Abstract
Background and Aims
Retrospective self-report and observational studies have yielded inconsistent findings regarding the capacity of negative affect (NA) to increase smoking motivation among dependent samples. Controlled laboratory studies offer an alternative paradigm for testing the role of affective state upon smoking motivation. The aim of the current study was to quantify cue-provoked cravings produced by affective manipulations in the published literature, and to identify theoretical and methodological moderators.
Methods
We conducted a systematic literature search to identify experimental studies that manipulated NA or positive affect (PA), and assessed post-manipulation craving. Separate random effects meta-analyses examined NA and PA cues as predictors of self-reported craving. Self-reported affect (NA and PA), nicotine deprivation, gender, nicotine dependence, order of cue presentation, single vs. multi-item craving assessment, and affect induction method were tested as moderators of affective cue-induced craving.
Results
NA manipulations produced a medium effect (g = .47; CI = .31 – .63) on craving, but no main effects were found for PA manipulations (g = .05; CI = −.09 – .20) on craving. Self-reported NA moderated the extent to which NA and PA manipulations elicited craving (p’s < .02 for each). That is, more effective NA manipulations produced greater cravings, and PA manipulations reduced cravings when they reduced NA.
Conclusions
Laboratory studies indicate that negative affect, but not positive affect, is a situational determinant of cravings to smoke among dependent smokers. Adverse emotional states increase craving to smoke among dependent smokers, but positive emotional states do not consistently reduce craving to smoke.
Keywords: tobacco, smoking, craving, conditioned stimuli, affect, cue-reactivity
Introduction
Affective processes have long been postulated to be essential mechanisms underlying the maintenance of drug consumption (1–8). Although these processes are quite complex (9), affect is generally differentiated in terms of duration (state vs. trait), valence (positive vs. negative), and arousal/activation (low vs. high). Particular attention has been devoted to negative affect (NA), as preclinical, epidemiological, clinical, experimental, observational, and longitudinal studies have attempted to delineate its role across developmental stages of drug use (i.e., initiation, maintenance, and relapse). Qualitative reviews have summarized these research efforts (10–15), but the relationship between controlled affective manipulations and drug use motivation has yet to be systematically quantified. Here we focus specifically on the maintenance phase of tobacco smoking.
Acute Negative Affect and Smoking Motivation
Dependent smokers consistently endorse affect regulation as a primary motive for smoking (16–19), and that NA is a precipitant to relapse (20–29). That is, retrospective self-report studies clearly suggest that NA is an antecedent of smoking during the maintenance and relapse phases. This may occur, in part, because smokers expect smoking to reduce NA, whether or not these reductions actually occur in a given situation (12).
In contrast, observational studies using ecological momentary assessment (EMA) have been equivocal, depending upon the phase of smoking. During a quit attempt, NA is the situational determinant most likely to precede a lapse, and these NA-related lapses most readily progress to relapse (30). However, no relationship between NA and ad lib smoking has been observed in the majority of uncontrolled observational EMA studies (31–33). Thus, there is inconsistency between most EMA findings and other self-report data during the maintenance phase.
Cue-Reactivity Paradigm and Smoking Motivation
Cue-reactivity paradigms are laboratory analogues of drug-seeking behavior (34–37), and perhaps the most widely-utilized method for examining the influence of situational stimuli on smoking motivation (38). A strength of these controlled designs is the experimental manipulation of the cue of interest relative to a neutral comparison condition, thereby eliminating potential confounds and providing an internally valid test of causality. The majority of cue-reactivity studies have focused on responses to environmental stimuli most proximal to the act of smoking (e.g., cigarettes); and the only meta-analysis on this topic found that these exteroceptive cues had small to medium effects (d = −.07 – .44) on psychophysiological indices and large effects (d = 1.18) on self-reported craving (39).
Given that the most robust response to smoking-related cues appears to occur in subjective reports of craving, it is not surprising that this is the most widely assessed measure of smoking motivation. Commonly operationalized as the desire to use a drug (40), craving has been conceptualized as an expression of drug use motivation that may mediate continued drug use and relapse (41). As such, it serves an integral role within the majority of contemporary models of drug dependence (4, 38, 42–44). Importantly, two types of craving should be distinguished: withdrawal-based or background craving and cue-based or cue-provoked craving (45).
The utility of cue-provoked craving for understanding nicotine dependence remains controversial (see Addiction, v. 104, issue 10). Perkins (46) pointed out that minimal evidence supported an association between cue-provoked craving and smoking relapse risk, as this relationship was only observed in one study at that time (47). However, recent empirical evidence suggests that cue-provoked cravings are clinically meaningful, as additional studies have since found relationships between cue-provoked cravings and smoking cessation outcomes (48–50).
The Perkins review and subsequent discourse also promulgated several areas of future inquiry for cue-reactivity paradigms. For example, Tiffany and Wray (51) pointed out that clarification is needed for many psychometric and methodological issues (e.g., procedural, measurement, sample characteristics). And Shiffman (52) suggested the need to better identify the influence of nonsmoking-specific cues (e.g., NA), which can be considered more distal than smoking-related cues (53). The current study was conducted with these recommendations in mind and to help clarify the equivocal findings on the role of NA as an antecedent of smoking motivation within current, minimally deprived, smokers (i.e., maintenance phase).
Current Study
The primary aim was to quantify the effect size of cue-provoked craving resultant from NA inductions by reviewing existing studies and using meta-analytic methods. Cravings induced by positive affect (PA) manipulations were also explored, given theoretical predictions that PA may increase smoking motivation (54, 55). Finally, we examined variables that may moderate the effects of affective manipulations on craving. Potential moderators were predicated upon their capacity to inform theory and/or cue-reactivity methodology (described below).
Methods
Study Acquisition
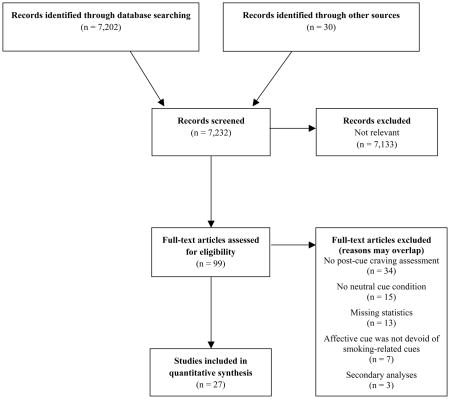
As part of a larger effort to identify all cue-reactivity studies the following terms were searched within PsycINFO, PubMed/MEDLINE, and Dissertation Abstracts: (smok* OR nicotine OR tobacco) AND (cue OR stimuli OR reactivity OR conditioned withdrawal OR conditioned responses OR craving OR urge OR stroop OR implicit OR response time OR dot-probe OR priming OR dual-task OR expectancy OR accessibility OR startle OR memory OR cognition OR affect OR mood OR topography OR psychophys* OR fMRI OR ERP). This search concluded in February of 2012 and yielded 7,202 abstracts that were then reviewed to determine relevance to the current study. Bibliographic searches were also conducted within pertinent studies and qualitative reviews.
