Abstract
Purpose
PET use for cancer care has increased unevenly, possibly because of regional health care market characteristics or underlying population characteristics. The aim of this study was to examine variation in advanced imaging use among individuals with cancer in relation to population and hospital service area (HSA) characteristics.
Methods
A retrospective national study of fee-for-service Medicare beneficiaries with diagnoses of 1 of 5 cancers covered by Medicare for PET (2004-2008) was conducted. Crude and adjusted rates of PET, CT, and MRI were estimated for HSAs and sociodemographic subgroups. Generalized linear mixed models were used to assess the effects of race/ethnicity, area-level income, and HSA-level physician supply and spending on imaging utilization.
Results
On the basis of an annual average of 116,452 beneficiaries with cancer, adjusted PET rates (imaging days per person-year) showed significantly higher use for whites compared with blacks in both 2004 (whites, 0.35 [95% confidence interval, 0.34-0.36]; blacks, 0.31 [95% confidence interval, 0.30-0.33]) and 2008 (whites, 0.64 [95% confidence interval, 0.63-0.65]; blacks, 0.57 [95% confidence interval, 0.55-0.59]). This trend was similar for the highest quartile of group-level median household income but was opposite for CT use, with blacks having higher rates than whites. The highest Medicare-spending HSAs had significantly higher adjusted PET rates compared with lower spending areas (0.57 [95% confidence interval, 0.55-0.60] vs 0.69 [95% confidence interval, 0.67-0.71] imaging days/person-year).
Conclusions
The use of PET among Medicare beneficiaries with cancer increased from 2004 to 2008, with higher rates observed among whites, among higher socioeconomic groups, and in higher Medicare spending areas. Sociodemographic differences in advanced imaging use are modality specific.
Keywords: PET, cancer, imaging, variation, Medicare, race
INTRODUCTION
The use of 2-[18F]fluoro-2-deoxyglucose PET and integrated PET/CT (hereafter collectively designated as PET) for cancer care is estimated to have increased >4-fold between 1999 and 2006 [1-3]. Although the use of other forms of advanced imaging (CT and MRI) has also increased, PET use has shown the greatest rise [1,4]. Between January 1998 and January 2005, CMS issued several national coverage decisions that progressively expanded Medicare coverage of PET to include various indications for 9 different types of cancer [5-7].
Recent studies have shown substantial geographic variation in use of PET for cancer patients [2,4]. For example, using 2007 Medicare claims, Parker et al [4] compared the 10 CMS administrative regions and found a 2.3-fold greater likelihood of body PET scans in highest vs lowest rate regions. That study suggests the need to examine PET use within smaller geographic areas, ideally those that represent health care markets rather than geopolitical boundaries. We still lack a national examination of PET use over time that accounts for more recent years, smaller and more appropriate geographic areas, and sociodemographic characteristics. Examining more recent years is important because the diffusion of PET use will have progressed since earlier reports. The importance of sociodemographic factors in PET use is important to examine given well-documented sociodemographic disparities in use of new technologies in general and, more specifically, on the basis of a recent study of PET use in patients with non–small-cell lung cancer. That study, using the Cancer Care Outcomes and Research Surveillance study population, found 13% lower use of PET among nonwhites and Hispanics compared to non-Hispanic whites after adjusting for income, education, insurance, and health care setting [8]. A national examination of sociodemographic patterns of PET use is compelling, particularly for cancers for which PET is covered by Medicare because that population accounts for the majority of cancers in the United States.
As is often seen with health care technologies, diffusion and adoption occur unevenly, resulting in notable variation in utilization patterns. Extensive literature demonstrates geographic and sociodemographic variation in the use of cancer screening, treatment, and endof-life care [9-14]. Variations in utilization may reflect underutilization, particularly for vulnerable populations [9-11], or overutilization, as has been noted in some health care markets [15-17]. Because of the rise in availability and use of PET for cancer care over the past decade [1,4], we sought to examine geographic and sociodemographic variation in PET use and to identify factors that may be contributing to observed variation in 5 cancers on the basis of Medicare claims. These cancers—head and neck, lung, esophageal, colorectal, and lymphoma—may follow different clinical courses but have common indications (initial staging, treatment monitoring, and restaging or detection of suspected recurrence) for the use of PET during active patient management. We examined PET use patterns from 2004 to 2008, several years after approval for use in these cancers. We specifically estimated rates of PET use in relation to hospital service area (HSA) characteristics—physician supply and Medicare spending—and to race and socioeconomic status. We also compared PET rates with those for CT and MRI among cancer patients to further understand geographic and sociodemographic patterns of advanced imaging use over time.
