Abstract
While fine needle aspiration (FNA) is certainly not a new biopsy technique, recent developments in advanced imaging techniques, molecular testing, and targeted therapies have coincided with a rapid increase in the number of FNA procedures being performed. Concurrently, the demand for on-site evaluation of adequacy (OSEA) has also increased, outstripping the capacity of available cytopathologists at some institutions. Among the several alternatives to cytopathologist-performed OSEA, cytotechnologist-attended OSEA stands out because it preserves the representation of the pathology service at the time of the procedure. Herein, we review the current literature about OSEA and the necessity of cytotechnologists to expand access of this useful pathology service to a broader patient population. We also examine how cytotechnologists are likely to fit into the emerging practice of telecytology.
Keywords: Biopsy, fine needle aspiration; Rapid on-site evaluation; On-site evaluation of adequacy; Cytotechnology; Telepathology; Telecytology
Fine-needle aspiration (FNA) is a well-established and widely used biopsy technique for the initial tissue diagnosis of many diseases. FNA is minimally invasive and usually safe. For this reason, it is the sampling method of choice for tumors in a variety of sites in the human body, including the head and neck, thyroid, liver, pancreas, bone, soft tissue, lung, and mediastinum.1-5 While FNA is certainly not new, parallel developments in advanced imaging techniques, molecular testing, and targeted therapies have coincided with a rapid increase in the number of FNA procedures. For example, endoscopic ultrasound (EUS)-guided FNA now provides adequate material to diagnose, characterize, and stage pancreatic, biliary and ampullary cancers without the need for diagnostic abdominal surgery.6-8 Similarly, endobronchial ultrasound (EBUS)-guided FNA has replaced mediastinoscopy as the method of choice for staging lung cancer.9
In addition to being less invasive, radiologically advanced sampling techniques that involve FNA are also cost-effective compared with their more invasive alternatives.10 However, their costs are non-trivial, and the expenses involved have required an increased emphasis on ensuring sample adequacy at many institutions. Several strategies proposed to ensure adequacy have included the upfront collection of a large number of passes11,12 and the non-microscopic examination of the direct smear.13-15 Alternatively, an on-site evaluation of adequacy (OSEA, also known as rapid on-site evaluation, or ROSE) has been used as a more consistent and potentially safer option. Traditionally, OSEA involved the physical presence of a cytopathologist at the procedure,16,17 although cytotechnologists have been shown to be capable of providing this service with equivalent accuracy.18-20 While telecytology is gaining popularity,21 this technology only partially solves the problem of increased cytopathologist demand; an individual capable of microscopy must still be present in or near the procedure room to prepare the direct smear and operate the microscope. Additionally, given the pauci-cellularity of many specimens and the low resolution of currently available real-time telepathology solutions, the microscope driver should also ideally possess diagnostic capabilities in order to maximize efficiency. Thus, with or without telecytology, cytotechnologists appear well-suited to meet the demands for OSEA of FNA biopsy procedures. In this article, we provide a brief review of the literature regarding OSEA and outline key areas in which cytotechnologists are necessary in order to meet the increasing demand of this useful pathology service.
