SUMMARY
Neuronal arborization is regulated by cell autonomous and non-autonomous mechanisms including endosomal signaling via BDNF/TrkB. The endosomal Na+/H+ exchanger 6 (NHE6) is mutated in a new autism-related disorder. NHE6 functions to permit proton leak from endosomes yet the mechanisms causing disease are unknown. We demonstrate that loss of NHE6 results in over-acidification of the endosomal compartment and attenuated TrkB signaling. Mouse brains with disrupted NHE6 display reduced axonal and dendritic branching, reduced synapse number and circuit strength. Site-directed mutagenesis shows that the proton leak function of NHE6 is required for neuronal arborization. We find that TrkB receptor co-localizes to NHE6-associated endosomes. TrkB protein and phosphorylation are reduced in NHE6 mutant neurons in response to BDNF signaling. Finally, exogenous BDNF rescues defects in neuronal arborization. We propose that NHE6 mutation leads to circuit defects that are in part due to impoverished neuronal arborization that may be treatable by enhanced TrkB signaling.
INTRODUCTION
Mutations in the X-linked, endosomal Na+/H+ Exchanger 6 (NHE6, also known as SLC9A6) represent a novel neurogenetic syndrome involving postnatal microcephaly, intellectual disability, epilepsy, non-verbal status, autistic features among other symptoms (Gilfillan et al., 2008; Morrow et al., 2013; Schroer et al., 2010). NHE6 is among the most commonly mutated genes causing X-linked developmental brain disorders (Tarpey et al., 2009). The first reports of mutations in NHE6 were associated with an Angelman-like syndrome (AS) (Gilfillan et al., 2008). One of the pedigrees determined to have an NHE6 mutation by Gilfillan et al. was a large South African pedigree previously reported by Christianson (Christianson et al., 1999). Christianson syndrome (CS) is now the commonly used term for the condition associated with NHE6 mutations. In addition to the symptoms described above, CS may also involve craniofacial dysmorphology, ataxia, ophthalmoplegia, and cerebellar and brainstem atrophy. Christianson also described an association with autistic symptoms, including autistic regressions, as has been reported subsequently (Christianson et al., 1999; Garbern et al., 2010; Morrow et al., 2013). In parallel to the description of autistic symptoms associated with mutations in NHE6, Morrow et al. published mutations in the highly related endosomal protein NHE9 in autism with epilepsy (Morrow et al., 2008). Recently, NHE9 mRNA has been shown to be significantly upregulated and NHE6 mRNA to be downregulated in postmortem brains from patients with idiopathic autism (Schwede et al., 2013).
Accumulating evidence indicates that a hallmark of a subset of intellectual and developmental disabilities (IDD) is altered axonal and dendritic growth and branching (Belichenko et al., 2009; Calderon de Anda et al., 2012; Dindot et al., 2008; Kwon et al., 2006; Zikopoulos and Barbas, 2010). Some mutations, such as those in PTEN appear to result in an excess of branching while others such as mutations in MeCP2 or TAOK2 lead to impoverished branching. Axonal branching phenotypes in postmortem studies in autism further support this hypothesis in human brain and in the more common, idiopathic forms of IDD (Zikopoulos and Barbas, 2010).
As classically delineated by Ramon y Cajal, the nervous system displays a fantastic diversity and complexity of neuronal arbors (Ramón y Cajal, 1909). Within the developing brain, strict control of axon and dendritic branching is critical for circuit development and function. This process may be influenced by a variety of signaling pathways amenable to environmental and pharmacologic intervention, including endosomal signaling via the BDNF/TrkB pathway (Chao and Lee, 2004; Danzer et al., 2002; Luikart et al., 2005; Reichardt, 2006; Segal, 2001). The endocytic machinery has an important role in governing neuronal arborization via functions including controlling receptor trafficking, recycling and degradation, and modulating signaling pathways essential for neurite growth and arborization (Jan and Jan, 2010). For example, the role of the endosomal pathway in neuronal morphogenesis is exemplified by the discovery of the Drosophila shrub mutations that demonstrate ectopic dendritic and axonal branching due to loss of a coiled-coil protein homologous to the yeast protein Snf7, a key component in the ESCRT-III (endosomal sorting complex required for transport) complex that is essential for endosomal to lysosomal sorting (Sweeney et al., 2006).
Endosomal biology has been well-studied in non-neuronal cells, and has been relatively less extensively investigated in neurons (Yap and Winckler, 2012). The endosomal compartment is divided into component parts with increasingly acidic luminal environment, specifically early endosome (pH ~ 6.3), recycling endosome (pH ~ 6.5), late endosome (pH ~ 5.5) and lysosome (pH ~4.7) (Casey et al., 2010). The vacuolar H+-ATPase (V-ATPase) is a pump that mediates acidification of endosomes and lysosomes (Mindell, 2012). The endosomal Na+/H+ exchangers (NHEs) allow movement of cations down their concentration gradients (Na+ and/or K+ in, and H+ out), and counter the V-ATPase by regulating relative alkalization of the lumen as well as endosomal size (Ohgaki et al., 2011). The gradation of intra-luminal acidity likely serves a number of critical functions (Mellman, 1992), yet this has also been scarcely investigated in developing neurons. Overly et al. demonstrated in differentiating sympathetic neurons a discontinuous gradient of endosome acidification along the axon with more high-pH endosomes, namely pH ~ 6.6 to 8.2, near growth cones or axonal branch points, and more low-pH endosomes, pH 4.2 to 6.0 proximally near the soma (Overly and Hollenbeck, 1996; Overly et al., 1995). The effect of pH on neurotrophin signaling has been studied in a single study in PC12 cells wherein NGF and NT3 binding and activation of TrkA are decreased by increasing lumen acidity (Harrington et al., 2011).
Ten NHE genes are known in vertebrate (Brett et al., 2002; Casey et al., 2010). The structure of NHE proteins generally involves a twelve-membrane spanning motif that harbors the Na+ and H+ exchange activity and is highly conserved across family members, and a large, less conserved carboxyl domain that is thought to involve protein interaction and regulation. In addition to showing distinct gene expression patterns across tissues, the NHE proteins also show distinct subcellular distributions. NHE1-5 are localized to the cell surface and NHE6-9 are organellar (Brett et al., 2002; Nakamura et al., 2005; Ohgaki et al., 2011). NHE6 and NHE9 are the “endosomal NHEs” and appear to be localized to early and recycling, and late endosomes respectively, in non-neuronal cell lines (Nakamura et al., 2005).
In our studies, we are dissecting the cellular mechanisms at the root of neurodevelopmental abnormalities in CS. We investigate early postnatal mechanisms in brain development such as neuronal arborization. Our data demonstrate that NHE6-associated endosomes localize within growing axons and dendrites and at branch points. We also report a cellular phenotype wherein NHE6-null neurons display over-acidification of the endosomal compartment, and impoverished axonal and dendritic arborization. Consistent with this observation, NHE6-null mice have impaired circuit function. Furthermore, we find that NHE6-null neurons demonstrate attenuated TrkB signaling, indicating that endosomal signaling through BDNF/TrkB may be perturbed in CS. These studies provide important insight into a mechanism underlying circuit dysfunction in CS and also a framework for developing potential treatments.
RESULTS
NHE6-associated endosomes are localized to growing axons and dendrites
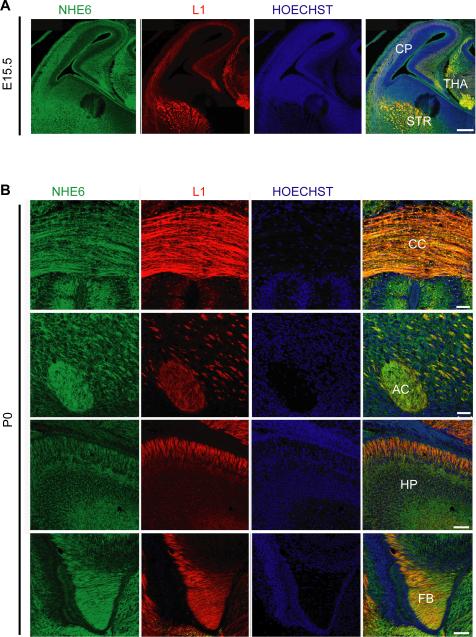
To examine NHE6 function in neuronal development, we generated antibodies against NHE6 (Supplementary Figure 1). NHE6 protein is prominent in growing axonal tracts in vivo during development including in cortical plate, striatum and thalamus at embryonic day 15.5 (E15.5), and in major fiber tracts such as corpus callosum, anterior commissure, fimbria, and other fiber tracts of hippocampus at postnatal day 0 (P0) (Figure 1). Staining was less prominent in embryonic stages prior to axon development (Supplementary Figure 2A). NHE6 is also expressed in glia (Supplementary Figure 2B).
Figure 1. NHE6 localizes to growing axon tracts during development.
(A) Anti-NHE6 antibodies label developing axonal tracts, embryonic mouse brain. Transverse section at E15.5. NHE6 (green), cortical plate (CP), striatum (STR), thalamus (THA). NHE6 co-localizes with L1 (red), a marker for growing axons. Scale bar, 100μm. (B) NHE6 labels growing fiber tracts in developing, postnatal mouse brain. Coronal, NHE6 (green) co-localizes with L1 (red) in corpus callosum (CC) (top row), anterior commissure (AC) (second row), hippocampal formation (HP) (third row), and fimbriae (FB) (bottom row). Nuclei, labeled with Hoechst (blue). Scale bar, CC and AC 50μm, HP and FB 100μm. See also Supplementary Figure 1 and 2.