For a study to be included the following criteria must have been met: 1) sample included smokers; 2) comparison of a neutral cue to an affective cue (PA or NA) devoid of smoking-related content; 3) assessment of post-cue craving; and 4) contained statistics necessary to compute an effect size. Attempts were made to contact authors of relevant studies without the final criterion (five provided this information). An enumerative flow diagram of study selection is presented in Appendix A, and a meta-analysis reporting checklist is presented in Appendix B. When studies included specific subsamples (56–60), each subsample was treated as an individual study.
Moderator Selection
Preliminary analyses tested the assumption that affective manipulations actually induced the intended affective state (i.e., NA or PA). Indeed, NA manipulations produced elevated levels of self-reported NA (g = .52, CI = .36 – .69) and significant reductions in self-reported PA (g = −.72, CI = −.95 – −.49). Also as expected, PA manipulations clearly induced PA (g = .74, CI = .31 – 1.18) and mitigated NA (g = −.20, CI = −.35 – .04). Effect size estimates for self-reported NA and PA were included as moderators of the craving analyses. Such moderation would provide additional evidence that these manipulations increased smoking motivation via their impact on affective state (i.e., representing a dose-response relationship).
Additional theoretical moderators included nicotine withdrawal, gender, and nicotine dependence. We hypothesized that nicotine withdrawal would exacerbate cue-reactivity, as it may activate craving networks and make cues more salient (54). A large literature base also suggests that females may be more reactive than males to non-pharmacological aspects of smoking, such as cues (61). Finally, the smoking behavior of those less nicotine dependent has been theorized to be more stimulus bound, and therefore, more reactive to cues (62); however, other theoretical perspectives suggest that cue-reactivity should be positively associated with dependence (43). The current study provided an avenue to clarify this relationship with respect to affective cues.
Order of cue presentation and how craving was assessed were methodological characteristics we hypothesized as potential moderators. Given the likelihood of carryover effects that may blur differences between neutral and smoking-related cues (63), we expected studies that employed fixed order cue presentation (neutral first) would evince higher magnitude effect sizes than other cue presentations (counterbalanced or randomized). It was also predicted that larger effect sizes would be observed for studies that assessed craving with validated multi-item measures, relative to those that used single items. This was based on the assumption that elevated reliability increases the power to detect craving fluctuations in response to experimental manipulations (40). Exploratory analyses were conducted for modality of affective induction (e.g., picture, mental imagery).
Study Codification
To conduct theoretical moderation analyses the following study characteristics were extracted, when available: post manipulation self-reported affect (PA and NA); hours of nicotine deprivation (via pre-session smoking instructional set); gender composition (calculated as percent male); nicotine dependence (via cigarettes per day [CPD] and Fagerström Test of Nicotine Dependence scores [FTND]; 64). Methodological moderator variables were dummy coded as follows: cue presentation (fixed vs. other); craving assessment (multi-item vs. single item measure); and cue presentation modality (picture vs. mental imagery).
We also codified various demographics and other aspects of the study (e.g., task duration), but these were generally unable to be analyzed (e.g., inadequate reporting, insufficient variability). The methodological quality (i.e., validity or risk of bias) for each study was scored using a modified scale informed by Cochrane, PRISMA, and PEDro guidelines. The scale consisted of 14 dichotomous items specific to procedural (e.g., randomization, sample size justification, description of inclusion criteria, manipulation check) and statistical (e.g., description of analyses, selective reporting) aspects of the study, with higher scores representing higher quality designs. The second and third authors independently coded each study (90% agreement), with any discrepancies resolved through discussion with the first author.
In the few cases where a single study implemented multiple affect manipulations (65, 66) or affect/craving assessments (i.e., subscales; 66, 67, 68) a composite score was computed across affective manipulations or measures (which was then used to calculate study effect sizes). However, when both multi- and single item craving indices were included, only the former was used (69, 70).
Quantitative Data Synthesis
Comprehensive Meta-Analysis (71) was used to calculate individual study effect sizes and meta-analytic statistics. Separate meta-analyses were conducted to assess experimental manipulations of NA and PA upon craving (56–60, 65–70, 72–82). Hedge’s g was calculated for all studies through the use of post-affective cue responses relative to post-neutral cue responses. As recommended by Rosenthal (83), a conservative correlation (.7) was assumed when calculating matched group effect sizes.
Primary Analyses
Summary statistics for each of the 2 primary meta-analyses (i.e., IV’s = PA and NA manipulations; DV = craving) were computed using a random effects approach. The Q statistic and I2 were used to explore heterogeneity between effect sizes. Multiple indices were utilized to examine the impact of publication bias. These included the classic fail-safe N, Orwin’s fail-safe N (trivial effect size set at .2 or −.2 and effect size of missing studies set at 0), Begg–Mazumdar rank correlation test (84), Egger’s test (85), and Duval and Tweedie’s trim and fill approach (86). Funnel plots were also visually inspected.
Moderation Analyses
All three methodological moderators (craving assessment, affect manipulation modality, and presentation order) were categorical and examined using mixed effects analyses. Meta-regression analyses (via method-of-moments parameter estimation) were conducted for the six continuous theoretical moderators (NA, PA, FTND, CPD, nicotine deprivation, and gender composition) and study quality scores. Thus, 10 single-predictor moderation analyses were conducted for each type of the affective cue (i.e., PA and NA).
Results
Sample Characteristics
Study characteristics are shown in Table 1. The entire sample consisted of 27 studies (N = 1,412), several of which contributed to multiple analyses (see Figures 1–2). Overall, the large majority of studies used within-subjects designs (93%) and few used fixed order cue presentation (12%). On average, study samples were 31.30 years of age, 57% male, smoked 20.60 CPD, and were moderately nicotine dependent (FTND = 5.26). There was a wide range of pre-session nicotine deprivation (0–18 hours), with a mean of 2.36 hours (median = 0 hours). Considerable variability was observed for type of affective manipulation employed, along with how affect and smoking motivation were assessed. No study reached the maximum methodological quality score, which averaged to 73% across studies (range = 64 – 86%).
Table 1.
