Abstract
Long-term potentiation (LTP), a well-characterized form of synaptic plasticity, has long been postulated as a cellular correlate of learning and memory. Although LTP can persist for long periods of time1, the mechanisms underlying LTP maintenance, in the midst of ongoing protein turnover and synaptic activity, remain elusive. Sustained activation of the brain-specific protein kinase C (PKC) isoform protein kinase M-ζ (PKM-ζ) has been reported to be necessary for both LTP maintenance and long-term memory2. Inhibiting PKM-ζ activity using a synthetic zeta inhibitory peptide (ZIP) based on the PKC-ζ pseudosubstrate sequence reverses established LTP in vitro and in vivo3,4. More notably, infusion of ZIP eliminates memories for a growing list of experience-dependent behaviours, including active place avoidance4, conditioned taste aversion5, fear conditioning and spatial learning6. However, most of the evidence supporting a role for PKM-ζ in LTP and memory relies heavily on pharmacological inhibition of PKM-ζ by ZIP. To further investigate the involvement of PKM-ζ in the maintenance of LTP and memory, we generated transgenic mice lacking PKC-ζ and PKM-ζ. We find that both conventional and conditional PKC-ζ/PKM-ζ knockout mice show normal synaptic transmission and LTP at Schaffer collateral–CA1 synapses, and have no deficits in several hippocampal-dependent learning and memory tasks. Notably, ZIP still reverses LTP in PKC-ζ/PKM-ζ knockout mice, indicating that the effects of ZIP are independent of PKM-ζ.
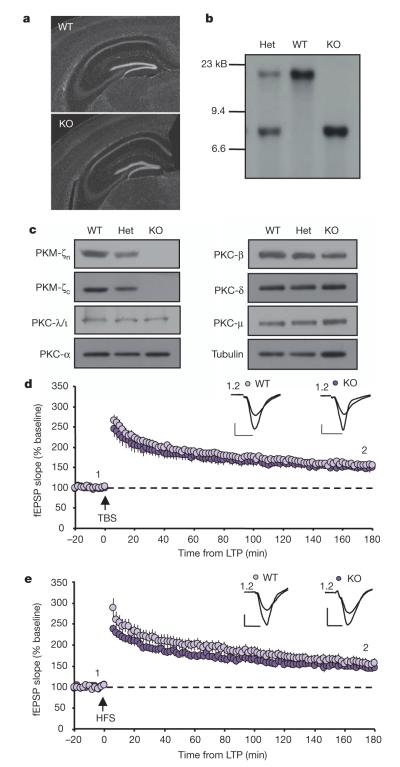
PKM-ζ is transcribed from an alternate internal promoter within the Prkcz gene encoding only the catalytic domain, rendering it free from pseudosubstrate auto-inhibition and PKC-ζ regulatory signal transduction pathways7,8. To generate mice lacking PKM-ζ we targeted exon 11 of the PKC-ζ catalytic domain for excision by Cre recombinase (Supplementary Fig. 1a). Crossing floxed PKC-ζ alleles (PKC-ζfl/fl) to cytomegalovirus (CMV)-Cre transgenic mice resulted in deletion of both PKC-ζ and PKM-ζ, as verified by southern and western blot analysis (Fig. 1b, c). PKC-ζ/PKM-ζ KO mice were both viable and fertile, and there were no anatomical abnormalities in the brains of PKC-ζ/PKM-ζ KO mice (Fig. 1a). Protein expression levels of a number of synaptic proteins as well as different PKC isozymes, including the other atypical isoform PKC-λ/Ι, were unchanged in PKC-ζ/PKM-ζ KO mice (Fig. 1c and Supplementary Fig. 1b–d). Analysis of basal synaptic transmission revealed no difference in input/output relationships in adult PKC-ζ/PKM-ζ KO mice compared to wild-type littermates (Supplementary Fig. 2a, b). Paired-pulse facilitation (PPF) was also unaffected, indicating normal presynaptic release probability in PKC-ζ/PKM-ζ KO mice (Supplementary Fig. 2c). Surprisingly, we found theta-burst stimulation (TBS) resulted in normal induction and maintenance of LTP in PKC-ζ/PKM-ζ KO mice, with a magnitude and time course similar to wild-type littermates (Fig. 1d). Some evidence suggests the dependence of LTP maintenance on PKM-ζ activity can vary with the induction protocol9. Therefore, we also tested high frequency stimulation (HFS) and again found no LTP deficit in PKC-ζ/PKM-ζ KO mice (Fig. 1e). As PKM-ζ is proposed to be necessary particularly for the late, protein-synthesis-dependent phase of LTP2, we used the protein-synthesis inhibitor emetine to verify that both stimulation protocols produce forms of LTP that are protein-synthesis-dependent in wild-type mice (Supplementary Fig. 3a, b). Furthermore, TBS-LTP in PKC-ζ/PKM-ζ KO mice is also protein-synthesis-dependent on a similar time scale as observed in wild-type mice (Supplementary Fig. 3c).
