Summary
Adoptive cellular therapy, involving the ex vivo enrichment and expansion of antigen-specific immune cells for adoptive transfer, has emerged as an increasingly effective modality for the treatment of patients with advanced cancer refractory to conventional therapy.
In this issue of CLINICAL CANCER RESEARCH, Besser and colleagues present an intent-to-treat (ITT) analysis of 80 patients enrolled on an adoptive therapy study using ex vivo expanded unselected tumor-infiltrating lymphocytes (1). Among this cohort of patients with advanced disease, the majority presenting with poor prognostic metastatic melanoma (Stage M1c), 57 were eventually treated, with about half of those not receiving treatment due to non-clinical reasons (no TIL growth or refusal to further participate) and half clinically progressing too rapidly to receive therapy. Overall response rate and median survival was about 40% and 15 months among the 57 treated patients and 30% and 10 months for all enrolled. Considering that all patients had had at least one previous treatment for metastatic disease, (often multiple prior lines of aggressive therapy), and the natural history of melanoma affecting visceral sites, these are very encouraging results for patients and for the field of immune-based therapies in general.
The development of immunotherapies for the treatment of refractory or recurrent disease has witnessed a renaissance of late in both cell-based and immunomodulatory approaches. Clinical trials using antibodies to establish immune checkpoint blockade against CTLA4 and the PD-1/PDL-1 axis report significant longlasting responses via in vivo activation and expansion of the endogenous anti-tumor immune response (2). As a means of providing an exogenous source of ex vivo expanded effector cell, adoptive cellular therapy, has also emerged as a highly effective modality capable of eliciting durable and complete responses.
Three forms of adoptive cellular therapy using T cells have been practiced- TIL therapy, using lymphocytes expanded from a tumor biopsy sample (3), antigen-specific T cell therapy, using endogenous T cells sourced from peripheral blood (4–6), and more recently, the use of gene-modified T cells engineered to express the desired TCR or chimeric antigen receptor (CAR) with occasional remarkable results (7) (Figure 1).
Figure 1.
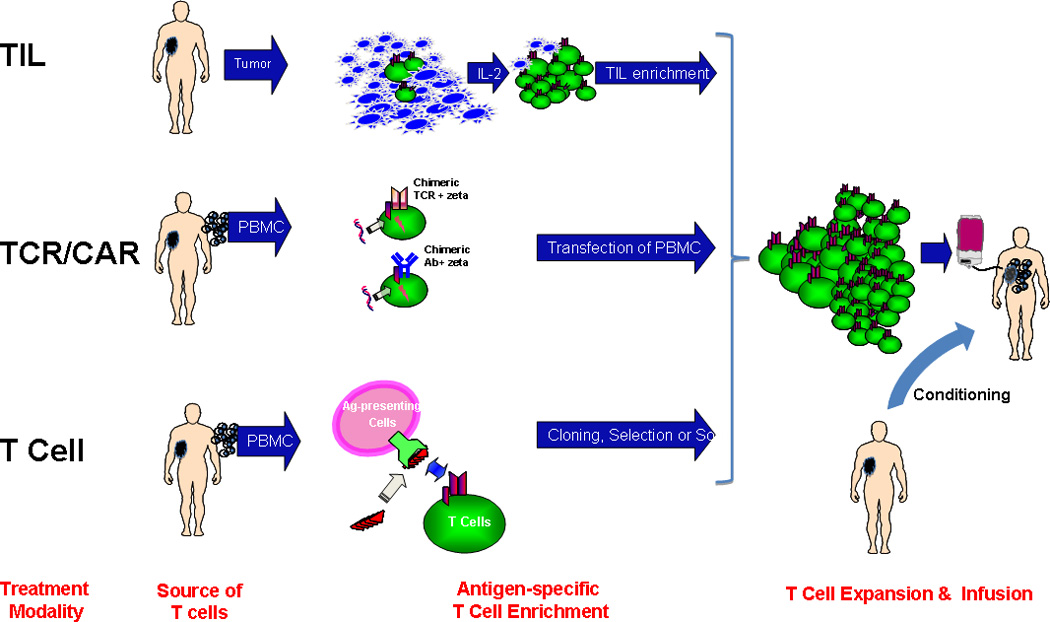
- Enrichment and expansion of tumor-infiltrating lymphocytes (TIL) from a disaggregated tumor biopsy sample
- Genetic transfer of T Cell Receptor (TCR) recognizing tumor antigen-derived peptide-MHC target or Chimeric Antibody Receptor (CAR) recognizing surface tumor protein
- Enrichment of endogenous antigen-specific T cells from peripheral blood mononuclear cells by in vitro stimulation followed by cell selection or cloning. PBMCs are a source of both antigen-presenting cells and T cells.
