Abstract
Increased body mass index (BMI) has been linked to various detrimental health outcomes, including cognitive dysfunction. Recent work investigating associations between obesity and the brain has revealed decreased white matter microstructural integrity in individuals with elevated BMI, independent of age or comorbid health conditions. However, the relationship between high BMI and white matter fiber bundle length (FBL), which represents a novel metric of microstructural brain integrity, remains unknown. The present study utilized quantitative tractography based on diffusion tensor imaging (DTI) to investigate the relationship between BMI and FBL in 72 otherwise healthy older adults (24 males, 48 females). All participants were between 51 and 85 years of age (M = 63.26, SD = 8.76). Results revealed that elevated BMI was associated with shorter FBL in the temporal lobe, independent of age (p < .01). In addition, increased age was associated with shorter frontal, temporal, and whole brain FBL (all p values < .01). These findings indicate that, while increased age is an important factor associated with reduced FBL, high BMI is uniquely associated with reduced FBL in the temporal lobe. These data offer evidence for additive adverse effects of high BMI on the brain, especially in areas already vulnerable to aging processes and age-related neurodegenerative diseases. Further research is necessary to determine the physiological mechanisms associated with the shortening of FBL in individuals with high BMI.
Keywords: Tractography, BMI, DTI, White Matter, Fiber Bundle Length, Aging
1. Introduction
Prevalence of obesity has become a growing public health concern in the past few decades, particularly in Western cultures (Flegal, Carroll, Ogden, & Curtin, 2010). Elevated body fat, measured using body mass index (BMI), leads to impairments in cognitive function as well as a host of other health concerns, including cardiovascular disease and type 2 diabetes (Mokdad, Marks, Stroup, & Gerberding, 2004). Individuals with cardiovascular disease show impaired cognitive function in executive function and memory and an increased risk for development of dementia, most notably Alzheimer’s disease (AD; Gunstad et al., 2007; Gustafson et al., 2003). Elevated BMI is also associated with cognitive decline across the adult lifespan, independent of aging processes (Gunstad et al., 2007) or cardiovascular disease (Jagust, 2007). Cognitive deficits appear to be exacerbated by obesity in older age, especially in executive function, memory, and processing speed (van den Berg, Kloppenborg, Kessels, Kappelle, & Biessels, 2009).
White matter integrity in the brain observed with diffusion tensor imaging (DTI) can also be adversely affected by increased body weight. Evidence suggests that the abundance of adipocytes seen in obese individuals leads to an over-activation of the inflammatory response to cellular injury, resulting in damage to oligodendrocytes which comprise myelin in the brain (Griffin, 2006; Roth, Ramírez, Alarcón, & Von Bernhardi, 2005). DTI provides information about the microstructural integrity of white matter (e.g. axons, myelin) and water movement within white matter fibers not captured by traditional magnetic resonance imaging (MRI; Basser & Pierpaoli, 1996). Indices of white matter fiber integrity such as fractional anisotropy (FA) show decreased fiber integrity in obese individuals compared to normal-weight and overweight individuals (Stanek et al., 2011). Studies have found specific declines in FA in the body of the corpus callosum, and increased diffusivity in the splenium of the corpus callosum and fornix in individuals with increased BMI (Xu, Li, Lin, Sinha, & Potenza, 2011). Other studies suggest that increased BMI is linked to alterations in myelin and neuronal structural damage in frontal white matter, a region already believed to be vulnerable to aging processes, leading to marked deficits in frontal lobe connectivity and increased atrophy (Gazdzinski, Kornak, Weiner, & Meyerhoff, 2008). Reduced FA has also been observed in temporal lobe structures associated with advanced age (Rogalski et al., 2012). Collectively, these studies suggest that DTI has the ability to capture white matter microstructural abnormalities associated with elevated BMI.
DTI tractography is a method that noninvasively traces neuronal fiber pathways in the brain (Conturo et al., 1999; Mori, Crain, Chacko, & Van Zijl, 1999). This method allows for visualization of coherent bundles of nerve fibers, represented as reconstructed track lines. It complements other scalar DTI metrics by providing additional detail about the direction and curvature of white matter pathways coursing through the brain (Correia et al., 2008). Histologic studies indicate that the lengths of white matter fibers may be a biomarker of age-related brain degradation (Marner, Nyengaard, Tang, & Pakkenberg, 2003; Tang, Nyengaard, Pakkenberg, & Gundersen, 1997). Also, evidence of reduced fiber bundle length (FBL) among individuals with severe vascular disease compared to healthy controls (Correia et al., 2008) suggests that FBL may represent a sensitive neuroimaging index to determine the integrity of white matter in the brain. Thus, measurement of white matter integrity using FBL may provide valuable information about changes in white matter associated with elevated BMI.
