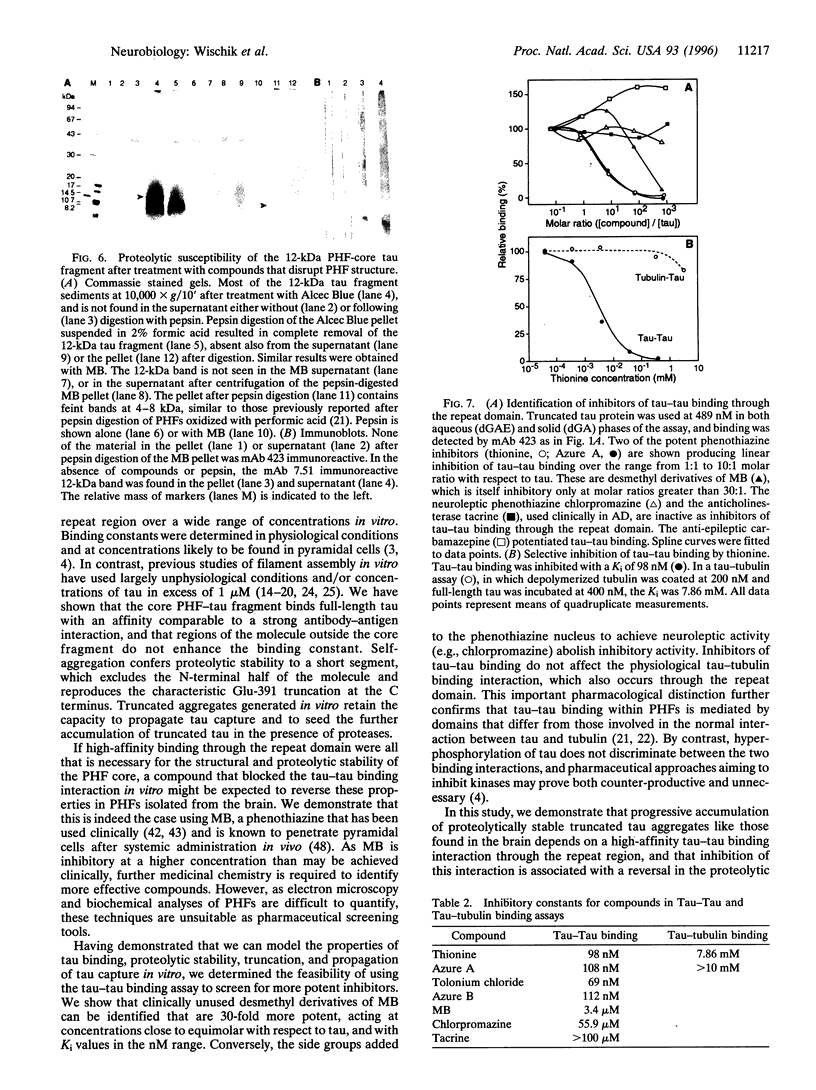
Abstract
In Alzheimer disease (AD) the microtubule-associated protein tau is redistributed exponentially into paired helical filaments (PHFs) forming neurofibrillary tangles, which correlate with pyramidal cell destruction and dementia. Amorphous neuronal deposits and PHFs in AD are characterized by aggregation through the repeat domain and C-terminal truncation at Glu-391 by endogenous proteases. We show that a similar proteolytically stable complex can be generated in vitro following the self-aggregation of tau protein through a high-affinity binding site in the repeat domain. Once started, tau capture can be propagated by seeding the further accumulation of truncated tau in the presence of proteases. We have identified a nonneuroleptic phenothiazine previously used in man (methylene blue, MB), which reverses the proteolytic stability of protease-resistant PHFs by blocking the tau-tau binding interaction through the repeat domain. Although MB is inhibitory at a higher concentration than may be achieved clinically, the tau-tau binding assay was used to identify desmethyl derivatives of MB that have Ki values in the nanomolar range. Neuroleptic phenothiazines are inactive. Tau aggregation inhibitors do not affect the tau-tubulin interaction, which also occurs through the repeat domain. Our findings demonstrate that biologically selective pharmaceutical agents could be developed to facilitate the proteolytic degradation of tau aggregates and prevent the further propagation of tau capture in AD.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arriagada P. V., Marzloff K., Hyman B. T. Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer's disease. Neurology. 1992 Sep;42(9):1681–1688. doi: 10.1212/wnl.42.9.1681. [DOI] [PubMed] [Google Scholar]
- Biernat J., Gustke N., Drewes G., Mandelkow E. M., Mandelkow E. Phosphorylation of Ser262 strongly reduces binding of tau to microtubules: distinction between PHF-like immunoreactivity and microtubule binding. Neuron. 1993 Jul;11(1):153–163. doi: 10.1016/0896-6273(93)90279-z. [DOI] [PubMed] [Google Scholar]
- Bondareff W., Mountjoy C. Q., Wischik C. M., Hauser D. L., LaBree L. D., Roth M. Evidence of subtypes of Alzheimer's disease and implications for etiology. Arch Gen Psychiatry. 1993 May;50(5):350–356. doi: 10.1001/archpsyc.1993.01820170028004. [DOI] [PubMed] [Google Scholar]
- Bondareff W., Wischik C. M., Novak M., Amos W. B., Klug A., Roth M. Molecular analysis of neurofibrillary degeneration in Alzheimer's disease. An immunohistochemical study. Am J Pathol. 1990 Sep;137(3):711–723. [PMC free article] [PubMed] [Google Scholar]
- Caputo C. B., Wischik C., Novak M., Scott C. W., Brunner W. F., De Garcini E. M., Lo M. M., Norris T. E., Salama A. I. Immunological characterization of the region of tau protein that is bound to Alzheimer paired helical filaments. Neurobiol Aging. 1992 Mar-Apr;13(2):267–274. doi: 10.1016/0197-4580(92)90039-z. [DOI] [PubMed] [Google Scholar]
- Crowther R. A., Olesen O. F., Jakes R., Goedert M. The microtubule binding repeats of tau protein assemble into filaments like those found in Alzheimer's disease. FEBS Lett. 1992 Sep 7;309(2):199–202. doi: 10.1016/0014-5793(92)81094-3. [DOI] [PubMed] [Google Scholar]
- Crowther R. A., Olesen O. F., Smith M. J., Jakes R., Goedert M. Assembly of Alzheimer-like filaments from full-length tau protein. FEBS Lett. 1994 Jan 10;337(2):135–138. doi: 10.1016/0014-5793(94)80260-2. [DOI] [PubMed] [Google Scholar]
