Abstract
Artemisia is an important genus of Iranian flora. Cytotoxic activities for some species of the genus have already been reported. In this study, we have investigated the cytotoxic effects of n-hexane, CH2Cl2, EtOAc, EtOH, and EtOH/H2O (1 : 1) extracts of A. turanica Krasch. on two human leukemic cancer cell lines (K562 and HL-60) and J774 as normal cells using alamarBlue (resazurin) assay. PI staining of the fragmented DNA and western blot analysis were used to evaluate the possible apoptotic effect of the extract. The CH2Cl2 extract of A. turanica showed the most antiproliferative effect on cancer cells among all tested extracts with IC50 values of 69 and 104 μg/mL on K562 and HL-60 cells, respectively, whereas the normal cells were not affected significantly by this extract. Sub-G1 peak in the flow cytometry histogram of the cells treated with CH2Cl2 extract of A. turanica and cleavage of PARP protein confirmed the induction of apoptosis with CH2Cl2 extract. Taken together, the findings of the present work suggest the anticancer potential of CH2Cl2 extract of A. turanica on human leukemic cancer cell lines.
1. Introduction
Cancer is a leading cause of life-threatening disease with limited efficient therapies [1]. Considering the significant levels of toxicity and drug resistance of current anticancer regimens, the challenge to develop highly effective drugs with little or no side effects is crucial.
Exploring the anticancer ability of novel compounds including plant derivatives provides a wealthy source of novel and potent bioactive compounds with minimal side effects. Artemisia is a promising natural source of phytochemicals with potent antimalarial and anticancer properties [2–7].
One of the largest genera in the tribe Anthemideae of the Asteraceae (Compositae) is the genus Artemisia, which grows mostly in the temperate zone of Asia, Europe, and North America [8]. There are 43 species of the genus in Iran [9], of which two are endemic [10]. Diverse chemical components in this genus such as flavonoids, coumarins, sterols, polyacetylenes, monoterpenes, sesquiterpenes, and sesquiterpene lactones have been reported so far [11, 12]. Artemisia turanica Krasch. with the Persian name of “Dermaneye ghermez” grows wildly in northeastern Iran [13]. One study has proved the effect of methanol extract of the aerial parts of the plant against Staphylococcus aureus, Bacillus subtilis and Pseudomonas aeruginosa [14]. Major volatiles from the aerial parts of A. turanica were identified as 1,8-cineole, chrysanthenone, and davanone [15]. Antimalarial activity of A. turanica during early infection as well as its in vitro leishmanicidal activity has been reported [16–18]. The crude hydroethanolic extract showed moderate toxicity against the HepG2 cell line [19].
In an effort to evaluate the potential anticancer effect of different extracts of A. turanica on human cancer cell lines, we have investigated the possible cytotoxic activity of the n-hexane, CH2Cl2, EtOAc, EtOH, and EtOH/H2O (1 : 1) extracts of A. turanica Krasch. on two human leukemic cancer cell lines (K562 and HL-60) and J774 as normal cells. Meanwhile, the possible mechanism(s) of the apoptosis mediated by the plant was also explored. Appearance of the apoptosis related protein and cleavage of PARP provided the first evidence that CH2Cl2 extract of A. turanica could induce apoptosis in human leukemia cells.
2. Methods
2.1. Reagents and Chemicals
AlamarBlue (resazurin) was obtained from Sigma (Saint Louis, MO, USA); RPMI-1640 and FCS were from Gibco; β-actin and PARP antibodies, anti-rabbit IgG, and HRP linked antibody were from Cell Signaling technology (Boston, USA); ECL Western blotting detection reagent was from Bio-Rad (USA); the fluorescent probe propidium iodide (PI), protease inhibitor cocktail, phosphatase inhibitor cocktail, sodium citrate, Triton X-100, phenylmethylsulfonyl fluoride, and Bio-Rad Protein Assay Kit (Hercules, CA, USA) were used; all the solvents used for extraction were purchased from Caledon and Scharlau.
2.2. Plant Materials
Aerial parts of the plant were collected from Sami' abad, Torbat Jam (Razavi Khorasan province, Iran) in September 2010. Sample was identified by Dr Valiollah Mozaffarian (Research Institute of Forest and Rangelands, Tehran, Iran). The voucher specimen (no. 12572) has been deposited in the herbarium, Department of Pharmacognosy, School of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran.