METHODS
Study Population and Data
Using retrospective data from CMS, we identified feefor-service beneficiaries from Medicare enrollment files (2004-2008) who were aged ≥ 65 years but < 100 years on January 1 of each of the 5 years, enrolled in Parts A and B, had no Medicare Advantage enrollment for any part of a given calendar year, and whose ZIP codes of residence matched those of HSAs [18]. CMS claims files used included Medicare Provider Analysis and Review 100% for all years, Outpatient 20% sample for all years, and Physician/Supplier 20% for 2004 to 2007 and 100% for 2008.
Definition of Cancer Cases
To identify a population of individuals actively treated for cancer in each of 5 years, ascertainment if treatment for 1 of the 5 cancers of interest was undertaken for each calendar year using the following criteria: (1) Any hospital admission with a primary diagnosis of 1 of the 5 cancers or (2) the first of 2 nonhospital claims, 7 days apart but within a calendar year, with a primary diagnosis of 1 of the 5 cancers. This annual cross-sectional sample of actively treated cancer patients provides a balance between temporally meaningful trends through the use of calendar years and the ability to ascertain person-time for patients actively undergoing cancer-related care. Inclusion and counting of person-years was based on time from first evidence of cancer codes until December 31 of that year or the date of death, whichever was sooner. Each beneficiary could be represented in any of the study years but was required to “requalify” for the study population each year as an active cancer patient on the basis of International Classification of Disease, ninth rev, codes for cancers of the head and neck (140-149, 160, and 161), esophagus (150), lung (162), or colon and rectum (153 and 154) and lymphoma (200-202). We excluded malignant melanoma to avoid potential racial bias related to the lower incidence of melanoma among black individuals.
Definition of Imaging Modalities
We included claims for PET, CT, and MRI that occurred on or after the date of the first qualifying cancer claim for any beneficiary in the study population for a given year. Specific Healthcare Common Procedure Coding System and Current Procedural Terminology® (CPT®) codes used to identify PET and PET/CT, CT, and MRI were taken from Outpatient and Physician/Supplier claims files (Table 1). We included PET-only and PET/CT in our definition of PET events, although PET/CT accounted for nearly 90% of claims. Because separate imaging events on the same day are difficult to distinguish, especially for CT or MRI, for which the claim for a single examination might consist of several CPT codes, we counted claim-days for each modality rather than number of imaging events.
Table 1.
Billing codes by anatomic site, used to identify PET, CT, and MRI events
| Imaging Type and Anatomic Site |
CPT® and HCPCS Codes |
|---|---|
| PET and PET/CT | G0210-G0234, G0252-G0254, G0330-G0336, 78608, 78810; 78811-78813 (specifically PET), 78814-78816 (specifically PET/ CT) |
| CT | |
| Head and neck | 70450, 70460, 70470, 70480-70492 |
| Chest | 71250, 71260, 71270 |
| Abdomen | 74150, 74160, 74170 |
| Pelvis | 72192-72194 |
| Other | 72125-72133, 73200-73205, 73700-73705, 76497 |
| MRI | |
| Brain | 70551-70553 |
| Abdomen | 74181-74183 |
| Spine | 72141-72158 |
| Other | 70540-70543, 71550-71552, 72195-72197, 75552-75556, 76093-76094, 76390, 76400, 76498 |
CPT® = Current Procedural Terminology®; HCPCS = Healthcare Common Procedure Coding System.
HSA Characteristics
We characterized geographic variation by HSAs as defined by the Dartmouth Atlas of Health Care [18]. Briefly, HSAs are geographic areas that represent health care markets for hospital-based care. Unlike geopolitical boundaries, such as counties, HSAs reflect health care utilization, having been derived from Medicare claims data, and so are more relevant units with which to examine variation in health care utilization. In the United States, there are 3,436 HSAs, most of which contain only one hospital (about 69%).
We used existing measures of supply for HSAs, including per capita supply of radiologists, oncologists, radiation oncologists, hospital-based physicians, and total physicians using AMA data [19] and linking physician counts at the ZIP code level to HSA data. Intensity of health care utilization in HSAs is reported through a variety of measures. We included 4 measures of Medicare spending by HSAs: (1) total Medicare reimbursements per enrollee (2007); (2) reimbursements for diagnostic, laboratory, and imaging services (2007); (3) Medicare reimbursements for inpatient short stays per enrollee (2007); and (4) Medicare reimbursements for outpatient services per enrollee (2007). We also defined the availability of PET (yes or no) in each HSA on the basis of presence of any paid claim for PET in the overall claims files (ie, not just in the study population) for each study year.
Statistical Analysis
Each year, demographic characteristics of the study population and HSAs were summarized with descriptive statistics. Multilevel generalized linear mixed models [20] were applied to individual PET, CT, and MRI counts using a Poisson link function with an offset for the number of person months of observation from the time of qualification for the cancer diagnosis to the end of the year. Hospital service area was used as a random effect, and age, gender, and year were used as fixed effects. Adjusted (age and sex) utilization rates for PET, CT, and MRI were then estimated for each HSA using empirical Bayes methods to account for potential rate instability due to variable population sizes.