Given that previous research suggests decreased white matter integrity in individuals with high BMI, possibly due to myelin alterations and neuronal damage (Gazdzinski et al., 2008), it is possible that FBL would be significantly reduced among individuals with high BMI. The purpose of the present study was to determine whether older individuals with high BMI exhibit reduced FBLs in the frontal and temporal lobes compared to older individuals with lower BMI. These regions were selected because they were shown to have changes associated with high BMI (van den Berg et al., 2009). We hypothesized that BMI and age would be significantly associated with mean length of white matter fiber bundles in each lobe, and that BMI would uniquely predict FBL in the frontal and temporal lobes.
2. Methods
2.1. Participants
Seventy-three (73) older adults (24 men, 49 women) between the ages of 51 and 85 who were enrolled in a study of cognitive aging were selected for the present study. Participants were recruited from the local community as well as the Research Participant Registry of the Washington University Institute of Clinical and Translational Sciences (ICTS). In order to be considered for the study, all participants were required to be English speaking and show no evidence of any medical or psychiatric condition that might affect cognition or mental status. Further, only participants whose BMI values were in the normal weight, overweight, or obese range (≥18.5 kg/m2) were included in the study. Reasons for exclusion from the study included history of neurological disease such as dementia, stroke, and Parkinson’s disease. The Mini-Mental State Examination (MMSE) was administered to identify individuals meeting criterion for dementia, excluding those with scores below 24. Participants were also excluded if they reported a history of diabetes, head injury (defined as loss of consciousness greater than 5 minutes), alcohol or drug abuse, or presence of an Axis I psychiatric condition (e.g., schizophrenia; bipolar disorder; or current severe, untreated depression). All participants provided informed consent and were financially compensated for their participation in the study. IRB approval was obtained from local institutional committees involved in the study.
2.2. Neuroimaging Acquisition
MRI acquisitions were obtained at Washington University in St. Louis using a head-only Magnetom Allegra 3T MRI scanner (Siemens Healthcare, Erlangen, Germany). For maximal stability and quality assurance, no modifications were made to scanner hardware or software during the course of the study, and quality assurance checks were carried out daily to ensure data fidelity. High-performance gradients (max strength 40 mT/m in a 100-microsecond rise time; maximum slew rate 400 T/m/s simultaneously on all 3 axes) were used to minimize scan times. Automated high-order shimming was utilized. The total time of the MRI scanning session was under one hour. Each scanning session began with a scout scan consisting of three orthogonal planes to confirm head positioning. Structural images were then obtained, consisting of T1-weighted magnetization-prepared rapid-acquisition gradient echo (MP-RAGE) imaging, T2-weighted turbo spin echo (TSE) imaging, and T2-weighted fluid-attenuated inversion-recovery (FLAIR) imaging, as described in Paul et al. (2011). Slice coverage and field of view (FOV) were determined from an initial pilot study of a subset of the same participants.
2.3 Diffusion-Weighted Imaging (DWI) Acquisition
Axial DWI was collected using a custom single-shot multislice echo-planar tensor-encoded pulse sequence. Diffusion gradients were applied in 31 non-collinear diffusion-encoded directions, which included 24 main directions (diffusion weighting of b=996 s/mm2). The pulse sequence and acquisition parameters were optimized for tractography. Acquisition parameters were designed for whole-brain coverage, high signal-to-noise ratio (SNR), and minimal artifact, with a TE of 86.2 ms, a TR of 7.82 s, and 64 contiguous 2.0-mm slices acquired for each contrast. The acquisition matrix was 128 × 128 with a 256 × 256 mm FOV (isotropic 2.0×2.0×2.0 mm voxels). Two scan repeats was acquired for signal averaging (72 total acquisitions).
