Abstract
Inflammatory myofibroblastic tumor of the urinary bladder is a rare mesenchymal tumor with uncertain malignant potential. It often mimics soft tissue sarcomas both clinically and radiologically. Surgical resection in the form of partial cystectomy or transurethral resection remains the mainstay of treatment. Herein we report the case of an inflammatory myofibroblastic tumor in a young girl, which was managed by laparoscopic partial cystectomy. To the best of our knowledge, this is the first reported case of laparoscopic management of an inflammatory myofibroblastic tumor of the urinary bladder.
Keywords: Cystectomy, Laparoscopy, Plasma cell granuloma, Urinary bladder
INTRODUCTION
Inflammatory myofibroblastic tumor (IMT) is a rare spindle cell tumor of unknown malignant potential that often masquerades as a soft tissue malignancy both clinically and radiologically. As such, preoperative differentiation from sarcomas is of utmost importance to avoid radical surgeries. The lung is the most common site, although IMT has been reported in the head and neck, gastrointestinal tract, retroperitoneum, breast, and central nervous system [1]. In the genitourinary tract, the urinary bladder is the most common site, although IMT has been reported in the kidney, ureter, prostate, urethra, uterus, and paratesticular tissue [1].
Herein we present the laparoscopic management of a case of IMT of the urinary bladder. Laparoscopic partial cystectomy (LPC) for IMT of the bladder has not yet been reported.
CASE REPORT
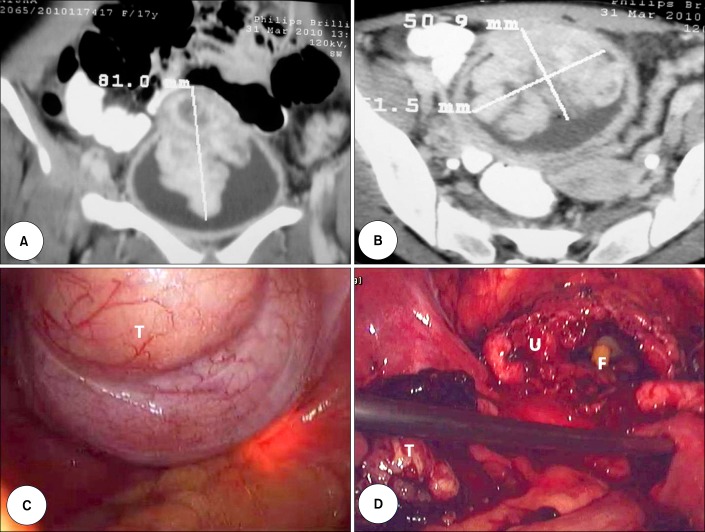
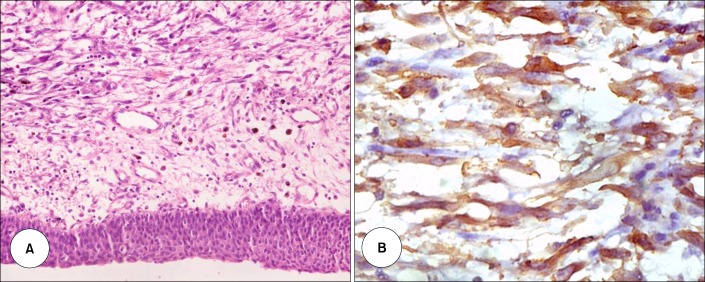
A 17-year-old unmarried nonsmoking female presented with burning micturition, increasing urinary frequency, suprapubic pain, and occasional hematuria for the past 1 year. The patient had no history of fever, pyuria, or flank pain. The results of general and systemic physical examinations were unremarkable. The results of routine hemogram and blood chemistry profiles were within normal limits. Ultrasonography of the abdomen and pelvis revealed a large heteroechoic mass arising from the dome and anterior wall of the urinary bladder. Contrast-enhanced computerized tomographic scans revealed a large, eccentric lobulated heterogeneously enhancing mass sized 8 cm×5 cm×5 cm and arising in the midline from the dome and anterior wall of the urinary bladder (Fig. 1A, B). Cystoscopy showed a broad-based, lobulated growth arising from the dome and anterior bladder wall. Biopsy of the lesion revealed oval to spindle-shaped cells accompanied by lymphocytes, neutrophils, and plasma cells on a myxoid stroma. The spindle cells had a high nuclear: cytoplasm ratio, a scant to moderate amount of eosinophilic cytoplasm, and oval to elongated hyperchromatic nuclei with frequent mitoses. Immunohistochemistry was positive for vimentin, smooth muscle actin, muscle-specific actin, cytokeratin, and anaplastic lymphoma kinase-1 (ALK-1) and negative for desmin (Fig. 2). A diagnosis of IMT was made and LPC was planned.
