Abstract
BACKGROUND
Left ventricular mass (LVM) and hypertrophy (LVH) are important parameters, but their use is surrounded by controversies. We compare LVM by echocardiography and cardiac magnetic resonance (CMR), investigating reproducibility aspects and the effect of echocardiography image quality. We also compare indexing methods within and between imaging modalities for classification of LVH and cardiovascular risk.
METHODS
MESA enrolled 880 participants in Baltimore City; 146 had echocardiograms and CMR on the same day. LVM was then assessed using standard techniques. Echocardiography image quality was rated (good/limited) according to the parasternal view. LVH was defined after indexing LVM to body surface area, height1.7, height2.7, or by the predicted LVM from a reference group. Participants were classified for cardiovascular risk according to Framingham score. Pearson’s correlation, Bland-Altman plots, percent agreement, and kappa coefficient assessed agreement within and between modalities.
RESULTS
LVM by echocardiography (140 ± 40 g) and by CMR were correlated (r = 0.8, p < 0.001) regardless of the echocardiography image quality. The reproducibility profile had strong correlations and agreement for both modalities. Image quality groups had similar characteristics; those with good images compared to CMR slightly superiorly. The prevalence of LVH tended to be higher with higher cardiovascular risk. The agreement for LVH between imaging modalities ranged from 77% to 98% and the kappa coefficient from 0.10 to 0.76.
CONCLUSIONS
Echocardiography has a reliable performance for LVM assessment and classification of LVH, with limited influence of image quality. Echocardiography and CMR differ in the assessment of LVH, and additional differences rise from the indexing methods.
Keywords: Left ventricular mass, left ventricular hypertrophy, echocardiography, image quality
Introduction
Echocardiography and cardiac magnetic resonance (CMR) are the two most frequent imaging modalities used to assess left ventricular mass (LVM). Although CMR is considered the gold standard method for LVM evaluation, echocardiography is well validated, harmless, and widely available. In fact, echocardiography-derived LVM is usually performed in clinical practice and has shown prediction ability for cardiovascular outcomes.[1–3]
Anthropometric parameters have been used to normalize myocardial mass, minimizing the influence of body size in the population distribution. LVM is usually indexed (LVMi) by height to some allometric power, by body surface area (BSA), or by comparing it to a reference group of healthy subjects. Left ventricular hypertrophy (LVH)—defined by an LVMi greater than some specified cut-off value (often the 95th percentile value estimated from a healthy sample)—has an important role in clinical practice. The definition for LVH and its performance as a cardiovascular risk predictor are strongly related to the LVM indexing method. [4–9]
Although LVMi and LVH are considered important markers for cardiovascular prognosis and therapeutic responses, the role of myocardial mass and hypertrophy in clinical practice has not been firmly established.[10] Echocardiography assessment has major limitations related to acoustic window quality, but how it affects the ability to assess LVM is unknown. Moreover, it is still unknown how concordant CMR and echocardiography are for the identification of hypertrophy. The controversies around echocardiography-derived LVM and LVH increase when different indexing methods and cut-off values are considered.[1, 10]
Our study compares LVM acquired by echocardiography and CMR, investigating reproducibility aspects of both modalities. We also explore the effect of echocardiography image quality in the assessment of LVM. We compare indexing methods within and between imaging modalities for the classification of LVH and cardiovascular risk.
Methods
Study design and population
The National Heart, Lung, and Blood Institute’s (NHLBI) Multi-Ethnic Study of Atherosclerosis (MESA) has been described in the literature.[11] In brief, between July 2000 and August 2002, 6,814 men and women who were free of clinically apparent cardiovascular disease were recruited from 6 U.S. communities: Baltimore City and Baltimore County, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; Northern Manhattan and the Bronx, New York; and St. Paul, Minnesota. The Baltimore City site included exclusively white and African-American participants. In the follow-up period between July 2005 and April 2007, a randomly selected subsample of participants from Baltimore City had echocardiography and CMR performed on the same day at the Johns Hopkins Hospital, Baltimore, MD. The institutional review boards at all centers approved the study, and all participants gave informed consent.
Echocardiography
Echocardiograms were performed by an experienced sonographer using an Aplio scanner (Toshiba Medical Systems Corp, Tochigi, Japan) and were recorded onto digital media. At the same site, experienced readers analyzed the images using an off-line system (Digiview 3.7.7.6, Digisonics Inc., Houston, Texas, USA). As recommended by the American Society of Echocardiography (ASE), from a two-dimensional (2D) parasternal view, LVM was calculated using linear measurements of interventricular septal thickness, left ventricular (LV) internal dimensions, and LV posterior wall thickness at end-diastole (Figure 1 – a).[2] Image quality was evaluated in the 2D parasternal view and rated according to the identification of the interfaces between cardiac blood pool and endocardium, and between the epicardium and pericardium. Images were rated as limited when at least one interface was not adequately assessed and as good when all interfaces were distinguished.
