Abstract
Effective weight management interventions could reduce race–sex disparities in cardiovascular disease (CVD), yet little is known about factors associated with successful weight loss maintenance in race–sex subgroups. In the Weight Loss Maintenance trial (WLM), overweight/obese (BMI 25–45 kg/m2) adults who lost ≥4 kg in a 6-month behavioral weight loss intervention (phase I) were randomized into one of three 30-month maintenance interventions (phase II). To investigate predictors in subgroups, randomized groups were combined for this analysis. Of 1,685 phase I participants, 1,032 (61%) entered phase II, including 12% black men (BM), 26% black women (BW), 25% white men (WM), and 37% white women (WW). Weight change over the 36-month study ranged from −2.3% (95% confidence interval = −3.1 to −1.5%) in BW to −4.5% (95% confidence interval = −5.7 to −4.0%) in WM, the result of differential weight loss during phase I. Within race, men lost significantly more weight than women, but within sex group, weight loss did not differ significantly between races. Although participants regained weight during phase II, regain did not differ by race–sex group, and mean weight at the end of the study was significantly lower than phase I entry weight for each subgroup. In regression models, phase I weight loss predicted overall 36-month weight loss in all race–sex groups. Healthy dietary pattern at entry, improvement in dietary pattern, or both were predictive in three of four race–sex groups. Few other variables other than initial weight loss and dietary pattern were predictive. Future research should identify additional modifiable influences on long-term maintenance after a modest weight loss.
INTRODUCTION
The obesity epidemic is a contributing factor in health disparities. Overweight and obesity are more prevalent in non-Hispanic black adults (74%) compared to non-Hispanic whites (67%), with the highest rates in black women (BW) (78%) (1). Disparities in obesity prevalence are likely to contribute to disparities in the prevalence and severity of hypertension, diabetes mellitus type 2, and dyslipidemia (2,3). These disparities in cardiovascular disease (CVD) risk factors contribute to excess rates of stroke and heart failure in blacks (3). Effective treatment of obesity with particular attention to at-risk demographic groups would improve the public health and reduce CVD disparities.
The mainstay of contemporary obesity treatment is lifestyle behavior change; i.e., reducing calorie intake, improving diet quality, and increasing physical activity (PA) (4). Weight loss interventions targeting these behaviors have shown considerable short-term (6 month) success (5,6). However, such interventions have generally been less effective in blacks, particularly in BW (6–8). In addition, relatively little data are available concerning race–sex differences in longer term weight loss interventions and on predictors of weight regain after significant initial weight loss. Perhaps most important, little is known concerning factors associated with successful long-term weight control in each of these demographic subgroups. Such information could help us to design more effective interventions for specific populations. To this end, we report the extent and predictors of weight loss maintenance both overall and in race–sex subgroups in the Weight Loss Maintenance trial (WLM).
METHODS AND PROCEDURES
WLM was a National Heart, Lung and Blood Institute-sponsored multicenter randomized controlled trial designed to test strategies for sustained weight loss in a diverse population with CVD risk factors (9,10). The study included an initial weight loss phase (phase I) in which 1,685 participants all received a 6-month intensive behavioral intervention. In phase II, 1,032 participants who lost at least 4 kg in phase I were randomly assigned to one of three 30-month behavioral maintenance conditions: a self-directed control condition without further intervention (SD), monthly personal counseling intervention (PC), or an interactive internet-based technology intervention (IT). WLM was approved by the institutional review board at each participating institution, and all participants provided written informed consent.
Participants
Participants were overweight or obese adults (BMI 25–45 kg/m2) who were taking medication for hypertension and/or dyslipidemia. Exclusion criteria included medical conditions that precluded full participation in the study; weight loss of >9 kg in the last 3 months; recent use of weight loss medications; history of weight loss surgery; and diabetes mellitus. Because this analysis focuses on weight change over the full 36-month study, only participants who lost at least 4 kg in phase I and were eligible for phase II are included here.
Measurements
Data collection occurred before the start of phase I (study entry), at randomization into phase II, and every 6 months thereafter for 30 months. Measurements were obtained by trained, certified staff masked to treatment assignment.
Weight was measured using a calibrated digital scale with the participant wearing light, indoor clothes without shoes. Height was measured once at entry using a wall-mounted stadiometer. Dietary intake was assessed by the Block Food Frequency Questionnaire (FFQ) (11), and summarized using the Healthy Eating Index (HEI) (12,13). The HEI ranges from 0 to 100, with higher scores indicating better diet quality.
To assess PA, participants wore a calibrated, triaxial accelerometer (RT3; Stayhealthy, Monrovia, CA) for at least 10 h/day for at least 4 days including one weekend day, and results were used to estimate total weekly minutes of moderate-to-vigorous PA (MVPA) (14,15).
Participants also completed questionnaires to assess demographic characteristics, medication usage, reported difference between current weight and desired weight (in pounds), prior weight loss history, and other health and behavior variables. In addition, they completed questionnaires measuring psychosocial variables including the following.
Social Support and Exercise and Social Support for Eating Habits were assessed by 13- and 18-item surveys, respectively, that use self-report to assess participants’ perceptions of social support for exercise and healthy eating, respectively. Specifically, participants report how often their friends or family members (separately rated) supported their exercise and healthy eating behaviors during the previous three 3 months. Responses are on a 5-point Likert scale and responses of each of four surveys (exercise or eating habits, friends or family) are summed, yielding friends-support and family support scores. These surveys have demonstrated adequate internal consistency (α = 0.76–0.85) (16).