2.3. Preparation of Extracts and Fractions
Air-dried and ground aerial parts (150 g) of A. turanica were extracted with n-hexane (40–60), CH2Cl2, EtOAc, EtOH, and EtOH/H2O (1 : 1 v/v), respectively (Sequential maceration with ca. 3 × 1.5 L of each solvent). The extracts were filtrated with filter paper and dried using rotary evaporator at a reduced pressure at a temperature below 45°C to yield 4.21, 18.25, 0.91, 5.94, and 28.26 g of each extract, respectively.
All of the isolated extracts were dissolved in dimethylsulfoxide (DMSO) and then were subjected to cytotoxic and apoptosis assays (Figure 1).
Figure 1.
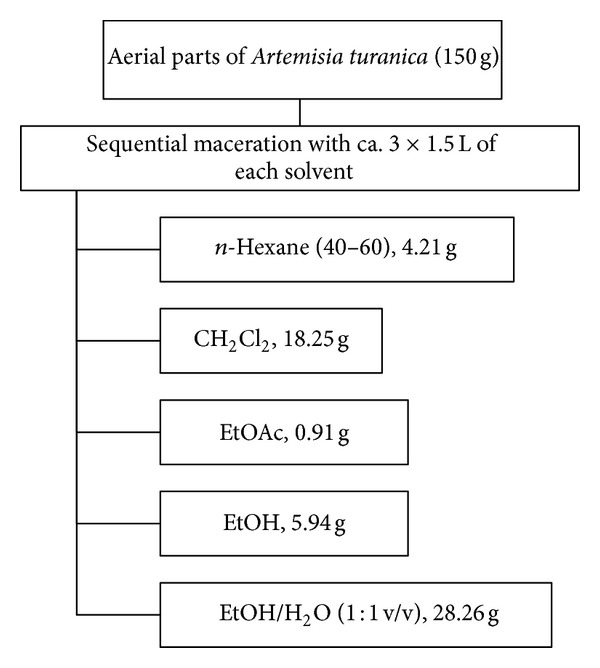
Extraction scheme of n-hexane, CH2Cl2, EtOAc, EtOH, and EtOH/H2O (1 : 1) extracts of A. turanica.
2.4. Cell Culture and Treatment Agents
The human leukemic cancer cell lines HL-60 and K562 were obtained from Pasteur Institute (Tehran, Iran) and maintained in RPMI-1640 medium with 10% v/v fetal bovine serum and 100 μ/mL penicillin and 100 mg/mL streptomycin at 37°C in a humidified atmosphere of 5% CO2 and 95% of air.
2.5. In Vitro Cell Proliferation
The AlamarBlue reagent is a cell viability indicator using the reducing power of living cells to quantify the proliferation of various cell lines, bacteria, plant, and fungi that allow to measure cytotoxicity of various chemicals. Upon entering cells, the blue and nonflorescent resazurin converts to the florescent and purple resorufin in viable cells [20].
About 5 × 104 K562 and 105 HL-60 cells were seeded in each well of 96-microwell plate and treated with various concentrations of each extract of A. turanica (0–200 μg/mL). J774 cell line was used as nonmalignant cells. After 48 incubations, 20 μL resazurin (0.01% w/v in PBS) was added to each well, and the plates were incubated at 37°C for 4 h before the absorbance was measured at 570 nm (test wavelength) and 600 nm (reference wavelength) in a Synergy H4 Hybrid Multi-Mode Microplate Reader (BioTek, Winooski, USA; Winooski is a city in Chittenden). The cytotoxicity of the A. turanica extracts was expressed as IC50, calculated using Prism 5 Software (GraphPad, La Jolla, CA, USA), and presented as mean ± SEM from three independent experiments (with three replicates for each concentration tested extract). For each study, a control sample remained untreated and received only medium in place of the text materials.