FIG. 1.
Coronal (A) and cross-sectional (B) view of the contrast-enhanced computerized tomographic scan of the pelvis showing the bladder mass. (C) Laparoscopic view of the urinary bladder with the tumor mass (T). The tumor outline was defined by cystoscopic-guided transillumination. (D) Laparoscopic view of the urinary bladder (U) after resection of the tumor mass (T). The tip of the Foley catheter (F) can be seen inside the bladder.
FIG. 2.
(A) Tumor composed of oval to spindle-shaped cells having a high nucleocytoplasmic ratio, prominent nucleoli, and a moderate amount of eosinophilic cytoplasm along with unremarkable urothelial lining epithelium and increased mitotic figures (H&E, ×400). (B) Anaplastic lymphoma kinase immunohistochemistry revealed strong cytoplasmic staining of the myofibroblasts (×400).
The bowel was prepared 1 day before surgery. A standard transperitoneal four-port technique was used with the patient in a modified lithotomy and 20-degree Trendelenburg position. First, a 12-mm camera port was placed 3 cm above the umbilicus. Two working ports 10 mm on the right side and 5 mm on the left side were placed at the midclavicular line 2 cm below the level of the umbilicus. Another 5-mm port was placed midway between the umbilicus and the symphysis pubis. The bladder was mobilized starting from just above the peritoneal reflection on the anterior abdominal wall and was extended down on both sides in an inverted-V shaped manner. Dissection was carried down to Retzius' space with mobilization of the bladder from all sides. With simultaneous cystoscopic guidance and transillumination, the tumor outline was defined (Fig. 1C). Cystotomy was done about 2 cm away from the tumor margin and was carried circumferentially around the tumor leaving a 2-cm healthy margin (Fig. 1D). The bladder was closed in two layers with 2-0 polyglactin suture and the specimen was retrieved in an EndoCatch bag (USCC, Norwalk, CT, USA). A Foley catheter and tube drain were placed in the bladder and Retzius' space, respectively. Operative time was 130 minutes and blood loss was 200 mL with no intraoperative complications. The drain was removed on the second postoperative day and the patient was discharged on the fourth day. The catheter was removed on the 10th postoperative day after a cystogram revealed no leaks.
Owing to the uncertain malignant potential of the IMT, the patient was kept on 3 monthly cystoscopic surveillance and has been free from any recurrence after 22 months of surgery.
DISCUSSION
From the time of its first description by Brunn in lungs in 1939, IMT has remained an enigma both for clinicians and pathologists. Genitourinary IMT is a rare entity that was first described by Roth [2] in 1980 as "an unusual pseudosarcomatous entity" in the bladder of a female. It has been referred to by various names, such as inflammatory pseudotumor, plasma cell pseudotumor, xanthomatous pseudotumor, pseudosarcomatous myofibroblastic proliferation, inflammatory myofibrohistiocytic proliferation, atypical fibromyxoid tumor, and atypical myofibroblastic tumor [1]. IMT is mostly seen in teens and young adults, with males affected more often than females, although children and elderly individuals have also been reported with the disease [3,4]. Vesical IMT most commonly presents as mild to moderate hematuria along with variable degrees of lower urinary tract symptoms. Other complaints include dysuria, frequency, suprapubic pain, or the discovery of a mass lesion. The reported sizes vary widely, ranging from a few centimeters up to 37.5 cm [5]. Constitutional symptoms are rare with IMT of the genitourinary tract, although they are found with such tumors at other body sites, especially the lungs. The etiology of IMT remains controversial though infective (bacterial and viral), autoimmune, and genetic mechanisms have been implicated [1].
Differential diagnosis of IMT includes benign diseases such as leiomyoma, postoperative spindle cell nodule, nodular fasciitis, and neurofibroma and malignant diseases such as leiomyosarcoma, embryonal rhabdomyosarcoma, and sarcomatoid carcinoma. Immunohistochemistry remains the cornerstone for diagnosis of IMT with positive stain for vimentin (95% to 100%), desmin (5% to 80%), smooth muscle actin (48% to 100%), muscle-specific actin (62%), and keratin (10% to 89%) and negative stains for epithelial membrane antigen, myogenin, p53, and h-caldesmon [4,6-8]. ALK stains positive in approximately half of these tumors and is a promising marker for differentiation of IMTs from other lesions [1].