Health-related quality of life was assessed with the 36-item self-report SF-36 questionnaire (17). The SF-36 is a generic (rather than disease-specific) measure appropriate for the general population. Norms-based scoring yields two composite scale t-scores (physical and mental health) and eight subscale t-scores related to functional health and well-being. Participants respond to questions formatted on a Likert scale. Excellent internal consistency has been demonstrated on the two composite scales (α = 0.90), and the eight subscales have displayed adequate internal consistency (α = 0.80) (17).
Patient Health Questionnaire Depression Scale (PHQ-8) (18) is a self-administered questionnaire consisting of eight of the nine criteria for Major Depressive Disorder (suicidality is omitted). Participants respond to questions using a Likert scale, and these values are summed to obtain a total depression score. Rohyans and Pressler reported adequate internal consistence (α = 0.83) (19).
Perceived Stress Scale (20) is a self-report measure that assesses the degree to which participants find their lives to be uncontrollable, unpredictable, and overloading. Participants respond to items assessing stressful thoughts and perceptions within the last month. The response format is a 5-point Likert. Good internal consistency and test–retest reliability (α = 0.85) has been established (20).
Initial weight loss intervention (phase I)
During phase I, a trained behavioral interventionist led 20-weekly group sessions conducted over ~6 months. Intervention goals were 180 min/week of MVPA, reduced caloric intake, consumption of the Dietary Approaches to Stop Hypertension (DASH) dietary pattern which reduces CVD risk factors (21–24), and weight loss of ~1–2 lb/week. The intervention was based on behavior change theory (25,26) and incorporated behavior change tools such as self-monitoring, goal-setting, social support, problem solving, relapse prevention, and motivational interviewing.
Maintenance interventions (phase II)
The goals of the internet-based technology intervention and personal counseling interventions were maintenance of the phase I weight loss (or loss of additional weight if desired), continued adherence to the recommended dietary pattern, and increasing MVPA to at least 225 min/week. Both active interventions reinforced the behavioral theory and tools of phase I. SD participants received lifestyle advice at randomization and again after the 12-month data collection visit (10). Treatment group results have been reported elsewhere (9). Briefly, the personal counseling intervention was superior to SD for 30 months following randomization. Internet-based technology intervention was superior to SD through 24 months, but not at 30 months. We observed no significant race–sex by treatment interactions and treatment effects were modest. Therefore in this secondary analysis to identify correlates of long-term weight loss within demographic subgroups, data from participants in personal counseling intervention, internet-based technology intervention, and SD have been combined.
Outcomes
The primary focus of this article is on predictors of weight change over the entire 3-year period from study entry to the end of phase II. We express weight change as a continuous variable (i.e., percent weight change from entry) and as two dichotomous variables (whether weight at end of study was (i) below entry weight and (ii) at least 5% below entry weight). Based on effects on blood pressure (5,27) and diabetes risk (28) shown in previous studies, we considered 5% weight loss to be clinically significant on an individual basis. In order to assess potential determinants of weight loss maintenance (i.e., after initial weight loss), we also evaluated the percent change in weight over the 30-month period from randomization to the end of phase II. We also report changes in diet and PA.
Statistical methods
Participants randomized into phase II are included in this analysis, stratified into four race–sex subgroups: Black men (BM), BW, and non-BM and women. Ninety-eight percent of non-blacks were self-identified white, and therefore for purposes of this analysis, non-blacks are referred to as white men (WM) and white women (WW).
We used multiple linear regression (continuous outcomes) or logistic regression (binary variables) to compare weight change outcomes across race–sex groups while adjusting for site and entry weight. Comparison of weight changes during phase II also adjusted for weight change during phase I.
We fit two reverse stepwise regression models to identify predictors of 3-year percent weight change. The first included only baseline (study entry) characteristics and phase I weight loss. The second included baseline characteristics and change in diet and PA from entry to end of the study. We fit these models both overall and within each race–sex subgroup. To identify predictors of percent weight change during phase II, we fit a reverse stepwise regression model that included entry characteristics and changes in diet, PA, and psychosocial variables during both phase I and phase II. Given the large number of variables included in the modeling process and our lack of a priori hypotheses regarding interactions, no attempt was made to formally test (i.e., via interactions) for differences in the models across race–sex subgroups.
One potential predictor (frequency of weighing) was self-reported retrospectively at the end of the study. Given the limitations of retrospective self-report, we calculated Pearson correlation coefficients between this variable and weight changes, but did not include it in regression models.
In order to adjust for potential biases due to nonrandom loss to follow-up, we used multiple imputation (29) to replace missing end of study weights (for 68 individuals) and selected other measures. Only data missing due to participant death (N = 3) were not imputed; these individuals are excluded from this analysis. The multiple imputation methodology involves creating not just one, but several (in our case five) imputations so that the analysis can properly reflect the added variability in our regression estimates that is due to the variation in the imputation process itself. For the stepwise regression modeling, we used just one of the imputed datasets to derive the final models, but all five imputation datasets to estimate the parameters and their standard errors in those models. We also performed the predictor analyses in those with follow-up data, without imputing any missing data. All analyses were conducted using SAS, version 9.1 (SAS Institute, Cary, NC), and all P values are two-sided. Unless otherwise noted, we used LSMEANS to calculate model-based means for the data in all figures and tables.
RESULTS
Approximately 12% of phase II participants were BM, 26% BW, 25% WM, and 37% WW (Table 1). Participants were middle-aged, with blacks younger than whites. Mean BMI at entry ranged from 33.4 kg/m2 in WM to 35.3 kg/m2 in BW. Most participants were on antihypertensive medication, taking an average of two medications. For both blacks and whites, the discrepancy at entry between self-identified “best weight for me” and measured weight was lower for men than women. Final data collection was complete in 93% of BM, 91% of BW, 96% of WM, and 93% of WW.
