Abstract
Telomerase reverse transcriptase (TERT) promoter mutations were recently shown to drive telomerase activity in various cancer types, including medulloblastoma. However, the clinical and biological implications of TERT mutations in medulloblastoma have not been described. Hence, we sought to describe these mutations and their impact in a subgroup-specific manner. We analyzed the TERT promoter by direct sequencing and genotyping in 466 medulloblastomas. The mutational distributions were determined according to subgroup affiliation, demographics, and clinical, prognostic, and molecular features. Integrated genomics approaches were used to identify specific somatic copy number alterations in TERT promoter-mutated and wild-type tumors. Overall, TERT promoter mutations were identified in 21 % of medulloblastomas. Strikingly, the highest frequencies of TERT mutations were observed in SHH (83 %; 55/66) and WNT (31 %; 4/13) medulloblastomas derived from adult patients. Group 3 and Group 4 harbored this alteration in <5 % of cases and showed no association with increased patient age. The prognostic implications of these mutations were highly subgroup-specific. TERT mutations identified a subset with good and poor prognosis in SHH and Group 4 tumors, respectively. Monosomy 6 was mostly restricted to WNT tumors without TERT mutations. Hallmark SHH focal copy number aberrations and chromosome 10q deletion were mutually exclusive with TERT mutations within SHH tumors. TERT promoter mutations are the most common recurrent somatic point mutation in medulloblastoma, and are very highly enriched in adult SHH and WNT tumors. TERT mutations define a subset of SHH medulloblastoma with distinct demographics, cytogenetics, and outcomes.
Electronic supplementary material
The online version of this article (doi:10.1007/s00401-013-1198-2) contains supplementary material, which is available to authorized users.
Keywords: TERT promoter mutations, SHH pathway, Adult, Medulloblastoma
Introduction
Medulloblastoma is a highly malignant embryonal brain tumor located in the posterior fossa [6, 29, 33, 35]. While this tumor comprises the most common malignant brain tumor in children, it only accounts for approximately 1 % of primary CNS tumors in adults [18, 20]. The current consensus recognizes four core molecular subgroups (WNT, SHH, Group 3, and Group 4) with distinct molecular, demographic, clinicopathological, and prognostic characteristics [5, 15, 16, 26, 27, 37, 38, 41, 42]. The defining features of medulloblastoma subgroups differ dramatically according to age at diagnosis [15, 27, 41]. Specifically, Group 3 tumors are largely confined to non-adults, SHH tumors are most frequent in infants and adults, while WNT and Group 4 medulloblastomas are mostly observed in pediatric cohorts [15, 24, 27, 38, 41]. Particularly within SHH tumors, age-associated heterogeneity was observed regarding the transcriptional characteristics, somatic copy number alterations (SCNA), and the prognostic implications of biomarkers [15, 18, 38, 40]. Delineation of tumorigenic features characteristic for these age-related differences, particularly within SHH tumors, are highly desirable to understand these clear biological and prognostic discrepancies.
Telomere maintenance is fundamentally important to normal self-renewing stem cells and cancer cells [3, 7, 9, 14, 22]. It has been suggested that tumors derived from cell populations with low self-renewal capacity generally rely on alterations that restore telomerase activity, while epigenetic mechanisms maintain telomerase activity in tumor types derived from self-renewing stem cells [13]. The identification of recurrent telomerase reverse transcriptase (TERT) promoter mutations in 21 % of 91 medulloblastomas [13] is intriguing, since other mechanisms converging on increased telomerase activity including alternative lengthening of telomeres (ALT) [8] or mutations affecting the ATRX/DAXX complex are excessively uncommon in medulloblastoma [12, 25, 32, 34, 39]. Although TERT mutations have been reported in several cancers [2, 10, 11, 13, 19, 43], their putative association with distinct biological behavior and clinical or even prognostic characteristics has not been comprehensively studied. The initial analyses of TERT mutations in medulloblastoma [12] mainly catalogued the mutational frequency rather than correlating the molecular and clinical features of these mutations in a subgroup-specific manner.
In this study, we analyzed a representative set of 466 medulloblastomas for TERT promoter mutations. Subsequently, we correlated the mutational distribution with clinicopathological features, outcome, and molecular characteristics in a subgroup-specific manner. We demonstrate that TERT promoter mutations comprise the most recurrent mutation in adult SHH tumors identified to date and potentially define distinct prognostic subgroups in SHH and Group 4 medulloblastoma patients.
Materials and methods
Tumor material and patient characteristics
All tissues and clinicopathological information were serially collected in accordance with institutional review boards from various contributing centers to this study. Nucleic acid extractions were carried out as previously described [28]. The clinicopathological characteristics of the investigated patient cohort are outlined in Table 1. The median follow-up was 44.06 months (range 0.7–301.5 months).
Table 1.
Clinicopathological and molecular characteristics according to TERT mutational status
| Characteristic | TERT MUT | TERT WT | p value |
|---|---|---|---|
| Age (years) | |||
| Median | 22.00 | 7.08 | <0.0001 # |
| Range | 0.66–49.00 | 0.24–56.32 | |
| NA | 1 | 0 | |
| Gender | |||
| Male | 56 | 236 | 0.47Φ |
| Female | 37 | 129 | |
| NA | 3 | 5 | |
| Histology | |||
| MBEN | 3 | 8 | 0.59χ |
| Desmoplastic | 10 | 59 | |
| Classic | 46 | 217 | |
| LC/A | 11 | 38 | |
| NA | 26 | 48 | |
| M-stage | |||
| M0 | 58 | 240 | 0.03 Φ |
| M1-3 | 12 | 103 | |
| NA | 26 | 27 | |
| TP53 status | |||
| MUT | 4 | 12 | 0.78Φ |
| WT | 42 | 97 | |
| NA | 50 | 261 | |
| Subgroup | |||
| WNT | 6 | 47 | <0.0001 χ |
| SHH | 80 | 133 | |
| Group 3 | 2 | 48 | |
| Group 4 | 8 | 142 | |
F female, LC/A large cell/anaplastic, M male, MB medulloblastomal, MBEN medulloblastoma with extensive nodularity, NA not available (data were excluded from statistical comparison)
Bold values indicate p < 0.05
#Mann–Whitney U test
ΦFisher’s exact test
χChi-square test
Gene expression and copy number analysis
Subgroup affiliation was determined using nanoString limited gene expression profiling as previously described [31]. Somatic copy number alterations were assessed on the Affymetrix Single Nucleotide Polymorphism (SNP) 6.0 array platform in 418 of 466 cases to identify SCNAs specific for TERT mutant and wild-type tumors. Raw copy number estimates were obtained in dChip, followed by CBS segmentation in R as previously described [30]. Somatic copy number alterations were identified using GISTIC2 [21]. TERT expression levels were compared using R2 (www.r2.amc.nl). Differences in expression were tested using one-way ANOVA.
