Abstract
Drug addiction has been conceptualized as a chronically relapsing disorder of compulsive drug seeking and taking that progresses through three stages: binge/intoxication, withdrawal/negative affect, and preoccupation/anticipation. Drug addiction impacts multiple motivational mechanisms and can be conceptualized as a disorder that progresses from positive reinforcement (binge/intoxication stage) to negative reinforcement (withdrawal/negative affect stage). The construct of negative reinforcement is defined as drug taking that alleviates a negative emotional state. Our hypothesis is that the negative emotional state that drives such negative reinforcement is derived from dysregulation of key neurochemical elements involved in the brain stress systems within the frontal cortex, ventral striatum, and extended amygdala. Specific neurochemical elements in these structures include not only recruitment of the classic stress axis mediated by corticotropin-releasing factor (CRF) in the extended amygdala as previously hypothesized but also recruitment of dynorphin-κ opioid aversive systems in the ventral striatum and extended amygdala. Additionally, we hypothesized that these brain stress systems may be engaged in the frontal cortex early in the addiction process. Excessive drug taking engages activation of CRF not only in the extended amygdala, accompanied by anxiety-like states, but also in the medial prefrontal cortex, accompanied by deficits in executive function that may facilitate the transition to compulsive-like responding. Excessive activation of the nucleus accumbens via the release of mesocorticolimbic dopamine or activation of opioid receptors has long been hypothesized to subsequently activate the dynorphin-κ opioid system, which in turn can decrease dopaminergic activity in the mesocorticolimbic dopamine system. Blockade of the κ opioid system can also block anxiety-like and reward deficits associated with withdrawal from drugs of abuse and block the development of compulsive-like responding during extended access to drugs of abuse, suggesting another powerful brain stress/anti-reward system that contributes to compulsive drug seeking. Thus, brain stress response systems are hypothesized to be activated by acute excessive drug intake, to be sensitized during repeated withdrawal, to persist into protracted abstinence, and to contribute to the development and persistence of addiction. The recruitment of anti-reward systems provides a powerful neurochemical basis for the negative emotional states that are responsible for the dark side of addiction.
Keywords: opponent process, extended amygdala, corticotropin-releasing factor, dynorphin, reward, compulsive, impulsive, sensitization, abstinence or withdrawal, prefrontal cortex
Conceptual framework for the dark side of addiction
Addiction has been conceptualized as a three-stage cycle—binge/intoxication, withdrawal/negative affect, and preoccupation/anticipation—with two primary sources of reinforcement: positive and negative reinforcement. Positive reinforcement is defined as the process by which presentation of a stimulus increases the probability of a response; negative reinforcement is defined as the process by which removal of an aversive stimulus (or aversive state in the case of addiction) increases the probability of a response. Secondary sources of reinforcement include conditioned positive and conditioned negative reinforcement. Different theoretical perspectives from experimental psychology (positive and negative reinforcement frameworks), social psychology (self-regulation failure framework), and neurobiology (counteradaptive and sensitization framework) can be superimposed on the stages of the addiction cycle (Koob and Le Moal, 1997). These stages are thought to feed into each other, become more intense, and ultimately lead to the pathological state known as addiction.
Motivation, withdrawal, and opponent process
Motivation is a state that involves arousal, emotion, and expectation, all of which direct behavior. William James, describing the far greater variety of impulses in humans, wrote, “some expectation of consequences must in every case like this be aroused; and this expectation, according as it is, that of something desired or of something disliked, must necessarily either reinforce or inhibit the mere impulse” (James, 1918, p. 390). Such motivational states are not constant but rather vary over time. The concept of motivation was inextricably linked with hedonic, affective, or emotional states in addiction in the context of temporal dynamics by Solomon's opponent process theory of motivation. Solomon and Corbit (1974) postulated that hedonic, affective, or emotional states, once initiated, are automatically modulated by the central nervous system through mechanisms that reduce the intensity of hedonic feelings. The a-process includes affective or hedonic habituation (or tolerance), and the b-process includes affective or hedonic withdrawal (abstinence). The a-process in drug use consists of positive hedonic responses, occurs shortly after the presentation of a stimulus, correlates closely with the intensity, quality, and duration of the reinforcer, and shows tolerance. In contrast, the b-process in drug use appears after the a-process has terminated, consists of negative hedonic responses, and is sluggish in onset, slow to build up to an asymptote, slow to decay, and gets larger with repeated exposure. Indeed, one can argue that in fact the b-process begins early in response to the a-process and opposes the manifestation of the a-process, yielding the phenomenom of “apparent tolerance” (Colpaert, 1996). The thesis we have elaborated is that there is a neurocircuitry change of specific neurochemical systems that account for the b-process. Thus, such opponent processes are hypothesized to begin early in drug taking, reflecting not only deficits in brain reward system function but also recruitment of function in the brain stress systems. Furthermore, we hypothesize that recruitment of the brain stress systems forms one of the major sources of negative reinforcement in addiction.
Thus, we define dependence, or the manifestation of a withdrawal syndrome after the removal of chronic drug administration, in terms of motivational symptoms, such as the emergence of a negative emotional state (e.g., dysphoria, anxiety, and irritability) when access to the drug is prevented (Koob and Le Moal, 2001), which can be exacerbated or even caused by the physical signs of withdrawal. Indeed, some have argued that the development of such a negative affective state can define dependence as it relates to addiction (Russell, 1976; Koob et al., 1989; Baker et al., 2004).
Negative emotional states have been characterized in humans by acute and protracted abstinence from all major drugs of abuse (American Psychiatric Association, 1994; Koob, 2012; Khantzian, 1997). Similar results have been observed in animal models with all major drugs of abuse using intracranial self-stimulation as a measure of hedonic tone. Withdrawal from chronic cocaine (Markou and Koob, 1991), amphetamine (Paterson et al., 2000), opioids (Schulteis et al., 1994), cannabinoids (Gardner and Vorel, 1998), nicotine (Epping-Jordan et al., 1998), and ethanol (Schulteis et al., 1995) leads to increases in reward threshold during acute abstinence, and some of these elevations in threshold can last for up to 1 week (Koob, 2009). These observations lend credence to the hypothesis that opponent processes in the hedonic domain have an identifiable neurobiological basis and provide an impetus for defining the mechanisms involved. Understanding the mechanisms that drive this increase in reward thresholds is key to understanding the mechanisms that drive negative reinforcement in addiction.
Such elevations in reward threshold begin rapidly and can be observed within a single session of self-administration (Kenny et al., 2003), bearing a striking resemblance to human subjective reports of acute withdrawal. Dysphoria-like responses also accompany acute opioid and ethanol withdrawal (Liu and Schulteis, 2004; Schulteis and Liu, 2006). Here, naloxone administration following single injections of morphine increased reward thresholds, measured by intracranial self-stimulation (ICSS), and increased thresholds with repeated morphine and naloxone-induced withdrawal experience (Liu and Schulteis, 2004). Similar results were observed during repeated acute withdrawal from ethanol (Schulteis and Liu, 2006).
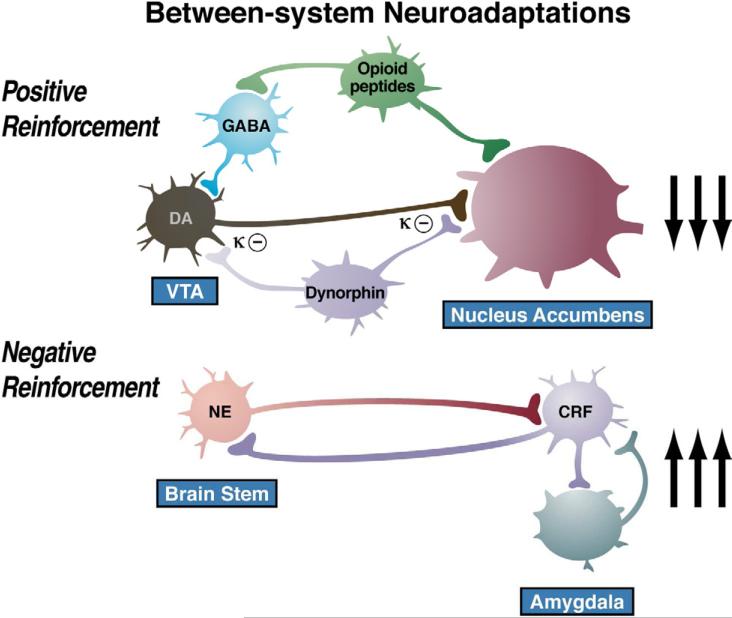
The development of the aversive emotional state that drives the negative reinforcement of addiction is defined here as the “dark side” of addiction. We have argued that drug addiction progresses from a source of positive reinforcement that may indeed involve a form of sensitization of incentive salience, as argued by Robinson and Berridge (1993), to sensitization of the brain stress and anti-reward systems that sets up a powerful negative reinforcement process. Anti-reward is a concept developed by Koob and Le Moal (2008), based on the hypothesis that brain systems are in place to limit reward (see footnote in Koob and Le Moal, 1997), with an opponent process concept that forms a general feature of biological systems. Our concept of an anti-reward system is derived from the hypothesis of both within- and between-system neuroadaptations to excessive activation of the reward system at the neurocircuitry level. Within-system neuroadaptations are defined as the process by which the primary cellular response element to the drug (circuit A) itself adapts to neutralize the drug's effects. Persistence of the opposing effects after the drug disappears produces adaptation. A between-system neuroadaptation is a circuitry change, in which circuit B (i.e., the anti-reward circuit) is activated by circuit A (i.e., the reward circuit). In the present treatise, we hypothesize that a within-system neuroadaptation can also result from a between-system neuroadaptation, in which circuit B (i.e., the anti-reward circuit) is activated either in parallel or in series to suppress the activity of circuit A (see below).
Animal models of the transition to an addiction-like state as defined by escalation in drug self-administration with prolonged access
A progressive increase in the frequency and intensity of drug use is one of the major behavioral phenomena that characterize the development of addiction and has face validity with the criteria of the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) “The substance is often taken in larger amounts and over a longer period than was intended” (American Psychological Association, 1994). A framework with which to model the transition from drug use to drug addiction can be found in recent animal models of prolonged access to intravenous cocaine self-administration. Historically, animal models of cocaine self-administration involved the establishment of stable behavior from day to day to allow the reliable interpretation of data provided by within-subject designs aimed at exploring the neuropharmacological and neurobiological bases of the reinforcing effects of acute cocaine. Up until 1998, after the acquisition of self-administration, rats were typically allowed access to cocaine for 3 h or less per day to establish highly stable levels of intake and patterns of responding between daily sessions. This was a useful paradigm for exploring the neurobiological substrates for the acute reinforcing effects of drugs of abuse.