OSEA IN PERSPECTIVE
Ultimately, the ability to make a pathologic diagnosis of either a malignant or benign lesion relies on the ability to obtain adequate cellular material during a procedure. Previous studies have shown that OSEA enhances the accuracy and efficiency of EUS-FNA.3,22,23 At institutions without immediate OSEA, up to 32% of FNAs in organs such as the thyroid, breast and lung are non-diagnostic due to scant cellularity and poor smear preparation.24,25 In a single institutional study, Iglesias-Garcia et al.26 showed that the introduction of OSEA decreased the number of inadequate samples from 12.6% to 1% (p=.002), increased their diagnostic sensitivity from 78.2% to 96.2% (p=.002) and increased their overall accuracy from 86.2% to 96.8% (p=.013).26 Chang et al.27 reported that OSEA during EUS-guided FNA of pancreatic lesions resulted in up to 100% adequacy, while 29% of procedures without OSEA required a second procedure to obtain an adequate specimen. Klapman et al.3 reported similar findings. Erickson et al.28 showed that OSEA for EUS-guided FNA of the pancreas increased the number of diagnostic cases by 10% to 15%. They also reported that OSEA resulted in a significant reduction in the number of needle passes in about one-third of cases.28 While OSEA almost uniformly increases adequacy rates, it does not consistently reduce the number of passes; in a different article, OSEA improved adequacy rates for EUS-guided FNA of pancreatic lesions independently of the number of passes, indicating that continuous feedback allows for prompt sampling of the diagnostic portion of the lesion.29 Similar reductions in additional procedures have been demonstrated for OSEA of EBUS-guided FNA for staging lung cancer.30
The two strongest studies arguing against OSEA both have arisen out of thyroid FNA. One institutional experience showed statistically insignificant adequacy gains for OSEA,31 and one cost-benefit analysis revealed that OSEA was only cost-effective on an individual level if the institutional adequacy rate was less than 85%.32 Even if taken at face value, these would not be compelling enough to discontinue OSEA programs because other studies have shown that small adequacy gains translate into tangible and significant savings on an institutional level.33,34 Cost-benefit analyses at the institutional level are the most relevant to consider given the increasing shift of healthcare expense management to accountable care organizations. Additionally, both of the studies arguing against OSEA involved thyroid sampling procedures, which usually have high adequacy rates; other organs have a priori adequacy rates that are significantly lower. Finally, the OSEA process involves non-adequacy benefits, including proper specimen preservation and procurement of tissue for appropriate ancillary testing based on informal preliminary diagnoses.19,20,33 These benefits have yet to be analyzed with cost-effectiveness modeling, and the multiplicity of diseases that can be encountered during procedures of the same organ make controlled trials impractical to perform.
CYTOTECHNOLOGIST-ATTENDED OSEA
Despite the compelling results for OSEA, many institutions do not have sufficient cytopathologist coverage to support its routine practice. The lack of availably of these professionals has led to the use of practitioners in other medical specialties for OSEA, often with suboptimal results.23,35 Thus, a clear opportunity exists for cytotechnologists to participate in this pathology service. The initial role of cytotechnologists in OSEA involved preparing direct and indirect specimens onsite with minimal diagnostic interpretation.36 This role has evolved over time so that cytotechnologists now perform OSEA in large academic centers without direct cytopathologist supervision.18-20 As cytotechnology training requires expertise in cellular morphology and attendance at OSEA, the participation of cytotechnologists as the primary onsite screener in OSEA is not unreasonable. Additionally, cytotechnologists play a significant role in the subsequent diagnostic process and therefore have a vested interest in specimen adequacy and integrity.
Many studies have shown the benefits of cytotechnologist performance of OSEA; these studies are summarized in Table 1. Cleveland et al.37 reported an association between cytotechnologist-attended OSEA with an increase in adequacy that was disproportionate to all other factors in their analysis, including needle gauge, number of passes, endoscopist, and biopsy site. One small study demonstrated a lack of difference with and without a cytotechnologist present for OSEA in EUS-guided FNA of the pancreas; however, this study lacked an adequate number of patients to assess clinically relevant differences.38 A larger study with more substantive data by Redman et al.16 showed that cytotechnologists were accurate in 93% of EUS-guided pancreas FNA as compared to the accuracy of cytopathologists, which was 97% (p=.0015). These results were contradicted by an equivalently small study that found that cytotechnologist-attended OSEA significantly improved the accuracy of EUS-FNA.29 Additionally, Petrone et al.39 reported that the diagnostic accuracy of a cytotechnologist can improve with advanced training in histology from a cytopathologist. A much larger retrospective study at our institution revealed that the accuracy of the cytotechnologist-attended OSEA of thyroid FNA biopsy (96%) is similar to that of the cytopathologist (95%); these accuracies are independent of experience.20 This publication also reported that the final adequacy was greaer than the OSEA adequacy for cytotechnologist-attended than for cytopathologist-attended OSEA: 26% versus 17%, p<.001. This latter finding was explained by the shift of OSEA from cytopathologists to cytotechnologists; this disparity in overall accuracy is likely related to operator variables and institutional experience with EUS-guided FNA.
Table 1.