Study characteristics
| Study | N | NA cue | PA cue | Neutral Cue | Smoking Motivation Assessment(s) |
|---|---|---|---|---|---|
| al’ Absi et al. (2003; non-deprived) | 17 | Public-speaking | n/a | Rest (video/magazine) | Single item (desire to smoke) |
| al’ Absi et al. (2003; deprived) | 21 | Public-speaking | n/a | Rest (video/magazine) | Single item (desire to smoke) |
| Beckham (1996) | 25 | Words (anxiety) | n/a | Words | 5-item craving subscale (SWQ) |
| Bradley et al. (2007) | 12 | Personal imagery & music (sad) | n/a | Personal imagery & music | QSU |
| Carpenter et al. (2009) | 78 | Personal imagery (stressful) | Personal imagery (relaxed) | In vivo (pencil and eraser) | QSU-B |
| Carter et al. (2006) | 63 | IAPS (high arousal) | IAPS (high arousal) | IAPS (low arousal) | Single item (craving) |
| Childs & de Wit (2010) | 17 | TSST | n/a | Describe book or film and computer game; no camera | VAS (craving) |
| Colamussi et al. (2007; males) | 72 | Standard imagery (stressful) | n/a | Standard imagery | 5-item (validated) |
| Colamussi et al. (2007; females) | 88 | Standard imagery (stressful) | n/a | Standard imagery | 5-item (validated) |
| Conklin & Tiffany (2001) | 60 | n/a | Composite imagery: standard; personal; other | Composite imagery: standard; personal; other | QSU-B |
| Ditre & Brandon (2008) | 66 | Cold pressor | n/a | Room temperature water | QSU-B |
| Drobes (2002; non-alcoholic) | 19 | Pictures (unpleasant) | Pictures (pleasant) | Pictures | VAS (craving) |
| Drobes (2002; alcoholic) | 21 | Pictures (unpleasant) | Pictures (pleasant) | Pictures | VAS (craving) |
| Elash et al. (1995) | 36 | Standard imagery | Standard imagery (relaxed) | Standard imagery | QSU-B |
| Litvin & Brandon (2010) | 88 | Speech preparation | n/a | Rate art (landscapes) | QSU-B |
| Maude-Griffin & Tiffany (1996; non-deprived) | 49 | Standard imagery | Standard imagery (relaxed) | Standard imagery | Single item (urge) |
| Maude-Griffin & Tiffany (1996; deprived) | 51 | Standard imagery | Standard imagery (relaxed) | Standard imagery | Single item (urge) |
| McKee et al. (2011) | 37 | Personal imagery (stressful) | n/a | Personal imagery (relaxing/neutral) | QSU-B |
| Perkins et al. (2008) | 200 | IAPS & music (high arousal) | IAPS & music (high arousal) | IAPS & music (low arousal) | QSU-4 item |
| Perkins et al. (2010) | 104 | Composite: digit recall; public-speaking; IAPS (high arousal) | n/a | IAPS (neutral) | QSU-B |
| Perkins & Grobe (1992)-(non-deprived) | 16 | Difficult digit recall | n/a | Simple digit recognition | VAS (desire to smoke) |
| Perkins & Grobe (1992)-(deprived) | 16 | Difficult digit recall | n/a | Simple digit recognition | VAS (desire to smoke) |
| Rehme et al. (2009)* | 36 | IAPS (high arousal) | IAPS (high arousal) | IAPS | VAS (desire to smoke) |
| Robinson et al. (2011) | 111 | IAPS (high arousal) | IAPS (high arousal) | IAPS (low arousal) | Single item (craving) |
| Taylor et al. (2000) | 18 | Standard imagery | Standard imagery | Standard imagery | QSU-B |
| Tiffany & Drobes (1990) | 60 | Standard imagery | Standard imagery | Standard imagery | 2 items (urge and craving) |
| Watson et al. (2010) | 90 | Personal imagery (stressful) | Personal imagery (relaxed) | In vivo (pencil and eraser) | QSU-B |
Figure 1.
Forest plot for comparisons of post-NA cue to post-neutral cue self-reported craving. This includes effect sizes (g), standard errors, variances, 95% confidence intervals, Z scores, and p-values. Effect sizes to the right of zero indicate larger craving in response to NA cues, relative to neutral cues. Confidence intervals that do not include zero reflect significant differences. Summary statistics are reported in the final row, computed via random effects meta-analysis.
Figure 2.
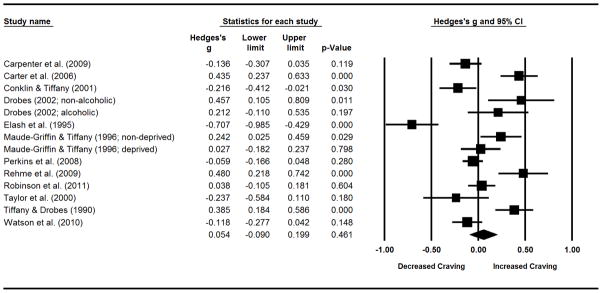
Forest plot for comparisons of post-PA cue to post-neutral cue self-reported craving. This includes effect sizes (g), standard errors, variances, 95% confidence intervals, Z scores, and p-values. Effect sizes to the right of zero indicate larger craving in response to PA cues, relative to neutral cues. Confidence intervals that do not include zero reflect significant differences. Summary statistics are reported in the final row, computed via random effects meta-analysis.
NA-Induced Craving Analyses
Primary
NA manipulations yielded medium effects for inducing cravings to smoke (see Table 2 and Figure 1). There was substantial heterogeneity in effect sizes, as seen by the significant Q statistic and large amount of true variance accounted for (i.e., I2). Of the five publication bias indices, only the trim and fill method indicated potential bias, and adjusting for imputed studies had little impact on the summary statistic (adjusted g = .35, CI = .18 – .52).
Table 2.