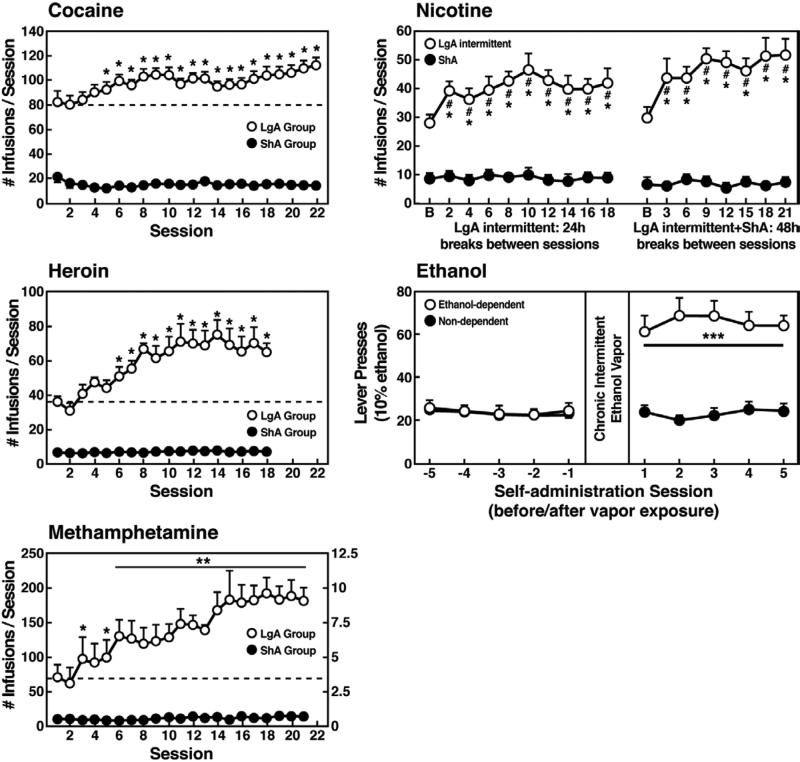
However, in an effort to explore the possibility that differential access to drugs of abuse may have more face validity for the compulsive-like responding observed in addiction, animals have been allowed extended access to all major drugs of abuse (Figure 1). Increased intake was observed in the extended access group for intravenous cocaine, methamphetamine, heroin, and nicotine and oral alcohol during extended access and dependence (Ahmed et al., 2000; Ahmed and Koob, 1998; Kitamura et al., 2006; O'Dell et al., 2004; George et al., 2007; Quadros and Miczek, 2009; Vendruscolo et al., 2011). For example, when animals were allowed access for 1 and 6 h to different doses of cocaine, both the long-access (LgA) and short-access (ShA) animals titrated their cocaine intake, but LgA rats consistently self-administered almost twice as much cocaine at any dose tested, further suggesting an upward shift in the set point for cocaine reward in the escalated animals (Ahmed and Koob, 1999; Deroche-Gamonet et al., 2004; Mantsch et al., 2004).
Figure 1.
(A) Effect of drug availability on cocaine intake (mean ± SEM). In long-access (LgA) rats (n = 12) but not short-access (ShA) rats (n = 12), the mean total cocaine intake started to increase significantly from session 5 (p < 0.05; sessions 5 to 22 compared with session 1) and continued to increase thereafter (p < 0.05; session 5 compared with sessions 8-10, 12, 13, and 17-22). [Taken with permission from Ahmed and Koob, 1998.] (B) Effect of drug availability on total intravenous heroin self-infusions (mean ± SEM). During the escalation phase, rats had access to heroin (40 μg per infusion) for 1 h (ShA rats, n = 5-6) or 11 h per session (LgA rats, n = 5-6). Regular 1-h (ShA rats) or 11-h (LgA rats) sessions of heroin self-administration were performed 6 days a week. The dotted line indicates the mean ± SEM number of heroin self-infusions in LgA rats during the first 11-h session. *p < 0.05, different from the first session (paired t-test). [Taken with permission from Ahmed et al., 2000.] (C) Effect of extended access to intravenous methamphetamine on self-administration as a function of daily sessions in rats trained to self-administer 0.05, 0.1, and 0.2 mg/kg/infusion of intravenous methamphetamine during the 6-h session. ShA, 1-h session (each unit dose, n = 6). LgA, 6-h session (0.05 mg/kg/infusion, n = 4; 0.1 mg/kg/infusion, n = 6; 0.2 mg/kg/infusion, n = 5). *p < 0.05, **p < 0.01, compared with day 1. [Taken with permission from Kitamura et al., 2006.] (D) Nicotine intake (mean ± SEM) in rats that self-administered nicotine under a fixed-ratio (FR) 1 schedule in either 21 h (long access [LgA]) or 1 h (short access [ShA]) sessions. LgA rats increased their nicotine intake on an intermittent schedule with 24-48 h breaks between sessions, whereas LgA rats on a daily schedule did not. The left shows the total number of nicotine infusions per session when the intermittent schedule included 24 h breaks between sessions. The right shows the total number of nicotine infusions per session when the intermittent schedule included 48 h breaks between sessions. #p < 0.05, compared with baseline; *p < 0.05, compared with daily self-administration group. n = 10 per group. [Taken with permission from Cohen et al., 2012.] (E) Ethanol self-administration in ethanol-dependent and nondependent animals. The induction of ethanol dependence and correlation of limited ethanol self-administration before and excessive drinking after dependence induction following chronic intermittent ethanol vapor exposure is shown. ***p < 0.001, significant group × test session interaction. With all drugs, escalation is defined as a significant increase in drug intake within-subjects in extended-access groups, with no significant changes within-subjects in limited-access groups. [Taken with permission from Edwards et al., 2011.]
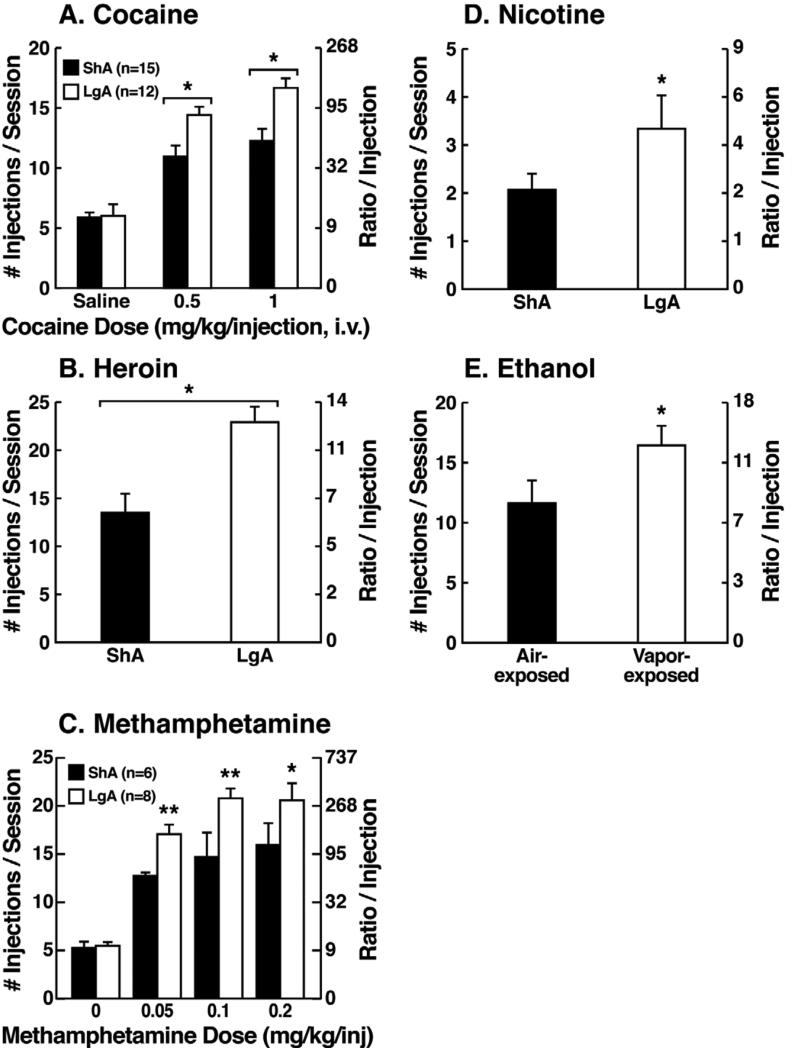
Consistent with the hypothesis that extended access to drugs of abuse produces compulsive-like responding, in which animals will “continue to respond in the face of adverse consequences” (another DSM-IV criteria for Substance Dependence), animals with extended access that show escalation in self-administration also show increased responding on a progress-ratio schedule of reinforcement (Paterson and Markou, 2003; Wee et al., 2008; Walker and Koob, 2007; Figure 2). Changes in the reinforcing and incentive effects of drug intake that are consistent with the increases in progressive-ratio responding have been observed following extended access and include increased drug-induced reinstatement after extinction, a decreased latency to goal time in a runway model for drug reward, and responding in the face of punishment (Deroche et al., 1999; Jonkman et al., 2012; Ben-Shahar et al., 2008; Vanderschuren and Everitt, 2004; Deroche-Gamonet et al., 2004; Pelloux et al., 2007; Vendruscolo et al., 2012). Altogether, these results suggest that drug taking with extended access changes the motivation to seek the drug. Some have argued that enhanced drug taking reflects a sensitization of reward (Vezina, 2004), but studies of locomotor sensitization suggest that locomotor sensitization occurs independently of escalation (Ben-Shahar et al., 2004; Knackstedt and Kalivas, 2007; Ahmed and Cador, 2006). The increased brain reward thresholds and neuropharmacological studies outlined below argue for a reward deficit state that drives the increased drug taking during extended access.
Figure 2.
(A) Dose-response function of cocaine by rats responding under a progressive-ratio schedule. Test sessions under a progressive-ratio schedule ended when rats did not achieve reinforcement within 1 h. The data are expressed as the number of injections per session on the left axis and ratio per injection on the right axis. *p < 0.05, compared with ShA rats at each dose of cocaine. [Taken with permission from Wee et al., 2008.] (B) Responding for heroin under a progressive-ratio schedule of reinforcement in ShA and LgA rats. *p < 0.05, LgA significantly different from LgA. [Modified with permission from Barbier et al., 2013.] (C) Dose-response for methamphetamine under a progressive-ratio schedule. Test sessions under a progressive-ratio schedule ended when rats did not achieve reinforcement within 1 h. *p < 0.05, **p < 0.01, LgA significantly different from ShA [Modified from Wee et al., 2007.] (D) Breakpoints on a progressive-ratio schedule in long access (LgA) rats that self-administered nicotine with 48 h abstinence between sessions. LgA rats on an intermittent schedule reached significantly higher breakpoints than LgA rats that self-administered nicotine daily. The data are expressed as mean ± SEM. *p < 0.05. n = 9 rats per group. [Taken with permission from Cohen et al., 2012.] (E) Mean (±SEM) breakpoints for ethanol while in nondependent and ethanol-dependent states. **p < 0.01, main effect of vapor exposure on ethanol self-administration. [Taken with permission from Walker and Koob, 2007.]