Figure 1. Illustrative representation of left ventricular mass (LVM) assessment by echocardiography (a) and cardiac magnetic resonance (b).
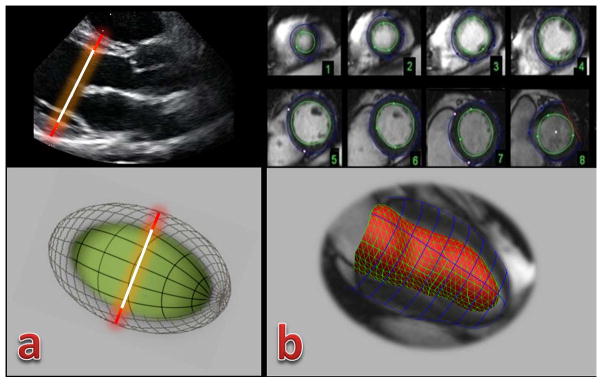
(a) Linear measurements for interventricular septum thickness, left ventricular internal dimension, and posterior wall thickness using 2D echocardiography in a parasternal view. LVM is then calculated assuming that the left ventricle has the shape of a prolate ellipsoid of revolution (below). In this participant, LVM by echocardiography was 134g;
(b) Cardiac magnetic resonance (CMR) using the Simpson method to assess LVM from short-axis views. CMR allows assessing cardiac geometry in its 3D shape (below). In this participant, LVM by CMR was 114g.
Cardiac Magnetic Resonance
The method used to assess LVM by CMR in MESA has been described in the literature.[12] Briefly, images were acquired on a 1.5 T scanner (Avanto, Siemens, Malvern, PA) using a 2D steady state free precession (SSFP) acquisition in vertical long axis, horizontal long axis and short axis orientations with the following parameters: TE 1.16 ms, TR 3.2 ms, flip angle 60°, receiver bandwidth ±1220 kHz, FOV 36 cm, slice thickness 8 mm, slice gap 2 mm, acquisition matrix 205×256, number of averages = 1, number of frames = 30. The endocardial and epicardial myocardial borders were contoured using a semi-automated 2D standard software (MASS 4.2, Medis, Leiden, Netherlands). The difference between the epicardial and endocardial areas for all slices was multiplied by the slice thickness and section gap, and then multiplied by the specific gravity of the myocardium (1.05 g/ml) to determine the ventricular mass (Figure 1 – b). Papillary muscle mass was included in the LV cavity and excluded from the LVM.[13]
Intra-reader, Inter-reader, and Inter-scan Reproducibility
Subsets of participants were randomly selected to have their echocardiography and CMR images re-read by the same reader, and by different readers. A subset of participants was also randomly selected to have a second echocardiogram performed by the same sonographer within one week of the primary (initial) echocardiogram and read by the same reader. Efforts were made to blind the readers to the primary results. All readers had appropriate training and experience in large cohort studies (such as MESA, EDIC, and CARDIA) using the imaging modalities they performed.
Left ventricular mass indices and hypertrophy definition
LVM was indexed by four methods: (1) dividing by body surface area, (2) dividing by height1.7, (3) dividing by height2.7, and (4) dividing by the predicted LVM based on a healthy sample. We used height to different allometric powers in #2 (height1.7) and #3 (height2.7), because these methods were found to perform differently for predicting CV outcomes in a previous MESA investigation.[6] Table 1 summarizes the indexing methods and cut-off values for LVH according to the imaging modality.
Table I.