Figure 1. Normal LTP, gross brain morphology, and PKC isoform expression in conventional PKC-ζ/PKM-ζ KO mice.
a, Hippocampal regions stained with DAPI. b, Southern blot analysis of wild-type, heterozygous (Het) and homozygous (KO) PKC-ζ/PKM-ζ mice. c, Protein expression of PKC isoforms from whole-brain tissue. d, e, TBS-LTP (d; WT, n=11, 156±6% at 175–180 min; KO, n=9, 149±8%; P>0.5) and HFS-LTP (e; WT, n=14, 155±11% at 175–180 min; KO, n=13, 148±6%; P>0.5) are intact and maintained for 3 h in mice lacking PKM-ζ. Data represent mean±s.e.m. Scale bars, 0.5 mV (vertical), 5 ms (horizontal).
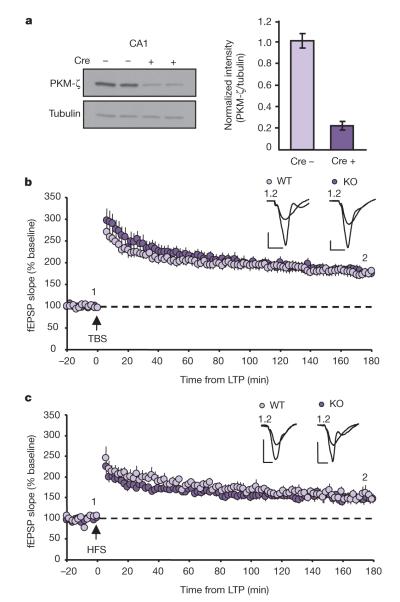
To limit possible compensatory effects resulting from PKC-ζ/PKM-ζ deletion during development, we also created conditional PKM-ζ knockout mice by crossing PKC-ζfl/fl to CaMKII-CreERT2 mice. These mice express Cre recombinase which becomes active only upon addition of tamoxifen driven by the CamkIIα (also known as Camk2a) promoter10. This inducible system allows us to delete PKM-ζ specifically in forebrain neurons of adult animals at will. Adult animals (8–10 weeks old) were injected with tamoxifen (2 mg per day for 5 days) and analysed 2 to 3 weeks later. We observed an ~80% reduction in PKM-ζ levels in the dorsal hippocampus of PKC-ζfl/fl; CaMKII-CreERT2 mice (Fig. 2a) compared to controls (PKM-ζfl/fl treated with tamoxifen or PKC-ζfl/fl; CaMKII-CreERT2 treated with vehicle). Remaining PKM-ζ expression is likely limited to interneurons, as evidence suggests PKM-ζ is not expressed in glia8 (and data not shown). In acute hippocampal slices from conditional PKM-ζ KO mice we saw no differences in input/output curves or PPF (Supplementary Fig. 2d–f), indicating that basal synaptic transmission is unaltered in these animals. Consistent with our findings in conventional PKC-ζ/PKM-ζ KO mice, we found no deficits in either TBS- or HFS-LTP in conditional PKM-ζ KO mice (Fig. 2b, c). As we saw no significant differences between the conventional and conditional knockout animals, we used conventional PKC-ζ/PKM-ζ KO mice for the remainder of our experiments.
Figure 2. LTP is intact in conditional PKC-ζ/PKM-ζ knockout mice.
a, Western blot (left) and quantification (right) of PKM-ζ protein reduction in conditional KO mice normalized to tubulin (WT, n=17, 101±6.5%; KO, n=20, 22±3.6%). b, c, TBS-LTP (b; WT, n=16, 180±10% at 175–180 min; KO, n=16, 177±9%; P>0.8) and HFS-LTP (c; WT, n=6, 154±11% at 175–180 min; KO, n=9, 148±10%; P>0.7) are intact and maintained for 3 h in mice with PKM-ζ conditionally deleted in adulthood. Data represent mean±s.e.m. Scale bars, 0.5 mV (vertical), 5 ms (horizontal).