2.4 Quantitative Diffusion Tensor Tractography
Using FSL FLIRT (mutual information metric; Jenkinson, Bannister, Brady, & Smith, 2002), each individual’s DWI images and diffusion-encoding vectors were registered to the I0 image in order to correct for subject motion within the scanner. Using Diffusion Toolkit’s dti_recon (Wang, Benner, Sorensen, & Wedeen, 2007), tensors and FA values were calculated from the DWIs, b values, and diffusion-encoding vectors. Trilinear interpolation was used to calculate the diffusion tensor field (Zhukov & Barr, 2002). From the tensor field, we then reconstructed tracks representing white matter fiber bundles, using a continuous tracking (FACT) algorithm (Mori et al., 1999), with one seed per voxel. The tracks were stopped when FA ≤ 0.15 or step angle ≥ 35 degrees. Tracks measuring less than 10 mm in length were excluded from the analysis. The tractography analyses were shown to be consistent in previous work by our group (Correia et al., 2008), and results in this study compared well with known white matter structures.
2.5 Measurement of Mean Fiber Bundle Length Associated with a Specific Lobe
The FA image was registered to the ICBM T1 atlas using FLIRT (mutual information metric; Mazziotta et al., 2001). ICBM T1 lobular labels were then used to transform this registration to the DWI space (Jenkinson et al., 2002) using nearest-neighbor interpolation. Custom software was utilized to segregate tracks connected to the frontal, parietal, temporal, and occipital lobes. A track was assigned to a lobe if it had at least one endpoint in either the left or right lobe. If a track was found to have endpoints in two different lobes, it was assigned to both lobes. Therefore, tracks could reside entirely in an individual lobe, could project to another ipsilateral lobe, or could cross to the contralateral side. The mean length of all the tracks associated with a given lobe was then calculated using custom software. This measure was based on each individual’s unique anatomy, so co-registration between multiple participants was not required. Prior to statistical comparison, all mean FBL measures were normalized to intracranial volume. Intracranial volume was estimated by adding three probability maps of voxel identity together. Measures of FBL then underwent head size correction using normalized algorithms for streamtube quantity, total sum of streamtube length, and weighted measures of total length for linear and fractional anisotropy. Details of normalized algorithms are explained in a previous paper (Correia et al., 2008).
2.6 Statistical Analysis
BMI was calculated for each participant based on the height and weight measured in our lab. One participant was identified as an outlier after comparing z-scores of FBL across all four brain lobes and whole-brain FBL and was removed from the statistical analyses. As a result, the total sample size was 72 participants (24 men, 48 women). Pearson’s correlation coefficients were computed to examine the relationships between age, gender, years of education, BMI, and white matter FBL by lobe to identify potential covariates in the subsequent analyses. Hierarchical regression analyses were performed to test the main hypothesis that BMI was related to FBL. In the first step, the demographic variables (age, gender, years of education) that were significantly correlated with FBL were entered as independent variables, with white matter FBL by lobe as the dependent variable. In the second step BMI was entered to determine its unique impact on FBL by lobe.
3. Results
Participant demographics and BMI values are listed in Table 1. Pearson’s correlation coefficients revealed moderate negative correlations between BMI and regional brain FBL (Table 2). BMI was negatively correlated with the mean FBL of tracks associated with the temporal lobe (p = .002). Age was negatively correlated with frontal FBL (p = .001), temporal FBL (p < .001), and whole brain FBL (p = .001), and a trend was observed for parietal FBL (p = .018).
Table 1.
Demographic Characteristics
|
N=72 (24 males, 48 females)
| ||||
|---|---|---|---|---|
| Variable | Mean | SD | Min | Max |
| Age | 63.26 | 8.76 | 51 | 85 |
| Education | 15.46 | 2.51 | 11 | 20 |
| BMI | 25.99 | 3.56 | 18.60 | 33.45 |
| MMSEa | 28.68 | 1.46 | 24 | 30 |
|
| ||||
| Cases (%) | ||||
| High Blood Pressure | 33.3 | |||
| High Cholesterol | 44.8 | |||
| Cardiovascular Disease | 23.5 | |||
Calculated from a total sample of 71 participants
Table 2.