To estimate the effects of geographic and sociodemographic factors, additional adjusting variables were included for cancer type and HSA-level quartiles of total Medicare spending and hematologist and oncologist per capita supply. We formed estimates of HSA PET rates by race (white vs black) and socioeconomic status using the HSA quartile of median household income and ZIP code of residence. We then summarized the distribution of rates by computing the means within deciles. To compare the geographic distributions of HSA rates by race, separate models were fit to obtain adjusted HSA rates, and the distributions were compared by examining the average adjusted rates within deciles.
RESULTS
The Medicare cancer study population included an annual average of 116,452 beneficiaries from 2004 to 2008, with an average of 74,977 person-years represented each year (Table 2). The median age was 75 years for all study years, and approximately 48% were women (Table 2). The racial composition of the cancer denominator was consistent from 2004 to 2008 (Table 2). The majority of HSAs did not have PET available in 2004 (72%); this proportion declined to 66% in 2008. Total Medicare reimbursements per beneficiary for the overall Medicare population in each HSA were $8,058 (2007 estimates: median, interquartile range, $6,894-$9,484), with the greatest variation in reimbursements seen in outpatient, diagnostic, laboratory, and imaging services (data not shown). Per capita physician supply within HSAs showed the greatest variability for hematologists or oncologists and radiation oncologists when comparing the differences in the 25th and 75th percentiles (data not shown).
Table 2.
Characteristics of the Medicare cancer study population* from 2004 to 2008
| Variable | 2004 | 2005 | 2006 | 2007 | 2008 |
|---|---|---|---|---|---|
| Study population | |||||
| Number of beneficiaries | 117,997 | 117,981 | 116,709 | 115,527 | 114,047 |
| Person-years | 74,747 | 75,117 | 75,195 | 75,023 | 74,805 |
| Beneficiary characteristics | |||||
| Median age (y) | 75.0 | 75.0 | 75.0 | 75.0 | 75.0 |
| Women (%) | 49.1 | 48.9 | 49.0 | 49.1 | 48.8 |
| Race/ethnicity† (%) | |||||
| White | 90.1 | 90.0 | 90.1 | 90.3 | 90.2 |
| Black | 6.9 | 6.8 | 6.7 | 6.5 | 6.4 |
| Asian | 1.0 | 1.0 | 1.0 | 1.1 | 1.1 |
| Hispanic | 1.0 | 1.0 | 1.0 | 0.9 | 1.0 |
| North American Native | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Cancer type (%) | |||||
| Lung | 33.6 | 33.8 | 33.9 | 34.3 | 34.6 |
| Esophagus | 2.9 | 2.9 | 3.0 | 2.9 | 3.0 |
| Colon | 33.9 | 32.9 | 32.2 | 31.5 | 30.7 |
| Lymphoma | 21.1 | 21.8 | 22.5 | 22.9 | 23.1 |
| Head and neck | 8.5 | 8.6 | 8.5 | 8.5 | 8.6 |
Based on eligible Medicare beneficiaries with active diagnoses of 1 of 5 cancers (lung, head and neck, esophagus, non-Hodgkin’s lymphoma, and colorectal) from 2004 to 2008.
Race and ethnicity were mutually exclusive categories. The racial/ethnic category of “other” had 0.8%, 0.8%, 0.9%, 1.0%, and 1.0% of the study population from 2004 to 2008, respectively.
The median crude HSA-level rate of PET use in 2004 was 0.31 PET imaging days/person-year (interquartile range, 0.15-0.49). In 2008, the median rate was 0.62 PET days/person-year (interquartile range, 0.38-0.92). From 2004 to 2008, the distribution of HSA-level crude imaging rates shifted notably higher for PET (2004: lowest quintile of HSAs crude median rate, 0.00, highest quintile, 0.74; 2008: lowest quintile, 0.00, highest quintile, 1.29), but there was minimal change in CT or MRI. Similar to crude rates, empirical Bayes adjusted PET rates for HSAs in 2004 and 2008 demonstrated an overall increase in use. The median adjusted HSA-level rate of PET use in 2004 was 0.31 PET imaging days/person-year (interquartile range, 0.15-0.49). In 2008, the median adjusted rate was 0.62 PET days/person-year (inter-quartile range, 0.38-0.92). These adjusted rates show that in 2004, the vast majority of HSAs (87%) had <0.5 PET imaging days/person-year, but by 2008, this pattern was almost reversed, with 79% of HSAs having >0.5 PET days/person-year (Figure 1).
Fig 1.
