Abstract
Nocturnal melatonin production in the pineal gland is under the control of norepinephrine released from superior cervical ganglia afferents in a rhythmic manner, and of cyclic AMP. Cyclic AMP increases the expression of serotonin N-acetyltransferase and of inducible cAMP early repressor that undergo circadian oscillations crucial for the maintenance and regulation of the biological clock. In the present study, we demonstrate a circadian pattern of expression of the calcium/calmodulin activated adenylyl cyclase type 1 (AC1) mRNA in the rat pineal gland. In situ hybridization revealed that maximal AC1 mRNA expression occurred at midday (12:00-15:00), with a very low signal at night (0:00-3:00). We established that this rhythmic pattern was controlled by the noradrenergic innervation of the pineal gland and by the environmental light conditions. Finally, we observed a circadian responsiveness of the pineal AC activity to calcium/calmodulin, with a lag due to the processing of the protein. At midday, AC activity was inhibited by calcium (40%) either in the presence or absence of calmodulin, while at night the enzyme was markedly (3-fold) activated by the calcium-calmodulin complex. These findings suggest (i) the involvement of AC1 acting as the center of a gating mechanism, between cyclic AMP and calcium signals, important for the fine tuning of the pineal circadian rhythm; and (ii) a possible regulation of cyclic AMP on the expression of AC1 in the rat pineal gland.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod J. The pineal gland: a neurochemical transducer. Science. 1974 Jun 28;184(4144):1341–1348. doi: 10.1126/science.184.4144.1341. [DOI] [PubMed] [Google Scholar]
- Baler R., Klein D. C. Circadian expression of transcription factor Fra-2 in the rat pineal gland. J Biol Chem. 1995 Nov 10;270(45):27319–27325. doi: 10.1074/jbc.270.45.27319. [DOI] [PubMed] [Google Scholar]
- Bentley J. K., Beavo J. A. Regulation and function of cyclic nucleotides. Curr Opin Cell Biol. 1992 Apr;4(2):233–240. doi: 10.1016/0955-0674(92)90038-e. [DOI] [PubMed] [Google Scholar]
- Borjigin J., Wang M. M., Snyder S. H. Diurnal variation in mRNA encoding serotonin N-acetyltransferase in pineal gland. Nature. 1995 Dec 21;378(6559):783–785. doi: 10.1038/378783a0. [DOI] [PubMed] [Google Scholar]
- Buda M., Klein D. C. A suspension culture of pinealocytes: regulation of N-acetyltransferase activity. Endocrinology. 1978 Oct;103(4):1483–1493. doi: 10.1210/endo-103-4-1483. [DOI] [PubMed] [Google Scholar]