Cytotechnologists in on-site evaluation of adequacy (OSEA)
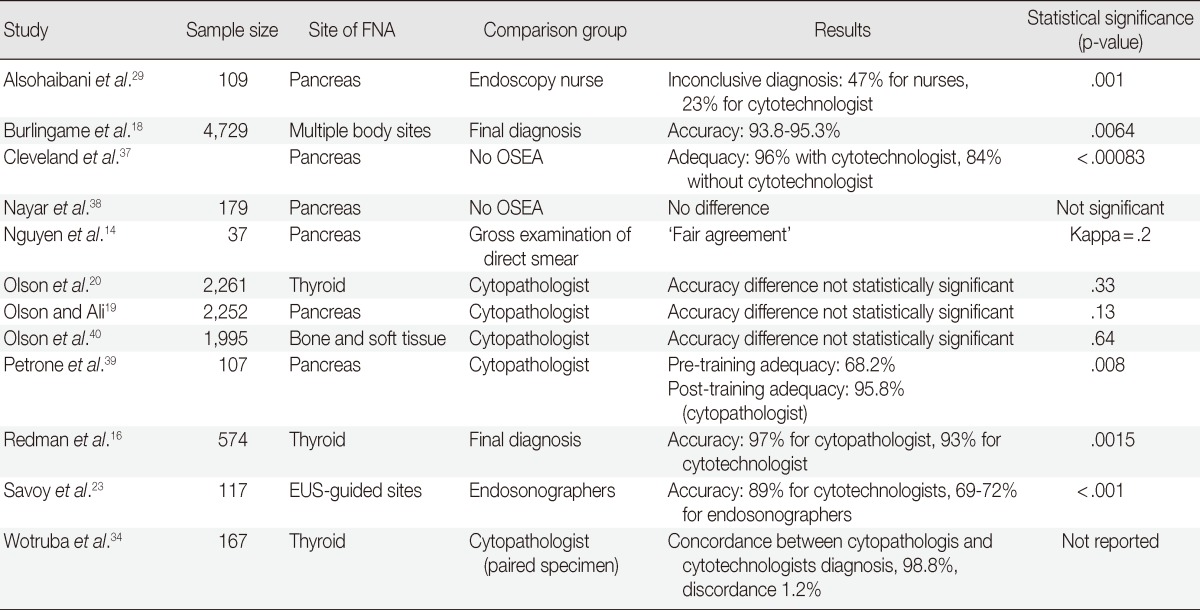
FNA, fine-needle aspiration; EUS, endoscopic ultrasound.
In one large retrospective analysis that included all body site FNAs, it was reported that cytotechnologist-performed OSEA had an acceptable level of adequacy regardless of body site or level of experience.18 This finding was correlated with our own institutional experiences in which we demonstrated through multiple studies that, regardless of body site, pancreas,19 thyroid,20 and non-sarcoma metastases found in the bone and soft tissue.40 OSEA is also important because it places a pathology representative in the procedure room to ensure proper tissue procurement, a detail that is extraordinarily important in cytopathology given the scant nature of most specimens. In a study by Alsohaibani et al.,29 there were 17% fewer crush artifacts and 24% fewer inconclusive diagnoses in the specimens prepared by the cytotechnologists relative to those prepared by endoscopic nurses. At our own institution, we showed that cytotechnologists can appropriately encourage the preservation of tissue for flow cytometric studies in lymphoma.40 Together, these studies demonstrated a cytotechnologist's capacity to independently utilize good specimen handling protocols on-site.
THE ECONOMICS OF CYTOTECHNOLOGIST-PERFORMED OSEA
There is a strong economic motivation for institutions to utilize cytotechnologists for OSEA assessments. The largest incentive is to avoid the absence of a pathologist for extended and unpredictable amounts of time. This absence can have a major impact on the number of cases signed out and also on the turn-around-time. These delays ultimately may have a tangible negative effect on the department or practice's billing potential and also an intangible effect on its reputation for making timely diagnoses, particularly for community hospitals, which have a more limited number of practicing pathologists. Furthermore, physician compensation for OSEA from Medicaid reimbursement fees is well below the professional fee required to cover the pathologist's time per procedure. In one study, the time expenditure on FNA adequacy ranged from 35 to 56 minutes and resulted in a revenue loss of up to $50 per procedure, which occurred at the expense of attaining additional revenue from performing other diagnostic services.41 Eedes and Wang11 also reported inefficient time expenditure, showing that it cost 220 minutes of a cytologist's time for each additional adequate case.
