Abstract
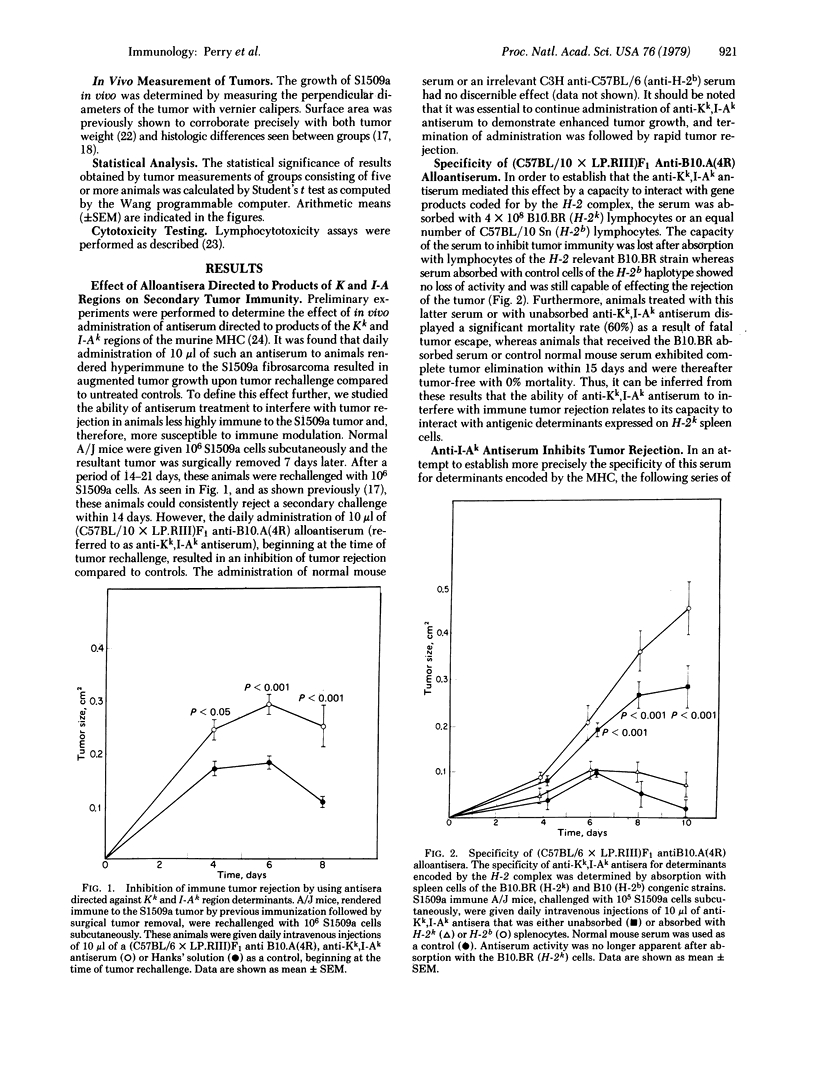
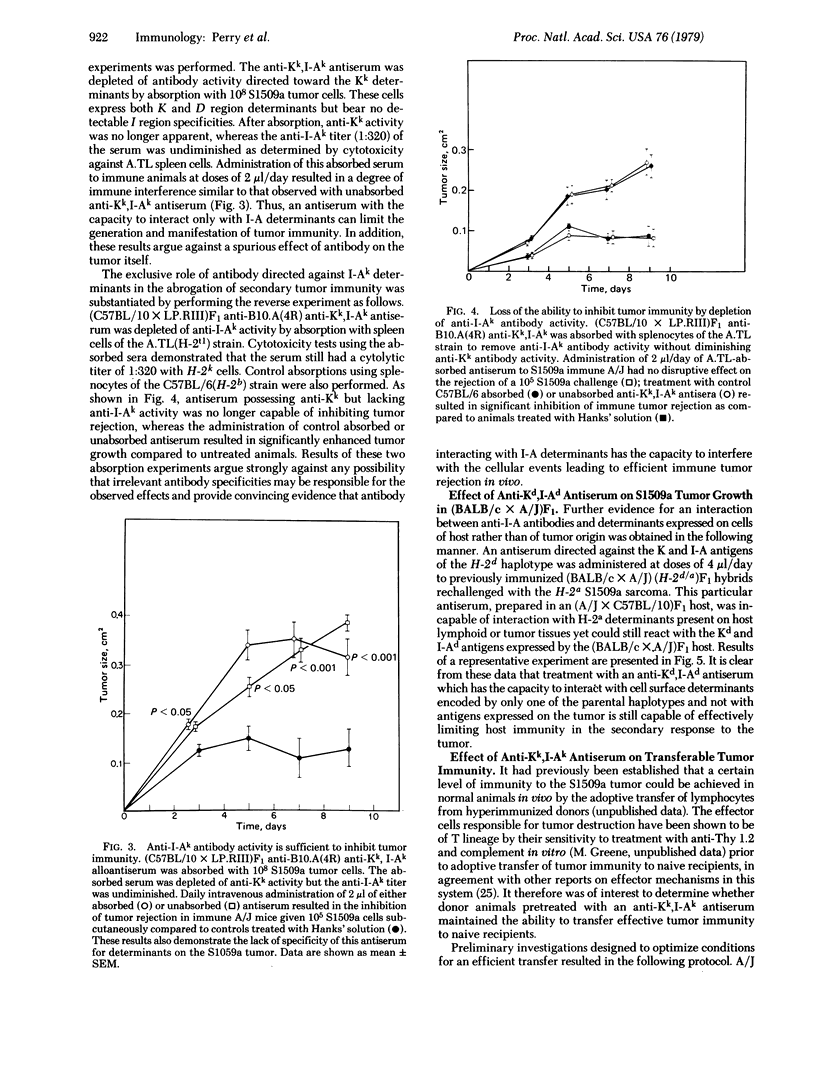
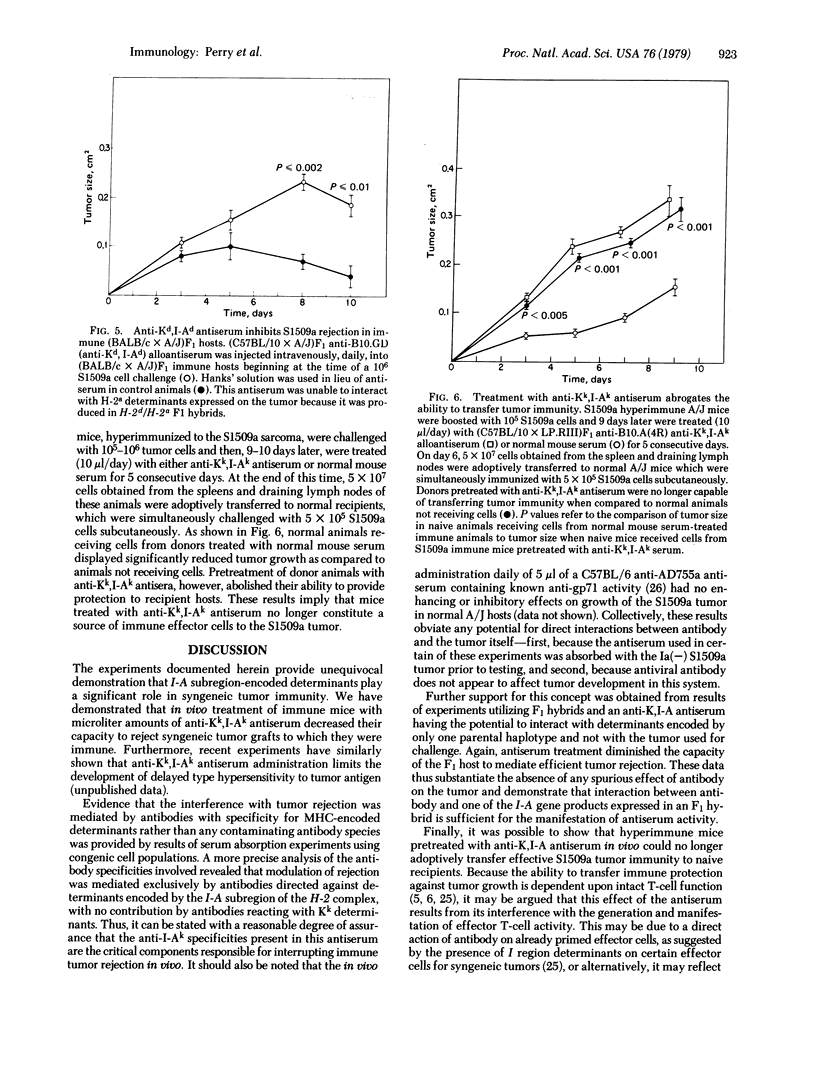
We present evidence for a role of I-A subregion-encoded determinants in syngeneic tumor immunity. In animals rendered immune to the S1509a fibrosarcoma, daily treatment with microliter quantities of antisera directed against Kk and I-Ak determinants expressed on lymphoid cells of host origin decreased the capacity for immune tumor rejection. Absorption studies revealed that anti-I-Ak antibody activity alone was sufficient for the manifestation of this effect. Furthermore, experiments utilizing F1 hybrids showed that an antiserum that was genetically unable to interact with H-2 determinants expressed on the tumor was equally effective in inhibiting tumor immunity. Suggestive evidence that the activity of this antiserum is related to interference with the generation of effector T cell function was provided by the observation that hyperimmune animals pretreated with an anti-Kk,I-Ak antiserum were no longer capable of adoptively transferring tumor immunity to naive recipients. Thus, it is possible to regulate the secondary immune response to tumor antigens by using antisera with specificity for I-A determinants expressed on cells or possibly on factors of the host lymphoid system.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benacerraf B., Germain R. N. The immune response genes of the major histocompatibility complex. Immunol Rev. 1978;38:70–119. doi: 10.1111/j.1600-065x.1978.tb00385.x. [DOI] [PubMed] [Google Scholar]
- Cerottini J. C., Brunner K. T. Cell-mediated cytotoxicity, allograft rejection, and tumor immunity. Adv Immunol. 1974;18:67–132. doi: 10.1016/s0065-2776(08)60308-9. [DOI] [PubMed] [Google Scholar]