Sanger sequencing
Isolated DNA (25 ng) from all 466 tumors and 7 matched germline samples (25 ng) was amplified by PCR. PCRs contained 1 μl DNA template, 10 μM forward (5′-CAG GGC ACG CAC ACC AG-3′) and reverse (5′-GTC CTG CCC CTT CAC CTT C-3′) TERT-specific primers, and 12.5 μl HotStar Taq Plus Master Mix (Qiagen, Gaithersburg, Maryland, USA) in a 25 μl total reaction volume. Cycle parameters comprised 95 °C × 15 min; 28 cycles of 98 °C × 40 s, 65 °C × 30 s, 72 °C × 1 min; 72 °C × 10 min. PCRs were carried out using the C1000 Thermal Cycler (BioRad, Hercules, CA, USA). PCR products were purified with the PureLink PCR Micro kit (Life Technologies, Burlington, ON, Canada). In all experiments, controls were included in the absence of DNA to rule out contamination by PCR products. Templates for Sanger sequencing were analyzed with forward (5′-CAG CGC TGC CTG AAA CTC-3′) and reverse (5′-GTC CTG CCC CTT CAC CTT C-3′) sequencing primers using dGTP BigDye Terminator v3.0 Cycle Sequencing Ready Reaction Kit (Life Technologies), and 5 % DMSO on the ABI3730XL capillary genetic analyzer (Life Technologies).
Genotyping assay
Two primers (forward primer, 5′-CAG CGC TGC CTG AAA CTC-3′; reverse primer, 5′-GTC CTG CCC CTT CAC CTT C-3′) were designed to amplify a 163-bp product encompassing C228T and C250T hotspot mutations in the TERT promoter—corresponding to the positions 124 and 146 bp, respectively, upstream of the ATG start site. Two fluorogenic LNA probes were designed with different fluorescent dyes to allow single-tube genotyping. One probe was targeted to the WT sequence (TERT WT, 5′-5HEX-CCC CTC CCG G-3IABkFQ-3′), and one was targeted to either of the two mutations (TERT mut, 5′-56FAM-CCC CTT CCG G-3IABkFQ). Primer and probes were custom designed by Integrated DNA Technologies (Coralville, Iowa, USA) using internal SNP design software, and sequence homogeneity was confirmed by comparison to all available sequences on the GenBank database using BLAST (http://www.ncbi.nlm.nih.gov/BLAST/). Primers were optimized to avoid for hairpins and homo- and heterodimers. Primers and probes were obtained from Integrated DNA Technologies.
Real-time PCR was performed in 25 μl reaction mixtures containing 12.5 μl of TaqMan Universal Master Mix II with UNG (Applied Biosystems), 900 nM concentrations of each primer, 250 nM TERT WT probe, 250 nM TERT MUT probe, and 1 μl (25 ng) of sample DNA. Thermocycling was performed on the StepOnePlus (Applied Biosystems) and consisted of 2 min at 50 °C, 10 min at 95 °C, and 40 cycles of 95 °C for 15 s and 60 °C for 1 min.
Analysis was performed using StepOne Software, version 2.1. Samples were considered mutant if they had CT values of ≤39 cycles. Each sample was verified visually by examining the PCR curves generated to eliminate false positives due to aberrant light emission. End-point allelic discrimination genotyping was performed by visually inspecting a plot of the fluorescence from the WT probe versus the MUT probe generated from the post-PCR fluorescence read.
Statistical analysis
Survival time according to TERT mutational status was assessed using the Kaplan–Meier estimate and a log-rank test. Comparisons of binary and categorical patient characteristics between subgroups and cohorts were performed using the two-sided Fisher’s exact test or Chi-squared test. Continuous variables were analyzed using the Mann–Whitney U test. p values <0.05 were considered statistically significant. Multivariate Cox proportional hazards regression was used to adjust for additional covariates using the survival R package (v.2.36). All other statistical analyses were performed using StataSE 12 (Stata Corp. College Station, TX, USA) and Graphpad Prism 5 (La Jolla, CA, USA).
Results
Characteristics of TERT-mutated medulloblastomas
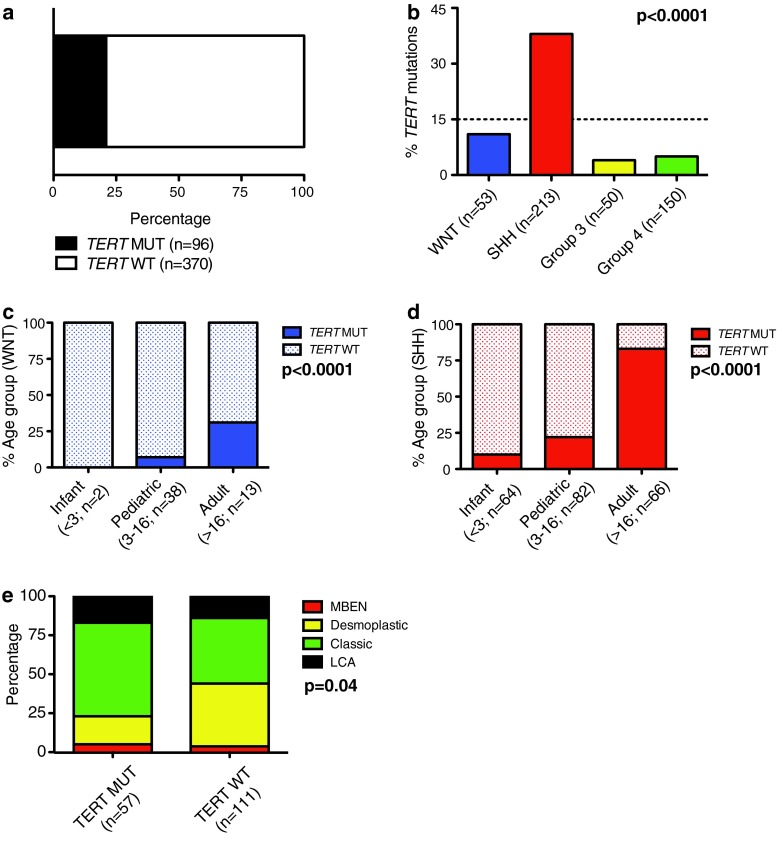
We performed Sanger sequencing on a clinically well-annotated medulloblastoma cohort (n = 466), reflecting the spectrum of demographics and histological subtypes of the disease (Table 1; Supplementary Figure 1A). Our results were verified using a Taqman-based genotyping assay that detects both of the most highly recurrent TERT promoter mutations (C228T and C250T). Since both mutational hotspots are located in highly homologous sequences, C228T and C250T mutations result in an identical binding sequence for the mutation-specific probe (CCCGGAAGGGG; Supplementary Figure 1B). A total of 21 % of medulloblastomas harbored TERT mutations (Fig. 1a). In line with a previous report, these mutations were enriched in older patients (Table 1; p < 0.0001), all mutations were heterozygous, and none of the available matched germline controls displayed this mutation [13]. Interestingly, we found that TERT-mutated medulloblastomas present less frequently with metastatic dissemination at diagnosis compared to TERT wild-type tumors (p = 0.03).
Fig. 1.
TERT promoter-mutated medulloblastomas display distinct demographics, histology, and subgroup affiliation. a Bar graph indicating the frequency of TERT mutations in 466 primary medulloblastomas. b Prevalence of TERT mutations according to medulloblastoma subgroups, and within, c WNT and d SHH subgroups according to age groups. Distribution of histological variants within SHH tumors according to TERT mutational status (e). MUT mutation, OS overall survival, WT wild-type
TERT mutations are specifically enriched in SHH medulloblastomas
In a subgroup-specific analysis, we revealed that TERT mutations were significantly enriched in SHH tumors (80/213; 38 %; p < 0.0001) compared to WNT (6/53; 11 %) and Group 3 (2/50; 4 %) or Group 4 tumors (8/150; 5 %). TERT mutations in both WNT and SHH medulloblastomas were positively correlated with age. TERT mutations were significantly enriched in adult patients (Fig. 1c, d, both p < 0.0001). Increasing age was not associated with increased mutational frequency in either Group 3 or Group 4 tumors (n.s.). While histopathological features were similar between TERT-mutated and wild-type tumors across subgroups, we observed that classic histology was more commonly observed in TERT mutant SHH tumors, and desmoplastic histology in wild-type SHH tumors (Fig. 1e; Table 2; p = 0.04), respectively.