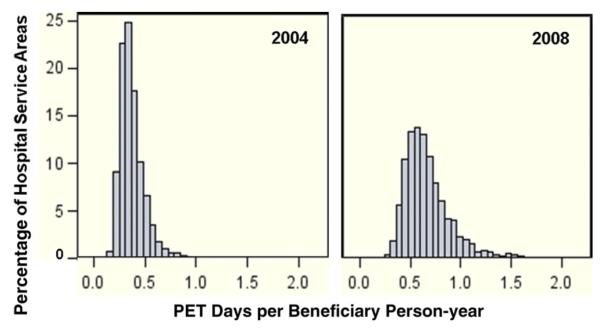
Empirical Bayes estimated hospital service area (HSA) rates for PET use among Medicare beneficiaries with 5 common cancers in 2004 and 2008, adjusting for age and sex.
Adjusted imaging rates showed higher PET use for whites compared with blacks in both 2004 (imaging days/person-year: whites, 0.35 [95% confidence interval (CI), 0.34-0.36]; blacks, 0.31 [95% CI, 0.30-0.33]) and 2008 (imaging days/person-year: whites, 0.64 [95% CI, 0.63-0.65]; blacks, 0.57 [95% CI, 0.55-0.59]) (Table 3). These modest differences in rates (12%-13%)—higher in whites compared with blacks—in 2004 and 2008, respectively, were highly statistically significant (P < .001).
Table 3.
Adjusted* PET rates (imaging days/person-year) by race, income, and Medicare spending
| PET Rate (Imaging Days/Person-Year) and 95% Confidence Interval |
|||||||
|---|---|---|---|---|---|---|---|
| 2004 |
2008 |
||||||
| Variable | Rate | Lower | Upper | Rate | Lower | Upper | P † |
| Race | <.0001 | ||||||
| White | 0.35 | 0.34 | 0.36 | 0.64 | 0.63 | 0.65 | |
| Black | 0.31 | 0.30 | 0.33 | 0.57 | 0.55 | 0.59 | |
| Asian | 0.37 | 0.33 | 0.42 | 0.65 | 0.60 | 0.71 | |
| Median household income ($)‡ | <.0001 | ||||||
| Quartile 1 (0-32,839) | 0.33 | 0.32 | 0.34 | 0.61 | 0.60 | 0.63 | |
| Quartile 2 (>32,839-40,925) | 0.34 | 0.33 | 0.35 | 0.63 | 0.62 | 0.65 | |
| Quartile 3 (>40,925-53,020) | 0.36 | 0.35 | 0.37 | 0.64 | 0.63 | 0.65 | |
| Quartile 4 (>53,020) | 0.36 | 0.35 | 0.37 | 0.64 | 0.63 | 0.66 | |
| Total Medicare spending per enrollee ($)§ | <.0001 | ||||||
| Quartile 1 (0-6,894) | 0.31 | 0.30 | 0.33 | 0.57 | 0.55 | 0.60 | |
| Quartile 2 (6,894-8,058) | 0.34 | 0.32 | 0.35 | 0.61 | 0.59 | 0.63 | |
| Quartile 3 (8,058-9,454) | 0.35 | 0.34 | 0.36 | 0.62 | 0.60 | 0.64 | |
| Quartile 4 (>9,454) | 0.37 | 0.35 | 0.38 | 0.69 | 0.67 | 0.71 | |
Adjusted for age, gender, cancer type, and hematologist and oncologist supply.
For average effect over 2004 to 2008.
Population-weighted quartiles of median ZIP code-level income (2000).
Total Medicare spending per enrollee was based on 2007 estimates from 5% Medicare claims data.
During these 5 years, PET rates sharply increased for all socioeconomic groups but with only modest differences across each successively higher HSA income quartile, with higher quartiles having higher rates (Table 3). The highest quartile of HSA-level total Medicare spending showed significantly higher PET rates than the lowest quartile (2008 imaging days/person-year: 0.69 [95% CI, 0.67-0.71] vs 0.57 [95% CI, 0.55-0.60]).
Modeled rates for CT and MRI also showed significant increases over time but smaller overall differences by race or income group (Table 4). In 2004, MRI rates for whites were significantly higher than for blacks, but in 2008, there were no significant differences. Rates of CT use in the black cancer populations were higher in both 2004 and 2008 compared with whites (imaging days/person year: 2004: blacks, 2.48 [95% CI, 2.43-2.53]; whites, 2.37 [95% CI, 2.36-2.39]; 2008: blacks, 2.50 [95% CI, 2.45-2.54]; whites, 2.32 [95% CI, 2.30-2.33]) (Table 4).
Table 4.