If laboratories could bill a reasonable fee for diverting cytotechnologists to OSEA duties, the economics of cytotechnologist-performed OSEA would be straightforward. However, there is currently no mechanism for billing cytotechnologist-attended OSEA, so the costs are borne at the institutional level in order to increase adequacy and reduce the need for re-biopsy. This procedure makes most sense in accountable care organizations that try to contain costs by billing per disorder rather than per procedure. In a large review, OSEA saved an institution approximately $404,525/yr as a result of decreasing repeat FNAs.33 A prospective study has also shown that using a cytotechnologist instead of a cytopathologist for OSEA procedures in a thyroid clinic provided the laboratory a cost savings of $464.10/nodule.34
TELECYTOLOGY AND CYTOTECHNOLOGISTS
One of the ways in which laboratories have demonstrated cost-effective use of cytotechnologists OSEA is through telecytology.34 Ideally, the time-intensive portions of the OSEA could be performed by the cytotechnologist with a billable adequacy assessment made remotely by a cytopathologist.42 Telecytology OSEAs have been shown to be equivalent to physical OSEAs in a number of contexts.43-46 However, current limitations in internet bandwidth hinder live streaming of high-resolution video feeds over an internet connection. In some practice settings, bandwidth may only be sufficient for low-resolution images. Additionally, technical malfunctions should be expected, and a cytopathologist with remote OSEA responsibilities in numerous locations may not be available to physically appear at a technically malfunctioning telecytology workstation in a timely fashion in order to assess adequacy. Thus, in order for telecytology to work effectively, the on-site cytology professional handling the tissue and operating the microscope should also be a diagnostic provider. With the exception of pathology trainees, cytotechnologists are the only logical members of the laboratory team who can fill this role.
CONCLUSIONS
As we have discussed, a growing number of practices and academic investigations have shown cytotechnologists to be an invaluable component of the OSEA service of a pathology laboratory. To date, there has been no randomized, controlled clinical trial to demonstrate the usefulness of cytotechnologists in this new role; there are also no published regulations, competencies, or proficiency testing mechanisms for these new duties. Therefore, cytotechnologist OSEA is currently an evolving field in which each laboratory must determine its own comfort level and financial exposure. The existing evidence strongly suggests that, if these obstacles can be overcome, cytotechnologists will be able to perform at a high level of competency and thus address the expanding utilization of OSEA in FNA procedures in this minimally invasive medical era. This new role will also ensure the preservation of the cytotechnology field in an era when most institutions are experiencing a decreasing gynecologic smear volume.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Harewood GC, Pascual J, Raimondo M, et al. Economic analysis of combined endoscopic and endobronchial ultrasound in the evaluation of patients with suspected non-small cell lung cancer. Lung Cancer. 2010;67:366–371. doi: 10.1016/j.lungcan.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khalbuss WE, Teot LA, Monaco SE. Diagnostic accuracy and limitations of fine-needle aspiration cytology of bone and soft tissue lesions: a review of 1,114 cases with cytological-histological correlation. Cancer Cytopathol. 2010;118:24–32. doi: 10.1002/cncy.20058. [DOI] [PubMed] [Google Scholar]
