Abstract
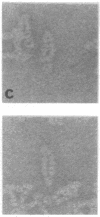
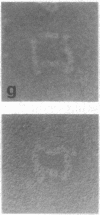
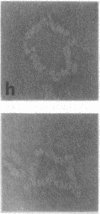
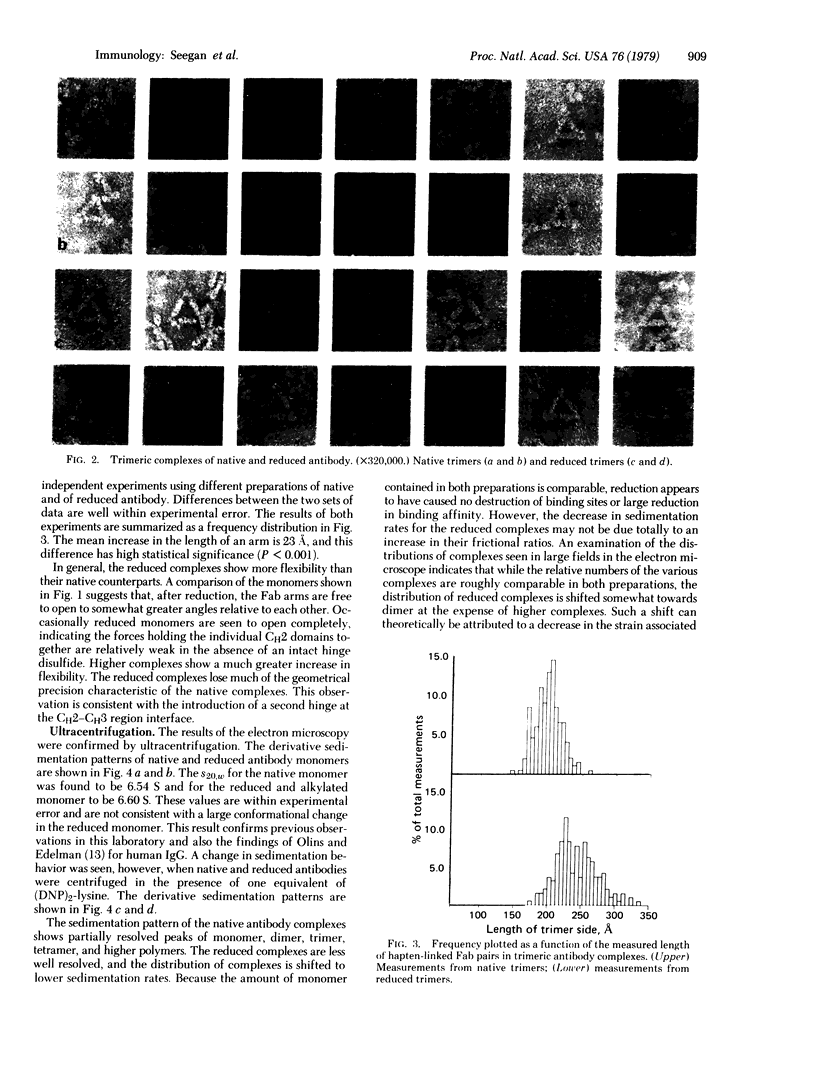
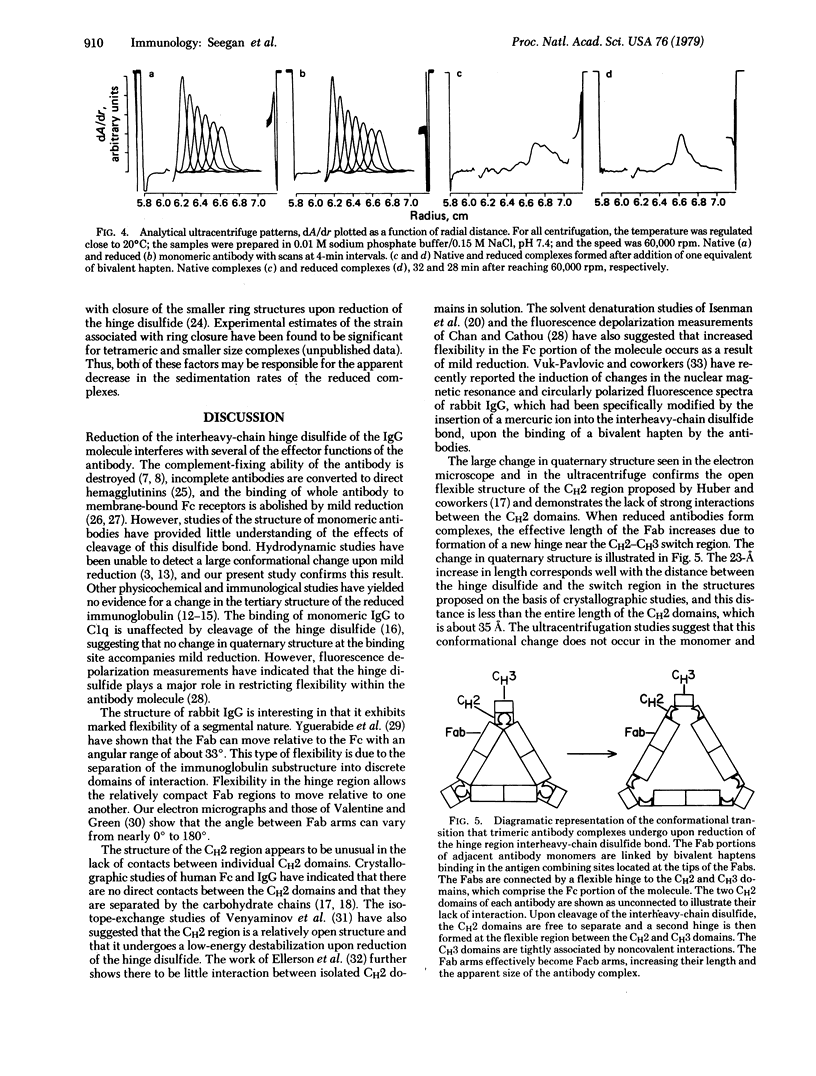
Reduction of the single disulfide bond between heavy chains in the hinge region of rabbit IgG antibody causes destabilization of the CH2 region of the molecule. Our studies indicate that reduced antibody molecules undergo a large change in quaternary structure in the CH2 region upon aggregation with a small bivalent hapten. The conformational change was observed both in hydrodynamic studies and by electron microscopy. The sizes of native and reduced antibody complexes were measured from electron micrographs. These measurements show that reduction of the hinge disulfide allows the CH2 domains of the antibody to separate under the strain induced by complex formation. The Fab arms, which are clearly seen in the electron micrographs of the native complexes, are extended by a portion of the Fc region to effectively become Facb arms in the reduced complexes. The length of the arms is effectively increased by 23 A. This results in a massive alteration in the quaternary structure of the CH2 region of the molecule, and this may be the basis of many of the effects of mild reduction on the various effector functions of the antibody molecule. These findings also support the open structure of the CH2 region proposed on the basis of crystallographic analyses, and they demonstrate how the interheavy-chain hinge disulfide restricts segmental flexibility in the Fc fragment of the IgG molecule.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björk I., Tanford C. Recovery of native conformation of rabbit immunoglobulin G upon recombination of separately renatured heavy and light chains at near-neutral pH. Biochemistry. 1971 Apr 13;10(8):1289–1295. doi: 10.1021/bi00784a003. [DOI] [PubMed] [Google Scholar]
- Chan L. M., Cathou R. E. The role of the inter-heavy chain disulfide bond in modulating the flexibility of immunoglobulin G antibody. J Mol Biol. 1977 Jun 5;112(4):653–656. doi: 10.1016/s0022-2836(77)80170-8. [DOI] [PubMed] [Google Scholar]