Left ventricular mass index (LVMi) and definition of left ventricular hypertrophy according to the LVMi cut-off value
| Imaging Modality | Indexation | Calculation Method | LVMi cut-off value | Reference for cut-off value |
|---|---|---|---|---|
| Echo | BSA | LVMi = LVM/BSA | > 115 g/m2 for men; > 95 g/m2 for women | ASE guidelines [2] |
| height1.7 | LVMi = LVM/height1.7 | ≥81g/m1.7 for men; ≥60g/m1.7 for women | Asklepius study[6] | |
| height2.7 | LVMi = LVM/height2.7 | ≥50g/m2.7 for men; ≥47g/m2.7 for women | deSimone[7] | |
| %predicted | LVMi = 100 × LVM/Predicted LVM: Men: 16.6 × [weight (kg)]0.51; women 13.9 × [weight (kg)]0.51 |
>1.45 | Cardiovascular Health Study[27] | |
| CMR | BSA | LVMi = LVM/BSA | >106.2g/m2 for men; >84.6g/m2 for women | MESA[28] |
| height1.7 | LVMi = LVM/height1.7 | ≥80g/m1.7 for men; ≥60g/m1.7 for women | MESA[6] | |
| height2.7 | LVMi = LVM/height2.7 | >45.1 g/m2.7 for men; >38 g/m2.7 for women | MESA[28] | |
| %predicted | 100 × LVM (g)/[a × height (m)0.54 × weight (Kg)0.61), where a = 6.82 for women or 8.25 for men | >1.31 | MESA[13] |
CMR - Cardiac magnetic resonance; Echo – echocardiography; LVMi – left ventricular mass index; BSA - Body-surface area; LVM – left ventricular mass; MESA - Multi-Ethnic Study of Atherosclerosis; %Predicted - percent of predicted LVM.
All reference populations are free of recognized cardiovascular disease
Statistical analysis
Continuous variables were reported in their mean values ± standard deviation (SD) and categorical variables in its proportions. Differences between mean values were evaluated with paired t-test and Fisher’s exact test to assess differences in proportions. Linear regression and Pearson’ s correlation coefficient (r) were used to evaluate the relationship between LVM as determined by echocardiography and by CMR, as well as to assess correlations between LVM and blood pressure in this population. Bland-Altman plots were also used to describe differences between LVM as determined by echocardiography and by CMR, reporting the mean differences and 95% limits of agreement (95% LA). Inter- and intra-reader and inter-scan reproducibility performances were also evaluated in both modalities using intra-class correlation coefficients (ICC) and Bland-Altman plots.
Participants were divided into 3 cardiovascular risk groups by their Framingham 10-year cardiovascular risk score: low risk (<10%), intermediate risk (10% – 20%), and high risk (>20%).[14] ANOVA was used to assess difference of the mean unindexed LVM and LVM indices among the cardiovascular risk groups (shown as supplemental material). Fisher’s exact test was used to assess differences in proportions for hypertrophy classification according to cardiovascular risk. The proportion of participants whose classification for hypertrophy (existence of hypertrophy or not) was concordant between indices was calculated, within and between imaging modalities. Cohen’s kappa coefficient was also used to evaluate agreement.
Results
A total of 880 subjects were enrolled in the site at Baltimore City; 155 were randomly selected to undergo echocardiography and CMR. From the total, 146 subjects had interpretable CMR and echocardiography on the same day and were included in the study; 100 (68%) of these had good quality echocardiography images. The mean value for LVM assessed by CMR was 128 ± 34 g and by echocardiography was 140 ± 40 g. Table 2 summarizes the clinical characteristics of all participants and the subsample with both interpretable echocardiography and CMR examinations.
Table II.
Characteristics of all subjects enrolled at the site and the sample for this study
| Variable | All participants (n = 880) | Included participants (n = 146) | ||
|---|---|---|---|---|
|
|
|
|||
| Mean | SD | Mean | SD | |
| Age (years) | 68 | 9.7 | 66 | 8.8 |
| Height (m) | 1.7 | 0.1 | 1.7 | 0.1 |
| Weight (Kg) | 83 | 18 | 82 | 18 |
| Creatinine (mg/dL) | 1.1 | 0.4 | 1.0 | 0.3 |
| EF by CMR (%) | 59 | 9.5 | 59 | 8.6 |
|
|
|
|||
| Proportion | Proportion | |||
| Male (gender) | 47% | 43% | ||
| Diabetes/IFG† | 38% | 31% | ||
| Hypertension‡ | 59% | 54% | ||
| African-Americans | 49% | 46% | ||
SD – standard deviation; EF – ejection fraction by the two-dimensional Simpson method; CMR – cardiac magnetic resonance; LVM – left ventricular mass; IFG – impaired fasting glucose.
Following 2003 ADA fasting criteria algorithm;
Hypertension by JNC VI (1997) criteria
The reproducibility profile had strong correlations and agreement for both CMR and echocardiography, with ICC ranging from 7.3 to 9.1 for inter-reader assessment by echocardiography and intra-reader assessment by CMR, respectively (Table 3). With all the investigated indexing methods and images modalities, the mean LVMi value was higher with higher cardiovascular risk category (Supplement Table S1) and a strong relationship was found between LVM and blood pressure (Supplement Table S2). Statistically significant differences among means by cardiovascular risk category exist for all LVMi except for the percent-predicted LVMi by either echocardiography or standard CMR.
