Abstract
Cerebral white matter damage is not only a commonly reported consequence of healthy aging, but is also associated with cognitive decline and dementia. The aetiology of this damage is unclear; however, individuals with hypertension have a greater burden of white matter signal abnormalities (WMSA) on MR imaging than those without hypertension. It is therefore possible that elevated blood pressure (BP) impacts white matter tissue structure which in turn has a negative impact on cognition. However, little information exists about whether vascular health indexed by BP mediates the relationship between cognition and white matter tissue structure. We used diffusion tensor imaging to examine the impact of vascular health on regional associations between white matter integrity and cognition in healthy older adults spanning the normotensive to moderate–severe hypertensive BP range (43–87 years; N = 128). We examined how white matter structure was associated with performance on tests of two cognitive domains, executive functioning (EF) and processing speed (PS), and how patterns of regional associations were modified by BP and WMSA. Multiple linear regression and structural equation models demonstrated associations between tissue structure, EF and PS in frontal, temporal, parietal, and occipital white matter regions. Radial diffusivity was more prominently associated with performance than axial diffusivity. BP only minimally influenced the relationship between white matter integrity, EF and PS. However, WMSA volume had a major impact on neurocognitive associations. This suggests that, although BP and WMSA are causally related, these differential metrics of vascular health may act via independent pathways to influence brain structure, EF and PS. Hum Brain Mapp, 2013. © 2011 Wiley Periodicals, Inc.
Keywords: blood pressure, hypertension, white matter signal abnormalities, diffusion tensor imaging, aging, executive function
INTRODUCTION
Hypertension is considered a risk factor for cognitive decline and dementia [Kilander et al., 1998; Qiu et al., 2005; Raz et al., 2007; Sepe‐Monti et al., 2007]. The neural mechanisms by which blood pressure may mediate cognitive decline are believed to be related to vascular changes affecting cerebral blood flow velocitiy, cerebrovascular reactivity and metabolic changes [Fujishima et al., 1995; Settakis et al., 2003; Sugimori et al., 1995]. These vascular changes may have an impact on brain tissue, as hypertension is associated with white matter signal abnormalities (WMSA) on magnetic resonance imaging [Artero et al., 2004; Firbank et al., 2007; Raz et al., 2007; Yoshita et al., 2005] which appear as hyperintense regions on T2‐weighted images or as hypointense regions on T1‐weighted images in the periventricular and deep white matter. Increased WMSA volume is associated with reduced cognitive performance and is apparent in cognitive disorders including Alzheimer's disease [Bombois et al., 2007; de Groot et al., 2000; DeCarli et al., 2001; Gunning‐Dixon and Raz 2000; Raz et al., 2007; Salat et al., 2010; van der Flier et al., 2005].
Previous work has demonstrated a likely link between hypertension and cognition, but the published findings are less than straightforward. Prior studies reported linear and nonlinear, as well as negative, positive or no relationships (for recent reviews see [Anson and Paran, 2005; Duron and Hanon, 2008]. Psychomotor speed, attention, executive functions, and working memory tend to be reported as the cognitive domains most affected by hypertension [Bucur and Madden, 2010; Kuo et al., 2004; Verdelho et al., 2007; Waldstein et al., 2005]. The same cognitive domains are negatively related to WMSA load [DeCarli et al., 2008; Esiri et al., 1999; Gunning‐Dixon et al., 2009; Gunning‐Dixon and Raz, 2000; Gunning‐Dixon and Raz, 2003; Prins et al., 2005; Raz and Rodrigue, 2006; Vernooij et al., 2009], confirming a potential influence of blood pressure on cognition through the promotion of WMSA. Recent reviews, as well as experimental studies, have shown consistently that executive functions and processing speed are the two domains most commonly affected by increased blood pressure and WMSA [Bucur and Madden 2010; Gunning‐Dixon and Raz, 2000; Gunning‐Dixon and Raz, 2003; Kuo et al., 2004; Prins et al., 2005; Raz and Rodrigue, 2006; Verdelho et al., 2007; Vernooij et al., 2009].
Diffusion tensor imaging (DTI) has seen a rapid expansion in use as an imaging method providing unique sensitivity to measure the microstructural properties of brain tissue, and this procedure could be useful in elucidating the potential impact of blood pressure on white matter integrity and cognition. Negative relationships between cognition and white matter integrity have been reported [Bucur et al., 2008; Charlton et al., 2006; Charlton et al., 2008; Duan et al., 2006; Hannesdottir et al., 2009; Head et al., 2004; Huang and Auchus, 2007; Kennedy and Raz, 2009a; Madden et al., 2009b; Medina et al., 2006; Rose et al., 2006; Schiavone et al., 2009; Sullivan and Pfefferbaum, 2006; Vernooij et al., 2009]. Prior work suggests that DTI may provide information about different pathophysiological processes and may be among the most sensitive neuroimaging indicators of vascular damage [Bucur et al., 2008; Burgmans et al., 2009; Leritz et al., 2010; Madden et al., 2009b; O'Sullivan et al., 2004; Raz and Rodrigue, 2006; Vernooij et al., 2009]. The microstructural diffusion metrics provide a more sensitive measure of degenerative changes compared with the macrostructural measures of volume loss and may be able to detect alterations before overt volume loss or a lesion is present, or may even provide additional information on white matter alterations not necessarily related to vascular pathology [Hugenschmidt et al., 2008; Salat et al., 2005]. Such comparisons however, must be performed with caution given that the sensitivity of any technique will depend on the specific manner in which it is implemented (e.g. regional versus global measurements).
Recent work has emphasized the potential for white matter damage to be a fundamental aspect of normal age‐associated cognitive decline as well as dementia [Bartzokis 2004; Bartzokis et al., 2004]. It is therefore striking that to date only few studies have examined the interactions among white matter integrity, cognition and blood pressure in older adults. The majority of prior studies focussed on extreme cases of cerebrovascular disease (CVD) or have classified individuals into hypertensive versus normotensive groups which may obscure the more quantitative, subtle effects that vascular physiology may have on neural health and cognition. There is accumulating evidence that even mildly elevated blood pressure levels within the normotensive range may affect cognitive functioning [Whitworth, 2003, 2005].
We [Leritz et al., 2010] and others [Kennedy and Raz, 2009b] have recently demonstrated negative associations between blood pressure and diffusion measures of white matter integrity. Importantly, these associations were observed across the range of blood pressure values sampled, including individuals in the normotensive range [Kennedy and Raz, 2009b; Leritz et al., 2010]. Another study among geriatric depressed patients [Hoptman et al., 2009] not only showed that blood pressure and diffusivity are negatively related, but also that white matter hyperintensities occur in areas with decreasing diffusivity. These data suggest that white matter may be influenced by subtle interindividual variation in vascular health which eventually results in a more overt lesion of the tissue, and that these associations extend to the preclinical population.
The aim of this study was to investigate the impact of vascular health indexed by blood pressure and WMSA burden on the association between executive functioning or processing speed and white matter integrity in individuals spanning the normotensive to moderate–severe hypertensive range [Levy et al., 1996]. This approach has to our knowledge not yet been taken. In contrast to the great majority of prior studies, we investigated blood pressure as a continuous metric from the normal to moderately hypertensive range. We hypothesized that there would be a negative relationship between white matter integrity and executive functioning that would be substantially mediated by blood pressure and/or by WMSA burden [de Groot et al., 2000]. Because white matter integrity measures tissue changes on a microstructural level, we expected partial mediation, indicating that WMSA and blood pressure do not fully capture the changes in white matter integrity associated with cognitive performance. Specifically, we used structural equation modelling [Bentler, 1998] to test whether elevations in mean arterial blood pressure contributed to increased WMSA volume and reduced regional DTI‐based white matter microstructural integrity with a corresponding decrease in performance on tests measuring executive functions and processing speed. Structural equation modeling (SEM) allows us to test multivariate associations, providing a statistical and theoretic conceptual advantage [Penke and Deary 2010]. This technique has been used in prior aging studies [Penke and Deary, 2010; Salthouse et al., 2003].
MATERIALS AND METHODS
Participants
The study sample consisted of 128 participants (50 men/78 women) recruited through two coinciding studies investigating the impact of cerebrovascular risk factors on brain structure and cognition. Forty‐one participants were recruited through the Harvard Cooperative Program on Aging Claude Pepper Older American Independence Center. The remaining 87 individuals were recruited through the Boston University Alzheimer's Disease Center as part of the Understanding Cerebrovascular and Alzheimer's Risk in the Elderly study. This aim of this project is to determine the impact of vascular risk on brain structure, cognition, and the development of dementia. Inclusion was based on the initial criteria of being neurologically healthy and having at least one first‐degree familial relative with a diagnosis of dementia. Both protocols were approved by the local Ethical Committees and a consent form for each participant was obtained according to the Declaration of Helsinki [Nylenna and Riis, 1991].
Participants ranged in age from 43 to 87 years. Individuals were excluded for a history of head trauma of “mild” severity or greater according to the criteria of Fortuny et al., [1980; loss of consciousness for greater than 10 min), diagnosis of any form of neurodegenerative disease (i.e., Parkinson's disease, Alzheimer's disease, vascular dementia), any severe psychiatric illness (including psychosis, delusions, and hallucinations), any hospitalization for psychiatric reasons or any history of brain surgery. Past history or current presence of neurodegenerative (including dementia) and psychiatric illness was assessed by the staff physician and a clinical neuropsychologist. All participants were literate with at least a 6th grade education. One hundred‐four participants claimed dominant right‐handedness. Mini‐Mental Status (MMSE) [Folstein 1975] scores ranged from 23 to 30. These scores are in a range outside of a dementia diagnosis, according to normative data based on the racial distribution of our sample [Bohnstedt et al., 1984]. A small percentage of participants had MMSE scores below 24 (2.5%) and were determined not to substantially influence the overall results. Earlier studies, excluding dementia, have used a comparable MMSE cut‐off [Euser et al., 2009; Firbank et al., 2007; Taylor et al., 2001].
MRI Acquisition
Imaging was performed on a 1.5T Siemens Avanto scanner. For structural imaging, two whole‐brain high‐resolution T1‐weighted MPRAGE scans were collected and averaged for each participant (TR = 2.73, TE = 3.39, flip angle = 7, slice thickness = 1.33 mm, 128 slices, FOV = 256 × 256 mm2) to create a single volume with a high contrast‐to‐noise ratio. DTI acquisition employed single shot echo planar imaging with a twice‐refocused spin echo pulse sequence, optimized to minimize eddy current‐induced image distortions [Reese et al., 2003] (TR/TE = 7,200/77 ms, b = 700 s/mm2, acquisition matrix = 128 × 128 mm2, 256 × 256 mm2 FOV, 2 mm slice thickness with 0‐mm gap for 2 mm3, isotropic voxels, 60 slices, 10 T2 + 60 DWI). The 60 diffusion weighted directions were obtained using the electrostatic shell method [Jones et al., 1999], providing a high signal‐to‐noise diffusion volume. The diffusion tensor was calculated on a voxel‐by‐voxel basis with conventional reconstruction methods [Basser et al., 1994]. Global and regional WM integrity was assessed using DTI measures of Fractional Anisotropy and diffusivity (comprised of axial and radial components [Budde et al., 2007; Song et al., 2003; Song et al., 2002], as well as through intervoxel coherence.