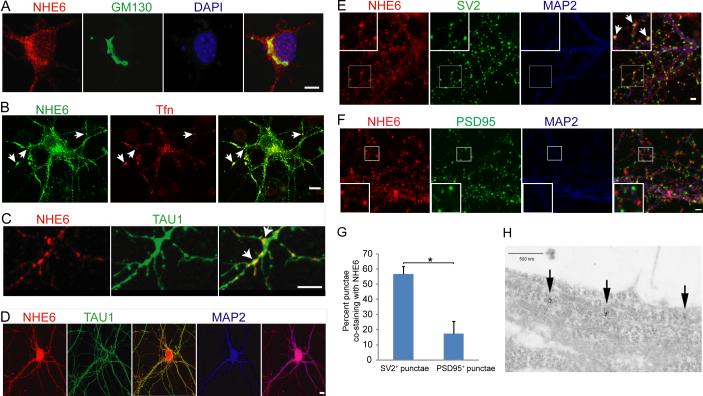
In dissociated hippocampal neurons in vitro, NHE6 staining appeared punctate with clusters localized to the perinuclear region, and also prominently within growing axons and dendrites (Figure 2). Although NHE6 punctae were notable in both axons and dendrites, there was a relatively greater prominence to the staining in axons over dendrites. The perinuclear staining appeared adjacent to anti-GM130 staining (a cis-Golgi marker), suggesting NHE6 localization adjacent to Golgi apparatus (Figure 2A). We functionally characterized NHE6-associated endosomes as including the early endosome compartment by loading neurons with fluorescently-tagged transferrin (Figure 2B). Ten minutes after loading neurons with Alexa568-conjugated transferrin (red), cells were fixed in order to mark early endosomes. NHE6 staining (green) indicated many double-labelled puncta (arrows, Figure 2B). Approximately 82.7 +/− 2.8 percent of transferrin-positive early endosomes were NHE6-positive, and 33.8 +/− 3.4 percent of NHE6-associated punctae stained positive for Alexa568-conjugated transferrin (Supplementary Figure 3).
Figure 2. NHE6 protein subcellular localization in dissociated hippocampal neurons.
(A) 4 days in vitro (DIV). Immunohistochemistry against NHE6 (red) and GM130 (green), a cis-Golgi marker. Scale bar, 5μm. (B) 7 DIV. Anti-NHE6 (green), co-localization with Alexa568-conjugated transferrin (red, incubated with neurons for 10min to indicate early endosomes). Arrows, early endosomes co-labelled (yellow) with NHE6 and transferrin. Scale bar, 10μm. (C) 10 DIV shows that NHE6 (red) is localized at the branching points (arrows) in phospho-Tau1-positive axons (green). Scale bar, 5μm (D) 9 DIV. Anti-NHE6 (red) co-labels phospho-Tau1-positive axons (green) and MAP2-positive dendrites (blue). Scale bar, 10μm. (E) 21 DIV. NHE6 (red), MAP2 (blue) and the presynaptic marker anti-SV2 (green). Insets are the higher magnification of the boxed regions. Arrows indicate regions of co-localization between NHE6 and SV2 suggesting presynaptic localization of NHE6. Scale bar, 2 μm. (F) 21 DIV. NHE6 (red), MAP2 (blue) and the postsynaptic marker anti-PSD95 (green). Insets are the higher magnification of the boxed regions. Scale bar, 2 μm. (G) Quantification of co-localization of NHE6 with SV2 or PSD95 at 21 DIV. (SV2: n=10 cells; PSD95: n=11 cells. Error bar, mean ± SEM. * p=0.014.) (H) Immunogold electron micrograph of neurites in culture. Arrows show clusters of NHE6 staining presumed to be endosomes along neurite. Scale bar, 500nm. See also Supplementary Figure 1 and 3.
To further characterized the NHE6 endosomal compartment, we quantified co-staining of NHE6 with Rab5- (early endosomes), Rab11- (recycling endosomes) and Rab7-associated endosomes (late endosomes). Using antibodies to endogenous Rab5 and NHE6, we discovered that 35.8 +/−2.6 percent of NHE6-associated endosomes co-stained with Rab5 antibodies, indicating a similar percentage of NHE6-associated early endosomes as suggested by the fluorescent-transferrin experiment. Also, 81.9 +/− 2.1 percent of Rab5-positive early endosomes stained for NHE6 (Supplementary Figure 3). Using antibodies to endogenous Rab11, we observed 40.6 +/− 4.2 percent of NHE6-associated endosomes are Rab11-positive, and approximately 85.8 +/− 1.7 percent of Rab11-associated recycling endosomes appear to be NHE6-positive (Supplementary Figure 3). Finally, using antibodies to endogenous Rab7, we observed that 34.9 +/− 1.8 percent of NHE6-associated endosomes are Rab7-positive, and approximately 50.9 +/− 2.8 percent of Rab7-associated, late endosomes are NHE6-positive. NHE6-staining in developing neurons was seen in the perinuclear region, and within growing neurites at tips and at branch points (Figure 2C arrows, with anti-Tau1 staining at branch points in growing axons; see also Figure 2D). Using immuno-electron microscopy on dissociated neuronal cultures, NHE6 staining appeared in discrete clusters within growing neurites (Figure 2H).
In older cultures, we continue to observe NHE6-positive punctae enriched in axons and dendrites (Figure 2D); however, in adult mouse brain, NHE6 staining is considerable less strong in mature axon tracts (data not shown), suggesting an active role of NHE6 endosomes in axon and dendrite development. Overall, our data were consistent with expression of NHE6 nearly equally distributed in early, late and recycling endosomes, with heavy staining in growing axons, and some staining in dendrites. Localization within growing neurites was frequently observed at branch points. As synapses appear in the culture, we observed NHE6-positive punctae co-localized with SV2 in 56.8 +/− 4.9 percent of SV2-positive punctae, a presynaptic marker (Figure 2E, quantified in 2G), and less commonly overlapped with the post-synaptic marker PSD95, wherein 17.4 +/− 8.0 percent of PSD95-positive structures were NHE6-positive (Figure 2F, quantified in 2G), suggesting that NHE6 localizes to a subset of synapses, with an apparently greater frequency at the presynapse.
Loss of NHE6 leads to impoverished axon and dendrite branching in vitro and in vivo
Given the strong staining in growing fiber tracts in vivo and the localization at branch points of growing neurites, we hypothesized that NHE6-associated endosomes play a role in neurite outgrowth and branching. Western blot analysis of proteins extracted from hippocampus on progressive days in vitro (DIV) and in vivo, revealed that NHE6 expression increases significantly when neurite growth accelerates, suggesting a role for NHE6 in neurite morphogenesis (Supplementary Figure 4A and 4B). To discern NHE6 function, we established an NHE6-null mouse line in which a LacZ-Neo cassette was inserted into exon 6 to inactivate the Nhe6 gene (Supplemental Figure 5 and Methods). We tested the mutant line by Western blot and demonstrate the absence of the anticipated bands specific to NHE6. NHE6-null mice showed an inconsistent increase in unexplained mortality in the first month (approximately 10-20% of pups). Otherwise mutant mice do not generally appear distinct from their wild-type littermates.
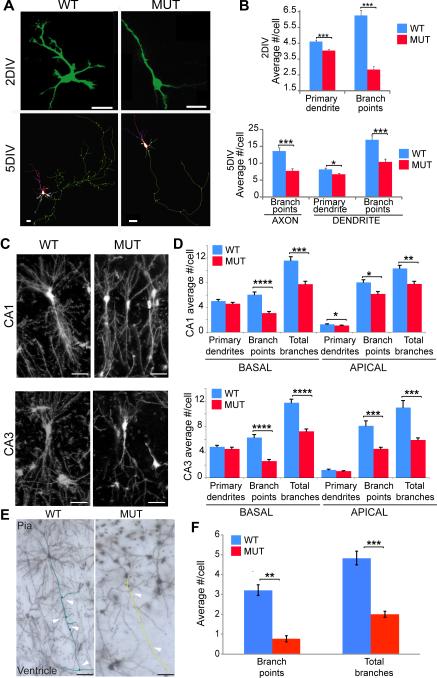
Mouse hippocampal cultures from postnatal day 0-1 NHE6-null and wild-type littermates were prepared, transfected with GFP-expressing vectors, and assayed at 2 DIV and 5 DIV for neuronal morphogenesis. As determined by computer tracing analysis (Figure 3A and 3B), hippocampal neurons from NHE6-null mice showed a significant reduction in axon branch points. Dendritic branch points and primary dendrite numbers were also reduced. Because these observations were made as early as 2 DIV, we hypothesize that these morphogenesis defects were primary failures in neuronal differentiation as opposed to secondary effects of over-pruning due to failures in synapses. Synapses are not substantially present prior to 5 DIV (Supplementary Figure 4B). The observation that there are defects in primary dendrite numbers and not simply branches also supports this interpretation.
Figure 3. NHE6 is required for dendrite and axonal branching.
(A) Cultured hippocampal neurons from P0-1 NHE6-null mice show fewer neurite branches compared to wild type littermates at 2 DIV (top panels), and reduced axonal and dendrite branching at 5 DIV (bottom panels). Scale bar, 20μm. Primary cultures were transfected with GFP-vector in order to label the processes. 5 DIV images show Neurolucida traces from reconstructed confocal Z-stacks of GFP-labeled neurons. Cell body, red line; axon, green line; and dendrites, various colors. Branch points are depicted as dots. (B) Quantification of the branching. At 2DIV (WT= 5 pups, n=299 cells; MUT= 4 pups, n=262 cells, 3 litters). At 5 DIV (WT=3 pups, n=31 cells; MUT= 5 pups, n=40 cells, 3 litters). Error bars are SEM. *p<0.05, **p<0.01, ***p<0.001. (C-D) NHE6-null mice have reduced branching in vivo in hippocampal CA1 and CA3 regions. (C) Hippocampal pyramidal neurons, Golgi-Cox stained coronal brain sections, P21 male mutant (MUT) and wild type (WT) littermates. Representative inverted micrographs of pyramidal neurons on CA1 (top) and CA3 (bottom) regions. Scale bars, 200 μm. (D) Quantification of branching defect using Neurolucida in hippocampal neurons, Mean ± SEM. CA1 (WT= 4 pups, 105 cells; MUT= 5 pups, 77 cells) and CA3 (WT = 4 pups, 64 cells; MUT= 5 pups, 52 cells) basal and apical dendrites. *p< 0.01, **p< 0.001, ***p< 0.0001, ****p<0.000001. (E-F) Cortical pyramidal neurons of cortical layer III show reduced axonal branching in vivo in NHE6-null mice. (E) Axonal branching in vivo. Golgi stained, coronal sections. Axons from layer III cortical pyramidal neurons were traced using Neurolucida. Left panel, wild type (WT) neuron, axon is highlighted in green. Right panel, mutant (MUT) neuron, axon highlighted in yellow. Arrowheads show branching points in WT neuron and the length of the MUT neuron. Scale bar is 12.5μm (F) Quantification of in vivo axonal branching. n=3 animals and 78 cells for wild type background, n=4 animals and 61 cells for mutant background. Error bar, mean ± SEM. **P=5.7E-09, ***P=1.6E-08. Also see Supplementary Figure 4, 5 and 6.