- Carter D. A. Neurotransmitter-stimulated immediate-early gene responses are organized through differential post-synaptic receptor mechanisms. Brain Res Mol Brain Res. 1992 Nov;16(1-2):111–118. doi: 10.1016/0169-328x(92)90200-u. [DOI] [PubMed] [Google Scholar]
- Carter D. A. Up-regulation of beta 1-adrenoceptor messenger ribonucleic acid in the rat pineal gland: nocturnally, through a beta-adrenoceptor-linked mechanism, and in vitro, through a novel posttranscriptional mechanism activated by specific protein synthesis inhibitors. Endocrinology. 1993 Nov;133(5):2263–2268. doi: 10.1210/endo.133.5.8404679. [DOI] [PubMed] [Google Scholar]
- Choi E. J., Wong S. T., Dittman A. H., Storm D. R. Phorbol ester stimulation of the type I and type III adenylyl cyclases in whole cells. Biochemistry. 1993 Mar 2;32(8):1891–1894. doi: 10.1021/bi00059a001. [DOI] [PubMed] [Google Scholar]
- Choi E. J., Wong S. T., Hinds T. R., Storm D. R. Calcium and muscarinic agonist stimulation of type I adenylylcyclase in whole cells. J Biol Chem. 1992 Jun 25;267(18):12440–12442. [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- Coon S. L., Roseboom P. H., Baler R., Weller J. L., Namboodiri M. A., Koonin E. V., Klein D. C. Pineal serotonin N-acetyltransferase: expression cloning and molecular analysis. Science. 1995 Dec 8;270(5242):1681–1683. doi: 10.1126/science.270.5242.1681. [DOI] [PubMed] [Google Scholar]
- Dash P. K., Karl K. A., Colicos M. A., Prywes R., Kandel E. R. cAMP response element-binding protein is activated by Ca2+/calmodulin- as well as cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):5061–5065. doi: 10.1073/pnas.88.11.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatt C. E., Snyder S. H. Cloning and expression of an adenylyl cyclase localized to the corpus striatum. Nature. 1993 Feb 11;361(6412):536–538. doi: 10.1038/361536a0. [DOI] [PubMed] [Google Scholar]
- Habecker B. A., Malec N. M., Landis S. C. Differential regulation of adrenergic receptor development by sympathetic innervation. J Neurosci. 1996 Jan;16(1):229–237. doi: 10.1523/JNEUROSCI.16-01-00229.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanoune J., Stengel D., Lacombe M. L., Feldmann G., Coudrier E. Proteolytic activation of rat liver adenylate cyclase by a contaminant of crude collagenase from Clostridium histolyticum. J Biol Chem. 1977 Mar 25;252(6):2039–2045. [PubMed] [Google Scholar]
- Impey S., Wayman G., Wu Z., Storm D. R. Type I adenylyl cyclase functions as a coincidence detector for control of cyclic AMP response element-mediated transcription: synergistic regulation of transcription by Ca2+ and isoproterenol. Mol Cell Biol. 1994 Dec;14(12):8272–8281. doi: 10.1128/mcb.14.12.8272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalli E., Sassone-Corsi P. Signal transduction and gene regulation: the nuclear response to cAMP. J Biol Chem. 1994 Jul 1;269(26):17359–17362. [PubMed] [Google Scholar]