MRI Analyses
For data processing, we used diffusion tools developed at the Martinos Center as well as tools available as part of the Freesurfer (http://surfer.nmr.mgh.harvard.edu) and FSL (http://www.fmrib.ox.ac.uk.ezp-prod1.hul.harvard.edu/fsl) processing streams. Diffusion volumes were eddy current and motion corrected using FSL's Eddy Correct tool. The diffusion tensor was calculated for each voxel using a least‐squares fit to the diffusion signal. The T2 weighted lowb volume was then skull stripped using FSL's Brain Extraction Tool (BET) [Smith, 2002], which served as a brain‐mask for all other diffusion maps. Microstructural maps of fractional anisotropy (FA) and axial (DA) and radial (RD) diffusivity were entered into group voxel based general linear models using Tract Based Spatial Statistics (TBSS) as the basis of interparticipant spatial normalization.
Nonlinear Registration and TBSS
Voxelwise processing of the DTI data was carried out using TBSS [Smith et al., 2006], which is part of the FSL data processing suite [Smith et al., 2004]. All participants' diffusion data were initially aligned into a common space using the nonlinear registration tool FNIRT, which uses a b‐spline representation of the registration warp field [Klein et al., 2009; Rueckert et al., 1999], resulting in all images transformed into 1 mm isotropic, MNI152 standard space. Next, the mean FA image was created by averaging all participants aligned FA volumes, and a mean FA skeleton was created from all voxels with a group mean FA of greater than 0.2 to reduce inclusion of voxels that are likely composed of multiple tissue types or fiber orientations. Each participants's aligned, common space FA data was then projected onto this skeleton to create a 4D skeletonized volume (3D skeletal volume × number of subjects), which was then fed into voxelwise group statistics. Data along the skeleton were smoothed using an anatomical constraint to limit the smoothing to neighboring data within adjacent voxels along the skeleton. The exact transformations derived for the FA maps were applied to the other diffusivity volumes for matched processing of image volumes per subject. Statistical maps were dilated from the TBSS skeleton for visualization purposes.
ROI Analysis
ROIs were created based on the results of the whole brain voxel based statistical GLMs analyses. Significant voxels that reached a minimum threshold of P < 0.05 from the GLMs examining the association between FA and the cognitive tasks, were binarized using minimum cluster sizes for contiguous significant voxels along the skeleton (>320 mm3 for Trail Making A and B and >680 mm3 for the Stroop Color Word Task). Regions of interest (ROIs) were labeled using the binarized, mean FA skeleton volume as a mask to assign each voxel along the skeleton a segmentation value based on structural white matter parcellations created via FreeSurfer [Salat et al., 2009] combined with predefined JHU White Matter Labels available through FSL.
White Matter Signal Abnormalities
T1 weighted MRI data were processed using the FreeSurfer version 4.1.0 (http://surfer.nmr.harvard.edu) morphometric analysis tools. Cortical surfaces were reconstructed using a semiautomated procedure that has been described and validated in previous work in detail [Dale et al., 1999; Fischl et al., 2001; Fischl et al., 1999]. Briefly, processing involves intensity normalization, skull stripping, segmentation of white matter, tessellation of the gray/white matter boundary, smoothing of the tessellated surface, and automatic topology correction. WMSA (i.e., T1 hypointensities within the WM) were labeled utilizing a probabilistic procedure [Fischl et al., 2002] subsequently extended to label white matter hypointensities. Total WMSA volume was calculated to examine the impact on the DTI metrics. This procedure has been shown to be sensitive to measure white matter damage in individuals with AD [Salat et al., 2010]. WMSA calculated on T1 images have been shown to be highly correlated with manual and semimanual measurements from T2/FLAIR (r > 0.93 when including extreme values; >0.72 when excluding extreme values), and WMSA from T1 images and similar procedures have been utilized in prior work [Bagnato et al., 2003; Burns et al., 2005; Camp et al., 2005; Salat et al., 2010].
Neuropsychological Tests
Executive functioning and processing speed were assessed using the Trail Making Test (TMT) and the Stroop Color Word Task (SCWT). These neuropsychological tests are commonly used to assess executive functioning [Lezak, 1995]. The TMT measures cognitive flexibility, selective attention, visual scanning, and visuo‐motor scanning [Chen et al., 2000; Giovagnoli et al., 1996; Greenlief et al., 1985; Zakzanis et al., 2005] and has demonstrated sensitivity in measuring decline in set‐shifting in older adults [Greenlief et al., 1985; Perry et al., 2009; Salthouse et al., 2000]. The TMT consists of two parts: (1) part A involves number sequencing and is thought to rely primarily on psychomotor speed and attention, (2) part B involves shifting between letter and number sequences, and results in slower performance times [Arbuthnott and Frank, 2000; Olivera‐Souza et al., 2000]. Previous studies have shown by using factor analytic procedures that the SCWT and the TMT‐B are related to executive functiong and the TMT‐A is related to information processing speed [Salthouse et al., 2003; Van der Elst et al., 2008]. The TMT is assumed to rely heavily on frontal lobe structures, as can be seen in lesion studies [Stuss and Levine, 2002].
However, functional MRI and DTI studies have shown that parietal and temporal areas might be involved in performance on the TMT, suggesting an underlying network [Moll et al., 2002; O'Sullivan et al., 2001; Olivera‐Souza et al., 2000; Perry et al., 2009; Zakzanis et al., 2005]. In this study, time to perform either TMT A or B in seconds was the dependent variable. The SCWT is considered as a general measure of cognitive flexibility and control [Uttl and Graf, 1997] or executive functioning [Moering et al., 2003]. The basic paradigm behind the SCWT involves the suppression of a habitual response in support of an unusual one (i.e., naming the ink color which is not congruent with the printed color words). These abilities decline with age [Ivnik et al., 1996] and in dementia [Houx et al., 1993]. Performance of the SCWT is also supported by frontal lobe function [Demakis, 2004; Stuss and Levine, 2002]. In this study the dependent measure was the number of incongruent color words correctly read within 2 min.
Blood Pressure
Systolic and diastolic blood pressure were measured in a seated and upright position after five minutes of rest, with the arm at rest at the level of the heart using a sphygmomanometer and a stethoscope. A second measurement was obtained 5 min later in the same position and at the same arm (thus in total four measurements were done), and the average of 2 values were calculated for both systolic and diastolic. Blood pressure was always measured by the same study physician. Blood pressure status was evaluated by a combination of antihypertensive medication use and current conventions considering a systolic BP of 120‐139 mm Hg as indicative of “mild” or “pre” hypertension, a systolic BP of 140–159 mm Hg to be “Stage 1” hypertension, and a systolic BP of 160 mm Hg or greater to be indicative of “Stage 2” (severe) hypertension [American Heart Association, 2009]. In our sample, 46 (40%) individuals would be classified as having “pre‐hypertension” under these guidelines. Thirty‐two (28%) would be classified as “Stage 1,” and nine (8%) would be classified as “Stage 2.” Thus, 28 (24%) would be considered to have normal‐mild BP readings. However, we did not classify participants in groups based on their blood pressure status, but used blood pressure as a continuous variable by considering systolic and diastolic blood pressure together and creating a mean arterial blood pressure (MABP) measure using the following formula: MABP: 1/3 (systolic − diastolic) + diastolic.
MABP is a metric commonly used in clinical settings to obtain an accurate metric of overall average BP, due to the fact that it contains both systolic and diastolic measurements in its formula. MABP is believed to indicate perfusion pressure, particularly in body organs. Thus, it is an appropriate metric to use when examining associations between blood pressure and brain structure, or blood pressure and function. Prior studies have utilized MABP, particularly when examining blood pressure in the context of cognition or brain structure in older adults [Brown et al., 2010; Guo et al., 2009; Leritz et al., 2010].
Another potential vascular risk factor, diabetes, was assessed according to current accepted criteria [American Diabetes Association, 2009] based on glucose levels and a measure of glycosylated haemoglobin HA1C. The majority of our sample had appropriately regulated glucose at the time of the assessment, suggesting that this did not likely have an effect on our data (data not presented, see [Leritz et al., 2011].
Statistical Analyses
For the behavioral data, we investigated the association between demographic characteristics, cognition, and MABP by means of zero‐order Pearson correlations. All analyses were performed with the Statistical Package for the Social Sciences (SPSS Inc., Chicago), version 15.0 for Windows.
For the DTI data, voxelwise statistics were performed for each diffusion measure (FA, ADC, DA, and RD) to test for significant correlations with performance on the trail making test, using FreeSurfer's general linear model tool. Before the analyses, all variables were checked for normality, linearity and influential outliers. TMT and SCWT performance and WMSA volume were all determined to not be normally distributed. TMT scores (in seconds) were converted using the inverse of the log transformation and SCWT was converted with a log transformation. The distribution of WMSA was highly skewed and could not be improved by log‐transformation. We therefore applied a cut‐off at 5000 mm3, dividing low versus high WMSA load. Age, education, ethnicity (Caucasian or African‐Americans, coded as 0 and 1, respectively) and use of blood pressure medication were used as covariates. To examine the mediating effect of either MABP or WMSA, these variables were added separately as a covariate to the model.
SEM analyses were performed to test hypothesized models and to determine how MABP and WMSA contributed to the association between white matter integrity and executive functioning, as qualified by the log(SCWT) measure. The SCWT was chosen because the regression analyses showed robust and consistent findings compared with the TMT (see results). A latent variable was specified for each diffusivity metric (FA, ADC, DA, and RD). This latent variable consisted of areas significant in the regression analyses, which are thought to be associated with performance on the SCWT. The advantage of a latent variable over the use of averages is that the latent variable only contains the variance shared by the various measured variables. This removes unsystematic measurement errors or other sources of variance and increased the reliability [Penke and Deary, 2010]. Structural equation models were implemented in AMOS (Analysis of Moment Structures) (SPSS Inc., Chicago). All structural models were evaluated via maximum likelihood estimation with several indices of model fit. Models were considered an acceptable fit with χ2/df of less than 2, a comparative fit index of more than 0.90 (CFI; [Bentler 1990]), and a root‐mean‐square error of approximation (RMSEA) of less than 0.08 provided that it included 0.05 within its 90% confidence interval [Browne and Cudeck, 1993]. The fit of nested models was compared by means of χ2 difference tests. In this procedure, the difference between the χ2 values of two models is calculated. This difference value is itself approximately χ2 distributed, with a number of the degrees of freedom that equals the difference in degrees of freedom between the two nested models [Reis and Judd, 2000]. The threshold for statistical significance was set at P < 0.05.
RESULTS
Basic Demographic Characteristics
Demographic characteristics of the complete study group are described in Table I. The sample had a mean age of 67.9 years (SD = 9.4; range, 43–87 years), mean education of 14.8 years (SD = 2.6) and 47.7% of the sample was on medication for hypertension.
Table I.
Characteristics of the study population (N = 128)
| Baseline | Mean (SD) |
|---|---|
| Age (years) | 67.9 (9.4) |
| Education (years) | 14.8 (2.6) |
| Female (n (%)) | 78 (60.9%) |
| MMSE score | 27.8 (1.8) |
| TMT A score (seconds) | 38.8 (13.4) |
| TMT B score (seconds) | 93.7 (43.6) |
| SCWT score (number of words) | 90.3 (25.2) |
| Mean Arterial Blood Pressure | 95.2 (10.7) |
| Baseline | n (%) |
| Ethnicity [Caucasian : African‐American) (n (%)] | 52 : 76 (40.6 : 59.4%) |
| Hypertension medication [n (%)] | 61 (47.7%) |
Note: Continuous variables are represented as mean (SD) and categorical variables as number (%). MMSE, Mini‐Mental State Examination; TMT A, Trail Making Test part A; TMT B, Trail Making Test part B; SCWT, Stroop Color Word Task.