Table 1.
Entry characteristics of randomized participants
| Black men | Black women | White men | White women | |
|---|---|---|---|---|
| N who started phase I | 196 | 540 | 355 | 594 |
| % who entered phase II | 62% | 49% | 72% | 65% |
| N who started phase II | 121 | 267 | 257 | 387 |
| Age in years, mean (s.d.) | 53 (9) | 53 (9) | 58 (9) | 57 (8) |
| BMI in kg/m2, mean (s.d.) | 34.4 (4.5) | 35.3 (5.0) | 33.4 (4.2) | 33.5 (4.9) |
| Weight in kg, mean (s.d.) | 108.3 (16.2) | 94.8 (15.2) | 104.2 (15.3) | 89.5 (14.5) |
| Overweight (BMI 25–29.9), % | 20 | 17 | 24 | 28 |
| Obese (BMI ≥30), % | 80 | 83 | 76 | 72 |
| College degree or higher, % | 69 | 56 | 72 | 56 |
| Household income ≥$60 k/year, % | 74 | 42 | 71 | 54 |
| On BP meds (%) | 89 | 94 | 83 | 84 |
| Number of BP meds (among those on BP meds), mean (s.d.) | 2.0 (0.9) | 1.9 (0.9) | 1.8 (0.8) | 1.7 (0.8) |
| On lipid-lowering meds, % | 42 | 24 | 53 | 41 |
| Current smoker, % | 3 | 5 | 3 | 5 |
| QOL: mental, mean (s.d.) | 54.8 (6.8) | 53.0 (8.6) | 53.7 (7.5) | 51.5 (9.3) |
| QOL: physical, mean (s.d.) | 51.2 (7.6) | 50.6 (8.0) | 51.5 (7.5) | 49.6 (8.0) |
| Social support for diet, mean (s.d.) | ||||
| Encouragement from family | 20.5 (8.3) | 18.1 (8.8) | 18.9 (7.4) | 16.4 (7.0) |
| Discouragement from familya | 14.7 (4.5) | 15.4 (5.4) | 13.9 (3.7) | 14.5 (4.8) |
| Encouragement from friends | 16.0 (6.9) | 17.7 (8.0) | 12.8 (5.2) | 14.1 (5.6) |
| Discouragement from friendsa | 13.7 (4.3) | 14.9 (5.1) | 12.7 (3.1) | 13.3 (3.5) |
| Social support for exercise score, mean score (s.d.) | ||||
| Encouragement from family | 29.0 (10.9) | 27.2 (11.2) | 29.4 (9.5) | 28.0 (10.3) |
| Encouragement from friends | 17.3 (8.2) | 19.4 (9.7) | 14.5 (6.3) | 17.1 (8.2) |
| Perceived stress, mean (s.d.) | 3.7 (2.5) | 4.1 (2.8) | 3.4 (2.2) | 4.6 (2.8) |
| Depression score, mean (s.d.) | 2.5 (3.0) | 3.3 (3.7) | 3.1 (3.1) | 4.4 (3.8) |
| Number of times participant previously lost ≥ 10 lbs, % | ||||
| Never | 27 | 9 | 11 | 5 |
| 1–5 times | 66 | 67 | 69 | 61 |
| 6 or more times | 7 | 24 | 20 | 34 |
| Maximum amount of weight loss from previous attempts, % | ||||
| Never tried | 1 | 2 | 5 | 1 |
| ≤20 lbs | 75 | 57 | 50 | 41 |
| 21–40 lbs | 17 | 32 | 32 | 39 |
| >40 lbs | 7 | 9 | 13 | 19 |
| Difference between current weight and “best weight for me,” mean lb (s.d.) |
19.6 (11.5) | 23.5 (11.7) | 20.1 (10.5) | 23.5 (11.8) |
BP, blood pressure; QOL, quality of life.
Coded so that higher discouragement score means less discouragement
Although by definition all phase II participants met the criterion of phase I weight loss of at least 4 kg, phase I weight loss was significantly greater in BM than BW, in WM than in WW, and in both WM and WW than in their black counterparts (Table 2). Following the initial weight loss (i.e., in phase II), mean weight increased in each race–sex group, but remained significantly lower than entry weight (Figure 1). Statistically significant differences among race–sex subgroups were evident at each time-point through 12 months. Percent weight change from entry to end of study ranged from −2.3% (95% confidence interval −3.1 to −1.5) in BW to −4.5% (95% confidence interval −5.7 to −4.0) in WM. Within each race group, men lost more weight than women (BM vs. BW, P = 0.02; WM vs. WW, P < 0.001), but within each sex group, weight loss did not differ significantly between races (BM vs. WM, P =0.25; BW vs. WW, P = 0.06). The data displayed in Table 2 also demonstrate that, despite these differences in weight change from entry to end of study, weight change from randomization to end of study (i.e., during phase II) did not differ significantly across race–sex groups (P = 0.22). Over 65% of participants in each race–sex group ended the study at or below their entry weight, and a substantial proportion in each race–sex group also maintained clinically significant weight loss (i.e., weight ≥5% lower than entry weight) through the end of the study (Table 2). As with percent weight change from entry, these dichotomous outcomes also varied significantly across the four race–sex groups.
Table 2.
