Abstract
Ocean surface CO2 levels are increasing in line with rising atmospheric CO2 and could exceed 900 μatm by year 2100, with extremes above 2000 μatm in some coastal habitats. The imminent increase in ocean pCO2 is predicted to have negative consequences for marine fishes, including reduced aerobic performance, but variability among species could be expected. Understanding interspecific responses to ocean acidification is important for predicting the consequences of ocean acidification on communities and ecosystems. In the present study, the effects of exposure to near-future seawater CO2 (860 μatm) on resting (Ṁ O2rest) and maximum (Ṁ O2max) oxygen consumption rates were determined for three tropical coral reef fish species interlinked through predator-prey relationships: juvenile Pomacentrus moluccensis and P. amboinensis, and one of their predators: adult Pseudochromis fuscus. Contrary to predictions, one of the prey species, P. amboinensis, displayed a 28 – 39 % increase in Ṁ O2max after both an acute and four-day exposure to near-future CO2 seawater, while maintaining Ṁ O2rest. By contrast, the same treatment had no significant effects on Ṁ O2rest or Ṁ O2max of the other two species. However, acute exposure of P. amboinensis to 1400 and 2400 μatm CO2 resulted in Ṁ O2max returning to control values. Overall, the findings suggest that: (1) the metabolic costs of living in a near-future CO2 seawater environment were insignificant for the species examined at rest; (2) the ṀO2max response of tropical reef species to near-future CO2 seawater can be dependent on the severity of external hypercapnia; and (3) near-future ocean pCO2 may not be detrimental to aerobic scope of all fish species and it may even augment aerobic scope of some species. The present results also highlight that close phylogenetic relatedness and living in the same environment, does not necessarily imply similar physiological responses to near-future CO2.
Keywords: Bioenergetics, aerobic metabolic scope, coral reef fishes, predator-prey relationship, climate change
1 Introduction
The average concentration of carbon dioxide (CO2) in the atmosphere has increased from approximately 280 ppm in pre-industrial times (Barnola et al., 1987) to >400 ppm in 2013 (Dlugokencky and Tans, 2013) and is projected to exceed 900 ppm by the year 2100 if the current emissions trajectory is maintained (Meinshausen et al., 2011). Because atmospheric and ocean surface pCO2 are in equilibrium, CO2 in the ocean is also increasing at approximately the same rate as in the atmosphere (Doney, 2010). Moreover, due to the hydrolysis of CO2 in seawater, ocean surface pH is 0.1 unit lower today than preindustrial values and is predicted to be a further 0.3–0.4 units lower by 2100, which translates to a 100–150% increase in [H+] (Solomon et al., 2007). Some coastal regions could experience changes to [H+] that are at least 2–3 times the global average due to CO2 enhancement from eutrophication (Melzner et al., 2012) and amplification of natural CO2 and pH variation (Shaw et al., 2013). Such changes in ocean chemistry are predicted to affect physiological functions of many marine organisms, with potentially far-reaching effects on marine diversity and ecosystem processes (Fabry et al., 2007; Gattuso and Hansson, 2011; Pörtner, 2008).
Ocean acidification has been hypothesized to have negative consequences for the performance of marine fishes, primarily through an effect of the capacity for oxygen supply and delivery (Pörtner et al., 2004). Aerobic scope, which represents the oxygen available for any activities beyond that required for basic maintenance (Fry and Hart, 1948; Fry, 1947, 1971), is expected to decline with increasing pCO2 (Pörtner and Farrell, 2008). Reduced aerobic scope could affect individual fitness, since less energy can be devoted to digestion, growth and reproduction (Munday et al., 2009b; 2012). Reduced aerobic scope could also affect the outcome of key ecological interactions and ultimately the structure of ecological communities (Nilsson et al., 2009; Pörtner, 2008).
In accordance with these predictions, the aerobic capacity of two cardinalfish species from the Great Barrier Reef (GBR) was significantly reduced by exposure to CO2-acidified water (Munday et al., 2009a). However, it may be expected that not all fish species of coral reef ecosystems will be similarly affected by CO2-induced ocean acidification. Fishes are the most diverse group of vertebrates and the ontogenetic and lifestyle traits of some species could provide pre-adaptation to high ambient CO2 (Ishimatsu et al., 2008; Melzner et al., 2009b). Indeed, the aerobic scope of some fish species appears to be unaffected by hypercapnia (Baker and Brauner, 2012; Ishimatsu et al., 2008; McKenzie et al., 2003). Interspecific differences in the response of aerobic scope to near-future seawater CO2 could have important ecological ramifications, especially for species that interact through competitive or predator-prey relationships (Munday et al., 2012). Many studies implicate species interactions to be an important proximate cause of extinction due to climate change, particularly due to decreases in food availability (Cahill et al., 2012).
The objective of the present study was to assess species-specific effects of near-future seawater CO2 on aerobic performance among tropical reef fish species involved in predator and prey relationships. The model predator species investigated was the brown dottyback (Pseudochromis fuscus Müller & Troschel 1849), a common meso-predator on the GBR. Two closely related damselfishes, the lemon damselfish (Pomacentrus moluccensis Bleeker 1853) and the Ambon damselfish (P. amboinensis Bleeker 1868) were chosen as the model prey species. P. fuscus is known to be a major predator of recently settled juveniles of these two damselfishes on the GBR (Holmes and McCormick, 2010). Respirometry was utilized to measure the effects of exposure to elevated CO2 on resting (ṀO2rest) and maximum (Ṁ O2max) oxygen consumption rates. In fish, physiological alterations in response to elevated ambient CO2 can occur within minutes (i.e., ventilatory responses; Gilmour and Perry, 2006), hours (i.e., blood acid-base regulation; Brauner and Baker, 2009; Heisler, 1993; Esbaugh et al., 2012) to days (i.e., neurological disruptions; Nilsson et al., 2012). In terms of neurological impairments, four days of high-CO2 exposure has been shown to disrupt a number of sensory systems and alter the behaviour of reef fishes, including the three species examined in the present study (Cripps et al., 2011; Ferrari et al., 2011a; Ferrari et al., 2011b; Nilsson et al., 2012). Longer exposure to elevated CO2 does not induce further behavioral effects (Munday et al., 2010). Therefore, in our first experiment, we measured oxygen consumption after exposing the three species to ambient or elevated CO2 for four days, to enable direct comparisons with previous studies on coral reef fish. In a second experiment, we measured oxygen consumption of the damselfishes, following acute exposure to near-future seawater CO2 to determine if exposure to elevated CO2 induces an immediate effect on aerobic performance. In both experiments, the elevated CO2 treatment (860 μatm) was selected to approximate the level predicted for the atmosphere and ocean surface in 2100 under the IPCC A2 emissions scenario (Meehl et al., 2007). Finally, a third experiment was conducted to understand the effects of more extreme fluctuations in seawater pCO2 and consequently pH that may occur in some coastal habitats, including shallow coral reef flats (Shaw et al., 2013). Four groups of P. amboinensis were exposed to one of four pH levels spanning from the present-day control pH of 8.1 down to pH 7.5 at 0.2 unit increments. The desired pH level was obtained by adding increasing volumes of 100 mM hydrochloric acid to the seawater. The addition of a strong acid to a closed system, such as a closed respirometer, has similar consequences on pCO2 and pH as equilibrating water of an open system with CO2 gas (Gattuso and Lavigne, 2009). Corresponding pCO2 levels were approximately 450 μatm at pH 8.1, 860 μatm at pH 7.9, 1400 μatm at pH 7.7 and 2400 μatm at pH 7.5.