- Crowther R. A., Wischik C. M. Image reconstruction of the Alzheimer paired helical filament. EMBO J. 1985 Dec 30;4(13B):3661–3665. doi: 10.1002/j.1460-2075.1985.tb04132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiSanto A. R., Wagner J. G. Pharmacokinetics of highly ionized drugs. II. Methylene blue--absorption, metabolism, and excretion in man and dog after oral administration. J Pharm Sci. 1972 Jul;61(7):1086–1090. doi: 10.1002/jps.2600610710. [DOI] [PubMed] [Google Scholar]
- García de Ancos J., Correas I., Avila J. Differences in microtubule binding and self-association abilities of bovine brain tau isoforms. J Biol Chem. 1993 Apr 15;268(11):7976–7982. [PubMed] [Google Scholar]
- Goedert M., Spillantini M. G., Jakes R., Rutherford D., Crowther R. A. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron. 1989 Oct;3(4):519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- Goedert M., Wischik C. M., Crowther R. A., Walker J. E., Klug A. Cloning and sequencing of the cDNA encoding a core protein of the paired helical filament of Alzheimer disease: identification as the microtubule-associated protein tau. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4051–4055. doi: 10.1073/pnas.85.11.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington C. R., Edwards P. C., Wischik C. M. Competitive ELISA for the measurement of tau protein in Alzheimer's disease. J Immunol Methods. 1990 Dec 5;134(2):261–271. doi: 10.1016/0022-1759(90)90388-c. [DOI] [PubMed] [Google Scholar]
- Harrington C. R., Louwagie J., Rossau R., Vanmechelen E., Perry R. H., Perry E. K., Xuereb J. H., Roth M., Wischik C. M. Influence of apolipoprotein E genotype on senile dementia of the Alzheimer and Lewy body types. Significance for etiological theories of Alzheimer's disease. Am J Pathol. 1994 Dec;145(6):1472–1484. [PMC free article] [PubMed] [Google Scholar]
- Harrington C. R. Lowry protein assay containing sodium dodecyl sulfate in microtiter plates for protein determinations on fractions from brain tissue. Anal Biochem. 1990 May 1;186(2):285–287. doi: 10.1016/0003-2697(90)90081-j. [DOI] [PubMed] [Google Scholar]
- Harrington C. R., Mukaetova-Ladinska E. B., Hills R., Edwards P. C., Montejo de Garcini E., Novak M., Wischik C. M. Measurement of distinct immunochemical presentations of tau protein in Alzheimer disease. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5842–5846. doi: 10.1073/pnas.88.13.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington C. R., Wischik C. M., McArthur F. K., Taylor G. A., Edwardson J. A., Candy J. M. Alzheimer's-disease-like changes in tau protein processing: association with aluminium accumulation in brains of renal dialysis patients. Lancet. 1994 Apr 23;343(8904):993–997. doi: 10.1016/s0140-6736(94)90124-4. [DOI] [PubMed] [Google Scholar]
- Jakes R., Novak M., Davison M., Wischik C. M. Identification of 3- and 4-repeat tau isoforms within the PHF in Alzheimer's disease. EMBO J. 1991 Oct;10(10):2725–2729. doi: 10.1002/j.1460-2075.1991.tb07820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobst K. A., Smith A. D., Szatmari M., Esiri M. M., Jaskowski A., Hindley N., McDonald B., Molyneux A. J. Rapidly progressing atrophy of medial temporal lobe in Alzheimer's disease. Lancet. 1994 Apr 2;343(8901):829–830. doi: 10.1016/s0140-6736(94)92028-1. [DOI] [PubMed] [Google Scholar]
