Abstract
Baeyer-Villiger monooxygenases (BVMOs) have been shown to play key roles for the biosynthesis of important natural products. MtmOIV, a homodimeric FAD- and NADPH-dependent BVMO, catalyzes the key frame-modifying steps of the mithramycin biosynthetic pathway, including an oxidative C-C bond cleavage, by converting its natural substrate premithramycin B into mithramycin DK, the immediate precursor of mithramycin. The drastically improved protein structure of MtmOIV along with the high-resolution structure of MtmOIV in complex with its natural substrate premithramycin B are reported here, revealing previously undetected key residues that are important for substrate recognition and catalysis. Kinetic analyses of selected mutants allowed us to probe the substrate binding pocket of MtmOIV, and also to discover the putative NADPH binding site. This is the first substrate-bound structure of MtmOIV providing new insights into substrate recognition and catalysis, which paves the way for the future design of a tailored enzyme for the chemo-enzymatic preparation of novel mithramycin analogues.
Baeyer-Villiger monooxygenases (BVMOs), an important subclass of flavoprotein monooxygenases, have not only proven to be potent biocatalysts for synthetic organic chemistry applications,(1) but also have been found to hold critical roles in the biosynthesis of many natural products. (2) Additionally, BVMO’s are known to be responsible for pro-drug activation (3) as well as biodegradation reactions.(4-6)
MtmOIV is a homodimeric BVMO from the mithramycin biosynthetic pathway that catalyzes the key frame-modifying step responsible for producing bioactivity. This FAD- and NADPH dependent enzyme reacts with its natural substrate premithramycin B to form mithramycin DK the substrate of the final enzymatic step of the mithramycin biosynthetic pathway (7, 8). Mithramycin (MTM, also known as aureolic acid, mithracin, LA-7017, PA-144, and plicamycin) is an aureolic acid-type polyketide anticancer antibiotic produced by the soil bacterium Streptomyces argillaceus (ATCC 12956) and various other species of streptomycetes. The small distinct group of aureolic acid-type anticancer antibiotics includes MTM, chromomycin, olivomycin, UCH9, and durhamycin, which all contain the same polyketide-derived tricyclic aromatic core with a highly functionalized pentyl side chain attached at C-3, but vary with respect to their saccharide patterns and side chains in 7-position.(9-11).
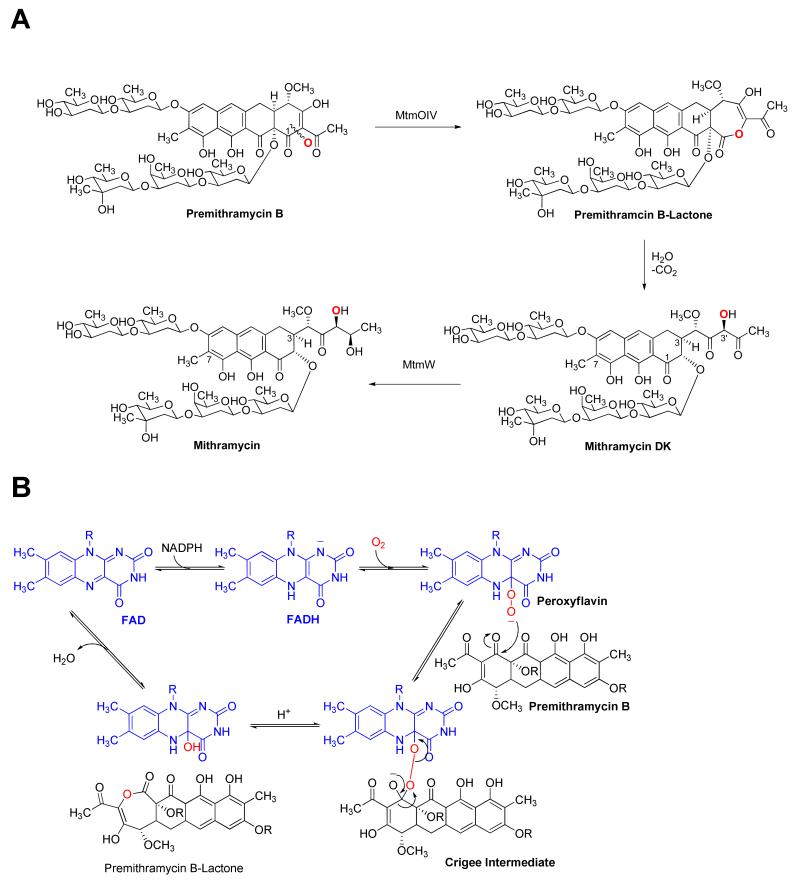
The structure of MTM consists of a tricyclic aglycone core, a highly functionalized pentyl side chain at the 3-position, and five deoxysugars linked as trisaccharide at the 2- position and a disaccharide at the 6-position, respectively. MTM biosynthesis proceeds through the polyketide-derived tetracyclic premithramycinone, to which the five sugar moieties and one C-methyl group are added leading to premithramycin B.(9-14) Then, MtmOIV oxidatively cleaves the fourth ring via a Baeyer-Villiger reaction, generating MTM’s characteristic tricyclic aglycone core and the highly functionalized pentyl side chain at position 3. The Baeyer–Villiger reaction precedes lactone opening, decarboxylation, and the final step of MTM biosynthesis, the reduction of the 4′-keto group catalyzed by ketoreductase MtmW (15) (Figure 1).
Figure 1. The mithramycin biosynthetic pathway.
A. The reaction catalyzed by the Baeyer-Villiger monooxygenase (BVMO) MtmOIV yields premithramycin B-lactone, which is further converted to mithramycin DK. Ketoreductase MtmW catalyzes the final step of mithramycin biosynthesis. B. Suggested Baeyer-Villiger oxidation of premithramycin B (sugar residues not shown) to premithramycin B-lactone involving co-factors FADH and NADPH (for FADH regeneration).
MTM exhibits anticancer activity by inhibiting replication and transcription by cross-linking of DNA strands predominantly in GC-rich regions used by Sp promoters.(16-18) MTM has been used clinically to treat certain cancers, such as testicular carcinoma,(19, 20) bone diseases, such as Paget’s disease,(21-24) and particularly to address cancer-related hypercalcemia.(25-28) Recent discoveries include MTM’s potential (i) as therapeutic to treat Huntington’s disease,(29-31) (ii) as a lead drug out of a screen of 50,000 compounds affecting Ewing sarcoma promotion factors that previously were believed to be “undruggable”,(32) and (iii) as a repressor of ABCG2-related stem cell signaling in lung and esophageal cancers.(33) Yet, the clinical use of MTM is still hindered because of its hardly manageable toxicity, which could potentially be reduced through the generation of new, less toxic analogues. In this context, the function of the Baeyer-Villiger oxygenase (BMVO) MtmOIV needs to be fully understood to pave the way for further chemo-enzymatic and/or combinatorial biosynthetic approaches towards new MTM analogues.
Besides our low resolution structure of MtmOIV (8) only a few crystal structures of Baeyer–Villiger reaction-performing enzymes are available to date,(1) including phenylacetone monooxygenase from the thermophilic bacterium Thermobifida fusca,(34) the oxygenating component of 3,6-diketocamphane monooxygenase,(35-37) a dimeric BVMO catalyzing the lactonization of 2-oxo-delta(3)-4,5,5-trimethylcyclopentenylacetyl-CoA, a key intermediate in the metabolism of camphor by Pseudomonas putida (5), and cyclohexanone monooxygenase (CHMO) from Rhodococcus sp. HI-31,(38) which could also be solved in complex with cyclohexanone as well as NADP and FAD.(39) Most recently, the toxoflavin lyase structure was solved, and the enzyme was found to catalyze a degradation reaction via a Baeyer-Villiger oxidation reaction.(6) Various BVMOs have been described and studied, and most of these appear to be involved in oxidative degradation pathways, for which substrates were identified ranging from simple ketones, such as phenylacetone or cyclohexanone and their derivatives, to steroids, such as progesterone.(1, 6, 40) In contrast to these mostly mono-functional molecules, the MtmOIV substrate premithramycin B (PMB) is a multiple functional and richly decorated molecule, and thus remains the most complex substrate and one of very few unambiguously proven natural BVMO substrates.(41)
We have previously reported the native structure of MtmOIV that provided initial insights into the intriguing enzymatic BV mechanism.(8) However, crystals for this structure suffered from a high solvent content which led to many disordered side chains. Here, we report the significantly improved crystal structure of native MtmOIV to 2.0 Å resolution, as well as the crystal structure of MtmOIV in complex with its natural substrate premithramycin B to 1.85 Å resolution, allowing us to identify key residues involved in substrate recognition and catalysis. This provides the structural information required to re-engineer MtmOIV to have altered substrate specificity for the generation of new bioactive analogues of MTM. Our structure of MtmOIV in complex with premithramycin B provides insight into substrate recognition and catalysis in MtmOIV and other important enzymes from this family. Furthermore, mutagenesis, kinetic assays, and docking studies based on a low resolution NADPH-bound co-crystal structure allowed us to probe the substrate binding pocket of MtmOIV and to discover the putative NADPH binding site which sits opposite the substrate binding site, yet still in close proximity to the FAD cofactor.