Weight outcomes by race–sex subgroup
| Black men | Black women | White men | White women | P valuea | |
|---|---|---|---|---|---|
| Mean (95% CI) % change during phase I | −8.1 (−8.8, −7.3)* | −7.7 (−8.1, −7.2)* | −10.3 (−10.8, −9.8)* | −8.7 (−9.1, −8.3)* | <0.0001 |
| Mean (95% CI) % change from entry to end of study |
−4.0 (−5.2, −2.8)* | −2.3 (−3.1, −1.5)* | −4.5 (−5.7, −4.0)* | −3.3 (−4.0, −2.6)* | 0.0002 |
| Mean (95% CI) % change, from randomization to end of study |
4.8 (3.6, 6.1)* | 6.3 (5.5, 7.1)* | 5.6 (4.7, 6.4)* | 6.1 (5.4, 6.8)* | 0.22 |
| Percentage of participants at or below entry weight at end of study |
71.9 | 65.7 | 78.9 | 68.8 | 0.003 |
| Percentage of participants at least 5% below entry weight at end of study |
34.5 | 28.8 | 48.3 | 36.2 | <0.0005 |
CI, confidence interval.
Three degree-of-freedom P value for comparing race–sex groups based on linear regression (continuous data) or logistic regression (binary data) after adjusting for site, entry weight, and (for % change from randomization).
P ≤ 0.01 that change from entry weight ≠ 0, within subgroup.
Figure 1.
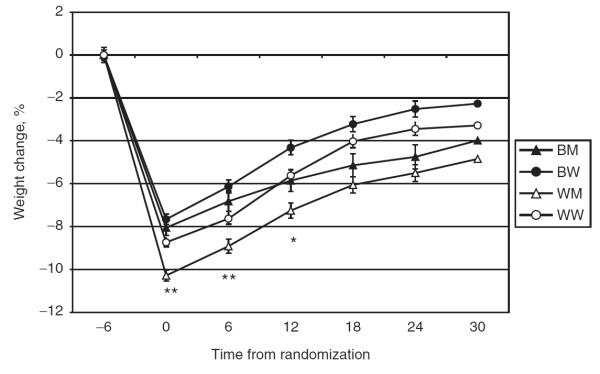
Percent weight change from entry by race–sex subgroup over time. Mean % with s.e. *P = 0.01; **P < 0.0001; P values for comparing race–sex groups at each time-point adjusted for site and entry weight. BM, black men; BW, black women; WM, white men; WW, white women.
Table 3 shows attendance and behavioral measures of intervention adherence for each race–sex subgroup. All subgroups increased their MVPA in phase I, with near complete recidivism in phase II. These changes did not differ significantly across race–sex groups. Dietary changes also showed improvements in phase I, with about half of the improvement reversed in phase II in all groups. In general, whites had greater dietary improvement in phase I, but also greater recidivism in phase II. Participants were asked at the final data collection visit to retrospectively report their frequency of self-weighing during the previous 30 days. Participants reported self-weighing approximately once per week.
Table 3.
Intervention adherence measures
| Black men | Black women | White men | White women | P valuea | ||
|---|---|---|---|---|---|---|
| Attendance at phase I groups, number of groups |
17.5 (0.2) | 17.6 (0.1) | 17.8 (0.1) | 17.9 (0.1) | 0.09 | |
| Food and PA records during phase I, number of weeks records kept |
14.0 (0.4) | 13.8 (0.3) | 15.7 (0.3) | 15.3 (0.2) | ≤0.0001 | |
| Physical activity, MVPA min/week | Phase I entry | 147.1 (10.1) | 85.5 (6.8) | 161.7 (7.0) | 100.3 (5.7) | ≤0.0001 |
| Change during phase I | 37.9 (13.1) | 60.1 (8.9) | 53.9 (9.1) | 34.1 (7.4) | 0.10 | |
| Change during phase II | −29.6 (14.5) | −40.1 (9.7) | −47.9 (10.0) | −32.6 (8.1) | 0.59 | |
| Dietary pattern | ||||||
| Fruits and vegetables, servings/day |
Phase I entry | 4.6 (0.3) | 5.1 (0.2) | 5.2 (0.2) | 5.7 (0.1) | 0.001 |
| Change during phase I | 3.3 (0.4) | 3.1 (0.2) | 3.8 (0.3) | 4.2 (0.2) | 0.01 | |
| Change during phase II | −1.5 (0.4) | −1.8 (0.2) | −1.9 (0.2) | −2.6 (0.2) | 0.02 | |
| Dairy, servings/day | Phase I entry | 0.77 (0.1) | 0.69 (0.1) | 1.4 (0.1) | 1.4 (0.1) | ≤0.0001 |
| Change during phase I | 0.3 (0.1) | 0.4 (0.1) | 0.4 (0.1) | 0.5 (0.1) | 0.38 | |
| Change during phase II | −0.1 (0.1) | −0.2 (0.1) | −0.2 (0.1) | −0.3 (0.1) | 0.06 | |
| %kcal fat | Phase I entry | 38.8 (0.7) | 38.4 (0.4) | 39.3 (0.5) | 38.5 (0.4) | 0.39 |
| Change during phase I | −6.6 (0.7) | −7.3 (0.5) | −9.3 (0.5) | −9.4 (0.4) | 0.001 | |
| Change during phase II | 3.0 (0.7) | 2.8 (0.5) | 4.3 (0.5) | 4.4 (0.4) | 0.03 | |
| Healthy Eating Index | Phase I entry | 59.8 (1.1) | 60.4 (0.7) | 61.9 (0.8) | 64.9 (0.6) | ≤0.0001 |
| Change during phase I | 13.1 (1.1) | 12.4 (0.8) | 15.8 (0.8) | 14.1 (0.6) | 0.01 | |
| Change during phase II | −6.0 (1.0) | −5.8 (0.7) | −5.8 (0.7) | −6.4 (0.6) | 0.77 | |
| Frequency of weighing, times/week | Based on retrospective questionnaire |
2.7 (0.1) | 3.0 (0.1) | 2.4 (0.1) | 2.6 (0.1) | ≤0.0001 |
Mean (s.e.m.).
