Abstract
Unresectable colorectal carcinomas (CRCs) as considered incurable even if the primary tumors and the metastatic ones can undergo resection are correlated with poor prognosis. We evaluated the association between micropapillary pattern at the invasive front and unresectable CRCs. Thirty-four out of 264 (12.9%) CRC patients with stages III and IV were unresectable cases. The patients with unresectable CRCs had significantly worse survival than those with resectable CRCs (P < 0.001). Micropapillary pattern was evident in 12 (4.5%) out of 264 cases. This pattern was observed in 6 of 34 (17.6%) unresectable CRCs and in 6 of 230 (2.6%) resectable cases (P = 0.002). Unresectable CRCs revealed more frequently deeper invasion (odds ratio (OR), 1.175; 95% confidence interval (CI), 1.113–1.241), lymph node metastasis (OR, 2.356; 95% CI, 1.132–4.905), and presence of micropapillary pattern at the invasive front (OR, 8.000; 95% CI, 2.415–26.504) as compared to resectable cases. By multivariable logistic regression analysis, only micropapillary pattern was shown to be an independent predictor of unresectable CRCs (OR, 9.451; 95% CI, 2.468–36.196; P < 0.001). In conclusion, micropapillary pattern at the invasive front is associated with unresectable CRCs, and detection of it could help identify unresectable CRC cases.
1. Introduction
Colorectal cancer (CRC) is a major cause of death in Japan, where it accounts for the largest number of deaths from malignant neoplasms in women and the third largest number in men [1]. The cumulative 5-year survival rates of Japanese CRC are 90.6% at stage I, 81.2% at stage II, 63.7% at stage III, and 13.2% at stage IV [1]. Unresectable CRCs are considered incurable even if the primary tumors as well as the metastatic ones are able to undergo resection, has been correlated with poor prognosis [2]. We previously reported that the preferential site and age of unresectable CRCs were not different from resectable CRCs, but K-ras mutations were more frequent in the latter [3]. The survival of unresectable CRC patients has dramatically improved with the progress in chemotherapeutic regimens such as new routes of administration and introduction of more potent cytotoxic agents. Biologic therapy in combination with chemotherapy leads to improved progression-free survival and overall survival in some cases such as the addition of cetuximab to cases with wild-type K-ras tumors [4]. For patients with an asymptomatic intact primary tumor, the initial prediction of metastatic potential is important for early intervention before disease progression to unresectable CRC.
Invasive micropapillary carcinoma (IMPC) was first described in the breast by Siriaunkgul and Tavassoli [5] and subsequently in other organs, including urinary bladder [6], lung [7], ovary [8], salivary gland [9], stomach [10], and colorectum [11–13]. Histologically, micropapillary features are characterized by tight neoplastic cell clusters surrounded by cleft-like spaces. The tumor cells have eosinophilic cytoplasm and pleomorphic nuclei. The inverted polarity of the cancer cells that compose the micropapillary nests with an inside-out growth pattern is probably related to its high invasive potential [14, 15]. Micropapillary CRC is rare with previously reported cases fewer than 130 [11–13]. In CRCs, micropapillary pattern is associated with a high risk of lymph node metastasis [13] and an unfavorable prognosis in CRC patients with stage I and II [16].
The purpose of this study was to clarify the association between micropapillary pattern at the invasive front and unresectable CRCs.
2. Materials and Methods
2.1. Study Cases and Tissue Samples
The subjects of our study were a consecutive series of 1165 patients diagnosed and undergoing surgery for CRC at the Dokkyo Medical University School of Medicine and affiliated hospitals between January 2004 and December 2009. Patients with multiple CRCs or those who had died within 30 days of surgery were not included in the analysis. Proximal cancers were classified as tumors proximal to the splenic flexure, and the remaining tumors were defined as distal. The depth of invasion (pT category), lymph node involvement (pN category), and pathological staging of all surgically resected tumors were assessed according to the UICC/TNMclassification. Out of 1165 patients, 264 cases (22.7%) were classified as stages III and IV, who were enrolled in our study. The patients comprised 138 males and 126 females, with a mean age of 65.4 years (median, 66 years; range, 32–88). Unresectable CRC was defined as a primary or locally recurrent tumor that is incurable even if the primary tumors as well as the metastatic ones are able to undergo resection. Remaining CRCs were resectable.