Meta-analyses of negative affect inductions on self-reported craving.
| k | g | LL | UL | Z | pZ | Q | pQ | I2 | |
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Overall Sample | 26 | 0.47 | 0.31 | 0.63 | 5.78 | 0.000 | 281.40 | 0.000 | 91.12 |
| Moderators | |||||||||
| Craving Assessment | - | - | - | - | - | - | 1.82 | 0.177 | - |
| Multi-item | 12 | 0.35 | 0.11 | 0.58 | 2.90 | 0.004 | 147.57 | 0.000 | 92.55 |
| Single-item | 14 | 0.57 | 0.35 | 0.79 | 5.09 | 0.000 | 120.75 | 0.000 | 89.23 |
| Induction Modality | - | - | - | - | - | - | 3.97 | 0.046 | - |
| Picture | 6 | 0.23 | −0.08 | 0.53 | 1.46 | 0.143 | 18.81 | 0.002 | 73.42 |
| Mental imagery | 11 | 0.61 | 0.38 | 0.83 | 5.29 | 0.000 | 152.57 | 0.000 | 93.45 |
| Cue Order | - | - | - | - | - | - | 0.10 | 0.748 | - |
| Fixed | 4 | 0.54 | 0.14 | 0.95 | 2.62 | 0.009 | 97.65 | 0.000 | 96.93 |
| Other | 20 | 0.47 | 0.28 | 0.66 | 4.90 | 0.000 | 170.24 | 0.000 | 91.42 |
Moderators
Self-reported NA significantly moderated the effects of the NA manipulations on craving ratings (slope = .74, p = .003), whereas moderation was not observed for self-reported PA (slope = −.21, p = .25). None of the other planned theoretical moderator analyses reached significance (i.e., gender, nicotine deprivation, nicotine dependence, or CPD; p’s > .10).
Modality was the only significant methodological moderator. Mental imagery manipulations produced large effect sizes, while picture presentation produced small effect sizes that did not reach statistical significance. Although not significant, studies that utilized single-item craving indices showed larger cue-provoked cravings than those with multi-item measures. Order of cue presentation and study quality scores were unrelated to NA-provoked craving.
PA-Induced Craving Analyses
Primary
No effect or publication bias was observed for PA inductions (see Table 3 and Figure 2). The Q statistic and I2 suggested heterogeneity across studies.
Table 3.
Meta-analyses of positive affect inductions on self-reported craving.
| k | g | LL | UL | Z | pZ | Q | pQ | I2 | |
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Overall Sample | 14 | 0.05 | −0.09 | 0.20 | 0.74 | 0.461 | 94.90 | 0.000 | 86.30 |
| Moderators | |||||||||
| Craving Assessment | - | - | - | - | - | - | 21.59 | 0.000 | - |
| Multi-item | 6 | −0.22 | −0.37 | −0.06 | −2.79 | 0.005 | 19.05 | 0.002 | 73.50 |
| Single-item | 8 | 0.27 | 0.13 | 0.41 | 3.84 | 0.000 | 22.05 | 0.002 | 68.25 |
| Induction Modality | - | - | - | - | - | - | 4.68 | 0.031 | - |
| Picture | 6 | 0.24 | 0.02 | 0.47 | 2.12 | 0.034 | 32.70 | 0.000 | 84.71 |
| Mental imagery | 8 | −0.08 | −0.28 | 0.11 | −0.85 | 0.394 | 52.49 | 0.000 | 86.66 |
| Cue Order | - | - | - | - | - | - | 0.18 | 0.676 | - |
| Fixed | 1 | −0.06 | −0.62 | 0.50 | −0.21 | 0.836 | 0.00 | 1.000 | 0.00 |
| Other | 13 | 0.07 | −0.10 | 0.23 | 0.77 | 0.442 | 91.80 | 0.000 | 86.93 |
Moderators
Again, self-reported NA significantly moderated craving ratings (slope = .71, p = .03), but self-reported PA did not (slope = .08, p = .62). That is, PA manipulations were found to reduce cravings as a function of their ability to reduce NA. No evidence of moderation was observed for gender, nicotine deprivation, nicotine dependence, or CPD (p’s > .34).
In contrast to the findings for NA inductions, PA mental imagery studies had little to no impact on craving. This was significantly different from the small positive effect size obtained by studies that used picture presentations. Craving assessment was also a significant moderator, as multi-item measures were associated with reductions in post-cue craving ratings, whereas single-item assessments tended to show craving increases. Additionally, PA-provoked craving was moderated by study quality scores (slope = −.11, p = .054), suggesting that PA decreased craving within higher quality studies.
Discussion
Affective Cues and Craving
To characterize the effects of affective stimuli on smoking motivation we quantitatively synthesized affective cue-reactivity studies that measured self-reported craving. With respect to controlled laboratory manipulations of NA (relative to neutral), as expected, we observed a clear cause-effect relationship with smoking motivation. Based on the quality and number of studies, there was strong evidence that NA inductions evoked cravings to smoke. Although NA manipulations increased cravings to smoke, it is noteworthy that the overall effect size was smaller than that previously reported for smoking-specific cue presentations (39). This is not surprising, as negative affect can be considered a distal cue to smoke, compared to the more proximal cues involving smoking-specific stimuli (53).
PA inductions did not have consistent effects on craving (although the methodologically stronger studies were more supportive of craving reduction). Thus, PA did not appear to act as a conditioned interoceptive stimulus for the maintenance of smoking motivation. This is at odds with addiction models that emphasize the role of PA, non-specific to drug use, as a motive for continued substance use (e.g., 54). The negligible role of PA found here converges with evidence that heightened PA is associated with a small proportion of smoking relapse (87).
Moderators
The relative importance of NA was further substantiated by analyses that tested self-reported affect as moderators for cue-provoked cravings. NA ratings were found to moderate the influence of NA and PA cues, such that craving was dependent upon the degree to which these stimuli altered NA. Although affective cues also had large effects on PA ratings, these did not modulate craving responses. That self-reported NA predicted cue-provoked cravings, regardless of cue type, supports the supposition that the interoceptive state of NA is a primary mechanism involved in the maintenance of drug dependence, including smoking (1). However, the interpretation of these analyses was limited by the number of studies that contributed both affective and craving outcomes.
None of the other moderation hypotheses was supported, but the power of these tests was limited by the few available studies. For example, fixed order of cue presentation was only used by four studies that investigated NA manipulations and one study that examined PA inductions. No clear pattern emerged across affective cues with respect to the use of single-item vs. multi-item craving assessments, but larger PA-induced cravings were observed when assessed via single-item scales. However, assessment approach may have been confounded with other design and methodological characteristics. For instance, five of the six studies that used multi-item craving measures also used mental imagery to induce PA, whereas this was only the case for two of the eight studies that assessed craving with a single-item. Thus, the induction modality may have driven the effects observed for craving assessment method, a possibility that a more highly powered study might be able to disentangle through multi-moderator analyses.
Indeed, significant differences were found when we explored which affective induction methods were most potent for inducing craving. Across affective cues, picture presentation produced small effect sizes. Mental imagery had no discernible impact as a PA cue, but was a more effective NA cue than were pictures. Although there were other types of NA manipulations, they were too sparsely used to be included within moderation analyses. However, pain induction and the Trier Social Stress Test (88) also appeared to have large effects. Thus, it seems as though active engagement in NA inductions (compared to passive viewing of pictures) results in higher smoking motivation.