Table III.
Reproducibility assessment for cardiac magnetic resonance and echocardiography
| Reproducibility | n | ICC (p value) | Mean difference (95% Limits of Agreement) |
|---|---|---|---|
| CMR | |||
| Intrareader (EC) | 15 | 0.91 (p<0.001) | 1.2 (−26.39, 28.81) |
| Interreader (EC vs. MN) | 22 | 0.88 (p<0.001) | 12.35 (18.29, 43.00) |
| Echocardiography | |||
| Intrareader (AA) | 15 | 0.84 (p<0.001) | 4.54 (−45.38, 54.46) |
| Interreader (AA vs. ES) | 85 | 0.73 (p<0.001) | 11.85 (−39.86, 63.56) |
| Interscan (ES vs. ES) | 15 | 0.85 (p<001) | 4.72 (−76.16, 85.63) |
CMR – cardiac magnetic resonance; 2D – two-dimension; 3D – three-dimension; ICC – intra-class correlation coefficient.
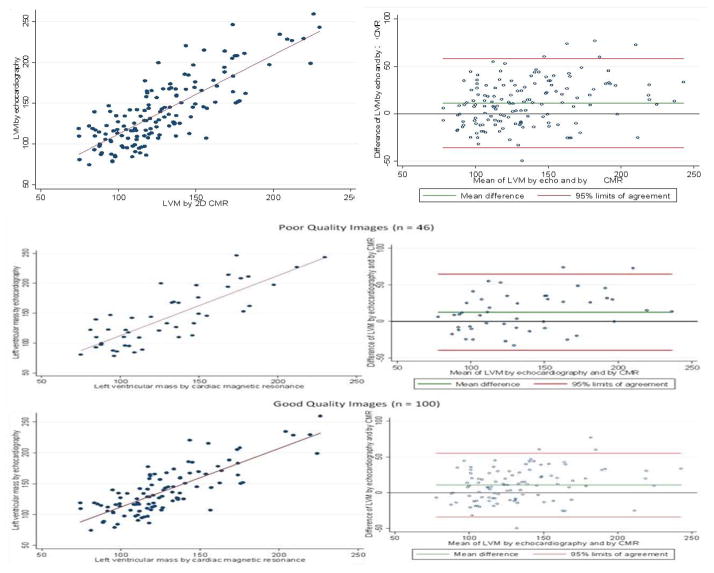
The mean value for echocardiography-derived LVM was 138±38g for participants with good quality images and 142±46g for those with limited quality, without significant difference (p = 0.61). Similarly, no statistically significant difference was found for anthropometrics comparing the imaging quality groups. For participants with limited or good image quality scores, we found 56% and 57% of females, respectively; 49% and 45% of African Americans; and mean BMI of 30 ± 6 g/m2 and 29 ± 5 g/m2. The overall correlation between LVM by echocardiography and CMR was consistent (Figure 2), regardless of echocardiography image quality scoring (r = 0.8; p<0.001 for the overall relation, good quality images, and limited quality echocardiography images). Compared to CMR, LVM was higher when assessed by echocardiography in 10.8 g (95% LA = −33.8, 55.4) in participants with good image quality. In those with limited image quality, the difference between echocardiography and CMR was slightly higher: 12.6 g (95% LA = −39.7, 64.8).
Figure 2. Scatter plots and Bland-Altman plots for left ventricular mass assessed by echocardiography and by cardiac magnetic resonance, overall and according to echocardiography image quality.
LVM - left ventricular mass; echo – echocardiography; CMR - cardiac magnetic resonance
For LVM assessment by CMR, the presence of hypertrophy ranged from 3.4% when indexed by predicted LVM to 6.8% when indexed by height1.7. For echocardiography-derived LVH, the range was from 3.4% when indexed by the predicted LVM to 24.0% when indexed by height1.7 (Table 4). The prevalence of hypertrophy did not differ significantly according to cardiovascular risk category for all 4 indexing methods in both imaging modalities.
Table IV.