The Ethics Committee of Dokkyo Medical University School of Medicine approved all protocols, and informed consent for tissue procurement was obtained from all patients. For the ethics procedure, this work was conducted in a blinded manner using a linkable anonymizing method. Samples used in this study were surgically resected materials for diagnosis or treatment but not for research purposes. Participation in the present study did not increase medical disadvantage or risk for patients.
2.2. Histologic Evaluation
Resected specimens were fixed in 10% formalin and processed for embedding in paraffin wax according to routine procedures, and then sections were cut and stained with hematoxylin and eosin (H&E). We selected H&E staining specimens of the invasive front (deepest part) of CRC and assessed whether they contained micropapillary pattern or not. Micropapillary component in CRC is usually a minor component of the entire tumor and is often present at the edge of the invasive tumor [13]. We thus focused on the presence of this pattern at the invasive front without taking into account the amount of micropapillary pattern. Micropapillary pattern was defined as a carcinoma composed of small clusters of tumor cells within stromal spaces that mimic vascular channels (Figure 1(a)), according to the previous description [11–13, 17]. We additionally used the MUC1 (clone Ma695, dilution 1 : 100; Novocastra, Newcastle, UK) and EMA (clone E29, dilution 1 : 200; Dako, Glostrup, Denmark) immunostaining to emphasize the inside-out pattern of micropapillary pattern. MUC1 expression was strongly and diffusely present in the stroma-facing (basal) surface of the neoplastic cell clusters, consisting of a thin band of staining in all carcinomas with micropapillary morphology invariably and regardless of the site (Figure 1(b)) [15].
Figure 1.
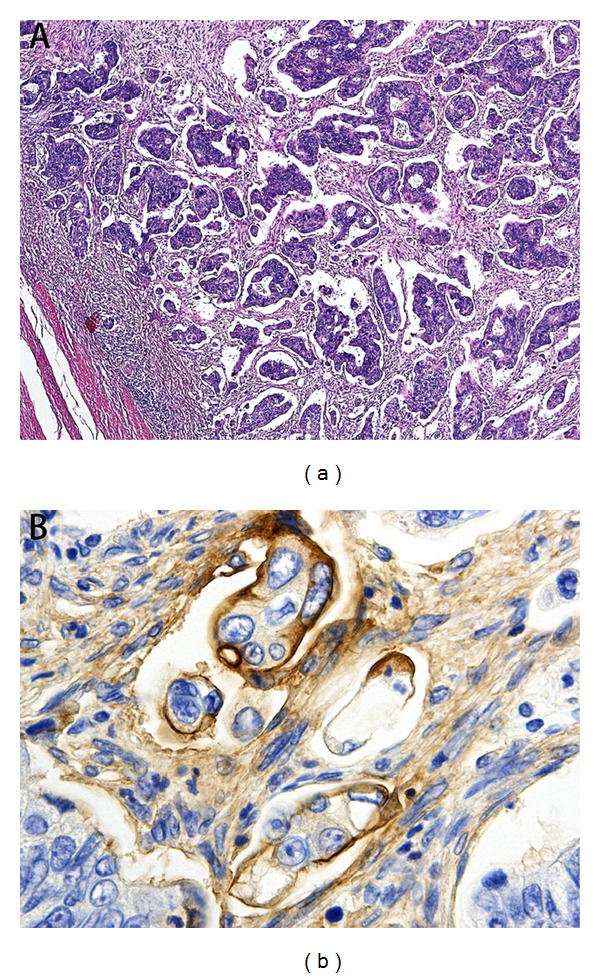
(a) Micropapillary pattern at the invasive front in CRC (H&E, ×100). (b) MUC1 immunoreactivity is present in the stroma-facing (basal) surface of the cancer cell clusters, indicating inside-out pattern (×400).
