Introduction
After 30 years of the HIV epidemic there are still an estimated two new infections for every individual starting treatment and no effective vaccine[1-2]. New interventions which address biological co-factors for HIV infection are urgently needed. There are established associations between sexually transmitted infections (STI), particularly Herpes simplex virus type 2 (HSV2), and HIV acquisition[3]. Recently, a number of descriptive studies have documented an association between human papillomavirus (HPV) infection and HIV acquisition.
HPV, the primary cause of cervical cancer, is acquired rapidly after sexual debut and infection with multiple genotypes is common, making HPV a highly prevalent STI world-wide[4-7]. Approximately 40 HPV genotypes infect the human genital tract and are classified into two groups depending on their oncogenic potential: high-risk oncogenic and low-risk non-oncogenic genotypes. Symptomatic infection is rare and usually manifests as ano-genital condylomata and cervical, vulvar, anal or penile precancers or cancers[8]. There are two extremely effective vaccines offering protection against HPV infection or precancerous lesions caused by vaccine HPV genotypes. The bivalent vaccine protects against HPV 16 and 18 and the quadrivalent vaccine against HPV 16, 18 and HPV 6 and 11[9-11]. Both vaccines also show evidence of cross-protection against non-vaccine types (particularly HPV 31, 33 and 45)[12-13]. A vaccine targeting 9 HPV genotypes has entered phase III clinical trials (NCT00943722).
Collection, appraisal and synthesis of available evidence for the association of HPV with HIV acquisition would provide an important resource to assess the potential role of HPV in the HIV pandemic. The objectives of the current study were to collate and appraise the observational evidence for any longitudinal association between prevalent HPV infection and HIV acquisition; and to estimate the proportion of HIV infections attributable to HPV infection.
Methods
Search
Pubmed and Embase were searched using search terms for HPV, genital warts and HIV (see supplementary material for full list). Only nested case-control and cohort studies were included, cross-sectional studies were excluded because of the risk of reverse causality: HPV prevalence increases rapidly after HIV seroconversion[14]. All abstracts available on-line from the International AIDS Society, the International Society for Sexually Transmitted Diseases Research, the British HIV Association conferences, the Conference on Retroviruses and Opportunistic Infections and the International Papillomavirus Conference were searched. Reference lists of review articles and all articles identified in the systematic search were checked. The search was carried out up to 29th July 2011. All abstracts were reviewed independently by two authors (CH and NL). Inconsistencies were discussed and consensus reached on potential relevance. Full text copies of potentially relevant papers were then obtained.
Included studies
Only human studies were included, with no language or date restrictions. Only studies which identified HPV DNA using hybrid capture II or PCR were included: other methods lack sensitivity and specificity[15-16]. HPV samples could be clinician or self-collected since these have high concordance[17]. Studies were restricted to those where HPV status was determined prior to HIV infection. In papers that presented analysis from the same population, the study that gave the most detailed description of the cohort and study design was selected.
Bias
Assessment of bias was made using a component approach, similar to the Cochrane Collaboration’s[18] and supported by PRISMA guidelines[19]. Studies were assessed on selection bias, timing of HPV test in relation to HIV, cohort retention, follow-up duration, adjustment for confounding and outcome reporting.
Data extraction
Data were independently extracted by two authors (CH and NL) using a piloted, standardised form. Either a hazard ratio (HR) or an odds ratio (OR) was extracted, since these approximate closely when the outcome is rare, as in this case. Extracted data included the association with HIV acquisition for: any HPV, high-risk and low-risk HPV genotypes, HPV 16/18, HPV 6/11/16/18 and HPV 6/11/16/18/31/33/45/52/58 and persistent and non-persistent HPV. Authors were contacted if the desired effect estimate was not published.
Meta-analysis
Studies conducted in (i) heterosexual male populations, (ii) men who have sex with men (MSM) and (iii) studies in women were considered separately. Meta-analyses were not performed if the effect estimates were not comparable (as in estimates for persistent and non-persistent HPV). Analyses were performed using STATA version 12.0 (StataCorp LP, Texas, USA). Random effects meta-analysis was used to produce summary effect estimates (presented as HR), which allow for between-study heterogeneity[20]. Meta-regression was not performed due to the large potential for false positive findings with a limited number of studies[21]. A sensitivity analysis was performed excluding unadjusted studies since lack of adjustment for confounders was not an exclusion criterion. Publication bias was assessed by funnel plot, which displays the log of the effect estimate against its standard error, and formally tested using Begg’s test[22]. Population attributable fractions (PAF) of HIV due to prior HPV infection were calculated using , where is the adjusted effect estimate for HIV acquisition in those with HPV, and pʹ is the prevalence of HPV prior to HIV acquisition in those who acquired HIV. PAFs were only calculated for infections with any HPV, since other effect estimates were either under-powered or unadjusted for the presence of other HPV genotypes.