Proportion of left ventricular hypertrophy (LVH) for diverse indexing methods and imaging modality in all participants (n = 146), and by Framingham 10-year cardiovascular risk score category (n = 136)
| Imaging modality and Normalization method | Hypertrophy (95% CI) | Hypertrophy proportion (95% CI) according to CV risk | p value§ | ||
|---|---|---|---|---|---|
|
| |||||
| Low (n = 48) | Intermediate (n = 42) | High (n = 46) | |||
| LVH by Echocardiography | |||||
| BSA (%) | 11.6 (6.4, 16.9) | 6.2 (−0.7, 13.2) | 9.5 (0.5, 18.6) | 17.4 (6.2, 28.6) | 0.2 |
| height1.7 (%) | 24.0 (17.0, 31.0) | 22.9 (10.8, 35.0) | 19.0 (6.9, 31.2) | 26.1 (13.1, 39.0) | 0.7 |
| height2.7 (%) | 7.5 (3.2, 12.0) | 4.2 (−1.6, 9.9) | 4.8 (−1.8, 11.3) | 13.0 (3.1, 23.0) | 0.3 |
| %predicted (%) | 3.4 (0.4, 6.4) | 2.1 (−2.0, 6.2) | 4.8 (−1.8, 11.3) | 4.4 (−1.7, 10.4) | 0.7 |
| LVH by CMR | |||||
| BSA (%) | 5.5 (1.7, 9.3) | 4.2 (−1.6, 9.9) | 4.8 (−1.8, 11.3) | 4.4 (−1.7, 10.4) | 1.0 |
| height1.7 (%) | 6.8 (2.7, 11.0) | 4.2 (−1.6, 9.9) | 7.1 (−0.8, 15.1) | 8.7 (0.4, 17.0) | 0.7 |
| height2.7 (%) | 6.2 (2.2, 10.1) | 4.2 (−1.6, 9.9) | 9.5 (0.5, 18.6) | 6.5 (−1.0, 13.8) | 0.6 |
| %predicted (%) | 3.4 (0.4, 6.4) | 2.1 (−2.0, 6.2) | 4.8 (−1.8, 11.3) | 4.4 (−1.7, 10.4) | 0.7 |
SD – standard deviation; CV – cardiovascular; CI – confidence interval; LVM – left ventricular mass; CMR – cardiac magnetic resonance; LVH – left ventricular hypertrophy; BSA – body-surface area; %predicted - percent of the predicted LVM from a reference group of healthy subjects.
The p value refers to Fisher’s exact test for difference in LVH classification according to cardiovascular risk group.
The percent agreement for the classification of LVH according to the image modality and index method ranged from 77% to 98%. The highest value was related to CMR-derived measurements normalized by BSA compared to normalization by percent-predicted LVM. The lowest values were found for echocardiography-derived LVM/height1.7 compared to CMR-derived normalization by BSA, by height2.7, or by the percent-predicted LVM. The Cohen’s kappa coefficient ranged from 0.10—comparing the CMR-derived percent-predicted LVM with echocardiography LVM/height1.7 — to 0.76 for the comparison between CMR LVM/BSA with the CMR percent-predicted LVM (Table 5).
Table V.
Proportion of agreed classification (below diagonal) and Cohen’s Kappa coefficient (above diagonal) for the classification of hypertrophy, according to the different image modalities and indices. In gray, results for inter-modality agreement
| Normalization Methodology | PLVH (%) | 2D CMR | Echocardiography | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| BSA | height1.7 | height2.7 | %predicted | BSA | height1.7 | height2.7 | %predicted | |||
| 2D CMR | BSA | 5.5 | * | 0.41† | 0.44† | 0.76† | 0.37† | 0.11§ | 0.29† | 0.44† |
| height1.7 | 3.4 | 93% | * | 0.61† | 0.37† | 0.23¥ | 0.23† | 0.23¥ | 0.23¥ | |
| height2.7 | 6.8 | 94% | 95% | * | 0.55† | 0.33† | 0.14¥ | 0.36† | 0.25† | |
| %predicted | 6.2 | 98% | 94% | 96% | * | 0.42† | 0.10§ | 0.34† | 0.59† | |
| Echo | BSA | 11.6 | 90% | 87% | 89% | 92% | * | 0.41† | 0.53† | 0.42† |
| height1.7 | 24.0 | 77% | 79% | 77% | 77% | 82% | * | 0.41† | 0.20† | |
| height2.7 | 7.5 | 92% | 90% | 92% | 93% | 92% | 84% | * | 0.34† | |
| %predicted | 3.4 | 95% | 92% | 93% | 97% | 92% | 79% | 93% | * | |
CMR – Cardiac Magnetic Resonance; BSA – body-surface area; Echo – echocardiography; PLVH – Prevalence of left ventricular hypertrophy; %predicted - percent of the predicted LVM from a reference group of healthy subjects.
p < 0.001,
p ≤0.01 and
p < 0.05 for test of null hypothesis that kappa=0
Discussion
Echocardiography is the most usual imaging method for assessing LVM in clinical practice, but CMR is well established as the gold standard modality.[1, 3] Our study included echocardiograms and CMR scans performed on the same day in a representative biracial sample of MESA participants to explore controversial aspects regarding the comparison between these imaging modalities for the assessment of LVM and LVH. To the best of our knowledge, our study is the first to evaluate the agreement for LVH classification within and between imaging modalities across diverse LVM indexing methods. Moreover, aspects related to echocardiography image quality were also explored when compared to CMR.