Associations between neuropsychological performance and demographic variables are presented in Table II. Performance on TMT A was significantly associated with age and ethnicity, while performance on TMT B was only associated with age. The SCWT was significantly associated with age, education, ethnicity, use of antihypertensive medication and WMH. MABP was significantly associated with ethnicity, WMH, TMT A, and SCWT. Because age, education, ethnicity, and use of blood pressure medication were each individually related to one or more of the cognitive variables, these demographic factors were utilized as covariates in statistical models.
Table II.
Correlations between demographic characteristics, cognition, and MABP
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | |
|---|---|---|---|---|---|---|---|---|---|
| 1. Age | |||||||||
| 2. Education | 0.038 | ||||||||
| 3. Gender | 0.020 | 0.094 | |||||||
| 4. Ethnicity | 0.311** | 0.343** | 0.088 | ||||||
| 5. Medication use | 0.230** | −0.108 | −0.209* | −0.016 | |||||
| 6. WMSA | 0.387** | 0.243** | −0.115 | 0.109 | 0.243** | ||||
| 7. TMT A | −0.203* | −0.139 | 0.052 | 0.325** | −0.139 | −0.032 | |||
| 8. TMT B | −0.106 | −0.112 | 0.139 | 0.421** | −0.112 | −0.094 | 0,437** | ||
| 9. SCWT | −0.379* | −0.199* | 0.145 | 0.185** | −0.199* | −0.396** | 0,382** | 0.573** | |
| 10. MABP | 0.145 | 0.163 | −0.100 | −0.206* | 0.163 | 0.278** | −0.189* | −0.094 | −0.189* |
P < 0.05,
P < 0.0.
Dark gray, correlations with MABP; light gray, correlations with the three cognitive measures. WMSA, White Matter Signal Abnormalities; TMT A, Trail Making Test part A; TMT B, Trail Making Test part B; SCWT, Stroop Color Word Task; MABP, Mean Arterial Blood Pressure.
Associations Between Cognitive Performance and DTI‐Based White Matter Integrity
Figure 1 demonstrates the whole brain associations between FA (left column) and RD (right column) and cognitive performance on all three neuropsychological measures (TMT A, TMT B, and SCWT), while taking the demographic covariates into account. Effects of ADC and DA were minimal and therefore not shown in this figure.
Figure 1.
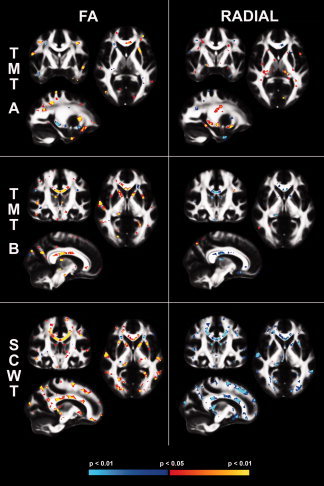
Association Between Cognition and DTI Measures. Associations between performance on the three different tasks, TMT A, TMT B, and SCWT and fractional anisotropy (FA) (left) and the same associations for radial diffusivity (right). Blue indicates a negative association, red indicates a positive association as indicated on the colorbar. TMT A, Trail Making Test part A; TMT B, Trail Making Test part B; SCWT, Stroop Color Word Task; FA, Fractional Anisotropy, P < 0.05. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Regional clusters showing a significant association between cognitive performance and FA are summarized in Table III. The major effects from these analyses are summarized below along with the assessment of the impact of blood pressure medication on these associations.
Table III.
Regional clusters showing a significant association between cognitive performance and FA
| Hemisphere | Cluster size (mm3) | Minimum P‐value (10−x) | Weight | |
|---|---|---|---|---|
| TMT A | ||||
| Frontal | ||||
| Precentral | LH | 840 | 4.76 | 3998.40 |
| Lateral orbitofrontal | LH | 1080 | 3.18 | 3434.40 |
| Pars triangularis | RH | 584 | 3.09 | 1804.56 |
| Superior frontal | LH | 572 | 3.14 | 1796.08 |
| Rostral middle frontal | RH | 476 | 2.54 | 1209.04 |
| Precentral | RH | 360 | 2.66 | 957.60 |
| Pars opercularis | RH | 344 | 2.30 | 791.20 |
| Pars opercularis | LH | 376 | 2.00 | 752.00 |
| Parietal | ||||
| Superior parietal | LH | 944 | 3.40 | 3209.60 |
| Superior parietal | RH | 896 | 3.26 | 2920.96 |
| Postcentral | RH | 360 | 2.58 | 928.80 |
| Temporal | ||||
| Superior temporal | LH | 698 | 3.52 | 2456.96 |
| Inferior temporal | RH | 336 | 3.98 | 1337.28 |
| Superior temporal | RH | 336 | 2.53 | 850.08 |
| Inferior temporal | LH | 360 | 2.15 | 774.00 |
| Occipital | ||||
| — | — | — | — | — |
| Other | ||||
| Anterior corona radiata | LH | 2192 | 3.65 | 8000.80 |
| Genu corpus callosum | — | 628 | 2.71 | 1701.88 |
| Superior corona radiata | RH | 352 | 3.01 | 1059.52 |
| Body corpus callosum | — | 328 | 2.43 | 797.04 |
| TMT B | ||||
| Frontal | ||||
| Rostral middle frontal | RH | 400 | 4.81 | 1924.00 |
| Lateral orbitofrontal | RH | 520 | 2.93 | 1523.60 |
| Precentral | LH | 424 | 3.36 | 1424.64 |
| Superior frontal | LH | 364 | 2.84 | 1033.76 |
| Parietal | ||||
| Postcentral | RH | 1020 | 4.60 | 4692.00 |
| Precuneus | RH | 848 | 3.87 | 3281.76 |
| Inferior parietal | RH | 592 | 3.22 | 1906.24 |
| Superior parietal | RH | 508 | 3.05 | 1549.40 |
| Temporal | ||||
| Superior temporal | RH | 516 | 3.13 | 1615.08 |
| Occipital | ||||
| — | — | — | — | — |
| Other | ||||
| Fornix stria terminalis | RH | 1000 | 3.38 | 3380.00 |
| Anterior limb of the internal capsule | RH | 784 | 2.47 | 1936.48 |
| Anterior limb of the internal capsule | LH | 424 | 2.58 | 1093.92 |
| Superior longitudinal fasciculus | LH | 408 | 2.23 | 909.84 |
| STROOP COLOR WORD TASK | ||||
| Frontal | ||||
| Caudal middle frontal | LH | 2072 | 4.57 | 9469.04 |
| Rostral middle frontal | LH | 1780 | 3.43 | 6105.40 |
| Lateral orbitofrontal | LH | 1648 | 3.08 | 5075.84 |
| Superior frontal | RH | 1088 | 3.48 | 3786.24 |
| Precentral | RH | 960 | 3.11 | 2985.60 |
| Superior frontal | LH | 880 | 3.14 | 2763.20 |
| Precentral | LH | 836 | 3.13 | 2616.68 |
| Parietal | ||||
| Precuneus | LH | 2224 | 5.16 | 11475.84 |
| Inferior parietal | LH | 1984 | 4.49 | 8908.16 |
| Superior parietal | RH | 1048 | 3.79 | 3971.92 |
| Postcentral | LH | 736 | 3.99 | 2936.64 |
| Superior parietal | LH | 848 | 3.14 | 2662.72 |
| Temporal | ||||
| Transverse temporal | LH | 888 | 3.92 | 3480.96 |
| Middle temporal | LH | 896 | 3.60 | 3225.60 |
| Middle temporal | RH | 712 | 4.18 | 2976.16 |
| Occipital | ||||
| Lateral occipital | LH | 1824 | 4.72 | 8609.28 |
| Pericalcarine | RH | 832 | 3.12 | 2595.84 |
| Other | ||||
| Superior corona radiata | RH | 25288 | 4.83 | 122141.00 |
| Superior longitudinal fasciculus | LH | 1832 | 2.89 | 5294.48 |
| Fornix stria terminalis | RH | 1152 | 4.09 | 4711.68 |
| Splenium corpus callosum | — | 1704 | 2.41 | 4106.64 |
| Superior longitudinal fasciculus | RH | 1440 | 2.83 | 4075.20 |
| Tapetum | LH | 1080 | 3.01 | 3250.80 |
| Posterior corona radiata | RH | 1136 | 2.81 | 3192.16 |
| Superior corona radiata | LH | 1136 | 2.50 | 2840.00 |
| External capsule | LH | 776 | 3.57 | 2770.32 |
| Anterior limb of internal capsule | LH | 816 | 3.35 | 2733.60 |
| Genu corpus callosum | — | 952 | 2.24 | 2132.48 |
Clusters were defined at P < 0.05 with a cluster threshold of 320mm3 for TMT A and B and a cluster threshold of 680 mm3 for the Stroop Color Word Task. Regions were ordered by region/lobe and by weighting calculated as the product of the cluster size by the minimum P‐value (expressed as 10−x). Regional definitions were based on proximity to neural labels described in (Desikan et al., 2006; Fischl et al., 2002) and in the JHU white matter atlas. TMT A, Trail Making Test part A; TMT B, Trail Making Test part B; SCWT, Stroop Color Word Task.
Associations Between the DTI Metrics and the TMT A
FA was significantly bilaterally associated with performance on TMT in the superior parietal, precentral, pars opercularis, superior temporal, and inferior temporal WM. ADC was not associated with performance on TMT A and ADC, and DA was only associated with performance in the left lateral orbitofrontal white matter (t = 2.823, P = 0.006). RD showed robust bilateral associations with TMT A performance in inferior temporal white matter (t = −2.614, P = 0.010, and t = −2.096, P = 0.038, respectively), in left precentral white matter (t = −2.107, P = 0.037), left superior parietal white matter (t = −2.328, P = 0.022), genu of the corpus callosum (−2.129, P = 0.035) and left superior frontal white matter (t = −2.052, P = 0.042). No region showed a significant interaction between FA and antihypertensive medication use.
Associations Between the DTI Metrics and the TMT B
Significant bilateral clusters were found for the association between performance on TMT B and FA in the anterior limb of the internal capsule. Performance on TMT B was associated with ADC values in the right fornix stria terminalis (t = −3.242, P = 0.002), the left anterior limb of the internal capsule (t = −2.267, P = 0.025) and the right postcentral white matter (t = −2.084, P = 0.039). DA was associated with TMT B performance in the right fornix stria terminalis (t = −2.980, P = 0.003), left superior longitudinal fasciculus (t = 2.284, P = 0.024) and right superior parietal white matter (t = 3.400, P = 0.001). Similar to associations with TMT A, RD showed greater regional associations than DA with TMT B performance, with effects in the right fornix stria terminalis (t = −3.316, P = 0.001), bilateral anterior limb of the internal capsule (t = −2.244, P = 0.027, and t = −2.419, P = 0.017, respectively), right inferior parietal white matter (t = −2.703, P = 0.008), right rostral middle frontal white matter (t = −1.986, P = 0.049), right postcentral white matter (t = −2.761, P = 0.007) and left superior frontal white matter (t = −2.155, P = 0.033). There were no regional interactions between those on and off medication on the associations between FA and cognitive performance.