- Dorf M. E., Toth E. K., Balner H. Cross-reactions of HL-A antibodies. V. Relationship between the human HL-A11 and chimpanzee ChW-11 specificities. Tissue Antigens. 1972;2(6):461–472. doi: 10.1111/j.1399-0039.1972.tb00067.x. [DOI] [PubMed] [Google Scholar]
- FOLEY E. J. Antigenic properties of methylcholanthrene-induced tumors in mice of the strain of origin. Cancer Res. 1953 Dec;13(12):835–837. [PubMed] [Google Scholar]
- Frbes J. T., Nakaw Y., Smith R. T. Tumor-secific immunity to chemically induced tumors. Evidence for immunologic specificity and shared antigenicity in lymphocyte responses to soluble tumor antigens. J Exp Med. 1975 May 1;141(5):1181–1200. doi: 10.1084/jem.141.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto S., Greene M. I., Sehon A. H. Regualtion of the immune response to tumor antigens. I. Immunosuppressor cells in tumor-bearing hosts. J Immunol. 1976 Mar;116(3):791–799. [PubMed] [Google Scholar]
- Fujimoto S., Greene M. I., Sehon A. H. Regulation of the immune response to tumor antigens. II. The nature of immunosuppressor cells in tumor-bearing hosts. J Immunol. 1976 Mar;116(3):800–806. [PubMed] [Google Scholar]
- Fujimoto S., Matsuzawa T., Nakagawa K., Tada T. Cellular interaction between cytotoxic and suppressor T cells against syngeneic tumors in the mouse. Cell Immunol. 1978 Jul;38(2):378–387. doi: 10.1016/0008-8749(78)90068-0. [DOI] [PubMed] [Google Scholar]
- Greene M. I., Dorf M. E., Pierres M., Benacerraf B. Reduction of syngeneic tumor growth by an anti-I-J-alloantiserum. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5118–5121. doi: 10.1073/pnas.74.11.5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene M. I., Fujimoto S., Sehon A. H. Regulation of the immune response to tumor antigens. III. Characterization of thymic suppressor factor(s) produced by tumor-bearing hosts. J Immunol. 1977 Aug;119(2):757–764. [PubMed] [Google Scholar]