We examined neuronal morphogenesis in vivo in both hippocampus and cortex. Abnormalities in neuronal morphogenesis that were observed in hippocampal neurons in vitro were corroborated by quantification of Golgi-Cox stained dendritic and axonal arbors in vivo (Figure 3C-3F). Dendritic branching points and total number of branches in both CA3 and CA1 hippocampal pyramidal neurons in vivo were significantly reduced in NHE6-null mice compared to littermate controls at postnatal day 21 in basal and apical dendrites (Figure 3C and 3D). We also examined axons in vivo. Axons of cortical pyramidal neurons from layer III were traced as these neurons establish intralaminar connections onto layer V cortical neurons. Our in vivo analysis demonstrated fewer branch points by Neurolucida computer reconstructions in the mutant (0.77+/−0.15 branches per cell; 61 cells counted, 4 animals) compared to control (3.21+/− 0.28 branches, 78 cells counted, 3 animals) (p value 5.7E-09) (Figure 3E and 3F).
In addition to the Golgi-Cox analysis, we generated NHE6-null mice expressing YFP from a Thy-1 promoter that were used to analyze further the branching defect in cortex in the absence of NHE6. Layer V cortical pyramidal neurons were analyzed from the lateral cortex, as single cells could be easily traced. Similar to our findings with Golgi-Cox stained brains, we found YFP-labeled neurons in NHE6-null mice had reduced apical and basal branching points, and reduced total number of branches and number of primary dendrites compared to controls (Supplementary Figure 6A and 6B).
Defects in neuronal morphogenesis are rescued by cell-autonomous expression of wild-type NHE6 in mutant neurons, but not by a cation-exchange deficient form of NHE6
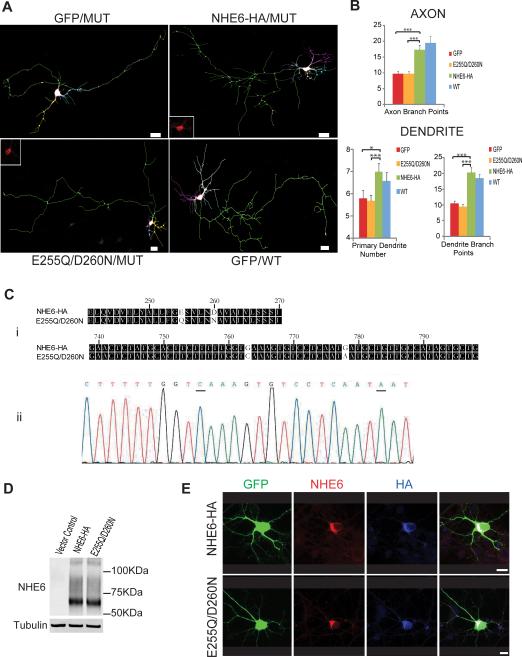
We established a rescue assay to dissect the function of NHE6 in greater molecular detail. Defects in neuronal morphogenesis in NHE6-null neurons were rescued through transfection of expression constructs for the human full length NHE6 protein in these cultures (Figure 4A). Quantification of this effect is shown in Figure 4B, wherein the average level of axonal branching is reduced by 50% in the mutant (9.8 +/− 0.7 branch points in mutant vs 19.5 +/− 2 in wild-type, p<0.001) and dendritic branching is reduced by 43% in the mutant (10.6+/− 0.7 branch points in mutant vs 18.5+/− 1.2 in wild-type, p<0.001) in the GFP-alone transfected mutant neurons. The level of branching in mutant cells is normalized by transfection with the construct expressing the full-length human NHE6.0 transcript (17.3+/−1.5 in axon branch points and 20.4 +/−1.5 in dendritic branch points in mutant cells).
Figure 4. NHE6 requires the protein cation exchange function to rescue neuronal morphogenesis.
(A-B) Overexpression of NHE6 but not an exchanger-defective NHE6 rescues neuronal morphogenesis in NHE6 mutant neurons. (A) P0-1 NHE6 mutant neurons were transfected after 1 DIV with constructs expressing GFP (top left), NHE6-HA (top right), or HA-tagged exchanger-defective NHE6 (E255Q/D260N) (bottom left). Also, neurons from wild-type littermates were transfected with GFP-expressing constructs alone. Neurons were imaged at 5DIV. Neurolucida traces of reconstructed confocal Z-stacks of GFP-labeled neurons. Insets show the expression of transfected E255Q/D260N or NHE6-HA (stained with anti-HA antibody) in the soma of neurons and demonstrates equivalent staining and localization of protein, appearing similar to endogenous protein distribution. Scale bar, 20μm. (B) Quantification of axonal and dendrite branching (MUT cells: GFP construct, 3 pups, n=50 cells; E255Q/D260N construct, 3 pups, n=49 cells; NHE6-HA construct, 3 pups, n=46 cells; WT cells: GFP construct, 3 pups, n=26 cells; 3 litters). Mean ± SEM (* p<0.05, *** p<0.001). (C-E) NHE6 E255Q/D260N exchanger deficient mutant construct. (Ci) Alignments of amino acid or nucleotide sequence of predicted ion transport region of NHE6-HA and E255Q/D260N. Mutated amino acids or nucleotides are unshaded. (Cii) Sequence trace of E255Q/D260N. Mutated nucleotides are underlined. Mutation strategy as previously described (Ohgaki et al., 2008)(Xinhan et al., 2011). (D) HeLa cells transfected with HA-tagged NHE6 WT, E255Q/D260N or vector. Lysates were subjected to Western blots probed with anti-NHE6 antibody. Equivalent level of protein expression and equivalent bands were observed between mutant NHE6 E255Q/D260N and NHE6 WT construct. (E) Hippocampal neurons expressing HA-tagged NHE6 WT or E255Q/D260N at 5 DIV and stained with anti-NHE6 and anti-HA antibody. E255Q/D260N showed the same subcellular localization as NHE6 WT construct. These experiments suggest that the point mutations that impair the exchanger domain do not affect protein stability or trafficking. GFP was used to visualize the morphology of hippocampal neurons. Scale bar, 10μm. See also Supplementary Figure 7.
To determine the functional domains of the NHE6 protein that are important for neuronal morphogenesis, we generated an NHE6 construct with mutations in the cation exchanger domain, an alteration known to impede proton transport (Xinhan et al., 2011) (Figure 4C). This exchanger-deficient NHE6 protein was stable and appeared to traffic normally (Figure 4D and 4E; also Supplementary Figure 7A and 7B). Also, the exchanger-deficient NHE6 co-localized with transferrin-associated early endosomes in neurons functionally loaded with transferrin (Supplementary Figure 7). However, the exchanger-deficient NHE6 completely failed to rescue neuronal morphogenesis in the NHE6-null cultures (Figure 4A and 4B). Neurons transfected with the mutant construct continued to show impairments in branching in a manner similar to mutant tissue, and without showing increases in cell death by analysis of pyknotic nuclei (data not shown). These data support an interpretation that the endosomal proton leak function of NHE6 may be required for the role of the protein in neuronal arborization.
NHE6-null mice have normal paired-pulse ratios, but reduced functional connectivity
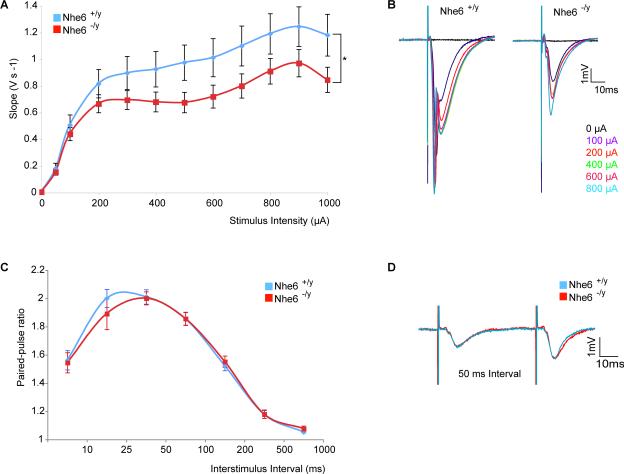
In order to understand further the functional consequences of NHE6 mutation on neuronal function, we compared extracellularly recorded synaptic field potentials in mutant versus wild-type animals in acute hippocampal slices. As demonstrated by the input/output curve, the extracellular synaptic potential was significantly reduced on average by 23.2% (p< 0.0001) in the NHE6-mutants compared to control animals between postnatal days 14 to 21 (Figure 5A and 5B). This result is consistent with at least three models: first, that the synapses within the given circuit are individually weaker; second, there are fewer axons and/or branches activated by a given presynaptic stimulus, and third, that there are fewer functional synapses. Either or both of the last two interpretations are consistent with our prior observations of reduced axonal and dendritic branching. To test these possible models we further examined the properties of synapses in acute hippocampal slice preparations. As NHE6 protein staining appeared quantitatively more presynaptic than postsynaptic (Figure 2G), we tested presynaptic function. We measured paired-pulse facilitation (PPF), a form of presynaptic short-term plasticity reflecting the synaptic vesicle probability of release over different time intervals (Figure 5C and 5D). The data show that mutant and wild-type synapses in hippocampal slices behaved identically, suggesting that the difference in the input/output curve is unlikely to be caused by difference in presynaptic release parameters. To examine if the reduced input/output curve in the NHE6 deficient mice was caused by a reduction in the number of axons, we analyzed the amplitude of the fiber volley in the presence of an AMPAR inhibitor. The fiber volley is directly related to the number of action potentials in stimulated presynaptic axons. In paired littermates we found a variable but significant decrease of 25% (p<0.001) in the fiber volley amplitude in the absence of NHE6 as compared to control (Supplementary Figure 8A and 8B). The electrophysiological data support a model involving circuit defects with a reduction in active synapses due to a reduction of afferent input without evidence thus far of perturbed individual synapse function. Overall, these circuit defects (reduced synaptic field strength and presynaptic fiber volley with intact paired-pulse facilitation) are consistent with the abnormalities in axonal and dendritic branching presented previously.