Animals escalate their intake of drugs with extended access, with a parallel increase in reward thresholds
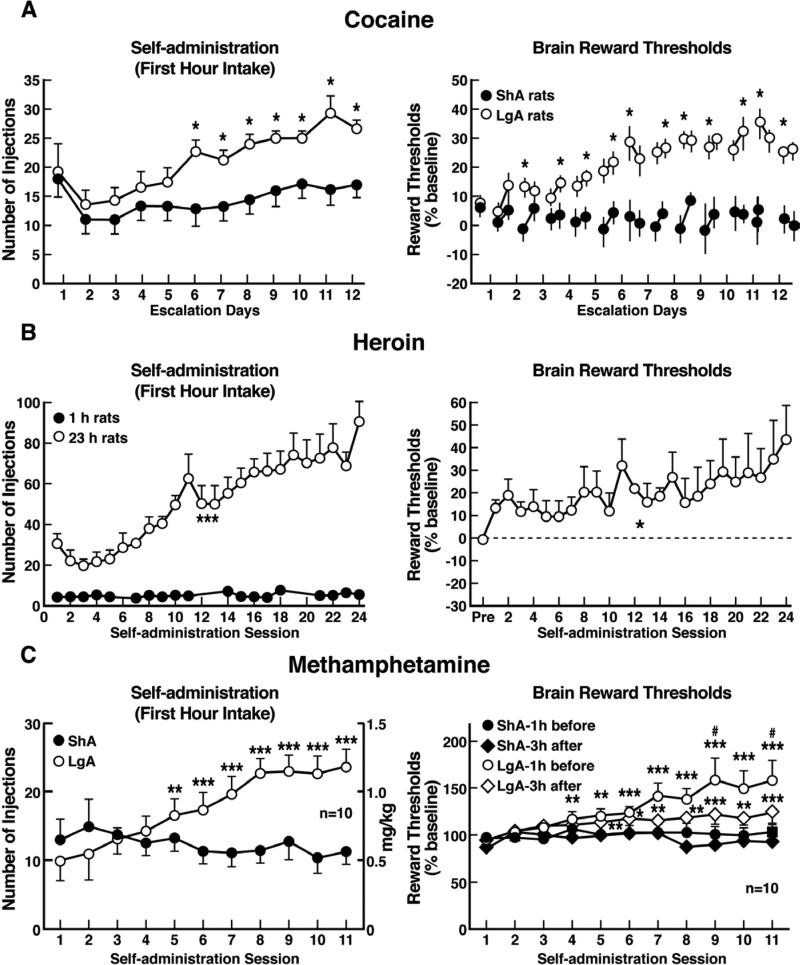
The hypothesis that compulsive cocaine use is accompanied by a chronic perturbation in brain reward homeostasis has been tested in animal models of escalation in drug intake with prolonged access combined with measures of brain stimulation reward thresholds. Animals implanted with intravenous catheters and allowed differential access to intravenous self-administration of cocaine showed increases in cocaine self-administration from day to day in the long-access group (6 h; LgA) but not in the short-access group (1 h; ShA). The differential exposure to cocaine self-administration had dramatic effects on reward thresholds that progressively increased in LgA rats but not ShA or control rats across successive self-administration sessions (Ahmed et al., 2002). Elevations in baseline reward thresholds temporally preceded and were highly correlated with escalation in cocaine intake (Figure 3). Post-session elevations in reward thresholds failed to return to baseline levels before the onset of each subsequent self-administration session, thereby deviating more and more from control levels. The progressive elevation in reward thresholds was associated with a dramatic escalation in cocaine consumption that was observed previously (Ahmed et al., 2002). Similar results have been observed with extended access to methamphetamine (Jang et al., 2013) and heroin (Kenny et al., 2006). Rats allowed 6 h access to methamphetamine or 23 h access to heroin also showed a time-dependent increase in reward thresholds that paralleled the increases in heroin intake (Figure 3). Similar results of parallel increases in brain reward thresholds with escalation of nicotine intake have also been observed with extended access to nicotine (Harris et al., 2011).
Figure 3.
(A) Relationship between elevation in ICSS reward thresholds and cocaine intake escalation. (Left) Percent change from baseline response latencies (3 h and 17-22 h after each self-administration session; first data point indicates 1 h before the first session). (Right) Percent change from baseline ICSS thresholds. *p < 0.05, compared with drug-naive and/or ShA rats (tests for simple main effects). [Taken with permission from Ahmed et al., 2002.]. (B) Unlimited daily access to heroin escalated heroin intake and decreased the excitability of brain reward systems. (Left) Heroin intake (± SEM; 20 μg per infusion) in rats during limited (1 h) or unlimited (23 h) self-administration sessions. ***p < 0.001, main effect of access (1 or 23 h). (Right) Percent change from baseline ICSS thresholds (± SEM) in 23 h rats. Reward thresholds, assessed immediately after each daily 23 h self-administration session, became progressively more elevated as exposure to self-administered heroin increased across sessions. *p < 0.05, main effect of heroin on reward thresholds. [Taken with permission from Kenny et al., 2006.]. (C) Escalation in methamphetamine self-administration and ICSS in rats. Rats were daily allowed to receive ICSS in the lateral hypothalamus 1 h before and 3 h after intravenous methamphetamine self-administration with either 1- or 6-h access. (Left) Methamphetamine self-administration during the first hour of each session. (Right) ICSS measured 1 h before and 3 h after methamphetamine self-administration. *p < 0.05, **p < 0.01, ***p < 0.001, compared with session 1. #p < 0.05, compared with LgA 3 h after. [Taken with permission from Jang et al., 2013.]
Brain stress system substrates for the dark side of addiction
Brain stress systems
The brain stress systems can be defined as neurochemical systems that are activated during exposure to acute stressors or in a chronic state of stress and mediate species-typical behavioral responses. These behavioral responses in animals range from freezing to flight and typically have face and predictive validity for similar behavior responses in humans. For example, animals exposed to a stressor will show an enhanced freezing response to a conditioned fear stimulus, an enhanced startle response to a startle stimulus, avoidance of open areas, open arms, or height, and enhanced species-typical responses to an aversive stimulus (e.g., burying a shock probe in the defensive burying test). Key neurotransmitter systems with circumscribed neurocircuitry that mediates behavioral responses to stressors include glucocorticoids, corticotropin-releasing factor (CRF), norepinephrine, and dynorphin, and key neurotransmitter systems that act in opposition to the brain stress systems include neuropeptide Y, nociceptin, and endocannabinoids (for reviews, see Koob, 2008; Sidhpura and Parsons, 2011; Heilig, 2004). For the purposes of this review, two brain stress systems with prominent roles in driving the dark side of addiction will be considered: CRF and dynorphin.
Corticotropin-releasing factor
Corticotropin-releasing factor is a 41-amino-acid polypeptide that controls hormonal, sympathetic, and behavioral responses to stressors (Rainnie et al., 2004; Lemos et al., 2012). Central administration of CRF mimics the behavioral response to activation and stress in rodents, and administration of competitive CRF receptor antagonists generally has anti-stress effects (for reviews, see Dunn and Berridge, 1990; Koob et al., 1994; Koob et al., 2001; Sarnyai et al., 2001). Two major CRF receptors have been identified, with CRF1 receptor activation associated with increased stress responsiveness (Koob and Heinrichs, 1999) and CRF2 receptor activation associated with decreases in feeding and decreases in stress responsiveness (Spina et al., 1996; Pelleymounter et al., 2000), although there is some controversy in this area (Takahashi et al., 2001). CRF neurons are present in the neocortex, the extended amygdala, the medial septum, the hypothalamus, the thalamus, the cerebellum, and autonomic midbrain and hindbrain nuclei (Swanson et al., 1983). Extensive research has been performed on CRF neurons in the paraventricular nucleus of the hypothalamus (PVN), central nucleus of the amygdala (CeA), and bed nucleus of the stria terminalis (BNST), demonstrating a key role for PVN CRF neurons in controlling the pituitary adrenal response to stress (Turnbull and Rivier, 1997) and a key role for BNST and CeA CRF in mediating the negative affective responses to stress and drug withdrawal (Koob and Kreek, 2007).
The neuroanatomical entity termed the extended amygdala (Heimer and Alheid, 1991) may represent a common anatomical substrate that integrates brain arousal-stress systems with hedonic processing systems to produce the between-system opponent process elaborated above. The extended amygdala is composed of the CeA, BNST, and a transition zone in the medial (shell) subregion of the nucleus accumbens. Each of these regions has cytoarchitectural and circuitry similarities (Heimer and Alheid, 1991). The extended amygdala receives numerous afferents from limbic structures, such as the basolateral amygdala and hippocampus, and sends efferents to the medial part of the ventral pallidum and a large projection to the lateral hypothalamus, thus further defining the specific brain areas that interface classical limbic (emotional) structures with the extrapyramidal motor system (Alheid et al., 1995). CRF in the extended amygdala has long been hypothesized to play a key role not only in fear conditioning (Maier and Watkins, 2005; Sink et al., 2013) but also in the emotional component of pain processing (Neugebauer et al., 2004).
Dynorphin-κ opioid system
Dynorphins are opioid peptides that derive from the prodynorphin precursor and contain the leucine (leu)-enkephalin sequence at the N-terminal portion of the molecule and are the presumed endogenous ligands for the κ opioid receptor (Chavkin et al., 1982). Dynorphins are widely distributed in the central nervous system (Watson et al., 1982) and play a role in neuroendocrine regulation, pain regulation, motor activity, cardiovascular function, respiration, temperature regulation, feeding behavior, and stress responsivity (Fallon and Leslie, 1986). Dynorphins bind to all three opioid receptors but show a preference for κ receptors (Chavkin et al., 1982). Dynorphin-κ receptor system activation produces actions that are similar to other opioids but often opposite to those of μ opioid receptors in the motivational domain. Dynorphins produce aversive dysphoric-like effects in animals and humans and have been hypothesized to mediate negative emotional states (Shippenberg et al., 2007; Wee and Koob, 2010; Mucha and Herz, 1985; Pfeiffer et al., 1986).
Dopamine receptor activation in the NAc shell stimulates a cascade of events that ultimately lead to cyclic adenosine monophosphate response element binding protein (CREB) phosphorylation and subsequent alterations in gene expression, notably the activation of the expression of prodynorphin mRNA. Subsequent activation of dynorphin systems has been hypothesized to feedback to decrease dopamine release in the mesolimbic dopamine system (Todtenkopf et al., 2004; Pliakas et al., 2001; Nestler, 2004; Mague et al., 2003; Knoll and Carlezon, 2010) and glutamate release in the nucleus accumbens (Hjelmstad and Fields, 2001; Gray et al., 1999). Both of these changes may contribute to the dysphoric syndrome associated with cocaine dependence. In vivo microdialysis studies have also provided evidence that κ opioid receptors located in the prefrontal cortex (PFC) and ventral tegmental area also regulate the basal activity of mesocortical dopamine neurons (Margolis et al., 2006; Tejeda, 2009).