Correlations between Age, BMI, and Fiber Bundle Length
| Brain Region | r | p value |
|---|---|---|
| Age | ||
| Whole Brain | −0.382 | 0.001* |
| Frontal Lobe | −0.376 | 0.001* |
| Temporal Lobe | −0.402 | <0.001* |
| Occipital Lobe | −0.225 | 0.058 |
| Parietal Lobe | −0.278 | 0.018 |
| BMI | ||
| Whole Brain | −0.230 | 0.052 |
| Frontal Lobe | −0.169 | 0.155 |
| Temporal Lobe | −0.360 | 0.002* |
| Occipital Lobe | −0.186 | 0.119 |
| Parietal Lobe | −0.035 | 0.768 |
Statistically significant at a Bonferroni-corrected p-value threshold of p<.01 (corrected for 5 brain regions)
Hierarchical regression analyses revealed that only temporal lobe FBL was significantly predicted by BMI after controlling for age (F(2, 69) = 11.816, p < .001, R2 = .255, R2 change attributed to BMI = .093). All other hierarchical regression models, including the relationship between BMI plus age and frontal lobe FBL, were significant except for the impact of BMI plus age on parietal lobe FBL. However, the unique contribution of BMI to these models did not reach significance (Table 3).
Table 3.
Hierarchical Regression Results for Fiber Bundle Length
| Variable | β | R2 | R2 change | F change |
|---|---|---|---|---|
| Whole Brain | ||||
| Age | −0.380 | 0.222 | 0.222 | 9.863*** |
| Gender | −0.297 | |||
| BMI | −0.207 | 0.264 | 0.042 | 3.840 |
| Frontal Lobe | ||||
| Age | −0.360 | 0.142 | 0.142 | 11.557** |
| BMI | −0.118 | 0.155 | 0.014 | 1.107 |
| Temporal Lobe | ||||
| Age | −0.358 | 0.162 | 0.162 | 13.498*** |
| BMI | −0.309 | 0.255 | 0.093 | 8.657** |
| Occipital Lobe | ||||
| Education | 0.253 | 0.071 | 0.071 | 5.346* |
| BMI | −0.164 | 0.098 | 0.027 | 2.049 |
| Parietal Lobe | ||||
| Age | −0.278 | 0.077 | 0.077 | 5.871* |
| BMI | 0.005 | 0.077 | 0.000 | 0.969 |
Note.
p<.05,
p<.01,
p<.001
4. Discussion
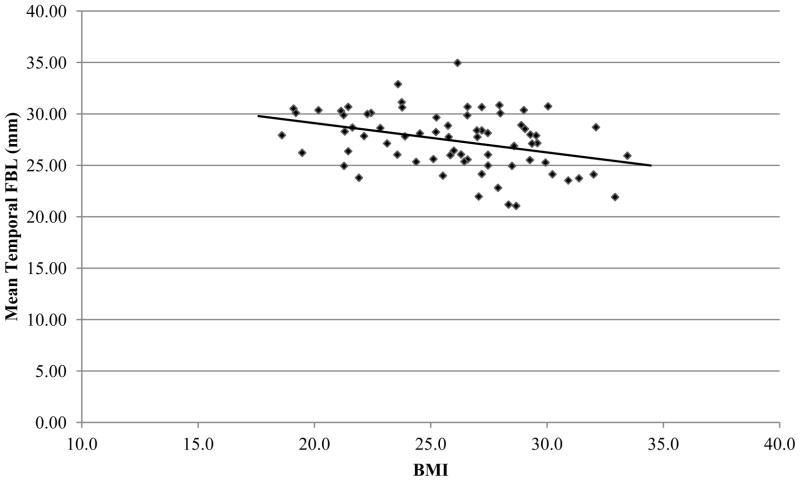
To our knowledge, this study is the first to utilize measurement of white matter fiber bundle lengths to investigate the impact of BMI on white matter in a sample of otherwise healthy older individuals. Increases in both BMI and age were found to predict decreases in white matter FBL throughout several regions of the brain, though these decreases were largely driven by the effects of age. After controlling for the robust influence of age, BMI was shown to significantly predict white matter FBL in the temporal lobe. Results of correlation analyses were consistent with this by demonstrating a significant relationship between BMI and temporal lobe FBL (Figure 1). Significant correlations were also observed between age and white matter FBL in two of the four major brain areas investigated in this study, as well as whole brain FBL. Overall, the results suggest that elevated BMI is associated with white matter changes evidenced by shorter temporal FBL, even after parsing out the effects of increased age. This finding suggests that elevated BMI may be associated with an accelerated course of “normal aging”, and an acceleration of age-related changes in microstructural integrity in these brain regions.