2.6. PI Staining
Apoptotic cells were detected by PI staining of small fragments of DNA in treated cells followed by flow cytometry. It has been reported that following DNA fragmentation the so-called sub-G1 peak can be noticed following incubation of cells in a hypotonic phosphate-citrate buffer containing quantitative DNA-binding dye such as PI. Apoptotic cells that have lost DNA will take up less stain and will show up in the left side of the G1 peak in the histogram. Briefly, 106 K562 and HL-60 cells were seeded in each well of a 24-well plate and treated with CH2Cl2 extract of A. turanica in different concentrations (25, 50 and 100 μg/mL) for 48 h. Floating and adherent cells were then harvested and incubated at 4°C overnight in the dark with 750 μL of a hypotonic buffer (50 μg/mL PI in 0.1% sodium citrate plus 0.1% Triton X-100) before flow cytometric analysis using a FACScan flow cytometer (Becton Dickinson, San Diego, CA) was performed. A minimum of 104 events were acquired for each sample. All data were then analyzed using WinMDI Version 2.8 software.
2.7. Western Blotting Analysis
About 107 HL-60 and K562 cells were treated with 25, 50, and 100 μg/mL of the CH2Cl2 extract of A. turanica for 48 h. The cells were rinsed and harvested with cool PBS for 3 times; the cell pellet was resuspended in a lysis buffer containing 50 mM tris-HCl (PH 7.4), 150 mM NaCl, 1% TritonX-100, 1 mM EDTA, 0.2% SDS, 1% Protease inhibitor cocktail, 1% phosphatase inhibitor cocktail, and 1 mM phenylmethylsulfonyl fluoride and left on ice for 30 min. After centrifugation at 10000 rpm for 20 min at 4°C, the cell lysate was collected, and protein concentration was determined according to the Bio-Rad Protein Assay kit. Equal amounts of proteins were subjected to 8% and 12.5% SDS-page (W/V). The proteins were transferred to a polyvinylidene fluoride (PVDF) membrane and subjected to immunoblotting using Bax, β-actin, and PARP antibody as primary antibodies and anti-rabbit IgG and HRP-linked antibody as secondary antibodies; Bax protein band and PARP cleavage in K562 and HL-60 cells were detected by enhanced chemiluminescence using the ECL western blotting detection reagent. Images were quantified using Gel-pro Analyser V.6.0 Gel Analysis software (Media Cybernetics, Inc, Bethesda, MD, USA).
2.8. Statistical Analysis
One way analysis of variance (ANOVA) and Bonferroni post hoc test were used for data analysis. All the results were expressed as mean ± SEM, and P values below 0.05 were considered statistically significant.
3. Results
3.1. Cytotoxicity of Various Fractions
n-Hexane, CH2Cl2, EtOAc, EtOH, and EtOH/H2O (1 : 1) extracts of A. turanica were examined for cytotoxic potential on K562, HL-60, and normal cells (J774). Cells were incubated at 37°C and 5% CO2 with various concentrations of the extract (0–200 μg/mL) for 48 h. Results demonstrated that extracts decreased cell viability in a concentration-dependent manner (Figure 2). Among all the samples, CH2Cl2 extract demonstrated the most cytotoxic effects on cancer cells but limited adverse effect on normal cells. IC50 values (μg/mL) for different extracts of A. turanica in HL-60 and K562 cells are presented in Table 1.
Figure 2.