Well-to-moderately differentiated tubular adenocarcinoma (tub) was characterized by large to small gland formation, and poorly differentiated adenocarcinoma (por) had little tendency to form glands or tubules but had intracellular mucus production. Mucinous carcinoma (muc) had at least 50% mucinous component. Venous or lymphatic invasion was categorized as either positive (v+/ly+) or negative (v−/ly−). A consensus diagnosis was reached for unequivocal cases by reviewing the slides together.
2.3. Statistical Analysis
Fisher's exact or chi-squire test was used to compare categorical variables. Survival curves were generated by the Kaplan-Meier method, and differences in survival were compared using the log-rank test. Multivariate analysis was performed using unconditional logistic regression models to calculate odds ratios (ORs) for unresectable CRCs, according to the status of the given clinicopathological variables and presence of micropapillary pattern. Differences were considered to be statistically significant if P < 0.05. Statistical analysis was performed with SPSS (version 20.0, Chicago, IL, USA).
3. Results
3.1. Clinicopathological Characteristics of Resectable and Unresectable CRCs
Thirty-four out of the 264 (12.9%) CRC patients with stages III and IV were unresectable cases. Micropapillary pattern was evident in 12 (4.5%) out of 264 CRC patients with stages III and IV. This pattern was observed in 6 of 34 (17.6%) unresectable CRCs and in 6 of 230 (2.6%) unresectable CRCs(P = 0.002). The percentages of the length of micropapillary pattern at the invasive front per the maximal length of the invasive carcinoma were calculated using H&E specimen as follows: resectable CRCs, 66.0/61.0 ± 30.0% (mean/median ± standard deviation); unresectable CRCs, 64.0/72.0 ± 30.7% (P = 0.905).
Unresectable CRCs revealed more frequently deeper invasion (odds ratio (OR), 1.175, 95% confidence interval (CI) 1.113–1.241), lymph node metastasis (OR, 2.356; 95% CI, 1.132–4.905), and presence of micropapillary pattern at the invasive front (OR, 8.000; 95% CI, 2.415–26.504) as compared to resectable cases (Table 1).
Table 1.
Clinicopathological characteristics of resectable and unresectable CRCs.
| Variable | Unresectable CRCs | Resectable CRCs | P value |
|---|---|---|---|
| (n = 34) | (n = 230) | ||
| Age (years) | |||
| ≤50 | 6 | 19 | 0.109 |
| >50 | 28 | 211 | |
| Sex | |||
| Male | 19 | 119 | 0.652 |
| Female | 15 | 111 | |
| Location | |||
| Left colon | 24 | 158 | 0.824 |
| Right colon | 10 | 72 | |
| Depth of tumor invasion | |||
| pT2 | 0 | 36 | 0.007 |
| pT3 | 34 | 194 | |
| Lymph node metastasis | |||
| pN1 | 18 | 167 | 0.019 |
| pN2 | 16 | 63 | |
| Lymphatic invasion | |||
| ly (−) | 0 | 8 | 0.602 |
| ly (+) | 34 | 222 | |
| Venous invasion | |||
| v (−) | 1 | 27 | 0.145 |
| v (+) | 33 | 203 | |
| Histologic type | |||
| tub | 31 | 213 | 0.730 |
| por/muc | 3 | 17 | |
| Micropapillary pattern | |||
| Presence | 6 | 6 | 0.002 |
| Absence | 28 | 224 |
CRCs: colorectal carcinomas.
3.2. Multivariable Logistic Regression Estimates for Risk Factors of Unresectable CRCs
In the multivariable logistic regression analysis, micropapillary pattern only proved to be independent predictors of unresectable CRCs (OR, 9.451; 95% CI, 2.468–36.196; P < 0.001) (Table 2).
Table 2.
Multivariable logistic regression estimates for risk factors of unresectable CRCs.
| Variable | OR | 95% CI | P value |
|---|---|---|---|
| Depth of tumor invasion | — | — | 0.998 |
| Lymph node metastasis | 1.94 | 0.898–4.198 | 0.092 |
| Presence of micropapillary pattern | 9.451 | 2.468–36.196 | <0.001 |
OR: odds ratio; CI: confidence interval.