Adjusted* MRI and CT rates (imaging days/person year) by race, income, and Medicare spending
| Rate (Imaging Days/Person-Year) and 95% Confidence Interval |
|||||||
|---|---|---|---|---|---|---|---|
| 2004 |
2008 |
||||||
| Variable | Rate | Lower | Upper | Rate | Lower | Upper | P † |
| MRI | |||||||
| Race | |||||||
| White | 0.39 | .38 | 0.40 | 0.43 | 0.41 | 0.44 | <.0001 |
| Black | 0.35 | .33 | 0.37 | 0.40 | 0.38 | 0.42 | |
| Asian | 0.34 | .31 | 0.39 | 0.42 | 0.38 | 0.46 | |
| Median household income ($)‡ | <.0001 | ||||||
| Quartile 1 (0-32,839) | 0.35 | .34 | 0.37 | 0.41 | 0.39 | 0.42 | |
| Quartile 2 (>32,839-40,925) | 0.38 | .37 | 0.39 | 0.41 | 0.39 | 0.42 | |
| Quartile 3 (>40,925-53,020) | 0.40 | .38 | 0.41 | 0.43 | 0.42 | 0.45 | |
| Quartile 4 (>53,020) | 0.41 | .40 | 0.43 | 0.45 | 0.43 | 0.46 | |
| Total Medicare spending per enrollee($)§ | <0.0001 | ||||||
| Quartile 1 (0-6,894) | 0.34 | .33 | 0.36 | 0.40 | 0.38 | 0.41 | |
| Quartile 2 (6,894-8,058) | 0.38 | .36 | 0.39 | 0.41 | 0.40 | 0.43 | |
| Quartile 3 (8,058-9,454) | 0.39 | .38 | 0.41 | 0.43 | 0.41 | 0.45 | |
| Quartile 4 (>9,454) | 0.40 | .39 | 0.42 | 0.44 | 0.42 | 0.45 | |
| CT | |||||||
| Race | |||||||
| White | 2.37 | 2.36 | 2.39 | 2.32 | 2.30 | 2.33 | <.0001 |
| Black | 2.48 | 2.43 | 2.53 | 2.50 | 2.45 | 2.54 | |
| Asian | 2.40 | 2.28 | 2.52 | 2.38 | 2.27 | 2.49 | |
| Median household income ($)‡ | .02 | ||||||
| Quartile 1 (0-32,839) | 2.39 | 2.36 | 2.41 | 2.34 | 2.32 | 2.36 | |
| Quartile 2 (>32,839-40,925) | 2.38 | 2.35 | 2.41 | 2.36 | 2.33 | 2.39 | |
| Quartile 3 (>40,925-53,020) | 2.39 | 2.36 | 2.41 | 2.31 | 2.29 | 2.34 | |
| Quartile 4 (>53,020) | 2.38 | 2.35 | 2.41 | 2.31 | 2.28 | 2.33 | |
| Total Medicare spending per enrollee($)§ | |||||||
| Quartile 1 (0-6,894) | 2.32 | 2.28 | 2.36 | 2.22 | 2.18 | 2.26 | <.0001 |
| Quartile 2 (6,894-8,058) | 2.35 | 2.32 | 2.39 | 2.27 | 2.24 | 2.30 | |
| Quartile 3 (8,058-9,454) | 2.38 | 2.34 | 2.42 | 2.36 | 2.33 | 2.40 | |
| Quartile 4 (>9,454) | 2.44 | 2.40 | 2.48 | 2.40 | 2.37 | 2.44 | |
Adjusted for age, gender, cancer type, and hematologist and oncologist supply.
For average effect over 2004 to 2008.
Population-weighted quartiles of median ZIP code-level income (2000).
Total Medicare spending per enrollee was based on 2007 estimates from 5% Medicare claims data.
PET use in HSAs with higher total Medicare spending per beneficiary was modestly but significantly greater for the highest spending quartile compared with the lowest in both 2004 and 2008 (imaging days/person year: 2004: lowest spending quartile, 0.31 [95% CI, 0.30-0.33]; highest, 0.37 [95% CI, 0.35-0.38]; 2008: lowest, 0.57 [95% CI, 0.55-0.60]; highest, 0.69 [95% CI, 0.67-0.71]) (Table 3). A similar pattern between the lowest and highest spending areas was noted for both MRI and CT in 2004 and 2008 (Table 4).
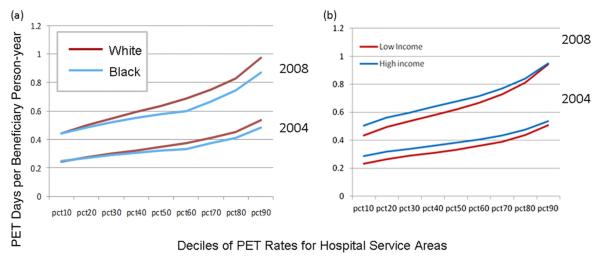
When examining deciles of HSA PET rates by race, we found that in 2004 and 2008, blacks consistently had lower rates of PET use than whites (Figure 2a). Furthermore, the difference in PET rates between blacks and whites across the HSA deciles increased in 2008 compared with 2004, even though overall, PET use was higher for both blacks and whites (Figure 2a). When comparing the 25th percentile of median household income with the 75th percentile, for both income groups, PET rates increased notably across HSA deciles from 2004 to 2008, but the gains were only modestly greater for the higher income group (Figure 2b).