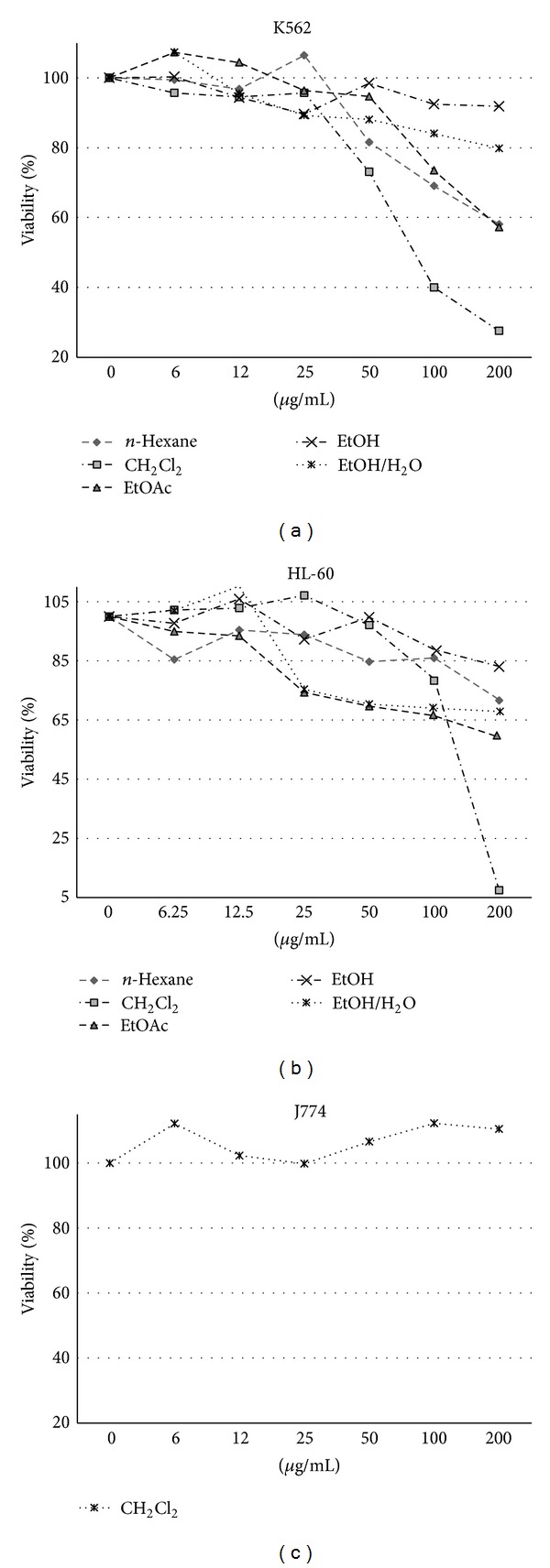
The dose-dependent effects of n-hexane, CH2Cl2, EtOAc, EtOH, and EtOH/H2O (1 : 1) extracts on the growth of K562 and HL-60 cells and normal J774 cells. All extracts exhibited cytotoxic activity against apoptosis-proficient HL-60 and apoptosis-resistant K562 cells, with IC50 values ranging from 68.83 to >450 μg/mL and with much less cytotoxic effects on normal J774 cells. Values were mean ± SEM of at least three independent experiments, each in triplicates.
Table 1.
IC50 values (μg/mL) for different extracts of A. turanica in HL-60 and K562 cell lines.
| Cell line | Fractions | ||||
|---|---|---|---|---|---|
| CH2CI2 | n-Hexane | EtOAc | EtOH | EtOH/H2O (1 : 1) | |
| K562 | 104.2 | 234.5 | 433.1 | >450 | >450 |
| HL-60 | 68.83 | >450 | 373.7 | >450 | >450 |
3.2. Apoptosis Induction by CH2Cl2 Fraction
Apoptosis in K562 and HL-60 cell lines was detected with flow cytometry using PI staining test. Cells incubated with various concentrations (0, 25, 50 and 100 μg/mL) of CH2Cl2 extract of A. turanica for 48 h. Sub-G1 peak of treated cells in flow cytometry histograms compared to that (Sub-G1 peak) of untreated control cells revealed the induction of apoptosis in treated cells (Figure 3).
Figure 3.
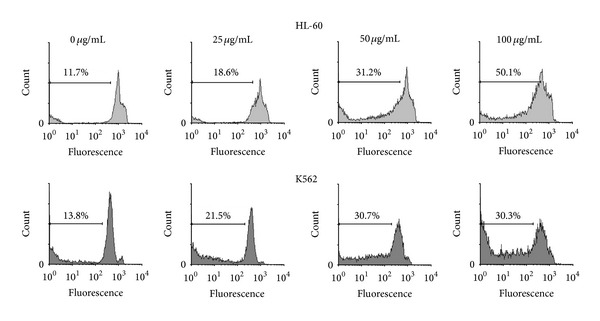
PI staining and flow cytometry analysis of CH2Cl2 extract (0, 25, 50, and 100 μg/mL) induced apoptosis in K562 and HL-60 cells.