Figure 5. NHE6 deficient mice have reduced synaptic field potentials for a given stimulus.
(A) Extracellular synaptic field potentials in acute hippocampal slices are diminished in the mutants (Nhe6 −/y) compared to wild type (Nhe6 +/y) littermates. Curves are shown as the mean ± SEM of the initial negative slope of the fEPSP as a function of increasing stimulation current (Nhe6 +/y, n=12 animals; Nhe6 −/y, n = 18 animals; p<0.0001. P value was obtained using a two way ANOVA non-matched analysis for the difference between the curves corresponding to each genotype). (B) Representative traces at increasing stimulation of slices from mutant (Nhe6 −/y) and control (Nhe6 +/y) males are shown at 0μA (black), 100μA (purple), 200μA (red). (C) Paired-pulse facilitation (PPF) in mutant and wild-type male littermates suggests no defects in presynaptic release probability. The average ratios of the initial negative fEPSP slopes after two synaptic stimuli elicited at different time intervals for Nhe6 −/y (n=9), ie PPF, is indistinguishable from Nhe6 +/y (n=17). Ratios are shown as mean ± SEM. (D) Superimposed representative traces of PPF responses at 50ms interval is shown for the control (blue) and mutant (red) paired littermates. Scale bar: 1mV, 10ms. See also Supplementary Figure 8.
NHE6 mutant mice display reduced synapse number and decreased mature spines
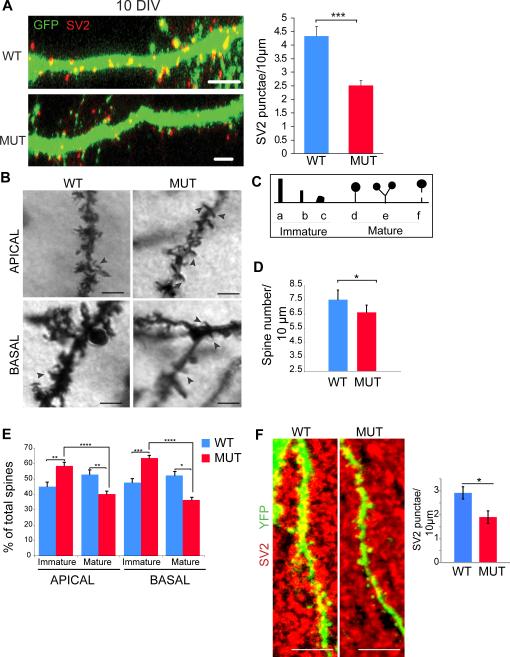
Reductions in axonal branches would predict corresponding reductions in synapses. We quantified the number of synapses and spines in NHE6 mutant mice. By quantifying SV2-positive punctae along the dendrites of GFP-transfected neurons in wild-type and mutant hippocampal cultures, we discovered that neurons in NHE6 mutant cultures displayed an approximately 42% decrease in SV2-positive presynapses per 10 μm of dendrite (4.3 +/− 0.4 SV2+ punctae in wild-type versus 2.5 +/− 0.2 in mutant, p < 0.001) at 10 DIV (Figure 6A). This observation was corroborated by the study of Golgi-Cox labeled spines in vivo at postnatal day 21. Using this method in CA1 hippocampal pyramidal neurons, we discovered a significant 12% decrease (p=0.03) in the total number of dendritic spines per 10μm length (Figure 6B to 6D). We also found NHE6-null mice had a very significant decrease in the percentage of mature synapses both in basal dendrites (mutant=36.4 ± 1.6% mature spines; control= 52.3 ± 2.5% mature spines, p=0.0009) and apical dendrites (mutant=40.2 ± 1.8% mature spines; control=52.9 ± 3.0% mature spines, p=0.0001) (Figure 6E). In addition, we observed a highly significant increase in the percentage of immature synapses in the mutant animals compared to controls both in basal dendrites (mutant=63.6 ± 1.6% immature spines; control= 47.7 ± 2.5% immature spines, p=0.000004) and apical dendrites (mutant=58.5 ± 2.3% immature spines; control=45.1 ± 2.8% immature spines, p=0.0005) dendrites. To corroborate the findings from the Golgi-Cox studies, we also quantified the number of SV2 punctae per length of apical dendrite in YFP labeled CA1 hippocampal pyramidal cells in vivo. We observed similar decreases in SV2+ punctae (a 35% decrease), ie 2.91 +/− 0.25 punctae per 10 μm in the wild type (n=210 synapses) as compared to 1.89 +/− 0.25 in the mutant (n=107 synapses) (p=0.012) (Figure 6F). Therefore, the electrophysiology data suggesting reduced synaptic field strength due to decreases in synaptic number is consistent with our direct measures both in vitro and in vivo of reduced synapse number. Altogether these data support the interpretation that NHE6 is required for proper circuit development in mouse hippocampus in vivo.
Figure 6. NHE6 mutant neurons show reduced synapses, decreased spine density and increased immature spines.
(A) Cultured hippocampal neurons from NHE6-null mice show reduced synapses compared to wild-type littermates at 10 DIV, as visualized by SV2 staining (red) (Left images). Neurons were transfected with GFP and immunostained with SV2. Scale bar, 5 μm. Quantification of SV2 densities per 10μm length in WT and MUT neurons (WT: n=21; MUT: n=24; 3 litters). Error bar, mean ± SEM. *** p<0.001. (B-E) In vivo analysis of Golgi stained dendritic spines shows reduced spine number as well as reduced maturation. (B) Basal (bottom) and apical (top) CA1 pyramidal dendrites in WT and MUT P21 littermates. Black arrows show immature spines on WT and MUT dendrites. Scale bar, 20μm. (C) Spine classification criteria for spine morphology analysis. Immature spines included (a) filopodia spines, (b) thin spines, and (c) stubby spines; mature spines included (d) mushroom spines, (e) branched spines, and (f) detached spines. (D) Reduction in total spine density in MUT pyramidal neurons compared to WT pyramidal neurons (WT= 3, N=38 cells, n=5292 spines; MUT=4, N=47 cells, n=5546 spines) of CA1 hippocampal region. Average spine number per 10μm of dendrite length, mean ± SEM, **p < 0.05. (E) Spine shape in apical and basal dendrites showed more immature spines in MUT animals compared to WT littermates. Immature and mature spines are shown as a percentage of total spine number, mean ± SEM. *p< 0.005, **p< 0.0006, ***p< 0.00001, ****p< 0.0000001. (WT= 3, N=38 cells, n=5292 spines; MUT=4, N=47 cells, n=5546 spines). (F) In vivo analysis of synapses. 40μm cortical sections of YFP+/WT or YFP+/MUT were stained with SV2. Left, representative WT and MUT images are shown for CA1 apical dendrites (YFP). Right, quantification of SV2 punctae colocalizing with YFP spines is shown per 10μm length, mean ± SEM. *P<0.015. Scale bar 5μm.
NHE6 mutant neurons show over-acidified endosomes and ectopic low-pH endosomes within growing axons and dendrites
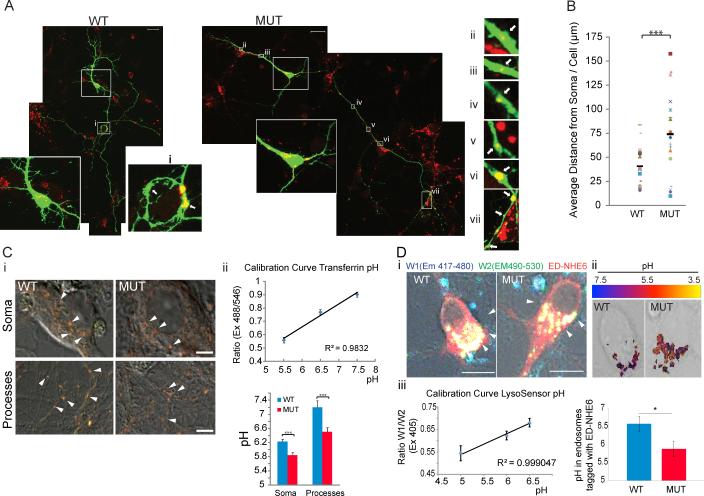
As Na+/H+ exchangers are generally construed as passively transporting selective cations down their concentration gradients, we hypothesize NHE6 to be a “proton leak” channel for endosomes. In the absence of NHE6, protons would be retained within endosomes and an expanded distribution of acidic (i.e. low-pH endosomes) would be evident. Using the low-pH dye LysoTracker DND-99, we visualized endosomes with low luminal pH (pH ≤ 6.0) in hippocampal neurons. Those LysoTracker-positive endosomes associated with cell soma likely reflect lysosomes. Intra-axonal LysoTracker-positive endosomes were rarely identified in controls (Figure 7Ai). Our results in controls are consistent with prior studies that have shown a proximal to distal gradient of low-pH endosomes. These studies have shown proximal intra-axonal endosomes to exhibit low luminal pH (pH ≤ 6.0) and distal intra-axonal endosomes representing higher luminal pH (pH ≥ 6.4) that would not be visualized by LysoTracker (Overly and Hollenbeck, 1996; Overly et al., 1995). In contrast to our control study, NHE6 mutant neurons showed an abundance of distally-placed LysoTracker-positive axonal and dendritic endosomes (Figure 7A). Ectopic low-pH endosomes were observed along the axon, at branches of developing axons and dendrites and at distances of up to 150 μm and further. On average, low-pH endosomes were 74 μm from the soma in NHE6 mutant neurons as compared to 41 μm for control neurons (p<0.001). These data are consistent with abnormal acidification of endosomes in the absence of NHE6.