Associations Between the DTI Metrics and the SCWT
Of all three neuropsychological tests, performance on the SCWT showed the most widespread associations with white matter microstructure. Bilateral clusters were found in the superior corona radiata, superior longitudinal fasciculus, and in the superior frontal, superior parietal, precentral, and middle temporal WM. ADC and RD were significantly associated with performance in almost all regions where FA was significant. However, for DA, only a few areas showed a strong relationship to the SCWT performance: the right superior corona radiata (t = −2.246, P = 0.027), the right fornix stria terminalis (t = −4.316, P < 0.001), the left tapetum (t = −3.081, P = 0.003) and the left postcentral white matter (t = −2.355, P = 0.020). The interaction between FA and blood pressure medication use was significant in only in the right superior longitudinal fasciculus (t = 2,162, P = 0.033).
Contribution of MABP and WMSA to the Association Between Cognition and DTI
We next examined the influence of vascular health, indexed by MABP and WMSA, on the associations between cognitive performances and white matter microstructure. To do so, associations between cognitive performance and white matter integrity were examined by voxel‐based general linear model while controlling for the effect of MABP and WMSA volume (separately). Similar analyses were performed by multiple regressions on the regional cluster data to investigate the adsded explained variance to the model when adding MABP or WMSA volume as a covariate. MABP had no significant effects on all models. In contrast, the addition of WMSA volume as a covariate substantially reduced associations between TMT A and SCWT performance and white matter microstructure. Figure 2 demonstrates that entering MABP as a nuisance covariate had a limited effect on the associations between cognitive performance and FA. In contrast, the addition of WMSA resulted in a dramatic reduction in the association between cognitive performance and white matter microstructure.
Figure 2.
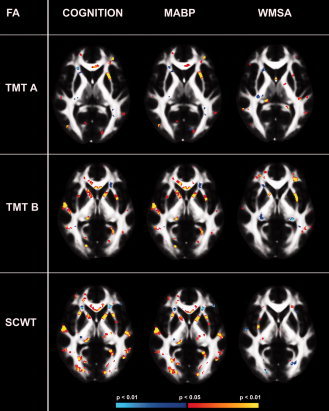
Influence of MABP or WMSA on the association between FA and cognition. The influence of MABP (middle) or WMSA (right) on the association between FA and the three cognitive tasks (left) TMT A, TMT B, and SCWT. TMT A, trail making Test part A; TMT B, Trail Making Test part B; SCWT, Stroop Color Word Task; MABP, mean arterial blood pressure, WMSA, white matter signal abnormalities. P < 0.05. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Table IV demonstrates the impact of MABP and WMSA volume on selected regions of interest assessed by stepwise regression. MABP had no significant effects on all models. In contrast, the addition of WMSA to the model increased the mean proportion of the explained variance with 2.6% as compared with a model which investigated associations between TMT A performance and FA (mean increase of 2.6% in explained variance) in the left pars opercularis, lateral orbitofrontal, superior parietal, and the right rostral middle frontal white matter (with covariates age, education, ethnicity, and use of antihypertensive medication). There was no significant change in the explained variance for ADC or DA in the areas examined. The association between performance and RD in the left superior parietal white matter was significantly mediated by adding WMSA to the model. Adding WMSA to the model of performance on TMT A resulted in stronger associations between performance and microstructural measures. This was not due to multi‐collinearity, which was checked by investigating the Variance Inflation Factor (VIF < 10).
Table IV.
Contribution of MABP and WMSA to the association between FA and cognition
| Cognition | Adding MABP | Adding WMSA | |||||
|---|---|---|---|---|---|---|---|
| Clustered regions of interest | Hemisphere | R 2 | P Fchange | R 2 | P Fchange | R 2 | P Fchange |
| Trail Making Test part A | |||||||
| Frontal | |||||||
| Lateral orbitofrontal | LH | 0.309 | 0.000*** | 0.310 | 0.851 | 0.340 | 0.022* |
| Rostral middle frontal | RH | 0.309 | 0.000*** | 0.309 | 0.902 | 0.332 | 0.044* |
| Pars opercularis | LH | 0.267 | 0.000*** | 0.267 | 0.981 | 0.297 | 0.029* |
| Parietal | |||||||
| Superior parietal | LH | 0.333 | 0.000*** | 0.333 | 0.716 | 0.360 | 0.028* |
| Stroop Color Word Task | |||||||
| Frontal | |||||||
| Superior frontal | RH | 0.323 | 0.000*** | 0.323 | 0.858 | 0.359 | 0.012* |
| Precentral | RH | 0.309 | 0.000*** | 0.309 | 0.849 | 0.359 | 0.004** |
| Superior frontal | LH | 0.314 | 0.000*** | 0.314 | 0.981 | 0.353 | 0.010** |
| Precentral | LH | 0.357 | 0.000*** | 0.357 | 0.924 | 0.376 | 0.029* |
| Parietal | |||||||
| Superior parietal | RH | 0.329 | 0.000*** | 0.329 | 0.994 | 0.356 | 0.033* |
| Superior parietal | LH | 0.329 | 0.000*** | 0.329 | 0.992 | 0.358 | 0.027* |
| Temporal | |||||||
| Middle temporal | LH | 0.353 | 0.000*** | 0.354 | 0.875 | 0.388 | 0.013* |
| Middle temporal | RH | 0.301 | 0.000*** | 0.301 | 0.806 | 0.350 | 0.004** |
| Other | |||||||
| Superior corona radiata | RH | 0.322 | 0.000*** | 0.322 | 0.850 | 0.350 | 0.029* |
| Superior long fasciculus | LH | 0.352 | 0.000*** | 0.353 | 0.799 | 0.377 | 0.036* |
| Superior long fasciculus | RH | 0.300 | 0.000*** | 0.301 | 0.816 | 0.338 | 0.013* |
| Superior corona radiata | LH | 0.310 | 0.000*** | 0.310 | 0.887 | 0.344 | 0.017* |
P < 0.05;
P < 0.01;
P < 0.001.
Overview of the significant ROI results from the stepwise multiple linear regression analyses with cognition as the dependent variable and regional FA as the independent variable with either MABP or WMSA volume added stepwise to the model. Age, education, ethnicity and use of antihypertensive medication were entered as covariates. MABP, Mean Arterial Blood Pressure; WMSA, White matter hyperintensities, R 2: the explained variance of the model.
For TMT B, there was not a substantial change in R 2 when adding MABP or WMSA to the model in any area or in any diffusivity metric.
For the SCWT, adding MABP to the model did not substantially affect the R 2 in any area or any metric. However, adding WMSA to the model changes the R 2 significantly in almost every area for each diffusivity metric (mean increase of 3.4% of the explained variance; see Table IV).
SEM Analyses
SEM was performed to test the hypothesis that elevations in blood pressure would contribute to WMSA burden that would have a mediating effect on the associations between DTI‐based white matter integrity and cognitive performance. To restrict the number of ROIs, three areas that were significantly related to SCWT and known to support executive functions were chosen for these analyses. Earlier fMRI studies showed that SCWT performance is associated with frontal and parietal activation [Egner and Hirsch, 2005; Kaufmann et al., 2005; Mathis et al., 2009; Pujol et al., 2001]. Therefore, the right and left superior longitudinal fasciculus and the left anterior limb of the internal capsule, regions that showed significant effects in our regression analyses and innervate frontal and parietal areas, were chosen for the SEM analysis. We chose to use the SCWT for these analyses, since the regression analyses were more robust and consistent in this task (based on the number of significant associations) compared with the TMT results. A latent variable was specified for each diffusivity metric (FA, ADC, DA, and RD). Each of these latent factors were represented by their corresponding values of the three ROIs. Next to this, age, MABP or WMSA, ethnicity, blood pressure medication, and SCWT score were entered in the model. Regressions with low standardized regression weights were removed from the model, if the model fit improved significantly. The full set of model variables and starting point for each subsequent model is shown in Figure 3 and presented in detail in Table V. As can be seen from Table V the fit of the model was relatively poor for each DTI metric in the full model. Removing MABP (and thus also medication use and ethnicity because these variables were modelled in association with MABP) improved the model fit substantially for FA, ADC, and for RD, but not for DA (χ2/df > 2). Based on the model fit indices and the χ2 difference tests, the most adequate model was stepwise defined for each metric. The reduced models for each diffusivity metric no longer contained MABP, ethnicity, or medication use. The model fit for DA was acceptable, but less adequate in terms of χ2 (χ2/df > 2). The most optimal models for each diffusion metric are presented in Figure 4.
Figure 3.
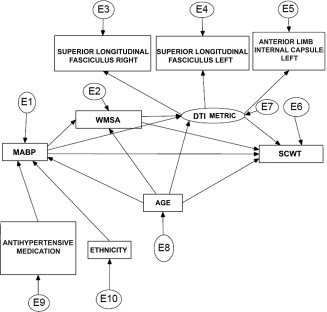
The full model for structural equation modeling analyses. The full model used for the SEM analyses for each diffusivity metric separately. E represents the error term for each included variable. MABP, mean arterial blood pressure; WMSA, white matter signal abnormalities; DTI, diffusion tensor imaging.
Table V.
Model fit indices for the full and final models on the different DTI metrics
| Model | χ2 (df) | CFI | RMSEA (90% CI) |
|---|---|---|---|
| Full model | |||
| FA | 82.70 (21)a | 0.75 | 0.15 (0.12–0.18) |
| ADC | 55.56 (21)a | 0.85 | 0.11 (0.08–0.15) |
| Axial diffusivity | 71.68 (21)a | 0.73 | 0.14 (0.10–0.17) |
| Radial diffusivity | 56.95 (21)a | 0.75 | 0.11 (0.08–0.15) |
| Without MABP | |||
| FA | 8.21 (6) | 0.98 | 0.05 (0.00–0.13) |
| ADC | 11.87 (6) | 0.97 | 0.09 (0.00–0.16) |
| Axial diffusivity | 16.17 (6)a | 0.92 | 0.11 (0.05–0.18) |
| Radial diffusivity | 8.92 (6) | 0.99 | 0.06 (0.00–0.14) |
| Reduced model | |||
| FA | 8.50 (8) | 0.99 | 0.02 (0.00–0.11) |
| ADC | 13.42 (8) | 0.97 | 0.07 (0.00–0.14) |
| Axial diffusivity | 17.32 (8)a | 0.93 | 0.09 (0.03–0.16) |
| Radial diffusivity | 9.67 (8) | 0.99 | 0.04 (0.00–0.12) |
Model fit indices for the different models from Structural Equation Modelling. FA, fractional anisotropy; ADC, apparent diffusion coefficient; CFI, comparative fit index; RMSEA, root mean‐square error of approximation; CI, confidence interval.
χ2/df > 2.
Figure 4.