2 Materials and Methods
2.1 Experimental fish
The experiments were conducted at Lizard Island Research Station (LIRS; 14°40′S, 145°28′E) between December and January (austral summer). Juvenile P. moluccensis (mean ±SD, 40.9 ±5.6 mg) and P. amboinensis (53.9 ±12.4 mg) were caught at night using light traps moored two meters below the surface and approximately 100 m off the reef (Meekan et al., 2001). In this location, the fish are trapped immediately before their arrival to the reef at the end of their planktonic larval stage (Meekan et al., 1993). Every morning, juveniles were collected from the traps and transferred to the laboratory where they were exposed to ambient or elevated CO2 for four days (see section 2.2). Adult P. fuscus (4.51 ±0.78 g) were collected from shallow reefs (<6 m) in the Lizard Island lagoon using a hand-net after lightly anaesthetizing them with a mixture of clove oil, ethanol and seawater (Munday and Wilson, 1997). Captured fish were transported to the research station where they were maintained for two days prior to exposure to CO2 treatments. Fish were maintained at ambient ocean temperatures, which ranged from 28.3 to 30.4°C (Table 1; 29.4 ±0.1°C) during the experimental period. Damselfishes were fed freshly hatched Artemia nauplii three times daily, and P. fuscus were fed twice daily to satiation with INVE Aquaculture Nutrition pellets. Feeding was discontinued 18 – 24 h prior to resting oxygen consumption measurements (see section 2.3.1). Animal care and experimental protocols complied with regulations at James Cook University and Lizard Island Research Station, and were approved by the James Cook University Ethic Committee (Approval # A1722). Fish were collected under permit G10/33239.1 from the GBR Marine Park Authority
Table 1.
Mean (±SE) seawater parameters in the experimental system. pCO2 was estimated in the program CO2SYS from measured pH, salinity and total alkalinity (AT) of water samples.
| Treatment | pHNBS | Temperature (°C) | Salinity (ppt) | AT (μmol kg−1SW) | pCO2 (μatm) |
|---|---|---|---|---|---|
| Control | 8.11–8.17 | 29.4 ±0.1 | 34.5 | 2272 ±13 | 451 ±15 |
| High-CO2 | 7.90–7.92 | 29.4 ±0.1 | 34.5 | 2267 ±2 | 860 ±14 |
2.2 CO2 exposure
Fish were exposed to either aerated control water (pCO2 = 451 μatm) or 860 μatm CO2 water (termed near-future seawater CO2 in the present study) for four days (Table 1). Near-future seawater CO2 concentrations were maintained by CO2-dosing to a set pHNBS (National Bureau of Standards) following standard techniques for ocean acidification research (Gattuso et al., 2010). Seawater was pumped from the ocean into two 60 l header tanks, one equilibrated with air (ambient control) and the other with CO2 to achieve the pH expected to correspond to the ocean CO2 concentration projected for 2100 (Meehl et al., 2007). The pH level was based upon preliminary observations of total alkalinity, salinity and temperature of seawater at Lizard Island. A pH-controller (Aqua Medic GmbH, Bissendorf, Germany) was attached to the CO2-treated header tank to maintain pH at the desired level. A solenoid injected a slow stream of CO2 into a submersible pump at the bottom of the header tank whenever the seawater pH rose above the set point. The pump ensured rapid dissolution of CO2 into the seawater and also served as a vigorous stirrer. The pump in the control seawater header tank was injected with a slow stream of air. Seawater from each header tank was supplied at a rate of ca 500 ml min−1 to four replicate 35 l aquaria for each species. pCO2 in the aquaria was checked twice daily with a CO2-permeable membrane connected to an infrared CO2 probe (Vaisala GMP343, Vaisala, Helsinki, Finland) in a closed loop (Hari et al., 2008). Water samples were collected at the start, middle and end of the experiment in order to precisely determine pCO2. Total alkalinity (AT) of seawater was estimated by Gran titration (Gran, 1950; 1952) using certified reference material from Dr. A. G. Dickson (Scripps Institution of Oceanography), and average seawater pCO2 was calculated in CO2SYS (http://cdiac.ornl.gov/oceans/co2rprt.html) from measured AT and pH and using the constants of Mehrbach et al. (1973) refit by Dickson and Millero (1987).
2.3 Experimental set-up and protocol
Ṁ O2rest and Ṁ O2max were used as proxies for resting and maximum metabolic rates and were measured by respirometry as previously utilized for assessing the effects of climate change variables on other fish (see Ishimatsu et al., 2008 for review), especially tropical reef fish species (Gardiner et al., 2010; Munday et al., 2009a; Nilsson et al., 2007a; Nilsson et al., 2010; Nilsson et al., 2007b; Nilsson and Östlund-Nilsson, 2004). Respirometry chambers were immersed in temperature-controlled (29°C) aquaria continuously supplied with either air- or near-future CO2-equilibrated seawater.
2.3.1 Resting oxygen consumption
Cylindrical 26.7-ml static respirometers were used for P. moluccensis and P. amboinensis juveniles. After four days of exposure to current day or near-future CO2 seawater, one fish was transferred to each respirometry chamber. The chamber was left open and the fish left undisturbed to habituate to the chamber for 1 – 2 h whereupon the chamber was cautiously closed without disturbing the fish. Previous experiments have shown that habituation periods longer than 2 h in the chamber do not further reduce Ṁ O2 (Nilsson et al., 2010; Nilsson and Östlund-Nilsson, 2004). All fishes included in this study settled down rapidly and remained virtually motionless during the measuring period. Once the chamber had been sealed, water oxygen concentration was recorded continuously with an oxygen probe (CellOx 325, WTW, Germany; calibrated daily) connected to an oxygen meter (OXI 340i, WTW, Germany). The oxygen probe was fitted with a magnetic propeller (BOD stirring accessory, WTW, Germany) set in motion with a magnetic stir plate situated outside the aquarium along the glass wall. The propeller ensured gentle water mixing inside the respirometer and water renewal along the O2 probe membrane during the habituation and recording periods. The oxygen meters were connected to a data acquisition system (PowerLab 4/20, ADInstruments, Colorado Springs, USA). Ṁ O2rest was calculated from the steady rate of oxygen consumption observed between 100 and 90% of air saturation. The decrease of water oxygen concentration was recorded until it reached approximately 10% of air saturation in order to calculate the critical oxygen concentration (O2crit), which is the lowest O2 concentration where the fish is still able to maintain Ṁ O2rest. O2crit was reached 2.5 – 3 h after the chamber was sealed. For each species, two parallel setups allowed for the simultaneous recording of one fish in high-CO2 water and another fish in control conditions. Similar to a number of other prior experiments conducted to determine the O2crit of a fish using this classic protocol, the measurement of O2crit required the respirometer to remain closed until almost all O2 was depleted from the system. Consequently, CO2 concomitantly increased in the respirometer due to the respiration of the fish and the fish simultaneously experienced hypoxia and increasing hypercapnia during the O2crit measurement. Assuming a respiratory quotient of between 0.7 and 1.0, the maximum build-up of CO2 within the respirometer for each 10% fall in O2 is estimated to be 400–600 μtam. However, most of the excreted CO2 would convert rapidly to bicarbonate, resulting in the build-up of pCO2 to be considerably less. Given that Ṁ O2rest did not differ significantly between the control and near-future CO2 exposed fish (see Fig. 1A), pCO2 would have increased by a similar magnitude and in parallel for both treatment groups. However, the near-future CO2 fish would have consistently experienced a greater level of hypercapnia (by 410 μatm) than the control fish at any point in time, including at O2crit, due to the elevated pCO2 of the water at the start of the experiment. For P. fuscus, Ṁ O2rest was measured in 1615-ml intermittent-flow respirometers. Fish were first habituated to the chambers for 90 min. Preliminary experiments determined that 90 min was ample time for this species to ensure O2 consumption rates had reached the lowest possible values under the experimental conditions. Beyond this time, O2 consumption rates did not significantly vary. Submersible pumps supplied a water flow (150 l h−1) from the aquaria through the chambers and after the habituation period, water flow to each chamber was stopped for 15 min every 30 min over a period of 90 min. The time the water flow was interrupted was short enough to ensure O2 did not fall below 80% of air saturation. Water oxygen concentration (mg l−1) was continuously recorded at a frequency of 1 Hz using oxygen-sensitive REDFLASH dye on contactless spots (2mm) adhered to the inside of each chamber and connected via fiber-optic cable to a Firesting Optical Oxygen Meter (Pyro Science e. K., Aachen, Germany). Data were analyzed using LabChart 6.1.3 (ADInstruments, Colorado Springs, USA).