- Haagensen D. E., Jr, Roloson G., Collins J. J., Wells S. A., Jr, Bolognesi D. P., Hansen H. J. Immunologic control of the ascites of murine adenocarcinoma 755. I. Protection with syngeneic immune serum or lymphoid cells. J Natl Cancer Inst. 1978 Jan;60(1):131–139. doi: 10.1093/jnci/60.1.131. [DOI] [PubMed] [Google Scholar]
- Haller O., Hansson M., Kiessling R., Wigzell H. Role of non-conventional natural killer cells in resistance against syngeneic tumour cells in vivo. Nature. 1977 Dec 15;270(5638):609–611. doi: 10.1038/270609a0. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Bartram S., Haskill J. S., Nunn M., Holden H. T., West W. H. Fc receptors on mouse effector cells mediating natural cytotoxicity against tumor cells. J Immunol. 1977 Jul;119(1):322–326. [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Holden H. T., Staal S., Djeu J. Y. Augmentation of natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic target cells. Int J Cancer. 1977 Apr 15;19(4):555–564. doi: 10.1002/ijc.2910190417. [DOI] [PubMed] [Google Scholar]
- Lamon E. W., Skurzak H. M., Andersson B., Whitten H. D., Klein E. Antibody-dependent lymphocyte cytotoxicity in the murine sarcoma virus system: activity of IgM and IgG with specificity for MLV determined antigen(s). J Immunol. 1975 Apr;114(4):1171–1176. [PubMed] [Google Scholar]
- PREHN R. T., MAIN J. M. Immunity to methylcholanthrene-induced sarcomas. J Natl Cancer Inst. 1957 Jun;18(6):769–778. [PubMed] [Google Scholar]
- Perry L. L., Benacerraf B., Greene M. I. Regulation of the immune response to tumor antigen. IV. Tumor antigen-specific suppressor factor(s) bear I-J determinants and induce suppressor T cells in vivo. J Immunol. 1978 Dec;121(6):2144–2147. [PubMed] [Google Scholar]
- Perry L. L., Benacerraf B., McCluskey R. T., Greene M. I. Enhanced syngeneic tumor destruction by in vivo inhibition of suppressor T cells using anti-I-J alloantiserum. Am J Pathol. 1978 Aug;92(2):491–506. [PMC free article] [PubMed] [Google Scholar]