In the extended amygdala, enhanced dynorphin action may also activate brain stress responses, such as CRF (Valdez et al., 2007), or CRF in turn may activate dynorphin (Land et al., 2008; McLaughlin et al., 2003). For example, Valdez showed that the reinstatement of cocaine seeking induced by the κ receptor agonist spiradoline in squirrel monkeys was antagonized by the CRF1 antagonist CP 154,526 (Valdez et al., 2007), and more recent data suggest that dynorphin in the central nucleus of the amygdala may mediate anxiety-like responses to cocaine by blocking cocaine escalation-induced increases in presynaptic GABA release (Kallupi et al., 2013). However, CRF also appears to activate dynorphin, in which nor-BNI pretreatment in mice blocked the stress-induced potentiation of cocaine-induced conditioned place preference (McLaughlin et al., 2003), and injection of CRF or urocortin 3, key mediators of the stress response, produced a place aversion that was also blocked by dynorphin gene deletion or κ receptor antagonism, suggesting that CRF drives dynorphin, possibly via the CRF2 receptor (Land et al., 2008).
Between-system neuroadaptations that contribute to the negative emotional state that drives negative reinforcement
Brain neurochemical systems involved in arousal-stress modulation have been hypothesized to be engaged within the neurocircuitry of the brain stress systems in an attempt to overcome the chronic presence of the perturbing drug and restore normal function despite the presence of drug (Koob, 2008). Both the hypothalamic-pituitary-adrenal (HPA) axis and extrahypothalamic brain stress system mediated by CRF are dysregulated by chronic administration of all major drugs with dependence or abuse potential, with a common response of elevated adrenocorticotropic hormone, corticosterone, and amygdala CRF during acute withdrawal (Rivier et al., 1984; Merlo-Pich et al., 1995; Koob et al., 1994; Rasmussen et al., 2000; Olive et al., 2002; Delfs et al., 2000; Koob, 2009). Indeed, activation of the HPA response may be an early dysregulation associated with excessive drug taking that ultimately “sensitizes” the extrahypothalamic CRF systems (Koob and Kreek, 2007; Vendruscolo et al., 2012).
As noted above, the excessive release of dopamine and opioid peptides produces subsequent activation of dynorphin systems, which has been hypothesized to feedback to decrease dopamine release and also contribute to the dysphoric syndrome associated with cocaine dependence (Nestler, 2004; Beardsley et al., 2005; Jackson et al., 2013; Tejeda et al., 2012). Dynorphins produce aversive dysphoric-like effects in animals and humans and have been hypothesized to mediate negative emotional states (Shippenberg et al., 2007; Wee and Koob, 2010; Mucha and Herz, 1985; Pfeiffer et al., 1986).
A common response to acute withdrawal and protracted abstinence from all major drugs of abuse is the manifestation of anxiety-like responses that are reversed by CRF antagonists. Withdrawal from repeated administration of cocaine produces an anxiogenic-like response in the elevated plus maze and defensive burying test, both of which are reversed by administration of CRF antagonists (Sarnyai et al., 1995; Basso et al., 1999). Opioid dependence also produces irritability-like effects that are reversed by CRF antagonists (Navarro-Zaragosa et al., 2010; Iredale et al., 2000). Ethanol withdrawal produces anxiety-like behavior that is reversed by intracerebroventricular administration of CRF1/CRF2 peptidergic antagonists (Baldwin et al., 1991), small molecule CRF1 antagonist (Knapp et al., 2004; Overstreet et al., 2004; Funk et al., 2007), and intracerebral administration of a peptidergic CRF1/CRF2 antagonist into the amygdala (Rassnick et al., 1993). The effects of CRF antagonists have been localized to the CeA (Rassnick et al., 1993). Precipitated withdrawal from nicotine produces anxiety-like responses that are also reversed by CRF antagonists (Tucci et al., 2003; George et al., 2007). CRF antagonists injected intracerebroventricularly or systemically also block the potentiated anxiety-like responses to stressors observed during protracted abstinence from chronic ethanol (Breese et al., 2005; Valdez et al., 2003; Huang et al., 2010; Overstreet et al., 2007; Wills et al., 2009).
Another measure of negative emotional states during drug withdrawal in animals is condition place aversion, in which animals avoid an environment previously paired with an aversive state. Such place aversions, when used to measure the aversive stimulus effects of withdrawal, have been observed largely in the context of opioids (Hand et al., 1988; Stinus et al., 1990). Systemic administration of a CRF1 receptor antagonist and direct intracerebral administration of a peptide CRF1/CRF2 antagonist also decreased opioid withdrawal-induced place aversions (Stinus et al., 2005; Heinrichs et al., 1995; Contarino and Papaleo, 2005). These effects have been hypothesized to be mediated by actions in the extended amygdala. The selective CRF1 antagonist antalarmin blocked the place aversion produced by naloxone in morphine-dependent rats (Stinus et al., 2005), and a CRF peptide antagonist injected into the CeA also reversed the place aversion produced by methylnaloxonium injected into the CeA (Heinrichs et al., 1995). CRF1 knockout mice failed to show conditioned place aversion to opioid withdrawal and failed to show an opioid-induced increase in dynorphin mRNA in the nucleus accumbens (Contarino and Papaleo, 2005).
A compelling test of the hypothesis that CRF-induced increases in anxiety-like responses during drug withdrawal has motivational significance in contributing to negative emotional states is the observation that CRF antagonists can reverse the elevation in reward thresholds produced by drug withdrawal. Nicotine and alcohol withdrawal-induced elevations in reward thresholds were reversed by a CRF antagonist (Bruijnzeel et al., 2007; Bruijnzeel et al., 2010). These effects have been localized to both the CeA and nucleus accumbens shell (Marcinkiewcz et al., 2009).
Enhanced dynorphin action is hypothesized to mediate the depression-like, aversive responses to stress and dysphoric-like responses during withdrawal from drugs of abuse (Chartoff et al., 2012; Schindler et al., 2010; Land et al., 2009; McLaughlin et al., 2003; Redila and Chavkin, 2008; Land et al., 2008; McLaughlin et al., 2006; Knoll et al., 2007; Mague et al., 2003; Beardsley et al 2005; Jackson et al., 2013: Tejeda et al, 2012). For example, pretreatment with a κ-opioid receptor antagonist blocked stress-induced analgesia and stress-induced immobility (McLaughlin et al., 2003), decreased anxiety-like behavior in the elevated plus maze and open field, decreased conditioned fear in fear-potentiated startle (Knoll et al., 2007), significantly reduced the footshock-induced reinstatement of responding previously reinforced by cocaine in rats (Beardley et al., 2005), attenuated stress-induced reinstatement of nicotine-induced conditioned place preference in rats (Jackson et al., 2013), and blocked depressive-like behavior induced by cocaine and nicotine withdrawal (Chartoff et al., 2012; Tejeda et al, 2012).
Brain Stress Substrates that Mediate Drug Taking with Extended Access
Corticotropin-releasing factor, compulsive-like drug seeking, and the extended amygdala
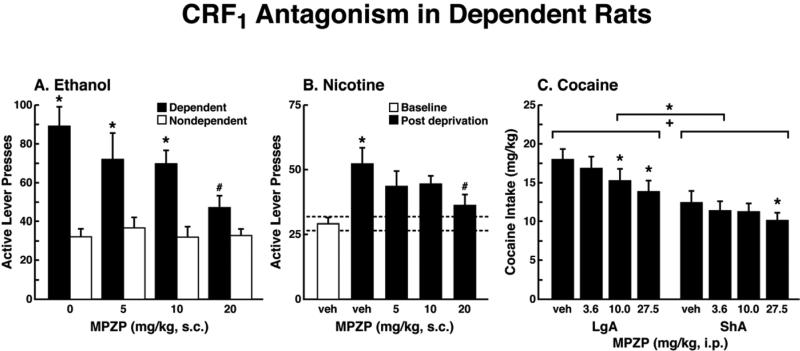
The ability of CRF antagonists to block the anxiogenic-like and aversive-like motivational effects of drug withdrawal predicted motivational effects of CRF antagonists in animal models of extended access to drugs. CRF antagonists selectively blocked the increased self-administration of drugs associated with extended access to intravenous self-administration of cocaine (Specio et al., 2008), nicotine (George et al., 2007), and heroin (Greenwell et al., 2009; Figure 4). For example, systemic administration of a CRF1 antagonist blocked the increased self-administration of nicotine associated with withdrawal in extended-access (23 h) animals (George et al., 2007).
Figure 4.
(A) The effect of the CRF1 receptor antagonist MPZP on operant self-administration of alcohol in dependent and nondependent rats. Testing was conducted when dependent animals were in acute withdrawal (6-8 h after removal from vapors). Dependent rats self-administered significantly more than nondependent animals, and MPZP dose-dependently reduced alcohol self-administration only in dependent animals. The data are expressed as mean + SEM lever presses for alcohol. [Taken with permission from Richardson et al., 2008.] (B) Abstinence-induced escalation of nicotine intake is blocked by a CRF1 receptor antagonist. Effect of MPZP (s.c., −1 h) on nicotine self-administration during the active period in rats given extended access to nicotine. *p < 0.05, compared with baseline; #p < 0.05, compared with after-abstinence vehicle treatment; n = 8). The data are expressed as mean + SEM lever presses for nicotine. [Taken with permission from George et al., 2007.] (C) MPZP reduces cocaine intake in ShA and LgA rats. The data are expressed as mean + SEM cocaine intake (mg/kg). *p < 0.05, **p < 0.01, compared with vehicle. [Taken with permission from Specio et al., 2008.]
CRF antagonists also blocked the increased self-administration of ethanol in dependent rats (Funk et al., 2007; Figure 4). For example, exposure to repeated cycles of chronic ethanol vapor produced substantial increases in ethanol intake in rats during both acute withdrawal and protracted abstinence (2 weeks post-acute withdrawal; O'Dell et al., 2004; Rimondini et al., 2002). Intracerebroventricular administration of a CRF1/CRF2 antagonist blocked the dependence-induced increase in ethanol self-administration during both acute withdrawal and protracted abstinence (Valdez et al., 2004). Systemic injections of small-molecule CRF1 antagonists also blocked the increased ethanol intake associated with acute withdrawal (Funk et al., 2007) and protracted abstinence (Gehlert et al., 2007). When administered directly into the CeA, a CRF1/CRF2 antagonist blocked ethanol self-administration in ethanol-dependent rats (Funk et al., 2006, 2007). Altogether, these results suggest that CRF in the basal forebrain may also play an important role in the development of the aversive motivational effects that drive the increased drug seeking associated with cocaine, heroin, nicotine, and alcohol dependence.
Dynorphin, compulsive-like drug seeking and the extended amygdala
Recent evidence suggests that the dynorphin-κ opioid system also mediates the compulsive-like drug responding (methamphetamine, heroin, and alcohol) with extended access and dependence. Evidence from our laboratory has shown that κ opioid receptor antagonism with a small-molecule κ antagonist selectively blocked responding on a progressive-ratio schedule for cocaine in rats with extended access (Wee et al., 2009). Even more compelling is that excessive drug self-administration can also be blocked by κ antagonists (Walker et al., 2010; Wee et al., 2009; Whitfield et al., 2011; Schlosburg et al., 2011) and may be mediated by the shell of the nucleus accumbens (Nealey et al., 2011). However, the neurobiological circuits involved in mediating the effects of activation of the dynorphin-κ opioid system on the escalation of methamphetamine intake with extended access, the specific sites of action for κ receptor antagonists to mediate dramatic effects of a κ antagonist on the escalation of methamphetamine self-administration with extended access, and the signaling cascades that mediate the activation of the dynorphin-κ opioid system on the escalation of methamphetamine self-administration remain unknown.