3.3. Prognosis in Patients with Resectable and Unresectable CRCs
Survival analysis was conducted for the patients who underwent surgery between 2005 and 2009 and were observed for more than two years. The median duration of follow-up was 31 months (range, 25–91) for the survivors who were alive at the date of their last visit. The patients with unresectable CRCs had significantly worse survival than those with resectable CRCs (P < 0.001; Figure 2).
Figure 2.
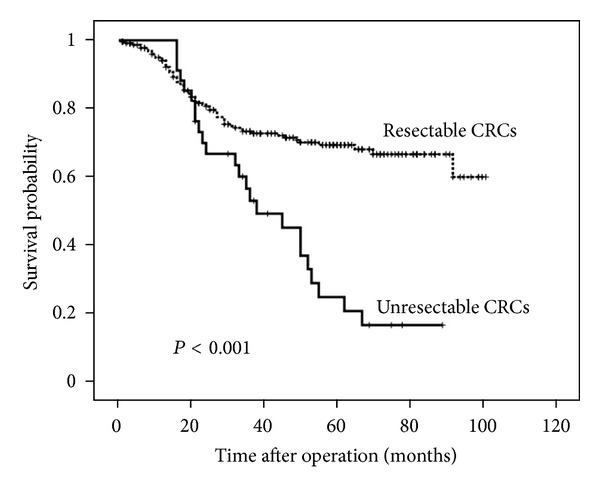
Survival curves of unresectable and resectable CRC patients.
4. Discussion
This report is the first to demonstrate the significant correlation between micropapillary pattern at the invasive front and unresectable CRCs. We show here that only micropapillary pattern was shown to be an independent predictor of unresectable CRCs by multivariable logistic regression analysis. Unexpectedly, our analysis revealed that depth of invasion (pT), nodal status (pN), and lymphovascular invasion were not associated with unresectable CRC.
Micropapillary pattern at the invasive front was identified in 12 of 264 (4.5%) CRC patients with stages III and IV. The age of the CRC patients with micropapillary pattern ranged from 41 to 75 years (mean/median, 62.0/66.0). Eight (66.7%) patients were men and 4 were women. Nine of 12 (75%) cases were located in the left colon and 3 were in the right colon. Two of 12 (16.6%) cases were pT2, and the remaining were pT3 or T4. Eight of 12 (66.7%) cases were pN1 and 4 were pN2. Histologically, there were 10 moderately differentiated tubular adenocarcinomas and 2 poorly differentiated adenocarcinomas. The percentages of length of micropapillary pattern per maximal length of the invasive carcinoma ranged from 24% to 100% (median, 66).
The incidence of micropapillary pattern in our study is much lower than the values (15–31%) reported by previous studies [13, 15, 16, 18]. This difference may due to different study patient populations (racial and environmental differences), different tumor stage, or different criteria used for identification of micropapillary component. Haupt et al. [13] stated that micropapillary component in CRC is usually a minor component of the entire tumor and is often present at the edge of invasive tumor. In CRC, the growth pattern at the invasive front represents a powerful prognostic tool related to the presence of small cancer cell clusters that invade the surrounding stromal compartment [19, 20]. Along this line, we focused on the presence of micropapillary pattern at the invasive front in CRC without taking into account the amount of micropapillary pattern. In fact, the relationship between the amount of micropapillary component and the prognosis remains inconclusive [13, 18].
We applied the term “micropapillary pattern” because the proportion of this component needed for the diagnosis of IMPC has not yet been decided. In some cases, small cancer clusters composed of micropapillary components, which are similar to lymphoid vessels, are difficult to distinguish from poorly differentiated adenocarcinomas. In our ancillary immunohistochemistry, MUC1 or EMA staining confirmed the inside-out pattern of IMPC, consistent with a previous report [15]. MUC1 is considered to be a key factor in the detachment of cells from the stroma, which is a morphological feature of the micropapillary pattern [14, 19]. It is also important not to overdiagnose cancer cells within the lymphovascular spaces as this pattern.