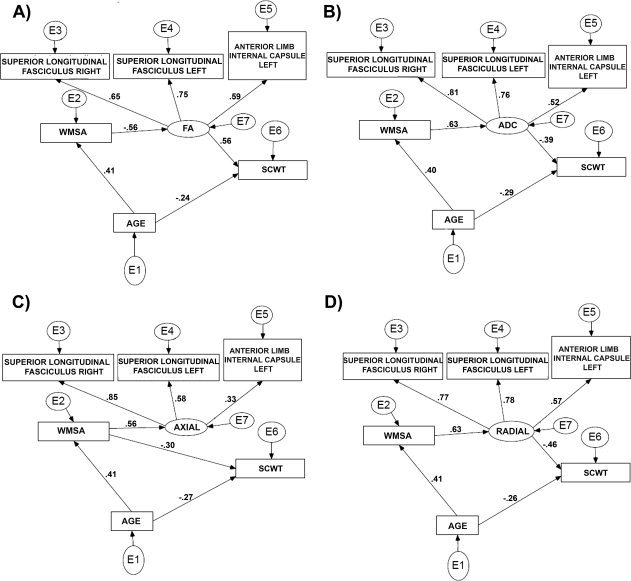
Final reduced models for the associations between DTI and cognition. The reduced models that explains best the relationship between the different diffusivity metric and cognition: A, FA; B, ADC, C, Axial diffusivity, D, Radial diffusivity. E represents the error term for each included variable. FA, fractional anisotropy; ADC, apparent diffusion coefficient; WMSA, white matter signal abnormalities.
Closer investigation of the final models showed that the direct relation between age and cognition is consistent across the four models, ranging between −0.24 and −0.29. However, this relationship was mediated by the DTI measures. As the SEM models showed, the indirect relation between age and cognition varied between −0.10 (ADC: 0.40 × 0.63 × −0.39) and −0.13 (FA: 0.41 × −0.56 × 0.56 and Radial: 0.41 × 0.63 × −0.46). And thus, the direct relation was stronger than the indirect relation. Adding the effect of the direct relationship age‐cognition with the indirect effect closely approximated the actual age‐cognition correlation of −0.379 (see Table II).
Furthermore, the relationship between the DTI metrics and cognition in an aged population was mediated by WMSA, which had been already suggested by the regression analyses and now confirmed by these SEM models. The direct relation between diffusion and cognition varied between −0.39 (ADC) and 0.56 (FA), while the actual relation between WMSA and cognition (direct and indirect) was estimated at −0.396 (see Table II). This indicated that the mediating effect of WMSA on the relationship between DTI and cognition was estimated between 0 (for ADC) and 0.16 (for FA). This roughly approximated the mean increase of 3.4% of the explained variance when adding WMSA to the DTI—Stroop model in the regression analyses.
The SEM model for the axial diffusivity analyses showed a different pattern. There was no significant mediation of axial diffusivity on the relation between age and cognition. But we did observe that WMSA mediates the relationship between age and cognition by −0.12 (0.41 × −0.30). Adding the direct effect between age and cognition of −0.27 to the indirect effect again approximated the estimated actual relationship shown in Table II.
The small added mediating effect of WMSA to the relation between ADC and cognition and the absence of a mediating effect of WMSA to the relationship between Axial diffusivity and cognition parallels the findings in the regression analyses, viz. that the mediating effect of WMSA were largest for FA and radial diffusivity.
Overlap Between Regions Showing Cognitive Associations With FA and WMSA
We mapped the colocalization of regions showing associations between cognition and white matter microstructure along with the distribution of WMSA in the sample to determine whether WMSA were responsible for the regional associations noted for FA. Figure 5 qualitatively demonstrates that the distribution of WMSA, viz. the number of participants with WMSA (threshold > 5), in our sample followed the common periventricular pattern. These regions are known to be vulnerable to ischemic events and white matter damage due to a large watershed area extending between 3 and 13 mm [Chalela et al., 2001]. Associations between DTI measures, executive functions and processing speed typically fell outside of these periventricular regions and in the deep white matter as well as in areas unlikely to show WMSA such as the corpus callosum.
Figure 5.
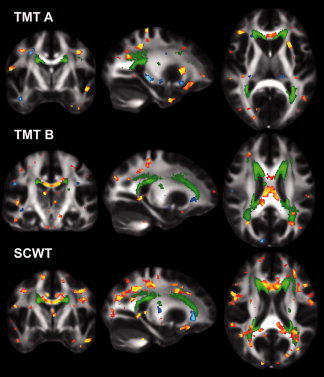
Spatial distribution of WMSA relative to the DTI clusters associated with cognition. The number of participants with WMSA (green, five participants—maximum participants) relative to the significant FA clusters associated with TMT A, TMT B or SCWT performance. TMT A, Trail Making Test part A; TMT B, Trail Making Test part B; SCWT, stroop color word task. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION
This work demonstrated that there are substantial associations between performance on executive functioning and speed of processing tests on the one hand and regional white matter integrity in generally healthy older adults on the other. Contrary to our expectations, although blood pressure was associated with several relevant variables, it did not have a substantial impact on the relationship between white matter integrity and cognition. On the other hand, WMSA burden, which has been associated with hypertension and is thought to be in part an indicator of vascular damage, contributed significantly to our model describing the relationship between blood pressure, white matter and executive functions and processing speed. These findings are consistent with prior studies demonstrating a complex pathway by which age‐associated changes in vascular health may influence neural health and contribute to cognitive decline. In the current study, although MABP and WMSA are both associated with the cognitive measures and with white matter microstructure, they each have a very different influence on the association between executive functions and processing speed, and white matter integrity. This study therefore suggests a new perspective, which should be tested in future studies; namely that blood pressure and WMSA work via different pathophysiological pathways to promote age‐associated cognitive decline. These analyses additionally suggest that DTI metrics reflect pathologies inherent to some degree in WMSA, at least in older adults. Additional work will be necessary to determine whether altered signal on DTI can differentiate tissue destined to become WMSA from more subtle and potentially independent pathological processes such as demyelination or other cellular changes that cannot be measured by WMSA.
Mediating Effect of Blood Pressure
There is little consensus on the relationship among blood pressure and cognition [Anson and Paran, 2005; de Groot et al., 2000; Duron and Hanon, 2008; Euser et al., 2009]. In this study, we found that variability in white matter integrity associated with executive functions or processing speed cannot be explained by variations in blood pressure. The lack of influence of blood pressure on the relationship between these cognition measures and white matter integrity was not dependent on the use of medication. We found no differences between participants off and on medication, similar to what we reported previously [Leritz et al., 2010]. Our recent work suggests two potential mechanisms by which blood pressure may influence cognition. We recently demonstrated an association between MABP and FA in the genu of the corpus callosum [Leritz et al., 2010] as well as a negative association between MABP and cortical thickness in bilateral frontal, temporal, and parietal regions [Leritz et al., 2010]. It is therefore possible that some cumulative effect of the subtle influence of blood pressure on both gray and white matter is necessary to account for the influence of blood pressure on cognition.
Blood pressure was examined as a continuous metric in the current study. This is in contrast to prior studies that have dichotomized participants into hypertensive or normotensive groups as the primary approach [Burgmans et al., 2009; Greenwald et al., 2001; Hannesdottir et al., 2009; Huang et al., 2006; Kennedy and Raz, 2009b]. Dichotomizing blood pressure in hypertensive and normotensive might conceal important variance and obscure the nature of the investigated relationships. The WHO suggested that a blood pressure of 140/90 mm Hg might already be considered as hypertension instead of 160/90 mm Hg. Therefore, blood pressure should better be viewed as a continuous distribution instead of a discrete one [Whitworth, 2003, 2005]. It is also possible that the relationship between blood pressure and cognition is curvilinear, in which low and high levels are associated with cognitive deficits and moderate blood pressure levels improve cognitive functioning as suggested earlier by Anson and Paran [2005]. This could be especially true in older individuals where reduced auto regulation contributes to decreased perfusion and orthostatic hypotension which may subsequently influence cerebral function. Our study adds to prior work utilizing an individual differences approach attempting to better capture the unique quantitative variance in vascular health which may influence specific cognitive domains [Glynn et al., 1999; Leritz et al., 2010; Waldstein et al., 2005; Yamada et al., 2003]. Finally, it should be noted that it is possible that resting blood pressure is a less sensitive measure than challenge‐based metrics of vascular health and reactivity for the examination of the influence of vascular health on brain tissue and cognition. Future studies will examine whether alternative metrics of systemic vascular health provide additional insight into these results.
Mediating Effects of White Matter Signal Abnormalities
Our results corroborate studies showing that the amount of WMSA is strongly associated with executive functions and processing speed [DeCarli et al., 2008; Gunning‐Dixon and Raz, 2003; Prins et al., 2005; Raz and Rodrigue, 2006; Verdelho et al., 2007; Vernooij et al., 2009]. White matter hypointensities on MRI are associated with a range of histopathologic mechnisms including reduction in myelin, gliosis, extracellular fluid, or vascular changes [de Groot et al., 1998; DeCarli, 2003; Pasquier and Leys, 1997] and have often been linked to hypertension and cognitive decline [Artero et al., 2004; de Groot et al., 2000; DeCarli et al., 2001; Firbank et al., 2007; Gunning‐Dixon and Raz, 2000; Raz et al., 2007; Salat et al., 2010; Yoshita et al., 2005]. WMSA may contribute to impaired cortical communication resulting in attenuated cognition.
Our results do not provide information on the underlying mechanisms, but we found a stronger relationship for RD than for DA. Given the animal literature relating DA to axonal integrity and RD to myelin integrity [Song et al., 2002, 2003], our results potentially suggest that the cognitive variables under investigation are affected by subtle quantitative regional demyelination and that the presence of white matter hypointensities can account for much of these associations. This inference must be taken cautiously since recent studies question the interpretation of RD and DA parameters in DTI studies [Wheeler‐Kingshott and Cercignani, 2009]. Nonetheless, our results indicate that DA and RD are differently related to executive functions and processing speed and that vascular factors such as blood pressure and WMSA have different effects upon them. The finding of stronger effects associations between cognitive performance and RD compared to DA is in accord with previous studies [Bhagat and Beaulieu, 2004; Madden et al., 2009a; Zhang et al., 2010]; however, changes in both DA and RD have been reported in the aged population [Sullivan et al., 2008; Vernooij et al., 2008].
Associations between white matter microstructure and executive functions or processing speed were found in regions outside of locations showing WMSA in this sample, however, WMSA contributed significantly to the relationship between diffusivity and cognition. Strong mediating effects of WMSA on the association between executive functions or processing speed and white matter integrity were found in areas such as the internal capsule and the corona radiate, but also distant from the periventricular areas. It is possible that periventricular damage contributes to diaschisis in other brain areas, which may appear normal on macrostrucutral level, but are altered in diffusivity at the microstructural level. Such a finding would support the disconnection hypothesis for age‐related cognitive decline, according to which white matter deterioration in older people disrupts the information flow in neural networks [Bartzokis et al., 2004; Hogan et al., 2006]. A slightly different interpretation is that WMSA are a general indicator of brain health, in tissue even remote from the lesion. In any case, these data add to a substantial literature demonstrating how altered white matter connectivity is likely an important contributor to cognitive decline [Bucur et al., 2008; Charlton et al., 2008; Kennedy and Raz 2009a; Kennedy and Raz 2009b; Sullivan and Pfefferbaum 2006].