Clinical Implications
The findings indicating a causal role of NA upon smoking motivation suggest that cessation treatments might benefit by including strategies that: 1) prevent NA or 2) extinguish the relationship between NA and drug seeking. The importance of targeting reactivity to NA is further highlighted by studies that indicate that NA-induced cravings predict ad lib smoking behavior and cocaine relapse better than cravings produced by drug-specific stimuli (73, 89). This may occur because cue-provoked cravings from drug-specific stimuli may extinguish rather quickly following cessation (90, 91), whereas reactivity to NA may be more resistant to change (92). Additionally, it may be more difficult to avoid internal cues that are devoid of environmental constraints (i.e., NA), relative to external cues that only occur in circumscribed environments (i.e., drug-specific stimuli). Therefore, the reduction of NA-induced cravings might serve as a proxy for treatment efficacy, and the cue-reactivity paradigm may offer a laboratory model for testing treatment effects (93) and explicating NA-related pathophysiology (14).
However, it is unclear to what extent our findings extend to relapse processes, as 90% of participants in the constituent studies were not attempting to quit smoking. Additionally, few studies examined affective cues in the context of extended nicotine deprivation. Manipulation of these sample characteristics may be a fruitful area for further exploration, and serve to establish more representative cessation laboratory analogues. Furthermore, it is imperative that laboratory models include ad lib smoking behavior (in addition to craving) as a dependent variable (94).
Limitations and Future Directions
The degree to which our results generalize to naturalistic settings is inherently limited by the cue-reactivity paradigms employed by the constituent studies. Examination of contrived affective manipulations in controlled environments thereby sacrificed external validity, but maximized internal validity and the ability to make causal inferences. These tradeoffs may, in part, account for the divergence between our findings and those of EMA studies that investigated smoking motivation via ad lib smoking. Alternatively, EMA studies may not detect a relationship between self-reported NA and smoking because naturalistic smoking may precede, and prevent, conscious awareness of NA (1).
Additionally, we could not delineate the influence of low arousal states of NA (e.g., sadness), or discrete aversive emotions (e.g., disgust, shame) upon smoking motivation, as all but one of the NA manipulations incorporated an element of high arousal (e.g., stress) and were nonspecific. Future studies may wish to consider the use of video presentations, which are evocative stimuli that have been validated to induce various forms of NA (95). A unique, and potentially informative, design would be to examine all combinations of valence and arousal within a single experimental study.
Central to the current study, and all cue-reactivity studies, is how cue-provoked craving is operationalized. We focused on studies in which responses to neutral cues could be compared to those of affective cues. Thus, we did not capture studies that examined only changes from baseline to post-cue. Our approach was designed to isolate cue-provoked craving from background craving, but methods that investigate these in conjunction may be more appropriate for understanding relapse processes (96).
An additional limitation of the current study is the large number of analyses conducted (2 primary analyses and 20 moderation analyses), without formal correction of statistical significance. Indeed, our approach may have increased the likelihood for Type I error and reduced the degree to which our results reflect “true” population values. However, we chose this approach because our primary aim was to investigate the role of affective manipulations on craving (only 2 analyses), and our moderation analyses were informed by previous theory or qualitative reviews. With the intention of guiding future research, we did not want to diminish the capacity to detect potential signals that may influence affective cue-provoked cravings (i.e., increase Type II error). As more studies accumulate, future meta-analyses will be better powered to use multi-predictor moderation analyses.
Conclusions
With the caveat that included studies focused primarily on the effects of high arousal NA upon dependent smokers, we found that NA inductions increase cravings to smoke. Consistent with previous theory and retrospective self-report findings, our analyses provide strong quantitative evidence in support of a causal relationship between NA and smoking motivation. This relationship is more readily observed when NA is induced via mental imagery, relative to picture presentation. The only theoretical moderator observed was self-reported NA, which influenced craving responses to NA and PA cues. These findings support negative reinforcement models of addiction that emphasize the role of aversive states upon drug-seeking behavior.
Acknowledgments
The authors thank Kamari Pasley for her work on the project.
Appendix A. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Flow Diagram
Appendix B. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Checklist
| Section/topic | # | Checklist item | Reported on page # |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both. | 1 |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. | 2 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. | 3–5 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS). | 5 |
| METHODS | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number. | n/a |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale. | 6 |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. | 5–6 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated. | 5–6 |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis). | 6 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators. | 7–8 |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made. | 7–8 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. | 8 |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means). | 8 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis. | 8 |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies). | 9 |
| Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified. | 9 |
| RESULTS | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram. | 6, 31 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations. | 9, 24–26 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12). | 10–11 |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot. | 29–30 |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency. | 27–28 |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15). | 10–11 |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression [see Item 16]). | 10–11, 27–28 |
| DISCUSSION | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users, and policy makers). | 11–12 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias). | 14–15 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research. | 15–16 |
| FUNDING | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review. | 1 |
Footnotes
Declaration of Interest: Funding for this research was provided by National Institute on Drug Abuse grant F31 DA033058 (BWH) and National Cancer Institute grants R01 CA137357 (THB) and R01 CA134347 (THB). Dr. Brandon has served on the Varenicline Advisory Board for Pfizer and consulted on the development of the online behavioral adjuvant for varenicline users. Dr. Drobes served as a reviewer for the Pfizer 2011 Global Research Awards for Nicotine Dependence (GRAND) program. Drs. Brandon and Drobes have also received research funding from Pfizer.