Fig. 1.
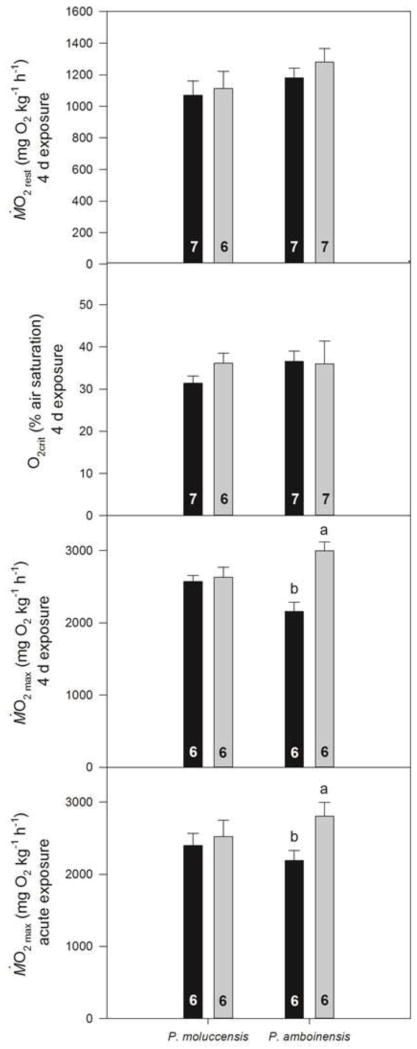
Respiratory performance (mean ±SE) of P. moluccensis and P. amboinensis. A. Resting oxygen consumption rate (Ṁ O2rest; mgO2 kg−1 h−1), B. Critical oxygen concentration (O2crit; % of air saturation), C. Maximum oxygen consumption rate (Ṁ O2max; mgO2 kg−1 h−1) after a 4 d exposure to control seawater (451 μatm;■) or near-future CO2 seawater (860 μatm;■) and D. Ṁ O2max after an acute exposure to near-future CO2 seawater. Letters that differ indicate statistically significant differences (see text for p values). N numbers are indicated at the bottom of each bar.
2.3.2 Maximum rate of oxygen consumption
Ṁ O2max was measured in custom made cylindrical swimming chambers previously utilized by a large number of studies for small tropical coral reef fish species (see Nilsson et al., 2007b for diagram and detailed description of the set-up; Gardiner et al., 2010; Nilsson et al., 2009, Munday et al., 2009a; Nilsson et al., 2007a; Nilsson et al., 2010; Nilsson and Östlund-Nilsson, 2004). The volume of the chambers ranged between 215 and 267 ml for P. moluccensis and P. amboinensis juveniles and was 1594 ml for P. fuscus. The decrease in [O2] was measured with an O2 electrode (WTW CellOx 325, as above). It has been suggested that the aerobically fuelled muscle mass in some fish is not large enough to force them to reach the maximum rate of oxygen uptake during maximal swimming performance (Goolish, 1991). Therefore, fish were fed ad libitum prior to estimating Ṁ O2max, since it is likely that the combined oxygen needs of digestion and maximal swimming would be high enough to engage the full capacity of the respiratory system (Bennett and Hicks, 2001; Gardiner et al., 2010). Up to three P. amboinensis (to increase the total fish weight so that reliable recordings could be made), one P. moluccensis or one P. fuscus (same fish as for Ṁ O2rest measurements) were placed in the swimming chamber. The water speed was regulated with a magnetic stirrer located beneath the chamber. As soon as the water was set in motion, the fishes started swimming against the current, apparently guided by landmarks provided by items such as the oxygen electrode and the edges of the surrounding aquarium. The speed was consistently increased to a point where it was assumed that the fishes swam at their maximum speed. The water speed corresponded to the point at which the fishes could just barely maintain a steady position in the chamber. At a slightly higher speed, the fishes were no longer able to maintain position for more than a few seconds and stopped swimming. Nilsson et al. (2007b) showed that water speeds of approximately 50 and 125 cm s−1 could be achieved near the inner and outer wall of the chamber, respectively. The speeds are more than sufficient for each of the species examined to reach their maximal swimming speed. Pre-settlement larvae of both Pomacentrus species as well as P. fuscus, which are more efficient swimmers than the post-settlement larvae and adults studied here (Nilsson and al., 2007b) exhibit an average maximum sustained swimming speed ranging from less than 30 cm s−1 to a maximum of 36 cm s−1 (Fisher et al., 2005). The decrease in oxygen concentration was recorded at a frequency of 1 Hz in the chamber at the maximum swimming speed for up to 6 min, during which time oxygen concentration remained above 90% of air saturation.
First, Ṁ O2max was measured in the three species that had been maintained in control or near-future CO2 seawater for four days. Then, in order to assess an acute effect of near-future CO2, Ṁ O2max was measured in P. moluccensis and P. amboinensis maintained in control conditions for four days and acutely exposed to near-future CO2 while in the respirometer. Finally, to further understand the effects of increased CO2 and low pH in more extreme habitats, Ṁ O2max of four groups of P. amboinensis was measured after acute exposure of the fish to pH levels of 8.1 (control, present day level, corresponding to approximately 450 μatm CO2), 7.9 (corresponding to approximately 860 μatm CO2), 7.7 (corresponding to approximately 1400 μatm CO2) and 7.5 (corresponding to approximately 2400 μatm CO2). The desired pH level was obtained by diluting increasing volumes of 100 mM hydrochloric acid in the seawater utilized in the experimental set-up. The water was prepared immediately prior to the experiment, and within 2 min, the fish was placed in the respirometry chamber, which was sealed, thereby preventing the water from equilibrating with the atmosphere. Ṁ O2max was then measured as described above. Average seawater pCO2 was calculated in CO2SYS (http://cdiac.ornl.gov/oceans/co2rprt.html) from measured pH and assuming the same AT as in the control conditions. The addition of a strong acid to a closed system like a closed respirometer has rather similar consequences on water chemistry as equilibrating water of an open system with CO2 gas, thereby allowing reasonable comparison between both techniques (Gattuso and Lavigne, 2009). However, without the addition of CO32− or HCO3−, the technique leads to a slightly lower AT. For example, Gattuso et al. (2010) report that the AT of seawater with a salinity of 35 ppt will decrease by 6% when pH is decreased from 8.1 to 7.8 via the addition of HCl in a closed system. This would reduce pCO2 estimates in our system by 6%. Therefore, our estimates of pCO2 from hydrochloric acid addition may be marginally higher than what was achieved in the respirometer prior to the start of the experiment and addition of respiratory CO2 from the fish.
Because the measurements of Ṁ O2max in the small damselfish (P. amboinensis) required the pooling of individuals (see above), Ṁ O2rest and Ṁ O2max had to be measured on different sets of fish. The procedure precluded the calculation of individual aerobic scope in damselfish. As for the measurement of Ṁ O2rest, two parallel setups allowed for the simultaneous measurement of Ṁ O2max of fish exposed to near-future or higher CO2, and those exposed to control conditions.