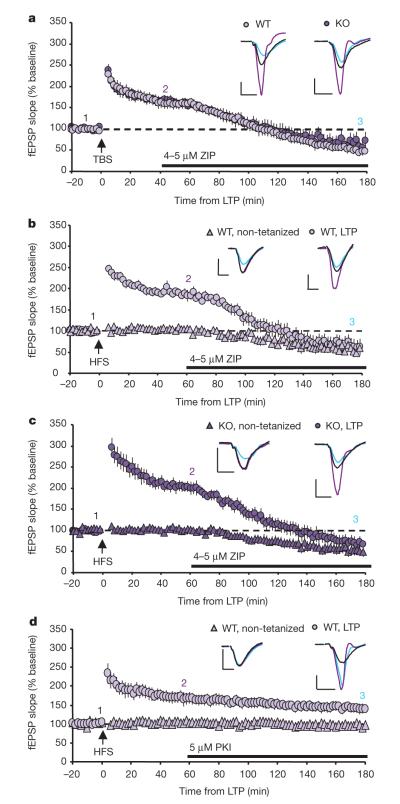
Recent studies have raised concerns regarding the specificity and appropriate use of ZIP11–14. We find that when added after LTP induction, ZIP was indeed able to reverse LTP in wild-type mice, confirming previously published results3,15. In our hands, however, not only were tetanized responses reduced below baseline (see also ref. 16), but ZIP also decreased synaptic transmission in non-tetanized control slices (Fig. 3b). The effects of ZIP were concentration-dependent, as neither LTP maintenance nor baseline transmission was reduced by 1 μM ZIP (Supplementary Fig. 4a–c) and 2–2.5 μM ZIP was effective in roughly half of our experiments (Supplementary Fig. 4d, e), while 5 μM ZIP reliably reduced both LTP and baseline transmission (Fig. 3). The reduction in field excitatory postsynaptic potential (fEPSP) amplitude was not due to changes in pre-synaptic release, as we did not observe changes in PPF following ZIP application (data not shown). Furthermore, whereas a scrambled version of ZIP (Scr-ZIP) is commonly used as a control peptide, some evidence suggests it may mimic the effects of ZIP in vivo17. To our surprise, Scr-ZIP was equally effective at reversing LTP in acute hippocampal slices (Supplementary Fig. 4f, g). Supporting these results, both peptides inhibit purified PKM-ζ and PKC-ζ activity in vitro, showing overlapping inhibition curves (Supplementary Fig. 4h, i). These effects of ZIP and scrambled ZIP were seen with peptides from several different vendors as well as with custom synthesized peptides. Importantly, the concentration of ZIP and Scr-ZIP used in our experiments is consistent with that used in previous studies (1–5 μM)13,15,18.
Figure 3. ZIP is not specific for PKM-ζ.
a, ZIP is equally effective at reversing established TBS-LTP in WT mice and mice lacking PKM-ζ. Before ZIP application (40 min post-LTP): WT, n=8, 166±12%; KO, n=6, 167±10%, P>0.9. 140 min after ZIP application: WT, 48±7%; KO, 60±18%, P>0.5. b, c, ZIP decreases both tetanized (HFS) and non-tetanized synaptic responses in WT mice (b) and mice lacking PKM-ζ (c). Before ZIP application (60 min post-LTP): WT, n=8, tetanized=181±8%, non-tetanized=102±4%; KO, n=8, tetanized=203±12%, non-tetanized=100±4%, P>0.15 WT vs KO tetanized or non-tetanized. 120 min after ZIP application: WT, tetanized=60±18%, non-tetanized=50±9%; KO, tetanized=67±14%, non-tetanized=52±11%, P>0.6 WT vs KO tetanized or non-tetanized. d, Myristoylated PKI peptide does not affect LTP or basal transmission (tetanized n=5, non-tetanized n=4). Data represent mean±s.e.m. Scale bars, 0.5 mV (vertical), 5 ms (horizontal).
ZIP and Scr-ZIP use an N-terminal myristoylation group to permeate cell membranes. Several studies in non-neuronal cells have demonstrated that the myristoylation group of ZIP can activate intracellular signalling cascades independent of PKC-ζ activity19,20. To control for possible non-specific effects of the myristoylation group, we tested whether myr-PKI, a myristoylated peptide inhibitor of protein kinase A (PKA), would give similar results. Whereas inhibition of PKA can prevent LTP induction, it has no effect on LTP that is already established21,22. Application of myr-PKI 1 h after LTP induction had no effect on LTP maintenance or baseline transmission (Fig. 3d). These data indicate that the reduction of LTP by ZIP is not due to the myristoyl group.
ZIP is reported to be highly specific for PKC-ζ/PKM-ζ3. It is therefore expected to have no effect on LTP in PKC-ζ/PKM-ζ KOmice. However, not only did ZIP reverse both TBS- and HFS-LTP in PKC-ζ/PKM-ζ KO mice (Fig. 3a, c), but the efficacy of ZIP in PKC-ζ/PKM-ζ KO mice was indistinguishable from that in wild-type (Fig. 3a–c). This raises the question of whether the other atypical PKC isoform, PKC-λ/Ι, which contains the same pseudosubstrate sequence, compensates for the lack of PKM-ζ. To date, there is no evidence that PKC-λ/Ι produces a truncated, constitutively active form in vivo. To test whether alternate products are generated in the absence of PKM-ζ under LTP conditions, we probed tetanized slices from PKC-ζ/PKM-ζ KO mice with a PKC-λ/Ι-specific antibody. We found no evidence for the formation of a truncated product 2 h after LTP induction in PKC-ζ/PKM-ζ KO mice (Supplementary Fig. 5a, b). Expression of full-length PKC-λ/Ι was also unchanged. Future work with conditional PKC-λ/Ι knockout mice is needed to validate whether PKC-λ/Ι has a role in LTP maintenance and could account for the effects of ZIP.