3.3. Western Blotting with HL-60 and K562 Cells
The cleavage of 116 kDa PARP-1 to 89 and 24 kDa fragments was used as an indicator of apoptosis. In HL-60 cells, PARP-1 was cleaved clearly to the 89 kDa and 24 kDa fragments after treatment with CH2Cl2 extract (25, 50 and 100 μg/mL) after 48 h (Figure 4). Bax proteins possess a crucial function in controlling cytochrome c release and apoptosis initiation via the mitochondrial pathway. CH2Cl2 extract (25, 50 and 100 μg/mL) could not change the level of Bax protein in both cells (Figure 4).
Figure 4.
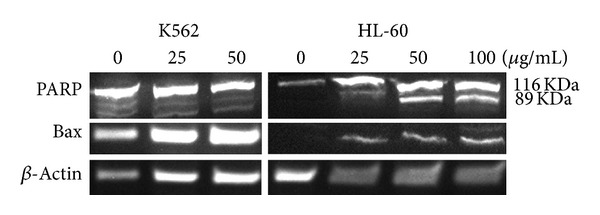
Proteolytic cleavage of poly(ADP-ribose) polymerase (PARP) in HL-60 cells and level of Bax protein in HL-60 and K562 cells after 48 h exposure to CH2Cl2 extract of A. turanica (25, 50 and 100 μg/mL). β-Actin was used as a loading control. All Western blots were representative of 3 independent experiments.
4. Discussion
Strong evidence supports the critical role of apoptosis in the pathology of many diseases including cancer. Thus, pharmacological modulation of apoptosis is likely to be the main strategy for searching for efficient anticancer therapeutics [21].
The result of the present study supports the cytotoxic and apoptotic activity of CH2Cl2 extract of A. turanica when compared with other extracts obtained from the plant on two human leukemic cancer cell lines (K562 and HL-60).
Using n-hexane, CH2Cl2, EtOAc, EtOH, and EtOH/H2O (1 : 1) solvents for extraction would afford different fractions extracts that contain the different groups of phytochemicals corresponding to the various polarity of the extractant [22]. Comparison of the results obtained with different extracts of the A. turanica confirmed the presence of potent non/semipolar phytochemicals in CH2Cl2 extract of the plant.
Apoptosis induction was validated using PI staining of fragmented DNA and western blot analysis of the proteins involved in programmed cell death pathway. PARP cleavage as an important indicator for apoptosis induction was consistent with other results obtained in this study. The unchanged level of Bax protein in K562 cells may reject the role of mitochondria in the apoptosis pathway. Full analysis of the proteins involved in the intrinsic pathway helps to recognize the role of the extract in apoptosis induction in cells.
Death receptor and mitochondria initiated apoptosis recruit caspases as the crucial enzyme in cell death. Caspase 8 and caspase 9 activation merged to caspase 3 stimulation, which leads to changes in the activity of some of the important enzymes involved in DNA repair. Cleavage of PARP is one of the examples of enzyme inactivation in apoptosis, which leads to unrepaired single-strand DNA breaks that accumulate in the absence of PARP activity [23].
The overcome of proapoptotic proteins like Bax to antiapoptotic proteins, located on the outer layer of the mitochondria, opens pores on the surface of the mitochondria leading to the release of cytochrome c, apoptosome formation, and caspase activation [24].
Due to some intrinsic differences, the cytotoxic results on HL-60 and K562 cells used in this study were different. The absence of the Fas/CD95/APO-1 receptor in K562 cells may be the main reason for lower IC50s in this cell line [25]. Accordingly, apoptosis was induced in lesser extent in apoptosis-resistant K562 cells when compared with apoptosis-proficient HL-60 cells. Since the role of mitochondria in the apoptosis-induction of the plant has not been proven, interaction of the extract with death receptors other than the Fas/CD95/APO-1 receptor in K562 cells has been speculated.
Plants serve as the important part of the antitumor regimen both in conventional and alternative medicine. Many plants of the genus Artemisia have been reported to possess promising effects in research also in treating cancer [26, 27].