Our results confirm studies which have shown that LVM by echocardiography linear measurements is higher on average compared to CMR measurements.[15–18] The PRESERVE study included echocardiography assessment of LVM to compare with CMR at baseline and after one-year follow-up, and found a mean overestimation of myocardial mass by echocardiography of 27.6±36.0 g and 37.1±27.6 g, respectively.[19] In patients undergoing mitral valve replacement, the assessment by M-mode echocardiography overestimated LVM values compared to CMR (mean differences ranged from 70 g to 108 g for post- and pre-operative assessments, respectively), but both provided reliable information of myocardial mass regression.[16] We observed a mean difference of 11.3 g which is statistically significantly different from 0 (95% confidence interval for the mean difference: 9.4–13.2); however, this difference is likely too small to have an impact on clinical decisions. Moreover, the mean difference between LVM by echocardiography and CMR is similar in magnitude to the mean difference between readers of echocardiography or CMR (inter-reader echocardiography mean difference = 11.85; inter-reader CMR mean difference = 12.35, Table 3).
As expected, the reproducibility of measurements by the same reader was better compared to the reproducibility of measurements by different readers, for both 2D CMR and echocardiography. Compared to our results (Table 5), the literature has shown similar findings for echocardiography and CMR reproducibility. For inter-scan reproducibility, Bottini et al. repeated echocardiograms in 22 hypertensive subjects and found a mean difference (95% limits of agreement) of 0.3 g (−96.3, 96.9). The same authors also had two readers independently assessing 24 echocardiography images and 34 CMR images, finding mean differences (95% limits of agreement) of 1.83 g (−48.8, 52.5) and 0.32 g (−20.1, 21.7) for echocardiography and CMR, respectively.[15] Using 20 hypertensive male subjects, Spratt at el. investigated echocardiography inter-reader reproducibility and found mean differences (95% limits of agreement) for LVM/BSA between 4.5 g/m2 (−24.9, 33.9) and 6.4 g/m2 (−23.0, 35.8) for harmonic and fundamental imaging, respectively.[20] For echocardiography intra-observer reproducibility, 21 subjects were assessed by Missouris et al., showing a mean coefficient of variation (95% CI) of 6.1% (3.9, 8.3). Using 9 normal young volunteers, the same study found CMR intra-reader reproducibility between LVM estimations of 0.5% with 95% limits of agreement of ±11%.[17]
Acoustic window and poor image quality are considered major limitations for the use of echocardiography in population studies and in clinical practice, but their real impact on the assessment of LVM is unclear. In our study, the ability to identify with confidence both blood/endocardium and epicardium/pericardium interfaces in a parasternal echocardiography window defined a good quality image. However, there is intrinsic subjectivity in this rating process, and more or less strict definitions for image quality may influence results. In our study, the echocardiography image quality did not appear to affect the correlation between LVM assessed by CMR and echocardiography.
Our study is the first using LVM assessed by echocardiography and by CMR on the same day to compare normalization by BSA, height1.7, height2.7, and as a proportion of the predicted LVM from a reference group of healthy subjects. The ASE recommends normalizing LVM by dividing by BSA,[2] but standard recommendations are lacking for CMR.[3] Indexing to an allometrically scaled height has been suggested as a better indexing method for heart size parameters,[21] with promising results for LVM predicting clinical outcomes.[6] For 2D-CMR-derived LVM, all 4 indexing methods and cut-offs for hypertrophy classifications were previously described for the MESA population (Table 1). For echocardiographic LVM, we used cut-offs for hypertrophy that reflect real practice and current echocardiography recommendations; however, the lack of cut-offs derived from the MESA sample could influence the agreement results.