References
*denotes studies included within the meta-analyses
- 1.Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- 2.Jellinek EM. Alcoholism, a genus and some of its species. Can Med Assoc J. 1960;83:1341–5. [PMC free article] [PubMed] [Google Scholar]
- 3.Khantzian EJ. The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harv Rev Psychiatry. 1997;4:231–44. doi: 10.3109/10673229709030550. [DOI] [PubMed] [Google Scholar]
- 4.Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- 5.Lindesmith AR. Opiate addiction. 1947. [Google Scholar]
- 6.Wikler A. Recent progress in research on the neurophysiologic basis of morphine addiction. Am J Psychiatry. 1948;105:329–38. doi: 10.1176/ajp.105.5.329. [DOI] [PubMed] [Google Scholar]
- 7.Wills TA, Shiffman S. Coping and substance use: A conceptual framework. Coping and substance use. 1985:3–24. [Google Scholar]
- 8.Solomon RL, Corbit JD. An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychol Rev. 1974;81:119–45. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- 9.Gross JJ, Thompson RA. Emotion Regulation: Conceptual Foundations. In: Gross JJ, editor. Handbook of emotion regulation. New York, NY US: Guilford Press; 2007. pp. 3–24. [Google Scholar]
- 10.Carmody TP, Vieten C, Astin JA. Negative affect, emotional acceptance, and smoking cessation. J Psychoactive Drugs. 2007;39:499–508. doi: 10.1080/02791072.2007.10399889. [DOI] [PubMed] [Google Scholar]
- 11.Cheetham A, Allen NB, Yucel M, Lubman DI. The role of affective dysregulation in drug addiction. Clin Psychol Rev. 2010;30:621–34. doi: 10.1016/j.cpr.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: correlation, causation, and context across stages of smoking. Psychol Bull. 2003;129:270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- 13.Kassel JD, Veilleux JC, Wardle MC, et al. Stress and Addiction: Biological and Psychological Mechanisms. San Diego, CA, US: Elsevier Academic Press; 2007. Negative Affect and Addiction; pp. 171–189. [Google Scholar]
- 14.Sinha R. Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addiction Biology. 2009;14:84–98. doi: 10.1111/j.1369-1600.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–30. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Copeland AL, Brandon TH, Quinn EP. The Smoking Consequences Questionnaire-Adult: Measurement of smoking outcome expectancies of experienced smokers. Psychological Assessment. 1995;7:484. [Google Scholar]
- 17.Frith CD. Smoking behaviour and its relation to the smoker’s immediate experience. Br J Soc Clin Psychol. 1971;10:73–8. doi: 10.1111/j.2044-8260.1971.tb00714.x. [DOI] [PubMed] [Google Scholar]
- 18.Ikard FF, Green DE, Horn D. A scale to differentiate between types of smoking as related to the management of affect. Substance Use & Misuse. 1969;4:649–659. [Google Scholar]
- 19.Shiffman S. Assessing smoking patterns and motives. J Consult Clin Psychol. 1993;61:732–42. doi: 10.1037//0022-006x.61.5.732. [DOI] [PubMed] [Google Scholar]
- 20.Baer JS, Kamarck T, Lichtenstein E, Ransom CC., Jr Prediction of smoking relapse: analyses of temptations and transgressions after initial cessation. J Consult Clin Psychol. 1989;57:623–7. doi: 10.1037//0022-006x.57.5.623. [DOI] [PubMed] [Google Scholar]
- 21.Baer JS, Lichtenstein E. Classification and prediction of smoking relapse episodes: an exploration of individual differences. J Consult Clin Psychol. 1988;56:104–10. doi: 10.1037//0022-006x.56.1.104. [DOI] [PubMed] [Google Scholar]
- 22.Borland R. Slip-ups and relapse in attempts to quit smoking. Addict Behav. 1990;15:235–45. doi: 10.1016/0306-4603(90)90066-7. [DOI] [PubMed] [Google Scholar]
- 23.Brandon TH. Negative affect as motivation to smoke. Current Directions in Psychological Science. 1994;3:33–37. [Google Scholar]
- 24.Cummings C, Gordon JR, Marlatt GA. Relapse: Prevention and prediction. The addictive behaviors. 1980:291–321. [Google Scholar]
- 25.Cummings KM, Giovino G, Jaen CR, Emrich LJ. Reports of smoking withdrawal symptoms over a 21 day period of abstinence. Addict Behav. 1985;10:373–81. doi: 10.1016/0306-4603(85)90034-6. [DOI] [PubMed] [Google Scholar]
- 26.O’Connell KA, Martin EJ. Highly tempting situations associated with abstinence, temporary lapse, and relapse among participants in smoking cessation programs. J Consult Clin Psychol. 1987;55:367–71. doi: 10.1037//0022-006x.55.3.367. [DOI] [PubMed] [Google Scholar]
- 27.Shiffman S. Relapse following smoking cessation: a situational analysis. J Consult Clin Psychol. 1982;50:71–86. doi: 10.1037//0022-006x.50.1.71. [DOI] [PubMed] [Google Scholar]
- 28.Swan GE, Denk CE, Parker SD, et al. Risk factors for late relapse in male and female ex-smokers. Addict Behav. 1988;13:253–66. doi: 10.1016/0306-4603(88)90052-4. [DOI] [PubMed] [Google Scholar]
- 29.Marlatt GA, Gordon JR. Determinants of relapse: Implications for the maintenance of behavior change. In: Davidson PO, Davidson SM, editors. Behavioral medicine: Changing health lifestyles. 1980. pp. 410–452. [Google Scholar]
- 30.Shiffman S, Hickcox M, Paty JA, et al. Progression from a smoking lapse to relapse: prediction from abstinence violation effects, nicotine dependence, and lapse characteristics. J Consult Clin Psychol. 1996;64:993–1002. doi: 10.1037//0022-006x.64.5.993. [DOI] [PubMed] [Google Scholar]
- 31.Carter BL, Lam CY, Robinson JD, et al. Real-time craving and mood assessments before and after smoking. Nicotine Tob Res. 2008;10:1165–9. doi: 10.1080/14622200802163084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shiffman S, Gwaltney CJ, Balabanis MH, et al. Immediate antecedents of cigarette smoking: an analysis from ecological momentary assessment. J Abnorm Psychol. 2002;111:531–45. doi: 10.1037//0021-843x.111.4.531. [DOI] [PubMed] [Google Scholar]
- 33.Shiffman S, Paty JA, Gwaltney CJ, Dang Q. Immediate antecedents of cigarette smoking: an analysis of unrestricted smoking patterns. Journal of abnormal psychology. 2004;113:166–71. doi: 10.1037/0021-843X.113.1.166. [DOI] [PubMed] [Google Scholar]
- 34.Donny EC, Griffin KM, Shiffman S, Sayette MA. The relationship between cigarette use, nicotine dependence, and craving in laboratory volunteers. Nicotine Tob Res. 2008;10:447–55. doi: 10.1080/14622200801901906. [DOI] [PubMed] [Google Scholar]