- Matthews R. P., Guthrie C. R., Wailes L. M., Zhao X., Means A. R., McKnight G. S. Calcium/calmodulin-dependent protein kinase types II and IV differentially regulate CREB-dependent gene expression. Mol Cell Biol. 1994 Sep;14(9):6107–6116. doi: 10.1128/mcb.14.9.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikuni M., Saito Y., Koyama T., Yamashita I. Circadian variation of cyclic AMP in the rat pineal gland. J Neurochem. 1981 Mar;36(3):1295–1297. doi: 10.1111/j.1471-4159.1981.tb01733.x. [DOI] [PubMed] [Google Scholar]
- Molina C. A., Foulkes N. S., Lalli E., Sassone-Corsi P. Inducibility and negative autoregulation of CREM: an alternative promoter directs the expression of ICER, an early response repressor. Cell. 1993 Dec 3;75(5):875–886. doi: 10.1016/0092-8674(93)90532-u. [DOI] [PubMed] [Google Scholar]
- Mons N., Cooper D. M. Adenylate cyclases: critical foci in neuronal signaling. Trends Neurosci. 1995 Dec;18(12):536–542. doi: 10.1016/0166-2236(95)98375-9. [DOI] [PubMed] [Google Scholar]
- Mons N., Yoshimura M., Cooper D. M. Discrete expression of Ca2+/calmodulin-sensitive and Ca(2+)-insensitive adenylyl cyclases in the rat brain. Synapse. 1993 May;14(1):51–59. doi: 10.1002/syn.890140108. [DOI] [PubMed] [Google Scholar]
- Moore R. Y. Neural control of the pineal gland. Behav Brain Res. 1996;73(1-2):125–130. doi: 10.1016/0166-4328(96)00083-6. [DOI] [PubMed] [Google Scholar]
- Pangerl B., Pangerl A., Reiter R. J. Circadian variations of adrenergic receptors in the mammalian pineal gland: a review. J Neural Transm Gen Sect. 1990;81(1):17–29. doi: 10.1007/BF01245442. [DOI] [PubMed] [Google Scholar]
- Post S. R., Hilal-Dandan R., Urasawa K., Brunton L. L., Insel P. A. Quantification of signalling components and amplification in the beta-adrenergic-receptor-adenylate cyclase pathway in isolated adult rat ventricular myocytes. Biochem J. 1995 Oct 1;311(Pt 1):75–80. doi: 10.1042/bj3110075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter R. J. Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr Rev. 1991 May;12(2):151–180. doi: 10.1210/edrv-12-2-151. [DOI] [PubMed] [Google Scholar]
- Romero J. A., Zatz M., Kebabian J. W., Axelrod J. Circadian cycles in binding of 3H-alprenolol to beta-adrenergic receptor sites in rat pineal. Nature. 1975 Dec 4;258(5534):435–436. doi: 10.1038/258435a0. [DOI] [PubMed] [Google Scholar]