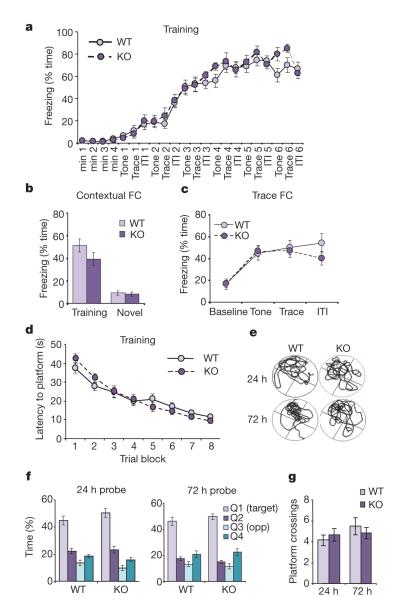
Although the lack of PKM-ζ does not appear to affect canonical forms of LTP in the hippocampus, plasticity mechanisms other than LTP clearly affect learning and memory23. Given the plethora of studies supporting the role of PKM-ζ in memory storage, we tested whether deletion of PKM-ζ could affect two different forms of hippocampal-dependent learning and memory. To assess classical associative conditioning, we chose auditory trace fear conditioning24. Freezing behaviour of both wild-type and PKC-ζ/PKM-ζ KO mice increased quickly and markedly during the training session (Fig. 4a). Placing the animals back into the training context 24 h later elicited context-evoked freezing behaviour in PKC-ζ/PKM-ζ KO mice that was not significantly different from wild-type littermates (Fig. 4b). On the following day, mice were placed in a novel context and presented with only the tone. Whereas neither wild-type or PKC-ζ/PKM-ζ KO mice exhibited significant freezing behaviour in the novel context, tone presentation resulted in similar levels of elevated freezing in both genotypes (Fig. 4b, c). These data show that PKC-ζ/PKM-ζ KO mice exhibit normal contextual and trace components of aversive conditioning.
Figure 4. Hippocampal-dependent learning and memory are intact in PKC-ζ/PKM-ζ KO mice.
a, Trace fear conditioning elicits freezing in PKC-ζ/PKM-ζ KO (n=13) and WT (n=15) mice with no significant main effect of genotype (F(1,546)=0.50, P>0.48). ITI, inter-trial interval. b, WT and PKC-ζ/PKM-ζ KO mice exhibit similar contextual freezing behaviour (24 h post-training, WT=51.5±5.7%, KO=39.3±5.7%, PWT vs KO>0.16; significant elevation in post-training vs pre-training freezing, P<0.05 for WT and KO), but show little freezing in a novel context (48 h post-training, WT±9.3±1.8%, KO=8.5±1.9%, WT and KO P>0.05 vs pre-training freezing). c, Trace fear conditioning is unaffected in PKC-ζ/PKM-ζ KO mice 48 h after training (no significant main effect of genotype, F(1, 78)=0.33, P>0.57). d, Mean escape latencies during Morris water maze training (WT n=21, KO n=17, no significant main effect of genotype, F(1,252)=0.00, P>0.98). e, Representative swim paths during probe trials for WT and PKC-ζ/PKM-ζ KO mice. f, Percentage of time spent in each quadrant during probe trials. Both genotypes showed a significant preference for the target quadrant at 24 (WT n=21, KO n=17) and 72 h (WT n=17, KO n=15); P<0.001 (target quadrant vs Q2, Q3 or Q4). There was no significant main effect of genotype at 24 (F(1,108)=0.79, P>0.38) or 72 h (F(1,90)=0.26, P>0.61). g, Number of platform crossings during probe trials (24 h: WT=4.14±0.52, KO=4.64±0.59, P>0.5, 72 h: WT=5.47±0.8, KO=4.80±0.54, P>0.5). Data represent mean±s.e.m.
To test spatial reference memory in PKC-ζ/PKM-ζ KO mice, we evaluated their performance in the Morris water maze, a task where rodents use spatial cues to locate a hidden platform in a pool of opaque water. Latency to find the platform gradually decreased over the course of eight training blocks for both genotypes, indicating PKC-ζ/PKM-ζ KO mice learned the task as well as wild-type mice (Fig. 4d). Memory retention tests were conducted 24 and 72 h after training. Both wild-type and PKC-ζ/PKM-ζ KO mice spent a significantly greater amount of time in the target quadrant (Fig. 4e, f). In addition, the number of times animals crossed through the platform location was not significantly different between the two groups (Fig. 4g). Contrary to results using ZIP infusion in rats6, our results demonstrate that global as well as specific location memory is unaffected by the deletion of PKM-ζ.