Fig 2.
Hospital service area rates adjusted for age and sex of PET use in deciles by race (a) and socioeconomic status (b) for Medicare beneficiaries with 5 common cancers in 2004 and 2008.
DISCUSSION
This study examined geographic and sociodemographic variation in PET use from 2004 to 2008 for Medicare beneficiaries with cancer in the United States within HSAs to better understand national patterns of variation in light of the rapid increase in PET utilization for cancer. We found modest but significant differences in PET use by race and group-level household income, with whites and the highest income quartile having higher PET rates compared with blacks and the lowest income quartile. Other advanced imaging modalities did not show this pattern. No notable differences by race or socioeconomic group were seen for MRI, whereas for CT, blacks had significantly higher rates of use than whites. We also found that PET, MRI, and CT use was higher in HSAs with higher spending. Black beneficiaries with cancer had lower rates of PET use compared with white beneficiaries in all study years. Although PET use increased for both blacks and whites, the gap in use between 2004 and 2008 widened somewhat. By furthering our understanding of PET use patterns, we inferred that there are areas and populations that could likely benefit from more PET, as well as areas that may be using this costly modality at a high rate relative to other geographic areas. Other advanced imaging modalities used in cancer care (CT and MRI) showed different relationships to race: MRI rates were not statistically significantly different by 2008, and CT use was significantly higher among black patients than whites. These findings suggest that the diffusion of new technologies does increase use overall across sociodemographic groups but may not narrow racial disparities. Furthermore, these findings suggest that racial or socioeconomic disparities in overall use of advanced imaging may not be notable, but differences by modality may be present. Modality-specific differences may reflect more rapid uptake of new technologies among whites and higher income groups compared with blacks and lower income groups. It is possible that these patterns represent incomplete diffusion of the new technology and that differences will diminish over time.
Other studies addressing the issue of racial disparities in technology diffusion have found significant differences among black compared with white patients in the use of many new therapies: implantable cardioverter defibrillators (14% lower rates among blacks) [21], tissue replacement of the aortic valve (55% less likely among blacks), and lumbar spinal fusion (17% less likely among blacks) [22]. Our results, demonstrating lower use of PET among black beneficiaries compared with white beneficiaries, are consistent with these prior reports of racial disparities in the use of new technologies and, of note, also with the study of PET use in non–small-cell lung cancer in the Cancer Care Outcomes and Research Surveillance population [8].
The literature suggests that the location of service mediates racial disparities in technology use. For example, hospitals with >20% black patients were less likely to use new technologies for either black or white patients compared with hospitals with <9% black patients [22]. However, as Bach [23] noted in his review of racial disparities and site of care, studies that disaggregate the effects of race and place of service are most useful in understanding potential mechanisms of observed racial differences. Our study used both adjusted analyses to examine the contribution of race in PET use while accounting for location. After stratifying or adjusting for HSA of residence, we demonstrated a modest but significantly lower use of PET among black cancer patients. This result may be due to or influenced within these geographic areas by racial bias, granular socioeconomic factors, referral patterns or physician characteristics, and clinical factors such as later stage at diagnosis [24]. We were not able to explicitly examine these factors. However, our study included an HSA-level measure of income, which did not significantly attenuate the observed racial differences.
On the basis of prior work showing that racial disparities in technology use between blacks and whites diminish significantly as diffusion progresses [25], we hypothesized that racial disparities in PET use would decrease over time. We found the opposite: that the gap in PET use between blacks and whites widened proportionally from 2004 to 2008. A benchmark does not exist to interpret whether this finding represents underutilization, overutilization, or adequate utilization within the racial groups. Nevertheless, the larger gap in PET use over time suggests that either PET may be underutilized among blacks or overutilized by whites, although there may be underlying differences in the need for PET between black and white patients with cancer, such as later stage at diagnosis among black patients. We are not able to address this in the current analysis, because indication for PET and cancer stage at diagnosis are not known.
The second key objective of our study was to describe variation of PET use among geographic areas that represent local health care markets. Among HSAs with PET capabilities, we found almost a 7-fold difference in PET rates in our study population. Geographic variation of health care utilization in HSAs has been shown to be related to a number of health care market characteristics, including supply of providers, services, and facilities, and local patterns of health care intensity and spending [16,17]. Our results suggest that HSA-level geographic variation is significantly associated with total per beneficiary Medicare spending but not with provider supply. The concern that a sizable portion of the increase in PET use represents overutilization has support from recent studies [1,2,26,27]. For example, 2 studies provide evidence that self-referral has contributed to the rapid growth of PET use [1,2]. Also, imaging costs among Medicare beneficiaries with cancer (1999-2006) rose by at least double the rate of total costs for that population [1]. These findings suggest that geographic variation in PET, and other advanced imaging modalities, may be attributed at least in part to measures of supply and spending within HSAs. We were not able to explicitly examine the role of regulatory practices (eg, certificate of need) that may influence the introduction of expensive nontherapeutic technologies, and that may be a potential source of variation.