- 35.Litt MD, Cooney NL, Morse P. Reactivity to alcohol-related stimuli in the laboratory and in the field: predictors of craving in treated alcoholics. Addiction. 2000;95:889–900. doi: 10.1046/j.1360-0443.2000.9568896.x. [DOI] [PubMed] [Google Scholar]
- 36.Niaura RS, Rohsenow DJ, Binkoff JA, et al. Relevance of cue reactivity to understanding alcohol and smoking relapse. J Abnorm Psychol. 1988;97:133–52. doi: 10.1037//0021-843x.97.2.133. [DOI] [PubMed] [Google Scholar]
- 37.Rohsenow DJ, Monti PM, Rubonis AV, et al. Cue reactivity as a predictor of drinking among male alcoholics. J Consult Clin Psychol. 1994;62:620–6. doi: 10.1037//0022-006x.62.3.620. [DOI] [PubMed] [Google Scholar]
- 38.Tiffany ST, Warthen MW, Goedeker KC. The functional significance of craving in nicotine dependence. Nebr Symp Motiv. 2009;55:171–97. doi: 10.1007/978-0-387-78748-0_10. [DOI] [PubMed] [Google Scholar]
- 39.Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–40. [PubMed] [Google Scholar]
- 40.Sayette MA, Shiffman S, Tiffany ST, et al. The measurement of drug craving. Addiction. 2000;95(Suppl 2):S189–210. doi: 10.1080/09652140050111762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferguson SG, Shiffman S. The relevance and treatment of cue-induced cravings in tobacco dependence. J Subst Abuse Treat. 2009;36:235–43. doi: 10.1016/j.jsat.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 42.Drummond DC. Theories of drug craving, ancient and modern. Addiction. 2001;96:33–46. doi: 10.1046/j.1360-0443.2001.961333.x. [DOI] [PubMed] [Google Scholar]
- 43.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 44.Witkiewitz K, Marlatt GA. Relapse prevention for alcohol and drug problems: that was Zen, this is Tao. Am Psychol. 2004;59:224–35. doi: 10.1037/0003-066X.59.4.224. [DOI] [PubMed] [Google Scholar]
- 45.Shiffman S. Relapse and addictive behaviour. New York, NY, US: Tavistock/Routledge; 1989. Conceptual issues in the study of relapse; pp. 149–179. [Google Scholar]
- 46.Perkins KA. Does smoking cue-induced craving tell us anything important about nicotine dependence? Addiction. 2009;104:1610–6. doi: 10.1111/j.1360-0443.2009.02550.x. [DOI] [PubMed] [Google Scholar]
- 47.Waters AJ, Shiffman S, Sayette MA, et al. Cue-provoked craving and nicotine replacement therapy in smoking cessation. J Consult Clin Psychol. 2004;72:1136–43. doi: 10.1037/0022-006X.72.6.1136. [DOI] [PubMed] [Google Scholar]
- 48.Ditre JW, Oliver JA, Myrick H, et al. Effects of divalproex on smoking cue reactivity and cessation outcomes among smokers achieving initial abstinence. Experimental and Clinical Psychopharmacology. 2012;20:293. doi: 10.1037/a0027789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Powell J, Dawkins L, West R, Powell J, Pickering A. Erratum to: Relapse to smoking during unaided cessation: clinical, cognitive and motivational predictors. Psychopharmacology. 2011;215:607–607. doi: 10.1007/s00213-010-1975-8. [DOI] [PubMed] [Google Scholar]
- 50.Powell J, Dawkins L, West R, Powell J, Pickering A. Relapse to smoking during unaided cessation: clinical, cognitive and motivational predictors. Psychopharmacology (Berl) 2010;212:537–49. doi: 10.1007/s00213-010-1975-8. [DOI] [PubMed] [Google Scholar]
- 51.Tiffany ST, Wray J. The continuing conundrum of craving. Addiction. 2009;104:1618–9. doi: 10.1111/j.1360-0443.2009.02588.x. [DOI] [PubMed] [Google Scholar]
- 52.Shiffman S. Responses to smoking cues are relevant to smoking and relapse. Addiction. 2009;104:1617–8. doi: 10.1111/j.1360-0443.2009.02580.x. [DOI] [PubMed] [Google Scholar]
- 53.Conklin CA, Robin N, Perkins KA, Salkeld RP, McClernon FJ. Proximal versus distal cues to smoke: the effects of environments on smokers’ cue-reactivity. Exp Clin Psychopharmacol. 2008;16:207–14. doi: 10.1037/1064-1297.16.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baker TB, Morse E, Sherman JE. The motivation to use drugs: a psychobiological analysis of urges. Nebr Symp Motiv. 1986;34:257–323. [PubMed] [Google Scholar]
- 55.Stewart J, De Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychological review. 1984;91:251. [PubMed] [Google Scholar]
- *56.al’Absi M, Wittmers LE, Erickson J, Hatsukami D, Crouse B. Attenuated adrenocortical and blood pressure responses to psychological stress in ad libitum and abstinent smokers. Pharmacology Biochemistry and Behavior. 2003;74:401–410. doi: 10.1016/s0091-3057(02)01011-0. [DOI] [PubMed] [Google Scholar]
- *57.Colamussi L, Bovbjerg DH, Erblich J. Stress- and cue-induced cigarette craving: effects of a family history of smoking. Drug Alcohol Depend. 2007;88:251–8. doi: 10.1016/j.drugalcdep.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *58.Drobes DJ. Cue reactivity in alcohol and tobacco dependence. Alcohol Clin Exp Res. 2002;26:1928–9. doi: 10.1097/01.ALC.0000040983.23182.3A. [DOI] [PubMed] [Google Scholar]
- *59.Maude-Griffin P, Tiffany ST. Production of smoking urges through imagery: The impact of affect and smoking abstinence. Experimental and Clinical Psychopharmacology. 1996;4:198. [Google Scholar]
- *60.Perkins KA, Grobe JE. Increased desire to smoke during acute stress. Br J Addict. 1992;87:1037–40. doi: 10.1111/j.1360-0443.1992.tb03121.x. [DOI] [PubMed] [Google Scholar]
- 61.Perkins KA. Sex differences in nicotine reinforcement and reward: influences on the persistence of tobacco smoking. Nebr Symp Motiv. 2009;55:143–69. doi: 10.1007/978-0-387-78748-0_9. [DOI] [PubMed] [Google Scholar]
- 62.Shiffman S, Paty J. Smoking patterns and dependence: contrasting chippers and heavy smokers. Journal of abnormal psychology. 2006;115:509. doi: 10.1037/0021-843X.115.3.509. [DOI] [PubMed] [Google Scholar]
- 63.Sayette MA, Griffin KM, Sayers WM. Counterbalancing in smoking cue research: a critical analysis. Nicotine Tob Res. 2010;12:1068–79. doi: 10.1093/ntr/ntq159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- *65.Conklin CA, Tiffany ST. The impact of imagining personalized versus standardized urge scenarios on cigarette craving and autonomic reactivity. Exp Clin Psychopharmacol. 2001;9:399–408. doi: 10.1037//1064-1297.9.4.399. [DOI] [PubMed] [Google Scholar]