2.4 Data analyses and statistics
Oxygen consumption (Ṁ O2 in mgO2 kg−1 h−1) was calculated for each fish or pool of fish using the following formula:
where Δ[O2] is the decrease in water oxygen concentration (Δ mgO2 l−1), Δt the recording time (h), VOLresp is the volume of the respirometer minus the volume of the fish (l), and M the mass of the fish (kg). Fish Ṁ O2 was corrected for background (microbial) respiration measured after each recording. For P. moluccensis and P. amboinensis, Ṁ O2rest was measured in a closed respirometer from Δ[O2] data above 90% of air saturation. O2crit was calculated for each damselfish as the concentration of O2 at the intersection of the regression lines from the data recorded >80% of air saturation (well above O2crit) and the data recorded <20% air saturation (well below O2crit as evident from the break in the curve at O2crit). For P. fuscus, three slopes (Δ[O2] × Δt−1) were averaged to calculate Ṁ O2rest. Ṁ O2max was calculated for all fish from within the first minute of recording. Unpaired t-tests were utilized to assess the effect of seawater pCO2 on Ṁ O2rest and Ṁ O2max for each species, and the effect of seawater CO2 concentration on O2crit in P. moluccensis and P. amboinensis. In the experiment examining the effect of extreme pH on Ṁ O2max in P. amboinensis, no significant differences were found between the three control groups (P = 0.236). Therefore, the control data were pooled. Then, a one-way ANOVA was used to test for the effect of pH on Ṁ O2max. In all instances, P<0.05 was considered significant.
3 Results
Ṁ O2rest of the two damselfish prey species was unaltered after exposure to near-future CO2 for four days (Fig 1A). Similarly, four days of exposure to near-future CO2 had no significant effect on O2crit for either of the two damselfish prey species (Fig. 1B).
Ṁ O2max of P. moluccensis was unaffected by four days exposure as well as acute exposure to near-future CO2 (Fig. 1C and D). In contrast, Ṁ O2max of P. amboinensis was significantly higher after four days (+39%; t[10] = 4.665; p = 0.009) as well as acute (+ 28%; t[10] = 2.597; p = 0.029) exposure to near-future CO2 when compared to controls (Fig. 1C and D). Furthermore, the two CO2 treatments resulted in a similar increase in Ṁ O2max. Ṁ O2max of P. amboinensis measured after four days exposure to near-future CO2 was not significantly different than Ṁ O2max measured after acute exposure to near-future CO2 (P = 0.417). Similarly, Ṁ O2max of the respective control groups did not differ significantly (P = 0.858).
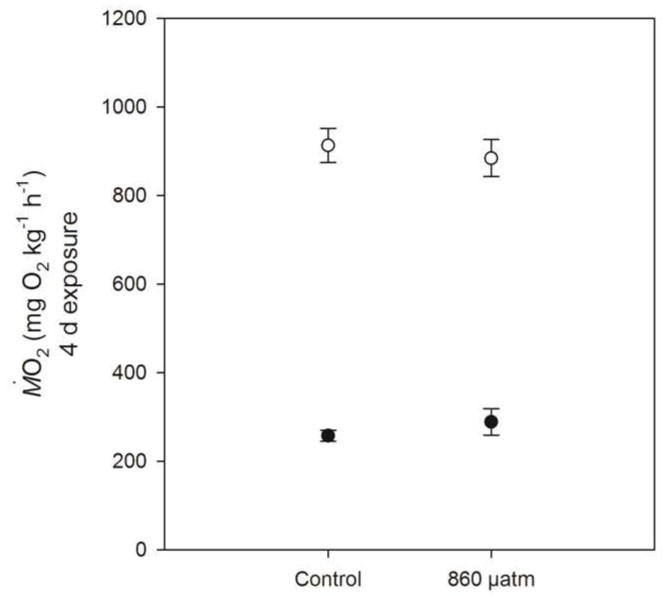
Exposure to near-future CO2 for four days had no effect on either Ṁ O2rest or Ṁ O2max of the predator, P. fuscus (Fig 2). Consequently, there was no significant effect of near-future CO2 on net aerobic scope or factorial aerobic scope in this species.
Fig. 2.
Respiratory performance (mean ±SE) of P. fuscus. Resting oxygen consumption rate (Ṁ O2rest; mgO2 kg−1 h−1) (○) and maximum oxygen consumption rate (Ṁ O2max; mgO2 kg−1 h−1) (○)after a 4 d exp&sure to control seawater (451 μatm; n = 8) or near-future CO2 seawater (860 μatm; n = 7).
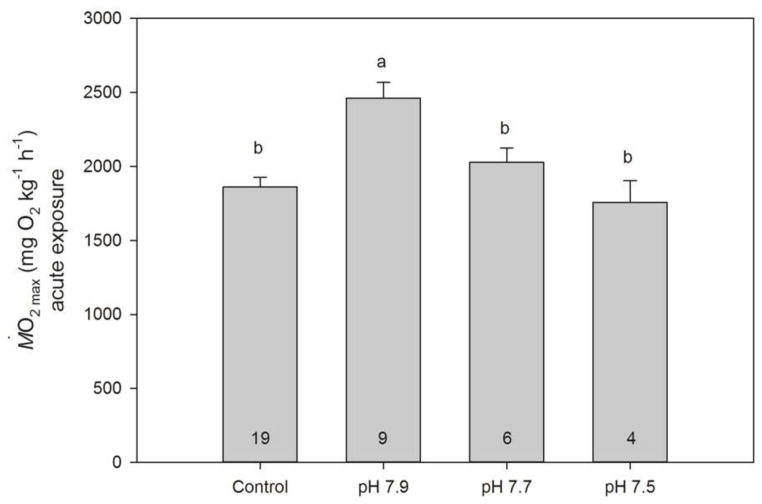
When acutely exposed to pH 7.9 (approximately 860 μatm pCO2), P. amboinensis exhibited a Ṁ O2max that was significantly higher than that of the control fish, as well that of fish acutely exposed to pH 7.7 (approximately 1400 μatm pCO2) or 7.5 (approximately 2400 μatm pCO2; Fig. 3; P <0.0001). The increase in Ṁ O2max (+ 32%) was quantitatively similar to the increased Ṁ O2max displayed by P. amboinensis after the four-day and acute exposures to near-future CO2. However, at pH 7.7 and 7.5, Ṁ O2max of P. amboinensis was no longer elevated and was not significantly different from the control (Fig. 3).
Fig. 3.
Maximum oxygen consumption rate (Ṁ O2max; mgO2 kg−1 h−1; mean ±SE) of P. amboinensis at different levels of seawater pH. N numbers are indicated at the bottom of each bar.
4 Discussion
CO2-driven ocean acidification has been predicted to have detrimental effects on marine organisms by reducing the scope for aerobic performance (Pörtner and Farrell, 2008; Pörtner and Knust, 2007; Seibel and Walsh, 2001). However, contrary to expectations, none of the three tropical reef fish examined here, P. moluccensis, P. amboinensis or P. fuscus, exhibited an elevated Ṁ O2rest or reduced Ṁ O2max when exposed to the average pCO2 projected to occur in the ocean surface by the year 2100. Rather, Ṁ O2rest of the two juvenile damselfish prey species (P. moluccensis and P. amboinensis) and the adult predator (P. fuscus) was maintained after a four-day exposure to near-future seawater CO2. Early life stages of fish and other marine organisms are believed to be more sensitive to pH changes because of their high metabolic demand (Brown and Sadler, 1989; Pörtner et al., 2005). The unchanged Ṁ O2rest of juvenile P. moluccensis and P. amboinensis after four days exposure to near-future CO2 thus suggests that the hypothesized metabolic costs of living in a high CO2 environment, namely altered acid-base balance, ionoregulation and cardiorespiratory function (Pörtner et al., 2004), were insignificant for these species at rest. Nevertheless, the unchanged Ṁ O2rest of the species examined after four days exposure to near-future CO2 does not preclude that physiological changes occurred, including compensatory ones. For example, gill ionoregulatory machinery is rapidly altered (within 8 h to 2 d) in response to hypercapnia exposure in the eelpout (Zoarces viviparous), without measurable effect on resting metabolic rate over the same time period (Deigweiher et al., 2008). Similarly, long-term (4–12 months) exposure to hypercapnia leads to upregulated gill Na+/K+-ATPase activity and protein expression in the Atlantic cod (Gadus morhua), but resting and low activity metabolic rates remain unaltered (Melzner et al., 2009a). Furthermore, the gilthead bream (Sparus auratus) exhibits a shift from aerobic to anaerobic metabolic pathways during hypercapnic exposure (Michaelidis et al., 2007). Whether similar compensatory physiological changes also occur in coral reef fish during exposure to near-future climate change CO2 levels remains to be investigated.