MVPA, moderate-to-vigorous physical activity; PA, physical activity.
Three degree-of-freedom P value for comparing race–sex groups based on linear regression after adjusting for site.
Table 4 shows the results of multivariate regression modeling to determine associations of demographic, behavioral, psychosocial, and physiological factors present at entry or subsequently with percent weight change. For each subgroup, phase I weight loss was strongly and positively associated with weight loss over the entire 3 years (model 1). Every 1% of weight loss during phase I was associated with 0.66% weight loss over the entire 3-year period, with parameter estimates ranging from 0.57 to 0.85% in the four race–sex subgroups (data not shown; see Supplementary Methods and Procedures for parameter estimates). Unexpectedly, the presence at entry of social support for diet and/or exercise was inversely associated with long-term weight loss in the population overall and in both WM and WW. No other factors present at entry were consistently associated with long-term weight loss.
Table 4.
Variables significantly associated with more weight loss from entry into phase I to end of study
| Model 1. Variables associated with more weight loss, entry into phase I to end of study (model includes phase I weight loss) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Entry | Phase I | ||||||||
|
|
|||||||||
| Model R2 (P value) |
Older age |
Lower entry weight |
Less social support |
Not taking BP meds |
Taking lipid meds |
Higher HEI score |
Lower depression score |
More phase I weight loss |
|
| All | 0.21 (<0.001) | √ | √ | √ | – | – | √ | – | √ |
| Black men | 0.18 (<0.001) | – | – | – | √ | – | – | – | √ |
| Black women | 0.23 (<0.001) | √ | – | – | – | – | – | – | √ |
| White men | 0.27 (<0.001) | – | √ | √ | – | √ | – | – | √ |
| White women | 0.21 (<0.001) | – | – | √ | – | – | √ | √ | √ |
| Model 2. Variables associated with more weight loss, from entry into phase I to end of study (model includes behavior change from entry into phase I to end of study) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry |
Change from entry to end of study |
|||||||
| Model R2 (P value) |
Older age |
male sex |
Less social support |
Higher HEI score |
More education |
Increase in HEI score |
Increase in MVPA | |
| All | 0.11 (<0.001) | √ | √ | √ | √ | – | √ | √ |
| Black men | 0.10 (0.02) | – | – | – | – | – | – | – |
| Black women | 0.08 (<0.001) | √ | – | – | √ | – | √ | – |
| White men | 0.19 (<0.001) | – | – | √ | √ | √ | √ | √ |
| White women | 0.08 (<0.001) | – | – | √ | √ | – | √ | – |
All models are based on backward selection and include the following variables measured at entry into phase I: age, weight, education, income, smoking status, treatment for hypertension and/or dyslipidemia, quality of life, social support for diet and exercise, perceived stress, depression, weight loss history, perceived discrepancy between current weight and desired weight, and participant’s expected weight loss during phase I. Model 1 also includes phase I weight change. Model 2 includes change in physical activity (PA) and change in Healthy Eating Index (HEI) over the entire course of the study (i.e., from phase I entry to 30 months post-randomization), but does not include phase I weight change.
Variables retained in model if P ≤ 0.10 but included in table only if P value in final model ≤0.05 (marked with √).
Coefficients based on 5-imputation datasets.
When we replaced phase I weight loss with changes in diet and MVPA from entry to end of study (model 2), we found that a healthier dietary pattern (either at study entry or improvements in the HEI over time) was predictive of long-term weight loss in the study population overall and in BW, WM, and WW. Increased MVPA over the course of the study predicted improved weight loss only in the group overall and in WM. In this model no factors were predictive of long-term weight loss in BM.
In models performed without imputing missing data (data not shown), predictors were similar, with some exceptions: in the group overall, entry-level HEI was no longer significant in model 1 but remained significant in model 2. In BW, entry-level HEI remained significant in model 2 but change in HEI was no longer significant in this model. In WM, entry-level MVPA became significant in model 1, but change in MVPA became marginally significant (P = 0.06) in model 2. Because of the potential for bias due to nonrandom loss to follow-up, we based our interpretation on the primary analysis with imputation of missing values (above).
Because it was based on retrospective reporting, we did not include frequency of self-weighing in the models. However, in a correlation analysis more self-reported weight monitoring was associated with more weight loss from entry to end of study, with correlation coefficients ranging from 0.21 to 0.25 across the four race–sex subgroups (P < 0.05 for each subgroup).
DISCUSSION
In a large, diverse cohort of adults who successfully lost weight during a 6-month intensive behavioral intervention, initial (phase I) weight loss was a consistent predictor of long-term weight loss in each race–sex group. Beyond initial weight loss, we identified few additional predictors of long-term weight loss either in the group overall or within race–sex subgroups. In the group of BM, no potentially modifiable variables beyond initial weight loss were associated with weight change from entry to end of study. Nonetheless, in each subgroup, over 65% ended the study at entry weight or lower, and over 34% ended at least 5% below entry weight.