Table 2.
Clinicopathological and molecular characteristics of SHH medulloblastoma according to TERT mutational status
| Characteristic | TERT MUT | TERT WT | p value |
|---|---|---|---|
| Age (years) | |||
| Median | 25.00 | 3.00 | <0.0001 # |
| Range | 0.66–49.00 | 0.24–52.00 | |
| NA | 1 | 0 | |
| Gender | |||
| Male | 46 | 80 | 0.77Φ |
| Female | 31 | 48 | |
| NA | 3 | 5 | |
| Histology | |||
| MBEN | 3 | 4 | 0.04 χ |
| Desmoplastic | 10 | 44 | |
| Classic | 34 | 47 | |
| LC/A | 10 | 16 | |
| NA | 23 | 22 | |
| M-stage | |||
| M0 | 46 | 87 | 0.84Φ |
| M1-3 | 10 | 22 | |
| NA | 24 | 24 | |
| TP53 status | |||
| MUT | 4 | 8 | 1Φ |
| WT | 38 | 71 | |
| NA | 38 | 54 | |
F female, LC/A large cell/anaplastic, M male, MB medulloblastoma, MBEN medulloblastoma with extensive nodularity, NA not available (data were excluded from statistical comparison)
Bold values indicate p < 0.05
#Mann–Whitney U test
ΦFisher’s exact test
χChi-square test
Prognostic implications of TERT mutations
When medulloblastoma patients across all subgroups were stratified by TERT mutational status, we observed no significant differences in survival (Fig. 2a; p = 0.45). Further after normalizing the subgroup composition to reported subgroup ratios, a statistical difference was still not revealed (data not shown; p = 0.36) [1, 15, 26, 41]. However, when TERT mutational status is re-analyzed in a subgroup-specific manner, several important survival associations are observed. TERT mutations had no prognostic impact within WNT tumors (Fig. 2b; p = 0.17). However, a significant association between TERT promoter mutations and outcomes was noted in SHH and Group 4 medulloblastomas. Specifically, the 5-year overall survival of SHH tumors with and without TERT mutations was 77.6 ± 7 % and 64.1 ± 5.1 %, respectively (Fig. 2c; p = 0.04). In contrast to the improved prognosis of TERT mutant SHH tumors, we observed the inverse pattern in Group 4 tumors where the 5-year overall survival for patients without and with TERT mutations was 73.3 % ± 4.3 % and 62.5 % ± 17.1 % (Fig. 2d; p = 0.04). Similar to the unfavorable prognosis of TERT mutations in Group 4 tumors, both of the patients with TERT-mutated Group 3 tumors died after 7 and 45 months of follow-up, respectively (Supplementary Table 1). Thus, we conclude that TERT mutations define distinct prognostic patient cohorts in a subgroup-specific fashion with good prognosis in SHH and poor prognosis in Group 4 medulloblastomas.
Fig. 2.
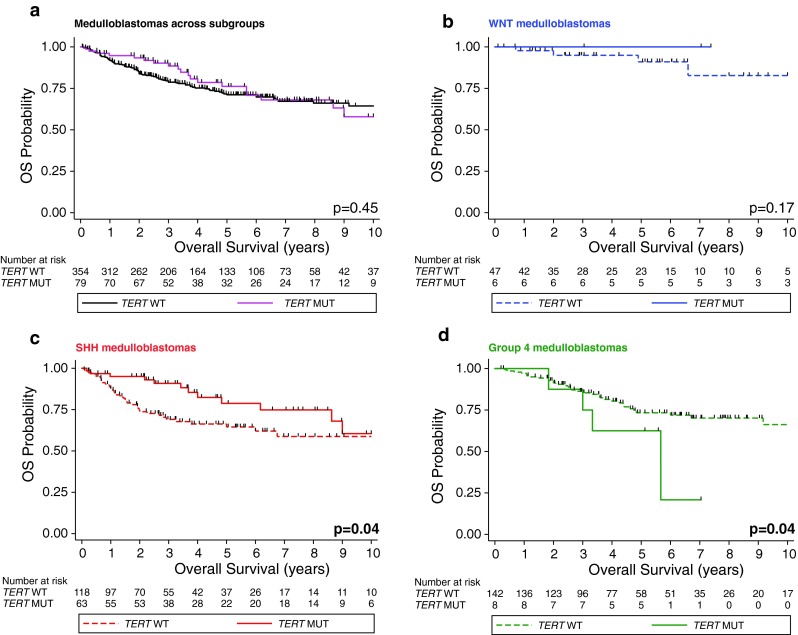
Prognostic impact of TERT promoter mutations varies according to medulloblastoma subgroups. Kaplan–Meier estimate displaying overall survival (OS) according to TERT mutational status in primary medulloblastomas (a), within WNT (b), SHH (c), and Group 4 (d) subgroups. Survival differences were calculated using continuous log-rank tests. MUT mutation, OS overall survival, WT wild-type
Survival analysis restricted to specific age groups
As TERT mutations are predominantly observed in non-infant medulloblastomas, we evaluated the prognostic implications of these promoter mutations across all four medulloblastoma subgroups in an age-dependent manner. TERT mutational status across subgroups had no prognostic impact among patients older than 3 years of age at diagnosis (Fig. 3a; p = 0.59). Interestingly, the prognostic impact of TERT mutation was more pronounced in the non-infant SHH population with a 5-year overall survival of 76.9 % ± 7.6 % and 59.3 % ± 6.9 % of non-infants with and without TERT promoter mutations, respectively (Fig. 3b; p = 0.019). These prognostic implications were similar in adult medulloblastoma patients and in the adult SHH subgroup (Supplementary Figure 2). In a subset of 76 SHH cases with known TP53 mutational status [44], we revealed that TP53 mutations identify non-infant SHH tumors with a particularly poor prognosis, while in contrast TERT mutations identify a subsets with good prognosis (Fig. 3c; p = 0.047). Mutations of both TERT and TP53 were observed in 4/12 SHH tumors (Supplementary Table 2). Non-infant Group 4 showed an inverse prognostic association with poor outcome of TERT-mutated cases (Fig. 3d; p = 0.024). Lastly, we analyzed the overall survival of SHH patients under a multivariate Cox proportional hazards model comprising age at diagnosis, TERT mutational status, M-stage, and histology. In addition to the known prognostic significance of M-stage (p < 0.001) and histology (p = 0.02), we revealed that TERT status continued to be associated with good prognosis (HR 0.17, CI 0.04–0.69, p = 0.01), independent of other prognostic factors including age at diagnosis (p = 0.35).
Fig. 3.