Like observed for Ṁ O2rest, Ṁ O2max of P. moluccensis and P. fuscus was also unaffected by exposure to near-future CO2. In contrast, Ṁ O2max of P. amboinensis was higher under near-future CO2 conditions than under control conditions. The lack of a detrimental effect of increased CO2 on Ṁ O2rest and Ṁ O2max, as reported here for P. moluccensis and P. fuscus, is not unprecedented for fish (Ishimatsu et al., 2008; McKenzie et al., 2003; Melzner et al., 2009a). However, to the best of our knowledge, an augmented aerobic capacity of a juvenile marine teleost in response to elevated CO2 has not been documented previously. The validity of the present results for P. amboinensis are supported by the findings that Ṁ O2max was increased with elevated CO2 in separate groups of fish that were exposed to near-future CO2 using three different experimental techniques. The specific treatments consisted of a four-day exposure to near-future CO2 maintained by CO2-dosing to a set pHNBS, an acute exposure to near-future CO2 maintained by CO2-dosing to a set pHNBS and an acute exposure to near-future CO2 obtained by the addition of strong acid into a closed system. Across the three methodologies, all 21 individuals exposed to near-future CO2 and the resulting acidosis exhibited an increased ṀO2max. Moreover, the magnitude of the increase in Ṁ O2max was consistent among the different experimental protocols (+28–39%). Finally, Ṁ O2max, Ṁ O2rest and O2crit of P. amboinensis in control conditions were comparable to data from a previous study using similar size fish (Nilsson et al., 2007b), indicating that the elevated Ṁ O2max under near-future CO2 did not arise from a comparison to control fish that were underperforming.
The physiological mechanism(s) underlying the increased Ṁ O2max of P. amboinensis under near-future seawater CO2 conditions remain to be elucidated. However, the consistent increase of Ṁ O2max exhibited by P. amboinensis across the three experimental protocols, two of which constituted an acute exposure to near-future CO2, suggests that the phenomenon did not arise from physiological acclimation to the elevated CO2, but rather from the consequences of an acute exposure to increased CO2 on the physiology of the fish. One explanation for the increased Ṁ O2max of P. amboinensis when exposed to near-future CO2 is that the maximum swimming speed of the fish was greater. Indeed, it is well established that Ṁ O2max of fishes is positively correlated with swimming speed (Fry, 1971; Bushnell et al., 1984; Lee et al. 2003; Smit et al., 1965; Torres and Childress, 1983). A greater swimming speed could arise from a number of possibilities.
A greater maximal swimming speed could stem from a change in the motivation of the fish to swim fast. Recent studies have revealed that tropical reef fish exhibit an array of behavioral and sensory disruptions when exposed near-future CO2 for several days. These range from reversal or loss of olfactory and auditory preferences, loss of behavioral lateralization to increased boldness and activity levels (Dixson et al., 2010; Domenici et al., 2011; Munday et al., 2010; Simpson et al., 2011; Nilsson et al., 2012). The behavioral changes are believed to arise from alterations of the normal flow of Cl− and HCO3− through GABA-A receptors caused by disruptions of transmembrane Cl− and HCO3− ion gradients (Nilsson et al. 2012). Nevertheless, we find it unlikely that the increased Ṁ O2max of P. amboinensis arises from a motivational drive to swim faster due to the effects of CO2 exposure on neurotransmitter function because the previously documented behavioral alterations only occurred after several days’ exposure to increased CO2 (Dixson et al., 2010; Domenici et al., 2012; Munday et al., 2010; Simpson et al., 2011; Nilsson et al., 2012). Short-term fluctuations in CO2 did not induce behavioral effects (Munday et al. 2010). In comparison, in the present study, P. amboinensis displayed an elevated Ṁ O2max immediately (within minutes) of exposure to near-future CO2. Moreover, a recent study investigating acid-base balance of the gulf toadfish (Opsanus beta) during exposure to near-future seawater CO2, reported that full compensation of the respiratory acidosis and elevation of plasma HCO3− did not occur until after two hours of exposure (Esbaugh et al., 2012). Again, the time course of physiological change is at odds with alacritous increase of Ṁ O2max displayed by P. amboinensis in the present study.
Alternatively, a greater swimming speed could arise from an increased oxygen delivery to the swimming muscles and oxygen uptake at the gills. Recent in vitro and in vivo studies have revealed that moderate acidosis can serve to increase the delivery of oxygen to the red muscle of teleost fish (Rummer and Brauner, 2011; Rummer et al., 2013). Briefly, in fish exposed to a stressor or mild acidosis, catecholamine release stimulates the activation of Na+/ H+ exchange across the erythrocyte membrane, thereby increasing red blood cell intracellular pH relative to the plasma and thus facilitating haemoglobin-O2 binding at the gills (Boutilier et al., 1986; Nikinmaa, 1986). However, if plasma accessible carbonic anhydrase, which catalyzes the reversible conversion fo HCO3− and H+ to CO2, is present in muscle capillaires, it serves to short-circuit the Na+/ H+ exchange, reduce red blood cell pH and haemoglobin-O2 affinity and enhance O2 unloading. For rainbow trout (Oncorhynchus mykiss) exposed to less than 1% CO2, the mechanism increased red muscle pO2 by 65% (Rummer et al., 2013). In the present study, the possibility exists that the combined exposure of P. amboinensis to near-future CO2 and maximal exercise led to catecholamine release. If carbonic anydrase is present in the red muscle of P. amboinensis, an increased oxygen delivery to the swimming muscles would have also likely ensued. Moreover, external CO2 can rapidly induce cardiorespiratory responses via gill chemoreceptors (Reid et al., 2005). In particular, environmental CO2 elicits increased ventilation in most water-breathing fishes. An increased oxygen delivery to the muscles combined with an increased ventilation rate could translate to the observed greater oxygen uptake.
In this regard, the species-specific responses to near-future CO2 in terms of Ṁ O2max may indicate different response times for catecholamine release, regulatory capacities or tolerance to changes in blood H+ and/or differences among the species with regards to the presence of plasma accessible carbonic anhydrase in the red muscle. The lack of increase of Ṁ O2max of P. amboinensis when the fish were exposed to pH 7.7 (corresponding to approximately 1400 μatm CO2) or pH 7.5 (corresponding to approximately 2400 μatm CO2) could arise from negative physiological effects of acidosis at the more extreme lower pH levels (Brauner and Baker, 2009) nullifying the enhanced physiological capacity to swim faster. This possibility could explain why many previous studies that exposed fish to much higher CO2 levels (3000 – 60000 μatm) than those employed in the present study did not report any positive effects on Ṁ O2max or aerobic scope (McKenzie et al., 2003; Melzner et al., 2009a). The juvenile damselfish and adult P. fuscus studied were much too small to enable blood sampling to test the above hypotheses. An alternate explanation for the increased Ṁ O2max of P. amboinensis under near-future CO2 conditions is that maximal swimming speed was unchanged at Ṁ O2max, but an additional demand for oxygen to maintain homeostasis arose that was not apparent at Ṁ O2rest or during maximal swimming under control conditions. For example, the ‘osmo-respiratory compromise’ almost doubles with exercise (Randall et al. 1972; Nilsson 1986). In this scenario, P. amboinensis would have incurred a greater cost to swim at its maximum speed under near-future CO2 conditions. Clearly, future studies incorporating the measurement of swimming speed at Ṁ O2max are needed to differentiate the possible explanations for the increased Ṁ O2max of P. amboinensis under near-future CO2. The use of a swimming flume was not feasible in the present study because such a system would have required a much larger volume of water than appropriate to accurately measure oxygen consumption of the extremely small juvenile fish (Steffensen, 1989). Rather, a cylindrical swimming chamber with a small volume, but with the capacity to swim the fish at their maximal swimming speed (Fisher et al. 2005; Gardiner et al., 2010; Nilsson et al., 2009, Munday et al., 2009a; Nilsson et al., 2007a; Nilsson et al., 2010; Nilsson and Östlund-Nilsson, 2004) was utilized to obtain reliable measurements of oxygen consumption.