Corticotropin-releasing factor, stress, and the frontal cortex
Converging lines of evidence suggest that impairment of medial PFC (mPFC) cognitive function and overactivation of the CeA may be linked to the development of compulsive-like responding for drugs of abuse during extended access (George et al., 2008; Briand et al., 2008a, b). Extended access to cocaine self-administration induced an escalated pattern of cocaine intake associated with an impairment of working memory and decrease in the density of dorsomedial PFC (dmPFC) neurons that lasts for months after cocaine cessation (George et al., 2008). Whereas long access (LgA) and short access (ShA) rats exhibited a high percentage of correct responses in the delayed-non-matching-to-sample task under low cognitive demand (delay < 10 s), increasing the working memory load (i.e., close to the capacity limit of working memory) by increasing the delay from 10 s to 70 and 130 s revealed a robust working memory deficit in LgA rats. Furthermore, the magnitude of escalation of cocaine intake was negatively correlated with working memory performance in ShA and LgA rats with the 70 s and 130 s delays but not with the 10 s delay or with baseline performance during training, demonstrating that the relationship between escalation of cocaine intake and behavioral performance in this task was restricted to working memory performance under high cognitive demand. The density of neurons and oligodendrocytes in the dmPFC was positively correlated with working memory performance. A lower density of neurons or oligodendrocytes in the dmPFC was associated with more severe working memory impairment. Working memory was also correlated with the density of oligodendrocytes in the orbitofrontal cortex (OFC), suggesting that OFC alterations after escalated drug intake may play a role in working memory deficits. However, no correlation was found between working memory performance and neuronal density in the OFC, suggesting that OFC neurons may be less vulnerable to the deleterious effects of chronic cocaine exposure than dmPFC neurons. Thus, PFC dysfunction may exacerbate the loss of control associated with compulsive drug use and facilitate the progression to drug addiction.
Similar results have been observed in an animal model of binge alcohol consumption, even before the development of dependence. Using an animal model of escalation of alcohol intake with chronic intermittent access to alcohol, in which rats are given continuous (24 h per day, 7 days per week) or intermittent (3 days per week) access to alcohol (20% vol/vol) using a two-bottle choice paradigm, FBJ murine osteosarcoma viral oncogene homolog (Fos) expression in the mPFC, CeA, hippocampus, and nucleus accumbens were measured and correlated with working memory and anxiety-like behavior (George et al., 2012). Abstinence from alcohol in rats with a history of escalation of alcohol intake specifically recruited γ-aminobutyric acid (GABA) and CRF neurons in the mPFC and produced working memory impairments associated with excessive alcohol drinking during acute (24-72 h) but not protracted (16-68 day) abstinence. The abstinence from alcohol was associated with a functional disconnection of the mPFC and CeA but not mPFC and nucleus accumbens. These results show that recruitment of a subset of GABA and CRF neurons in the mPFC during withdrawal and disconnection of the PFC CeA pathway may be critical for impaired executive control over motivated behavior, suggesting that dysregulation of mPFC interneurons may be an early index of neuroadaptation in alcohol dependence.
Brain Stress Systems in Addiction: An Allostatic View
For the binge/intoxication stage of the addiction cycle, studies of the acute reinforcing effects of drugs of abuse per se have identified key neurobiological substrates. Evidence is strong for a role for dopamine in the acute reinforcing actions of psychostimulants, opioid peptide receptors in the acute reinforcing effects of opioids, and GABA and opioid peptides in the acute reinforcing actions of alcohol. Important anatomical circuits include the mesocorticolimbic dopamine system that originates in the ventral tegmental area and projects to the nucleus accumbens. However, acute drug use, even binge drug use, is not addiction. The withdrawal/negative affect stage can be defined as the presence of motivational signs of withdrawal in humans, including chronic irritability, physical pain, emotional pain (i.e., hyperkatifeia; Shurman et al., 2010), malaise, dysphoria, alexithymia, and loss of motivation for natural rewards. It is characterized in animals by increases in reward thresholds during withdrawal from all major drugs of abuse. More compelling, in animal models of the transition to addiction, similar changes in brain reward thresholds occur that temporally precede and are highly correlated with escalation in drug intake (Ahmed et al., 2002; Kenny et al., 2006; Jang et al., 2013). Such acute withdrawal is associated with decreased activity of the mesocorticolimbic dopamine system, reflected by electrophysiological recordings and in vivo microdialysis. Even more compelling are recent data that show a blunted phasic dopamine response in the nucleus accumbens and dorsal striatum, measured by in vivo voltammetry in animals that exhibit escalation with extended access (Willuhn et al., 2012). Human imaging studies of individuals with addiction during withdrawal or protracted abstinence have generated results that are consistent with animal studies. There are decreases in dopamine D2 receptors (hypothesized to reflect hypodopaminergic functioning), hyporesponsiveness to dopamine challenge (Volkow et al., 2003), and hypoactivity of the orbitofrontal-infralimbic cortex system (Volkow et al., 2003). These are hypothesized to be within-system neuroadaptations that may reflect presynaptic release or postsynaptic receptor plasticity.
More importantly for the present thesis, as dependence and withdrawal develop, brain anti-reward systems, such as CRF and dynorphin, are recruited in the extended amygdala. We hypothesize that this brain stress neurotransmitter that is known to be activated during the development of excessive drug taking comprises a between-system opponent process, and this activation is manifest when the drug in removed, producing anxiety, hyperkatifeia, and irritability symptoms associated with acute and protracted abstinence. Notably, however, there is evidence of CRF immunoreactivity in the ventral tegmental area, and a CRF1 receptor antagonist injected directly into the ventral tegmental area blocked social stress-induced escalation of cocaine self-administration (Boyson et al., 2011). Altogether, these observations suggest between-system/within-system neuroadaptations that were originally hypothesized for dynorphin by Carlezon and Nestler (Carlezon et al., 1998), in which activation of CREB by excessive dopamine and opioid peptide receptor activation in the nucleus accumbens triggers the induction of dynorphin to feedback to suppress dopamine release. Thus, we hypothesize that anti-reward circuits are recruited as between-system neuroadaptations (Koob and Bloom, 1988) during the development of addiction and produce aversive or stress-like states (Nestler, 2001; Koob, 2003; Aston-Jones et al., 1999) via two mechanisms: direct activation of stress-like, fear-like states in the extended amygdala (CRF) and indirect activation of a depression-like state by suppressing dopamine (dynorphin).
A critical problem in drug addiction is chronic relapse, in which addicted individuals return to compulsive drug taking long after acute withdrawal. This corresponds to the preoccupation/anticipation stage of the addiction cycle outlined above. Koob and Le Moal also hypothesized that the dysregulations that comprise the “dark side” of drug addiction persist during protracted abstinence to set the tone for vulnerability to “craving” by activating drug-, cue-, and stress-induced reinstatement neurocircuits that are now driven by a reorganized and possibly hypofunctioning prefrontal system. Here, a hypofunctioning cortical system would be argued to play a permissive role, in which subcortical structures that drive addiction are not inhibited. Thus, the hypothesized allostatic, dysregulated reward and sensitized stress state produces the motivational symptoms of acute withdrawal and protracted abstinence and provides the basis by which drug priming, drug cues, and acute stressors acquire even more power to elicit drug-seeking behavior (Vendruscolo et al., 2012). The combination of decreases in reward system function and recruitment of anti-reward systems provides a powerful source of negative reinforcement that contributes to compulsive drug-seeking behavior and addiction. A compelling argument can be made that the neuroplasticity that charges the CRF stress system may indeed begin much earlier that previously thought via stress actions in the PFC.
The overall conceptual theme argued here is that drug addiction represents an excessive and prolonged engagement of homeostatic brain regulatory mechanisms that regulate the response of the body to stressors. The dysregulation of the stress axis begins with the binge and subsequent acute withdrawal, triggering a cascade of changes from activation of the HPA axis to activation of CRF in the prefrontal cortex, to activation of CRF in the extended amygdala to activation of dynorphin in the ventral striatum (Figure 5). This cascade of overactivation of the stress axis represents more than simply a transient homeostatic dysregulation; it also represents a dynamic homeostatic dysregulation that has been termed allostasis.
Figure 5.
Neurocircuitry framework for the between-systems neuroadaptations hypothesized to mediate the transition to dependence in addiction. (Top) The hypothesis outlined here is that excessive activation of elements of the brain reward system (dopamine and opioid peptides via μ opioid receptors) in turn activates dynorphin in the ventral striatum, which in turn suppresses dopamine release to contribute to the negative emotional (dysphoric-like) effects of drug withdrawal. (Bottom) The hypothesis outlined here is that activation of elements of the brain stress systems in the extended amygdala during withdrawal (CRF, norepinephrine, and dynorphin) sensitize via feed-forward mechanisms and also contribute to the negative emotional (anxiety-like) effects of drug withdrawal.
Allostasis, originally conceptualized to explain persistent morbidity of arousal and autonomic function, can be defined as “stability through change.” Allostasis involves a feed-forward mechanism rather than the negative feedback mechanisms of homeostasis, with continuous re-evaluation of need and continuous readjustment of all parameters toward new set points. An allostatic state has been defined as a state of chronic deviation of the regulatory system from its normal (homeostatic) operating level (Koob and Le Moal, 2001). Allostatic load was defined as the “long-term cost of allostasis that accumulates over time and reflects the accumulation of damage that can lead to pathological states” (McEwen, 2000).
Repeated challenges, such as with drugs of abuse, lead to attempts of the brain stress systems at the molecular, cellular, and neurocircuitry level to maintain stability but at a cost. For the drug addiction framework elaborated here, the residual activation of the brain stress systems to produce the consequent negative emotional state is termed an allostatic state. This state represents a combination of recruitment of anti-reward systems and consequent chronic decreased function of reward circuits, both of which lead to the compulsive drug seeking and loss of control over intake. How these systems are modulated by other known brain emotional systems localized to the basal forebrain, where the ventral striatum and extended amygdala project to convey emotional valence, how frontal cortex dysregulations in the cognitive domain linked to impairments in executive function contribute to the dysregulation of the extended amygdala, and how individuals differ at the molecular-genetic level of analysis to convey loading on these circuits remain challenges for future research.
Addiction involves elements of impulsivity and compulsivity.
Excessive drug use leads to within-reward-system neuroadaptations.
Excessive drug use leads to between-system neuroadaptations.
Compulsive drug seeking recruits NE and CRF stress systems in the extended amygdala.
Excessive drug taking produces PFC-mediated deficits in executive function.
Figure 6.