- Kosik K. S., Orecchio L. D., Binder L., Trojanowski J. Q., Lee V. M., Lee G. Epitopes that span the tau molecule are shared with paired helical filaments. Neuron. 1988 Nov;1(9):817–825. doi: 10.1016/0896-6273(88)90129-8. [DOI] [PubMed] [Google Scholar]
- Ksiezak-Reding H., Yen S. H. Structural stability of paired helical filaments requires microtubule-binding domains of tau: a model for self-association. Neuron. 1991 May;6(5):717–728. doi: 10.1016/0896-6273(91)90169-z. [DOI] [PubMed] [Google Scholar]
- Lai R. Y., Gertz H. N., Wischik D. J., Xuereb J. H., Mukaetova-Ladinska E. B., Harrington C. R., Edwards P. C., Mena R., Paykel E. S., Brayne C. Examination of phosphorylated tau protein as a PHF-precursor at early stage Alzheimer's disease. Neurobiol Aging. 1995 May-Jun;16(3):433–445. doi: 10.1016/0197-4580(95)00041-c. [DOI] [PubMed] [Google Scholar]
- Levy-Lahad E., Wasco W., Poorkaj P., Romano D. M., Oshima J., Pettingell W. H., Yu C. E., Jondro P. D., Schmidt S. D., Wang K. Candidate gene for the chromosome 1 familial Alzheimer's disease locus. Science. 1995 Aug 18;269(5226):973–977. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- McKee A. C., Kowall N. W., Kosik K. S. Microtubular reorganization and dendritic growth response in Alzheimer's disease. Ann Neurol. 1989 Nov;26(5):652–659. doi: 10.1002/ana.410260511. [DOI] [PubMed] [Google Scholar]
- Mena R., Edwards P. C., Harrington C. R., Mukaetova-Ladinska E. B., Wischik C. M. Staging the pathological assembly of truncated tau protein into paired helical filaments in Alzheimer's disease. Acta Neuropathol. 1996;91(6):633–641. doi: 10.1007/s004010050477. [DOI] [PubMed] [Google Scholar]
- Mena R., Wischik C. M., Novak M., Milstein C., Cuello A. C. A progressive deposition of paired helical filaments (PHF) in the brain characterizes the evolution of dementia in Alzheimer's disease. An immunocytochemical study with a monoclonal antibody against the PHF core. J Neuropathol Exp Neurol. 1991 Jul;50(4):474–490. doi: 10.1097/00005072-199107000-00008. [DOI] [PubMed] [Google Scholar]
- Montejo de Garcini E., Serrano L., Avila J. Self assembly of microtubule associated protein tau into filaments resembling those found in Alzheimer disease. Biochem Biophys Res Commun. 1986 Dec 15;141(2):790–796. doi: 10.1016/s0006-291x(86)80242-x. [DOI] [PubMed] [Google Scholar]
- Mukaetova-Ladinska E. B., Harrington C. R., Roth M., Wischik C. M. Biochemical and anatomical redistribution of tau protein in Alzheimer's disease. Am J Pathol. 1993 Aug;143(2):565–578. [PMC free article] [PubMed] [Google Scholar]
- Müller T. Light-microscopic demonstration of methylene blue accumulation sites in mouse brain after supravital staining. Acta Anat (Basel) 1992;144(1):39–44. [PubMed] [Google Scholar]
- Naylor G. J., Martin B., Hopwood S. E., Watson Y. A two-year double-blind crossover trial of the prophylactic effect of methylene blue in manic-depressive psychosis. Biol Psychiatry. 1986 Aug;21(10):915–920. doi: 10.1016/0006-3223(86)90265-9. [DOI] [PubMed] [Google Scholar]