Regardless of the possible mechanistic determinant(s) of the elevated Ṁ O2max under near-future CO2, the differing response to near-future CO2 between P. amboinensis and its congener P. moluccensis could foreseeably have consequences for ecological interactions and the relative abundance of species within coral reef fish communities. With a higher aerobic metabolic capacity (and potentially maximum swimming speed), P. amboinensis would have the potential for increased individual performance in any energetically demanding behavior, such as swimming against a current, repaying O2 debt after repeated anaerobic burst-swimming escapes from a threat, foraging or digesting (i.e., specific dynamic action). Concurrently, P. amboinensis should still be able to enter and remain in the hypoxic waters found deep inside coral colonies at night in order to escape predation (Nilsson et al., 2007a). The unchanged O2crit of P. amboinensis after exposure to near-future CO2 suggests that no trade-off exists for this species between its higher aerobic capacity and its hypoxia tolerance. In addition, in face of warming ocean surface temperatures, the enhanced Ṁ O2max of juvenile P. amboinensis under near-future CO2 may enable it to maintain its thermal tolerance window, and perhaps geographical distribution, as thermal tolerance is thought to be guided by aerobic scope in many species (Pörtner and Knust, 2007). On the contrary, if P. amboinensis incurs a greater metabolic cost while swimming at its maximum speed under near-future CO2 conditions, energy expenditure for any other energetically demanding process would be reduced. Consequently, individual performance would be decreased in any energetically demanding behavior, leading to potentially negative consequences for the species.
Interestingly, the results of a recent experiment that examined mortality rate of a number of juvenile damselfish species when facing P. fuscus in a mesocosm after a four-day exposure to CO2-acidified water found that P. amboinensis showed a similar mortality rate to P. moluccensis, as well as to other damselfish species (Ferrari et al., 2011b). The findings suggest no benefit or disadvantage of the increased Ṁ O2max displayed by P. amboinensis under near-future CO2 conditions. It may be that larger scale and longer term studies that encompass a variety of other variables such as temperature, current, life stages and the presence of other predators are required to reveal the implications of species-specific Ṁ O2max responses to near-future CO2 on fitness and mortality rate. Another recent study reported that in CO2 acidified water, P. fuscus had a slower response to prey detection than in control water, but higher activity levels (Cripps et al., 2011). The higher activity level had been suggested to compensate for slower prey detection by increasing the chance of prey encounter (Cripps et al., 2011). The present findings indicate that a higher activity level in P. fuscus in CO2-acidified water is not linked to a higher aerobic performance (aerobic scope). The higher activity levels of P. fuscus might be one of the consequences of increased neural excitation in CO2-acidified water (Nilsson et al., 2012).
5. Concluding remarks
In summary, the present study reports an increased aerobic capacity of a juvenile marine teleost, P. amboinensis, in response to near-future pCO2, but no effect on its congener P. moluccensis or on their predator P. fuscus. While the mechanistic basis for the species-specific responses and potential for differences in the swimming performance of the fish under near-future pCO2 remains to be investigated, the results emphasize that being of the same genus, sharing similar ecology and life history, or living in the same environment, does not necessarily imply similar physiological responses to near-future CO2. The results highlight that understanding interspecific variability is an important component of predicting the consequences of ocean acidification on marine communities and ecosystems. Additional studies assessing the effects of a number of other environmental factors in conjunction with near-future CO2 exposure are required to more fully understand the interesting finding of the increased Ṁ O2max of P. amboinensis in response to near-future seawater CO2. Most importantly, future experiments should assess how maximum swimming ability is affected and if species-specific responses to elevated CO2 persist with elevated temperature. Elevated pCO2 in the future will not occur independently of temperature. Likewise, investigations into the effects of near-future CO2 and temperature on different life stages of tropical coral reef species, as well as the capacity of both prey and predators to adapt to ocean acidification over the long-term, are needed to understand key ecological interactions and ultimately how the structure of ecological communities will be affected by climate change variables.
Acknowledgments
This research was financially supported by the University of Oslo, the Australian Research Council, the ARC Centre of Excellence for Coral Reef Studies and the U.S. National Institute of General Medical Sciences of the National Institutes of Health under Award Number P20GM103395 (to J.A.W.S). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors would like to thank Sue-Ann Watson for measuring the water chemistry parameters and technical assistance with the CO2 system, Yoland Bosiger, Oona Lönnstedt and members of Mark McCormick’s research group for aiding in the collection of the experimental fish, and Hans Borg, Johan Erland, Mads Peter Granberg and Bjørn Langrekken from the University of Oslo - Department of Biology workshop, for construction of the respirometers. We are very grateful to the enthusiastic support of the personnel at Lizard Island Research Station.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker DW, Brauner CJ. Metabolic changes associated with acid–base regulation during hypercarbia in the CO2-tolerant chondrostean, white sturgeon (Acipenser transmontanus) Comp Biochem Physiol A-Mol Integr Physiol. 2012;161:61–68. doi: 10.1016/j.cbpa.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Barnola JM, Raynaud D, Korotkevich YS, Lorius C. Vostok ice core provides 160,000-year record of atmospheric CO2. Nature. 1987;329:408–414. [Google Scholar]
- Bennett AF, Hicks JW. Postprandial exercise: prioritization or additivity of the metabolic responses? J Exp Biol. 2001;204:2127–2132. doi: 10.1242/jeb.204.12.2127. [DOI] [PubMed] [Google Scholar]
- Boutilier RG, Iwama GK, Randall DJ. The promotion of catecholamine release in rainbow trout, Salmo gairdneri, by acute acidosis: interactions between red cell pH and haemoglobin oxygen-carrying capacity. J Exp Biol. 1986;123:145–157. doi: 10.1242/jeb.123.1.145. [DOI] [PubMed] [Google Scholar]
- Brauner CJ, Baker DW. Patterns of acid–base regulation during exposure to hypercarbia in fishes. In: Glass ML, Wood SC, editors. Cardio-respiratory control in vertebrates. Springer; Berlin, Germany: 2009. pp. 43–63. [Google Scholar]
- Brown DJA, Sadler K. Fish survival in acid waters. In: Morris R, Taylor EW, Brown DJA, Brown JA, editors. Acid toxicity and aquatic animals. Cambridge University Press; Cambridge, UK: 1989. pp. 31–44. [Google Scholar]
- Bushnell PG, Steffensen JF, Johansen K. Oxygen consumption and swimming performance in hypoxia-acclimated rainbow trout Salmo gairdneri. J Exp Biol. 1984;113:225–235. [Google Scholar]
- Cahill AE, Aiello-Lammens ME, Fisher-Reid MC, Hua X, Karanewsky CJ, Yeong Ryu H, Sbeglia GC, Spagnolo F, Waldron JB, Warsi O, Wiens JJ. How does climate change cause extinction? Proc R Soc B-Biol Sci. 2012;280:20121890. doi: 10.1098/rspb.2012.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron JN. Regulation of blood-pH in teleost fish. Respir Physiol. 1978;33:129–144. doi: 10.1016/0034-5687(78)90092-0. [DOI] [PubMed] [Google Scholar]
- Cripps IL, Munday PL, McCormick MI. Ocean acidification affects prey detection by a predatory reef fish. PLoS ONE. 2011;6:e22736. doi: 10.1371/journal.pone.0022736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deigweiher K, Koschnick N, Portner HO, Lucassen M. Acclimation of ion regulatory capacities in gills of marine fish under environmental hypercapnia. Am J Physiol-Regul Integr Comp Physiol. 2008;295:R1660–R1670. doi: 10.1152/ajpregu.90403.2008. [DOI] [PubMed] [Google Scholar]
- Dickson AG, Millero FJ. A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep-Sea Res Pt I. 1987;34:1733–1743. [Google Scholar]
- Dixson DL, Munday PL, Jones GP. Ocean acidification disrupts the innate ability of fish to detect predator olfactory cues. Ecol Lett. 2010;13:68–75. doi: 10.1111/j.1461-0248.2009.01400.x. [DOI] [PubMed] [Google Scholar]
- Dlugokencky E, Tans P. Trends in atmospheric carbon dioxide. 2013 http://www.esrl.noaa.gov/gmd/ccgg/trends/global.html#global.