In conclusion, to investigate the role of PKM-ζ in the maintenance of LTP and learning and memory we generated constitutive and conditional PKC-ζ/PKM-ζ knockout mice. Using both prolonged and acute genetic deletion we find that PKM-ζ is not required for the induction or maintenance of LTP in the CA1 region of the hippocampus. The inhibitory peptide ZIP still reversed LTP in the PKC-ζ/PKM-ζ knockout, indicating that ZIP either has non-specific effects or its action is independent of PKM-ζ. Further studies will be necessary to determine ZIP’s specificity and site of action. Moreover, deletion of PKC-ζ/PKM-ζ had no effect on two forms of hippocampal-dependent learning and memory. Intracellular perfusion of PKM-ζ has been shown to enhance synaptic transmission by increasing the number of synaptic AMPA (alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid)-type glutamate receptors3,25. PKM-ζ overexpression has also been shown to enhance memory for conditioned taste aversion26, suggesting that under some conditions or in select brain regions overexpression of PKM-ζ may regulate synaptic plasticity and memory. However, our results demonstrate that normal synaptic plasticity, learning, and memory can occur in the absence of PKM-ζ and call into question an essential, general role for PKM-ζ in the maintenance of synaptic plasticity and memory.
METHODS
Animals
Wild-type and PKC-ζ/PKM-ζ knockout (KO) mice were 129/C57BL6 hybrid background or backcrossed with C57BL6 (N10). No differences were observed between backgrounds or sex in either biochemistry or electrophysiology experiments. Behavioural experiments were performed using only the C57BL6 background. All animals were housed in standard conditions and treated in accordance with the Johns Hopkins University Animal Care and Use Committee guidelines.
Generation of mouse lines: the PKC-ζ/PKM-ζ KO mouse was generated by targeting exon 11 for excision by Cre recombinase. A bacterial artificial chromosome (BAC) genomic clone containing Prkcz was obtained from RPCI. A fragment of ~12 kb was subcloned into pBlueScript, and the targeting vector was created by inserting an orphan loxP site upstream of exon 11 and a neomycin resistance cassette flanked by Flippase Recognition Target sequences (FRT-neoR) and a loxP site downstream of exon 11 (Supplementary Fig. 1a). Upon verification of homologous recombination by Southern blot analysis, targeted 129 embryonic stem cells were injected into C57BL6 blastocysts at the Transgenic Facility of the Johns Hopkins University School of Medicine. Southern blot analysis using an outer probe confirmed germline transmission in the F1 generation of chimaeric mice. Conventional PKC-ζ/PKM-ζ KOs were then generated by breeding to a CMV-Cre transgenic mouse line. For conditional PKM-ζ knockout mice, the FRT-neoR cassette was first removed by breeding to a transgenic Flippase mouse line28. PKC-ζ/PKM-ζfl/fl animals were then crossed with aCaMKII-CreERT2 14. Conditional knockout of Prkcz was induced in adult mice by intraperitoneal injection of tamoxifen (Sigma) dissolved in sunflower seed oil. Control mice were either PKC-ζ/PKM-ζfl/fl injected with tamoxifen or PKC-ζ/PKM-ζfl/fl; aCaMKII-CreERT2 injected with sunflower seed oil.
Histology
Following deep anaesthesia mice were transcardially perfused with 4% paraformaldehyde (w/v) in phosphate-buffered saline (PBS). Brains were removed and post-fixed for an additional 24 h followed by overnight incubations in 10, 20 and 30% sucrose (w/v) in PBS. Brains were frozen on dry ice and sectioned coronally at 50 μm using a standard microtome. Slices containing the hippocampus were rinsed twice 5 min in PBS, blocked and permeabilized for 3 h at room temperature in PBS containing 0.3% Triton-X-100 (PBST) and 10% normal goat serum (NGS; Vector Laboratories) then incubated for 2 h at room temperature in PBST with 2% NGS and DAPI stain (4′,6-diamidino-2-phenylindole, 1:300; Invitrogen). Tissue was then rinsed twice 10 min in PBST followed by twice 10 min in PBS and mounted using PermaFluor (ThermoScientific). Images were acquired using a Zeiss Axiophot microscope with a ×10 objective.
Biochemistry
Western blot analysis: brains were dissected and submerged in 5 ml of ice-cold RIPA buffer (25 mM Tris pH 7.4, 1% Triton-X-100, 0.5% deoxycholate, 0.1% SDS, 100 mM NaCl, 2 mM EDTA, 2 mM EGTA, 50 mM NaF, 10 mM NaPPi, 1 mM Na orthovanadate) supplemented with 1 μM microcystin (Roche) and complete protease inhibitor cocktail (Roche). Tissue was homogenized using a Dounce-homogenizer, rotated for an additional 30 min at 4 °C then spun at 10,000g for 30 min at 4 °C. Protein concentration of the supernatant was determined by a bicinchoninic acid assay (Pierce). Samples were denatured by adding 5× SDS-loading sample buffer and incubated at 95 °C for 10 min before loading for SDS–PAGE. Hippocampal slices were homogenized and lysed directly in 100 μl of 2× SDS-loading sample buffer. Proteins were transferred onto PVDF membranes (Millipore), blocked in Tris-buffered saline (TBS) containing 3% non-fat dry milk and incubated with the indicated antibodies diluted in either 3% non-fat dry milk or 5% BSA in TBS with 0.1% Tween-20. Antibodies used are as follows: monoclonal GluA1 (clone 4.9D) and GluA2 (clone 6A) were generated in-house, as were polyclonal anti-C-terminal PKM-ζ (JH6066), anti-N-terminal PKM-ζ (JH6056), and anti-GluN1 (JH4762) after antisera for each was purchased from Covance. Mouse monoclonal anti-PKC-α, PKC-β, PKC-Ι, PKC-λ and anti-GRIP1 were from BD Transduction Laboratories. Anti-PKC-δ and anti-PKC-μ were from Cell Signaling. Anti-tubulin and anti-synaptophysin (SVP-38) were from Sigma and anti-PSD95 (K28/43) was from NeuroMab.