- 3.Klapman JB, Logrono R, Dye CE, Waxman I. Clinical impact of on-site cytopathology interpretation on endoscopic ultrasound-guided fine needle aspiration. Am J Gastroenterol. 2003;98:1289–1294. doi: 10.1111/j.1572-0241.2003.07472.x. [DOI] [PubMed] [Google Scholar]
- 4.Rimm DL, Stastny JF, Rimm EB, Ayer S, Frable WJ. Comparison of the costs of fine-needle aspiration and open surgical biopsy as methods for obtaining a pathologic diagnosis. Cancer. 1997;81:51–56. doi: 10.1002/(sici)1097-0142(19970225)81:1<51::aid-cncr11>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 5.Ylagan LR, Edmundowicz S, Kasal K, Walsh D, Lu DW. Endoscopic ultrasound guided fine-needle aspiration cytology of pancreatic carcinoma: a 3-year experience and review of the literature. Cancer. 2002;96:362–369. doi: 10.1002/cncr.10759. [DOI] [PubMed] [Google Scholar]
- 6.Eloubeidi MA, Cerfolio RJ, Bryant AS, Varadarajulu S. Efficacy of endoscopic ultrasound in patients with esophageal cancer predicted to have N0 disease. Eur J Cardiothorac Surg. 2011;40:636–641. doi: 10.1016/j.ejcts.2010.12.054. [DOI] [PubMed] [Google Scholar]
- 7.Eloubeidi MA, Buxbaum JL. Improving endoscopic ultrasound-guided fine needle aspiration specimens in the absence of rapid onsite evaluation: does cytotechnologist training provide the solution? Dig Liver Dis. 2012;44:273–274. doi: 10.1016/j.dld.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Olson MT, Siddiqui MT, Ali SZ. The differential diagnosis of squamous cells in pancreatic aspirates: from contamination to adenosquamous carcinoma. Acta Cytol. 2013;57:139–146. doi: 10.1159/000346326. [DOI] [PubMed] [Google Scholar]
- 9.Block MI. Transition from mediastinoscopy to endoscopic ultrasound for lung cancer staging. Ann Thorac Surg. 2010;89:885–890. doi: 10.1016/j.athoracsur.2009.11.034. [DOI] [PubMed] [Google Scholar]
- 10.Eloubeidi MA, Tamhane A, Jhala N, et al. Agreement between rapid onsite and final cytologic interpretations of EUS-guided FNA specimens: implications for the endosonographer and patient management. Am J Gastroenterol. 2006;101:2841–2847. doi: 10.1111/j.1572-0241.2006.00852.x. [DOI] [PubMed] [Google Scholar]
- 11.Eedes CR, Wang HH. Cost-effectiveness of immediate specimen adequacy assessment of thyroid fine-needle aspirations. Am J Clin Pathol. 2004;121:64–69. doi: 10.1309/XLND-TE28-9WAQ-YK0Y. [DOI] [PubMed] [Google Scholar]
- 12.Rossi ED, Morassi F, Santeusanio G, Zannoni GF, Fadda G. Thyroid fine needle aspiration cytology processed by ThinPrep: an additional slide decreased the number of inadequate results. Cytopathology. 2010;21:97–102. doi: 10.1111/j.1365-2303.2009.00659.x. [DOI] [PubMed] [Google Scholar]
- 13.Mayall F, Cormack A, Slater S, McAnulty K. The utility of assessing the gross appearances of FNA specimens. Cytopathology. 2010;21:395–397. doi: 10.1111/j.1365-2303.2009.00733.x. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen YP, Maple JT, Zhang Q, et al. Reliability of gross visual assessment of specimen adequacy during EUS-guided FNA of pancreatic masses. Gastrointest Endosc. 2009;69:1264–1270. doi: 10.1016/j.gie.2008.08.030. [DOI] [PubMed] [Google Scholar]
- 15.Prayaga AK, Vijaya K. Role of unstained smears in determining sample adequacy. Acta Cytol. 2004;48:321–324. doi: 10.1159/000326380. [DOI] [PubMed] [Google Scholar]
- 16.Redman R, Zalaznick H, Mazzaferri EL, Massoll NA. The impact of assessing specimen adequacy and number of needle passes for fine-needle aspiration biopsy of thyroid nodules. Thyroid. 2006;16:55–60. doi: 10.1089/thy.2006.16.55. [DOI] [PubMed] [Google Scholar]
- 17.Zhu W, Michael CW. How important is on-site adequacy assessment for thyroid FNA? An evaluation of 883 cases. Diagn Cytopathol. 2007;35:183–186. doi: 10.1002/dc.20552. [DOI] [PubMed] [Google Scholar]