When it became apparent that the duration of T cell survival after adoptive transfer correlated with clinical response; strategies to enhance in vivo persistence were implemented involving both extrinsic modification of the host environment, through the use of conditioning lymphodepletion or intrinsic manipulation of the effector T cell itself, by enhancing cellular replicative potential via cytokine modulation (8), phenotype-based selection or genetic engineering (7). By incorporating these approaches into the TIL therapy protocol, a significant increase in clinical response rates was achieved (> 50% in select cases) and durable complete remissions in the setting of significant tumor burden (9).
In the field of adoptive T cell therapy using TIL, two important milestones were attained, in large part through pioneering efforts of the Surgery Branch at the NCI, enabling its promulgation into the clinical arena as a feasible therapeutic option : one was a means of expanding the TIL population 1000–5000 fold based on methods originally established for antigen-specific T cell expansion using a TCR trigger (anti-CD3) and irradiated feeder cells, and the second, was inclusion of a lymphodepleting conditioning regimen for patients prior to TIL infusion. This regimen was initially nonmyeloablative, and later advanced to a TBI-containing ablative regimen with a commensurate increase in serious adverse toxicities, but also dramatic and durable clinical responses (up to 40%). The nonmyeloablative regimen used in this study is the most established; coupled with an expedited protocol to generate ‘young’ TIL that was successfully expanded and infused in more than 90% of patients, this represented a potential ‘standardized’ protocol with which to move forward to a randomized controlled clinical trial given the encouraging ITT results.
The impetus to develop improved and simpler TIL protocols arose from prior work at the NCI and other centers involved in larger scale TIL trials such as those conducted at MD Anderson Cancer Center (10) and at Sheba Medical Center (1) where response rates of 40% or more were consistently achieved among patients who eventually received treatment. Although these studies corroborated the original promising results, only 40% to as few as 27% of patients who underwent resection for TIL generation ultimately received TIL therapy (11) this attrition due in part to disease progression, protocol-specific and product-related exclusion criteria - features which could be addressed by a shortened time to therapy from enrollment and modification of product release criteria. While the original TIL protocol, commonly practiced at the NCI required 7–8 weeks from resection to TIL product, the young TIL protocol developed by Tran et al shortened the pre-expansion phase, eliminated exclusion of TIL cultures based on absent in vitro activity and produced a TIL product in 4 weeks (12); implementation of this protocol in the study presented by Besser et al here, led to > 70% treatment : enrollment ratio, with response rates among treated patients at least as favorable as those shown in prior studies. Only 10 % of patients failed to yield a useable TIL product and 14% were excluded due to clinical deterioration.
There remain however a number of issues yet to be resolved: should ablative radiation therapy be added to the conditioning regimen and which patients should be considered for this life-threatening but highly effective treatment? Can a superior TIL effector population be defined on the basis of in vitro phenotype selection or cytokine modulation? Is there a clinical or immunological biomarker profile that can identify patients predicted to respond to therapy? Furthermore, the treatment landscape for patients with metastatic melanoma has changed in a very positive and dramatic fashion over the last 5 years. With the advent of more and more positive data arising from the use of immune checkpoint inhibitors and targeted therapies, alone, in combination with each other, or in combination with conventional modalities, it is becoming less and less obvious which algorithmic endpoint cellular therapies will eventually find its niche; more than likely, combinational therapies involving the use of clinically-approved immunomodulators together with adoptive cellular therapies will be established as the standard of practice for clinical trials and hopefully, a standard of care.