RESULTS AND DISCUSSION
Overall Structure of MtmOIV
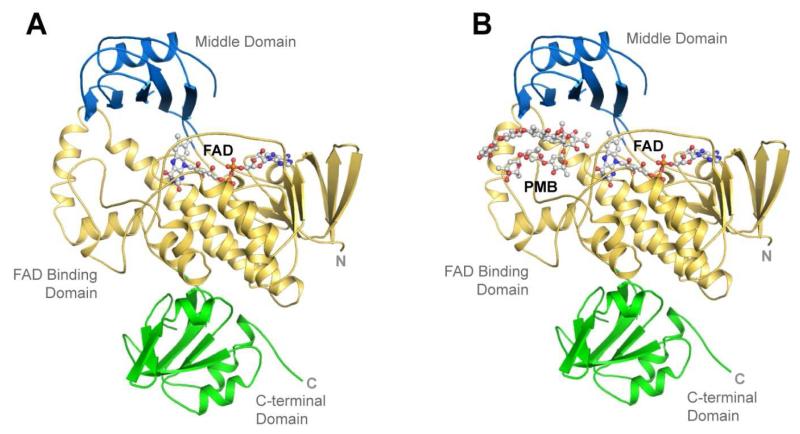
The crystal structure of native MtmOIV has previously been described.(8) However, the N-terminal fusion tag was not removed and the structure suffered from high solvent content (~70%) which led to a large number of disordered side chains. Here, we have removed the N-terminal fusion tag which led to crystal conditions with overall improved quality and significantly improved electron density quality and side chain order with ~50% solvent content. The new structure was solved to 2.0 Å resolution in space group P2 with one molecule per asymmetric unit. The final structure contains 499 of 533 total residues (Ala10-Ala509) for MtmOIV and a single non-covalently bound FAD cofactor (Figure 2A). Final data collection and refinement statistics are summarized in Table 1. Figure 2A shows the three domains of MtmOIV: the FAD binding domain (residues 10-183 and 276-394), the middle domain (residues 184-275), and the C-terminal domain (residues 395-510). A loop containing residues 218-224, which lies at the perimeter of the substrate binding pocket, was found ordered in the new structure. However, the loop containing residues 233-239, which has been suggested to be involved in NADPH binding (8), was again found disordered along with a small loop within the C-terminal domain containing residues 402-403.
Figure 2. Overall structures of MtmOIV and the MtmOIV-premithramycin B complex.
A.The improved native MtmOIV crystal structure to 2.0 Å resolution. FAD is shown in ball and stick representation. B. The MtmOIV-premithramycin B complex crystal structure showing FAD and premitramycin B in ball and stick representation. For both panels, the FAD domain is shown in gold, the middle domain in blue, and the C-terminal domain in green.
Table 1.
Data Collection and Refinement Summary
| Native | PMB | |
|---|---|---|
| Data Collection | ||
| λ (Å) | 1.0 | 1.0 |
| Space group | P2 | P2221 |
| Mol/ASU | 2 | 1 |
| a (Å) | 83.30 | 55.72 |
| b (Å) | 56.05 | 79.75 |
| c (Å) | 124.90 | 154.26 |
| α (°) | 90.0 | 90 |
| β (°) | 95.0 | 90 |
| γ (°) | 90.0 | 90 |
| Resolution (Å) | 50-2.0 (2.07-2.0) | 50-1.85 (1.92-1.85) |
| Completeness (%)* | 99.4 (97.7) | 96.1 (91.5) |
| Redundancy | 3.8 (3.0) | 5.6 (4.4) |
| Rsym†* | 0.10 (0.66) | 0.09 (0.71) |
| I / σ (I)* | 12.2 (1.7) | 15.1 (1.8) |
| Refinement | ||
| Resolution (Å) | 20-2.0 | 20-1.85 |
| R§/Rfree¶ | 0.21/0.25 | 0.18/0.22 |
| Bonds(Å) | 0.010 | 0.007 |
| Angles (°) | 1.138 | 1.139 |
| Protein atoms | 7238 | 3734 |
| Ligand atoms | 106 | 130 |
| Water molecules | 358 | 508 |
| B-factors | ||
| Protein | 48.7 | 20.7 |
| Ligand | 34.6 | 22.8 |
| Water molecules | 42.7 | 31.8 |
| Ramachandran Analysis ¥ | ||
| Core (%) | 90.9 | 93.1 |
| Allowed (%) | 8.9 | 6.6 |
| Generously Allowed (%) | 0.1 | 0.3 |
| Disallowed (%) | 0 | 0 |
| PDB ID | 4K5R | 4K5S |
Rsym = Σhkl,j (|Ihkl-<Ihkl>|) / Σhkl,j Ihkl, where <Ihkl> is the average intensity for a set of j symmetry related reflections and Ihkl is the value of the intensity for a single reflection within a set of symmetry-related reflections.
R factor = Σhkl(||Fo| - |Fc||)/Σhkl|Fo| where Fo is the observed structure factor amplitude and Fc is the calculated structure factor amplitude.
Rfree = Σhkl,T(||Fo| - |Fc||)/Σhkl,T|Fo|, where a test set, T (5% of the data), is omitted from the refinement.
Performed using Procheck.
Indicates statistics for last resolution shell shown in parenthesis.
Structure of MtmOIV-PMB complex
For the structure of MtmOIV in complex with its substrate premithramycin B (PMB), PMB was added to MtmOIV prior to concentrating the protein for crystallization trials. Co-crystals were grown in final conditions consisting of 200 mM ammonium acetate and 30% PEG (polyethylene glycol) 1000, and the structure was solved to 1.85 Å resolution in space group P2221, with two molecules per asymmetric unit. The final structure contains 500 of 533 total residues (Ala10-Asn510) for MtmOIV and a single non-covalently bound FAD cofactor per subunit (Figure 2B) with the same domain architecture as the native structure (RMSD, = root mean square deviation, of 0.266 Å for 2933 aligned atoms). A difference density map revealed the presence of un-modeled density along the putative substrate binding site in which PMB was modeled and refined (Figures 2B and 3). Final data collection and refinement statistics are summarized in Table 1.
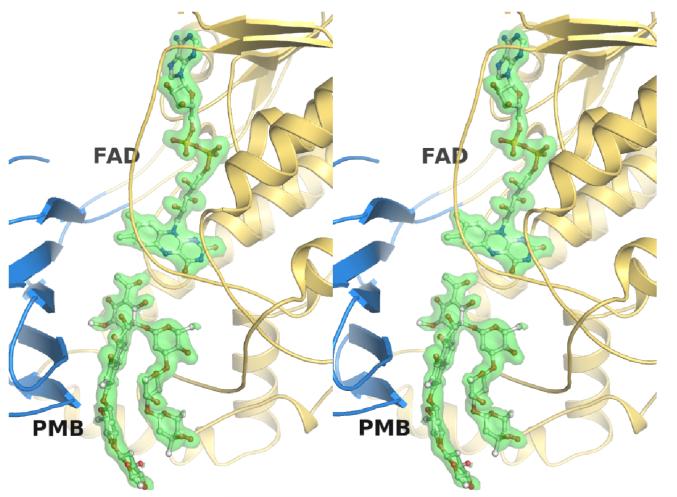
Figure 3. Wall-eye stereoview showing the electron density for FAD and premitramycin B.
The middle domain in shown in blue, the FAD domain in gold, both FAD and premithramycin B (PMB) are shown in ball and stick representation, and the electron density (SA-omit Fo-Fc map contoured to 2.5 σ) is shown as a green transparent isosurface.