Figure 1.
Scatter plot illustrating correlation between BMI and temporal lobe FBL
The observed reductions in temporal FBL seen in the current study may represent early indicators of temporal lobe dysfunction. Impairments in cognitive functioning related to the temporal lobe (especially memory) occur in both the elderly population and in adults with elevated BMI (Brickman et al., 2006; DeCarli et al., 2005; Gunstad et al., 2007). While cognitive domains related to the frontal lobe are commonly affected in both elderly individuals (Greenwood, 2000) and in adults with high BMI (Gunstad et al., 2007), no statistically significant relationship between BMI and frontal FBL was seen in the current study. This may be due to under-representation of obese participants in the current study; only 11% (n = 8) of our sample were classified as “obese” based on BMI value. This small sample of participants on the high end of the BMI spectrum may have attenuated the potential association between BMI and frontal lobe FBL. Another reason for this discrepancy might be that the cohort in the current study was relatively young, especially in the context of cognitive impairment and dementia (Evans et al., 1989). These disorders tend to proliferate later in life and mainly affect temporal structures before later affecting the frontal lobes (Braak & Braak, 1991; van Hoesen, Hyman, & Damasio, 2004). Overall, evidence from past research combined with results of the current study suggest that high BMI in older adult populations is associated with evidence of accelerated aging processes on the brain, which are manifested by the shortening of fiber bundles, especially fiber bundles associated with the temporal lobe. Future studies are necessary to investigate the relationships between FBL, BMI, and cognitive function.
Past research using DTI to investigate the effect of elevated BMI has shown similar reductions in white matter microstructural integrity. Studies have shown reduced FA in the corpus callosum in individuals with elevated BMI, indicating a decrease in the directionality of diffusion along white matter tracks (Stanek et al., 2011; Xu et al., 2011). Other studies have measured higher radial diffusivity (RD) associated with increased BMI (Mueller et al., 2011). These prior DTI findings suggest a reduction in myelination in individuals with high BMI, consistent with evidence of brain tissue injury (Mueller et al., 2011). Results of the current study, which exhibited shorter FBL in temporal white matter, provide support for these past findings. A mechanism of histologic fiber length shortening has been suggested (Marner et al., 2003; Tang et al., 1997), evidenced by a pattern of patchy microscopic areas of myelin loss and/or axonal damage that collapse, thereby shortening the overall fiber length. Our data extend the findings of prior research by showing that elevated BMI is related to alterations in white matter integrity that result in the observed reduction in FBL, even after controlling for the effect of age. In general, a process of chronic progressive myelin loss and fiber bundle shortening may lead to impaired neural transmission and connectivity between brain regions, which may contribute to the pattern of global functioning and cognitive deficits commonly seen in individuals with elevated BMI (Gunstad et al., 2007).
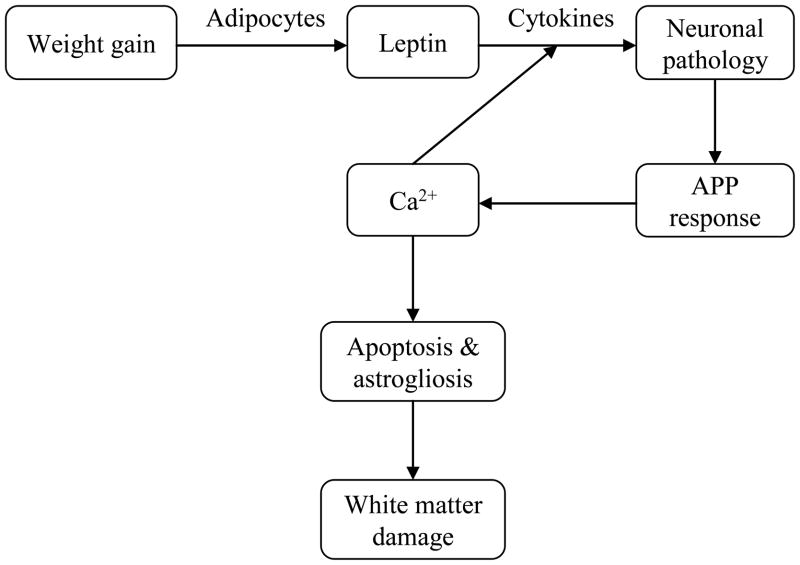
Previous research has identified several mechanisms by which obesity leads to white matter abnormalities (Figure 2). Normal-weight individuals have relatively low numbers of adipocytes, leading to lower levels of the hormone leptin (Wisse, 2004). Leptin plays a key role in regulation of the inflammatory response, and is specifically involved in the expression of pro-inflammatory cytokines (Wisse, 2004). In obese individuals, the increased quantity of adipocytes leads to excessive levels of circulating leptin (Considine et al., 1996). Since obesity is a chronic disease of leptin hypersecretion, production of