A cytotoxic evaluation of the isolated dimeric guaianolides from A. anomala showed significant inhibitory effects against the cell growth of BGC-823 tumor cell lines [28]. Two new eudesmane sesquiterpenoids from the same species exhibited cytotoxicity against HCT-8 and A549 cell lines [29]. 5/7-fused bicyclic guaianolides isolated from A. myriantha and A. absinthium are classified in one of the major categories of α-methylene-γ-lactones with anticancer potential [30]. A naturally occurring flavonoid, eupatilin, isolated from A. princeps inhibited the growth of human endometrial cancer cells via G2/M phase cell cycle arrest [31]. RXF-393 renal cancer cell line displayed high sensibility to the organic extract from the leaves of A. verlotiorum, which induced a significant dose-dependent increase in the lipid peroxidation [32]. Drimartol A, a sesquiterpene coumarin ether, and two other new sesquiterpenes could efficiently induce apoptosis of a human lung cancer cell line (95-D) through the mitochondrial-dependent pathway. The compounds were isolated from the cultured hairy roots of A. annua [33–35]. Isoscopoletin from A. argyi and artemisinin from A. annua have shown great cytotoxicity against lung and colon cancers [35]. A sesquiterpene lactone purified from A. diffusa inhibited spontaneous mouse mammary tumor growth in vivo [36]. The essential oil of A. capillaris is believed to be a good resource for searching new drugs, especially anticancer drugs because of its ability to induce apoptosis in human oral cancer cells [37].
The biological evaluation of the whole plant is provided to assess the synergistic and antagonistic interactions of mixture of phytochemicals existing in the extract [38].
Taken together, cytotoxicity and DNA fragmentation along with cleavage of PARP confirmed the apoptotic activity of the CH2Cl2 extract of A. turanica. These results indicated the presence of non/semipolar nature of the phytochemical responsible for the observed effects. Further analytical experiments on CH2Cl2 extract of A. turanica and structure elucidation should be performed to recognize the pure component responsible for the cytotoxic activity of the plant.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgments
The authors would like to thank Mr. M. Malaekeh for his assistance in flow cytometry. This work was supported by Grants (no. 910238) from Research Affairs of Mashhad University of Medical Sciences as a part of Pharm. D. thesis.
References
- 1.Hsiao WLW, Liu L. The role of traditional Chinese herbal medicines in cancer therapy from TCM theory to mechanistic insights. Planta Medica. 2010;76(11):1118–1131. doi: 10.1055/s-0030-1250186. [DOI] [PubMed] [Google Scholar]
- 2.Efferth T, Dunstan H, Sauerbrey A, Miyachi H, Chitambar CR. The anti-malarial artesunate is also active against cancer. International Journal of Oncology. 2001;18(4):767–773. doi: 10.3892/ijo.18.4.767. [DOI] [PubMed] [Google Scholar]
- 3.Choi E, Park H, Lee J, Kim G. Anticancer, antiobesity, and anti-inflammatory activity of Artemisia species in vitro . Journal of Traditional Chinese Medicine. 2013;33:92–97. doi: 10.1016/s0254-6272(13)60107-7. [DOI] [PubMed] [Google Scholar]
- 4.Taghizadeh Rabe SZ, Mahmoudi M, Ahi A, Emami SA. Antiproliferative effects of extracts from Iranian Artemisia species on cancer cell lines. Pharmaceutical Biology. 2011;49(9):962–969. doi: 10.3109/13880209.2011.559251. [DOI] [PubMed] [Google Scholar]
- 5.Tin AS, Sundar SN, Tran KQ, Park AH, Poindexter KM, Firestone GL. Antiproliferative effects of artemisinin on human breast cancer cells requires the downregulated expression of the E2F1 transcription factor and loss of E2F1-target cell cycle genes. Anti-Cancer Drugs. 2012;23(4):370–379. doi: 10.1097/CAD.0b013e32834f6ea8. [DOI] [PubMed] [Google Scholar]