In vitro kinase assays: Glutathione-S-transferase (GST)-conjugated PKM-ζ was expressed in HEK cells and purified to near-homogeneity using glutathione Sepharose-4B (GE Healthcare). In vitro kinase reactions (50 μl final volume) consisted of: 50 mM Tris (pH 7.4), 5 mM MgCl2, 10 μM peptide ε (Anaspec), 0.5 μg BSA, 25 μM dithiothreitol, GST–PKM-ζ or GST–PKC-ζ (Stressgen) (within linear range for substrate concentration and reaction time), and varying concentrations of ZIP, Scr-ZIP (Sigma, Tocris, Invitrogen, and Johns Hopkins University School of Medicine Biosynthesis and Sequencing Facility), or Myr-PKI (Tocris). Reactions were initiated by the addition of 50 μM [32P]ATP (1 μCi per assay) and proceeded for 10 min at 30 °C. Reactions were stopped by pipetting 40 μl onto Whatman P81 paper, and immediately washed in 0.5% o-phosphoric acid in excess and counted using a Beckman scintillation counter. PKM-ζ activity was measured as the difference between counts incorporated in the presence and absence of enzyme. In the absence of substrate, minimal counts were recorded, indicating purified GST–PKM-ζ was maximally auto-phosphorylated.
Electrophysiology
Slice preparation: mice were anaesthetized with the inhalation anaesthetic isofluorane followed by rapid decapitation. 380-μm transverse hippocampal slices were prepared with a vibratome (Leica VT1200S) after dissection of the hippocampus in ice-cold oxygenated (95% O2/5% CO2) dissection buffer containing in mM: 2.6 KCl, 1.25 NaH2PO4, 26 NaHCO3, 211 sucrose, 10 glucose, 0.75 CaCl2, 7 MgCl2. Slices were recovered submerged in ACSF (in mM: 125 NaCl, 3.25 KCl, 25 NaHCO3, 1.25 NaH2PO4•H2O, 11 glucose, 2 CaCl2, 1 MgCl2) at 30 °C for at least 2 h before recording.
Extracellular recordings: field excitatory postsynaptic potentials (fEPSPs) were evoked at 0.033 Hz as previously described27. Theta burst LTP: 4 trains of 10 bursts at 5 Hz, with each burst consisting of 4 stimuli given at 100 Hz, 10 s inter-train interval. High frequency stimulation: 4 trains of 100 stimuli given at 100 Hz with a 20 s inter-train interval. Stimuli during HFS were set at 75% maximum response. Because we observed effects of ZIP on basal transmission, non-tetanized experiments were conducted in separate slices (from the same animal and the same time/recording chamber as tetanized slices) to rule out the possibility of overlapping pathways in the small mouse stratum radiatum. myr-ZIP, myr-Scr-ZIP, myr-PKI and emetine (Sigma) were bath-applied at the indicated time points.
Data analysis and statistics: all plasticity experiments are presented as responses normalized to the average of the 20 min baseline. Every fourth trace (2-min intervals) is shown in graphs due to size limitations. 5 min averages taken at the indicated time were used to calculate the magnitude of plasticity and for statistical tests. A two-tailed, unpaired student’s t-test was used for determining significance in synaptic plasticity and input–output experiments. All error bars represent standard error of the mean. Sample traces are averages of 6 consecutive traces (3 min), with the stimulation artefact removed for clarity.
Behaviour
Trace fear conditioning: trace fear conditioning was performed using adult (3–4 month old) male mice with the experimenter blind to genotype. Experimental design is illustrated in Supplementary Fig. 6 and was conducted as previously described27 with the exception that the tone test was conducted on day 4. Data shown in Fig. 4 are from the first testing block. Percentage of time spent freezing was quantified using automated motion detection software (CleverSys). Genotype differences for training and tone-evoked (trace) fear conditioning were analysed with a two-way repeated measures ANOVA (GraphPad Prism). Different time points within each genotype were analysed with repeated measures one-way ANOVA. Contextual fear conditioning between wild type and KO was analysed using two-tailed, unpaired student’s t-test. Comparisons of contextual freezing across training sessions/contexts within genotype were made using a repeated measures one-way ANOVA. Bonferroni post-hoc tests were used to make pair-wise comparisons where appropriate. P was set at 0.05 for all tests.