pro-inflammatory cytokines is continuously elevated in these individuals (Wisse, 2004). Elevated levels of cytokines lead to hyperphosphorylated tau protein and neurofibrillary tangles, influencing dystrophic neurite growth (Griffin, 2006). Dystrophic neurite formation stimulates the production of β-amyloid precursor protein, which further stimulates expression of the interleukin (IL-1 and IL-6) and S100β cytokines (Mrak, Sheng, & Griffin, 1996). This continuous cycle induces increased intracellular calcium concentrations, promoting premature apoptosis and astrogliosis (Griffin, 2006). These microphysiological changes have been shown to cause disruptions in white matter via damage to oligodendrocytes (Roth et al., 2005), which offers support for the alterations in white matter integrity seen in our study.
Figure 2.
Flow chart illustrating the theorized mechanistic model behind reductions in fiber bundle length associated with elevated BMI
The model depicts a cascade of events initiated by weight gain and leads to white matter damage.
Though the shorter temporal fiber bundles observed in the current study provides a possible mechanism for the progression from “normal aging” to pathological aging in people with high BMI, the use of FBL as a measure of brain pathology requires additional study. Our tractography data were limited by the use of only one seed per voxel, despite literature suggesting that tractography computations benefit most from using 10–15 seeds per voxel (Cheng et al., 2012). Further, while MR spectroscopy measures of axonal viability are associated with BMI (Gazdzinski et al., 2008), the causal nature of the relationship between elevated BMI and shorter FBL, and the reason for the selective regional track shortening seen in the current study have yet to be determined. However, the combination of findings from healthy aging studies (e.g. Barrick, Charlton, Clark, & Markus, 2010) and BMI studies (e.g. Stanek et al., 2011) suggests that examining white matter using FBL may provide important information regarding overall brain health. Delineating the relationships between high BMI, aging processes, cognitive functioning, and FBL represent important areas of future research in brain aging, and will further evaluate the validity of FBL as a measure of white matter integrity and connectivity. Additionally, investigating the potential role of genetic status on this relationship represents an important area of future research. Research has implicated several genetic risk factors involved in the inflammatory response associated with high BMI, including CRP, IL-1β, and IL-6 (Brull et al., 2003; D’Aiuto, Parkar, Brett, Ready, & Tonetti, 2004; Latkovskis, Licis, & Kalnins, 2004). Genetic polymorphisms of the renin-angiotensinogen system and other genetic risks for vascular pathology (e.g. ApoE) have also been linked to elevated BMI levels (Schmidt, Freudenberger, Seiler, & Schmidt, 2012).
Overall, results of this study suggest that temporal lobe FBL provides a sensitive marker of brain integrity and may serve as an early indicator of age-related brain changes, especially in individuals with elevated BMI. The observed association between BMI and temporal FBL has potential relationship to the known increased prevalence of AD in individuals with high BMI (Gustafson et al., 2003), and the appearance of pathological changes in the temporal lobes in the earliest stages of AD. If reductions in lengths of fiber bundles seen in the current study are indicative of BMI-related neuropathology, then FBL measurements may have a role in predicting cognitive decline even in a pre-clinical, relatively healthy population.
Acknowledgments
Study Funding: Supported by NIH/NINDS grant number R01 NS052470 and R01 NS039538, and NIH/NIMH grant R21 MH090494. Recruitment database searches were supported in part by NIH/NCRR grant UL1 TR000448.
Footnotes
Disclosure Statement
There are no actual or potential conflicts of interest for any of the authors on this manuscript.