In the parlance, then, of today’s Youtube generation, is adoptive cellular therapy ready to go ‘viral’ as in the case of a recently popularized Korean music video star, or is it limited still to an eclectic group of diehard believers? The answer lies somewhere in between. While there remains much to be addressed by taking a reductionist approach to adoptive cellular therapy - by isolating and expanding a uniform population of antigen-specific T cells, epigenetically modulating or genetically engineering an ideal central memory / stem cell effector population, limiting toxicities and fine tuning affinities - there is reason to believe that cellular therapy is now poised to make the leap from ‘boutique therapy’ to ‘treatment modality’. The report presented here describes one significant step towards this goal-and now, may just be the time for adoptive cellular therapy to go mainstream.
Footnotes
Conflict of Interest Statement:
The author has no conflicts relevant to the content of this manuscript
References
- 1.Besser MJ, Shapira-Frommer R, Itzhaki O, Treves AJ, Zippel D, Levy D, et al. Adoptive Transfer of Tumor Infiltrating Lymphocytes in Metastatic Melanoma Patients: Intent-to-Treat Analysis and Efficacy after Failure to Prior Immunotherapies. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013 doi: 10.1158/1078-0432.CCR-13-0380. [DOI] [PubMed] [Google Scholar]
- 2.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature reviews Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. The New England journal of medicine. 1988;319:1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 4.Hunder NN, Wallen H, Cao J, Hendricks DW, Reilly JZ, Rodmyre R, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ES-O-1. The New England journal of medicine. 2008;358:2698–2703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler MO, Friedlander P, Milstein MI, Mooney MM, Metzler G, Murray AP, et al. Establishment of antitumor memory in humans using in vitro-educated CD8+ T cells. Science translational medicine. 2011;3:80ra34. doi: 10.1126/scitranslmed.3002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapuis AG, Thompson JA, Margolin KA, Rodmyre R, Lai IP, Dowdy K, et al. Transferred melanoma-specific CD8+ T cells persist, mediate tumor regression, and acquire central memory phenotype. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:4592–4597. doi: 10.1073/pnas.1113748109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. The New England journal of medicine. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y, Bleakley M, Yee C. IL-21 influences the frequency, phenotype, and affinity of the antigen-specific CD8 T cell response. Journal of immunology. 2005;175:2261–2269. doi: 10.4049/jimmunol.175.4.2261. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radvanyi LG, Bernatchez C, Zhang M, Fox PS, Miller P, Chacon J, et al. Specific lymphocyte subsets predict response to adoptive cell therapy using expanded autologous tumor-infiltrating lymphocytes in metastatic melanoma patients. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:6758–6770. doi: 10.1158/1078-0432.CCR-12-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartzentruber DJ, Hom SS, Dadmarz R, White DE, Yannelli JR, Steinberg SM, et al. In vitro predictors of therapeutic response in melanoma patients receiving tumor-infiltrating lymphocytes and interleukin-2. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1994;12:1475–1483. doi: 10.1200/JCO.1994.12.7.1475. [DOI] [PubMed] [Google Scholar]
- 12.Tran KQ, Zhou J, Durflinger KH, Langhan MM, Shelton TE, Wunderlich JR, et al. Minimally cultured tumor-infiltrating lymphocytes display optimal characteristics for adoptive cell therapy. Journal of immunotherapy. 2008;31:742–751. doi: 10.1097/CJI.0b013e31818403d5. [DOI] [PMC free article] [PubMed] [Google Scholar]