Results
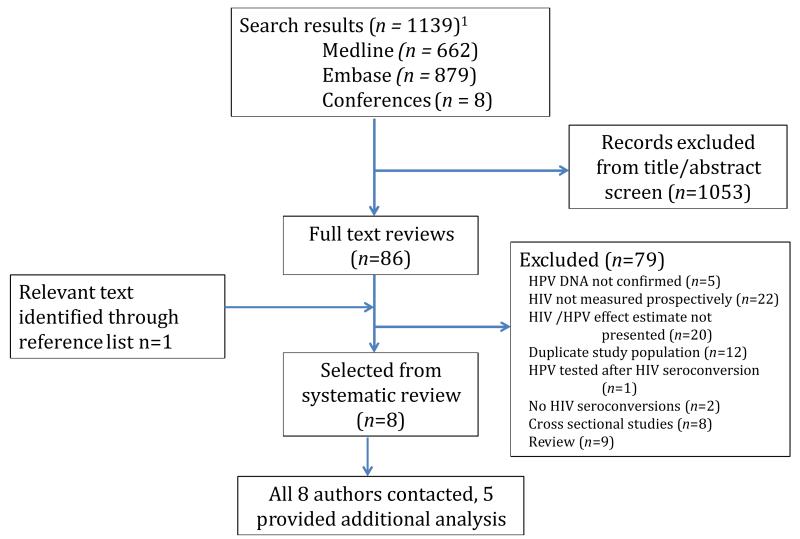
After duplicate removal, a total of 1139 titles and abstracts were identified (Figure 1). 1053 were excluded after abstract review, due to non-relevance or clear exclusion criteria, leaving 86 papers for full text review. Eight relevant studies were identified and data were extracted. Characteristics of included studies are summarised in Table 1. The 8 studies provided data from a combined total of 12,750 individuals. Studies included one nested case-control study and seven cohort studies. Six studies were in women, one in heterosexual men, and one in MSM. Meta-analysis was therefore only possible for studies in women. One study was conducted in the USA[23] and seven in sub-Saharan Africa. Of eight authors contacted for further analysis, five responded. No study had the primary aim of measuring the association between prevalent HPV and HIV acquisition.
Figure 1. Results from the systematic search.
1After removal of duplicates
Table 1. Summary of studies of the association of HPV and HIV acquisition.
| First Author, year |
Study type (median follow-up per participant) |
Participants, location | HPV prevalence |
Number of HIV sero- conversions (cohort size or controls) |
HPV genotypes | Comparison group | Confounding factors adjusted for1 |
Unadjusted HR (95 % CI) |
Adjusted HR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Studies in Women | |||||||||
| Auvert, 2011[29] |
Cohort (2.5yrs) |
Microbicides trial in sex workers, South Africa |
70.5% at baseline5,18 |
25 (88) | ≥2 high-risk15 | ≤1 high-risk HPV | TV, NG, CT, TP and sexual behavior |
- | 4.0 (1.2-14.0) |
| Averbach, 2010[24] |
Nested case control study (21.9 months, 3 months between HPV and HIV) |
Hormonal contraception observational study, Zimbabwe |
48.7% at visit before sero- conversion 19 |
145 (446) | Any16 High-risk16 Low-risk16 Non-persistent16 Persistent16 16/1816 6/11/16/1816 |
No HPV No HPV No HPV No HPV No HPV No HPV 16/18 No HPV 6/11/16/18 |
BV, TV, NG, CT, HSV2, TP and sexual behavior |
2.7 (1.7 – 4.1) 2.7 (1.7 – 4.3) 2.5 (1.3 – 4.6) 5.3 (3.2–9.0) 1.12 (0.6–2.0) 1.65 (0.96-2.72)10 1.63 (0.98-2.72)10 |
2.4 (1.5-4.0) 2.3 (1.4-3.9) 2.8 (1.3-5.9) 5.4 (2.9–9.9) 0.97 (0.51-1.85) 0.94 (0.46-1.92)10 0.94 (0.47 - 1.84)10 |
| Low, 2011[25] |
Cohort (1.7 years) |