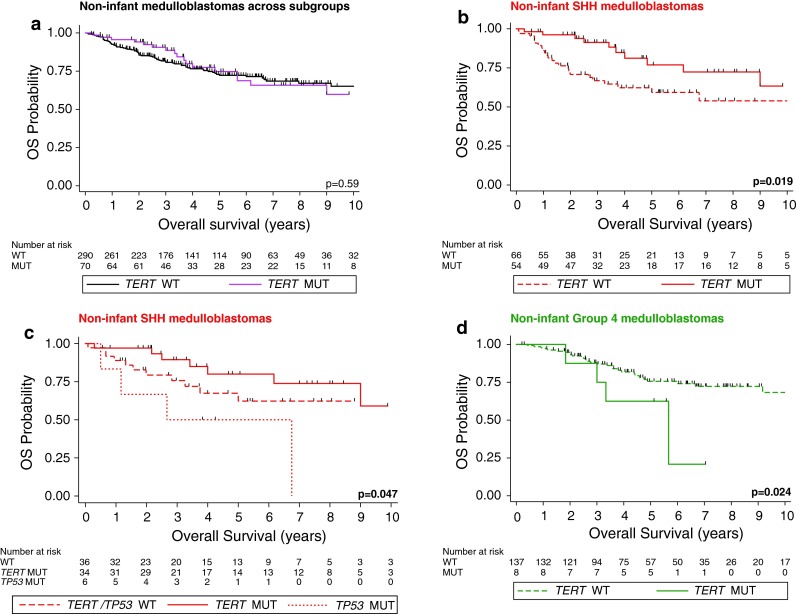
TERT promoter mutations delineate prognostic subsets within non-infant SHH and Group 4 medulloblastomas. Kaplan–Meier estimate displaying overall survival (OS) in non-infant medulloblastomas (>3 years of age at diagnosis) according to TERT mutational status across subgroups (a), in SHH tumors (b), in SHH tumors (TP53 mutated/wild-type) (c), and Group 4 (d). Survival differences were calculated using continuous log-rank tests
Distinct somatic copy number alterations of TERT-mutated medulloblastomas
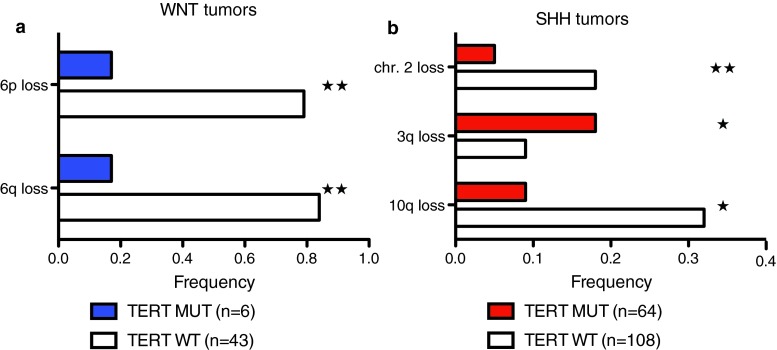
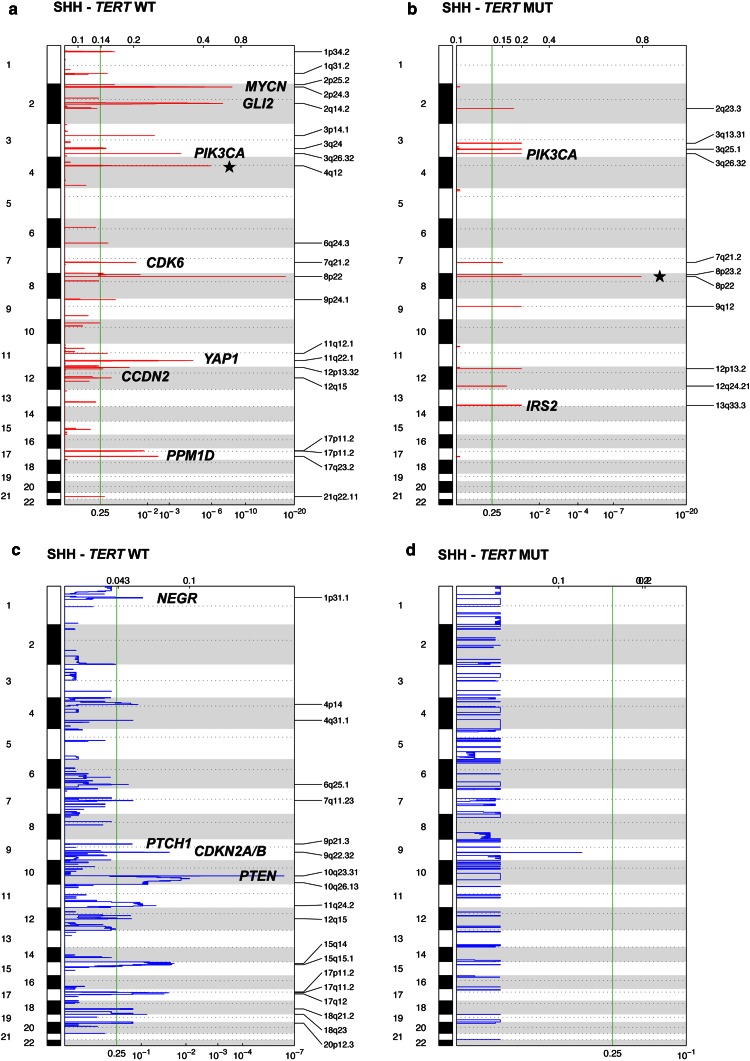
To identify additional genetic features associated with these distinct demographic and clinical differences, we evaluated broad and focal copy number alterations according to subgroup affiliation and TERT promoter mutations. Notably, only 1/6 (17 %) of TERT-mutated WNT tumors harbored monosomy 6, while this alteration is observed in approximately 80 % of TERT wild-type medulloblastomas of the WNT subgroup (Fig. 4a; p = 0.005). Loss of chromosome 2 and 10q loss were significantly enriched in TERT wild-type SHH tumors, while 3q loss was more frequently observed in their TERT mutant counterparts (Fig. 4b). Previously described focal alterations characteristic for SHH tumors including amplification of MYCN/GLI2/CDK6/YAP1/PPM1D, and deletions targeting PTCH1/CDKN2A/CDKN2B/PTEN were largely confined to TERT wild-type SHH medulloblastomas, while TERT mutant SHH (Fig. 5) and Group 4 (Supplementary Figure 3) showed very few focal SCNAs. Consistent with the higher frequency of TERT mutations in SHH tumors, we observed increased TERT expression in the SHH subgroup compared to Group 4 tumors in two independent gene expression profiling studies (p < 0.001; Supplementary Figure 4). Furthermore, we observed TERT amplification in two tumors included in the entire cohort of 1,088 previously studied tumors [30]. Both of these cases with TERT amplification were SHH-driven medulloblastomas with wild-type TERT status, which were derived from pediatric patients who were both alive after 15 and 83 months of follow-up (Supplementary Figure 5). Thus, broad and focal SCNAs underline that TERT mutations define a genetically distinct subset within SHH tumors and possibly within the WNT and Group 4 tumors.
Fig. 4.
WNT and SHH medulloblastoma harbor distinct broad genomic imbalances depending on the mutational status of TERT. Bar graphs indicating the frequency of broad cytogenetic alterations in WNT (a), and SHH (b) tumors. ★★ p < 0.01; ★ p < 0.05; MUT mutation, WT wild-type
Fig. 5.
Focal somatic copy number alterations are largely confined to TERT wild-type SHH medulloblastomas. GISTIC2 analysis indicating focal amplifications/deletions in 108 wild-type (a, c) and 64 mutant (b, d) SHH tumors, respectively. Star regions enriched for reported DNA copy number variations
Discussion
The underlying biology of adult medulloblastomas remains poorly understood. Next-generation sequencing studies have revealed a broad spectrum of novel, potentially tumorigenic mutations in the recent past, but none of these studies focused on adult medulloblastomas [12, 25, 32, 34, 39]. In addition, the vast majority of these mutations are not recurrent enough to stratify patients into distinct clinical and prognostic subgroups.