- Colomb M., Porter R. R. Characterization of a plasmin-digest fragment of rabbit immunoglobulin gamma that binds antigen and complement. Biochem J. 1975 Feb;145(2):177–183. doi: 10.1042/bj1450177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrington K. J., Smith B. R. Conformational changes accompanying the dissociation and association of immunoglobulin-G subunits. Biochim Biophys Acta. 1972 Mar 15;263(1):70–81. doi: 10.1016/0005-2795(72)90160-2. [DOI] [PubMed] [Google Scholar]
- Dorrington K. J., Zarlengo M. H., Tanford C. Conformational change and complementarity in the combination of H and L chains of immunoglobulin-G. Proc Natl Acad Sci U S A. 1967 Sep;58(3):996–1003. doi: 10.1073/pnas.58.3.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellerson J. R., Yasmeen D., Painter R. H., Dorrington K. J. Structure and function of immunoglobulin domains. III. Isolation and characterization of a fragment corresponding to the Cgamma2 homology region of human immunoglobin G1. J Immunol. 1976 Feb;116(2):510–517. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Goers J. W., Schumaker V. N., Glovsky M. M., Rebek J., Müller-Eberhard H. J. Complement activation by a univalent hapten-antibody complex. J Biol Chem. 1975 Jul 10;250(13):4918–4925. [PubMed] [Google Scholar]
- Goers J. W., Ziccardi R. J., Schumaker V. N., Glovsky M. M. The mechanism of activation of the first component of complement by a univalent hapten-IgG antibody complex. J Immunol. 1977 Jun;118(6):2182–2191. [PubMed] [Google Scholar]
- Griffith J., Kornberg A. Mini M13 bacteriophage: circular fragments of M13 DNA are replicated and packaged during normal infections. Virology. 1974 May;59(1):139–152. doi: 10.1016/0042-6822(74)90211-6. [DOI] [PubMed] [Google Scholar]
- Hong R., Nisonoff A. Relative labilities of the two types of interchain disulfide bond of rabbit gamma G-immunoglobulin. J Biol Chem. 1965 Oct;240(10):3883–3891. [PubMed] [Google Scholar]
- Huber R., Deisenhofer J., Colman P. M., Matsushima M., Palm W. Crystallographic structure studies of an IgG molecule and an Fc fragment. Nature. 1976 Dec 2;264(5585):415–420. doi: 10.1038/264415a0. [DOI] [PubMed] [Google Scholar]
- ISHIZAKA K., ISHIZAKA T., BANOVITZ J. BIOLOGICAL ACTIVITIES OF AGGREGATED GAMMA-GLOBULIN. VII. MINIMUM SIZE OF AGGREGATED GAMMA-GLOBULIN OR ITS PIECE 3 REQUIRED FOR THE INDUCTION OF SKIN REACTIVITY AND COMPLEMENT FIXATION. J Immunol. 1965 Jun;94:825–832. [PubMed] [Google Scholar]