References
- Barrick TR, Charlton RA, Clark CA, Markus HS. White matter structural decline in normal ageing: A prospective longitudinal study using tract-based spatial statistics. Neuroimage. 2010;51(2):565–577. doi: 10.1016/j.neuroimage.2010.02.033. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. Journal of Magnetic Resonance. Series B. 1996;111(3):209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathologica. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Brickman AM, Zimmerman ME, Paul RH, Grieve SM, Tate DF, Cohen RA, Gordon E. Regional white matter and neuropsychological functioning across the adult lifespan. Biological Psychiatry. 2006;60(5):444–453. doi: 10.1016/j.biopsych.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Brull DJ, Serrano N, Zito F, Jones L, Montgomery HE, Rumley A, Hingorani AD. Human CRP gene polymorphism influences CRP levels implications for the prediction and pathogenesis of coronary heart disease. Arteriosclerosis, Thrombosis, and Vascular Biology. 2003;23(11):2063–2069. doi: 10.1161/01.ATV.0000084640.21712.9C. [DOI] [PubMed] [Google Scholar]
- Cheng H, Wang Y, Sheng J, Sporns O, Kronenberger WG, Mathews VP, Saykin AJ. Optimization of seed density in DTI tractography for structural networks. Journal of Neuroscience Methods. 2012;203(1):264–272. doi: 10.1016/j.jneumeth.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Caro JF. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. New England Journal of Medicine. 1996;334(5):292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- Conturo TE, Lori NF, Cull TS, Akbudak E, Snyder AZ, Shimony JS, Raichle ME. Tracking neuronal fiber pathways in the living human brain. Proceedings of the National Academy of Sciences. 1999;96(18):10422–10427. doi: 10.1073/pnas.96.18.10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia S, Lee SY, Voorn T, Tate DF, Paul RH, Zhang S, Laidlaw DH. Quantitative tractography metrics of white matter integrity in diffusion-tensor MRI. Neuroimage. 2008;42(2):568. doi: 10.1016/j.neuroimage.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Aiuto F, Parkar M, Brett PM, Ready D, Tonetti MS. Gene polymorphisms in pro-inflammatory cytokines are associated with systemic inflammation in patients with severe periodontal infections. Cytokine. 2004;28(1):29–34. doi: 10.1016/j.cyto.2004.06.005. [DOI] [PubMed] [Google Scholar]
- DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, Wolf PA. Measures of brain morphology and infarction in the Framingham Heart Study: Establishing what is normal. Neurobiology of Aging. 2005;26(4):491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Evans DA, Funkenstein HH, Albert MS, Scherr PA, Cook NR, Chown MJ, Taylor JO. Prevalence of Alzheimer’s disease in a community population of older persons. JAMA: The Journal of the American Medical Association. 1989;262(18):2551–2556. [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA: The Journal of the American Medical Association. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Kornak J, Weiner MW, Meyerhoff DJ. Body mass index and magnetic resonance markers of brain integrity in adults. Annals of Neurology. 2008;63(5):652–657. doi: 10.1002/ana.21377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood PM. The frontal aging hypothesis evaluated. Journal of the International Neuropsychological Society. 2000;6(6):705–726. doi: 10.1017/s1355617700666092. [DOI] [PubMed] [Google Scholar]
- Griffin WST. Inflammation and neurodegenerative diseases. The American Journal of Clinical Nutrition. 2006;83(2):470S–474S. doi: 10.1093/ajcn/83.2.470S. [DOI] [PubMed] [Google Scholar]
- Gunstad J, Paul RH, Cohen RA, Tate DF, Spitznagel MB, Gordon E. Elevated body mass index is associated with executive dysfunction in otherwise healthy adults. Comprehensive Psychiatry. 2007;48(1):57–61. doi: 10.1016/j.comppsych.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Archives of Internal Medicine. 2003;163(13):1524. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- Jagust W. What can imaging reveal about obesity and the brain? Current Alzheimer Research. 2007;4(2):135–139. doi: 10.2174/156720507780362146. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Latkovskis G, Licis N, Kalnins U. C-reactive protein levels and common polymorphisms of the interleukin-1 gene cluster and interleukin-6 gene in patients with coronary heart disease. European Journal of Immunogenetics. 2004;31(5):207–213. doi: 10.1111/j.1365-2370.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- Marner L, Nyengaard JR, Tang Y, Pakkenberg B. Marked loss of myelinated nerve fibers in the human brain with age. The Journal of Comparative Neurology. 2003;462(2):144–152. doi: 10.1002/cne.10714. [DOI] [PubMed] [Google Scholar]