The study population is a particular strength of this study, consisting of large numbers of both men and women, blacks and whites. The eligibility criteria defined a population at high risk of CVD events, and thus defined a population who may derive the greatest CVD risk reduction from successful weight loss. The focus on predictors of weight loss maintenance in individuals who successfully lost weight during phase I is an additional strength. Although predictors in this population may not necessarily apply to all high-risk adults who are over-weight/obese and attempt weight loss, they may generalize to the substantial numbers who initiate weight loss and are successful over the short term. Weight regain is common, with presumed reversal of health benefits. Sustained weight control is the ultimate goal of all weight loss interventions.
In BW as well as WM and WW, healthier eating habits (i.e., similar to the DASH dietary pattern (21)) at entry, and improvement in dietary pattern over time, were associated with more weight loss over the entire 3 years, and with less weight regain in phase II. Randomized trials of different dietary patterns in weight loss interventions suggest that in general, diet composition is not a strong determinant of weight loss (30,31). However, in WLM, we stressed using the DASH dietary pattern while restricting calories to lose weight because the DASH dietary pattern also directly improves CVD risk factors independent of weight loss (23,32). The association between higher HEI score, which reflects a dietary pattern similar to DASH (12) and weight loss maintenance suggests that individuals who are adherent to recommendations concerning dietary pattern may also be more adherent to recommendations concerning caloric restriction (33). It also suggests that weight loss can be maintained in the context of a dietary pattern consistent with current dietary guidelines (34).
Surprisingly, MVPA was not related to either long-term weight loss from entry to end of study or weight regain during phase II, except in WM. Self-report data from a registry of successful weight losers suggest that MVPA is a critical factor in long-term weight loss maintenance (35). However, these registry data indicate an association between weight loss maintenance and high levels of MVPA (e.g., 225 min/week), and the majority of WLM participants did not achieve this level of activity. In addition, on average, there was almost complete MVPA recidivism during phase II of WLM, so that MVPA at the end of the study was similar to entry levels. Our data suggest that the general adherence to a higher quality diet even in the setting of lower levels of PA helped to maintain moderate energy balance. However, it is possible that an even greater proportion would have sustained this level of weight loss if increased MVPA had been sustained. Future prospective trials are needed to determine if increased adoption of current PA recommendations leads to more sustained weight loss, and if effects differ based on patient characteristics.
Observed associations between social support and weight loss were complex and inconsistent across race–sex subgroups. If anything, contrary to expectation, our data suggest that entry-level social support from family and friends may have inhibiting effects on long-term weight loss efforts. However, cause and effect cannot be determined from this study. It may be that the social support questionnaires used in this study do not adequately detect nuances of the social interactions that affect long-term behavior change. Alternatively, higher levels of social support at entry may identify people who rely more heavily on social support and who therefore may be more vulnerable to weight regain if the support wanes over time, compared to those with lower levels of social support at entry who nonetheless succeed at initial weight loss. In addition, because our social support measures assessed support for PA and dietary behaviors rather than social support for maintaining weight loss, it may not have been the ideal assessment tool. And finally, in a study of African Americans, Kumanyika et al. reported that involving family/friend partners in a weight loss program was favorable to success only when the family/friend partners also lost weight or attended personal counseling sessions with the participant (36). Gorin et al. reported a similar finding in a primarily white population (37). More research is needed to correctly specify appropriate social support for weight loss maintenance.
Our finding that self-weighing is associated with weight loss maintenance must be interpreted with caution since it is based on retrospective self-report. However, it is consistent with other evidence that self-monitoring in general is associated with greater weight control. For example, in WLM, self-monitoring of diet and MVPA was associated with phase I weight loss (6), and observational studies have suggested a similar effect for self-monitoring of weight. Future research should prospectively establish the effectiveness of self-weighing and the optimal frequency of weight self-monitoring for sustained weight loss.
The absence of significant predictors in BM is likely due to several factors, including the lower power in this relatively small subgroup, which was less than half the size of the other race–sex groups. It is also likely that we did not measure other variables of particular relevance in this subgroup. In addition, there is little data on the reliability and validity of some of our potential predictors in race–sex subgroups, although what little data exist suggest comparable reliability in blacks and whites (16,38–40). Some observational studies have reported better weight loss maintenance in blacks than whites and have identified some predictors that may apply to this subgroup as well as others (41–43). Although observational data provide clues to other potentially useful variables or assessment instruments, it is also possible that predictors of weight loss maintenance in observational studies differ from those for some or all subgroups of clinical trial participants, who by definition meet particular eligibility criteria and are exposed to a particular intervention. It is important to note that although we did not identify predictors of maintenance in BM, they were nonetheless successful at maintaining weight loss over the course of the study, with 71.9% remaining below entry weight and 34.5% remaining at least 5% below entry weight.
Our lack of assessment of either environmental variables (such as food marketing and the built environment) or of family context is a limitation of the study that may have been particularly relevant to blacks. In an ancillary study to WLM, Samuel-Hodge found that family emotional involvement and family cohesion were associated with greater phase I weight loss in blacks but not in whites (44). The role of family in long-term maintenance is unknown. The broader social context may also play a role in all subgroups: the majority of adults in America are overweight or obese (45), and social norms may both influence and be influenced by the prevalence of obesity. (46). For example, the fact that most social interaction is likely to be with other overweight/obese individuals may make weight loss more challenging. Understanding the influence of environmental, family, and social context on weight loss efforts overall and within race–sex subgroups can potentially improve behavioral intervention strategies.