Several other limitations of our study should be noted. First, the presence of PET use in some HSAs may have been underestimated because our analyses used a 20% sample of claims. Second, the additional coverage for PET provided via the National Oncologic PET Registry beginning in May 2006 may have influenced the rate of diffusion or use patterns, although National Oncologic PET Registry scans were estimated to have constituted only 10% of all Medicare PET studies in 2007 [3]. Also, we were not able to describe access to PET in terms of radiopharmaceutical availability or by distinguishing installed vs mobile PET units within geographic areas. Furthermore, total Medicare spending may be endogenous to PET rates; however, the model-estimated rates did not appreciably change when spending was added to the models. Finally, as with most Medicare claims analyses, clinical variation, performance status, cancer stage at diagnosis, and disease severity could not be measured. Differences in these clinical factors and patient preferences between blacks and whites could potentially account for differences in PET use. A study designed to address the influence of these potential differences on PET use would be an important next step.
PET use among Medicare beneficiaries with cancer increased from 2004 to 2008, but this growth was not uniform across health care markets or patient populations. Geographic factors related to health care spending patterns seem to explain some of the variation. Sociodemographic differences in PET use showed consistently higher rates among whites compared to blacks, but the increase was greater for whites. The higher rates of CT in black compared with white cancer patients suggest that overall disparities in advanced imaging in cancer may not be evident; rather, the uptake of or access to PET may be occurring faster at sites where whites get their care, while blacks are receiving more CT in part from less access.
TAKE-HOME POINTS.
PET use is higher among white cancer patients compared with black cancer patients and for those in the highest quartile of ZIP code-level income.
MRI use does not differ by race or income for cancer patients, but CT use is higher among black patients compared with white patients.
PET use among Medicare beneficiaries with cancer increased from 2004 to 2008, but this increase widened the differences in use by race.
Sociodemographic differences in advanced imaging use are modality specific and may reflect patterns of technology diffusion.
Acknowledgments
This work was supported by grant 1RC2CA148259 from the National Cancer Institute, National Institutes of Health (Bethesda, Md).
REFERENCES
- 1.Dinan MA, Curtis LH, Hammill BG, et al. Changes in the use and costs of diagnostic imaging among Medicare beneficiaries with cancer 1999-2006. JAMA. 2010;303:1625–31. doi: 10.1001/jama.2010.460. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell JM. Utilization trends for advanced imaging procedures: evidence from individuals with private insurance coverage in California. Med Care. 2008;46:460–6. doi: 10.1097/MLR.0b013e31815dc5ae. [DOI] [PubMed] [Google Scholar]
- 3.Hillner BE, Tosteson AN, Song Y, Tosteson TD, Onega T, Siegel BA. Growth in the use of PET for six cancer types after coverage by Medicare: additive or replacement? additive or replacement? J Am Coll Radiol. 2012;9:33–41. doi: 10.1016/j.jacr.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parker L, Levin DC, Frangos A, Rao VM. Geographic variation in the utilization of noninvasive diagnostic imaging: national Medicare data 1998-2007. AJR Am J Roentgenol. 2010;194:1034–9. doi: 10.2214/AJR.09.3528. [DOI] [PubMed] [Google Scholar]
- 5.Hillner BE, Liu D, Coleman RE, et al. The National Oncologic PET Registry (NOPR): design and analysis plan. J Nucl Med. 2007;48:1901–8. doi: 10.2967/jnumed.107.043687. [DOI] [PubMed] [Google Scholar]
- 6.Podoloff A, Advani RH, Allred C, et al. NCCN task force report: positron emission tomography (PET)/computed tomography (CT) scanning in cancer. J Natl Compr Canc Netw. 2007;5(suppl):S1–22. [PubMed] [Google Scholar]
- 7.Lindsay MJ, Siegel BA, Tunis SR, et al. The National Oncologic PET Registry: expanded Medicare coverage for PET under coverage with evidence development. AJR Am J Roentgenol. 2007;188:1109–13. doi: 10.2214/AJR.06.1175. [DOI] [PubMed] [Google Scholar]