- Novak M., Jakes R., Edwards P. C., Milstein C., Wischik C. M. Difference between the tau protein of Alzheimer paired helical filament core and normal tau revealed by epitope analysis of monoclonal antibodies 423 and 7.51. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5837–5841. doi: 10.1073/pnas.88.13.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak M., Kabat J., Wischik C. M. Molecular characterization of the minimal protease resistant tau unit of the Alzheimer's disease paired helical filament. EMBO J. 1993 Jan;12(1):365–370. doi: 10.1002/j.1460-2075.1993.tb05665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruben G. C., Iqbal K., Grundke-Iqbal I., Johnson J. E., Jr The organization of the microtubule associated protein tau in Alzheimer paired helical filaments. Brain Res. 1993 Jan 29;602(1):1–13. doi: 10.1016/0006-8993(93)90234-e. [DOI] [PubMed] [Google Scholar]
- Schweers O., Mandelkow E. M., Biernat J., Mandelkow E. Oxidation of cysteine-322 in the repeat domain of microtubule-associated protein tau controls the in vitro assembly of paired helical filaments. Proc Natl Acad Sci U S A. 1995 Aug 29;92(18):8463–8467. doi: 10.1073/pnas.92.18.8463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington R., Rogaev E. I., Liang Y., Rogaeva E. A., Levesque G., Ikeda M., Chi H., Lin C., Li G., Holman K. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature. 1995 Jun 29;375(6534):754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- Trojanowski J. Q., Lee V. M. Phosphorylation of paired helical filament tau in Alzheimer's disease neurofibrillary lesions: focusing on phosphatases. FASEB J. 1995 Dec;9(15):1570–1576. doi: 10.1096/fasebj.9.15.8529836. [DOI] [PubMed] [Google Scholar]
- Troncoso J. C., Costello A., Watson A. L., Jr, Johnson G. V. In vitro polymerization of oxidized tau into filaments. Brain Res. 1993 Jun 11;613(2):313–316. doi: 10.1016/0006-8993(93)90918-d. [DOI] [PubMed] [Google Scholar]
- Wilcock G. K., Esiri M. M., Bowen D. M., Smith C. C. Alzheimer's disease. Correlation of cortical choline acetyltransferase activity with the severity of dementia and histological abnormalities. J Neurol Sci. 1982 Dec;57(2-3):407–417. doi: 10.1016/0022-510x(82)90045-4. [DOI] [PubMed] [Google Scholar]
- Wille H., Drewes G., Biernat J., Mandelkow E. M., Mandelkow E. Alzheimer-like paired helical filaments and antiparallel dimers formed from microtubule-associated protein tau in vitro. J Cell Biol. 1992 Aug;118(3):573–584. doi: 10.1083/jcb.118.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. M., Binder L. I. Polymerization of microtubule-associated protein tau under near-physiological conditions. J Biol Chem. 1995 Oct 13;270(41):24306–24314. doi: 10.1074/jbc.270.41.24306. [DOI] [PubMed] [Google Scholar]
- Wischik C. M., Crowther R. A., Stewart M., Roth M. Subunit structure of paired helical filaments in Alzheimer's disease. J Cell Biol. 1985 Jun;100(6):1905–1912. doi: 10.1083/jcb.100.6.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischik C. M., Novak M., Edwards P. C., Klug A., Tichelaar W., Crowther R. A. Structural characterization of the core of the paired helical filament of Alzheimer disease. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4884–4888. doi: 10.1073/pnas.85.13.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischik C. M., Novak M., Thøgersen H. C., Edwards P. C., Runswick M. J., Jakes R., Walker J. E., Milstein C., Roth M., Klug A. Isolation of a fragment of tau derived from the core of the paired helical filament of Alzheimer disease. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4506–4510. doi: 10.1073/pnas.85.12.4506. [DOI] [PMC free article] [PubMed] [Google Scholar]