Limitations and Future Directions
The current study has limitations which must be addressed in future work. First, the sample consisted of a large proportion of African Americans as well as cognitively healthy individuals with a family history of dementia. It is possible that this unique composition of the sample influenced the results and its generalizibility. Recent work suggests that first‐degree relatives of patients with AD have an increased risk for developing dementia [Huang et al., 2004], and may be characterized by more vascular risk factors, such as hypertension [Abdullah et al., 2009]. Thus, inclusion of first‐degree relatives of AD patients may limit the generalizibility of our results by increasing the influence of vascular risk factors on the association between cognition and white matter integrity. On the other hand, this inclusion criterion could also be considered being strength, as this allowed us to investigate the mediating effects in a sample likely enriched with cerebrovascular disease factors, such as blood pressure. This sample provided a population that, although cognitively healthy at recruitment, may be of greater risk for subsequent complications, and examination of this cohort longitudinally will of great interest for future work. The mechanisms of how WMSA mediate the association between white matter integrity and executive functioning or speed of processing needs further investigation. Therefore, we are currently in the process of replicating this work in a more normative sample.
Second, a few limitations with regards to the measurement of blood pressure should be noted. We did not have information of the actual duration of hypertension, an important risk factor when assessing the impact of vascular factors on brain structure. However, acquiring this information correctly is difficult, as many people are not aware of being hypertensive. This would require a prospective study with multiple blood pressure assessments and a larger population sample, and was therefore considered not feasible. Furthermore, a continuous monitoring of blood pressure levels would be the ideal method. Recent work showed that a 30 min office blood pressure measurement (six measurements) agrees well with daytime ambulatory blood pressure measurement and has also the potential to detect white‐coat and masked hypertension [van der Wel et al., 2011]. The participants in our sample received four blood pressure measurements (two seated and two standing), which is comparable to the procedure described in van der Wel et al., [2011]. We therefore can conclude that our blood pressure measurement can be considered reliable and we should thus be able to detect potential effects. Furthermore, we did not investigate any differences between the various medications used to treat blood pressure, as we do not have this information. However, we have corrected our analyses for medication use and the interaction of medication use and the relationship between cognition and white matter integrity revealed no significant differences between participants on or off blood pressure medication, suggesting that the effect is not significant in our sample.
Third, we only included the SCWT and the TMT in order to limit the number of analyses. These tests were chosen as an indication for executive functions and processing speed. Several studies have shown that these areas are most likely to be affected by hypertension or white matter damage [Bucur and Madden, 2010; Gunning‐Dixon and Raz, 2000; Gunning‐Dixon and Raz, 2003; Kuo et al., 2004; Prins et al., 2005; Raz and Rodrigue, 2006; Verdelho et al., 2007; Vernooij et al., 2009]. However, working memory is also an important cognitive domain that seems to be related to vascular health [Raz and Rodrigue, 2006]. Future work preferably should replicate our findings on other cognitive domains and therefore should include additional tests which tap into these domains. Related to this limitation, is the fact that we did not include the baseline trial for the SCWT reflecting perceptual motor speed. Although, the results on the TMT‐A suggest that influence from speed processes would be minimal, this should be investigated in more depth.
Given the large number of associations in the multiple regression analysis, it is possible that chance findings may have occurred. However, each regression showed the same trend across regions. Specifically, that the association between cognitive performance and white matter microstructure is mediated by WMSA. Results must be interpreted with caution given the cross‐sectional design which precludes assessment of causality. Although we used SEM analyses to investigate associations among vascular, cerebral and cognitive changes, and evaluated the contribution of individual differences in MABP or WMSA to the association between diffusion and cognition, only longitudinal designs can investigate the mutual associations between these variables. We employed an automated procedure for the labeling of white matter hypointensities on T1 weighted MRI. The use of T1 images in this manner has been described in ours and other prior work [Bagnato et al., 2003; Burns et al., 2005; Camp et al., 2005; Salat et al., 2010]. These studies found the T1 WMSA measures to be clinically relevant, altered in patient populations, and as demonstrated in this study, associated with cognition and white matter microstructure. Recent discussions in the literature of patients with Multiple Sclerosis have indeed noted that FLAIR or T2 weighted images, which are more sensitive for the detection of changes in white matter signal, may also be less clinically significant [Bagnato et al., 2003; Miller et al., 1998; Sailer et al., 2001]. Most importantly, measurements of white matter hypointensities on T1‐weighted images correlate with measurements from FLAIR, and thus provides a reliable metric of white matter lesions that is on par with more traditional techniques [Benedict et al., 2004; Rovaris et al., 1999].
We note that certain regions measured by DTI are highly prone to artifactual results given their size and location in the brain. We therefore note that results regarding the fornix stria terminalis should be interpreted cautiously as this is a thin structure surrounded by cerebrospinal fluid. Despite these limitations, the current data showed an important relationship among vascular, neural, and cognitive health in older adults, and may be important towards future understanding of the clinical management of vascular health in older adults.
CONCLUSION
In summary, our results show that variability in white matter integrity associated with executive functions or processing speed can be partially explained by WMSA and not by variations in blood pressure. Greatest associations between executive functions or processing speed performance and microstructure were found for radial diffusivity and performance on the stroop task which may suggest that the breakdown of myelin and the suggested associated loss of connectivity in older adults has an important influence on executive function. Future work must aim to understand precise mechanisms by which vascular physiology influences white matter and contributes to age‐associated cognitive decline.
Acknowledgements
The authors would like to thank Joost M. Riphagen, MD (Radboud University Medical Center, Nijmegen, The Netherlands) for his comments on our manuscript.
REFERENCES
- Abdullah L, Luis C, Paris D, Ait‐ghezala G, Mouzon B, Allen E, Parrish J, Mullan MA, Ferguson S, Wood M, et al. ( 2009): High serum Abeta and vascular risk factors in first‐degree relatives of Alzheimer's disease patients. Mol Med 15: 95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association , 2009.
- American Heart Association , 2009.
- Anson O, Paran E ( 2005): Hypertension and cognitive functioning among the elderly: An overview. Am J Ther 12: 359–365. [PubMed] [Google Scholar]
- Arbuthnott K, Frank J ( 2000): Trail making test, part B as a measure of executive control: Validation using a set‐switching paradigm. J Clin Exp Neuropsychol 22: 518–528. [DOI] [PubMed] [Google Scholar]
- Artero S, Tiemeier H, Prins ND, Sabatier R, Breteler MM, Ritchie K ( 2004): Neuroanatomical localisation and clinical correlates of white matter lesions in the elderly. J Neurol Neurosurg Psychiatry 75: 1304–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnato F, Jeffries N, Richert ND, Stone RD, Ohayon JM, McFarland HF, Frank JA ( 2003): Evolution of T1 black holes in patients with multiple sclerosis imaged monthly for 4 years. Brain 126( Part 8): 1782–1789. [DOI] [PubMed] [Google Scholar]
- Bartzokis G ( 2004): Age‐related myelin breakdown: A developmental model of cognitive decline and Alzheimer's disease. Neurobiol Aging 25: 5–18; author reply 49‐62. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Sultzer D, Lu PH, Nuechterlein KH, Mintz J, Cummings JL ( 2004): Heterogeneous age‐related breakdown of white matter structural integrity: implications for cortical “disconnection” in aging and Alzheimer's disease. Neurobiol Aging 25: 843–851. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D ( 1994): MR diffusion tensor spectroscopy and imaging. Biophys J 66: 259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict RH, Weinstock‐Guttman B, Fishman I, Sharma J, Tjoa CW, Bakshi R ( 2004): Prediction of neuropsychological impairment in multiple sclerosis: comparison of conventional magnetic resonance imaging measures of atrophy and lesion burden. Arch Neurol 61: 226–230. [DOI] [PubMed] [Google Scholar]
- Bentler PM ( 1990): Comparative fit indexes in structural models. Psychol Bull 107: 238–246. [DOI] [PubMed] [Google Scholar]
- Bhagat YA, Beaulieu C ( 2004): Diffusion anisotropy in subcortical white matter and cortical gray matter: Changes with aging and the role of CSF‐suppression. J Magn Reson Imaging 20: 216–227. [DOI] [PubMed] [Google Scholar]
- Bombois S, Debette S, Delbeuck X, Bruandet A, Lepoittevin S, Delmaire C, Leys D, Pasquier F ( 2007): Prevalence of subcortical vascular lesions and association with executive function in mild cognitive impairment subtypes. Stroke 38: 2595–2597. [DOI] [PubMed] [Google Scholar]
- Brown AD, McMorris CA, Longman RS, Leigh R, Hill MD, Friedenreich CM, Poulin MJ ( 2010): Effects of cardiorespiratory fitness and cerebral blood flow on cognitive outcomes in older women. Neurobiol Aging 31: 2047–2057. [DOI] [PubMed] [Google Scholar]
- Browne MW, Cudeck R. 1993. Alternative ways of assessing model fit In: Bollen KA, Long JS, editors. Testing Structural Equation Models. Newbury Park, CA: Sage; pp 136–162. [Google Scholar]
- Bucur B, Madden DJ ( 2010): Effects of adult age and blood pressure on executive function and speed of processing. Exp Aging Res 36: 153–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucur B, Madden DJ, Spaniol J, Provenzale JM, Cabeza R, White LE, Huettel SA ( 2008): Age‐related slowing of memory retrieval: Contributions of perceptual speed and cerebral white matter integrity. Neurobiol Aging 29: 1070–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde MD, Kim JH, Liang HF, Schmidt RE, Russell JH, Cross AH, Song SK ( 2007): Toward accurate diagnosis of white matter pathology using diffusion tensor imaging. Magn Reson Med 57: 688–695. [DOI] [PubMed] [Google Scholar]
- Burgmans S, van Boxtel MP, Gronenschild EH, Vuurman EF, Hofman P, Uylings HB, Jolles J, Raz N ( 2009): Multiple indicators of age‐related differences in cerebral white matter and the modifying effects of hypertension. Neuroimage 49: 2083–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JM, Church JA, Johnson DK, Xiong C, Marcus D, Fotenos AF, Snyder AZ, Morris JC, Buckner RL ( 2005): White matter lesions are prevalent but differentially related with cognition in aging and early Alzheimer disease. Arch Neurol 62: 1870–1876. [DOI] [PubMed] [Google Scholar]