In this study, we demonstrate that TERT promoter mutations, initially described in melanoma [10, 11], comprise the most recurrent mutation described so far across medulloblastoma subgroups, with a particular enrichment in older patient cohorts. These somatic mutations are especially common in older patients with SHH tumors (83 %) and to a lesser extent in adults with WNT medulloblastomas (11 %). Based on the transcriptional heterogeneity of SHH tumors in infant and adult patients, we suspect that the adult cluster mainly comprised TERT-mutated medulloblastomas [24]. According to the initial classification of tumor types with TERT mutations at frequencies over 15 % (TERT-high) vs. below this threshold (TERT-low) [13], our report suggests distinct baseline telomerase activity of the cell of origin in each of the subgroups (Group 3 ≥ Group 4 > WNT >> SHH). Furthermore, the identification of recurrent TERT promoter mutations makes a compelling argument that the increasing availability of whole-genome sequencing results may substantially add to a refined understanding of the mutational landscape of different biological and age-driven medulloblastoma subgroups, since earlier next-generation sequencing studies focusing on the protein-coding regions had not encompassed gene-regulatory regions including promoter mutations.
In this study, we demonstrate that the mutational status of the TERT promoter can segregate individuals with SHH and Group 4 medulloblastomas with distinct prognostic outcomes, while a prognostic impact of this mutation was not observed in glioblastomas [23]. Molecular mechanisms converging on TERT up-regulation were recently reported to be associated with dismal prognosis in pediatric brain cancers [4]. Our findings in Group 4 tumors with TERT mutations follow this pattern, while SHH tumors with TERT mutations comprise a prognostically favorable subgroup. Notably, survival curves of SHH tumors increasingly approximate with extended follow-up. We hypothesize that this pattern might be due to secondary malignancies and late relapses in older SHH tumors [36–38]. Since virtually all of the TERT promoter mutations encompass the mutational hotspots C228T and C250T, patient stratification can be carried out using a single PCR followed up with Sanger sequencing or with a single experiment using our newly designed Taqman-based genotyping assay. The latter assay is particularly suitable for routine clinical applications as it is highly sensitive and specific (5 ng DNA input is sufficient). Furthermore, our Taqman-based genotyping assay can be used on DNA derived from fresh-frozen and formalin-fixed paraffin-embedded tissue, since it only amplifies a short DNA fragment.
Both hotspot mutations C228T and C250T create an E-twenty-six (ETS) binding motif [10, 11] resulting in up-regulation of TERT expression at the mRNA level [2], which was not observed at the protein level in glioblastomas [43]. We now demonstrate that SHH tumors with TERT mutations are mostly mutually exclusive with those harboring 10q loss (p = 0.017) Notably, the relatively favorable prognosis of TERT-mutated SHH medulloblastomas may be explained by the relative lack of high-risk biomarkers [17, 18, 24, 44].
In summary, we describe the demographic, clinicopathological, and biological implications of TERT promoter mutations in a subgroup-specific fashion. This study underlines the dependence of adult WNT and SHH tumors to reacquire telomerase activity and suggests a potential prognostic utility of TERT mutational analysis in an era of individualized therapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Representative electropherograms (a) and genotyping results (b) of the wild-type and mutated TERT promoter sequence (JPEG 1454 kb)
Overall survival (OS) in adult medulloblastomas (a), and adult SHH-driven tumors (b) according to TERT mutational status. Survival differences were calculated using continuous log-rank tests. Abbreviations: MUT, mutation; WT, wild-type (EPS 650 kb)
Focal somatic copy number alterations are largely confined to TERT wild-type Group 4 tumors. GISTIC2 analysis indicating focal amplifications/deletions in 140 wild-type (a/c) and 7 mutant (b/d) Group 4 tumors, respectively. Legend: ★, regions enriched for reported DNA copy number variations (JPEG 1392 kb)
Transcriptional profiling reveals high TERT expression in SHH medulloblastoma. Boxplots illustrate significantly higher levels of TERT expression in SHH tumors compared to Group 4 medulloblastomas (EPS 869 kb)
Minimally overlapping region including the TERT gene on chromosome arm 5p. DNA copy number gains and losses are indicated by red and blue, respectively (EPS 21771 kb)
Patient characteristics of non-SHH TERT-mutated medulloblastoma (DOCX 153 kb)
Acknowledgments
We thank Susan Archer for technical writing, and Nick Downey from Integrated DNA Technologies for support with probe/primer design. We acknowledge CRB HCL—Neurobiotec tumor bank (Hospices Civils de Lyon, Lyon, France). MDT is supported by a CIHR Clinician Scientist Phase II award, funds from the Garron Family Chair in Childhood Cancer Research at The Hospital for Sick Children and the University of Toronto, and operating funds from the Canadian Institutes of Health Research, the National Institutes of Health (R01CA159859 and R01CA148699) and the Pediatric Brain Tumor Foundation. MR is supported by a fellowship from the Dr. Mildred Scheel Foundation for Cancer Research/German Cancer Aid and funds from the Baden-Wurttemberg Foundation. VR is supported by a CIHR fellowship and an Alberta Innovates-Health Solutions Clinical Fellowship. KZ acknowledges research support from MH CZ-DRO FNBr 65269705. AK was supported by the TAMOP-4.2.2A-11/1/KONV-2012-0025 project and the János Bolyai Scholarship of the Hungarian Academy of Sciences.
Conflict of interest
The authors declare no conflicts of interest.
Contributor Information
Andreas von Deimling, Phone: +49-6221-564651, FAX: +49-6221-564566, Email: andreas.vondeimling@med.uni-heidelberg.de.