Echocardiography seems to classify a larger number of participants with LVH, particularly when LVM is indexed to BSA; however, the long-term clinical implications of these differences are unknown. Although the prevalence of LVH was not statistically significant among risk categories in our study, the prevalence of LVH tended to be higher with the higher cardiovascular risk category. We also found that the mean LVM and LVM indices are higher with higher cardiovascular risk category. In fact, LVM and LVH have been shown to have a relationship with risk factors.[22] In the Northern Manhattan Study, the prevalence of LVH based on LVM indexed to BSA was 18%, 23%, and 35% for low, intermediate, and high-risk groups, respectively.[23]
The proportion of agreement and the kappa coefficient were generally better for comparisons between indices within imaging modality than between imaging modality. The proportion agreement and kappa coefficient were each relatively similar for comparisons among indices except for comparisons with height1.7, where they tended to be lower. Height1.7 was first described by Chirinos et al. as the best description of the relationship between LVM determined by echocardiography and body size in European Caucasian subjects.[6] Further investigation of this index is needed.
Conclusions
Left ventricular mass and hypertrophy are of high relevance in clinical and research settings,[24, 25] but there are still important technical controversies.[26] Echocardiography has a reliable performance for LVM assessment and classification of LVH, with limited influence of image quality in our population. These findings support the use of LVM and LVH assessed by echocardiography in population studies and clinical practice. Compared to echocardiography, CMR seems to be appropriate for population studies aiming to find small differences in LVM or LVH using a lower number of examinations or for clinical conditions where small LVM changes over time are expected for a given patient. Echocardiography and CMR are not interchangeable techniques for the assessment of LVH and additional differences rise from the indexing methods. Direct comparisons between imaging modalities using long-term follow-up periods could clarify the clinical impact of these differences. In addition, efforts to standardize techniques and normalization methods are important to promote the use of LVM and LVH on a clinical basis.
Supplementary Material
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. Particularly, Mrs. Elzbieta Chamera and Mrs. Ellen Stengel, readers at the MRI reading center, for their collaborative efforts in this study. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding Sources
The study was funded by the National Heart, Lung, and Blood Institute (NHLBI); supported by the MESA study contracts RO1-HL-66075, NO1-HC-9808, N01-HC-95162, NO1-HC-95168, and NO1-HC-95169. Dr. Armstrong was funded by Universidade Federal do Vale do São Francisco (UNIVASF), Petrolina, PE, Brazil.
Footnotes
Potential Conflict of Interest: The authors have no competing interests in this study.
Authors’ contributions
Dr. Armstrong, Dr. Bluemke, and Dr. Lima contributed in all procedures of this study. Dr. Gjesdal, Dr. Almeida, and Dr. Nacif contributed to concepts, design, data collection, interpretation, and critical revision of article. Dr. Wu and Dr. Brumback contributed to design, data statistical analysis, interpretation, and critical revision of article.
References
- 1.Armstrong AC, Gjesdal O, Wu C, et al. Left ventricular mass assessed by Echocardiography and Cardiac Magnetic Resonance, cardiovascular outcomes, and medical practice. J Am Coll Cardiol Img. 2012;5:11. [Google Scholar]
- 2.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Hundley WG, Bluemke DA, Finn JP, et al. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation. 2010;121:2462–2508. doi: 10.1161/CIR.0b013e3181d44a8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2007;28:1462–1536. doi: 10.1093/eurheartj/ehm236. [DOI] [PubMed] [Google Scholar]
- 5.The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 6.Chirinos JA, Segers P, De Buyzere ML, et al. Left ventricular mass: allometric scaling, normative values, effect of obesity, and prognostic performance. Hypertension. 2010;56:91–98. doi: 10.1161/HYPERTENSIONAHA.110.150250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Simone G, Devereux RB, Daniels SR, et al. Effect of growth on variability of left ventricular mass: assessment of allometric signals in adults and children and their capacity to predict cardiovascular risk. J Am Coll Cardiol. 1995;25:1056–1062. doi: 10.1016/0735-1097(94)00540-7. [DOI] [PubMed] [Google Scholar]
- 8.Jafary FH, Jafar TH. Disproportionately high risk of left ventricular hypertrophy in Indo-Asian women: a call for more studies. Echocardiography. 2008;25:812–819. doi: 10.1111/j.1540-8175.2008.00713.x. [DOI] [PubMed] [Google Scholar]
