective mPGES-1 inhibitor. Biochem Pharmacol. 2010;79:1445–54. doi: 10.1016/j.bcp.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 70.Rorsch F, Buscato E, Deckmann K, Schneider G, Schubert-Zsilavecz M, Geisslinger G, et al. Structure-activity relationship of nonacidic quinazolinone inhibitors of human microsomal prostaglandin synthase 1 (mPGES 1) J Med Chem. 2012;55:3792–803. doi: 10.1021/jm201687d. [DOI] [PubMed] [Google Scholar]
- 71.Xu D, Rowland SE, Clark P, Giroux A, Cote B, Guiral S, et al. MF63 [2-(6-chloro-1H-phenanthro[9,10-d]imidazol-2-yl)-isophthalonitrile], a selective microsomal prostaglandin E synthase-1 inhibitor, relieves pyresis and pain in preclinical models of inflammation. J Pharmacol Exp Ther. 2008;326:754–63. doi: 10.1124/jpet.108.138776. [DOI] [PubMed] [Google Scholar]