Michael D. Taylor, Phone: +416-813-6427, FAX: +416-813-4975, Email: mdtaylor@sickkids.ca
References
- 1.Aaron RH, Elion GB, Colvin OM, Graham M, Keir S, Bigner DD, Friedman HS. Busulfan therapy of central nervous system xenografts in athymic mice. Cancer Chemother Pharmacol. 1994;35(2):127–131. doi: 10.1007/BF00686634. [DOI] [PubMed] [Google Scholar]
- 2.Arita H, Narita Y, Fukushima S, Tateishi K, Matsushita Y, Yoshida A, Miyakita Y, Ohno M, Collins VP, Kawahara N, Shibui S, Ichimura K. Upregulating mutations in the TERT promoter commonly occur in adult malignant gliomas and are strongly associated with total 1p19q loss. Acta Neuropathol. 2013;126(2):267–276. doi: 10.1007/s00401-013-1141-6. [DOI] [PubMed] [Google Scholar]
- 3.Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet. 2005;6(8):611–622. doi: 10.1038/nrg1656. [DOI] [PubMed] [Google Scholar]
- 4.Castelo-Branco P, Choufani S, Mack S, Gallagher D, Zhang C, Lipman T, Zhukova N, Walker EJ, Martin D, Merino D, Wasserman JD, Elizabeth C, Alon N, Zhang L, Hovestadt V, Kool M, Jones DT, Zadeh G, Croul S, Hawkins C, Hitzler J, Wang JC, Baruchel S, Dirks PB, Malkin D, Pfister S, Taylor MD, Weksberg R, Tabori U. Methylation of the TERT promoter and risk stratification of childhood brain tumours: an integrative genomic and molecular study. Lancet Oncol. 2013;14(6):534–542. doi: 10.1016/S1470-2045(13)70110-4. [DOI] [PubMed] [Google Scholar]
- 5.Cho YJ, Tsherniak A, Tamayo P, Santagata S, Ligon A, Greulich H, Berhoukim R, Amani V, Goumnerova L, Eberhart CG, Lau CC, Olson JM, Gilbertson RJ, Gajjar A, Delattre O, Kool M, Ligon K, Meyerson M, Mesirov JP, Pomeroy SL. Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J Clin Oncol. 2011;29(11):1424–1430. doi: 10.1200/JCO.2010.28.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubuc AM, Northcott PA, Mack S, Witt H, Pfister S, Taylor MD. The genetics of pediatric brain tumors. Curr Neurol Neurosci Rep. 2010;10(3):215–223. doi: 10.1007/s11910-010-0103-9. [DOI] [PubMed] [Google Scholar]
- 7.Forsyth NR, Wright WE, Shay JW. Telomerase and differentiation in multicellular organisms: turn it off, turn it on, and turn it off again. Differentiation. 2002;69(4–5):188–197. doi: 10.1046/j.1432-0436.2002.690412.x. [DOI] [PubMed] [Google Scholar]
- 8.Heaphy CM, Subhawong AP, Hong SM, Goggins MG, Montgomery EA, Gabrielson E, Netto GJ, Epstein JI, Lotan TL, Westra WH, Shih Ie M, Iacobuzio-Donahue CA, Maitra A, Li QK, Eberhart CG, Taube JM, Rakheja D, Kurman RJ, Wu TC, Roden RB, Argani P, De Marzo AM, Terracciano L, Torbenson M, Meeker AK. Prevalence of the alternative lengthening of telomeres telomere maintenance mechanism in human cancer subtypes. Am J Pathol. 2011;179(4):1608–1615. doi: 10.1016/j.ajpath.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hiyama E, Hiyama K. Telomere and telomerase in stem cells. Br J Cancer. 2007;96(7):1020–1024. doi: 10.1038/sj.bjc.6603671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horn S, Figl A, Rachakonda PS, Fischer C, Sucker A, Gast A, Kadel S, Moll I, Nagore E, Hemminki K, Schadendorf D, Kumar R. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339(6122):959–961. doi: 10.1126/science.1230062. [DOI] [PubMed] [Google Scholar]
- 11.Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin L, Garraway LA. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339(6122):957–959. doi: 10.1126/science.1229259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones DT, Jager N, Kool M, Zichner T, Hutter B, Sultan M, Cho YJ, Pugh TJ, Hovestadt V, Stutz AM, Rausch T, Warnatz HJ, Ryzhova M, Bender S, Sturm D, Pleier S, Cin H, Pfaff E, Sieber L, Wittmann A, Remke M, Witt H, Hutter S, Tzaridis T, Weischenfeldt J, Raeder B, Avci M, Amstislavskiy V, Zapatka M, Weber UD, Wang Q, Lasitschka B, Bartholomae CC, Schmidt M, von Kalle C, Ast V, Lawerenz C, Eils J, Kabbe R, Benes V, van Sluis P, Koster J, Volckmann R, Shih D, Betts MJ, Russell RB, Coco S, Tonini GP, Schuller U, Hans V, Graf N, Kim YJ, Monoranu C, Roggendorf W, Unterberg A, Herold-Mende C, Milde T, Kulozik AE, von Deimling A, Witt O, Maass E, Rossler J, Ebinger M, Schuhmann MU, Fruhwald MC, Hasselblatt M, Jabado N, Rutkowski S, von Bueren AO, Williamson D, Clifford SC, McCabe MG, Collins VP, Wolf S, Wiemann S, Lehrach H, Brors B, Scheurlen W, Felsberg J, Reifenberger G, Northcott PA, Taylor MD, Meyerson M, Pomeroy SL, Yaspo ML, Korbel JO, Korshunov A, Eils R, Pfister SM, Lichter P. Dissecting the genomic complexity underlying medulloblastoma. Nature. 2012;488(7409):100–105. doi: 10.1038/nature11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Killela PJ, Reitman ZJ, Jiao Y, Bettegowda C, Agrawal N, Diaz LA, Jr, Friedman AH, Friedman H, Gallia GL, Giovanella BC, Grollman AP, He TC, He Y, Hruban RH, Jallo GI, Mandahl N, Meeker AK, Mertens F, Netto GJ, Rasheed BA, Riggins GJ, Rosenquist TA, Schiffman M, Shih Ie M, Theodorescu D, Torbenson MS, Velculescu VE, Wang TL, Wentzensen N, Wood LD, Zhang M, McLendon RE, Bigner DD, Kinzler KW, Vogelstein B, Papadopoulos N, Yan H. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci USA. 2013;110(15):6021–6026. doi: 10.1073/pnas.1303607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266(5193):2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 15.Kool M, Korshunov A, Remke M, Jones DT, Schlanstein M, Northcott PA, Cho YJ, Koster J, Schouten-van Meeteren A, van Vuurden D, Clifford SC, Pietsch T, von Bueren AO, Rutkowski S, McCabe M, Collins VP, Backlund ML, Haberler C, Bourdeaut F, Delattre O, Doz F, Ellison DW, Gilbertson RJ, Pomeroy SL, Taylor MD, Lichter P, Pfister SM. Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol. 2012;123(4):473–484. doi: 10.1007/s00401-012-0958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kool M, Koster J, Bunt J, Hasselt NE, Lakeman A, van Sluis P, Troost D, Meeteren NS, Caron HN, Cloos J, Mrsic A, Ylstra B, Grajkowska W, Hartmann W, Pietsch T, Ellison D, Clifford SC, Versteeg R. Integrated genomics identifies five medulloblastoma subtypes with distinct genetic profiles, pathway signatures and clinicopathological features. PLoS One. 2008;3(8):e3088. doi: 10.1371/journal.pone.0003088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korshunov A, Remke M, Kool M, Hielscher T, Northcott PA, Williamson D, Pfaff E, Witt H, Jones DT, Ryzhova M, Cho YJ, Wittmann A, Benner A, Weiss WA, von Deimling A, Scheurlen W, Kulozik AE, Clifford SC, Peter Collins V, Westermann F, Taylor MD, Lichter P, Pfister SM. Biological and clinical heterogeneity of MYCN-amplified medulloblastoma. Acta Neuropathol. 2012;123(4):515–527. doi: 10.1007/s00401-011-0918-8. [DOI] [PubMed] [Google Scholar]