- 6.Lee JG, Kim JH, Ahn JH, Lee KT, Baek NI, Choi JH. Jaceosidin, isolated from dietary mugwort (Artemisia princeps), induces G2/M cell cycle arrest byinactivating cdc25C-cdc2 via ATM-Chk1/2 activation. Food and Chemical Toxicology. 2013;55:214–221. doi: 10.1016/j.fct.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 7.Park EY, Lee KW, Lee HW, et al. The ethanol extract from Artemisia princeps Pampanini induces p53-mediated G1 phase arrest in A172 human neuroblastoma cells. Journal of Medicinal Food. 2008;11(2):237–245. doi: 10.1089/jmf.2007.609. [DOI] [PubMed] [Google Scholar]
- 8.Mucciarelli M, Maffei M. Introduction of the genus. In: Wright CW, editor. Artemisia. London, UK: Taylor and Francis; 2002. [Google Scholar]
- 9.Mozaffarian V. Compositae (Anthemideae & Ehinopeae) no. 59. Tehran: Research Institute of Forest and Rangelands; Flora of Iran; pp. 199–261. 2008 (Persian) [Google Scholar]
- 10.Emami SA, Aghazari F. Les Phanerogames Endemiques De La Flore D’Iran. Téhéran, Iran: Publications de I’Université de Téhéran des Sciences Médicales; 2011. [Google Scholar]
- 11.Tan RX, Tang HQ, Hu J, Shuai B. Lignans and sesquiterpene lactones from Artemisia sieversiana and Inula racemosa . Phytochemistry. 1998;49(1):157–161. doi: 10.1016/s0031-9422(97)00889-3. [DOI] [PubMed] [Google Scholar]
- 12.Bora KS, Sharma A. The genus Artemisia: a comprehensive review. Pharmaceutical Biology. 2011;49(1):101–109. doi: 10.3109/13880209.2010.497815. [DOI] [PubMed] [Google Scholar]
- 13.Mozaffarian V. A Dictionary of Iranian Plant Names. Tehran, Iran: Farhang Moaser; 1998 (Persian) [Google Scholar]
- 14.Ramezani M, Fazli-Bazzaz BS, Saghafi-Khadem F, Dabaghian A. Antimicrobial activity of four Artemisiaspecies of Iran. Fitoterapia. 2004;75(2):201–203. doi: 10.1016/j.fitote.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Firouznia A, Akbari MT, Rustaiyan A, Masoudi S, Bigdeli M, Tabatabaei-Anaraki M. Composition of the essential oils of Artemisia turanica Krasch., Helichrysum oocephalum Boiss. and Centaurea ispahanica Boiss. three asteraceae herbs growing wild in Iran. Journal of Essential Oil-Bearing Plants. 2007;10(2):88–93. [Google Scholar]
- 16.Nahrevanian H, Aboufazeli F, Kazemi SM, Hajihosseini R, Naeimi S. Phytochemical evaluation and antimalarial effects of artemisia turanica herbal extracts as an iranian flora on Plasmodium berghei in vivo . Journal of Natural Remedies. 2011;11(2):167–176. [Google Scholar]
- 17.Emami SA, Rabe SZT, Ahi A, Mahmoudi M. Inhibitory activity of eleven Artemisia species from Iran against Leishmania major parasites. Iranian Journal of Basic Medical Sciences. 2012;15(2):807–811. [PMC free article] [PubMed] [Google Scholar]
- 18.Taherkhani M, Rustaiyan A, Nahrevanian H, Naeimi S, Taherkhani T. Comparison of antimalarial activity of Artemisia turanica extract with current drugs in vivo . Journal of Vector Borne Diseases. 2013;50:51–56. [PubMed] [Google Scholar]
- 19.Emami SA, Vahdati-Mashhadian N, Vosough R, Oghazian MB. The anticancer activity of five species of Artemisia on Hep2 and HepG2 cell lines. Pharmacologyonline. 2009;3:327–339. [Google Scholar]
- 20.O’Brien J, Wilson I, Orton T, Pognan F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. European Journal of Biochemistry. 2000;267(17):5421–5426. doi: 10.1046/j.1432-1327.2000.01606.x. [DOI] [PubMed] [Google Scholar]
- 21.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 22.Mälkiä A, Murtomäki L, Urtti A, Kontturi K. Drug permeation in biomembranes: in vitro and in silico prediction and influence of physicochemical properties. European Journal of Pharmaceutical Sciences. 2004;23(1):13–47. doi: 10.1016/j.ejps.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 23.Kreuzaler P, Watson CJ. Killing a cancer: what are the alternatives? Nature Reviews. Cancer. 2012;11(6):411–424. doi: 10.1038/nrc3264. [DOI] [PubMed] [Google Scholar]