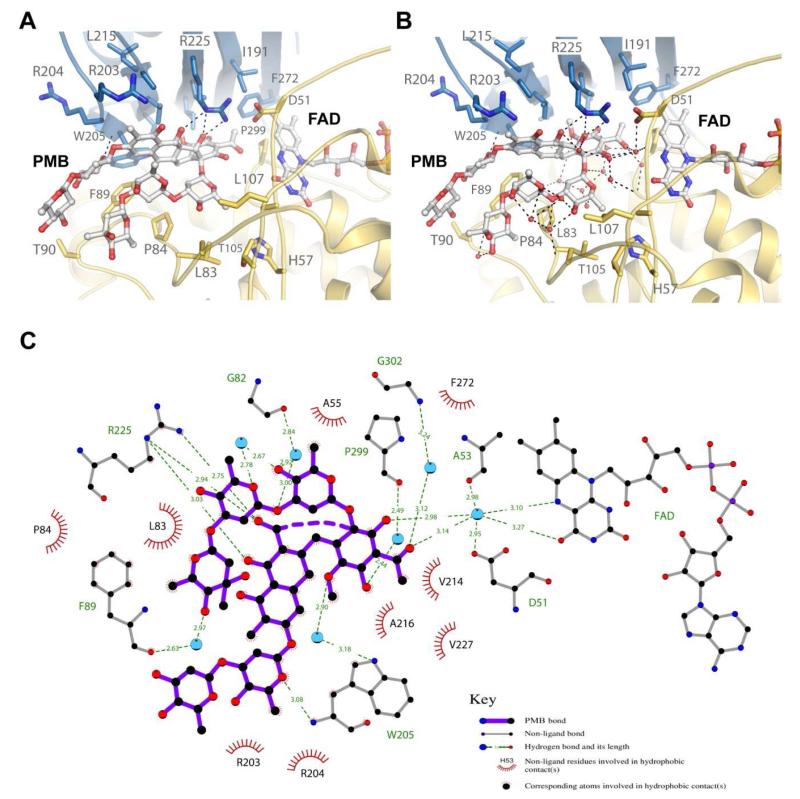
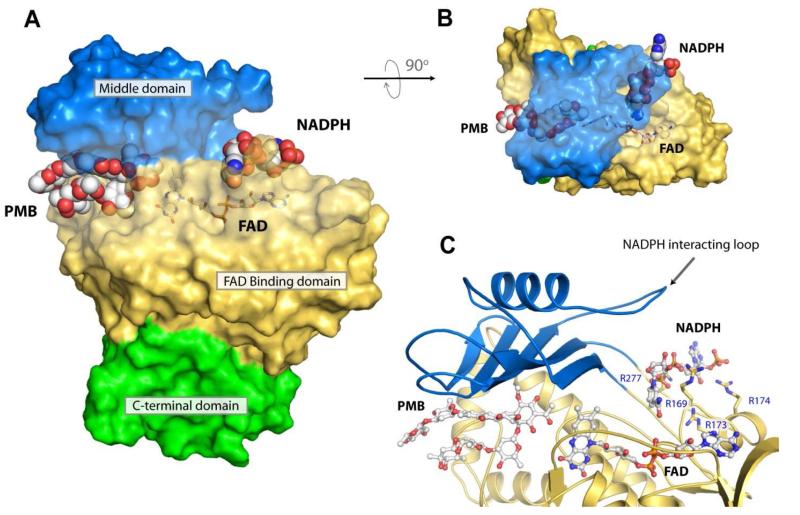
As shown in Figure 2B, PMB binds MtmOIV within the large binding pocket formed between the FAD binding domain and the middle domain and the sugar chains of PMB interact with the inner walls of the middle and the FAD binding domains within the pocket. Ignoring any ordered water molecules found in the structure, only a few residues from MtmOIV appear to make direct hydrogen bonding interactions with PMB including R225 and W205 (Figure 4A), indicating that van-der-Waals and hydrophobic interactions may play a major role in substrate binding and selectivity. When we include the ordered water molecules found within the crystal structure, it becomes clear that the ordered solvent may also play a major role in substrate binding by forming a hydrogen bonding network that bridges PMB binding within the binding pocket of MtmOIV (Figure 4B). A Ligplot analysis of the interactions between PMB and MtmOIV depicts best the vast number of interactions involved in substrate binding involving not only protein side chains but also many water molecules that are crucial for binding (Figure 4C).
Figure 4. The premithramycin B binding site in MtmOIV.
A. Shown are the interactions (dashed lines) of MtmOIV with premithramycin B (PMB) bound along the active site with water molecules removed from structure. Without water molecules, there appear to be two primary interactions with W205 and R225, with van der Waals and hydrophobic interactions contributing a large part to the binding energy. B. Shown are the interactions of MtmOIV with PMB, including the water molcules that were observed in the crystal structure (1.85 Å resolution). Once solvation is included, a vast number of interactions (dashed lines, distance cutoff of 3.3 Å) are observed which are bridged by ordered water molecules, indicating that hydrogen bonding networks play a major role in substrate binding. C. Ligplot of PMB bound to MtmOIV indicating important interactions (dashed lines, distance cutoff of 3.3 Å). For clarity, atom names for PMB have been removed and only water molecules (cyan spheres) having two or more hydrogen bonding interactions are shown.
The improved native (substrate-free) MtmOIV crystal structure described above allowed us to compare the substrate-free and substrate-bound crystal structures. The overall conformation of MtmOIV in the complex structure was the same as in the native structure, however a minimal domain shift was observed within the middle domain relative to the FAD binding domain (more open) which likely serves to accommodate the bound substrate. While only minimal rearrangements were observed for most of the side chains of residues lining the substrate binding pocket, the largest shifts appear in W205 (1.6 Å along atom CH2) and F89 (0.9 Å along atom CHζ), which sit on opposite sides at the entrance to the substrate binding pocket. Aside from these slight shifts, no major conformational differences were observed between the substrate-free and substrate-bound states of MtmOIV.
Mutagenesis and steady state kinetics assays
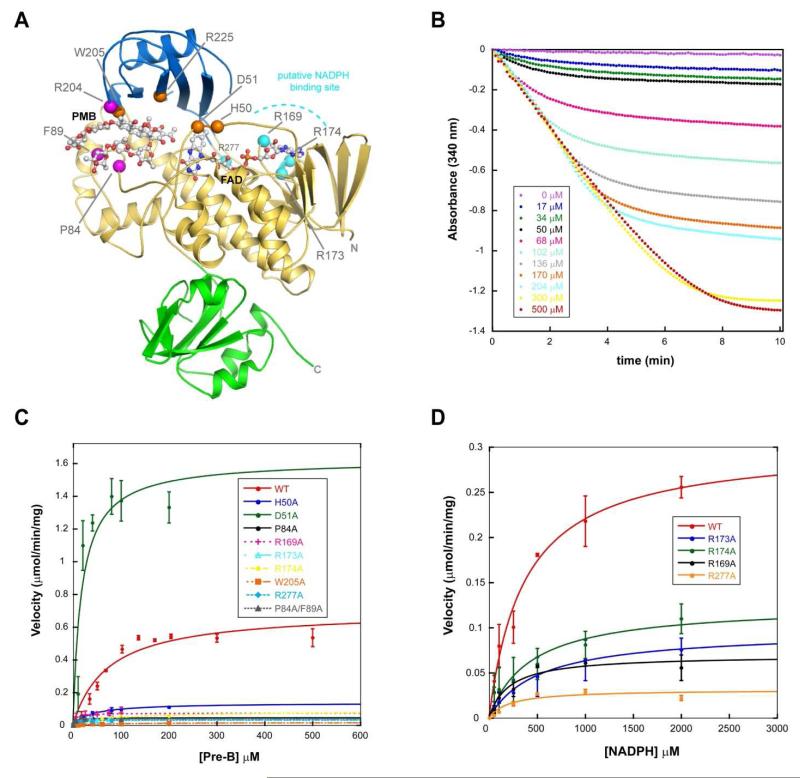
Using the structure of MtmOIV in complex with PMB, we were able to identify residues important for substrate recognition (P84, F89, R204) and catalysis (H50, D51, W205, R225) (Figure 5A). We mutated these residues to alanine and then used a kinetic assay to determine the effect each mutation had on substrate binding and catalysis (Figures 5B, 5C, 5D, and Table 2). While the substrate binding of mutants was relatively tolerant to most of the mutations, turnover rates for several of the mutants were severely affected. Interestingly, none of the substrate recognition site mutants significantly affect substrate binding except the double-mutant P84A/F89A with a ~50-fold decrease in KM compared to the wild-type enzyme, leading to a 3-fold increased catalytic efficiency. The single mutants F89A, P84A, and R204A decrease KM only by 3 ~ 10-fold, however, their catalytic efficiencies are even greater than of the double mutant due to a less severe decrease in kcat. The active site mutant W205A is interesting since its catalytic efficiency is drastically decreased (~ 30-fold), although the substrate binding is comparable to the wild-type enzyme, likely because of its role as gate-keeper for the entry into the binding pocket (see below). Arginine-225 appears crucial, its mutation led to a non-catalytic enzyme. Figure 4 illustrates that R225 stabilizes the polyketide core of PMB. Interestingly, the D51A mutant –like the previously reported F89A mutant– showed a ~ 2-fold increase in kcat and a drastically increased catalytic efficiency, which proves the crucial role of this amino acid for MtmOIV’s catalysis of the BV reaction. It is possible that changing the carboxylate residue of aspartate into the simple methyl group of alanine may decrease both sterically and electronically disadvantageous interactions between the substrate PMB and the peroxyflavin (Table 2, Figures 1 and 4).