- 18.Burlingame OO, Kessé KO, Silverman SG, Cibas ES. On-site adequacy evaluations performed by cytotechnologists: correlation with final interpretations of 5,241 image-guided fine-needle aspiration biopsies. Cancer Cytopathol. 2012;120:177–184. doi: 10.1002/cncy.20184. [DOI] [PubMed] [Google Scholar]
- 19.Olson MT, Ali SZ. Cytotechnologist on-site evaluation of pancreas fine needle aspiration adequacy: comparison with cytopathologists and correlation with the final interpretation. Acta Cytol. 2012;56:340–346. doi: 10.1159/000338646. [DOI] [PubMed] [Google Scholar]
- 20.Olson MT, Tatsas AD, Ali SZ. Cytotechnologist-attended on-site adequacy evaluation of thyroid fine-needle aspiration: comparison with cytopathologists and correlation with the final interpretation. Am J Clin Pathol. 2012;138:90–95. doi: 10.1309/AJCP84AXSRABZCTZ. [DOI] [PubMed] [Google Scholar]
- 21.Collins BT. Telepathology in cytopathology: challenges and opportunities. Acta Cytol. 2013;57:221–232. doi: 10.1159/000350718. [DOI] [PubMed] [Google Scholar]
- 22.Erickson RA, Tretjak Z. Clinical utility of endoscopic ultrasound and endscopic ultrasound-guided fine needle aspiration in retroperitoneal neoplasms. Am J Gastroenterol. 2000;95:1188–1194. doi: 10.1111/j.1572-0241.2000.02008.x. [DOI] [PubMed] [Google Scholar]
- 23.Savoy AD, Raimondo M, Woodward TA, et al. Can endosonographers evaluate on-site cytologic adequacy? A comparison with cytotechnologists. Gastrointest Endosc. 2007;65:953–957. doi: 10.1016/j.gie.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Austin JH, Cohen MB. Value of having a cytopathologist present during percutaneous fine-needle aspiration biopsy of lung: report of 55 cancer patients and metaanalysis of the literature. AJR Am J Roentgenol. 1993;160:175–177. doi: 10.2214/ajr.160.1.8416620. [DOI] [PubMed] [Google Scholar]
- 25.Miller DA, Carrasco CH, Katz RL, Cramer FM, Wallace S, Charnsangavej C. Fine needle aspiration biopsy: the role of immediate cytologic assessment. AJR Am J Roentgenol. 1986;147:155–158. doi: 10.2214/ajr.147.1.155. [DOI] [PubMed] [Google Scholar]
- 26.Iglesias-Garcia J, Dominguez-Munoz JE, Abdulkader I, et al. Influence of on-site cytopathology evaluation on the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) of solid pancreatic masses. Am J Gastroenterol. 2011;106:1705–1710. doi: 10.1038/ajg.2011.119. [DOI] [PubMed] [Google Scholar]
- 27.Chang KJ, Nguyen P, Erickson RA, Durbin TE, Katz KD. The clinical utility of endoscopic ultrasound-guided fine-needle aspiration in the diagnosis and staging of pancreatic carcinoma. Gastrointest Endosc. 1997;45:387–393. doi: 10.1016/s0016-5107(97)70149-4. [DOI] [PubMed] [Google Scholar]
- 28.Erickson RA, Sayage-Rabie L, Avots-Avotins A. Clinical utility of endoscopic ultrasound-guided fine needle aspiration. Acta Cytol. 1997;41:1647–1653. doi: 10.1159/000333155. [DOI] [PubMed] [Google Scholar]
- 29.Alsohaibani F, Girgis S, Sandha GS. Does onsite cytotechnology evaluation improve the accuracy of endoscopic ultrasound-guided fine-needle aspiration biopsy? Can J Gastroenterol. 2009;23:26–30. doi: 10.1155/2009/194351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tournoy KG, Praet MM, Van Maele G, Van Meerbeeck JP. Esophageal endoscopic ultrasound with fine-needle aspiration with an on-site cytopathologist: high accuracy for the diagnosis of mediastinal lymphadenopathy. Chest. 2005;128:3004–3009. doi: 10.1378/chest.128.4.3004. [DOI] [PubMed] [Google Scholar]
- 31.O'Malley ME, Weir MM, Hahn PF, Misdraji J, Wood BJ, Mueller PR. US-guided fine-needle aspiration biopsy of thyroid nodules: adequacy of cytologic material and procedure time with and without immediate cytologic analysis. Radiology. 2002;222:383–387. doi: 10.1148/radiol.2222010201. [DOI] [PubMed] [Google Scholar]