- *66.Perkins KA, Karelitz JL, Conklin CA, Sayette MA, Giedgowd GE. Acute negative affect relief from smoking depends on the affect situation and measure but not on nicotine. Biological psychiatry. 2010;67:707–14. doi: 10.1016/j.biopsych.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *67.Childs E, de Wit H. Effects of acute psychosocial stress on cigarette craving and smoking. Nicotine Tob Res. 2010;12:449–53. doi: 10.1093/ntr/ntp214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *68.McKee SA, Sinha R, Weinberger AH, et al. Stress decreases the ability to resist smoking and potentiates smoking intensity and reward. J Psychopharmacol. 2011;25:490–502. doi: 10.1177/0269881110376694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *69.Bradley BP, Garner M, Hudson L, Mogg K. Influence of negative affect on selective attention to smoking-related cues and urge to smoke in cigarette smokers. Behav Pharmacol. 2007;18:255–63. doi: 10.1097/FBP.0b013e328173969b. [DOI] [PubMed] [Google Scholar]
- *70.Taylor RC, Harris NA, Singleton EG, Moolchan ET, Heishman SJ. Tobacco craving: intensity-related effects of imagery scripts in drug abusers. Exp Clin Psychopharmacol. 2000;8:75–87. doi: 10.1037//1064-1297.8.1.75. [DOI] [PubMed] [Google Scholar]
- 71.Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive Meta-Analysis Version 2.2. Englewood Cliffs: Biostat; 2010. [Google Scholar]
- *72.Beckham JC, Lytle BL, Vrana SR, et al. Smoking withdrawal symptoms in response to a trauma-related stressor among Vietnam combat veterans with posttraumatic stress disorder. Addict Behav. 1996;21:93–101. doi: 10.1016/0306-4603(95)00038-0. [DOI] [PubMed] [Google Scholar]
- *73.Carpenter MJ, Saladin ME, DeSantis S, et al. Laboratory-based, cue-elicited craving and cue reactivity as predictors of naturally occurring smoking behavior. Addictive Behaviors. 2009;34:536–541. doi: 10.1016/j.addbeh.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *74.Carter BL, Robinson JD, Lam CY, et al. A psychometric evaluation of cigarette stimuli used in a cue reactivity study. Nicotine Tob Res. 2006;8:361–9. doi: 10.1080/14622200600670215. [DOI] [PubMed] [Google Scholar]
- *75.Ditre JW, Brandon TH. Pain as a motivator of smoking: effects of pain induction on smoking urge and behavior. J Abnorm Psychol. 2008;117:467–72. doi: 10.1037/0021-843X.117.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *76.Elash CA, Tiffany ST, Vrana SR. Manipulation of smoking urges and affect through a brief-imagery procedure: Self-report, psychophysiological, and startle probe responses. Experimental and Clinical Psychopharmacology. 1995;3:156. [Google Scholar]
- *77.Litvin EB, Brandon TH. Testing the influence of external and internal cues on smoking motivation using a community sample. Exp Clin Psychopharmacol. 2010;18:61–70. doi: 10.1037/a0017414. [DOI] [PubMed] [Google Scholar]
- *78.Perkins KA, Ciccocioppo M, Conklin CA, et al. Mood influences on acute smoking responses are independent of nicotine intake and dose expectancy. Journal of abnormal psychology. 2008;117:79–93. doi: 10.1037/0021-843X.117.1.79. [DOI] [PubMed] [Google Scholar]
- *79.Rehme AK, Frommann I, Peters S, et al. Startle cue-reactivity differentiates between light and heavy smokers. Addiction. 2009;104:1757–64. doi: 10.1111/j.1360-0443.2009.02668.x. [DOI] [PubMed] [Google Scholar]
- *80.Robinson JD, Lam CY, Carter BL, et al. A multimodal approach to assessing the impact of nicotine dependence, nicotine abstinence, and craving on negative affect in smokers. Exp Clin Psychopharmacol. 2011;19:40–52. doi: 10.1037/a0022114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *81.Tiffany ST, Drobes DJ. Imagery and smoking urges: the manipulation of affective content. Addict Behav. 1990;15:531–9. doi: 10.1016/0306-4603(90)90053-z. [DOI] [PubMed] [Google Scholar]
- *82.Watson NL, Carpenter MJ, Saladin ME, Gray KM, Upadhyaya HP. Evidence for greater cue reactivity among low-dependent vs. high-dependent smokers. Addict Behav. 2010;35:673–7. doi: 10.1016/j.addbeh.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rosenthal R. Meta-analytic procedures for social research / Robert Rosenthal. Newbury Park: Sage Publications; 1991. c1991. Rev. ed. [Google Scholar]
- 84.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994:1088–1101. [PubMed] [Google Scholar]
- 85.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Duval S, Tweedie R. Trim and Fill: A Simple Funnel–Plot–Based Method of Testing and Adjusting for Publication Bias in Meta–Analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 87.Brandon TH, Tiffany ST, Obremski KM, Baker TB. Postcessation cigarette use: the process of relapse. Addictive behaviors. 1990;15:105–14. doi: 10.1016/0306-4603(90)90013-n. [DOI] [PubMed] [Google Scholar]
- 88.Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- 89.Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63:324–31. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- 90.Collins BN, Nair US, Komaroff E. Smoking cue reactivity across massed extinction trials: negative affect and gender effects. Addict Behav. 2011;36:308–14. doi: 10.1016/j.addbeh.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 91.O’Connell KA, Shiffman S, Decarlo LT. Does extinction of responses to cigarette cues occur during smoking cessation? Addiction. 2011;106:410–7. doi: 10.1111/j.1360-0443.2010.03172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vinci C, Copeland AL, Carrigan MH. Exposure to negative affect cues and urge to smoke. Exp Clin Psychopharmacol. 2012;20:47–55. doi: 10.1037/a0025267. [DOI] [PubMed] [Google Scholar]
- 93.McKee SA. Developing human laboratory models of smoking lapse behavior for medication screening. Addiction Biology. 2009;14:99–107. doi: 10.1111/j.1369-1600.2008.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Perkins KA. Cues must increase smoking behaviour to be clinically relevant. Addiction. 2009;104:1620–2. doi: 10.1111/j.1360-0443.2009.02670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rottenberg J, Ray RD, Gross JJ. Emotion elicitation using films. In: Coan JA, Allen JJB, editors. Handbook of emotion elicitation and assessment. New York, NY US: Oxford University Press; 2007. pp. 9–28. [Google Scholar]
- 96.Sayette MA, Tiffany ST. Peak-provoked craving: An alternative to smoking cue reactivity. Addiction. doi: 10.1111/j.1360-0443.2012.04013.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]