Despite advances in chemotherapy and surgical techniques, only a highly selective subgroup of stage IV CRC patients are eligible to receive resection of the primary tumor with concomitant metastatic lesion followed by chemotherapy as curative treatment and to prolong survival [1, 21]. In the remaining patients with unresectable stage IV CRC, the sequential introduction of new cytotoxic agents (irinotecan and oxaliplatin) and targeted therapies (bevacizumab and cetuximab) has contributed to significant improvement in the response rate and survival [22–24].
In conclusion, the micropapillary pattern at the invasive front is associated with unresectable CRCs. Therefore, detection of this pattern could facilitate the identification of unresectable CRC cases. Subsequently, predicting unresectable CRC may allow the administration of adjuvant chemotherapy including molecular target therapy in the early stage for better prognosis.
Conflict of Interests
The authors declare no conflict of interests.
Acknowledgments
The authors would like to thank C. Matsuyama, A. Shimizu, T. Ono, M. Katayama, N. Nagashima, S. Kidate, and A. Kikuchi (Department of Surgical and Molecular Pathology, Dokkyo University School of Medicine, Tochigi, Japan) for their excellent technical and secretarial assistance. The authors are grateful to Y. Okamoto, T. Yamaguchi, Y. Otake, K. Okamoto, S. Kobayashi, and Y. Shida for their invaluable comments on the paper.
References
- 1.Watanabe T, Itabashi M, Shimada Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. International Journal of Clinical Oncology. 2011;17:1–29. doi: 10.1007/s10147-011-0315-2. [DOI] [PubMed] [Google Scholar]
- 2.Shao YC, Chang YY, Lin JK, et al. Neoadjuvant chemotherapy can improve outcome of colorectal cancer patients with unresectable metastasis. International Journal of Colorectal Disease. 2013;28(10):1359–1365. doi: 10.1007/s00384-013-1713-x. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka T, Ichikawa K, Muraoka T, et al. Analysis of the anatomic subsites, gender and age in unresectable advanced colorectal carcinomas in Tochigi, Japan suggests a shift in location towards the right side colon in elderly patients treated with cetuximab. Molecular Clinical Oncology. 2013;1(2):291–296. doi: 10.3892/mco.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adam R, Haller DG, Poston G, et al. Toward optimized front-line therapeutic strategies in patients with metastatic colorectal cancer-an expert review from the international congress on anti-cancer treatment (ICACT) 2009. Annals of Oncology. 2010;21(8):1579–1584. doi: 10.1093/annonc/mdq043. [DOI] [PubMed] [Google Scholar]
- 5.Siriaunkgul S, Tavassoli FA. Invasive micropapillary carcinoma of the breast. Modern Pathology. 1993;6(6):660–662. [PubMed] [Google Scholar]
- 6.Amin MB, Ro JY, El-Sharkawy T, et al. Micropapillary variant of transitional cell carcinoma of the urinary bladder: histologic pattern resembling ovarian papillary serous carcinoma. American Journal of Surgical Pathology. 1994;18(12):1224–1232. doi: 10.1097/00000478-199412000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Amin MB, Tamboli P, Merchant SH, et al. Micropapillary component in lung adenocarcinoma: a distinctive histologic feature with possible prognostic significance. American Journal of Surgical Pathology. 2002;26(3):358–364. doi: 10.1097/00000478-200203000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Sehdev AES, Sehdev PS, Kurman RJ. Noninvasive and invasive micropapillary (low-grade) serous carcinoma of the ovary: a clinicopathologic analysis of 135 cases. American Journal of Surgical Pathology. 2003;27(6):725–736. doi: 10.1097/00000478-200306000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Michal M, Skálová A, Mukenšnabl P. Micropapillary carcinoma of the parotid gland arising in mucinous cystadenoma. Virchows Archiv. 2000;437(4):465–468. doi: 10.1007/s004280000274. [DOI] [PubMed] [Google Scholar]