- Roseboom P. H., Klein D. C. Norepinephrine stimulation of pineal cyclic AMP response element-binding protein phosphorylation: primary role of a beta-adrenergic receptor/cyclic AMP mechanism. Mol Pharmacol. 1995 Mar;47(3):439–449. [PubMed] [Google Scholar]
- Sassone-Corsi P. Rhythmic transcription and autoregulatory loops: winding up the biological clock. Cell. 1994 Aug 12;78(3):361–364. doi: 10.1016/0092-8674(94)90415-4. [DOI] [PubMed] [Google Scholar]
- Spessert R., Albertowski K., Reuss S., Layes E., Vollrath L. Down-regulation of the nocturnally elevated guanylyl cyclase activity in the rat pineal gland. Neurosci Lett. 1995 Apr 28;190(1):61–64. doi: 10.1016/0304-3940(95)11500-v. [DOI] [PubMed] [Google Scholar]
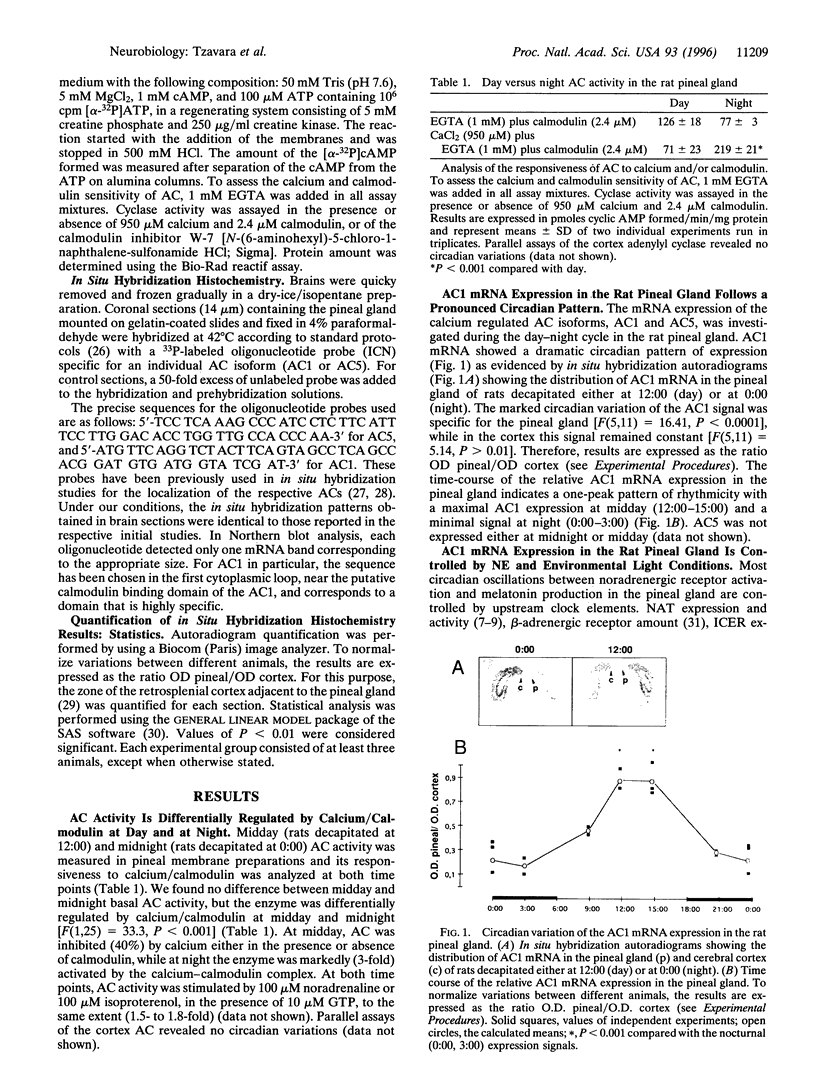
- Stehle J. H., Foulkes N. S., Molina C. A., Simonneaux V., Pévet P., Sassone-Corsi P. Adrenergic signals direct rhythmic expression of transcriptional repressor CREM in the pineal gland. Nature. 1993 Sep 23;365(6444):314–320. doi: 10.1038/365314a0. [DOI] [PubMed] [Google Scholar]
- Sugden D., Vanecek J., Klein D. C., Thomas T. P., Anderson W. B. Activation of protein kinase C potentiates isoprenaline-induced cyclic AMP accumulation in rat pinealocytes. 1985 Mar 28-Apr 3Nature. 314(6009):359–361. doi: 10.1038/314359a0. [DOI] [PubMed] [Google Scholar]
- Sun P., Lou L., Maurer R. A. Regulation of activating transcription factor-1 and the cAMP response element-binding protein by Ca2+/calmodulin-dependent protein kinases type I, II, and IV. J Biol Chem. 1996 Feb 9;271(6):3066–3073. doi: 10.1074/jbc.271.6.3066. [DOI] [PubMed] [Google Scholar]
- Vanecek J., Sugden D., Weller J., Klein D. C. Atypical synergistic alpha 1- and beta-adrenergic regulation of adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate in rat pinealocytes. Endocrinology. 1985 Jun;116(6):2167–2173. doi: 10.1210/endo-116-6-2167. [DOI] [PubMed] [Google Scholar]
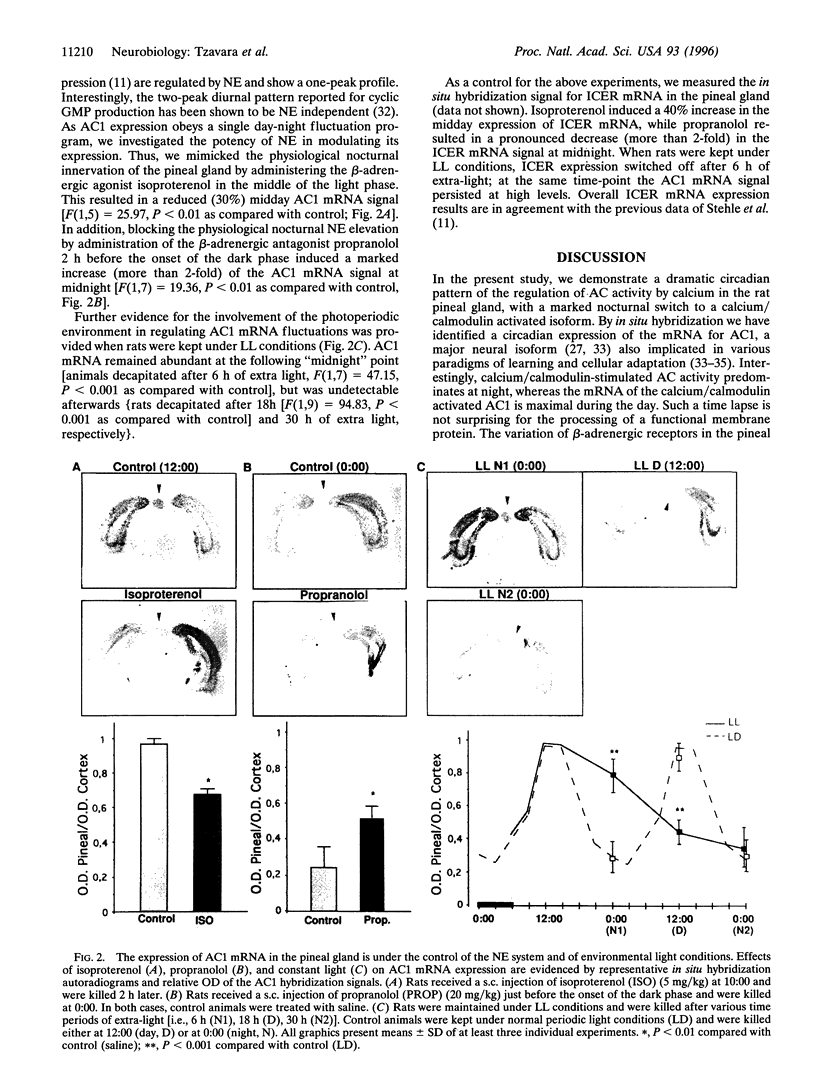
- Wayman G. A., Impey S., Wu Z., Kindsvogel W., Prichard L., Storm D. R. Synergistic activation of the type I adenylyl cyclase by Ca2+ and Gs-coupled receptors in vivo. J Biol Chem. 1994 Oct 14;269(41):25400–25405. [PubMed] [Google Scholar]
- Wu Z. L., Thomas S. A., Villacres E. C., Xia Z., Simmons M. L., Chavkin C., Palmiter R. D., Storm D. R. Altered behavior and long-term potentiation in type I adenylyl cyclase mutant mice. Proc Natl Acad Sci U S A. 1995 Jan 3;92(1):220–224. doi: 10.1073/pnas.92.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z., Choi E. J., Wang F., Blazynski C., Storm D. R. Type I calmodulin-sensitive adenylyl cyclase is neural specific. J Neurochem. 1993 Jan;60(1):305–311. doi: 10.1111/j.1471-4159.1993.tb05852.x. [DOI] [PubMed] [Google Scholar]