- 32.Zanocco K, Pitelka-Zengou L, Dalal S, Elaraj D, Nayar R, Sturgeon C. Routine on-site evaluation of specimen adequacy during initial ultrasound-guided fine needle aspiration of thyroid nodules: a cost-effectiveness analysis. Ann Surg Oncol. 2013;20:2462–2467. doi: 10.1245/s10434-013-2954-1. [DOI] [PubMed] [Google Scholar]
- 33.Nasuti JF, Gupta PK, Baloch ZW. Diagnostic value and cost-effectiveness of on-site evaluation of fine-needle aspiration specimens: review of 5,688 cases. Diagn Cytopathol. 2002;27:1–4. doi: 10.1002/dc.10065. [DOI] [PubMed] [Google Scholar]
- 34.Wotruba AL, Stewart J, 3rd, Scheberl T, Selvaggi SM. Added value, decreased cost: the evolving role of the cytotechnologist for preliminary screening and triage of thyroid aspirates. Diagn Cytopathol. 2011;39:896–899. doi: 10.1002/dc.21487. [DOI] [PubMed] [Google Scholar]
- 35.Renshaw AA. 88172 is more than counting cells: ensuring the quality of immediate assessment of fine-needle aspiration material. Am J Clin Pathol. 2012;138:27–28. doi: 10.1309/AJCPP8KTFT3OARGT. [DOI] [PubMed] [Google Scholar]
- 36.Ducatman BS, Hogan CL, Wang HH. A triage system for processing fine needle aspiration cytology specimens. Acta Cytol. 1989;33:797–799. [PubMed] [Google Scholar]
- 37.Cleveland P, Gill KR, Coe SG, et al. An evaluation of risk factors for inadequate cytology in EUS-guided FNA of pancreatic tumors and lymph nodes. Gastrointest Endosc. 2010;71:1194–1199. doi: 10.1016/j.gie.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 38.Nayar MK, Chatterjee S, Wadehra V, Cunningham J, Leeds J, Oppong K. Does on-site adequacy assessment by cytotechnologists improve results of EUS guided FNA of solid pancreaticobiliary lesions? JOP. 2013;14:44–49. doi: 10.6092/1590-8577/1277. [DOI] [PubMed] [Google Scholar]
- 39.Petrone MC, Arcidiacono PG, Carrara S, Mezzi G, Doglioni C, Testoni PA. Does cytotechnician training influence the accuracy of EUS-guided fine-needle aspiration of pancreatic masses? Dig Liver Dis. 2012;44:311–314. doi: 10.1016/j.dld.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Olson MT, Novak A, Kirby J, Shahid H, Boonyaarunnate T, Ali SZ. Acta Cytol. 2013. Oct 1, Cytotechnologist attended on-site evaluation of adequacy for metastatic disease involving bone and soft tissue. [Epub] http://dx.doi.org/10.1159/000354079. [DOI] [PubMed] [Google Scholar]
- 41.Layfield LJ, Bentz JS, Gopez EV. Immediate on-site interpretation of fine-needle aspiration smears: a cost and compensation analysis. Cancer. 2001;93:319–322. doi: 10.1002/cncr.9046. [DOI] [PubMed] [Google Scholar]
- 42.Goyal A, Jhala N, Gupta P. TeleCyP (Telecytopathology): real-time fine-needle aspiration interpretation. Acta Cytol. 2012;56:669–677. doi: 10.1159/000339791. [DOI] [PubMed] [Google Scholar]
- 43.Khurana KK, Kovalovsky A, Wang D, Lenox R. Feasibility of dynamic telecytopathology for rapid on-site evaluation of endobronchial ultrasound-guided transbronchial fine needle aspiration. Telemed J E Health. 2013;19:265–271. doi: 10.1089/tmj.2012.0168. [DOI] [PubMed] [Google Scholar]
- 44.Khurana KK, Rong R, Wang D, Roy A. Dynamic telecytopathology for on-site preliminary diagnosis of endoscopic ultrasound-guided fine needle aspiration of pancreatic masses. J Telemed Telecare. 2012;18:253–259. doi: 10.1258/jtt.2011.110706. [DOI] [PubMed] [Google Scholar]
- 45.Khurana KK, Swati I, Kasturi R, Lambert R, Izquierdo R. Telecyto-pathology for rapid preliminary diagnosis of ultrasound-guided fine-needle aspiration of thyroid nodules. Telemed J E Health. 2011;17:763–767. doi: 10.1089/tmj.2011.0052. [DOI] [PubMed] [Google Scholar]
- 46.Khurana KK, Kovalovsky A, Masrani D. Feasibility of telecytopathology for rapid preliminary diagnosis of ultrasound-guided fine needle aspiration of axillary lymph nodes in a remote breast care center. J Pathol Inform. 2012;3:36. doi: 10.4103/2153-3539.101803. [DOI] [PMC free article] [PubMed] [Google Scholar]