- ISHIZAKA T., ISHIZAKA K. Biological activities of aggregated gamma globulin. I. Skin reactive and complement-fixing properties of heat denatured gamma globulin. Proc Soc Exp Biol Med. 1959 Aug-Sep;101:845–850. doi: 10.3181/00379727-101-25116. [DOI] [PubMed] [Google Scholar]
- Isenman D. E., Dorrington K. J., Painter R. H. The structure and function of immunoglobulin domains. II. The importance of interchain disulfide bonds and the possible role of molecular flexibility in the interaction between immunoglobulin G and complement. J Immunol. 1975 Jun;114(6):1726–1729. [PubMed] [Google Scholar]
- Isenman D. E., Ellerson J. R., Painter R. H., Dorrington K. J. Correlation between the exposure of aromatic chromophores at the surface of the Fc domains of immunoglobulin G and their ability to bind complement. Biochemistry. 1977 Jan 25;16(2):233–240. doi: 10.1021/bi00621a012. [DOI] [PubMed] [Google Scholar]
- Lawrence D. A., Weigle W. O., Spiegelberg H. L. Immunoglobulins cytophilic for human lymphocytes, monocytes, and neutrophils. J Clin Invest. 1975 Feb;55(2):368–376. doi: 10.1172/JCI107940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULLER-EBERHARD H. J., KUNKEL H. G. Isolation of a thermolabile serum protein which precipitates gamma-globulin aggregates and participates in immune hemolysis. Proc Soc Exp Biol Med. 1961 Feb;106:291–295. doi: 10.3181/00379727-106-26313. [DOI] [PubMed] [Google Scholar]
- Michaelsen T. E., Wisloff F., Natvig J. B. Structural requirements in the Fc region of rabbit IgG antibodies necessary to induce cytotoxicity by human lymphocytes. Scand J Immunol. 1975;4(1):71–78. doi: 10.1111/j.1365-3083.1975.tb02601.x. [DOI] [PubMed] [Google Scholar]
- OLINS D. E., EDELMAN G. M. RECONSTITUTION OF 7S MOLECULES FROM L AND H POLYPEPTIDE CHAINS OF ANTIBODIES AND GAMMA-GLOBULINS. J Exp Med. 1964 May 1;119:789–815. doi: 10.1084/jem.119.5.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecht I., Ehrenberg B., Calef E., Arnon R. Conformational changes and complement activation induced upon antigen binding to antibodies. Biochem Biophys Res Commun. 1977 Feb 21;74(4):1302–1310. doi: 10.1016/0006-291x(77)90584-8. [DOI] [PubMed] [Google Scholar]