- 10.Shimoda M, Okada Y, Hayashi Y, et al. Primary invasive micropapillary carcinoma of the stomach. Pathology International. 2008;58(8):513–517. doi: 10.1111/j.1440-1827.2008.02265.x. [DOI] [PubMed] [Google Scholar]
- 11.Sakamoto K, Watanabe M, de La Cruz C, et al. Primary invasive micropapillary carcinoma of the colon. Histopathology. 2005;47(5):479–484. doi: 10.1111/j.1365-2559.2005.02241.x. [DOI] [PubMed] [Google Scholar]
- 12.Trabelsi A, Ali AB, Yacoub-Abid LB, Stita W, Mokni M, Korbi S. Primary invasive micropapillary carcinoma of the colon: case report and review of the literature. Pathologica. 2008;100(5):428–430. [PubMed] [Google Scholar]
- 13.Haupt B, Ro JY, Schwartz MR, Shen SS. Colorectal adenocarcinoma with micropapillary pattern and its association with lymph node metastasis. Modern Pathology. 2007;20(7):729–733. doi: 10.1038/modpathol.3800790. [DOI] [PubMed] [Google Scholar]
- 14.Verdu M, Roman R, Calvo M, et al. Clinicopathological and molecular characterization of colorectal micropapillary carcinoma. Modern Pathology. 2011;24:729–738. doi: 10.1038/modpathol.2011.1. [DOI] [PubMed] [Google Scholar]
- 15.Nassar H, Pansare V, Zhang H, et al. Pathogenesis of invasive micropapillary carcinoma: role of MUC1 glycoprotein. Modern Pathology. 2004;17(9):1045–1050. doi: 10.1038/modpathol.3800166. [DOI] [PubMed] [Google Scholar]
- 16.Xu F, Xu J, Lou Z, et al. Micropapillary component in colorectal carcinoma is associated with lymph node metastasis in T1 and T2 stages and decreased survival time in TNM stages I and II. American Journal of Surgical Pathology. 2009;33(9):1287–1292. doi: 10.1097/PAS.0b013e3181a5387b. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton SR, Bosman FT, Boffetta P, Ilyas M. Carcinoma of the colon and rectum. In: Bostman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of Tumours of the Digestive System. Vol. 3. Lyon, France: IARC; 2010. p. p. 138. [Google Scholar]
- 18.Kim M-J, Hong S-M, Jang SJ, et al. Invasive colorectal micropapillary carcinoma: an aggressive variant of adenocarcinoma. Human Pathology. 2006;37(7):809–815. doi: 10.1016/j.humpath.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 19.Cianchi F, Messerini L, Palomba A, et al. Character of the invasive margin in colorectal cancer: does it improve prognostic information of dukes staging? Diseases of the Colon and Rectum. 1997;40(10):1170–1175. doi: 10.1007/BF02055162. [DOI] [PubMed] [Google Scholar]
- 20.Palmqvist R, Stenling R, Oberg A, Landberg G. Prognostic significance of p27(Kip1) expression in colorectal cancer: a clinico-pathological characterization. The Journal of Pathology. 1999;188:18–23. doi: 10.1002/(SICI)1096-9896(199905)188:1<18::AID-PATH311>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 21.Martin R, Paty PB, Fong Y, et al. Simultaneous liver and colorectal resections are safe for synchronous colorectal liver metastasis. Journal of the American College of Surgeons. 2003;197(2):241–242. doi: 10.1016/S1072-7515(03)00390-9. [DOI] [PubMed] [Google Scholar]
- 22.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. New England Journal of Medicine. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 23.Falcone A, Ricci S, Brunetti I, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the gruppo oncologico nord ovest. Journal of Clinical Oncology. 2007;25(13):1670–1676. doi: 10.1200/JCO.2006.09.0928. [DOI] [PubMed] [Google Scholar]
- 24.Van Cutsem E, Köhne C-H, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. New England Journal of Medicine. 2009;360(14):1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]