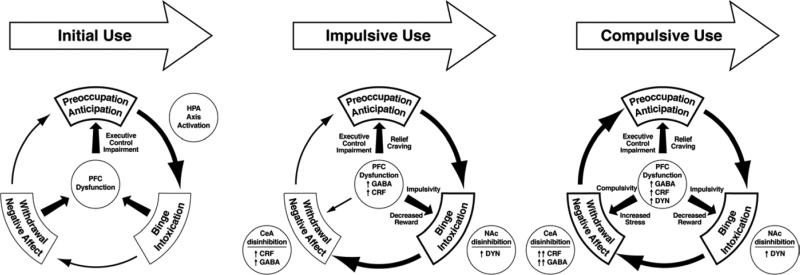
The progression of compulsive drug use over time mediates recruitment of brain stress systems. The schematic illustrates the shift in underlying motivational mechanisms. As the addictive process progresses over time, the initial positively reinforcing, pleasurable drug effects are augmented by negatively reinforcing relief from a negative emotional state. The data summarized in the present treatise suggest that the neuroadaptations that encompass the recruitment of extrahypothalamic CRF and dynorphin brain stress systems are key to this shift. Initial use is characterized by activation of the hypothalamic-pituitary-adrenal axis to drive the binge/intoxication stage. Impulsive use is characterized by prefrontal cortex dysfunction with activation of corticotropin-releasing factor (CRF), γ-aminobutyric acid (GABA), and possibly dynorphin in the prefrontal cortex and subsequent disinhibition of the nucleus accumbens and extended amygdala that in turn drive increases in dynorphin in the nucleus accumbens and CRF in the extended amygdala. Compulsive use involves a pronounced activation of CRF in the extended amygdala, dynorphin-induced decreases in dopamine in the nucleus accumbens, and a pronounced loss of executive control in the prefrontal cortex, all leading to the spiralling distress and loss of control associated with full-blown dependence mediated by negative reinforcement.
Acknowledgements
The author would like to thank Michael Arends and Mellany Santos for their assistance with the preparation of this manuscript. Research was supported by National Institutes of Health grants AA006420, AA020608, and AA008459 from the National Institute on Alcohol Abuse and Alcoholism, DA010072, DA004043, DA023597, and DA004398 from the National Institute on Drug Abuse, and DK26741 from the National Institute of Diabetes and Digestive and Kidney Diseases. Research also was supported by the Pearson Center for Alcoholism and Addiction Research. This is publication number 23022 from The Scripps Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SH, Cador M. Dissociation of psychomotor sensitization from compulsive cocaine consumption. Neuropsychopharmacology. 2006;31:563–571. doi: 10.1038/sj.npp.1300834. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat. Neurosci. 2002;5:625–626. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology. 1999;146:303–312. doi: 10.1007/s002130051121. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22:413–421. doi: 10.1016/S0893-133X(99)00133-5. [DOI] [PubMed] [Google Scholar]
- Alheid GF, De Olmos JS, Beltramino CA. Amygdala and extended amygdala. In: Paxinos G, editor. The Rat Nervous System. Academic Press; San Diego: 1995. pp. 495–578. [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th edition American Psychiatric Press; Washington DC: 1994. [Google Scholar]
- Aston-Jones G, Delfs JM, Druhan J, Zhu Y. The bed nucleus of the stria terminalis: a target site for noradrenergic actions in opiate withdrawal. In: McGinty JF, editor. Advancing from the Ventral Striatum to the Extended Amygdala: Implications for Neuropsychiatry and Drug Abuse (series title: Annals of the New York Academy of Sciences, vol. 877) New York Academy of Sciences; New York: 1999. pp. 486–498. [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol. Rev. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Baldwin HA, Rassnick S, Rivier J, Koob GF, Britton KT. CRF antagonist reverses the “anxiogenic” response to ethanol withdrawal in the rat. Psychopharmacology. 1991;103:227–232. doi: 10.1007/BF02244208. [DOI] [PubMed] [Google Scholar]
- Barbier E, Vendruscolo LF, Schlosburg JE, Edwards S, Juergens N, Park PE, Misra KK, Cheng K, Rice KC, Schank J, Schulteis G, Koob GF, Heilig M. The NK1 receptor antagonist L822429 reduces heroin reinforcement. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2012.261. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso AM, Spina M, Rivier J, Vale W, Koob GF. Corticotropin-releasing factor antagonist attenuates the “anxiogenic-like” effect in the defensive burying paradigm but not in the elevated plus-maze following chronic cocaine in rats. Psychopharmacology. 1999;145:21–30. doi: 10.1007/s002130051028. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Howard JL, Shelton KL, Carroll FI. Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacology. 2005;183:118–126. doi: 10.1007/s00213-005-0167-4. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Ahmed SH, Koob GF, Ettenberg A. The transition from controlled to compulsive drug use is associated with a loss of sensitization. Brain Res. 2004;995:46–54. doi: 10.1016/j.brainres.2003.09.053. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Posthumus EJ, Waldroup SA, Ettenberg A. Heightened drug-seeking motivation following extended daily access to self-administered cocaine. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32:863–869. doi: 10.1016/j.pnpbp.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyson CO, Miguel TT, Quadros IM, Debold JF, Miczek KA. Prevention of social stress-escalated cocaine self-administration by CRF-R1 antagonist in the rat VTA. Psychopharmacology. 2011;218:257–269. doi: 10.1007/s00213-011-2266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ, Navarro M. Prior multiple ethanol withdrawals enhance stress-induced anxiety-like behavior: inhibition by CRF1- and benzodiazepine-receptor antagonists and a 5-HT1a-receptor agonist. Neuropsychopharmacology. 2005;30:1662–1669. doi: 10.1038/sj.npp.1300706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand LA, Flagel SB, Garcia-Fuster MJ, Watson SJ, Akil H, Sarter M, Robinson TE. Persistent alterations in cognitive function and prefrontal dopamine D2 receptors following extended, but not limited, access to self-administered cocaine. Neuropsychopharmacology. 2008a;33:2969–2980. doi: 10.1038/npp.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand LA, Gross JP, Robinson TE. Impaired object recognition following prolonged withdrawal from extended-access cocaine self-administration. Neuroscience. 2008b;155:1–6. doi: 10.1016/j.neuroscience.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Small E, Pasek TM, Yamada H. Corticotropin-releasing factor mediates the dysphoria-like state associated with alcohol withdrawal in rats. Behav. Brain Res. 2010;210:288–291. doi: 10.1016/j.bbr.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Zislis G, Wilson C, Gold MS. Antagonism of CRF receptors prevents the deficit in brain reward function associated with precipitated nicotine withdrawal in rats. Neuropsychopharmacology. 2007;32:955–963. doi: 10.1038/sj.npp.1301192. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr., Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, Duman RS, Neve RL, Nestler EJ. Regulation of cocaine reward by CREB. Science. 1998;282:2272–2275. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- Chartoff E, Sawyer A, Rachlin A, Potter D, Pliakas A, Carlezon WA. Blockade of kappa opioid receptors attenuates the development of depressive-like behaviors induced by cocaine withdrawal in rats. Neuropharmacology. 2012;62:1167–1176. doi: 10.1016/j.neuropharm.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C, James IF, Goldstein A. Dynorphin is a specific endogenous ligand of the κ opioid receptor. Science. 1982;215:413–415. doi: 10.1126/science.6120570. [DOI] [PubMed] [Google Scholar]
- Cohen A, Koob GF, George O. Robust escalation of nicotine intake with extended access to nicotine self-administration and intermittent periods of abstinence. Neuropsychopharmacology. 2012;37:2153–2160. doi: 10.1038/npp.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colpaert FC. System theory of pain and of opiate analgesia: no tolerance to opiates. Pharmacol. Rev. 1996;48:355–402. [PubMed] [Google Scholar]
- Contarino A, Papaleo F. The corticotropin-releasing factor receptor-1 pathway mediates the negative affective states of opiate withdrawal. Proc. Natl Acad. Sci. U. S. A. 2005;102:18649–18654. doi: 10.1073/pnas.0506999102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403:430–434. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- Deroche V, Le Moal M, Piazza PV. Cocaine self-administration increases the incentive motivational properties of the drug in rats. Eur. J. Neurosci. 1999;11:2731–2736. doi: 10.1046/j.1460-9568.1999.00696.x. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res. Rev. 1990;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- Edwards S, Guerrero M, Ghoneim OM, Roberts E, Koob GF. Evidence that vasopressin V1b receptors mediate the transition to excessive drinking in ethanol-dependent rats. Addict. Biol. 2011;17:76–85. doi: 10.1111/j.1369-1600.2010.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Fallon JH, Leslie FM. Distribution of dynorphin and enkephalin peptides in the rat brain. J. Comp. Neurol. 1986;249:293–336. doi: 10.1002/cne.902490302. [DOI] [PubMed] [Google Scholar]
- Funk CK, O'Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J. Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol. Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner EL, Vorel SR. Cannabinoid transmission and reward-related events. Neurobiol. Dis. 1998;5:502–533. doi: 10.1006/nbdi.1998.0219. [DOI] [PubMed] [Google Scholar]
- Gehlert DR, Cippitelli A, Thorsell A, Le AD, Hipskind PA, Hamdouchi C, Lu J, Hembre EJ, Cramer J, Song M, McKinzie D, Morin M, Ciccocioppo R, Heilig M. 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethylimidazo[1,2-b]pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. J. Neurosci. 2007;27:2718–2726. doi: 10.1523/JNEUROSCI.4985-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, O'Dell LE, Richardson HN, Koob GF. CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc. Natl Acad. Sci. U. S. A. 2007;104:17198–17203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Mandyam CD, Wee S, Koob GF. Extended access to cocaine self-administration produces long-lasting prefrontal cortex-dependent working memory impairments. Neuropsychopharmacology. 2008;33:2474–2482. doi: 10.1038/sj.npp.1301626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Sanders C, Freiling J, Grigoryan E, Vu S, Allen CD, Crawford E, Mandyam CD, Koob GF. Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking. Proc. Natl Acad. Sci. U. S. A. 2012;109:18156–18161. doi: 10.1073/pnas.1116523109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray AM, Rawls SM, Shippenberg TS, McGinty JF. The kappa-opioid agonist, U-69593, decreases acute amphetamine-evoked behaviors and calcium-dependent dialysate levels of dopamine and glutamate in the ventral striatum. J. Neurochem. 1999;73:1066–1074. doi: 10.1046/j.1471-4159.1999.0731066.x. [DOI] [PubMed] [Google Scholar]
- Greenwell TN, Funk CK, Cottone P, Richardson HN, Chen SA, Rice K, Zorrilla EP, Koob GF. Corticotropin-releasing factor-1 receptor antagonists decrease heroin self-administration in long-, but not short-access rats. Addict. Biol. 2009;14:130–143. doi: 10.1111/j.1369-1600.2008.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand TH, Koob GF, Stinus L, Le Moal M. Aversive properties of opiate receptor blockade: Evidence for exclusively central mediation in naive and morphine-dependent rats. Brain Res. 1988;474:364–368. doi: 10.1016/0006-8993(88)90452-0. [DOI] [PubMed] [Google Scholar]