Morris water maze: the Morris water maze was performed using adult (3–4 month old) male and female mice with the experimenter blind to genotype. Mice were handled for 5 min each for 5 consecutive days before beginning experiments. The arena consisted of a circular pool (diameter of 120 cm) filled with water that was temperature-regulated (24 °C) and made opaque with non-toxic white tempera paint. A square, plexiglass platform (length of 10 cm) was submerged 1 cm below the surface of the water and four local cues were provided to allow spatial map generation. Mice were trained on a total of 40 trials over 5 days, with 4 trials per session and 2 sessions per day separated by approximately 2 h. Prior to the first training trial, mice were given a single habituation trial without the platform to assess any spatial bias. Trials were 60 s and mice that did not find the platform within that time were guided to the platform by the experimenter. Once on top of the platform, mice were left for an additional 10 s before being removed. Start locations (north, south, east and west) were pseudo-randomized so that each start location was used once per session and the sequence of start locations in any session was never used twice. On the fifth day the first trial consisted of a 60 s probe trial in which the platform was removed (24 h probe trial). The remaining 8 training trials were conducted after the 24 h probe trial. A second probe trial was performed 72 h after the first probe trial. Following the second probe trial, visual and sensorimotor skills were assessed by 6 visible platform trials given in 2 sessions of 3 trials each. The top of the platform was positioned 0.5–1 cm above the surface of the water and the sides of the platform were wrapped in black electrical tape to provide a strong visible cue. The position of the visible platform varied from trial to trial. Tracking and analysis of animal movement was done using the ANY-maze tracking system (SD instruments). Data were analysed by comparing quadrant preferences and escape latencies averaged across animals within groups (wild type or KO) using one-way repeated measures ANOVA and between groups using two-way repeated measures ANOVA with α set at 0.5. Bonferroni post-hoc tests were used for pairwise comparisons. The number of platform crossings was analysed using a two-tailed, unpaired student’s t-test.
Supplementary Material
Acknowledgements
We thank G. Schütz for providing the CaMKII Cre-ERT2 mice, M. Gallagher and D. Smith for advice on behaviour and M. Coulter for technical support. We also thank all members of the Huganir lab for discussion and support. This work was supported by grants from the National Institute of Health (NS36715) and the Howard Hughes Medical Institute (to R.L.H.). L.J.V. is supported by a training grant from the National Institute of Health (T32MH15330).
Footnotes
Supplementary Information is available in the online version of the paper.
Author Contributions L.J.V., J.L.B. and R.L.H. designed experiments. L.J.V. and J.L.B performed experiments and analysed data. R.J. designed and generated the knockout animals. Y.Y. assisted in mating and genotyping mouse lines. L.J.V., J.L.B. and R.L.H. wrote the manuscript.
Author Information Reprints and permissions information is available at www.nature.com/reprints. The authors declare competing financial interests: details are available in the online version of the paper. Readers are welcome to comment on the online version of the paper.
References
- 1.Abraham WC, Logan B, Greenwood JM, Dragunow M. Induction and experience-dependent consolidation of stable long-term potentiation lasting months in the hippocampus. J. Neurosci. 2002;22:9626–9634. doi: 10.1523/JNEUROSCI.22-21-09626.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sacktor TC. PKMζ, LTP maintenance, and the dynamic molecular biology of memory storage. Prog. Brain Res. 2008;169:27–40. doi: 10.1016/S0079-6123(07)00002-7. [DOI] [PubMed] [Google Scholar]
- 3.Ling DS, et al. Protein kinase Mζ is necessary and sufficient for LTP maintenance. Nature Neurosci. 2002;5:295–296. doi: 10.1038/nn829. [DOI] [PubMed] [Google Scholar]
- 4.Pastalkova E, et al. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313:1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- 5.Shema R, Sacktor TC, Dudai Y. Rapid erasure of long-term memory associations in the cortex by an inhibitor of PKMζ. Science. 2007;317:951–953. doi: 10.1126/science.1144334. [DOI] [PubMed] [Google Scholar]
- 6.Serrano P, et al. PKMζ maintains spatial, instrumental, and classically conditioned long-term memories. PLoS Biol. 2008;6:e318. doi: 10.1371/journal.pbio.0060318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernandez AI, et al. Protein kinase Mζ synthesis from a brain mRNA encoding an independent protein kinase Cζ catalytic domain. Implications for the molecular mechanism of memory. J. Biol. Chem. 2003;278:40305–40316. doi: 10.1074/jbc.M307065200. [DOI] [PubMed] [Google Scholar]