Figure 5. Probing substrate recognition and catalysis in MtmOIV.
Based on structural analysis, three regions were initially targeted for this study to determine their contribution to substrate binding and catalysis (see Table 2). A. Shown is the MtmOIV-premithramycin B (PMB) crystal structure with the location of the mutations that were made shown as spheres and color coded by their predicted role in catalysis, (i) substrate binding (magenta), (ii) active site (orange), and (iii) putative NADPH binding (cyan). The FAD domain is shown in gold, the middle domain in blue, the C-terminal domain in green, and both FAD and PMB are shown in ball and stick representation. B. Sample of raw kinetic data collected at λ=340 nm. C. Michaelis-Menton curve fitting of data for WT MtmOIV and mutants. D. Curve fitting of data for WT MtmOIV and the mutants R169A, R173A, R174A, and R277A.
Table 2.
Summary table of MtmOIV mutagenesis and kinetics assays.
| NADPH | |||||
|---|---|---|---|---|---|
| Mutation(s) | Target Region | KM (μM) | Vmax (μmol min−1 mg−1) |
kcat (s−1) | kcat/KM (s−1mM−1) |
| wild type | 372 ± 68 | 0.3 ± 0.02 | 0.29 ± 0.02 | 0.8 | |
| R169A | NADPH site | 211 ± 71 | 0.07 ± 0.007 | 0.07 ± 0.006 | 0.3 |
| R173A | NADPH site | 528 ± 63 | 0.1 ± 0.004 | 0.09 ± 0.0042 | 0.2 |
| R174A | NADPH site | 437 ± 159 | 0.1 ± 0.017 | 0.12 ± 0.02 | 0.3 |
| R277A | NADPH site | 221 ± 118 | 0.03 ± 0.004 | 0.03 ± 0.0005 | 0.1 |
| Pre-B | |||||
| Mutation(s) | Target Region | Km (μM) | Vmax (μmol min−1 mg−1 |
kcat (s−1) | kcat/KM (s−1mM−1) |
| wild type | 73 ± 20 | 0.7 ± 0.07 | 0.7 ± 0.06 | 9 | |
| P84A | substrate binding | 7.8 ± 2.4 | 0.04 ± 0.002 | 0.07 ± 0.007 | 9 |
| F89A8 | substrate binding | 27.1 ± 8.9 | 1.3 ± 0.1 | 1.27 ± 0.11 | 47 |
| P84A/F89A | substrate binding | 1.5 ± 0.9 | 0.04 ± 0.005 | 0.04 ± 0.004 | 26 |
| R204A8 | substrate binding | 18.0 ± 2.6 | 0.77 ± 0.02 | 0.73 ± 0.02 | 41 |
| H50A | active site | 40.4 ± 15.3 | 0.14 ± 0.02 | 0.13 ± 0.02 | 3 |
| D51A | active site | 18.1 ± 9.9 | 1.62 ± 0. 2 | 1.55 ± 0.2 | 86 |
| W205A | active site | 62.1 ± 29.0 | 0.02 ± 0.003 | 0.02 ± 0.003 | 0.3 |
| R225A | active site | no activity | no activity | no activity | no activity |
| R169A | NADPH site | 80.2 ± 42.3 | 0.08 ± 0.02 | 0.08 ± 0.02 | 1 |
| R173A | NADPH site | 5.3 ± 4.0 | 0.04 ± 0.005 | 0.02 ± 0.003 | 4 |
| R174A | NADPH site | 5.4 ± 3.0 | 0.09 ± 0.02 | 0.07 ± 0.007 | 13 |
| R277A | NADPH site | 4.8 ± 1.4 | 0.03 ± 0.001 | 0.03 ± 0.004 | 6 |
Identification of the putative NADPH binding site of MtmOIV
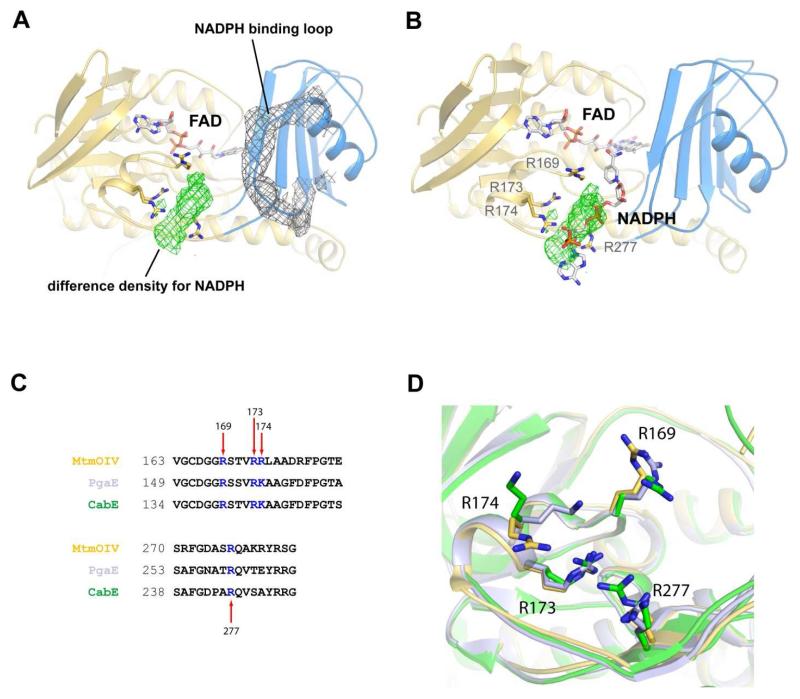
We recently grew crystals of MtmOIV in the presence of 5 mM NADPH that diffracted to ~3.5 Å (data not shown). We were able to solve the structure and observed density for the putative NADPH binding loop (residues 233-239), which had previously always been found disordered, as well as for a possible NADPH molecule found at a novel binding site along the 2nd identified Rossmann-type fold (residues 163-180 correspond to a single β-α-β super secondary structural motif commonly found in Rossmann folds), which is distinct from either the FAD or the substrate binding sites (Figures 7A and B). However, due to the low resolution of the data and partial disorder within the ligand, we were not able to model the entire NADPH molecule and our efforts to improve the resolution of the crystals have so far been unsuccessful. Yet, based on the location of the density, we were able to identify four residues (R169, R173, R174, R277) which seem well positioned to interact with NADPH at this site (Figures 6A and B). Mutating these residues to alanine significantly altered the catalysis of MtmOIV, although the KM values of the mutants still indicate similar NADPH binding as found for the wild type enzyme. We assume that inactivating single arginine residues leads to a distorted NADPH binding that precludes efficient reduction of FAD and therefore efficient enzyme turnover. Unexpectedly, PMB binding seems to be enhanced upon changing the latter three of these four arginine residues (Table 2),(42) possibly because the access of substrate PMB is facilitated if NADPH is bound less efficiently to the enzyme. All four arginine residues are fully conserved in other FAD and NADPH dependent monooxygenases, e.g., PgaE and CabE,(43) two enzymes with a similar fold as MtmOIV (Figures 6C and D). These R to A mutants seem not to affect FAD binding, since they keep the same bright yellow color as the wild type enzyme.
Figure 7. NADPH docked into the putative NADPH binding pocket in MtmOIV.