A key finding was that a substantial proportion of each race–sex subgroup maintained a clinically significant amount of weight loss over 3 years. Though the weight loss was modest, this finding is consistent with weight loss in the Lifestyle Intervention arm of the Diabetes Prevention Project (7). Even relatively modest weight loss leads to substantial improvements in cardiovascular risk factors. For example, 5% weight loss in a high-risk population leads to a 58% reduction in diabetes incidence (7), a 42% reduction in hypertension incidence (47), and an estimated 13% reduction in 10-year risk of coronary heart disease (48). Given the high CVD risk of the WLM participants (based on presence of risk factors), the proportion of participants with sustained weight loss suggests the potential for significant reduction in morbidity and mortality.
These results must be generalized with caution: the WLM participants successfully lost at least 4 kg during phase I of the study. Therefore, strictly speaking, the results of this analysis are only generalizable to high-risk adults who have already achieved weight loss. Predictors in this population may or may not generalize to all adults who are overweight/obese. However, large numbers of overweight/obese individuals achieve short-term weight loss, and our results can inform strategies for helping them to sustain that weight loss. However, we recognize that sustaining weight loss over 3 years after initial weight loss, as in WLM, may not be long enough for optimal health outcomes (49).
In summary, this analysis suggests that intervention strategies for achieving and sustaining clinically significant long-term weight loss in diverse populations should focus on achieving greater initial weight loss and eating a healthy diet such as the DASH dietary pattern. Future research should clarify the potential for MVPA and self-monitoring to improve weight loss maintenance, and should identify other modifiable factors that promote long-term weight management in general and in each race–sex subgroup.
Supplementary Material
ACKNOWLEDGMENTS
The Weight Loss Maintenance trial was sponsored by National Heart, Lung, Blood Institute grants 5-U01 HL68734, 5-U01 HL68676, 5-U01 HL68790, 5-U01 HL68920, and 5-HL68955.
Footnotes
SUPPLEMENTARY MATERIAL Supplementary material is linked to the online version of the paper at http://www.nature.com/oby
DISCLOSURE The authors declared no conflict of interest.
REFERENCES
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Wright JT, Jr, Harris-Haywood S, Pressel S, et al. Clinical outcomes by race in hypertensive patients with and without the metabolic syndrome: Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Arch Intern Med. 2008;168:207–217. doi: 10.1001/archinternmed.2007.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lloyd-Jones D, Adams R, Carnethon M, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics–2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 4.Wadden TA, Butryn ML, Wilson C. Lifestyle modification for the management of obesity. Gastroenterology. 2007;132:2226–2238. doi: 10.1053/j.gastro.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 5.Appel LJ, Champagne CM, Harsha DW, et al. Writing Group of the PREMIER Collaborative Research Group Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA. 2003;289:2083–2093. doi: 10.1001/jama.289.16.2083. [DOI] [PubMed] [Google Scholar]
- 6.Hollis JF, Gullion CM, Stevens VJ, et al. Weight Loss Maintenance Trial Research Group Weight loss during the intensive intervention phase of the weight-loss maintenance trial. Am J Prev Med. 2008;35:118–126. doi: 10.1016/j.amepre.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.West DS, Elaine Prewitt T, Bursac Z, Felix HC. Weight loss of black, white, and Hispanic men and women in the Diabetes Prevention Program. Obesity (Silver Spring) 2008;16:1413–1420. doi: 10.1038/oby.2008.224. [DOI] [PubMed] [Google Scholar]
- 8.Kumanyika S. Ethnic minorities and weight control research priorities: where are we now and where do we need to be? Prev Med. 2008;47:583–586. doi: 10.1016/j.ypmed.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Svetkey LP, Stevens VJ, Brantley PJ, et al. Weight Loss Maintenance Collaborative Research Group Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA. 2008;299:1139–1148. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]
- 10.Brantley P, Appel L, Hollis J, et al. Design considerations and rationale of a multi-center trial to sustain weight loss: the Weight Loss Maintenance Trial. Clin Trials. 2008;5:546–556. doi: 10.1177/1740774508096315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Block G, Subar AF. Estimates of nutrient intake from a food frequency questionnaire: the 1987 National Health Interview Survey. J Am Diet Assoc. 1992;92:969–977. [PubMed] [Google Scholar]
- 12.McCullough ML, Feskanich D, Stampfer MJ, et al. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr. 2002;76:1261–1271. doi: 10.1093/ajcn/76.6.1261. [DOI] [PubMed] [Google Scholar]
- 13.Guenther PM, Reedy J, Krebs-Smith SM. Development of the Healthy Eating Index-2005. J Am Diet Assoc. 2008;108:1896–1901. doi: 10.1016/j.jada.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Chen C, Jerome GJ, Laferriere D, Young DR, Vollmer WM. Procedures used to standardize data collected by RT3 triaxial accelerometers in a large-scale weight-loss trial. J Phys Act Health. 2009;6:354–359. doi: 10.1123/jpah.6.3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jerome GJ, Young DR, Laferriere D, Chen C, Vollmer WM. Reliability of RT3 accelerometers among overweight and obese adults. Med Sci Sports Exerc. 2009;41:110–114. doi: 10.1249/MSS.0b013e3181846cd8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sallis JF, Grossman RM, Pinski RB, Patterson TL, Nader PR. The development of scales to measure social support for diet and exercise behaviors. Prev Med. 1987;16:825–836. doi: 10.1016/0091-7435(87)90022-3. [DOI] [PubMed] [Google Scholar]