- 18.Korshunov A, Remke M, Werft W, Benner A, Ryzhova M, Witt H, Sturm D, Wittmann A, Schottler A, Felsberg J, Reifenberger G, Rutkowski S, Scheurlen W, Kulozik AE, von Deimling A, Lichter P, Pfister SM. Adult and pediatric medulloblastomas are genetically distinct and require different algorithms for molecular risk stratification. J Clin Oncol. 2010;28(18):3054–3060. doi: 10.1200/JCO.2009.25.7121. [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Bishop J, Shan Y, Pai S, Liu D, Murugan AK, Sun H, El-Naggar AK, Xing M. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr Relat Cancer. 2013;20(4):603–610. doi: 10.1530/ERC-13-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mermel CH, Schumacher SE, Hill B, Meyerson ML, Beroukhim R, Getz G. GISTIC2.0 facilitates sensitive and confident localization of the targets of focal somatic copy-number alteration in human cancers. Genome Biol. 2011;12(4):R41. doi: 10.1186/gb-2011-12-4-r41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrison SJ, Prowse KR, Ho P, Weissman IL. Telomerase activity in hematopoietic cells is associated with self-renewal potential. Immunity. 1996;5(3):207–216. doi: 10.1016/S1074-7613(00)80316-7. [DOI] [PubMed] [Google Scholar]
- 23.Nonoguchi N, Ohta T, Oh JE, Kim YH, Kleihues P, Ohgaki H. TERT promoter mutations in primary and secondary glioblastomas. Acta Neuropathol. 2013 doi: 10.1007/s00401-013-1163-0. [DOI] [PubMed] [Google Scholar]
- 24.Northcott PA, Hielscher T, Dubuc A, Mack S, Shih D, Remke M, Al-Halabi H, Albrecht S, Jabado N, Eberhart CG, Grajkowska W, Weiss WA, Clifford SC, Bouffet E, Rutka JT, Korshunov A, Pfister S, Taylor MD. Pediatric and adult sonic hedgehog medulloblastomas are clinically and molecularly distinct. Acta Neuropathol. 2011;122(2):231–240. doi: 10.1007/s00401-011-0846-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Northcott PA, Jones DT, Kool M, Robinson GW, Gilbertson RJ, Cho YJ, Pomeroy SL, Korshunov A, Lichter P, Taylor MD, Pfister SM. Medulloblastomics: the end of the beginning. Nat Rev Cancer. 2012;12(12):818–834. doi: 10.1038/nrc3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Northcott PA, Korshunov A, Pfister SM, Taylor MD. The clinical implications of medulloblastoma subgroups. Nat Rev Neurol. 2012;8(6):340–351. doi: 10.1038/nrneurol.2012.78. [DOI] [PubMed] [Google Scholar]
- 27.Northcott PA, Korshunov A, Witt H, Hielscher T, Eberhart CG, Mack S, Bouffet E, Clifford SC, Hawkins CE, French P, Rutka JT, Pfister S, Taylor MD. Medulloblastoma comprises four distinct molecular variants. J Clin Oncol. 2011;29(11):1408–1414. doi: 10.1200/JCO.2009.27.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Northcott PA, Nakahara Y, Wu X, Feuk L, Ellison DW, Croul S, Mack S, Kongkham PN, Peacock J, Dubuc A, Ra YS, Zilberberg K, McLeod J, Scherer SW, Sunil Rao J, Eberhart CG, Grajkowska W, Gillespie Y, Lach B, Grundy R, Pollack IF, Hamilton RL, Van Meter T, Carlotti CG, Boop F, Bigner D, Gilbertson RJ, Rutka JT, Taylor MD. Multiple recurrent genetic events converge on control of histone lysine methylation in medulloblastoma. Nat Genet. 2009;41(4):465–472. doi: 10.1038/ng.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Northcott PA, Rutka JT, Taylor MD. Genomics of medulloblastoma: from Giemsa-banding to next-generation sequencing in 20 years. Neurosurg Focus. 2010;28(1):E6. doi: 10.3171/2009.10.FOCUS09218. [DOI] [PubMed] [Google Scholar]
- 30.Northcott PA, Shih DJ, Peacock J, Garzia L, Morrissy AS, Zichner T, Stutz AM, Korshunov A, Reimand J, Schumacher SE, Beroukhim R, Ellison DW, Marshall CR, Lionel AC, Mack S, Dubuc A, Yao Y, Ramaswamy V, Luu B, Rolider A, Cavalli FM, Wang X, Remke M, Wu X, Chiu RY, Chu A, Chuah E, Corbett RD, Hoad GR, Jackman SD, Li Y, Lo A, Mungall KL, Nip KM, Qian JQ, Raymond AG, Thiessen NT, Varhol RJ, Birol I, Moore RA, Mungall AJ, Holt R, Kawauchi D, Roussel MF, Kool M, Jones DT, Witt H, Fernandez LA, Kenney AM, Wechsler-Reya RJ, Dirks P, Aviv T, Grajkowska WA, Perek-Polnik M, Haberler CC, Delattre O, Reynaud SS, Doz FF, Pernet-Fattet SS, Cho BK, Kim SK, Wang KC, Scheurlen W, Eberhart CG, Fevre-Montange M, Jouvet A, Pollack IF, Fan X, Muraszko KM, Gillespie GY, Di Rocco C, Massimi L, Michiels EM, Kloosterhof NK, French PJ, Kros JM, Olson JM, Ellenbogen RG, Zitterbart K, Kren L, Thompson RC, Cooper MK, Lach B, McLendon RE, Bigner DD, Fontebasso A, Albrecht S, Jabado N, Lindsey JC, Bailey S, Gupta N, Weiss WA, Bognar L, Klekner A, Van Meter TE, Kumabe T, Tominaga T, Elbabaa SK, Leonard JR, Rubin JB, Liau LM, Van Meir EG, Fouladi M, Nakamura H, Cinalli G, Garami M, Hauser P, Saad AG, Iolascon A, Jung S, Carlotti CG, Vibhakar R, Ra YS, Robinson S, Zollo M, Faria CC, Chan JA, Levy ML, Sorensen PH, Meyerson M, Pomeroy SL, Cho YJ, Bader GD, Tabori U, Hawkins CE, Bouffet E, Scherer SW, Rutka JT, Malkin D, Clifford SC, Jones SJ, Korbel JO, Pfister SM, Marra MA, Taylor MD. Subgroup-specific structural variation across 1,000 medulloblastoma genomes. Nature. 2012;488(7409):49–56. doi: 10.1038/nature11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Northcott PA, Shih DJ, Remke M, Cho YJ, Kool M, Hawkins C, Eberhart CG, Dubuc A, Guettouche T, Cardentey Y, Bouffet E, Pomeroy SL, Marra M, Malkin D, Rutka JT, Korshunov A, Pfister S, Taylor MD. Rapid, reliable, and reproducible molecular sub-grouping of clinical medulloblastoma samples. Acta Neuropathol. 2011 doi: 10.1007/s00401-011-0899-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parsons DW, Li M, Zhang X, Jones S, Leary RJ, Lin JC, Boca SM, Carter H, Samayoa J, Bettegowda C, Gallia GL, Jallo GI, Binder ZA, Nikolsky Y, Hartigan J, Smith DR, Gerhard DS, Fults DW, VandenBerg S, Berger MS, Marie SK, Shinjo SM, Clara C, Phillips PC, Minturn JE, Biegel JA, Judkins AR, Resnick AC, Storm PB, Curran T, He Y, Rasheed BA, Friedman HS, Keir ST, McLendon R, Northcott PA, Taylor MD, Burger PC, Riggins GJ, Karchin R, Parmigiani G, Bigner DD, Yan H, Papadopoulos N, Vogelstein B, Kinzler KW, Velculescu VE. The genetic landscape of the childhood cancer medulloblastoma. Science. 2011;331(6016):435–439. doi: 10.1126/science.1198056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pfister SM, Korshunov A, Kool M, Hasselblatt M, Eberhart C, Taylor MD. Molecular diagnostics of CNS embryonal tumors. Acta Neuropathol. 