A. The MtmOIV structure showing the bound premithramycin B in the substrate binding pocket and the lowest energy docked model (−2.8 kcal/mol) of NADPH positioned within the putative NADPH binding pocket. B. Top-down view of the MtmOIV structure shown in panel A through the middle domain (blue). The binding pockets in panel A and B are shown as transparent surface and for clarity, FAD is shown in stick. C. Close-up view of the putative NADPH binding site shown in proximity to FAD and PMB (ball and stick) which was co-crystallized within the substrate binding pocket. Middle domain loop consisting of residues 233-239 has been postulated to participate in binding NADPH (ball and stick) and was found disordered in all known structures of MtmOIV except our low resolution NADPH co-crystal structure. Those residues that were identified from our low resolution crystal structure to interact with NADPH (R169, R173, R174, R277) are shown in stick representation.
Figure 6. The low resolution crystal structure of NADPH bound to MtmOIV.
A. The ordered NADPH binding loop of MtmOIV showing 2Fo-Fc electron density (gray) contoured at 0.8 σ. Difference density (green) contoured at 2.5 σ for NADPH that was observed only in this crystal structure and not within any of the previous MtmOIV structures. The space group for the NADPH-bound co-crystal structure was P1 with 6 molecules in the asymmetric unit. The resolution was 3.5 Å and final R/Rfree values are 0.22/0.26. B. Rigid body placement of NADPH along the difference density within the MtmOIV structure which was used as a starting point for subsequent docking studies. C. Sequence alignment showing the conservation of basic residues at the putative NADPH binding site. D. Structural alignment of MtmOIV (gold), PgaE (light purple), and CabE (green) depicting residues proposed to interact with NADPH shown in stick representation.
Using this information along with the recently reported protein structure of CHMO in complex with NADP(39) and the suggested role of the disordered loop containing residues 233-239 for NADPH binding, we performed steered docking studies using AutoDock 4 to form an in silico model for the NADPH-bound state of MtmOIV (Figure 7). The putative NADPH binding site is formed by the outer edge of the FAD binding domain and the middle domain and is located on the opposite side of the substrate binding pocket, but in close proximity to the FAD cofactor. Together, our structural and mutagenesis studies have not only allowed us to identify both the exact FAD and substrate binding sites, but also to identify the putative NADPH binding site.
Conclusions
Our new refined structure is the first substrate bound crystal structure of MtmOIV. The MtmOIV structure with its bound substrate PMB revealed significant differences to the previous computer model, in which PMB was docked into the low resolution structure of MtmOIV.(8) Most important, two glutamine residues (Q78 and Q91) and a proline residue (P84) were recognized as critical to hold the trisaccharide chain in place. The trisaccharide chain is essential for the DNA interaction of the anticancer drug mithramycin (MTM) with DNA,(44, 45) and BVMO MtmOIV, which catalyzes the second last step in MTM biosynthesis, seems to be optimized to exclusively process PMB, i.e. a molecule already fully glycosylated with the 5 sugars found in the final mithramycin (MTM) molecule. The three above mentioned trisaccharide stabilizing residues were not identified in the previous PMB-MtmOIV binding site model, but are likely critical for our future attempts to further modify MTM’s trisaccharide side chain. Preliminary SAR studies showed that modifications of this trisaccharide chain can significantly change the biological activity of mithramycins,(46, 47) and it was shown that changing the terminal d-mycarose of the trisaccharide chain into d-digitoxose led to a significant reduction of toxicity.(48) A re-engineered MtmOIV will be essential for future approaches to bio-engineer MTM variants with alternate trisaccharide residues. Proline residue (P84) appears to be pivotal to accommodate the trisaccharide chain of the substrate PMB. Interestingly, a P84A mutation increased the catalytic activity of MtmOIV almost 1.5-fold, but seems less effective than the previously generated F89A mutant, which led to a 5-fold activity increase. Combining these successful mutations in a P84A/F89A double mutant failed, since the double mutant only led to a 3-fold increased catalytic activity. In contrast to the longer trisaccharide chain that interacts well inside the PMB binding pocket, the shorter disaccharide chain of PMB seems to establish no particular interaction with that pocket (Figure 4), and may serve as a mechanical block along with the gatekeeper residues W205 and F89 (see below) to allow only a certain degree of penetration of the PMB molecule into its binding pocket.
The new MtmOIV protein structures discussed here also revealed that a tryptophan (W205) and an arginine (R204) residue along with phenylalanine 89 (F89) play key roles to guard the entry loop for the substrate binding process. Comparing the substrate free and the substrate bound structures on MtmOIV showed the largest shifts for W205 (1.6 Å) and F89 (0.9 Å), which sit on opposite sides at the entrance to the substrate binding pocket. To confirm our hypothesis regarding the W205 residue as key gatekeeper, we prepared the W205A mutant, which turned out to be the only mutant that negatively affects substrate binding, while the previously published F89A and R204A mutants had only marginal effects on the substrate binding and slightly increased catalytic activity. In contrast, the W205A mutation decreased the catalytic activity about 30-fold compared to the wild type enzyme (see Table 2), corroborating W205’s role as key gatekeeper which needs to stay intact to correctly place the PMB molecule. The new structures also revealed that a histidine (H50) and an aspartate (D51) residue seem to be important to fine-tune the positioning of the FAD co-factor. Modifying these could affect the catalytic activity of the enzyme. Indeed, in the H50A and D51A mutants an increased catalytic activity of 1.5 and 3-fold, respectively, was observed.
Based on a low resolution complex structure of MtmOIV in complex with NADPH we identified four arginine residues (R169, R173, R174, R277) potentially important for binding NADPH. These residues are quite distant from the substrate binding site and mutants were made to confirm the putative NADPH binding site. The mutants showed a clearly diminished catalytic activity of 10% ~ 50% relative to the wild type enzyme in their ability to reduce NADPH, supportive of the hypothesis that these residues contribute to the NADPH binding. Future ligand binding studies to completely delineate this enzyme’s complex mechanism may shed greater light on the role each NADPH binding arginine plays, especially if product inhibition plays a role and becomes the rate limiting step of catalysis.
In summary, the high resolution structures of MtmOIV revealed new details of this important bottle-neck enzyme of the MTM biosynthesis pathway and the subsequent structural mutations pointed out possible strategies for the broadening of its substrate specificity and enhancement of its catalytic activity.
METHODS
Protein Expression, Purification, and Crystallization
The cloning and overexpression of MtmOIV was performed basically as previously reported (49) except that we chose here to remove the N-terminal His6-tag leading to better crystallization conditions. Briefly, the mtmOIV coding sequence (1.5 kb) was amplified and subcloned into pET28a(+) and expressed with an N-terminal His6 fusion tag for purification and removed used a Thrombin Cleavage Capture Kit (Novagen) prior to crystallization. This construct was then transformed into BL21(DE3) cells. A 1L culture LB medium (Lysogeny Broth, Fisher) supplemented with 0.1 mM kanamycin was grown at 37 °C until the OD600 reached 0.5–0.7, and then induced with 1mM IPTG (isopropyl-β-d-1-thiogalactopyranoside) and allowed to grow at 18 °C for an additional 12 h.
For purification, cells were collected by centrifugation, re-suspended in lysis buffer (50mM KH2PO4, 300 mM KCl, 10mM imidazole, pH 8.0), lysed by two passes through a French press and then centrifuged for 45 minutes at 18000 rpm at 4 °C. The supernatant was then passed through a 0.45 μm syringe driven multigrade glassfiber filter (Millipore), applied to a Ni-NTA (nitrilotriacetic acid) column using a Profinia protein purifier (Bio-Rad) and eluted with 250 mM imidazole. Fractions containing MtmOIV were pooled and incubated with biotinylated thrombin (Thrombine Cleavage Capture Kit, Novagen) overnight at 4 °C with gentle rocking to remove the His6 fusion tag. Thrombin was then removed by treatment with streptavidin-linked agarose beads at 4 °C with gentle rocking for 30 min. The beads were separated from the cleaved protein by centrifugation at 500×g for 5 min at room temperature using spin filters (Novagen). Free cleaved His-tags and un-cleaved protein were then separated by passage across a 2mL column of Talon metal affinity resin (Clontech) using lysis buffer. As a final step for crystallization, the sample was concentrated to ~200 μL using a Ultracel 30K filter (Millipore) and further purified by gel filtration across a Superose 6/300 GL Tricorn high performance column (Amersham Biosciences) on a BioLogic DuoFlow HPLC system (Bio-Rad) and crystallization buffer (20mM Tris, pH 7.5, 200mM NaCl). Fractions were collected and concentrated to a final concentration of ~10mg/mL. For the MtmOIV-premithramycin B (PMB) complex structure, PMB (5 mM) was added to the protein solution ~200 μL 1 h prior to concentrating to 10 mg/mL for crystallization. Protein concentrations were determined by Bradford assay (Sigma) for crystallization and kinetic studies.