- Harris AC, Pentel PR, Burroughs D, Staley MD, Lesage MG. A lack of association between severity of nicotine withdrawal and individual differences in compensatory nicotine self-administration in rats. Psychopharmacology. 2011;217:153–166. doi: 10.1007/s00213-011-2273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M. The NPY system in stress, anxiety and depression. Neuropeptides. 2004;38:213–224. doi: 10.1016/j.npep.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Heimer L, Alheid G. Piecing together the puzzle of basal forebrain anatomy. In: Napier TC, Kalivas PW, Hanin I, editors. The Basal Forebrain: Anatomy to Function (series title: Advances in Experimental Medicine and Biology, vol. 295) Plenum Press; New York: 1991. pp. 1–42. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Menzaghi F, Schulteis G, Koob GF, Stinus L. Suppression of corticotropin-releasing factor in the amygdala attenuates aversive consequences of morphine withdrawal. Behav. Pharmacol. 1995;6:74–80. [PubMed] [Google Scholar]
- Hjelmstad GO, Fields HL. Kappa opioid receptor inhibition of glutamatergic transmission in the nucleus accumbens shell. J. Neurophysiol. 2001;85:1153–1158. doi: 10.1152/jn.2001.85.3.1153. [DOI] [PubMed] [Google Scholar]
- Huang MM, Overstreet DH, Knapp DJ, Angel R, Wills TA, Navarro M, Rivier J, Vale W, Breese GR. Corticotropin-releasing factor (CRF) sensitization of ethanol withdrawal-induced anxiety-like behavior is brain site specific and mediated by CRF-1 receptors: relation to stress-induced sensitization. J. Pharmacol. Exp. Ther. 2010;332:298–307. doi: 10.1124/jpet.109.159186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iredale PA, Alvaro JD, Lee Y, Terwilliger R, Chen YL, Duman RS. Role of corticotropin-releasing factor receptor-1 in opiate withdrawal. J. Neurochem. 2000;74:199–208. doi: 10.1046/j.1471-4159.2000.0740199.x. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, McLaughlin JP, Carroll FI, Damaj MI. Effects of the kappa opioid receptor antagonist, norbinaltorphimine, on stress and drug-induced reinstatement of nicotine-conditioned place preference in mice. Psychopharmacology (Berl) 2013;226:763–768. doi: 10.1007/s00213-012-2716-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W. The Principles of Psychology. Dover Publications; New York: 1918. [Google Scholar]
- Jang CG, Whitfield T, Schulteis G, Koob GF, Wee S. Sensitization of a negative emotional-like state during repeated withdrawal from extended access methamphetamine self-administration in rats. Psychopharmacology. 2013;225:753–763. doi: 10.1007/s00213-012-2864-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkman S, Pelloux Y, Everitt BJ. Drug intake is sufficient, but conditioning is not necessary for the emergence of compulsive cocaine seeking after extended self-administration. Neuropsychopharmacology. 2012;37:1612–1619. doi: 10.1038/npp.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallupi M, Wee S, Edwards S, Whitfield TW, Jr., Oleata CS, Luu G, Schmeichel BE, Koob GF, Roberto M. Kappa opioid receptor-mediated dysregulation of GABAergic transmission in the central amygdala in cocaine addiction. Biol. Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.04.028. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Chen SA, Kitamura O, Markou A, Koob GF. Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. J. Neurosci. 2006;26:5894–5900. doi: 10.1523/JNEUROSCI.0740-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Polis I, Koob GF, Markou A. Low dose cocaine self-administration transiently increases but high dose cocaine persistently decreases brain reward function in rats. Eur. J. Neurosci. 2003;17:191–195. doi: 10.1046/j.1460-9568.2003.02443.x. [DOI] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harvard Rev. Psychiatry. 1997;4:231–244. doi: 10.3109/10673229709030550. [DOI] [PubMed] [Google Scholar]
- Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L. Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology. 2006;186:48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Kalivas PW. Extended access to cocaine self-administration enhances drug-primed reinstatement but not behavioral sensitization. J. Pharmacol. Exp. Ther. 2007;322:1103–1109. doi: 10.1124/jpet.107.122861. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Moy SS, Breese GR. SB242084, flumazenil, and CRA1000 block ethanol withdrawal-induced anxiety in rats. Alcohol. 2004;32:101–111. doi: 10.1016/j.alcohol.2003.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA., Jr. Dynorphin, stress, and depression. Brain Res. 2010;1314:56–73. doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll AT, Meloni EG, Thomas JB, Carroll FI, Carlezon WA., Jr. Anxiolytic-like effects of kappa-opioid receptor antagonists in models of unlearned and learned fear in rats. J. Pharmacol. Exp. Ther. 2007;323:838–845. doi: 10.1124/jpet.107.127415. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neuroadaptive mechanisms of addiction: studies on the extended amygdala. Eur. Neuropsychopharmacol. 2003;13:442–452. doi: 10.1016/j.euroneuro.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009;56:18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction: alcohol addiction as a reward deficit disorder. In: Sommer WH, Spanagel R, editors. Behavioral Neurobiology of Alcohol Addiction (series title: Current Topics in Behavioral Neuroscience, vol. 13) Springer-Verlag; Berlin: 2012. pp. 3–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Bartfai T, Roberts AJ. The use of molecular genetic approaches in the neuropharmacology of corticotropin-releasing factor. Int. J. Comp. Psychol. 2001;14:90–110. [Google Scholar]
- Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- Koob GF, Heinrichs SC. A role for corticotropin-releasing factor and urocortin in behavioral responses to stressors. Brain Res. 1999;848:141–152. doi: 10.1016/s0006-8993(99)01991-5. [DOI] [PubMed] [Google Scholar]
- Koob GF, Heinrichs SC, Menzaghi F, Pich EM, Britton KT. Corticotropin releasing factor, stress and behavior. Semin. Neurosci. 1994;6:221–229. [Google Scholar]
- Koob GF, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am. J. Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu. Rev. Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Koob GF, Stinus L, Le Moal M, Bloom FE. Opponent process theory of motivation: neurobiological evidence from studies of opiate dependence. Neurosci. Biobehav. Rev. 1989;13:135–140. doi: 10.1016/s0149-7634(89)80022-3. [DOI] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J. Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Schattauer S, Giardino WJ, Aita M, Messinger D, Hnasko TS, Palmiter RD, Chavkin C. Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc. Natl Acad. Sci. U. S. A. 2009;106:19168–19173. doi: 10.1073/pnas.0910705106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos JC, Wanat MJ, Smith JS, Reyes BA, Hollon NG, Van Bockstaele EJ, Chavkin C, Phillips PE. Severe stress switches CRF action in the nucleus accumbens from appetitive to aversive. Nature. 2012;490:402–406. doi: 10.1038/nature11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Schulteis G. Brain reward deficits accompany naloxone-precipitated withdrawal from acute opioid dependence. Pharmacol. Biochem. Behav. 2004;79:101–108. doi: 10.1016/j.pbb.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jr., Jones RM, Portoghese PS, Carlezon WA., Jr. Antidepressant-like effects of κ-opioid receptor antagonists in the forced swim test in rats. J. Pharmacol. Exp. Ther. 2003;305:323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci. Biobehav. Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ. Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacology. 2004;175:26–36. doi: 10.1007/s00213-004-1778-x. [DOI] [PubMed] [Google Scholar]
- Marcinkiewcz CA, Prado MM, Isaac SK, Marshall A, Rylkova D, Bruijnzeel AW. Corticotropin-releasing factor within the central nucleus of the amygdala and the nucleus accumbens shell mediates the negative affective state of nicotine withdrawal in rats. Neuropsychopharmacology. 2009;34:1743–1752. doi: 10.1038/npp.2008.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Chefer VI, Shippenberg TS, Hjelmstad GO, Fields HL. Kappa opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proc. Natl Acad. Sci. U. S. A. 2006;103:2938–2942. doi: 10.1073/pnas.0511159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Koob GF. Post-cocaine anhedonia: an animal model of cocaine withdrawal. Neuropsychopharmacology. 1991;4:17–26. [PubMed] [Google Scholar]
- McEwen BS. Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22:108–124. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology. 2006;31:1241–1248. doi: 10.1038/sj.npp.1300872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, Chavkin C. κ Opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J. Neurosci. 2003;23:5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo-Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J. Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucha RF, Herz A. Motivational properties of kappa and mu opioid receptor agonists studied with place and taste preference conditioning. Psychopharmacology. 1985;86:274–280. doi: 10.1007/BF00432213. [DOI] [PubMed] [Google Scholar]
- Navarro-Zaragoza J, Núñez C, Laorden ML, Milanés MV. Effects of corticotropin-releasing factor receptor-1 antagonists on the brain stress system responses to morphine withdrawal. Mol. Pharmacol. 2010;77:864–873. doi: 10.1124/mol.109.062463. [DOI] [PubMed] [Google Scholar]
- Nealey KA, Smith AW, Davis SM, Smith DG, Walker BM. Kappa-opioid receptors are implicated in the increased potency of intra-accumbens nalmefene in ethanol- dependent rats. Neuropharmacology. 2011;61:35–42. doi: 10.1016/j.neuropharm.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat. Rev. Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Historical review: molecular and cellular mechanisms of opiate and cocaine addiction. Trends Pharmacol. Sci. 2004;25:210–218. doi: 10.1016/j.tips.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. Neuroscientist. 2004;10:221–234. doi: 10.1177/1073858403261077. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol. Clin. Exp. Res. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW. Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacol. Biochem. Behav. 2002;72:213–220. doi: 10.1016/s0091-3057(01)00748-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacol. Biochem. Behav. 2004;77:405–413. doi: 10.1016/j.pbb.2003.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Drug challenges reveal differences in mediation of stress facilitation of voluntary alcohol drinking and withdrawal-induced anxiety in alcohol-preferring P rats. Alcohol. Clin. Exp. Res. 2007;31:1473–1481. doi: 10.1111/j.1530-0277.2007.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, Markou A. Increased motivation for self-administered cocaine after escalated cocaine intake. Neuroreport. 2003;14:2229–2232. doi: 10.1097/00001756-200312020-00019. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Myers C, Markou A. Effects of repeated withdrawal from continuous amphetamine administration on brain reward function in rats. Psychopharmacology. 2000;152:440–446. doi: 10.1007/s002130000559. [DOI] [PubMed] [Google Scholar]
- Pelleymounter MA, Joppa M, Carmouche M, Cullen MJ, Brown B, Murphy B, Grigoriadis DE, Ling N, Foster AC. Role of corticotropin-releasing factor (CRF) receptors in the anorexic syndrome induced by CRF. J. Pharmacol. Exp. Ther. 2000;293:799–806. [PubMed] [Google Scholar]