- 9.Wong RC, Yip JW, Gupta A, et al. Echocardiographic left ventricular mass in a multiethnic Southeast Asian population: proposed new gender and age-specific norms. Echocardiography. 2008;25:805–811. doi: 10.1111/j.1540-8175.2008.00709.x. [DOI] [PubMed] [Google Scholar]
- 10.Gidding SS. Controversies in the assessment of left ventricular mass. Hypertension. 2010;56:26–28. doi: 10.1161/HYPERTENSIONAHA.110.153346. [DOI] [PubMed] [Google Scholar]
- 11.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 12.Natori S, Lai S, Finn JP, et al. Cardiovascular function in multi-ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol. 2006;186:S357–365. doi: 10.2214/AJR.04.1868. [DOI] [PubMed] [Google Scholar]
- 13.Bluemke DA, Kronmal RA, Lima JA, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–2155. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 15.Bottini PB, Carr AA, Prisant LM, et al. Magnetic resonance imaging compared to echocardiography to assess left ventricular mass in the hypertensive patient. Am J Hypertens. 1995;8:221–228. doi: 10.1016/0895-7061(94)00178-E. [DOI] [PubMed] [Google Scholar]
- 16.Guenzinger R, Wildhirt SM, Voegele K, et al. Comparison of magnetic resonance imaging and transthoracic echocardiography for the identification of LV mass and volume regression indices 6 months after mitral valve repair. J Card Surg. 2008;23:126–132. doi: 10.1111/j.1540-8191.2007.00558.x. [DOI] [PubMed] [Google Scholar]
- 17.Missouris CG, Forbat SM, Singer DR, et al. Echocardiography overestimates left ventricular mass: a comparative study with magnetic resonance imaging in patients with hypertension. J Hypertens. 1996;14:1005–1010. [PubMed] [Google Scholar]
- 18.Breitenbach I, Harringer W, Tsui S, et al. Magnetic resonance imaging versus echocardiography to ascertain the regression of left ventricular hypertrophy after bioprosthetic aortic valve replacement: Results of the REST study. J Thorac Cardiovasc Surg. 2011 doi: 10.1016/j.jtcvs.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 19.Tse HF, Cheung BM, Ng W, et al. Regression of left ventricular hypertrophy after treatment of hypertension: comparison of directed M-echocardiography with magnetic resonance imaging in quantification of serial mass changes. J Card Fail. 2003;9:122–127. doi: 10.1054/jcaf.2003.12. [DOI] [PubMed] [Google Scholar]
- 20.Spratt JC, Leslie SJ, White A, et al. Harmonic imaging improves estimation of left ventricular mass. Int J Cardiovasc Imaging. 2004;20:107–111. doi: 10.1023/b:caim.0000014047.59389.1f. [DOI] [PubMed] [Google Scholar]
- 21.Dewey FE, Rosenthal D, Murphy DJ, Jr, et al. Does size matter? Clinical applications of scaling cardiac size and function for body size. Circulation. 2008;117:2279–2287. doi: 10.1161/CIRCULATIONAHA.107.736785. [DOI] [PubMed] [Google Scholar]
- 22.Movahed MR, Bates S, Strootman D, et al. Obesity in adolescence is associated with left ventricular hypertrophy and hypertension. Echocardiography. 2011;28:150–153. doi: 10.1111/j.1540-8175.2010.01289.x. [DOI] [PubMed] [Google Scholar]
- 23.Abe Y, Rundek T, Sciacca RR, et al. Ultrasound assessment of subclinical cardiovascular disease in a community-based multiethnic population and comparison to the Framingham score. Am J Cardiol. 2006;98:1374–1378. doi: 10.1016/j.amjcard.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 24.Mortensen KH, Gravholt CH, Hjerrild BE, et al. Left ventricular hypertrophy in Turner syndrome: a prospective echocardiographic study. Echocardiography. 2012;29:1022–1030. doi: 10.1111/j.1540-8175.2012.01754.x. [DOI] [PubMed] [Google Scholar]
- 25.Konstam MA, Kramer DG, Patel AR, et al. Left ventricular remodeling in heart failure: current concepts in clinical significance and assessment. JACC Cardiovasc Imaging. 2011;4:98–108. doi: 10.1016/j.jcmg.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Cuspidi C, Negri F, Giudici V, et al. Echocardiography in clinical practice: the burden of arterial hypertension. A multicenter Italian survey. J Hum Hypertens. 2010;24:395–402. doi: 10.1038/jhh.2009.78. [DOI] [PubMed] [Google Scholar]
- 27.Gardin JM, Siscovick D, Anton-Culver H, et al. Sex, age, and disease affect echocardiographic left ventricular mass and systolic function in the free-living elderly. The Cardiovascular Health Study. Circulation. 1995;91:1739–1748. doi: 10.1161/01.cir.91.6.1739. [DOI] [PubMed] [Google Scholar]
- 28.Brumback LC, Kronmal R, Heckbert SR, et al. Body size adjustments for left ventricular mass by cardiovascular magnetic resonance and their impact on left ventricular hypertrophy classification. Int J Cardiovasc Imaging. 2010;26:459–468. doi: 10.1007/s10554-010-9584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.