For crystallization, broad matrix screening was performed using a TTP LabTech Mosquito crystallization robot and lead conditions optimized accordingly. Final conditions for the native MtmOIV crystals consisted of 100 mM Bis-Tris 6.5 and 28% PEG MME (polyethylene glycol monomethyl ether) 2000 while the final conditions for the MtmOIV-PMB co-crystals consisted of 200 mM ammonium acetate and 30% PEG 1000. The best crystals typically grew within 3–5 days at 21 °C and were harvested directly from the crystallization drop, and then were plunged into liquid nitrogen for storage until data collection.
Data Collection and Structure Determination
Lead conditions were initially screened using our in-house x-ray diffractometer (Rigaku MicroMax-007 HF microfocus x-ray generator, Raxis IV++ detector). Final datasets were collected at the SER-CAT (Southeast Regional Collaborative Access Team) beamline at the Advanced Photon Source at Argonne National Laboratory. All data were processed using HKL2000 (50) and statistics are summarized in Table 1. Both the native MtmOIV and the MtmOIV-premithramycin B (PMB) complex structures were solved by molecular replacement using PHASER (51) within CCP4 (Collaborative Computational Project 4).(52) An initial search model was prepared using the previously deposited MtmOIV coordinates (PDB code 3FMW). A difference map clearly indicated the locations of the FAD molecules within the native MtmOIV structure and the locations of FAD and PMB molecules within the MtmOIV-PMB complex structure.
All model building was performed using COOT (Crystallographic Object-Oriented Toolkit),(53) and refinement (including automated water picking followed by visual inspection) was performed using PHENIX (Python-based Hierarchical Environment for Integrated X-ray crystallography) (54) and CCP4.(52) All figures were prepared using PyMOL (Schrödinger), and final editing was done in Adobe Illustrator. Root mean square deviation (RMSD) analysis was performed within PyMOL (Schrödinger).
Modeling the putative NADPH binding site
We were able to grow crystals of MtmOIV in the presence of NADPH. These crystals diffracted to ~3.5 Å and while we were able to observe extra density indicating the presence of NADPH, the density was mostly disordered and incomplete preventing us from modeling the ligand accurately and with confidence. Nevertheless, based on the location of the difference density, we were able to identify four residues at this site that interacted with the putative NADPH molecule, namely R169, R1-3, R1-4, and R277. Mutagenesis of three of these residues proved to have a major impact on catalysis. Further, a disordered loop containing residues 233-239 is located along this putative NADPH binding site and has been suggested to play a role in binding NADPH. This information has allowed us to propose the putative NADPH binding site, which –in comparison with the substrate binding site–sits on the opposite side of the MtmOIV structure, yet still in proximity to the FAD cofactor. To model this, we used the NADP(+)-bound crystal structure of cyclohexanone monooxygenase (PDB code 3GWF) and aligned to MtmOIV along the FAD cofactor, which positioned the NADP(+) molecule in the proximity of the putative NADPH binding pocket. We then added NADPH to our structure at this site and manually optimized the fit to agree with the observable density in our low resolution NADPH-bound structure. This was used as a starting point for docking studies to optimize the NADPH position within the binding pocket. Here, we used AutoDock 4.2 (55-57) and the AutoDockTools (56, 58-60) to perform the docking analysis using the FAD-bound MtmOIV crystal structure as the receptor molecule and NADPH as the ligand molecule. Based on the low resolution co-crystal structure with NADPH, the putative NADPH binding loop was added to the receptor molecule prior to the analysis. A grid was calculated about the placed ligand and an autodocking procedure was performed using the Local Search algorithm. The docking analysis produced coordinates for the top fifty solutions which were further sorted based on lowest energy, with the top solution, having the lowest energy of −2.8 kcal/mol, being used for subsequent analysis.
Mutagenesis and kinetics assays
MtmOIV mutants were prepared using an Agilent Quikchange site-directed mutagenesis kit. Primers used for mutagenesis are available upon request. All kinetic assays were repeated in triplicate with no less than two separate purifications of the enzyme. The kinetic assays utilized here with respect to the premithramycin-B substrate have been previously reported.(8) Briefly, we monitored the conversion (100mM Tris-HCl pH 8.25, 30 °C, 10 min) of premithramycin B by MtmOIV in a continuous assay measuring the oxidation of NADPH at 340 nm (ε 340 = 6220 M−1 cm−1) in the presence of FAD and O2 (0.25 mM NADPH/NADH; 0.1 mM FAD added; open cuvette/96 well plate). The reactions were initiated by addition of 0.25, 1, 5, or 10μM wild type or mutant MtmOIV. Kinetic parameters were determined by fitting with Kaleidagraph 4.0 (Synergy).
The kinetic assays utilized with respect to the substrate NADPH were monitored via a fluorescent assay due to the inability to monitor the reaction at high concentrations of NADPH by absorbance because of absorbance values exceeding the detection limits of available spectrophotometers. Fluorescence spectroscopy was used to monitor the oxidation of NADPH in the presence of FAD and O2 (2mM Pre-B; 0.1 mM FAD added; open 96 well plate). This conversion was continuously monitored at an excitation λ=355nm and an emission λ=460nm (100mM Tris-HCl pH 8.25, 30 °C, 10 min). The excitation λ=355nm was used instead of the typical λ=340nm due to the available equipment filter selection. Assays were preformed utilizing the FLOUstar Omega plate reader (BMG LABTECH) and non-treated white optical flat bottom non-sterile 96 well plates (Nunc). The reactions were initiated by addition of 0.25, 1, 5, or 10μM wild type or mutant MtmOIV. To determine the value of NADPH oxidation a standard curve was created utilizing known concentrations of NADPH. Kinetic parameters were determined by fitting with Kaleidagraph 4.0 (Synergy).