Figure 7. Ectopic low-pH endosomes in axons and dendrites, and over-acidification of endosomes are observed in the absence of NHE6.
(A) Hippocampal neurons from NHE6-null and wild type mice were transfected with GFP constructs at 1DIV and stained with LysoTracker red at 5DIV. i-vii are high-magnification images of the boxed regions. Boxes on left reflect cell body. Arrows in boxes on the right indicate LysoTracker (yellow) labeled, low-pH endosomes along the dendrite and axon of neurons. Scale bar, 20μm. (B) The average positions of LysoTracker red puncta in neurites were measured as a distance from the center of the soma. (WT: 3 pups, n=18 cells; MUT: 3 pups, n=17 cells). Black line indicates mean distance. (*** p<0.001). (C) Luminal pH in transferrin-positive early endosomes, fluorescence ratio imaging. NHE6 WT and MUT neurons incubated with a mixture of fluorescein- and Alexa Fluor 546-transferrin at 37°C for 10min. Observed under live conditions in Neurobasal-A solution (pH7.4) with 5% CO2. Arrows in (Ci) indicate transferrin-positive early endosomes. pH was calculated from the ratio of fluorescence intensity (Fluorescein/Alexa Fluor 546) with a calibration curve (ii). Mut, n=53 cells; WT, n=57 cells; ***, p=0.0004. (D) LysoSensor analysis of luminal pH in endosomes tagged with exchanger-deficient (ED) NHE6. DIV2 WT and MUT hippocampal neurons transfected with exchanger deficient NHE6-mCherry (red), analyzed on DIV6 using LysoSensor yellow/blue DND-160 (Excitation 405nm). (i) Images of exchanger-deficient NHE6-mCherry in WT and MUT neurons, also labeled with LysoSensor yellow/blue DND160 (yellow). Scale bar 10μm. (ii) Pseudo color representation of the pH values in ED-NHE6 endosomes in WT and MUT hippocampal neurons (see Supplementary Methods). (iii) pH was calculated from a calibration curve generated from the ratio of LysoSensor W1 (Emission 417-480)/LysoSensor W2 (Emission 490-530). Quantification of pH from four independent experiments shows a decrease in the average pH per endosome in mutant cells (N animals = 7; endosomes =190) compared to controls (N animals= 4; endosomes=131). Error bars are S.E.M. *P=0.03 See also Supplementary Figure 9.
We also observed that endosomes in the NHE6 mutant cells were over-acidified by two additional independent studies. First, we measured the luminal pH in wild-type and NHE6 mutant neurons in transferrin-positive early endosomes using fluorescent ratio imaging of fluorescein-conjugated (pH sensitive) transferrin to Alexa-Fluor-546-conjugated (pH non-sensitive) transferrin. Cells were loaded with the transferrin cocktail for 10 minutes and then live-imaged. The luminal pH of these early endosomes was measured to be pH of 6.2 +/− 0.08 in wild-type cells and a pH of 5.8 +/− 0.07 in mutant cells (p value 0.0004) for endosomes in the soma, and pH of 7.2 +/− 0.2 in wild-type cells as compared to pH of 6.5 +/− 0.1 in mutant cells (p value 5.7E-06) for endosomes along the processes (Figure 7C).
Second, we characterized the intra-luminal pH of NHE6-associated endosomes directly in wild-type and mutant hippocampal neurons by expressing a fluorescently-tagged exchanger-deficient NHE6 construct in these cells. We used the exchanger-deficient construct in order to tag endosomes that are likely to be associated with NHE6 without expressing an active form of the protein which may change (ie alkalinize) the endosome lumen. Using LysoSensor DND-160 to generate an accurate measurement of intraluminal pH, we discovered that on average the pH within endosomes tagged with exchanger-deficient NHE6 protein is 6.57+/−0.23 in the wild-type neurons (131 vesicles counted) and 5.88+/−0.21 in the mutant neurons (190 vesicles analyzed) (p value 0.03) (Figure 7D). Finally, in addition to over-acidification of early endosomes, we also observed that the lysosome compartment is modestly expanded in NHE6 mutant neurons (Supplementary Figure 9).
TrkB and phospho-Trk levels are reduced in response to BDNF signaling in the absence of NHE6
Our data taken together support a model wherein NHE6 is required for regulation of intra-endosomal proton concentration, which is required for axon and dendritic branching. We next hypothesized that abnormal endosomal acidification may perturb endosomal signaling mechanisms relevant to neuronal arborization. Given the well-known role for BDNF/TrkB endosomal signaling in neuronal arborization (Chao and Lee, 2004; Danzer et al., 2002; Luikart et al., 2005; Reichardt, 2006; Segal, 2001), we set out to test the role of this signaling pathway in the pathophysiology and/or treatment for NHE6 mutations.
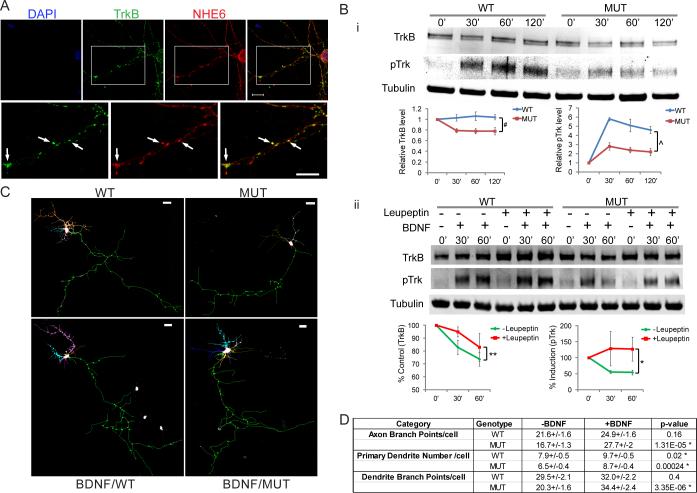
We initially investigated to see if NHE6 and TrkB protein, the BDNF receptor, co-localized to the same endosomes (Figure 8A). Using immunohistochemical co-staining against endogenous epitopes of NHE6 and TrkB, we discovered a very high degree of overlap of these two proteins in a distribution of punctate staining in the perinuclear area but also along growing axons and dendrites (Figure 8A).
Figure 8. Branching defects in NHE6 mutant neuron are rescued by BDNF.
(A) NHE6 and TrkB protein co-localized to the same endosomes. Cultured hippocampal neurons at 5 DIV were treated with BDNF and then fixed and immunostained with antibodies to NHE6 (red) and TrkB (green). Arrows indicated the co-localization of NHE6 and TrkB in the same endosomes. Scale bar, 10 μm. (Bi) TrkB levels and phospho-Trk induction after BDNF are decreased in NHE6 mutant neurons. Top, representative Western blot analysis of total TrkB and phosphorylated Trk protein levels after BDNF administration in cultured hippocampal neurons from NHE6 mutant and wild type littermates. Tubulin as loading control. Bottom, Quantitative analysis of the immunoblot bands. Values were normalized to the respective tubulin levels and then were normalized to time 0min (#, single factor ANOVA, p=8.36E-05, n=8 experimental replicates; ^, single factor ANOVA, p=2.44E-08, n=4 replicates). Means +/− SEM. (Bii) Reduction in TrkB and phospho-Trk after BDNF is rescued by leupeptin, a protease inhibitor. Top, representative Western blot analysis. Bottom, Quantitative analysis of the immunoblot bands. Values were normalized to the respective tubulin levels and then were normalized to time 0min. % control and % induction were the ratio of mutant /WT. (+, with 100μg/ml leupeptin; **, single factor ANOVA, p=0.001; *, single factor ANOVA, p=0.03, n=14 replicates for without leupeptin and n=6 replicates for with leupeptin). (C) Defects in arborization in NHE6 mutant neurons are rescued by exogenous administration of BDNF. Neurolucida traces of reconstructed confocal Z-stacks of GFP-labeled WT and MUT neurons with or without 50ng/ml BDNF. Scale bar, 20 μm. (D) Quantification of axonal and dendrite branching with or without 50ng/ml BDNF (MUT cells: without BDNF, 4 pups, n=47 cells; with BDNF, 4 pups, n=44 cells; WT cells: without BDNF, 3 pups, n=46 cells; with BDNF, 3 pups, n=38 cells; 3 litters). Mean ± SEM (* p<0.05).