- Mazziotta J, Toga A, Evans A, Fox P, Lancaster J, Zilles K, Pike B. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM) Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 2001;356(1412):1293–1322. doi: 10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA: The Journal of the American Medical Association. 2004;291(10):1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, Van Zijl P. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Annals of Neurology. 1999;45(2):265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Mrak RE, Sheng JG, Griffin WST. Correlation of astrocytic S100 [beta] expression with dystrophic neurites in amyloid plaques of Alzheimer’s disease. Journal of Neuropathology & Experimental Neurology. 1996;55(4):273–279. doi: 10.1097/00005072-199603000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller K, Anwander A, Möller HE, Horstmann A, Lepsien J, Busse F, Pleger B. Sex-dependent influences of obesity on cerebral white matter investigated by diffusion-tensor imaging. PLoS ONE. 2011;6(4):e18544. doi: 10.1371/journal.pone.0018544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Lane EM, Tate DF, Heaps J, Romo DM, Akbudak E, Conturo TE. Neuroimaging signatures and cognitive correlates of the Montreal cognitive assessment screen in a nonclinical elderly sample. Archives of Clinical Neuropsychology. 2011;26:454–60. doi: 10.1093/arclin/acr017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski E, Stebbins GT, Barnes CA, Murphy CM, Stoub TR, George S, deToledo-Morrell L. Age-related changes in parahippocampal white matter integrity: A diffusion tensor imaging study. Neuropsychologia. 2012;50(8):1759–1765. doi: 10.1016/j.neuropsychologia.2012.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth AD, Ramírez G, Alarcón R, Von Bernhardi R. Oligodendrocytes damage in Alzheimer’s disease: Beta amyloid toxicity and inflammation. Biological Research. 2005;38(4):381. doi: 10.4067/s0716-97602005000400011. [DOI] [PubMed] [Google Scholar]
- Schmidt H, Freudenberger P, Seiler S, Schmidt R. Genetics of subcortical vascular dementia. Experimental Gerontology. 2012;47(11):873–877. doi: 10.1016/j.exger.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanek KM, Grieve SM, Brickman AM, Korgaonkar MS, Paul RH, Cohen RA, Gunstad JJ. Obesity is associated with reduced white matter integrity in otherwise healthy adults. Obesity. 2011;19(3):500–504. doi: 10.1038/oby.2010.312. [DOI] [PubMed] [Google Scholar]
- Tang Y, Nyengaard J, Pakkenberg B, Gundersen HJ. Age-induced white matter changes in the human brain: A stereological investigation. Neurobiology of Aging. 1997;18(6):609–615. doi: 10.1016/s0197-4580(97)00155-3. [DOI] [PubMed] [Google Scholar]
- Van den Berg E, Kloppenborg RP, Kessels RPC, Kappelle LJ, Biessels GJ. Type 2 diabetes mellitus, hypertension, dyslipidemia and obesity: A systematic comparison of their impact on cognition. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2009;1792(5):470–481. doi: 10.1016/j.bbadis.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Hyman BT, Damasio AR. Entorhinal cortex pathology in Alzheimer’s disease. Hippocampus. 1991;1(1):1–8. doi: 10.1002/hipo.450010102. [DOI] [PubMed] [Google Scholar]
- Wang R, Benner T, Sorensen AG, Wedeen VJ. Diffusion toolkit: A software package for diffusion imaging data processing and tractography. Proc Intl Soc Mag Reson Med. 2007;15:3720. [Google Scholar]
- Wisse BE. The inflammatory syndrome: The role of adipose tissue cytokines in metabolic disorders linked to obesity. Journal of the American Society of Nephrology. 2004;15(11):2792–2800. doi: 10.1097/01.ASN.0000141966.69934.21. [DOI] [PubMed] [Google Scholar]
- Xu J, Li Y, Lin H, Sinha R, Potenza MN. Body mass index correlates negatively with white matter integrity in the fornix and corpus callosum: A diffusion tensor imaging study. Human Brain Mapping. 2011 Dec 3; doi: 10.1002/hbm.21491. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhukov L, Barr AH. Visualization, 2002. VIS 2002. IEEE; 2002. Oriented tensor reconstruction: Tracing neural pathways from diffusion tensor MRI; pp. 387–394. [Google Scholar]