ACKNOWLEDGMENTS
This work was supported by NIH grant CA 091901 to JR. NN and SKB were supported by the Intramural Research Program of the NIH, National Institute of Diabetes and Digestive and Kidney Diseases. Data were collected at Southeast Regional Collaborative Access Team (SER-CAT) beamline 22-ID at the Advanced Photon Source, Argonne National Laboratory. Use of the Advanced Photon Source was supported by the U. S. Department of Energy. We also wish to acknowledge S. G. Van Lanen and J. S. Thorson for sharing lab instrumentation with us.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- 1.Leisch H, Morley K, Lau PC. Baeyer-Villiger monooxygenases: more than just green chemistry. Chem. Rev. 2011;111:4165–4222. doi: 10.1021/cr1003437. [DOI] [PubMed] [Google Scholar]
- 2.Walsh CT, Wencewicz TA. Flavoenzymes: versatile catalysts in biosynthetic pathways. Nat. Prod. Rep. 2013;30:175–200. doi: 10.1039/c2np20069d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dover LG, Corsino PE, Daniels IR, Cocklin SL, Tatituri V, Besra GS, Futterer K. Crystal structure of the TetR/CamR family repressor Mycobacterium tuberculosis EthR implicated in ethionamide resistance. J. Mol. Biol. 2004;340:1095–1105. doi: 10.1016/j.jmb.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Koma D, Sakashita Y, Kubota K, Fujii Y, Hasumi F, Chung SY, Kubo M. Degradation pathways of cyclic alkanes in Rhodococcus sp. NDKK48. Appl. Microbiol. Biotechnol. 2004;66:92–99. doi: 10.1007/s00253-004-1623-5. [DOI] [PubMed] [Google Scholar]
- 5.Leisch H, Shi R, Grosse S, Morley K, Bergeron H, Cygler M, Iwaki H, Hasegawa Y, Lau PC. Camphor Pathway 2-Oxo-{Delta}3-4,5,5-trimethylcyclopentenylacetyl-CoA Monooxygenase of Pseudomonas putida ATCC 17453: Cloning, Baeyer-Villiger Biooxidations, and Structures. Appl. Environ. Microbiol. 2012;78:2200–2212. doi: 10.1128/AEM.07694-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Philmus B, Abdelwahed S, Williams HJ, Fenwick MK, Ealick SE, Begley TP. Identification of the product of toxolyase: degradation via Baeyer-Villiger oxidation. J. Am. Chem. Soc. 2012;134:5326–5330. doi: 10.1021/ja211759n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibson M, Nur-e-Alam M, Lipata F, Oliveira MA, Rohr J. Characterization of Kinetics and Products of the Baeyer-Villiger Oxygenase MtmOIV, The Key Enzyme of the Biosynthetic Pathway toward the Natural Product Anticancer Drug Mithramycin from Streptomyces argillaceus. J. Am. Chem. Soc. 2005;127:17594–17595. doi: 10.1021/ja055750t. [DOI] [PubMed] [Google Scholar]
- 8.Beam MP, Bosserman MA, Noinaj N, Wehenkel M, Rohr J. Crystal structure of Baeyer-Villiger monooxygenase MtmOIV, the key enzyme of the mithramycin biosynthetic pathway. Biochemistry. 2009;48:4476–4487. doi: 10.1021/bi8023509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lombó F, Menendez N, Salas JA, Méndez C. The aureolic acid family of antitumor compounds: structure, mode of action, biosynthesis, and novel derivatives. Appl. Microbiol. Biotechnol. 2006;73:1–14. doi: 10.1007/s00253-006-0511-6. [DOI] [PubMed] [Google Scholar]
- 10.Rohr J, Méndez C, Salas JA. The biosynthesis of aureolic acid group antibiotics. Bioorg. Chem. 1999;27:41–54. [Google Scholar]
- 11.Rawlings BJ. Biosynthesis of polyketides (other than actinomycete macrolides) Nat. Prod. Rep. 1999;16:425–484. doi: 10.1039/a900566h. [DOI] [PubMed] [Google Scholar]
- 12.Wang G, Pahari P, Kharel MK, Chen J, Zhu H, Van Lanen SG, Rohr J. Cooperation of Two Bifunctional Enzymes in the Biosynthesis and Attachment of Deoxysugars of the Antitumor Antibiotic Mithramycin. Angew. Chem. Int. Ed. 2012;51:10638–10642. doi: 10.1002/anie.201205414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang G, Kharel MK, Pahari P, Rohr J. Investigating Mithramycin Deoxysugar Biosynthesis: Enzymatic Total Synthesis of TDP-D-Olivose. ChemBioChem. 2011;12:2568–2571. doi: 10.1002/cbic.201100540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Connor SE. Aureolic acids: similar antibiotics with different biosynthetic gene clusters. Chem. Biol. 2004;11:8–10. doi: 10.1016/j.chembiol.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Remsing LL, Gonzalez AM, Nur-e-Alam M, Fernandez-Lozano MJ, Brana AF, Rix U, Oliveira MA, Mendez C, Salas JA, Rohr J. Mithramycin SK, a novel antitumor drug with improved therapeutic index, mithramycin SA, and demycarosyl-mithramycin SK: three new products generated in the mithramycin producer Streptomyces argillaceus through combinatorial biosynthesis. J. Am. Chem. Soc. 2003;125:5745–5753. doi: 10.1021/ja034162h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nehls MC, Brenner DA, Gruss HJ, Dierbach H, Mertelsmann R, Herrmann F. Mithramycin selectively inhibits collagen-alpha 1(I) gene expression in human fibroblast. J. Clin. Invest. 1993;92:2916–2921. doi: 10.1172/JCI116914. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Seznec J, Silkenstedt B, Naumann U. Therapeutic effects of the Sp1 inhibitor mithramycin A in glioblastoma. J. Neurooncol. 2011;101:365–377. doi: 10.1007/s11060-010-0266-x. [DOI] [PubMed] [Google Scholar]
- 18.Sachrajda I, Ratajewski M. Mithramycin A suppresses expression of the human melanoma-associated gene ABCB8. Mol. Genet. Genomics. 2011;285:57–65. doi: 10.1007/s00438-010-0586-8. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy BJ. Mithramycin therapy in advanced testicular neoplasms. Cancer. 1970;26:755–766. doi: 10.1002/1097-0142(197010)26:4<755::aid-cncr2820260403>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 20.Kennedy BJ, Torkelson JL. Long-term follow-up of stage III testicular carcinoma treated with mithramycin (plicamycin) Med. Pediatr. Oncol. 1995;24:327–328. doi: 10.1002/mpo.2950240511. [DOI] [PubMed] [Google Scholar]
- 21.Elias EG, Evans JT. Mithramycin in the treatment of Paget’s disease of bone. J.Bone Joint Surg. 1972;54-A:1730–1736. [PubMed] [Google Scholar]
- 22.Albertini V, Jain A, Vignati S, Napoli S, Rinaldi A, Kwee I, Nur-e-Alam M, Bergant J, Bertoni F, Carbone GM, Rohr J, Catapano CV. Novel GC-rich DNA-binding compound produced by a genetically engineered mutant of the mithramycin producer Streptomyces argillaceus exhibits improved transcriptional repressor activity: implications for cancer therapy. Nucl. Acids Res. 2006;34:1721–1734. doi: 10.1093/nar/gkl063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee TJ, Jung EM, Lee JT, Kim S, Park JW, Choi KS, Kwon TK. Mithramycin A sensitizes cancer cells to TRAIL-mediated apoptosis by down-regulation of XIAP gene promoter through Sp1 sites. Mol. Cancer Ther. 2006;5:2737–2746. doi: 10.1158/1535-7163.MCT-06-0426. [DOI] [PubMed] [Google Scholar]
- 24.Hadjipavlou AG, Gaitanis LN, Katonis PG, Lander P. Paget’s disease of the spine and its management. Eur. Spine J. 2001;10:370–384. doi: 10.1007/s005860100329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiang DT, Loken MK, Kennedy BJ. Mechanism of the hypocalcemic effect of mithramycin. J. Clin. Endocrinol. Metabol. 1979;48:341–344. doi: 10.1210/jcem-48-2-341. [DOI] [PubMed] [Google Scholar]
- 26.Lumachi F, Brunello A, Roma A, Basso U. Cancer-induced hypercalcemia. Anticancer Res. 2009;29:1551–1555. [PubMed] [Google Scholar]
- 27.Perlia CP, Gubisch NJ, Wolter J, Edelberg D, Dederick MM, Taylor SG., 3rd Mithramycin treatment of hypercalcemia. Cancer. 1970;25:389–394. doi: 10.1002/1097-0142(197002)25:2<389::aid-cncr2820250217>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 28.Zojer N, Keck AV, Pecherstorfer M. Comparative tolerability of drug therapies for hypercalcaemia of malignancy. Drug Saf. 1999;21:389–406. doi: 10.2165/00002018-199921050-00004. [DOI] [PubMed] [Google Scholar]
- 29.Ferrante RJ, Ryu H, Kubilus JK, D’Mello S, Sugars KL, Lee J, Lu P, Smith K, Browne S, Beal MF, Kristal BS, Stavrovskaya IG, Hewett S, Rubinsztein DC, Langley B, Ratan RR. Chemotherapy for the Brain: The Antitumor Antibiotic Mithramycin Prolongs Survival in a Mouse Model of Huntington’s Disease. J. Neurosci. 2004;24:10335–10342. doi: 10.1523/JNEUROSCI.2599-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiu Z, Norflus F, Singh B, Swindell MK, Buzescu R, Bejarano M, Chopra R, Zucker B, Benn CL, DiRocco DP, Cha JH, Ferrante RJ, Hersch SM. Sp1 is up-regulated in cellular and transgenic models of Huntington disease, and its reduction is neuroprotective. J. Biol. Chem. 2006;281:16672–16680. doi: 10.1074/jbc.M511648200. [DOI] [PubMed] [Google Scholar]