2010;120(5):553–566. doi: 10.1007/s00401-010-0751-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pugh TJ, Weeraratne SD, Archer TC, Pomeranz Krummel DA, Auclair D, Bochicchio J, Carneiro MO, Carter SL, Cibulskis K, Erlich RL, Greulich H, Lawrence MS, Lennon NJ, McKenna A, Meldrim J, Ramos AH, Ross MG, Russ C, Shefler E, Sivachenko A, Sogoloff B, Stojanov P, Tamayo P, Mesirov JP, Amani V, Teider N, Sengupta S, Francois JP, Northcott PA, Taylor MD, Yu F, Crabtree GR, Kautzman AG, Gabriel SB, Getz G, Jager N, Jones DT, Lichter P, Pfister SM, Roberts TM, Meyerson M, Pomeroy SL, Cho YJ. Medulloblastoma exome sequencing uncovers subtype-specific somatic mutations. Nature. 2012;488(7409):106–110. doi: 10.1038/nature11329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramaswamy V, Northcott PA, Taylor MD. FISH and chips: the recipe for improved prognostication and outcomes for children with medulloblastoma. Cancer Genet. 2011;204(11):577–588. doi: 10.1016/j.cancergen.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 36.Ramaswamy V, Remke M, Bouffet E, Faria CC, Perreault S, Cho YJ, Shih DJ, Luu B, Dubuc AM, Northcott PA, Schüller U, Gururangan S, McLendon R, Bigner D, Fouladi M, Ligon KL, Pomeroy SL, Dunn S, Triscott J, Jabado N, Fontebasso A, Jones DT, Kool M, Karajannis MA, Gardner SL, Zagzag D, Nunes S, Pimentel J, Mora J, Lipp E, Walter AW, Ryzhova M, Zheludkova O, Kumirova E, Alshami J, Croul SE, Rutka JT, Hawkins C, Tabori U, Codispoti KE, Packer RJ, Pfister SM, Korshunov A, Taylor MD (2013) Recurrence patterns across medulloblastoma subgroups: an integrated clinical and molecular analysis. Lancet Oncol. doi:10.1016/S1470-2045(13)70449-2 [DOI] [PMC free article] [PubMed]
- 37.Remke M, Hielscher T, Korshunov A, Northcott PA, Bender S, Kool M, Westermann F, Benner A, Cin H, Ryzhova M, Sturm D, Witt H, Haag D, Toedt G, Wittmann A, Schottler A, von Bueren AO, von Deimling A, Rutkowski S, Scheurlen W, Kulozik AE, Taylor MD, Lichter P, Pfister SM. FSTL5 Is a marker of poor prognosis in Non-WNT/Non-SHH medulloblastoma. J Clin Oncol. 2011;29(29):3852–3861. doi: 10.1200/JCO.2011.36.2798. [DOI] [PubMed] [Google Scholar]
- 38.Remke M, Hielscher T, Northcott PA, Witt H, Ryzhova M, Wittmann A, Benner A, von Deimling A, Scheurlen W, Perry A, Croul S, Kulozik AE, Lichter P, Taylor MD, Pfister SM, Korshunov A. Adult medulloblastoma comprises three major molecular variants. J Clin Oncol. 2011;29(19):2717–2723. doi: 10.1200/JCO.2011.34.9373. [DOI] [PubMed] [Google Scholar]
- 39.Robinson G, Parker M, Kranenburg TA, Lu C, Chen X, Ding L, Phoenix TN, Hedlund E, Wei L, Zhu X, Chalhoub N, Baker SJ, Huether R, Kriwacki R, Curley N, Thiruvenkatam R, Wang J, Wu G, Rusch M, Hong X, Becksfort J, Gupta P, Ma J, Easton J, Vadodaria B, Onar-Thomas A, Lin T, Li S, Pounds S, Paugh S, Zhao D, Kawauchi D, Roussel MF, Finkelstein D, Ellison DW, Lau CC, Bouffet E, Hassall T, Gururangan S, Cohn R, Fulton RS, Fulton LL, Dooling DJ, Ochoa K, Gajjar A, Mardis ER, Wilson RK, Downing JR, Zhang J, Gilbertson RJ. Novel mutations target distinct subgroups of medulloblastoma. Nature. 2012;488(7409):43–48. doi: 10.1038/nature11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rutkowski S, von Hoff K, Emser A, Zwiener I, Pietsch T, Figarella-Branger D, Giangaspero F, Ellison DW, Garre ML, Biassoni V, Grundy RG, Finlay JL, Dhall G, Raquin MA, Grill J. Survival and prognostic factors of early childhood medulloblastoma: an international meta-analysis. J Clin Oncol. 2010;28(33):4961–4968. doi: 10.1200/JCO.2010.30.2299. [DOI] [PubMed] [Google Scholar]
- 41.Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC, Eberhart CG, Parsons DW, Rutkowski S, Gajjar A, Ellison DW, Lichter P, Gilbertson RJ, Pomeroy SL, Kool M, Pfister SM. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012;123(4):465–472. doi: 10.1007/s00401-011-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thompson MC, Fuller C, Hogg TL, Dalton J, Finkelstein D, Lau CC, Chintagumpala M, Adesina A, Ashley DM, Kellie SJ, Taylor MD, Curran T, Gajjar A, Gilbertson RJ. Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. J Clin Oncol. 2006;24(12):1924–1931. doi: 10.1200/JCO.2005.04.4974. [DOI] [PubMed] [Google Scholar]
- 43.Vinagre J, Almeida A, Populo H, Batista R, Lyra J, Pinto V, Coelho R, Celestino R, Prazeres H, Lima L, Melo M, Rocha AG, Preto A, Castro P, Castro L, Pardal F, Lopes JM, Santos LL, Reis RM, Cameselle-Teijeiro J, Sobrinho-Simoes M, Lima J, Maximo V, Soares P. Frequency of TERT promoter mutations in human cancers. Nature Commun. 2013;4:2185. doi: 10.1038/ncomms3185. [DOI] [PubMed] [Google Scholar]
- 44.Zhukova N, Ramaswamy V, Remke M, Pfaff E, Shih DJ, Martin DC, Castelo-Branco P, Baskin B, Ray PN, Bouffet E, von Bueren AO, Jones DT, Northcott PA, Kool M, Sturm D, Pugh TJ, Pomeroy SL, Cho YJ, Pietsch T, Gessi M, Rutkowski S, Bognar L, Klekner A, Cho BK, Kim SK, Wang KC, Eberhart CG, Fevre-Montange M, Fouladi M, French PJ, Kros M, Grajkowska WA, Gupta N, Weiss WA, Hauser P, Jabado N, Jouvet A, Jung S, Kumabe T, Lach B, Leonard JR, Rubin JB, Liau LM, Massimi L, Pollack IF, Shin Ra Y, Van Meir EG, Zitterbart K, Schuller U, Hill RM, Lindsey JC, Schwalbe EC, Bailey S, Ellison DW, Hawkins C, Malkin D, Clifford SC, Korshunov A, Pfister S, Taylor MD, Tabori U. Subgroup-specific prognostic implications of TP53 mutation in medulloblastoma. J Clin Oncol. 2013;31(23):2927–2935. doi: 10.1200/JCO.2012.48.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative electropherograms (a) and genotyping results (b) of the wild-type and mutated TERT promoter sequence (JPEG 1454 kb)
Overall survival (OS) in adult medulloblastomas (a), and adult SHH-driven tumors (b) according to TERT mutational status. Survival differences were calculated using continuous log-rank tests. Abbreviations: MUT, mutation; WT, wild-type (EPS 650 kb)
Focal somatic copy number alterations are largely confined to TERT wild-type Group 4 tumors. GISTIC2 analysis indicating focal amplifications/deletions in 140 wild-type (a/c) and 7 mutant (b/d) Group 4 tumors, respectively. Legend: ★, regions enriched for reported DNA copy number variations (JPEG 1392 kb)
Transcriptional profiling reveals high TERT expression in SHH medulloblastoma. Boxplots illustrate significantly higher levels of TERT expression in SHH tumors compared to Group 4 medulloblastomas (EPS 869 kb)
Minimally overlapping region including the TERT gene on chromosome arm 5p. DNA copy number gains and losses are indicated by red and blue, respectively (EPS 21771 kb)
Patient characteristics of non-SHH TERT-mutated medulloblastoma (DOCX 153 kb)