Next, we examined directly the levels of TrkB receptor in response to signaling. TrkB is well-known to be endocytosed upon binding BDNF and a significant degree of signaling has been proposed to occur within signaling endosomes upon endocytosis (Chao and Lee, 2004; Cosker et al., 2008; Harrington et al., 2011; Philippidou et al., 2011; Rajagopal et al., 2004). Given the possibility of abnormal acidification of early endosomes in the absence of NHE6, we hypothesized that TrkB protein may show increased rates of degradation and consequently reduced signaling. To test this hypothesis, we examined TrkB receptor levels and phospho-Trk levels in response to BDNF signaling in NHE6 mutant and wild type hippocampal cultures at 4 DIV. Western blot analysis indicated that after adding 50ng/ml of BDNF for 30min, TrkB receptor level in mutant neurons was significantly reduced to 76.6% of the control (0.79+/−0.04 in mutant versus 1.03+/−0.06 in wild type where receptor levels are normalized to time of BDNF administration). The reduction effect in mutant neurons was maintained throughout 2h of BDNF treatment (73.3% and 75.0% of wild type at 60min and 2h, respectively; p=8.35E-05). The induction of phospho-Trk was also significantly reduced in mutant after adding BDNF (induction was approximately 48% compared to control throughout the whole treatment duration; p=2.44E-08) (Figure 8Bi). Collectively, these findings indicate that depletion of NHE6 has an effect on TrkB protein turnover and endosomal signaling, and suggest that the failures in dendrite and axon growth and branching in NHE6 mutants may be in part due to deficiencies in BDNF/TrkB signaling. In order to probe in greater depth the mechanism by which endosomal signaling via BDNF/TrkB may be attenuated in the mutant cells, we treated wild-type and mutant cultures with leupeptin, a reversible inhibitor of proteases generally found in the endo-lysosome system and activated by low-pH in the lysosomes. We observed that treatment of cultures with leupeptin prior to BDNF treatment partially reversed the decrease in TrkB levels. Attenuation in phospho-Trk signal was completely reversed in the mutant with leupeptin pre-treatment (Figure 8Bii). At 30 minutes after BDNF treatment, TrkB levels were approximately equal to wild-type cells (95% of wild-type levels) and phospho-Trk induction was 128.5% wild-type levels as compared to 55.3% wild-type levels without leupeptin treatment (p=0.001 for comparison of TrkB levels in mutant with and without leupeptin; p=0.03 for comparison of phospho-Trk levels in mutant with and without leupeptin). These data support the mechanism that diminished TrkB levels and signaling in the mutant result, at least in part, from excess degradation of the receptor due to over-acidification of the endosome compartment, premature activation of pH-sensitive proteases and/or expansion of the lysosome compartment.
Rescue of branching defects by exogenous administration of BDNF
Taken together our data support a model wherein due to loss of NHE6 there is an abnormal acidification of early endosomes resulting in impoverished branching as a result of decreases in BDNF/TrkB signaling. Given this model, we predicted that exogenous and high levels of BDNF may rescue the branching defects through enhancing TrkB signaling. To investigate the ability of exogenous BDNF to improve the cellular phenotype seen, we added BDNF (50ng/ml) to wild-type and mutant cultures at 2 DIV and examined cultures on day 5 for effects on axonal and dendritic branching associated with NHE6 mutation. We discovered that exogenous BDNF increased both axonal and dendritic branching in mutant cells to a level approaching wild-type levels (Figure 8C and 8D). We saw effects on both branching as well as an increase in the number of primary dendrites. In NHE6 mutant neurons exposed to exogenous BDNF, axonal branching increased from 16.7 +/− 1.3 branch points per cell to 27.7 +/− 2.0 branch points per cell (p < 0.000013). Dendritic branching increased from 20.3 +/− 1.6 branches per cell to 34.4 +/− 2.4 branches per cell. Primary dendrites increased from an average of 6.5 +/− 0.4 dendrites to 8.7 +/− 0.4. Our data are consistent with the notion that BDNF/TrkB endosomal signaling is attenuated with loss of NHE6 yet this loss may be overcome through addition of exogenous levels of BDNF.
DISCUSSION
Impoverished arborization and reduced synapse numbers weakens circuit strength in CS
Mutations in endosomal NHE6 represent a new neurogenetic disorder named Christianson syndrome (Gilfillan et al., 2008; Morrow et al., 2013; Schroer et al., 2010). Large-scale resequencing of X-chromosome genes in pedigrees with X-linked developmental brain disorders revealed that NHE6 is one of the most commonly mutated genes (Tarpey et al., 2009). Specifically, out of 208 pedigrees with possible X-linked inheritance, two truncating and likely null mutations in NHE6 were discovered; that is, approximately, 1% of families with X-linked inheritance of developmental brain disorders may harbor null alleles in NHE6. As the majority of disease-associated variants found in NHE6 appear to be null alleles, the mouse knockout presented in this study has excellent genetic construct validity for investigating the molecular and neurodevelopmental mechanisms at the root of Christianson syndrome.
While CS appears to be a novel and important developmental brain disorder, little is known about the mechanisms of disease. In addition to the role the NHE6 plays in CS, NHE6 and NHE9 also play roles in idiopathic autism, as we have previously reported mutations in NHE9 in severe forms of autism with epilepsy (Morrow et al., 2008). Also the gene expression of NHE6 and NHE9 is misregulated in postmortem autism brain (Schwede et al., 2013). In this study, we demonstrate that NHE6-associated endosomes play a critical role in the arborization of axons and dendrites. This cellular phenotype appears to be shared by a number of severe forms of autism-related conditions including those associated with MeCP2 mutations and TAOK2 mutations (Belichenko et al., 2009; Calderon de Anda et al., 2012).
Failures in axonal and dendritic branching will significantly impair neuronal connectivity which is likely responsible, at least in part, for cognitive and language impairments in children with NHE6 mutations. The protein localization data presented here are consistent with NHE6 localization in early endosomes as has been previously published for non-neuronal cell lines (Nakamura et al., 2005). We also showed additional evidence to support additional localization of NHE6 with recycling and late endosomes. Localization to late endosomes stands in contrast to previous studies in non-neuronal cell lines where NHE6 was reported to be associated only with early and recycling endosomes (Nakamura et al., 2005). This new finding may reflect differences between at least some types of neurons and other types of cells, or species differences.
Also, the localization of NHE6-associated endosomes within growing, long-range axons and in dendrites and localized to branches is consistent with the cellular phenotype of impoverished arbors observed in mouse mutant neurons. NHE6 localization and the function in axonal growth and branching are consistent with a leading hypothesis in autism related to failures in long-range connectivity (Geschwind and Levitt, 2007). In addition to impoverished arbors in vitro, we also observed reduced complexity of neuronal arborization in vivo in both hippocampus and cortex. Our electrophysiological studies in acute hippocampal slices demonstrate reduced synaptic connectivity in mutant animals, indicating either impaired synapses or reduced number of synapses. However, the observation of normal paired-pulse facilitation argues for unimpaired presynaptic release probability in NHE6-null neurons. Moreover, the diminished fiber volley in the mutants reflects fewer presynaptic action potentials for a given stimulus, consistent with fewer functional axonal inputs. Overall, our observations of decreased circuit strength and presynaptic action potentials are entirely consistent with a primary defect in axonal and dendritic branching, leading in turn to fewer functional synapses. We do observe reduced synapse numbers both in vitro and in vivo which may simply be the result of abnormalities in axonal and dendritic branching, although we also observe an increase in immature spines. This may suggest additional abnormalities in other synaptic mechanisms. Further work will be necessary to elucidate additional possible synaptic perturbations. Goh et al. have suggested an NHE activity involved in the loading of glutamate into synaptic vesicles (Goh et al., 2011). Although this may contribute to the reduced input-output relationship, it cannot explain the reduction in fiber volley amplitude observed here.
Loss of NHE6 leads to over-acidification of endosome compartment and attenuated endosomal signaling
In this study, we dissect the cellular mechanism by which loss of endosomal NHE6 may result in the cellular phenotype of impoverished neuronal arborization. Our data are consistent with a model wherein in the absence of NHE6 endosomes are more rapidly acidified that leads to hastened degradation of TrkB and attenuated endosomal signaling in response to BDNF (Figure 9). Endosomal signaling is a critical cellular mechanism that regulates a variety of cellular processes including neurotrophin signaling which is well known to govern neuronal arborization (Cosker et al., 2008; Danzer et al., 2002; Gorski et al., 2003; Marshak et al., 2007; Rajagopal et al., 2004; Wu et al., 2009). Other studies have modulated NGF/TrkA signaling via molecular manipulation of endosome maturation in PC12 cells. Specifically, dominant-negative forms of Rab5 that putatively forestall transit through early endosomes enhanced signaling, while overexpression of a constitutively active Rab5 leads to more rapid endosome maturation and attenuated signaling (Liu et al., 2007).
Figure 9. NHE6/TrkB signaling endosome pathway model.
(A) NHE6/TrkB signaling endosome in a wild-type neuron. Left. Schema of an endosome. Acidification is regulated by the vacuolar-type H+-ATPase (V-ATPase) which pumps protons into the endosome lumen, and NHE6 which allows for proton exit via exchange with Na+. On the right, TrkB endosome signaling (radio-waves) is depicted. With binding of BDNF, TrkB is endocytosed and signals from within endosomes. Endosomes are either recycled or traffic through a series of increasingly acidic endosomal compartments leading to degradation in the lysosome. This signaling process contributes substantially to promoting arborization in developing neurons. (B) Depiction of NHE6/TrkB signaling endosome in the NHE6 null neuron. Left. Without NHE6 proton exit from the endosome is impeded resulting in accelerated acidification of the endosome. On the right, upon BDNF binding and endocytosis, the endosomal lumen has accelerated acidification which results in increased degradation of TrkB and attenuated TrkB signaling which leads to an impoverishment of axonal and dendritic arbors.
Early studies by Hollenbeck and colleagues using endosome pH indicators characterized the distribution of low-pH endosomes along the growing axon in chick sympathetic neurons demonstrating a paucity of low-pH endosomes distally with an increase proximally near the soma (Overly and Hollenbeck, 1996; Overly et al., 1995). Our studies generally recapitulated this distribution of low-pH endosomes in the axons and dendrites of wild-type cultured hippocampal neurons; however, in NHE6 mutant neurons, we directly demonstrate an over-acidification of endosomes. Interestingly, we also see a modest expansion of the lysosome compartment distally along neurites in the mutant.