- Pelloux Y, Everitt BJ, Dickinson A. Compulsive drug seeking by rats under punishment: effects of drug taking history. Psychopharmacology. 2007;194:127–137. doi: 10.1007/s00213-007-0805-0. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by κ opiate receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr. Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J. Neurosci. 2001;21:7397–7403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadros IM, Miczek KA. Two modes of intense cocaine bingeing: increased persistence after social defeat stress and increased rate of intake due to extended access conditions in rats. Psychopharmacology. 2009;206:109–120. doi: 10.1007/s00213-009-1584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainnie DG, Bergeron R, Sajdyk TJ, Patil M, Gehlert DR, Shekhar A. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. J. Neurosci. 2004;24:3471–3479. doi: 10.1523/JNEUROSCI.5740-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, Boldt BM, Bryant CA, Mitton DR, Larsen SA, Wilkinson CW. Chronic daily ethanol and withdrawal: 1. Long-term changes in the hypothalamo-pituitary-adrenal axis. Alcohol. Clin. Exp. Res. 2000;24:1836–1849. [PubMed] [Google Scholar]
- Rassnick S, Heinrichs SC, Britton KT, Koob GF. Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Res. 1993;605:25–32. doi: 10.1016/0006-8993(93)91352-s. [DOI] [PubMed] [Google Scholar]
- Redila VA, Chavkin C. Stress-induced reinstatement of cocaine seeking is mediated by the kappa opioid system. Psychopharmacology. 2008;200:59–70. doi: 10.1007/s00213-008-1122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson HN, Zhao Y, Fekete EM, Funk CK, Wirsching P, Janda KD, Zorrilla EP, Koob GF. MPZP: a novel small molecule corticotropin-releasing factor type 1 receptor (CRF1) antagonist. Pharmacol. Biochem. Behav. 2008;88:497–510. doi: 10.1016/j.pbb.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimondini R, Arlinde C, Sommer W, Heilig M. Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. FASEB J. 2002;16:27–35. doi: 10.1096/fj.01-0593com. [DOI] [PubMed] [Google Scholar]
- Rivier C, Bruhn T, Vale W. Effect of ethanol on the hypothalamic-pituitary-adrenal axis in the rat: role of corticotropin-releasing factor (CRF). J. Pharmacol. Exp. Ther. 1984;229:127–131. [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res. Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Russell MAH. What is dependence? In: Edwards G, editor. Drugs and Drug Dependence. Lexington Books; Lexington MA: 1976. pp. 182–187. [Google Scholar]
- Sarnyai Z, Biro E, Gardi J, Vecsernyes M, Julesz J, Telegdy G. Brain corticotropin-releasing factor mediates “anxiety-like” behavior induced by cocaine withdrawal in rats. Brain Res. 1995;675:89–97. doi: 10.1016/0006-8993(95)00043-p. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Shaham Y, Heinrichs SC. The role of corticotropin-releasing factor in drug addiction. Pharmacol. Rev. 2001;53:209–243. [PubMed] [Google Scholar]
- Schindler AG, Li S, Chavkin C. Behavioral stress may increase the rewarding valence of cocaine-associated cues through a dynorphin/kappa-opioid receptor-mediated mechanism without affecting associative learning or memory retrieval mechanisms. Neuropsychopharmacology. 2010;35:1932–1942. doi: 10.1038/npp.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg JE, Vendruscolo LF, Park PE, Whitfield TW, Jr., Koob GF. Long-term antagonism of kappa opioid receptors prevents escalation of, and increased motivation for, heroin intake. Soc. Neurosci. Abstracts, abstr# 16.07. 2011 doi: 10.1523/JNEUROSCI.1979-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulteis G, Liu J. Brain reward deficits accompany withdrawal (hangover) from acute ethanol in rats. Alcohol. 2006;39:21–28. doi: 10.1016/j.alcohol.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Cole M, Koob G. Decreased brain reward produced by ethanol withdrawal. Proc. Natl Acad. Sci. U. S. A. 1995;92:5880–5884. doi: 10.1073/pnas.92.13.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Gold LH, Stinus L, Koob GF. Relative sensitivity to naloxone of multiple indices of opiate withdrawal: a quantitative dose-response analysis. J. Pharmacol. Exp. Ther. 1994;271:1391–1398. [PubMed] [Google Scholar]
- Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol. Ther. 2007;116:306–321. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurman J, Koob GF, Gutstein HB. Opioids, pain, the brain, and hyperkatifeia: a framework for the rational use of opioids for pain. Pain Med. 2010;11:1092–1098. doi: 10.1111/j.1526-4637.2010.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhpura N, Parsons LH. Endocannabinoid-mediated synaptic plasticity and addiction-related behavior. Neuropharmacology. 2011;61:1070–1087. doi: 10.1016/j.neuropharm.2011.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, Walker DL, Freeman SM, Flandreau EI, Ressler KJ, Davis M. Effects of continuously enhanced corticotropin releasing factor expression within the bed nucleus of the stria terminalis on conditioned and unconditioned anxiety. Mol. Psychiatry. 2013;18:308–319. doi: 10.1038/mp.2011.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation: 1. Temporal dynamics of affect. Psychological Rev. 1974;81:119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Specio SE, Wee S, O'Dell LE, Boutrel B, Zorrilla EP, Koob GF. CRF1 receptor antagonists attenuate escalated cocaine self-administration in rats. Psychopharmacology. 2008;196:473–482. doi: 10.1007/s00213-007-0983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spina M, Merlo-Pich E, Chan RKW, Basso AM, Rivier J, Vale W, Koob GF. Appetite-suppressing effects of urocortin, a CRF-related neuropeptide. Science. 1996;273:1561–1564. doi: 10.1126/science.273.5281.1561. [DOI] [PubMed] [Google Scholar]
- Stinus L, Cador M, Zorrilla EP, Koob GF. Buprenorphine and a CRF1 antagonist block the acquisition of opiate withdrawal-induced conditioned place aversion in rats. Neuropsychopharmacology. 2005;30:90–98. doi: 10.1038/sj.npp.1300487. [DOI] [PubMed] [Google Scholar]
- Stinus L, Le Moal M, Koob GF. Nucleus accumbens and amygdala are possible substrates for the aversive stimulus effects of opiate withdrawal. Neuroscience. 1990;37:767–773. doi: 10.1016/0306-4522(90)90106-e. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale W. The organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Ho SP, Livanov V, Graciani N, Arneric SP. Antagonism of CRF2 receptors produces anxiolytic behavior in animal models of anxiety. Brain Res. 2001;902:135–142. doi: 10.1016/s0006-8993(01)02405-2. [DOI] [PubMed] [Google Scholar]
- Tejeda HA. Modulation of extracellular dopamine in the prefrontal cortex by local and ventral tegmental area kappa-opioid receptors. Soc. Neurosci. Abstr. abstr# 751.7. 2009 [Google Scholar]
- Tejeda HA, Natividad LA, Orfila JE, Torres OV, O'Dell LE. Dysregulation of kappa-opioid receptor systems by chronic nicotine modulate the nicotine withdrawal syndrome in an age-dependent manner. Psychopharmacology (Berl) 2012;224:289–301. doi: 10.1007/s00213-012-2752-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA., Jr. Effects of κ-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology. 2004;172:463–470. doi: 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- Tucci S, Cheeta S, Seth P, File SE. Corticotropin releasing factor antagonist, α-helical CRF9-41, reverses nicotine-induced conditioned, but not unconditioned, anxiety. Psychopharmacology. 2003;167:251–256. doi: 10.1007/s00213-003-1403-4. [DOI] [PubMed] [Google Scholar]
- Turnbull AV, Rivier C. Corticotropin-releasing factor (CRF) and endocrine responses to stress: CRF receptors, binding protein, and related peptides. Proc. Natl Acad. Sci. U. S. A. 1997;215:1–10. doi: 10.3181/00379727-215-44108. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Platt DM, Rowlett JK, Rüedi-Bettschen D, Spealman RD. κ Agonist-induced reinstatement of cocaine seeking in squirrel monkeys: a role for opioid and stress-related mechanisms. J. Pharmacol. Exp. Ther. 2007;323:525–533. doi: 10.1124/jpet.107.125484. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Sabino V, Koob GF. Increased anxiety-like behavior and ethanol self-administration in dependent rats: reversal via corticotropin-releasing factor-2 receptor activation. Alcohol. Clin. Exp. Res. 2004;28:865–872. doi: 10.1097/01.alc.0000128222.29875.40. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Zorrilla EP, Roberts AJ, Koob GF. Antagonism of corticotropin-releasing factor attenuates the enhanced responsiveness to stress observed during protracted ethanol abstinence. Alcohol. 2003;29:55–60. doi: 10.1016/s0741-8329(03)00020-x. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- Vendruscolo L, Schlosburg JE, Misra KK, Chen SA, Greenwell TN, Koob GF. Escalation patterns of varying periods of heroin access. Pharmacol. Biochem. Behav. 2011;98:570–574. doi: 10.1016/j.pbb.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield T, Jr., Logrip ML, Rivier CL, Repunte-Canonigo V, Zorrilla EP, Sanna PP, Heilig M, Koob GF. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J. Neurosci. 2012;32:7563–7571. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci. Biobehav. Rev. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain: insights from imaging studies. J. Clin. Invest. 2003;111:1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Koob GF. The γ-aminobutyric acid-B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcohol. Clin. Exp. Res. 2007;31:11–18. doi: 10.1111/j.1530-0277.2006.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Zorrilla EP, Koob GF. Systemic κ-opioid receptor antagonism by norbinaltorphimine reduces dependence-induced excessive alcohol self-administration in rats. Addict. Biol. 2010;16:116–119. doi: 10.1111/j.1369-1600.2010.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson SJ, Khachaturian H, Akil H, Coy DH, Goldstein A. Comparison of the distribution of dynorphin systems and enkephalin systems in brain. Science. 1982;218:1134–1136. doi: 10.1126/science.6128790. [DOI] [PubMed] [Google Scholar]
- Wee S, Koob GF. The role of the dynorphin-κ opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology. 2010;210:121–135. doi: 10.1007/s00213-010-1825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Mandyam CD, Lekic DM, Koob GF. α1-Noradrenergic system role in increased motivation for cocaine intake in rats with prolonged access. Eur. Neuropsychopharmacol. 2008;18:303–311. doi: 10.1016/j.euroneuro.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Orio L, Ghirmai S, Cashman JR, Koob GF. Inhibition of kappa opioid receptors attenuated increased cocaine intake in rats with extended access to cocaine. Psychopharmacology. 2009;205:565–575. doi: 10.1007/s00213-009-1563-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Wang Z, Woolverton WL, Pulvirenti L, Koob GF. Effect of aripiprazole, a partial D2 receptor agonist, on increased rate of methamphetamine self-administration in rats with prolonged access. Neuropsychopharmacology. 2007;32:2238–2247. doi: 10.1038/sj.npp.1301353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield TW, Jr., Wee S, Gould A, Schlosburg J, Vendruscolo L, Koob GF. Kappa receptor activation underlies compulsive methamphetamine intake. Soc. Neurosci. Abstracts abstr# 797.19. 2011 [Google Scholar]
- Wills TA, Knapp DJ, Overstreet DH, Breese GR. Sensitization, duration, and pharmacological blockade of anxiety-like behavior following repeated ethanol withdrawal in adolescent and adult rats. Alcohol. Clin. Exp. Res. 2009;33:455–463. doi: 10.1111/j.1530-0277.2008.00856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willuhn I, Burgeno LM, Everitt BJ, Phillips PE. Hierarchical recruitment of phasic dopamine signaling in the striatum during the progression of cocaine use. Proc. Natl Acad. Sci. U. S. A. 2012;109:20703–20708. doi: 10.1073/pnas.1213460109. [DOI] [PMC free article] [PubMed] [Google Scholar]