Sex workers observational HIV study, Burkina Faso |
1.6% at baseline6,19 |
4 (183) | Any17 | No HPV | HSV2 | 2.45 (0.26-24.85)10 | 2.26 (0.23-22.60)10 |
| Myer, 2007[27] |
Cohort (14.3 months) |
Cervical cancer screening Study, South Africa |
17.5% at baseline19 |
111 (4200) | High-risk17 | No HPV | NG, CT and sexual behavior |
1.72 (1.25-2.35)10 | 1.66 (1.21-2.28)10 |
| Smith- McCune, 2010[26] |
Cohort (21 months) |
HIV prevention trial of diaphragm and gel, Zimbabwe |
24.5% baseline18 |
88 (2040) | Any16 High-risk16 Low-risk16 Non-persistent high-risk16 Non-persistent low-risk16 Persistent high-risk16 Persistent low-risk16 16/1816 6/11/16/18 6/11/16/18/31/ 33/45/52/588,16 |
No HPV No HPV No HPV No HPV No HPV No HPV No HPV No HPV 16/18 No HPV 6/11/16/18 No HPV 6/11 /16/18/31/ 33/45/52/588 |
TV, NG, CT, HSV2, TP, MC9 and sexual behavior |
1.50 (0.92–2.43) 1.95 (1.19–3.21) 2.02 (1.47-3.98) 2.42 (1.26-2.35) 1.01 (0.72-3.15) 1.50 (0.56-1.84) - - - |
1.71 (1.00-2.92)11 1.96 (1.16-3.3) 1.7 (1.02-2.85) 1.67 (1.03-2.74) 2.09 (1.27-3.44) 0.82 (0.45-1.50) 1.24 (0.59-2.60) 0.84 (0.32-2.18)10 2.00 (1.00-3.99)10 2.57 (1.48-4.46)10 |
| Veldhuijzen, 2010[28] |
Cohort (16.6 months2) | Sex workers observational HIV study, Rwanda |
70% at baseline7,19 |
10 (366) | High-risk17 | No HPV | None | 4.9 (1.2-19.7) | - |
|
| |||||||||
| Studies in men who have sex with men | |||||||||
| Chin-Hong, 2009[23] |
Cohort (36 months3) |
Behavioural intervention study, Multicentre, USA |
56.8% at baseline12 |
51(1409) | 1type12,17 2 or more types12,17 |
No HPV No HPV |
Self-reported STIs and sexual behavior |
2.8 (1.04-7.4) 3.6 (1.5-8.4) |
2.0 (0.61-6.5) 3.5 (1.2-10.6) |
|
| |||||||||
| Studies in heterosexual men | |||||||||
| Smith, 2010[30] |
Cohort4 | Male circumcision trial, Kenya |
50% at baseline14 |
63(2168) | Any type13,17 High-risk14,17 Low-risk14,17 16/1814,17 6/11/16/1814,17 6/11/16/18/31/ 33/45/52/588,14,17 |
No HPV No HPV No HPV No HPV 16/18 No HPV 6/11/16/18 No HPV 6/11 /16/18/31/ 33/45/52/588 |
HSV2 and MC | - - - - - - |
1.8 (1.1-2.9) 1.5 (0.9-2.6) 1.8 (0.9-3.6) 1.8 (0.9-3.4)10 1.3 (0.7-2.5)10 1.4(0.8-2.7)10 |
TV is Trichomonas vaginalis, NG is Neisseria gonorrhoeae, HSV2 is Herpes simplex virus type 2, TP is Treponema pallidum, CT is Chlamydia trachomatis, BV is Bacterial vaginosis and MC is male circumcision.