To our knowledge, only one prior study has examined the level of acidification of endosomes relative to endosomal neurotrophin signaling (Harrington et al., 2011). By altering the acidity of the culture media, Harrington et al. argue that precocious acidification of endosomes may lead to attenuated TrkA signaling in a ligand-dependent fashion. The authors demonstrate that NT-3 and NGF signaling is attenuated at low pH, and they demonstrate a reduction in ligand-affinity for TrkA receptor at low pH. The authors did not examine the stability of the TrkA receptor itself. In the experiments here, we directly observed decreases in TrkB and phospho-Trk levels which appear reversed by treatment with the lysosome protease inhibitor leupeptin. In addition to expansion of the LAMP-1 compartment, it is possible that over-acidification of the endosome compartment may lead to precocious activation of degradative enzymes within endosomes. However, TrkB levels in response to leupeptin treatment may be partially and not completely rescued in the mutant. One possible interpretation for this is there is a component of reduced TrkB synthesis in the mutant. Overall, our data are consistent with a significant excess of degradation of TrkB as contributing at least in part to reduced TrkB signaling in the mutant. Finally, we propose that the effect of NHE6 on TrkB signaling is one of relative attenuation (and not absolute blockage) such that this handicapped signaling pathway may be rescued through exogenous and high concentrations of BDNF as we achieved in our rescue experiment wherein application of exogenous BDNF resulted in wild-type levels of arborization in mutant cells.
The function of NHEs has been examined in a variety of cellular functions and organisms but has been scarcely studied in the developing nervous system. The first organellar NHE was identified in yeast, nhx1, and conferred salt-tolerant growth in Saccharomyces cerevisiae (Nass et al., 1997). A relative of NHE6, nhx1 protein was shown to be localized to the prevacuolar compartment where proteins are sorted before delivery to the vacuole (the equivalent of the lysosome). Relative acidification of the prevacuolar compartment results in nhx1-delta mutants (Brett et al., 2005), and proteolytic cleavage of select proteins is more rapid in mutants (Bowers et al., 2000). In non-neuronal cell lines, Xinhan et al. knocked-down NHE6 using RNAi strategies and demonstrate a relative acidification of the early endosome compartment (Xinhan et al., 2011). In our study, we find a similar finding however in primary differentiating neurons. We observe over-acidification of endosomes in growing axons and dendrites, and we observe an associated neuronal phenotype that is the impoverishment of axon and dendrite growth and branching. We extend our observations to include mechanistic data demonstrating abnormalities in endosomal signaling. We cannot rule out the possibility that other endosomal processes may be involved in the cellular pathology underlying CS, and we anticipate that there are likely to be several such consequences yet to be explored that result from NHE6 mutation. For example, by employing a variety of tissue staining techniques, Stromme et al. report abnormal accumulation of GM2 ganglioside and unesterified cholesterol in the endolysosome compartment in select regions of the adult brain of NHE6 mutant mice (Stromme et al., 2011). Yet we contend that our observations regarding neuronal arborization are likely to be a primary and early process in CS leading to early failures in brain growth and in formation of functional neurocircuitry. As patients with CS show both regressions and potentially neurodegenerative features with age, we anticipate that the observations of Stromme et al. may have significance relative to these aging processes; however, further studies are required to begin to understand the regressions and neurodegeneration in more mechanistic detail.
Summary
In conclusion, we report a novel mechanism of attenuated endosomal signaling and a cellular phenotype involving impoverished arbors in CS, a new neurogenetic disorder. These observations are also relevant to conditions such as autism wherein NHE6 may be down-regulated. Our study examines how regulation of intra-endosomal pH modulates a well-studied and important signaling pathway, ie neurotrophin endosome signaling (Figure 9). We have been able to rescue the mutant arborization phenotype using the application of exogenous BDNF treatment. As BDNF secretion and TrkB activation may be amenable to pharmacologic targeting (Nagahara and Tuszynski, 2011), these data provide a plausible approach for potential interventions in CS and related forms of severe autistic disorders.
EXPERIMENTAL PROCEDURES
MICE
The Nhe6 knockout mice were obtained from Jackson Laboratories. A LacZ-Neo cassette was inserted into exon 6 to inactivate the Nhe6 gene (Supplementary Figure 5). See Supplemental Information for genotyping protocol. All experiments involving mice were carried out in accordance with the US National Institutes of Health Guide for the Care and Use of Animals under the protocols approved by the Brown University Institutional Animal Care and Use Committee.
PRIMARY NEURON CULTURE AND TRANSFECTION
Hippocampi were dissected from P0-P1 mice, dissociated with papain (20units/ml) in Earle's Balanced Salt Solution (EBSS) with bicarbonate at 37°C for 30min and triturated with a 1ml pipette. Hippocampal neurons were placed on 8-well chamber slides coated with 1mg/ml poly-D-lysine at a cell density of 3 × 105 cells/ml. For neuronal morphology, neurons at 1DIV were transfected with EGFP constructs or together with testing constructs at a ratio of 1:1 using Lipofectamine 2000 (Invitrogen). For BDNF treatment, 50ng/ml BDNF was added into medium right after transfection.
MORPHOMETRIC ANALYSES
Quantification of the branch number of axons and dendrites per cell was done on images acquired with 20X or 40X objectives. Images of cultured hippocampal neurons (2 DIV) were captured from randomly chosen fields on a Zeiss LSM710 confocal laser scanning microscope, blinded to genotypes, and traced using Metamorph Neurite Outgrowth software. We determined the number of processes (i.e. neurites emanating directly from the cell body) per cell, the total number of all neurite branches per cell. Z-series images of individual GFP-filled neurons at 5 DIV were obtained on a Carl Zeiss 710 confocal microscope and traced manually using Neurolucida software. Analysis of in vivo branching of hippocampal pyramidal neurons in Golgi stained brains was conducted using Neurolucida software. Neurons were traced manually and the number of dendrites and branch points we obtained per cell in images acquire with 20x objective. Similarly analysis of branching in YFP labeled cortical neurons was done as described above using images acquired at 20x and 63x objective in a Carl Zeiss 710 confocal microscope. Analysis of spine density and spine morphology in hippocampal pyramidal neurons of Golgi stained brains was done using z-stack images of 0.4μm stack thickness acquired with a 100x objective of a Zeiss microscope driven by Axiovision software. Images were analyzed as described above, tracing manually and using Neurolucida spine morphology selection criteria.
Supplementary Material
HIGHLIGHTS.
NHE6-associated endosomes govern neuronal arborization and circuit strength.
Loss of NHE6 leads to over-acidification of the endosome compartment.
In NHE6 mutant neurons, BDNF signal is attenuated and TrkB degradation is increased.
Defects in neuronal arborization are reversed by enhanced BDNF treatment in mutant.
ACKNOWLEDGMENTS
We thank Dr. Robbert Creton at Brown University Leduc Bioimaging Facility for advice regarding microscopy, Dr. Peter Davies for the kind gift of Phospho-Tau1 antibody. EMM has received support from Career Award in Medical Science, Burroughs Wellcome Fund and NIH NIGMS COBRE 8P20GM103537-10. SBL has received support from NIH 5T32MH019118-21. This work was supported by a grant from the Simons Foundation (SFARI #239834 to EMM), and also generous support to EMM from the Nancy Lurie Marks Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTERESTS
The authors declare no competing financial interests or conflicts of interest.