- 24.Peixoto PM, Dejean LM, Kinnally KW. The therapeutic potential of mitochondrial channels in cancer, ischemia-reperfusion injury, and neurodegeneration. Mitochondrion. 2012;12(1):14–23. doi: 10.1016/j.mito.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munker R, Andreeff M. Induction of death (CD95/FAS), activation and adhesion (CD54) molecules on blast cells of acute myelogenous leukemias by TNF-α and IFN-γ . Cytokines and Molecular Therapy. 1996;2(3):147–160. [PubMed] [Google Scholar]
- 26.Firestone GL, Sundar SN. Anticancer activities of artemisinin and its bioactive derivatives. Expert Reviews in Molecular Medicine. 2009;11:p. 32. doi: 10.1017/S1462399409001239. [DOI] [PubMed] [Google Scholar]
- 27.Efferth T. Willmar Schwabe Award 2006: antiplasmodial and antitumor activity of artemisinin—from bench to bedside. Planta Medica. 2007;73(4):299–309. doi: 10.1055/s-2007-967138. [DOI] [PubMed] [Google Scholar]
- 28.Zan K, Chai XY, Chen XQ, et al. Artanomadimers A-F: six new dimeric guaianolides from Artemisia anomala . Tetrahedron. 2012;68:5060–5065. [Google Scholar]
- 29.Zan K, Chen XQ, Chai XY, et al. Two new cytotoxic eudesmane sesquiterpenoids from Artemisia anomala . Phytochemistry Letters. 2012;5(2):313–315. [Google Scholar]
- 30.Janecka A, Wyrebska A, Gach K, Fichna J, Janecki T. Natural and synthetic α-methylenelactones and α-methylenelactams with anticancer potential. Drug Discovery Today. 2012;17:561–572. doi: 10.1016/j.drudis.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 31.Cho JH, Lee JG, Yang YI, et al. Eupatilin, a dietary flavonoid, induces G2/M cell cycle arrest in human endometrial cancer cells. Food and Chemical Toxicology. 2011;49(8):1737–1744. doi: 10.1016/j.fct.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 32.Marx C, Kayser GB, Schunemann DP, Regner A, da Rocha AB, Grivicich I. Cytotoxic effect and oxidative damage of organic extract from Artemisia verlotiorum in human cancer cell lines. Latin American Journal of Pharmacy. 2010;29(7):1061–1066. [Google Scholar]
- 33.Zhai DD, Supaibulwatana K, Zhong JJ. Inhibition of tumor cell proliferation and induction of apoptosis in 95-D lung cancer cells by Drimartol A from hairy root cultures of Artemisia annua . Latin American Journal of Pharmacy. 2010;29(7):1159–1165. doi: 10.1016/j.phymed.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Zhai DD, Supaibulwatana K, Zhong JJ. Inhibition of tumor cell proliferation and induction of apoptosis in human lung carcinoma 95-D cells by a new sesquiterpene from hairy root cultures of Artemisia annua . Phytomedicine. 2010;17(11):856–861. doi: 10.1016/j.phymed.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 35.Zhai DD, Jin HZ, Zhong JJ. A new sesquiterpene from hairy root culture of Artemisia annua . Chinese Chemical Letters. 2010;21(5):590–592. [Google Scholar]
- 36.Noori S, Taghikhani M, Hassan ZM, Allameha A, Mostafaei A. Tehranolide molecule modulates the immune response, reduce regulatory T cell and inhibits tumor growth in vivo . Molecular Immunology. 2010;47(7-8):1579–1584. doi: 10.1016/j.molimm.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 37.Cha JD, Moon SE, Kim HY, Cha IH, Lee KY. Essential oil of artemisia capillaris induces apoptosis in KB cells via mitochondrial stress and caspase activation mediated by MAPK-stimulated signaling pathway. Journal of Food Science. 2009;74(9):75–81. doi: 10.1111/j.1750-3841.2009.01355.x. [DOI] [PubMed] [Google Scholar]
- 38.Bandaranayake WM. Quality control, screening, toxicity, and regulation of herbal drugs. In: Ahmad I, Aqil F, Owais M, editors. Modern Phytomedicine: Turning Medicinal Plants into Drugs. Weinheim, Germany: Wiley-VCH & Co. KGaA; 2006. pp. 25–58. [Google Scholar]