- 31.Sleiman SF, Langley BC, Basso M, Berlin J, Xia L, Payappilly JB, Kharel MK, Guo H, Marsh JL, Thompson LM, Mahishi L, Ahuja P, MacLellan WR, Geschwind DH, Coppola G, Rohr J, Ratan RR. Mithramycin is a gene-selective Sp1 inhibitor that identifies a biological intersection between cancer and neurodegeneration. J. Neurosci. 2011;31:6858–6870. doi: 10.1523/JNEUROSCI.0710-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grohar PJ, Woldemichael GM, Griffin LB, Mendoza A, Chen QR, Yeung C, Currier DG, Davis S, Khanna C, Khan J, McMahon JB, Helman LJ. Identification of an inhibitor of the EWS-FLI1 oncogenic transcription factor by high-throughput screening. J. Natl. Cancer. Inst. 2011;103:962–978. doi: 10.1093/jnci/djr156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang M, Mathur A, Zhang Y, Xi S, Atay S, Hong JA, Datrice N, Upham T, Kemp CD, Ripley RT, Wiegand G, Avital I, Fetsch P, Mani H, Zlott D, Robey R, Bates SE, Li X, Rao M, Schrump DS. Mithramycin Represses Basal and Cigarette Smoke-Induced Expression of ABCG2 and Inhibits Stem Cell Signaling in Lung and Esophageal Cancer Cells. Cancer Res. 2012;72:4178–4192. doi: 10.1158/0008-5472.CAN-11-3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torres Pazmino DE, Baas B-J, Janssen DB, Fraaije MW. Kinetic Mechanism of Phenylacetone Monooxygenase from Thermobifida fusca. Biochemistry. 2008;47:4082–4093. doi: 10.1021/bi702296k. [DOI] [PubMed] [Google Scholar]
- 35.Beecher J, Grogan G, Roberts S, Willetts A. Enantioselective oxidations by the diketocamphane monooxygenase isozymes from pseudomonas putida. Biotechnology Letters. 1996;18:571–576. [Google Scholar]
- 36.McGhie EJ, Isupov MN, Schroder E, Littlechild JA. Crystallization and preliminary X-ray diffraction studies of the oxygenating subunit of 3,6-diketocamphane monooxygenase from Pseudomonas putida. Acta Crystallogr. D Biol. Crystallogr. 1998;54:1035–1038. doi: 10.1107/s0907444998004946. [DOI] [PubMed] [Google Scholar]
- 37.Pasta P, Carrea G, Gaggero N, Grogan G, Willetts A. Enantioselective oxidations catalyzed by diketocamphane monooxygenase from pseudomonas putida with macromolecular nad in a membrane reactor. Biotech. Lett. 1996;18:1123–1128. [Google Scholar]
- 38.Mirza IA, Yachnin BJ, Wang S, Grosse S, Bergeron H, Imura A, Iwaki H, Hasegawa Y, Lau PC, Berghuis AM. Crystal structures of cyclohexanone monooxygenase reveal complex domain movements and a sliding cofactor. J. Am. Chem. Soc. 2009;131:8848–8854. doi: 10.1021/ja9010578. [DOI] [PubMed] [Google Scholar]
- 39.Yachnin BJ, Sprules T, McEvoy MB, Lau PC, Berghuis AM. The substrate-bound crystal structure of a Baeyer-Villiger monooxygenase exhibits a Criegee-like conformation. J. Am. Chem. Soc. 2012;134:7788–7795. doi: 10.1021/ja211876p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bucko M, Schenkmayerova A, Gemeiner P, Vikartovska A, Mihovilovic MD, Lacik I. Continuous testing system for Baeyer-Villiger biooxidation using recombinant Escherichia coli expressing cyclohexanone monooxygenase encapsulated in polyelectrolyte complex capsules. Enzyme Microb. Technol. 2011;49:284–248. doi: 10.1016/j.enzmictec.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 41.Tibrewal N, Pahari P, Wang G, Kharel MK, Morris C, Downey T, Hou Y, Bugni TS, Rohr J. Baeyer-Villiger C-C bond cleavage reaction in gilvocarcin and jadomycin biosynthesis. J. Am. Chem. Soc. 2012;134:18181–18184. doi: 10.1021/ja3081154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leadbeater C, McIver L, Campopiano DJ, Webster SP, Baxter RL, Kelly SM, Price NC, Lysek DA, Noble MA, Chapman SK, Munro AW. Probing the NADPH-binding site of Escherichia coli flavodoxin oxidoreductase. Biochem. J. 2000;352(Pt 2):257–266. [PMC free article] [PubMed] [Google Scholar]
- 43.Koskiniemi H, Metsä-Ketelä M, Dobritzsch D, Kallio P, Korhonen H, Mantsälä P, Schneider G, Niemi J. Crystal structures of two aromatic hydroxylases involved in the early tailoring steps of angucycline biosynthesis. J.Mol. Biol. 2007;372:633–648. doi: 10.1016/j.jmb.2007.06.087. [DOI] [PubMed] [Google Scholar]
- 44.Sastry M, Fiala R, Patel DJ. Solution structure of mithramycin dimers bound to partially overlapping sites on DNA. J. Mol. Biol. 1995;251:674–689. doi: 10.1006/jmbi.1995.0464. [DOI] [PubMed] [Google Scholar]
- 45.Sastry M, Patel DJ. Solution structure of the mithramycin dimer-DNA complex. Biochemistry. 1993;32:6588–6604. doi: 10.1021/bi00077a012. [DOI] [PubMed] [Google Scholar]
- 46.Baig I, Pérez M, Braña AF, Gomathinayagam R, Damodaran C, Salas JA, Méndez C, Rohr J. Mithramycin analogues generated by combinatorial biosynthesis show improved bioactivity. J. Nat. Prod. 2008;71:199–207. doi: 10.1021/np0705763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pérez M, Baig I, Braña AF, Salas JA, Rohr J, Méndez C. Generation of new derivatives of the antitumor antibiotic mithramycin by altering the glycosylation pattern through combinatorial biosynthesis. ChemBioChem. 2008;9:2295–2304. doi: 10.1002/cbic.200800299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nuñez LE, Nybo SE, Gonzalez-Sabin J, Pérez M, Ménendez N, Braña AF, He M, Morís F, Salas JA, Rohr J, Méndez C. A Novel Mithramycin Analogue with High Antitumor Activity and Less Toxicity Generated by Combinatorial Biosynthesis. J. Med. Chem. 2012;55:5813–5825. doi: 10.1021/jm300234t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang C, Gibson M, Rohr J, Oliveira MA. Crystallization and X-ray diffraction properties of Baeyer-Villiger monooxygenase MtmOIV from the mithramycin biosynthetic pathway in Streptomyces argillaceus. Acta Cryst. 2005;F61:1023–1026. doi: 10.1107/S1744309105033221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Otwinowski Z, Minor W. Processing of X-Ray Diffraction Data Collected in Oscillation Mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 51.McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. Phaser crystallographic software. J. Appl. Cryst. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Winn MD, Ballard CC, Cowtan KD, Dodson EJ, Emsley P, Evans PR, Keegan RM, Krissinel EB, Leslie AG, McCoy A, McNicholas SJ, Murshudov GN, Pannu NS, Potterton EA, Powell HR, Read RJ, Vagin A, Wilson KS. Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 2011;67:235–242. doi: 10.1107/S0907444910045749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Cryst. 2004;D60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 54.Adams PD, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, Moriarty NW, Read RJ, Sacchettini JC, Sauter NK, Terwilliger TC. PHENIX: building new software for automated crystallographic structure determination. Acta Crystr. 2002;D58:1948–1954. doi: 10.1107/s0907444902016657. [DOI] [PubMed] [Google Scholar]
- 55.Goodsell DS, Morris GM, Olson AJ. Automated docking of flexible ligands: applications of AutoDock. J. Mol. Recogn. 1996;9:1–5. doi: 10.1002/(sici)1099-1352(199601)9:1<1::aid-jmr241>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 56.Goodsell DS. Computational docking of biomolecular complexes with AutoDock. Cold Spring Harb. Protoc. 2009. 2009 doi: 10.1101/pdb.prot5200. pdb prot5200. [DOI] [PubMed] [Google Scholar]
- 57.Norgan AP, Coffman PK, Kocher JP, Katzmann DJ, Sosa CP. Multilevel Parallelization of AutoDock 4.2. J. Cheminform. 2011;3:12. doi: 10.1186/1758-2946-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baba N, Akaho E. VSDK: Virtual screening of small molecules using AutoDock Vina on Windows platform. Bioinformation. 2011;6:387–388. doi: 10.6026/97320630006387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cosconati S, Forli S, Perryman AL, Harris R, Goodsell DS, Olson AJ. Virtual Screening with AutoDock: Theory and Practice. Expert Opin. Drug Discov. 2010;5:597–607. doi: 10.1517/17460441.2010.484460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vistoli G, Pedretti A, Mazzolari A, Testa B. Homology modeling and metabolism prediction of human carboxylesterase-2 using docking analyses by GriDock: a parallelized tool based on AutoDock 4.0. J. Comput. Aided Mol. Des. 2010;24:771–787. doi: 10.1007/s10822-010-9373-1. [DOI] [PubMed] [Google Scholar]