- 8.Hirai T, Niino YS, Chida K. PKCζII, a small molecule of protein kinase Cζ, specifically expressed in the mouse brain. Neurosci. Lett. 2003;348:151–154. doi: 10.1016/s0304-3940(03)00780-8. [DOI] [PubMed] [Google Scholar]
- 9.Sajikumar S, Korte M. Metaplasticity governs compartmentalization of synaptic tagging and capture through brain-derived neurotrophic factor (BDNF) and protein kinase Mζ (PKMζ) Proc. Natl Acad. Sci. USA. 2011;108:2551–2556. doi: 10.1073/pnas.1016849108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erdmann G, Schutz G, Berger S. Inducible gene inactivation in neurons of the adult mouse forebrain. BMC Neurosci. 2007;8:63. doi: 10.1186/1471-2202-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lisman J. Memory erasure by very high concentrations of ZIP may not be due to PKM-zeta. Hippocampus. 2012;22:648–649. doi: 10.1002/hipo.20980. [DOI] [PubMed] [Google Scholar]
- 12.Wu-Zhang AX, Schramm CL, Nabavi S, Malinow R, Newton AC. Cellular pharmacology of protein kinase Mζ (PKMζ) contrasts with its in vitro profile. J. Biol. Chem. 2012;287:12879–12885. doi: 10.1074/jbc.M112.357244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sacktor TC, Fenton AA. Appropriate application of ZIP for PKMζ inhibition, LTP reversal, and memory erasure. Hippocampus. 2012;22:645–647. doi: 10.1002/hipo.20978. [DOI] [PubMed] [Google Scholar]
- 14.Yao Y, et al. Matching biochemical and functional efficacies confirm ZIP as a potent competitive inhibitor of PKMζ in neurons. Neuropharmacol. 2013;64:37–44. doi: 10.1016/j.neuropharm.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serrano P, Yao Y, Sacktor TC. Persistent phosphorylation by protein kinase Mζ maintains late-phase long-term potentiation. J. Neurosci. 2005;25:1979–1984. doi: 10.1523/JNEUROSCI.5132-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sajikumar S, Navakkode S, Sacktor TC, Frey JU. Synaptic tagging and cross-tagging: the role of protein kinase Mζ in maintaining long-term potentiation but not long-term depression. J. Neurosci. 2005;25:5750–5756. doi: 10.1523/JNEUROSCI.1104-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwapis JL, Jarome T, Lonegran M, Helmstetter F. Protein kinase Mzeta maintains fear memory in the amygdala but not in the hippocampus. Behav. Neurosci. 2009;123:844–850. doi: 10.1037/a0016343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mei F, Nagappan G, Ke Y, Sacktor TC, Lu B. BDNF facilitates L–LTP maintenance in the absence of protein synthesis through PKMζ. PLoS ONE. 2011;6:e21568. doi: 10.1371/journal.pone.0021568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krotova K, et al. Peptides modified by myristoylation activate eNOS in endothelial cells through Akt phosphorylation. Br. J. Pharmacol. 2006;148:732–740. doi: 10.1038/sj.bjp.0706777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim S, et al. AmyristoylatedpseudosubstratepeptideofPKC-ζinducesdegranulation in HMC-1 cells independently of PKC-ζ activity. Life Sci. 2008;82:733–740. doi: 10.1016/j.lfs.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Denny JB, Polan-Curtain J, Rodriguez S, Wayner MJ, Armstrong DL. Evidence that protein kinase M does not maintain long-term potentiation. Brain Res. 1990;534:201–208. doi: 10.1016/0006-8993(90)90130-4. [DOI] [PubMed] [Google Scholar]
- 22.Huang YY, Kandel ER. Recruitment of long-lasting and protein kinase A-dependent long-term potentiation in the CA1 region of hippocampus requires repeated tetanization. Learn. Mem. 1994;1:74–82. [PubMed] [Google Scholar]
- 23.Kim SJ, Linden DJ. Ubiquitous plasticity and memory storage. Neuron. 2007;56:582–592. doi: 10.1016/j.neuron.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 24.McEchron MD, Bouwmeester H, Tseng W, Weiss C, Disterhoft JF. Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus. 1998;8:638–646. doi: 10.1002/(SICI)1098-1063(1998)8:6<638::AID-HIPO6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 25.Ling DS, Benardo LS, Sacktor TC. Protein kinase Mζ enhances excitatory synaptic transmission by increasing the number of active postsynaptic AMPA receptors. Hippocampus. 2006;16:443–452. doi: 10.1002/hipo.20171. [DOI] [PubMed] [Google Scholar]
- 26.Shema R, et al. Enhancement of consolidated long-term memory by overexpression of protein kinase Mζ in the neocortex. Science. 2011;331:1207–1210. doi: 10.1126/science.1200215. [DOI] [PubMed] [Google Scholar]
- 27.Makuch L, et al. Regulation of AMPA receptor function by the human memory-associated gene KIBRA. Neuron. 2011;71:1022–1029. doi: 10.1016/j.neuron.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodríguez CI, et al. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nature Genet. 2000;25:139–140. doi: 10.1038/75973. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.