- Doney SC. The growing human footprint on coastal and open-ocean biogeochemistry. Science. 2010;328:1512–1516. doi: 10.1126/science.1185198. [DOI] [PubMed] [Google Scholar]
- Domenici P, Allan B, McCormick MI, Munday PL. Elevated carbon dioxide affects behavioural lateralization in a coral reef fish. Biol Lett. 2012;8:78–81. doi: 10.1098/rsbl.2011.0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esbaugh A, Heuer R, Grosell M. Impacts of ocean acidification on respiratory gas exchange and acid–base balance in a marine teleost, Opsanus beta. J Comp Physiol B-Biochem Syst Environ Physiol. 2012:1–14. doi: 10.1007/s00360-012-0668-5. [DOI] [PubMed] [Google Scholar]
- Fabry VJ, Seibel BA, Feely RA, Orr JC. 4th International Zooplankton Production Symposium. Oxford Univ Press; Hiroshima, Japan: 2007. Impacts of ocean acidification on marine fauna and ecosystem processes; pp. 414–432. [Google Scholar]
- Ferrari MCO, Dixson DL, Munday PL, McCormick MI, Meekan MG, Sih A, Chivers DP. Intrageneric variation in antipredator responses of coral reef fishes affected by ocean acidification: implications for climate change projections on marine communities. Glob Change Biol. 2011a;17:2980–2986. [Google Scholar]
- Ferrari MCO, McCormick MI, Munday PL, Meekan MG, Dixson DL, Lonnstedt O, Chivers DP. Putting prey and predator into the CO2 equation - qualitative and quantitative effects of ocean acidification on predator-prey interactions. Ecol Lett. 2011b;14:1143–1148. doi: 10.1111/j.1461-0248.2011.01683.x. [DOI] [PubMed] [Google Scholar]
- Fisher R, Leis JM, Clark DL, Wilson SK. Critical swimming speeds of late-stage coral reef fish larvae: variation within species, among species and between locations. Mar Biol. 2005;147:1201–1212. [Google Scholar]
- Fry FEJ. Publications of the Ontario Fisheries Research Laboratory, Biological services. 55. University of Toronto studies; 1947. Effects of the environment on animal activity. [Google Scholar]
- Fry FEJ. The effect of environmental factors on the physiology of fish. In: Hoar WS, Randall DJ, editors. Environmental Relations and Behavior. Academic Press; London: 1971. pp. 1–98. [Google Scholar]
- Fry FEJ, Hart JS. The relation of temperature to oxygen consumption in the goldfish. Biol Bull. 1948;94:66–77. [PubMed] [Google Scholar]
- Gardiner NM, Munday PL, Nilsson GE. Counter-gradient variation in respiratory performance of coral reef fishes at elevated temperatures. PLoS ONE. 2010;5:e13299, 13291–13213. doi: 10.1371/journal.pone.0013299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattuso J-P, Hansson L. Ocean acidification. Oxford University Press; London, UK: 2011. p. 352. [Google Scholar]
- Gattuso J-P, Kunshan G, Lee K, Rost B, Schulz KG. Approaches and tools to manipulate the carbonate chemistry. In: Riebesell U, Fabry VJ, Hansson L, Gattuso J-P, editors. Guide to best practices for ocean acidification research and data reporting. Publications Office of the European Union; Luxembourg: 2010. pp. 41–52. [Google Scholar]
- Gattuso JP, Lavigne H. Technical note: Approaches and software tools to investigate the impact of ocean acidification. Biogeosciences. 2009;6:2121–2133. [Google Scholar]
- Gilmour KM, Perry SF. Branchial chemoreceptor regulation of cardiorespiratory function. In: Toshiaki JH, Barbara SZ, editors. Fish Physiology 25. Academic Press; 2006. pp. 97–151. [Google Scholar]
- Goolish EM. Aerobic and anaerobic scaling in fish. Biol Rev. 1991;66:33–56. [Google Scholar]
- Gran G. Determination of the equivalence point in potentiometric titrations. Acta Chem Scand. 1950;4:559–577. [Google Scholar]
- Gran G. Determination of the equivalence point in potentiometric titrations. Part II Analyst. 1952;77:661–671. [Google Scholar]
- Hari P, Pumpanen J, Huotari J, Kolari P, Grace J, Vesala T, Ojala A. High-frequency measurements of photosynthesis of planktonic algae using rugged nondispersive infrared carbon dioxide probes. Limnol Oceanogr: Methods. 2008;6:347–354. [Google Scholar]
- Heisler N. Acid–base regulation. In: Evans DH, editor. The physiology of fishes. CRC Press Inc; Boca Raton, FL: 1993. pp. 343–377. [Google Scholar]
- Holmes TH, McCormick MI. Size-selectivity of predatory reef fish on juvenile prey. Mar Ecol-Prog Ser. 2010;399:273–283. [Google Scholar]
- Ishimatsu A, Hayashi M, Kikkawa T. Fishes in high-CO2, acidified oceans. Mar Ecol-Prog Ser. 2008;373:295–302. [Google Scholar]
- Lee CG, Farrell AP, Lotto A, MacNutt MJ, Hinch SG, Healey MC. The effect of temperature on swimming performance and oxygen consumption in adult sockeye (Oncorhynchus nerka) and coho (O. kisutch) salmon stocks. J Exp Biol. 2003;206:3239–3251. doi: 10.1242/jeb.00547. [DOI] [PubMed] [Google Scholar]
- McKenzie DJ, Piccolella M, Dalla Valle AZ, Taylor EW, Bolis CL, Steffensen JF. Tolerance of chronic hypercapnia by the European eel Anguilla anguilla. J Exp Biol. 2003;206:1717–1726. doi: 10.1242/jeb.00352. [DOI] [PubMed] [Google Scholar]
- Meehl GA, Stocker TF, Collins WD, Friedlingstein P, Gaye AT, Gregory JM, Kitoh A, Knutti R, Murphy JM, Noda A, Raper SCB, Watterson IG, Weaver AJ, Zhao Z-C. Global climate projections. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL, editors. Climate change 2007: the physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press; Cambridge, UK: 2007. pp. 747–846. [Google Scholar]
- Meekan MG, Milicich MJ, Doherty PJ. Larval production drives temporal patterns of larval supply and recruitment of a coral-reef damselfish. Mar Ecol-Prog Ser. 1993;93:217–225. [Google Scholar]
- Meekan MM, Wilson SW, Halford AH, Retzel AR. A comparison of catches of fishes and invertebrates by two light trap designs, in tropical NW Australia. Mar Biol. 2001;139:373–381. [Google Scholar]
- Mehrbach C, Culberso CH, Hawley JE, Pytkowic RM. Measurement of apparent dissociation-constants of carbonic-acid in seawater at atmospheric pressure. Limnol Oceanogr. 1973;18:897–907. [Google Scholar]
- Meinshausen M, Smith S, Calvin K, Daniel J, Kainuma M, Lamarque JF, Matsumoto K, Montzka S, Raper S, Riahi K, Thomson A, Velders G, van Vuuren DP. The RCP greenhouse gas concentrations and their extensions from 1765 to 2300. Climatic Change. 2011;109:213–241. [Google Scholar]
- Melzner F, Gobel S, Langenbuch M, Gutowska MA, Portner HO, Lucassen M. Swimming performance in Atlantic cod (Gadus morhua) following long-term (4–12 months) acclimation to elevated seawater pCO2. Aquat Toxicol. 2009a;92:30–37. doi: 10.1016/j.aquatox.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Melzner F, Gutowska MA, Langenbuch M, Dupont S, Lucassen M, Thorndyke MC, Bleich M, Poertner HO. Physiological basis for high CO2 tolerance in marine ectothermic animals: pre-adaptation through lifestyle and ontogeny? Biogeosciences. 2009b;6:2313–2331. [Google Scholar]