- Plata F., Gomard E., Leclerc J. C., Levy J. P. Further evidence for the involvement of thymus-processed lymphocytes in syngeneic tumor cell cytolysis. J Immunol. 1973 Sep;111(3):667–671. [PubMed] [Google Scholar]
- Rouse B. T., Röllinghoff M., Warner N. L. Anti-theta serum-induced supression of the cellular transfer of tumour-specific immunity to a syngeneic plasma cell tumour. Nat New Biol. 1972 Jul 26;238(82):116–117. doi: 10.1038/newbio238116a0. [DOI] [PubMed] [Google Scholar]
- Rouse B. T., Röllinghoff M., Warner N. L. Tumor immunity to murine plasma cell tumors. II. Essential role of T lymphocytes in immune response. Eur J Immunol. 1973 Apr;3(4):218–224. doi: 10.1002/eji.1830030408. [DOI] [PubMed] [Google Scholar]
- Russell S. W., Gillespie G. Y., McIntosh A. T. Inflammatory cells in solid murine neoplasms. III. Cytotoxicity mediated in vitro by macrophages recovered from disaggregated regressing Moloney sarcomas. J Immunol. 1977 May;118(5):1574–1579. [PubMed] [Google Scholar]
- Röllinghoff M., Wagner H. In vitro induction of tumor specific immunity: requirements for T lymphocytes and tumor growth inhibition in vivo. Eur J Immunol. 1973 Aug;3(8):471–476. doi: 10.1002/eji.1830030804. [DOI] [PubMed] [Google Scholar]
- Shin H. S., Hayden M., Langley S., Kaliss N., Smith M. R. Antibody-mediated suppression of grafted lymphoma. III. Evaluation of the role of thymic function, non-thymus-derived lymphocytes, macrophages, platelets, and polymorphonuclear leukocytes in syngeneic and allogeneic hosts. J Immunol. 1975 Apr;114(4):1255–1263. [PubMed] [Google Scholar]
- Takei F., Levy J. G., Kilburn D. G. Characterization of suppressor cells in mice bearing syngeneic mastocytoma. J Immunol. 1977 Feb;118(2):412–417. [PubMed] [Google Scholar]