- Camp SJ, Stevenson VL, Thompson AJ, Ingle GT, Miller DH, Borras C, Brochet B, Dousset V, Falautano M, Filippi M, et al. ( 2005): A longitudinal study of cognition in primary progressive multiple sclerosis. Brain 128( Part 12): 2891–2898. [DOI] [PubMed] [Google Scholar]
- Charlton RA, Barrick TR, McIntyre DJ, Shen Y, O'Sullivan M, Howe FA, Clark CA, Morris RG, Markus HS ( 2006): White matter damage on diffusion tensor imaging correlates with age‐related cognitive decline. Neurology 66: 217–222. [DOI] [PubMed] [Google Scholar]
- Charlton RA, Landau S, Schiavone F, Barrick TR, Clark CA, Markus HS, Morris RG ( 2008): A structural equation modeling investigation of age‐related variance in executive function and DTI measured white matter damage. Neurobiol Aging 29: 1547–1555. [DOI] [PubMed] [Google Scholar]
- Chen P, Ratcliff G, Belle SH, Cauley JA, DeKosky ST, Ganguli M ( 2000): Cognitive tests that best discriminate between presymptomatic AD and those who remain nondemented. Neurology 55: 1847–1853. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI ( 1999): Cortical surface‐based analysis. I. Segmentation and surface reconstruction. Neuroimage 9: 179–194. [DOI] [PubMed] [Google Scholar]
- de Groot JC, de Leeuw FE, Breteler MM ( 1998): Cognitive correlates of cerebral white matter changes. J Neural Transm Suppl 53: 41–67. [DOI] [PubMed] [Google Scholar]
- de Groot JC, de Leeuw FE, Oudkerk M, van Gijn J, Hofman A, Jolles J, Breteler MM ( 2000): Cerebral white matter lesions and cognitive function: The Rotterdam Scan Study. Ann Neurol 47: 145–151. [DOI] [PubMed] [Google Scholar]
- DeCarli C ( 2003): The role of cerebrovascular disease in dementia. Neurologist 9: 123–136. [DOI] [PubMed] [Google Scholar]
- DeCarli C, Miller BL, Swan GE, Reed T, Wolf PA, Carmelli D ( 2001): Cerebrovascular and brain morphologic correlates of mild cognitive impairment in the National Heart, Lung, and Blood Institute Twin Study. Arch Neurol 58: 643–647. [DOI] [PubMed] [Google Scholar]
- DeCarli C, Reed BR, Jagust W, Martinez O, Ortega M, Mungas D ( 2008): Brain behavior relationships among African Americans, whites, and Hispanics. Alzheimer Dis Assoc Disord 22: 382–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demakis GJ ( 2004): Frontal lobe damage and tests of executive processing: A meta‐analysis of the category test, stroop test, and trail‐making test. J Clin Exp Neuropsychol 26: 441–450. [DOI] [PubMed] [Google Scholar]
- Duan JH, Wang HQ, Xu J, Lin X, Chen SQ, Kang Z, Yao ZB ( 2006): White matter damage of patients with Alzheimer's disease correlated with the decreased cognitive function. Surg Radiol Anat 28: 150–156. [DOI] [PubMed] [Google Scholar]
- Duron E, Hanon O ( 2008): Hypertension, cognitive decline and dementia. Arch Cardiovasc Dis 101: 181–189. [DOI] [PubMed] [Google Scholar]
- Egner T, Hirsch J ( 2005): The neural correlates and functional integration of cognitive control in a Stroop task. Neuroimage 24: 539–547. [DOI] [PubMed] [Google Scholar]
- Esiri MM, Nagy Z, Smith MZ, Barnetson L, Smith AD ( 1999): Cerebrovascular disease and threshold for dementia in the early stages of Alzheimer's disease. Lancet 354: 919–920. [DOI] [PubMed] [Google Scholar]
- Euser SM, van Bemmel T, Schram MT, Gussekloo J, Hofman A, Westendorp RG, Breteler MM ( 2009): The effect of age on the association between blood pressure and cognitive function later in life. J Am Geriatr Soc 57: 1232–1237. [DOI] [PubMed] [Google Scholar]
- Firbank MJ, Wiseman RM, Burton EJ, Saxby BK, O'Brien JT, Ford GA ( 2007): Brain atrophy and white matter hyperintensity change in older adults and relationship to blood pressure. Brain atrophy, WMH change and blood pressure. J Neurol 254: 713–721. [DOI] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM ( 2001): Automated manifold surgery: Constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging 20: 70–80. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, et al. ( 2002): Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 33: 341–355. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM ( 1999): Cortical surface‐based analysis. II. Inflation, flattening, and a surface‐based coordinate system. Neuroimage 9: 195–207. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR ( 1975): “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatric Res 12: 189–198. [DOI] [PubMed] [Google Scholar]
- Fujishima M, Ibayashi S, Fujii K, Mori S ( 1995): Cerebral blood flow and brain function in hypertension. Hypertens Res 18: 111–117. [DOI] [PubMed] [Google Scholar]
- Giovagnoli AR, Del Pesce M, Mascheroni S, Simoncelli M, Laiacona M, Capitani E ( 1996): Trail making test: Normative values from 287 normal adult controls. Ital J Neurol Sci 17: 305–309. [DOI] [PubMed] [Google Scholar]
- Glynn RJ, Beckett LA, Hebert LE, Morris MC, Scherr PA, Evans DA ( 1999): Current and remote blood pressure and cognitive decline. JAMA 281: 438–445. [DOI] [PubMed] [Google Scholar]
- Greenlief CL, Margolis RB, Erker GJ ( 1985): Application of the Trail Making Test in differentiating neuropsychological impairment of elderly persons. Percept Mot Skills 61( Part 2): 1283–1289. [DOI] [PubMed] [Google Scholar]
- Greenwald BS, Kramer‐Ginsberg E, Krishnan KR, Hu J, Ashtari M, Wu H, Aupperle P, Patel M, Pollack S ( 2001): A controlled study of MRI signal hyperintensities in older depressed patients with and without hypertension. J Am Geriatr Soc 49: 1218–1225. [DOI] [PubMed] [Google Scholar]
- Gunning‐Dixon FM, Brickman AM, Cheng JC, Alexopoulos GS ( 2009): Aging of cerebral white matter: A review of MRI findings. Int J Geriatr Psychiatry 24: 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning‐Dixon FM, Raz N ( 2000): The cognitive correlates of white matter abnormalities in normal aging: A quantitative review. Neuropsychology 14: 224–232. [DOI] [PubMed] [Google Scholar]
- Gunning‐Dixon FM, Raz N ( 2003): Neuroanatomical correlates of selected executive functions in middle‐aged and older adults: A prospective MRI study. Neuropsychologia 41: 1929–1941. [DOI] [PubMed] [Google Scholar]
- Guo X, Pantoni L, Simoni M, Bengtsson C, Bjorkelund C, Lissner L, Gustafson D, Skoog I ( 2009): Blood pressure components and changes in relation to white matter lesions: A 32‐year prospective population study. Hypertension 54: 57–62. [DOI] [PubMed] [Google Scholar]
- Hannesdottir K, Nitkunan A, Charlton RA, Barrick TR, MacGregor GA, Markus HS ( 2009): Cognitive impairment and white matter damage in hypertension: A pilot study. Acta Neurol Scand 119: 261–268. [DOI] [PubMed] [Google Scholar]
- Head D, Buckner RL, Shimony JS, Williams LE, Akbudak E, Conturo TE, McAvoy M, Morris JC, Snyder AZ ( 2004): Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: Evidence from diffusion tensor imaging. Cereb Cortex 14: 410–423. [DOI] [PubMed] [Google Scholar]
- Hogan AM, Vargha‐Khadem F, Saunders DE, Kirkham FJ, Baldeweg T. ( 2006): Impact of frontal white matter lesions on performance monitoring: ERP evidence for cortical disconnection. Brain 129( Part 8): 2177–2188. [DOI] [PubMed] [Google Scholar]
- Hoptman MJ, Gunning‐Dixon FM, Murphy CF, Ardekani BA, Hrabe J, Lim KO, Etwaroo GR, Kanellopoulos D, Alexopoulos GS ( 2009): Blood pressure and white matter integrity in geriatric depression. J Affect Disord 115( 1‐2): 171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Auchus AP ( 2007): Diffusion tensor imaging of normal appearing white matter and its correlation with cognitive functioning in mild cognitive impairment and Alzheimer's disease. Ann NY Acad Sci 1097: 259–264. [DOI] [PubMed] [Google Scholar]
- Huang L, Ling XY, Liu SR ( 2006): Diffusion tensor imaging on white matter in normal adults and elderly patients with hypertension. Chin Med J (Engl) 119: 1304–1307. [PubMed] [Google Scholar]
- Huang W, Qiu C, von Strauss E, Winblad B, Fratiglioni L ( 2004): APOE genotype, family history of dementia, and Alzheimer disease risk: A 6‐year follow‐up study. Arch Neurol 61: 1930–1934. [DOI] [PubMed] [Google Scholar]
- Hugenschmidt CE, Peiffer AM, Kraft RA, Casanova R, Deibler AR, Burdette JH, Maldjian JA, Laurienti PJ ( 2008): Relating imaging indices of white matter integrity and volume in healthy older adults. Cereb Cortex 18: 433–442. [DOI] [PubMed] [Google Scholar]
- Jones DK, Horsfield MA, Simmons A ( 1999): Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magn Reson Med 42: 515–525. [PubMed] [Google Scholar]
- Kaufmann L, Koppelstaetter F, Delazer M, Siedentopf C, Rhomberg P, Golaszewski S, Felber S, Ischebeck A ( 2005): Neural correlates of distance and congruity effects in a numerical Stroop task: An event‐related fMRI study. Neuroimage 25: 888–898. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Raz N ( 2009a): Aging white matter and cognition: differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia 47: 916–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KM, Raz N ( 2009b): Pattern of normal age‐related regional differences in white matter microstructure is modified by vascular risk. Brain Res 1297: 41–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilander L, Nyman H, Boberg M, Hansson L, Lithell H ( 1998): Hypertension is related to cognitive impairment: A 20‐year follow‐up of 999 men. Hypertension 31: 780–786. [DOI] [PubMed] [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, Christensen GE, Collins DL, Gee J, Hellier P, et al. ( 2009): Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage 46: 786–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo HK, Sorond F, Iloputaife I, Gagnon M, Milberg W, Lipsitz LA ( 2004): Effect of blood pressure on cognitive functions in elderly persons. J Gerontol A Biol Sci Med Sci 59: 1191–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leritz EC, Salat DH, Milberg WP, Williams VJ, Chapman CE, Grande LJ, Rudolph JL, Schnyer DM, Barber CE, Lipsitz LA, et al. ( 2010): Variation in blood pressure is associated with white matter microstructure but not cognition in African Americans. Neuropsychology 24: 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leritz EC, Salat DH, Williams VJ, Schnyer DM, Rudolph JL, Lipsitz L, Fischl B, McGlinchey RE, Milberg WP ( 2011): Thickness of the human cerebral cortex is associated with metrics of cerebrovascular health in a normative sample of community dwelling older adults. Neuroimage 54: 2659–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK ( 1996): The progression from hypertension to congestive heart failure. JAMA 275: 1557–1562. [PubMed] [Google Scholar]
- Lezak MD ( 1995): Neuropsychological ASSESSMENT. New York Oxford: Oxford University Press. [Google Scholar]
- Madden DJ, Bennett IJ, Song AW ( 2009a): Cerebral white matter integrity and cognitive aging: Contributions from diffusion tensor imaging. Neuropsychol Rev 19: 415–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Costello MC, Bucur B, White LE, Cabeza R, Davis SW, Dennis NA, Provenzale JM, Huettel SA ( 2009b): Cerebral white matter integrity mediates adult age differences in cognitive performance. J Cogn Neurosci 21: 289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis A, Schunck T, Erb G, Namer IJ, Luthringer R ( 2009): The effect of aging on the inhibitory function in middle‐aged subjects: A functional MRI study coupled with a color‐matched Stroop task. Int J Geriatr Psychiatry 24: 1062–1071. [DOI] [PubMed] [Google Scholar]