- Melzner F, Thomsen J, Koeve W, Oschlies A, Gutowska MA, Bange HW, Hansen HP, Körtzinger A. Future ocean acidification will be amplified by hypoxia in coastal habitats. Mar Biol. 2012 doi: 10.1007/s00227-012-1954-1. Epub ahead of print. [DOI] [Google Scholar]
- Michaelidis B, Spring A, Pörtner H. Effects of long-term acclimation to environmental hypercapnia on extracellular acid–base status and metabolic capacity in Mediterranean fish Sparus aurata. Mar Biol. 2007;150:1417–1429. [Google Scholar]
- Munday PL, Crawley NE, Nilsson GE. Interacting effects of elevated temperature and ocean acidification on the aerobic performance of coral reef fishes Mar. Ecol-Prog Ser. 2009a;388:235–242. [Google Scholar]
- Munday PL, Dixson DL, McCormick MI, Meekan M, Ferrari MCO, Chivers DP. Replenishment of fish populations is threatened by ocean acidification. Proc Natl Acad Sci U S A. 2010;107:12930–12934. doi: 10.1073/pnas.1004519107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday PL, Donelson JM, Dixson DL, Endo GGK. Effects of ocean acidification on the early life history of a tropical marine fish. Proc R Soc B-Biol Sci. 2009b;276:3275–3283. doi: 10.1098/rspb.2009.0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday PL, McCormick MI, Nilsson GE. Impact of global warming and rising CO2 levels on coral reef fishes: what hope for the future? J Exp Biol. 2012;215:3865–3873. doi: 10.1242/jeb.074765. [DOI] [PubMed] [Google Scholar]
- Munday PL, Wilson SK. Comparative efficacy of clove oil and other chemicals in anaesthetization of Pomacentrus amboinensis, a coral reef fish. J Fish Biol. 1997;51:931–938. [Google Scholar]
- Nikinmaa M. Control of red cell pH in teleost fishes. Ann Zool Fennici. 1986;23:223–235. [Google Scholar]
- Nilsson GE, Crawley N, Lunde IG, Munday PL. Elevated temperature reduces the respiratory scope of coral reef fishes. Glob Change Biol. 2009;15:1405–1412. [Google Scholar]
- Nilsson GE, Dixson DL, Domenici P, McCormick MI, Sorensen C, Watson SA, Munday PL. Near-future carbon dioxide levels alter fish behaviour by interfering with neurotransmitter function. Nature Clim Change. 2012;2:201–204. [Google Scholar]
- Nilsson GE, Hobbs JPA, Östlund-Nilsson S. Tribute to P. L. Lutz: respiratory ecophysiology of coral-reef teleosts. J Exp Biol. 2007a;210:1673–1686. doi: 10.1242/jeb.02718. [DOI] [PubMed] [Google Scholar]
- Nilsson GE, Ostlund-Nilsson S, Munday PL. Effects of elevated temperature on coral reef fishes: loss of hypoxia tolerance and inability to acclimate. Comp Biochem Physiol A-Mol Integr Physiol. 2010;156:389–393. doi: 10.1016/j.cbpa.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Nilsson GE, Ostlund-Nilsson S, Penfold R, Grutter AS. From record performance to hypoxia tolerance: respiratory transition in damselfish larvae settling on a coral reef. Proc R Soc B-Biol Sci. 2007b;274:79–85. doi: 10.1098/rspb.2006.3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson GE, Östlund-Nilsson S. Hypoxia in paradise: widespread hypoxia tolerance in coral reef fishes. Proc R Soc B-Biol Sci. 2004;271:S30–33. doi: 10.1098/rsbl.2003.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson S. Control of gill blood flow. In: Nilsson S, Holmgren S, editors. Fish Physiology: Recent advances. Cromm Helm; London: 1986. pp. 87–101. [Google Scholar]
- Pörtner HO. Ecosystem effects of ocean acidification in times of ocean warming: a physiologist’s view. Mar Ecol-Prog Ser. 2008;373:203–217. [Google Scholar]
- Pörtner HO, Farrell AP. Physiology and climate change. Science. 2008;322:690–692. doi: 10.1126/science.1163156. [DOI] [PubMed] [Google Scholar]
- Pörtner HO, Knust R. Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science. 2007;315:95–97. doi: 10.1126/science.1135471. [DOI] [PubMed] [Google Scholar]
- Pörtner HO, Langenbuch M, Michaelidis B. Synergistic effects of temperature extremes, hypoxia, and increases in CO2 on marine animals: from Earth history to global change. J Geohys Res C. 2005;110:C09S10. [Google Scholar]
- Pörtner HO, Langenbuch M, Reipschläger A. Biological impact of elevated ocean CO2 concentrations: lessons from animal physiology and earth history. J Oceanogr. 2004;60:705–718. [Google Scholar]
- Reid SG, Sundin L, Milsom WK. The cardiorespiratory system in tropical fishes: structure, function, and control. Fish Physiol. 2005;21:225–275. [Google Scholar]
- Randall DJ, Baumgarten D, Malyusz M. The relationship between gas and ion transfer across the gills of fishes. Comp Biochem Physiol A-Physiol. 1972;41:629–637. doi: 10.1016/0300-9629(72)90017-5. [DOI] [PubMed] [Google Scholar]
- Rummer JL, Brauner CJ. Plasma-accessible carbonic anhydrase at the tissue of a teleost fish may greatly enhance oxygen delivery: In vitro evidence in rainbow trout, Oncorhynchus mykiss. J Exp Biol. 2011;214:2319–2328. doi: 10.1242/jeb.054049. [DOI] [PubMed] [Google Scholar]
- Rummer JL, McKenzie DJ, Innocenti A, Supuran CT, Brauner CJ. Root effect hemoglobin may have evolved to enhance general tissue oxygen delivery. Science. 2013;340:1327–1329. doi: 10.1126/science.1233692. [DOI] [PubMed] [Google Scholar]
- Shaw EC, McNeil BI, Tilbrook B, Matear R, Bates ML. Anthropogenic changes to seawater buffer capacity combined with natural reef metabolism induce extreme future coral reef CO2 conditions. Glob Change Biol. 2013 doi: 10.1111/gcb.12154. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Simpson SD, Munday PL, Wittenrich ML, Manassa R, Dixson DL, Gagliano M, Yan HY. Ocean acidification erodes crucial auditory behaviour in a marine fish. Biol Lett. 2011 doi: 10.1098/rsbl.2011.0293. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. Some experiments on the oxygen consumption of goldfish (Carassius auratus L.) in relation to swimming speed. Can J Zool-Rev Can Zool. 1965;43:623–633. doi: 10.1139/z65-063. [DOI] [PubMed] [Google Scholar]
- Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL. Contribution of working group I to the fourth assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press; Cambridge, United Kingdom and New York, NY, USA: 2007. p. 996. [Google Scholar]
- Steffensen JF. Some errors in respirometry of aquatic breathers: how to avoid and correct for them ? Fish Physiol Biochem. 1989;6:49–59. doi: 10.1007/BF02995809. [DOI] [PubMed] [Google Scholar]
- Torres JJ, Childress JJ. Relationship of oxygen consumption to swimming speed in Euphausia pacifica - 1. Effects of temperature and pressure. Mar Biol. 1983;74:79–86. [Google Scholar]