- 17.Ware JE. SF-36 Health Survey: Manual and Interpretation Guide. The Health Institute, New England Medical Center; Boston, MA: 1993. [Google Scholar]
- 18.Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med. 2002;64:258–266. doi: 10.1097/00006842-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Rohyans LM, Pressler SJ. Depressive symptoms and heart failure: examining the sociodemographic variables. Clin Nurse Spec. 2009;23:138–144. doi: 10.1097/NUR.0b013e3181a443b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 21.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 22.Sacks FM, Svetkey LP, Vollmer WM, et al. DASH-Sodium Collaborative Research Group Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 23.Harsha DW, Sacks FM, Obarzanek E, et al. Effect of dietary sodium intake on blood lipids: results from the DASH-sodium trial. Hypertension. 2004;43:393–398. doi: 10.1161/01.HYP.0000113046.83819.a2. [DOI] [PubMed] [Google Scholar]
- 24.Appel LJ, Sacks FM, Carey VJ, et al. OmniHeart Collaborative Research Group Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA. 2005;294:2455–2464. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- 25.Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Prentice Hall; Englewood Cliffs, NJ: 1986. [Google Scholar]
- 26.Prochaska JM, Prochaska JO, Levesque DA. A transtheoretical approach to changing organizations. Adm Policy Ment Health. 2001;28:247–261. doi: 10.1023/a:1011155212811. [DOI] [PubMed] [Google Scholar]
- 27.The effects of nonpharmacologic interventions on blood pressure of persons with high normal levels. Results of the Trials of Hypertension Prevention, Phase I. JAMA. 1992;267:1213–1220. doi: 10.1001/jama.1992.03480090061028. [DOI] [PubMed] [Google Scholar]
- 28.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- 30.Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA. 2005;293:43–53. doi: 10.1001/jama.293.1.43. [DOI] [PubMed] [Google Scholar]
- 31.Gardner CD, Kiazand A, Alhassan S, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA. 2007;297:969–977. doi: 10.1001/jama.297.9.969. [DOI] [PubMed] [Google Scholar]
- 32.Svetkey LP, Simons-Morton D, Vollmer WM, et al. Effects of dietary patterns on blood pressure: subgroup analysis of the Dietary Approaches to Stop Hypertension (DASH) randomized clinical trial. Arch Intern Med. 1999;159:285–293. doi: 10.1001/archinte.159.3.285. [DOI] [PubMed] [Google Scholar]
- 33.Young DR, Vollmer WM, King AC, et al. Can individuals meet multiple physical activity and dietary behavior goals? Am J Health Behav. 2009;33:277–286. doi: 10.5993/ajhb.33.3.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicklas TA, Weaver C, Britten P, Stitzel KF. The 2005 Dietary Guidelines Advisory Committee: developing a key message. J Am Diet Assoc. 2005;105:1418–1424. doi: 10.1016/j.jada.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 35.Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005;82:222S–225S. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]
- 36.Kumanyika SK, Wadden TA, Shults J, et al. Trial of family and friend support for weight loss in African American adults. Arch Intern Med. 2009;169:1795–1804. doi: 10.1001/archinternmed.2009.337. [DOI] [PubMed] [Google Scholar]
- 37.Gorin A, Phelan S, Tate D, et al. Involving support partners in obesity treatment. J Consult Clin Psychol. 2005;73:341–343. doi: 10.1037/0022-006X.73.2.341. [DOI] [PubMed] [Google Scholar]
- 38.Young DR, Stewart KJ. A church-based physical activity intervention for African American women. Fam Community Health. 2006;29:103–117. doi: 10.1097/00003727-200604000-00006. [DOI] [PubMed] [Google Scholar]
- 39.McHorney CA, Ware JE, Jr, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32:40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 40.John EW, Snow K, Kosinski K, Gandek B. SF-36 Health Survey Manual and Interpretation Guide. New England Medical Center, The Health Institute; Boston: 1993. [Google Scholar]
- 41.Kruger J, Blanck HM, Gillespie C. Dietary practices, dining out behavior, and physical activity correlates of weight loss maintenance. Prev Chronic Dis. 2008;5 A11. [PMC free article] [PubMed] [Google Scholar]
- 42.Phelan S, Liu T, Gorin A, et al. What distinguishes weight-loss maintainers from the treatment-seeking obese? Analysis of environmental, behavioral, and psychosocial variables in diverse populations. Ann Behav Med. 2009;38:94–104. doi: 10.1007/s12160-009-9135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phelan S, Wing RR, Loria CM, Kim Y, Lewis CE. Prevalence and predictors of weight-loss maintenance in a biracial cohort: results from the coronary artery risk development in young adults study. Am J Prev Med. 2010;39:546–554. doi: 10.1016/j.amepre.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samuel-Hodge CD, Gizlice Z, Cai J, et al. Family functioning and weight loss in a sample of african americans and whites. Ann Behav Med. 2010;40:294–301. doi: 10.1007/s12160-010-9219-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 46.Christakis NA, Fowler JH. The spread of obesity in a large social network over 32 years. N Engl J Med. 2007;357:370–379. doi: 10.1056/NEJMsa066082. [DOI] [PubMed] [Google Scholar]
- 47.Stevens VJ, Obarzanek E, Cook NR, et al. Trials for the Hypertension Prevention Research Group Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med. 2001;134:1–11. doi: 10.7326/0003-4819-134-1-200101020-00007. [DOI] [PubMed] [Google Scholar]
- 48.Maruthur NM, Wang NY, Appel LJ. Lifestyle interventions reduce coronary heart disease risk: results from the PREMIER Trial. Circulation. 2009;119:2026–2031. doi: 10.1161/CIRCULATIONAHA.108.809491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Initiative NOE . Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in adults: The Evidence Report. US Department of Health and Human Services; Bethesda: 1998. Report No.: NIH Publication No. 98–4083. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