- Medina D, DeToledo‐Morrell L, Urresta F, Gabrieli JD, Moseley M, Fleischman D, Bennett DA, Leurgans S, Turner DA, Stebbins GT ( 2006): White matter changes in mild cognitive impairment and AD: A diffusion tensor imaging study. Neurobiol Aging 27: 663–672. [DOI] [PubMed] [Google Scholar]
- Miller DH, Grossman RI, Reingold SC, McFarland HF ( 1998): The role of magnetic resonance techniques in understanding and managing multiple sclerosis. Brain 121( Part 1): 3–24. [DOI] [PubMed] [Google Scholar]
- Moll J, de Oliveira‐Souza R, Moll FT, Bramati IE, Andreiuolo PA ( 2002): The cerebral correlates of set‐shifting: an fMRI study of the trail making test. Arq Neuropsiquiatr 60: 900–905. [DOI] [PubMed] [Google Scholar]
- Nylenna M, Riis P ( 1991): Identification of patients in medical publications: need for informed consent. Bmj 302: 1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan M, Jones DK, Summers PE, Morris RG, Williams SC, Markus HS ( 2001): Evidence for cortical “disconnection” as a mechanism of age‐related cognitive decline. Neurology 57: 632–638. [DOI] [PubMed] [Google Scholar]
- O'Sullivan M, Morris RG, Huckstep B, Jones DK, Williams SC, Markus HS ( 2004): Diffusion tensor MRI correlates with executive dysfunction in patients with ischaemic leukoaraiosis. J Neurol Neurosurg Psychiatry 75: 441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera‐Souza RD, Moll J, Passman LJ, Cunha FC, Paes F, Adriano MV, Ignacio FA, Marrocos RP ( 2000): Trail making and cognitive set‐shifting. Arq Neuropsiquiatr 58: 826–829. [DOI] [PubMed] [Google Scholar]
- Pasquier F, Leys D ( 1997): Why are stroke patients prone to develop dementia? J Neurol 244: 135–142. [DOI] [PubMed] [Google Scholar]
- Penke L, Deary IJ ( 2010): Some guidelines for structural equation modelling in cognitive neuroscience: The case of Charlton et al.'s study on white matter integrity and cognitive ageing. Neurobiol Aging 31: 1656–1660; discussion 1561–1566. [DOI] [PubMed] [Google Scholar]
- Perry ME, McDonald CR, Hagler DJ Jr, Gharapetian L, Kuperman JM, Koyama AK, Dale AM, McEvoy LK ( 2009): White matter tracts associated with set‐shifting in healthy aging. Neuropsychologia 47: 2835–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins ND, van Dijk EJ, den Heijer T, Vermeer SE, Jolles J, Koudstaal PJ, Hofman A, Breteler MM. ( 2005): Cerebral small‐vessel disease and decline in information processing speed, executive function and memory. Brain 128( Part 9): 2034–2041. [DOI] [PubMed] [Google Scholar]
- Pujol J, Vendrell P, Deus J, Junque C, Bello J, Marti‐Vilalta JL, Capdevila A ( 2001): The effect of medial frontal and posterior parietal demyelinating lesions on stroop interference. Neuroimage 13: 68–75. [DOI] [PubMed] [Google Scholar]
- Qiu C, Winblad B, Fratiglioni L ( 2005): The age‐dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol 4: 487–499. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM ( 2006): Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev 30: 730–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Acker JD ( 2007): Vascular health and longitudinal changes in brain and cognition in middle‐aged and older adults. Neuropsychology 21: 149–157. [DOI] [PubMed] [Google Scholar]
- Reese TG, Heid O, Weisskoff RM, Wedeen VJ ( 2003): Reduction of eddy‐current‐induced distortion in diffusion MRI using a twice‐refocused spin echo. Magn Reson Med 49: 177–182. [DOI] [PubMed] [Google Scholar]
- Reis HT, Judd CM ( 2000): Handbook of Research Methods in Social and Personality Psychology. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Rose SE, McMahon KL, Janke AL, O'Dowd B, de Zubicaray G, Strudwick MW, Chalk JB ( 2006): Diffusion indices on magnetic resonance imaging and neuropsychological performance in amnestic mild cognitive impairment. J Neurol Neurosurg Psychiatry 77: 1122–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovaris M, Comi G, Rocca MA, Cercignani M, Colombo B, Santuccio G, Filippi M ( 1999): Relevance of hypointense lesions on fast fluid‐attenuated inversion recovery MR images as a marker of disease severity in cases of multiple sclerosis. AJNR Am J Neuroradiol 20: 813–820. [PMC free article] [PubMed] [Google Scholar]
- Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ ( 1999): Nonrigid registration using free‐form deformations: Application to breast MR images. IEEE Trans Med Imaging 18: 712–721. [DOI] [PubMed] [Google Scholar]
- Sailer M, Losseff NA, Wang L, Gawne‐Cain ML, Thompson AJ, Miller DH ( 2001): T1 lesion load and cerebral atrophy as a marker for clinical progression in patients with multiple sclerosis. A prospective 18 months follow‐up study. Eur J Neurol 8: 37–42. [DOI] [PubMed] [Google Scholar]
- Salat DH, Greve DN, Pacheco JL, Quinn BT, Helmer KG, Buckner RL, Fischl B ( 2009): Regional white matter volume differences in nondemented aging and Alzheimer's disease. Neuroimage 44: 1247–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, Zaleta AK, Rosen BR, Fischl B, Corkin S, Rosas HD, et al. ( 2005): Age‐related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging 26: 1215–1227. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, van der Kouwe AJ, Greve DN, Pappu V, Lee SY, Hevelone ND, Zaleta AK, Growdon JH, Corkin S, et al. ( 2010): White matter pathology isolates the hippocampal formation in Alzheimer's disease. Neurobiol Aging 31: 244–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Atkinson TM, Berish DE ( 2003): Executive functioning as a potential mediator of age‐related cognitive decline in normal adults. J Exp Psychol Gen 132: 566–594. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Toth J, Daniels K, Parks C, Pak R, Wolbrette M, Hocking KJ ( 2000): Effects of aging on efficiency of task switching in a variant of the trail making test. Neuropsychology 14: 102–111. [PubMed] [Google Scholar]
- Schiavone F, Charlton RA, Barrick TR, Morris RG, Markus HS ( 2009): Imaging age‐related cognitive decline: A comparison of diffusion tensor and magnetization transfer MRI. J Magn Reson Imaging 29: 23–30. [DOI] [PubMed] [Google Scholar]
- Sepe‐Monti M, Pantano P, Vanacore N, De Carolis A, Bianchi V, Antonini G, Guidoni SV, Giubilei F ( 2007): Vascular risk factors and white matter hyperintensities in patients with amnestic mild cognitive impairment. Acta Neurol Scand 115: 419–424. [DOI] [PubMed] [Google Scholar]
- Settakis G, Pall D, Molnar C, Bereczki D, Csiba L, Fulesdi B ( 2003): Cerebrovascular reactivity in hypertensive and healthy adolescents: TCD with vasodilatory challenge. J Neuroimaging 13: 106–112. [PubMed] [Google Scholar]
- Smith SM ( 2002): Fast robust automated brain extraction. Hum Brain Mapp 17: 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen‐Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, et al. ( 2006): Tract‐based spatial statistics: Voxelwise analysis of multi‐subject diffusion data. Neuroimage 31: 1487–1505. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen‐Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, et al. ( 2004): Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23( Suppl 1): S208–S219. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH ( 2003): Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 20: 1714–1722. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH ( 2002): Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 17: 1429–1436. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Levine B ( 2002): Adult clinical neuropsychology: lessons from studies of the frontal lobes. Annu Rev Psychol 53: 401–433. [DOI] [PubMed] [Google Scholar]
- Sugimori H, Ibayashi S, Fujii K, Sadoshima S, Kuwabara Y, Fujishima M ( 1995): Can transcranial Doppler really detect reduced cerebral perfusion states? Stroke 26: 2053–2060. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A ( 2006): Diffusion tensor imaging and aging. Neurosci Biobehav Rev 30: 749–761. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rohlfing T, Pfefferbaum A ( 2008): Quantitative fiber tracking of lateral and interhemispheric white matter systems in normal aging: Relations to timed performance. Neurobiol Aging 31: 464–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WD, Payne ME, Krishnan KR, Wagner HR, Provenzale JM, Steffens DC, MacFall JR ( 2001): Evidence of white matter tract disruption in MRI hyperintensities. Biol Psychiatry 50: 179–183. [DOI] [PubMed] [Google Scholar]
- Van der Elst W, van Boxtel MPJ, van Breukelen GJP, Jolles J ( 2008): A large‐scale cross‐sectional and longitudinal study into the ecological validity of neuropsychological test measures in neurologically intact people. Arch Clin Neuropsychol 23 ( 7‐8): 787–800. [DOI] [PubMed] [Google Scholar]
- van der Flier WM, van der Vlies AE, Weverling‐Rijnsburger AW, de Boer NL, Admiraal‐Behloul F, Bollen EL, Westendorp RG, van Buchem MA, Middelkoop HA ( 2005): MRI measures and progression of cognitive decline in nondemented elderly attending a memory clinic. Int J Geriatr Psychiatry 20: 1060–1066. [DOI] [PubMed] [Google Scholar]
- van der Wel MC, Buunk IE, van Weel C, Thien TA, Bakx JC ( 2011): A novel approach to office blood pressure measurement: 30‐minute office blood pressure vs daytime ambulatory blood pressure. Ann Fam Med 9: 128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdelho A, Madureira S, Ferro JM, Basile AM, Chabriat H, Erkinjuntti T, Fazekas F, Hennerici M, O'Brien J, Pantoni L, et al. ( 2007): Differential impact of cerebral white matter changes, diabetes, hypertension and stroke on cognitive performance among non‐disabled elderly. The LADIS study. J Neurol Neurosurg Psychiatry 78: 1325–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernooij MW, de Groot M, van der Lugt A, Ikram MA, Krestin GP, Hofman A, Niessen WJ, Breteler MM ( 2008): White matter atrophy and lesion formation explain the loss of structural integrity of white matter in aging. Neuroimage 43: 470–477. [DOI] [PubMed] [Google Scholar]
- Vernooij MW, Ikram MA, Vrooman HA, Wielopolski PA, Krestin GP, Hofman A, Niessen WJ, Van der Lugt A, Breteler MM ( 2009): White matter microstructural integrity and cognitive function in a general elderly population. Arch Gen Psychiatry 66: 545–553. [DOI] [PubMed] [Google Scholar]
- Waldstein SR, Giggey PP, Thayer JF, Zonderman AB ( 2005): Nonlinear relations of blood pressure to cognitive function: The Baltimore Longitudinal Study of Aging. Hypertension 45: 374–379. [DOI] [PubMed] [Google Scholar]
- Wheeler‐Kingshott CA, Cercignani M ( 2009): About “axial” and “radial” diffusivities. Magn Reson Med 61( 5), 1255–60. [DOI] [PubMed] [Google Scholar]
- Whitworth JA ( 2003): 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens 21: 1983–1992. [DOI] [PubMed] [Google Scholar]
- Whitworth JA ( 2005): Blood pressure and control of cardiovascular risk. Vasc Health Risk Manag 1: 257–260. [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Kasagi F, Sasaki H, Masunari N, Mimori Y, Suzuki G ( 2003): Association between dementia and midlife risk factors: The Radiation Effects Research Foundation Adult Health Study. J Am Geriatr Soc 51: 410–414. [DOI] [PubMed] [Google Scholar]
- Yoshita M, Fletcher E, DeCarli C ( 2005): Current concepts of analysis of cerebral white matter hyperintensities on magnetic resonance imaging. Top Magn Reson Imaging 16: 399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakzanis KK, Mraz R, Graham SJ ( 2005): An fMRI study of the Trail Making Test. Neuropsychologia 43: 1878–1886. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Du AT, Hayasaka S, Jahng GH, Hlavin J, Zhan W, Weiner MW, Schuff N ( 2010): Patterns of age‐related water diffusion changes in human brain by concordance and discordance analysis. Neurobiol Aging 31: 1991–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]