- Press E. M. Fixation of the first component of complement by immune complexes: effect of reduction and fragmentation of antibody. Biochem J. 1975 Jul;149(1):285–288. doi: 10.1042/bj1490285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romans D. G., Tilley C. A., Crookston M. C., Falk R. E., Dorrington K. J. Conversion of incomplete antibodies to direct agglutinins by mild reduction: evidence for segmental flexibility within the Fc fragment of immunoglobulin G. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2531–2535. doi: 10.1073/pnas.74.6.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHUR P. H., CHRISTIAN G. D. THE ROLE OF DISULFIDE BONDS IN THE COMPLEMENT-FIXING AND PRECIPITATING PROPERTIES OF 7S RABBIT AND SHEEP ANTIBODIES. J Exp Med. 1964 Oct 1;120:531–545. doi: 10.1084/jem.120.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumaker V. N., Calcott M. A., Spiegelberg H. L., Müller-Eberhard H. J. Ultracentifuge studies of the binding of IgG of different subclasses to the Clq subunit of the first component of complement. Biochemistry. 1976 Nov 16;15(23):5175–5181. doi: 10.1021/bi00668a035. [DOI] [PubMed] [Google Scholar]
- Schumaker V. N., Green G., Wilder R. L. A theory of bivalent antibody-bivalent hapten interactions. Immunochemistry. 1973 Aug;10(8):521–528. doi: 10.1016/0019-2791(73)90224-3. [DOI] [PubMed] [Google Scholar]
- Silverton E. W., Navia M. A., Davies D. R. Three-dimensional structure of an intact human immunoglobulin. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5140–5144. doi: 10.1073/pnas.74.11.5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine R. C., Green N. M. Electron microscopy of an antibody-hapten complex. J Mol Biol. 1967 Aug 14;27(3):615–617. doi: 10.1016/0022-2836(67)90063-0. [DOI] [PubMed] [Google Scholar]
- Venyaminov S. Y., Rajnavölgyi E., Medgyesi G. A., Gergely J., Závodszky P. The role of interchain disulphide bridges in the conformational stability of human immunoglobulin G1 subclass. Hydrogen-deuterium exchange studies. Eur J Biochem. 1976 Aug 1;67(1):81–86. doi: 10.1111/j.1432-1033.1976.tb10635.x. [DOI] [PubMed] [Google Scholar]
- WIEDERMANN G., MIESCHER P. A., FRANKLIN E. C. Effect of mercaptoethanol on complement binding ability of human 7 S gammaglobulin. Proc Soc Exp Biol Med. 1963 Jul;113:609–613. doi: 10.3181/00379727-113-28440. [DOI] [PubMed] [Google Scholar]
- Yguerabide J., Epstein H. F., Stryer L. Segmental flexibility in an antibody molecule. J Mol Biol. 1970 Aug;51(3):573–590. doi: 10.1016/0022-2836(70)90009-4. [DOI] [PubMed] [Google Scholar]