REFERENCES
- Belichenko PV, Wright EE, Belichenko NP, Masliah E, Li HH, Mobley WC, Francke U. Widespread changes in dendritic and axonal morphology in Mecp2-mutant mouse models of Rett syndrome: evidence for disruption of neuronal networks. J Comp Neurol. 2009;514:240–258. doi: 10.1002/cne.22009. [DOI] [PubMed] [Google Scholar]
- Bowers K, Levi BP, Patel FI, Stevens TH. The sodium/proton exchanger Nhx1p is required for endosomal protein trafficking in the yeast Saccharomyces cerevisiae. Mol Biol Cell. 2000;11:4277–4294. doi: 10.1091/mbc.11.12.4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett CL, Tukaye DN, Mukherjee S, Rao R. The yeast endosomal Na+K+/H+ exchanger Nhx1 regulates cellular pH to control vesicle trafficking. Mol Biol Cell. 2005;16:1396–1405. doi: 10.1091/mbc.E04-11-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett CL, Wei Y, Donowitz M, Rao R. Human Na(+)/H(+) exchanger isoform 6 is found in recycling endosomes of cells, not in mitochondria. Am J Physiol Cell Physiol. 2002;282:C1031–1041. doi: 10.1152/ajpcell.00420.2001. [DOI] [PubMed] [Google Scholar]
- Calderon de Anda F, Rosario AL, Durak O, Tran T, Graff J, Meletis K, Rei D, Soda T, Madabhushi R, Ginty DD, et al. Autism spectrum disorder susceptibility gene TAOK2 affects basal dendrite formation in the neocortex. Nat Neurosci. 2012;15:1022–1031. doi: 10.1038/nn.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey JR, Grinstein S, Orlowski J. Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol. 2010;11:50–61. doi: 10.1038/nrm2820. [DOI] [PubMed] [Google Scholar]
- Chao MV, Lee FS. Neurotrophin survival signaling mechanisms. J Alzheimers Dis. 2004;6:S7–11. doi: 10.3233/jad-2004-6s611. [DOI] [PubMed] [Google Scholar]
- Christianson AL, Stevenson RE, van der Meyden CH, Pelser J, Theron FW, van Rensburg PL, Chandler M, Schwartz CE. X linked severe mental retardation, craniofacial dysmorphology, epilepsy, ophthalmoplegia, and cerebellar atrophy in a large South African kindred is localised to Xq24-q27. J Med Genet. 1999;36:759–766. doi: 10.1136/jmg.36.10.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosker KE, Courchesne SL, Segal RA. Action in the axon: generation and transport of signaling endosomes. Curr Opin Neurobiol. 2008;18:270–275. doi: 10.1016/j.conb.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzer SC, Crooks KR, Lo DC, McNamara JO. Increased expression of brain-derived neurotrophic factor induces formation of basal dendrites and axonal branching in dentate granule cells in hippocampal explant cultures. J Neurosci. 2002;22:9754–9763. doi: 10.1523/JNEUROSCI.22-22-09754.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dindot SV, Antalffy BA, Bhattacharjee MB, Beaudet AL. The Angelman syndrome ubiquitin ligase localizes to the synapse and nucleus, and maternal deficiency results in abnormal dendritic spine morphology. Hum Mol Genet. 2008;17:111–118. doi: 10.1093/hmg/ddm288. [DOI] [PubMed] [Google Scholar]
- Garbern JY, Neumann M, Trojanowski JQ, Lee VM, Feldman G, Norris JW, Friez MJ, Schwartz CE, Stevenson R, Sima AA. A mutation affecting the sodium/proton exchanger, SLC9A6, causes mental retardation with tau deposition. Brain. 2010;133:1391–1402. doi: 10.1093/brain/awq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17:103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Gilfillan GD, Selmer KK, Roxrud I, Smith R, Kyllerman M, Eiklid K, Kroken M, Mattingsdal M, Egeland T, Stenmark H, et al. SLC9A6 mutations cause X-linked mental retardation, microcephaly, epilepsy, and ataxia, a phenotype mimicking Angelman syndrome. Am J Hum Genet. 2008;82:1003–1010. doi: 10.1016/j.ajhg.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh GY, Huang H, Ullman J, Borre L, Hnasko TS, Trussell LO, Edwards RH. Presynaptic regulation of quantal size: K+/H+ exchange stimulates vesicular glutamate transport. Nat Neurosci. 2011;14:1285–1292. doi: 10.1038/nn.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski JA, Zeiler SR, Tamowski S, Jones KR. Brain-derived neurotrophic factor is required for the maintenance of cortical dendrites. J Neurosci. 2003;23:6856–6865. doi: 10.1523/JNEUROSCI.23-17-06856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington AW, St Hillaire C, Zweifel LS, Glebova NO, Philippidou P, Halegoua S, Ginty DD. Recruitment of actin modifiers to TrkA endosomes governs retrograde NGF signaling and survival. Cell. 2011;146:421–434. doi: 10.1016/j.cell.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan YN, Jan LY. Branching out: mechanisms of dendritic arborization. Nat Rev Neurosci. 2010;11:316–328. doi: 10.1038/nrn2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon CH, Luikart BW, Powell CM, Zhou J, Matheny SA, Zhang W, Li Y, Baker SJ, Parada LF. Pten regulates neuronal arborization and social interaction in mice. Neuron. 2006;50:377–388. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Lamb D, Chou MM, Liu YJ, Li G. Nerve growth factor-mediated neurite outgrowth via regulation of Rab5. Mol Biol Cell. 2007;18:1375–1384. doi: 10.1091/mbc.E06-08-0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luikart BW, Nef S, Virmani T, Lush ME, Liu Y, Kavalali ET, Parada LF. TrkB has a cell-autonomous role in the establishment of hippocampal Schaffer collateral synapses. J Neurosci. 2005;25:3774–3786. doi: 10.1523/JNEUROSCI.0041-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshak S, Nikolakopoulou AM, Dirks R, Martens GJ, Cohen-Cory S. Cell-autonomous TrkB signaling in presynaptic retinal ganglion cells mediates axon arbor growth and synapse maturation during the establishment of retinotectal synaptic connectivity. J Neurosci. 2007;27:2444–2456. doi: 10.1523/JNEUROSCI.4434-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I. The importance of being acid: the role of acidification in intracellular membrane traffic. J Exp Biol. 1992;172:39–45. doi: 10.1242/jeb.172.1.39. [DOI] [PubMed] [Google Scholar]
- Mindell JA. Lysosomal acidification mechanisms. Annu Rev Physiol. 2012;74:69–86. doi: 10.1146/annurev-physiol-012110-142317. [DOI] [PubMed] [Google Scholar]
- Morrow EM, Pescosolido M, Schmidt M, Stein D, Sabbagh M, McLean R. Genetic and clinical diversity of NHE6 mutations in Christianson syndrome. 2013 doi: 10.1002/ana.24225. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow EM, Yoo SY, Flavell SW, Kim TK, Lin Y, Hill RS, Mukaddes NM, Balkhy S, Gascon G, Hashmi A, et al. Identifying autism loci and genes by tracing recent shared ancestry. Science. 2008;321:218–223. doi: 10.1126/science.1157657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahara AH, Tuszynski MH. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov. 2011;10:209–219. doi: 10.1038/nrd3366. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Tanaka S, Teko Y, Mitsui K, Kanazawa H. Four Na+/H+ exchanger isoforms are distributed to Golgi and post-Golgi compartments and are involved in organelle pH regulation. J Biol Chem. 2005;280:1561–1572. doi: 10.1074/jbc.M410041200. [DOI] [PubMed] [Google Scholar]
- Nass R, Cunningham KW, Rao R. Intracellular sequestration of sodium by a novel Na+/H+ exchanger in yeast is enhanced by mutations in the plasma membrane H+- ATPase. Insights into mechanisms of sodium tolerance. J Biol Chem. 1997;272:26145–26152. doi: 10.1074/jbc.272.42.26145. [DOI] [PubMed] [Google Scholar]
- Ohgaki R, Fukura N, Matsushita M, Mitsui K, Kanazawa H. Cell surface levels of organellar Na+/H+ exchanger isoform 6 are regulated by interaction with RACK1. J Biol Chem. 2008;283:4417–4429. doi: 10.1074/jbc.M705146200. [DOI] [PubMed] [Google Scholar]
- Ohgaki R, van ISC, Matsushita M, Hoekstra D, Kanazawa H. Organellar Na+/H+ exchangers: novel players in organelle pH regulation and their emerging functions. Biochemistry. 2011;50:443–450. doi: 10.1021/bi101082e. [DOI] [PubMed] [Google Scholar]
- Overly CC, Hollenbeck PJ. Dynamic organization of endocytic pathways in axons of cultured sympathetic neurons. J Neurosci. 1996;16:6056–6064. doi: 10.1523/JNEUROSCI.16-19-06056.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overly CC, Lee KD, Berthiaume E, Hollenbeck PJ. Quantitative measurement of intraorganelle pH in the endosomal-lysosomal pathway in neurons by using ratiometric imaging with pyranine. Proc Natl Acad Sci U S A. 1995;92:3156–3160. doi: 10.1073/pnas.92.8.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippidou P, Valdez G, Akmentin W, Bowers WJ, Federoff HJ, Halegoua S. Trk retrograde signaling requires persistent, Pincher-directed endosomes. Proc Natl Acad Sci U S A. 2011;108:852–857. doi: 10.1073/pnas.1015981108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal R, Chen ZY, Lee FS, Chao MV. Transactivation of Trk neurotrophin receptors by G-protein-coupled receptor ligands occurs on intracellular membranes. J Neurosci. 2004;24:6650–6658. doi: 10.1523/JNEUROSCI.0010-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramón y Cajal S. Histologie du système nerveux de l'homme & des vertébrés, Ed. française rev. & mise à jour par l'auteur, tr. de l'espagnol par L. Azoulay. edn. Maloine; Paris: 1909. [Google Scholar]
- Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroer RJ, Holden KR, Tarpey PS, Matheus MG, Griesemer DA, Friez MJ, Fan JZ, Simensen RJ, Stromme P, Stevenson RE, et al. Natural history of Christianson syndrome. Am J Med Genet A. 2010;152A:2775–2783. doi: 10.1002/ajmg.a.33093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwede M, Garbett K, Mirnics K, Geschwind DH, Morrow EM. Genes for endosomal NHE6 and NHE9 are misregulated in autism brains. Mol Psychiatry. 2013 doi: 10.1038/mp.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal R. Neurotrophins: which way did they go? Sci STKE. 2001;2001:pe1. doi: 10.1126/stke.2001.84.pe1. [DOI] [PubMed] [Google Scholar]
- Stromme P, Dobrenis K, Sillitoe RV, Gulinello M, Ali NF, Davidson C, Micsenyi MC, Stephney G, Ellevog L, Klungland A, et al. X-linked Angelman-like syndrome caused by Slc9a6 knockout in mice exhibits evidence of endosomal-lysosomal dysfunction. Brain. 2011;134:3369–3383. doi: 10.1093/brain/awr250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney NT, Brenman JE, Jan YN, Gao FB. The coiled-coil protein shrub controls neuronal morphogenesis in Drosophila. Curr Biol. 2006;16:1006–1011. doi: 10.1016/j.cub.2006.03.067. [DOI] [PubMed] [Google Scholar]
- Tarpey PS, Smith R, Pleasance E, Whibley A, Edkins S, Hardy C, O'Meara S, Latimer C, Dicks E, Menzies A, et al. A systematic, large-scale resequencing screen of X-chromosome coding exons in mental retardation. Nat Genet. 2009;41:535–543. doi: 10.1038/ng.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Cui B, He L, Chen L, Mobley WC. The coming of age of axonal neurotrophin signaling endosomes. J Proteomics. 2009;72:46–55. doi: 10.1016/j.jprot.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xinhan L, Matsushita M, Numaza M, Taguchi A, Mitsui K, Kanazawa H. Na+/H+ exchanger isoform 6 (NHE6/SLC9A6) is involved in clathrin-dependent endocytosis of transferrin. Am J Physiol Cell Physiol. 2011;301:C1431–1444. doi: 10.1152/ajpcell.00154.2011. [DOI] [PubMed] [Google Scholar]
- Yap CC, Winckler B. Harnessing the power of the endosome to regulate neural development. Neuron. 2012;74:440–451. doi: 10.1016/j.neuron.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H. Changes in prefrontal axons may disrupt the network in autism. J Neurosci. 2010;30:14595–14609. doi: 10.1523/JNEUROSCI.2257-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.