Owing to the predominantly benign course of the tumor, vesical IMT is usually treated by transurethral resection or partial cystectomy, although radical cystectomy has also been reported [9]. Rare malignant transformation has been seen but no metastasis has been reported [9]. Because a significant recurrence rate of 10% to 25% has been reported with genitourinary IMT, routine cystoscopic surveillance and close clinical follow-up are necessary [3].
LPC was first described by Nezhat and Nezhat [10] in 1993 and has since been used mainly in cases of bladder endometriosis, pheochromocytoma, urothelial carcinoma, and urachal adenocarcinoma. It has the advantage of rapid recovery, decreased postoperative morbidity, and better cosmetic outcome compared with its open counterpart. Simultaneous cystoscopic guidance helps in precise delineation of the tumor margin. Although bladder capacity decreases to a variable extent after LPC, most of the patients have normal or marginally increased urinary frequency. Our patient had normal daytime and nighttime frequency after surgery.
Footnotes
The authors have nothing to disclose.
References
- 1.Cheng L, Foster SR, MacLennan GT, Lopez-Beltran A, Zhang S, Montironi R. Inflammatory myofibroblastic tumors of the genitourinary tract: single entity or continuum. J Urol. 2008;180:1235–1240. doi: 10.1016/j.juro.2008.06.049. [DOI] [PubMed] [Google Scholar]
- 2.Roth JA. Reactive pseudosarcomatous response in urinary bladder. Urology. 1980;16:635–637. doi: 10.1016/0090-4295(80)90578-6. [DOI] [PubMed] [Google Scholar]
- 3.Montgomery EA, Shuster DD, Burkart AL, Esteban JM, Sgrignoli A, Elwood L, et al. Inflammatory myofibroblastic tumors of the urinary tract: a clinicopathologic study of 46 cases, including a malignant example inflammatory fibrosarcoma and a subset associated with high-grade urothelial carcinoma. Am J Surg Pathol. 2006;30:1502–1512. doi: 10.1097/01.pas.0000213280.35413.1b. [DOI] [PubMed] [Google Scholar]
- 4.Coffin CM, Humphrey PA, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor: a clinical and pathological survey. Semin Diagn Pathol. 1998;15:85–101. [PubMed] [Google Scholar]
- 5.Watanabe K, Baba K, Saito A, Hoshi N, Suzuki T. Pseudosarcomatous myofibroblastic tumor and myosarcoma of the urogenital tract. Arch Pathol Lab Med. 2001;125:1070–1073. doi: 10.5858/2001-125-1070-PMTAMO. [DOI] [PubMed] [Google Scholar]
- 6.Emerson RE, Cheng L. Immunohistochemical markers in the evaluation of tumors of the urinary bladder: a review. Anal Quant Cytol Histol. 2005;27:301–316. [PubMed] [Google Scholar]
- 7.Iczkowski KA, Shanks JH, Gadaleanu V, Cheng L, Jones EC, Neumann R, et al. Inflammatory pseudotumor and sarcoma of urinary bladder: differential diagnosis and outcome in thirty-eight spindle cell neoplasms. Mod Pathol. 2001;14:1043–1051. doi: 10.1038/modpathol.3880434. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe K, Kusakabe T, Hoshi N, Saito A, Suzuki T. h-Caldesmon in leiomyosarcoma and tumors with smooth muscle cell-like differentiation: its specific expression in the smooth muscle cell tumor. Hum Pathol. 1999;30:392–396. doi: 10.1016/s0046-8177(99)90113-2. [DOI] [PubMed] [Google Scholar]
- 9.Harik LR, Merino C, Coindre JM, Amin MB, Pedeutour F, Weiss SW. Pseudosarcomatous myofibroblastic proliferations of the bladder: a clinicopathologic study of 42 cases. Am J Surg Pathol. 2006;30:787–794. doi: 10.1097/01.pas.0000208903.46354.6f. [DOI] [PubMed] [Google Scholar]
- 10.Nezhat CR, Nezhat FR. Laparoscopic segmental bladder resection for endometriosis: a report of two cases. Obstet Gynecol. 1993;81:882–884. [PubMed] [Google Scholar]