- 8.Gould MK, Schultz EM, Wagner TH, et al. Disparities in lung cancer staging with positron emission tomography in the Cancer Care Outcomes Research and Surveillance (CanCORS) study. J Thoracic Oncol. 2011;6:875–83. doi: 10.1097/JTO.0b013e31821671b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabatino SA, Coates RJ, Uhler RJ, Breen N, Tangka F, Shaw KM. Disparities in mammography use among US women aged 40-64 years, by race, ethnicity, income, and health insurance status 1993 and 2005. Med Care. 2008;46:692–700. doi: 10.1097/MLR.0b013e31817893b1. [DOI] [PubMed] [Google Scholar]
- 10.Haggstrom DA, Quale C, Smith-Bindman R. Differences in the quality of breast cancer care among vulnerable populations. Cancer. 2005;104:2347–58. doi: 10.1002/cncr.21443. [DOI] [PubMed] [Google Scholar]
- 11.Li CI, Malone KE, Daling JR. Differences in breast cancer stage, treatment, and survival by race and ethnicity. Arch Intern Med. 2003;163:49–56. doi: 10.1001/archinte.163.1.49. [DOI] [PubMed] [Google Scholar]
- 12.Cooper GS, Yuan Z, Chak A, Rimm AA. Geographic and patient variation among Medicare beneficiaries in the use of follow-up testing after surgery for nonmetastatic colorectal carcinoma. Cancer. 1999;85:2124–31. [PubMed] [Google Scholar]
- 13.Virnig BA, Marshall McBean A, Kind S, Dholakia R. Hospice use before death: variability across cancer diagnoses. Med Care. 2002;40:73–8. doi: 10.1097/00005650-200201000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Virnig BA, Kind S, McBean M, Fisher E. Geographic variation in hospice use prior to death. J Am Geriatr Soc. 2000;48:1117–25. doi: 10.1111/j.1532-5415.2000.tb04789.x. [DOI] [PubMed] [Google Scholar]
- 15.Baicker K, Chandra A, Skinner JS, Wennberg JE. Who you are and where you live: how race and geography affect the treatment of Medicare beneficiaries. Health Aff (Millwood) 2004;(Suppl Variation):VAR33–44. doi: 10.1377/hlthaff.var.33. [DOI] [PubMed] [Google Scholar]
- 16.Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder EL. The implications of regional variations in Medicare spending part 1: the content, quality, and accessibility of care. Ann Intern Med. 2003;138:273–87. doi: 10.7326/0003-4819-138-4-200302180-00006. [DOI] [PubMed] [Google Scholar]
- 17.Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder EL. The implications of regional variations in Medicare spending part 2: health outcomes and satisfaction with care. Ann Intern Med. 2003;138:288–98. doi: 10.7326/0003-4819-138-4-200302180-00007. [DOI] [PubMed] [Google Scholar]
- 18.Wennberg JE. The Dartmouth atlas of healthcare. American Hospital Publishing; Chicago, Ill: 1996. [Google Scholar]
- 19.The Trustees of Dartmouth College [Accessed October 3, 2011];The Dartmouth atlas of health care. Available at: http://www.dartmouthatlas.org/tools/downloads.aspx#reimbursements.
- 20.SAS Institute, Inc . SAS/STAT 9.2 user’s guide. SAS Institute Inc; Cary, NC: [Google Scholar]
- 21.Stanley A, DeLia D, Cantor JC. Racial disparity and technology diffusion: the case of the cardioverter defibrillator implants 1996-2001. J Natl Med Assoc. 2007;99:201–7. [PMC free article] [PubMed] [Google Scholar]
- 22.Groeneveld PW, Laufer SB, Garber AM. Technology diffusion, hospital variation, and racial disparities among elderly Medicare beneficiaries 1989-2000. Med Care. 2005;43:320–9. doi: 10.1097/01.mlr.0000156849.15166.ec. [DOI] [PubMed] [Google Scholar]
- 23.Bach PB. Racial disparities and site of care. Ethnicity Dis. 2005;15:S2–33. [PubMed] [Google Scholar]
- 24.Schwartz KL, Crossley-May H, Vigneau FD, Brown K, Banerjee M. Race, socioeconomic statusand stage at diagnosis for five common malignancies. Cancer Causes Control. 2003;14:761–6. doi: 10.1023/a:1026321923883. [DOI] [PubMed] [Google Scholar]
- 25.Ferris TG, Kuhlthau K, Ausiello J, Perrin J, Kahn R. Are minority children the last to benefit from a new technology? Med Care. 2006;44:81–6. doi: 10.1097/01.mlr.0000188914.47033.cd. [DOI] [PubMed] [Google Scholar]
- 26.Agarwal R, Levin DC, Parker L, Rao VM. Trends in PET scanner ownership and leasing by nonradiologist physicians. J Am Coll Radiol. 2010;7:187–91. doi: 10.1016/j.jacr.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Hendee WR, Becker GJ, Borgstede JP, et al. Addressing overutilization in medical imaging. Radiology. 2010;257:240–5. doi: 10.1148/radiol.10100063. [DOI] [PubMed] [Google Scholar]