Median time from HPV visit (at month 6 of the study) to HIV test follow-up
Calculated from person years follow-up and number of participants
In the original trial follow up was for 24 months. 1550 (71%) entered prolonged follow-up for 42 months. No median follow-up time was provided
Prevalence of oncogenic at baseline, prevalence of non-oncogenic HPV was 60.2%
In those who later became HIV positive
Prevalence of oncogenic HPV In those who later became HIV positive, the prevalence of oncogenic HPV in those who remained HIV negative was 32%
These HPV types are those covered by the nonovalent vaccine
In this study, MC relates to circumcision status of regular partner at baseline or of new partner during return visits
These unpublished data were provided by the authors and are additionally adjusted for the presence of other HPV genotypes
Unpublished data provided by the authors
Anal sample
Penile sample from glans/coronal sulcus
Penile sample, any site
HPV measured at two time points, and most recent used
At visit prior to seroconversion
At baseline
Cervico-vaginal
Cervical
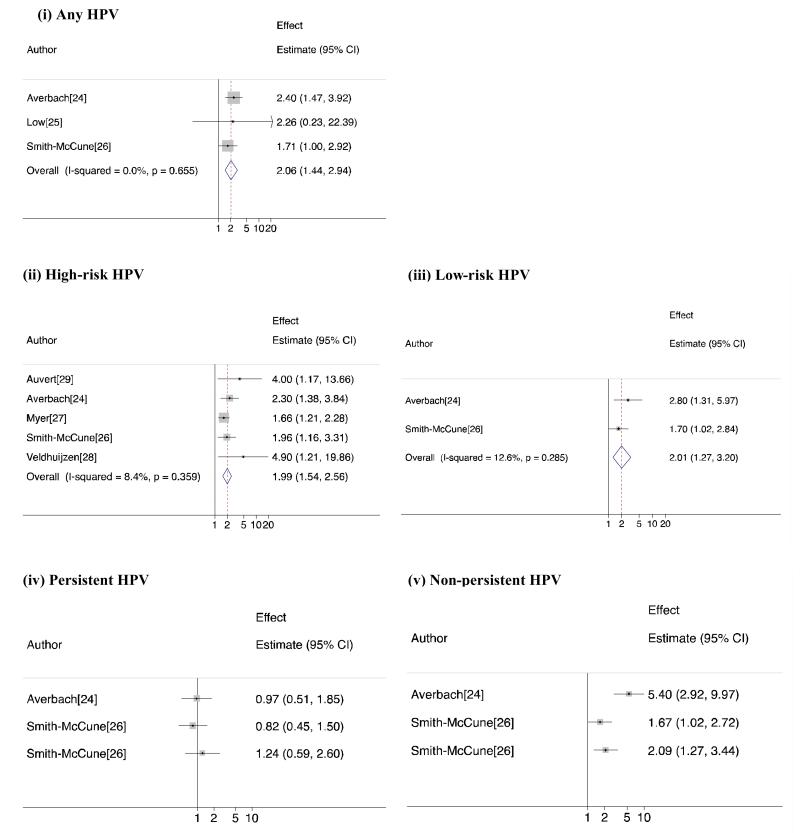
Results of the meta-analysis showing HR and 95% CI for the individual studies and pooled measures of effect are shown in Figure 2(i-v).
Figure 2. Meta-analysis of HIV risk in women associated with (i) Any HPV (ii) High-risk HPV (iii) Low-risk HPV (iv) Persistent HPV and (v) Non-persistent HPV.
HPV infection and HIV acquisition in women
Averbach[24], Low[25] and Smith-McCune[26] et al increased risk of HIV acquisition associated with infection with any HPV genotype compared to no HPV infection in women. The point estimate from all three were consistent with a harmful effect of HPV infection (aHR=1.71, aHR=2.40 and aHR=2.26 respectively) although the association was only statistically significant for two[24, 26]: the third had low power[25] Figure 2(i). There was strong evidence of an increased risk of HIV acquisition with any prevalent HPV genotype from the meta-analysis (summary HR=2.06 (95%CI=1.44-2.94), I2=0%, P heterogeneity=0.66).
All but one[25] of six studies in women presented a separate effect measure for HIV acquisition associated with high-risk HPV. In these studies (Figure 2 (ii)), Averbach[24], Myer[27], Smith-McCune[26] and Veldhuijzen[28] et al, presented the effect estimate for infection with high-risk HPV compared to no HPV and Auvert et al[29] presented the effect estimate for infection with 2 or more high-risk HPV genotypes compared to infection with one or zero high-risk genotypes. These five effect estimates combine to show a doubling of HIV risk with prevalent high-risk HPV infection (summary HR=1.99 (95%CI=1.54-2.56), I2=8.4%, P heterogeneity=0.36). Excluding the unadjusted study by Veldhuijzen et al[28], a strong association persisted (summary HR=1.90 (95%CI=1.50-2.40), I2=0%, P heterogeneity=0.48, Forest Plot not presented).
Averbach[24], Smith-McCune[26] and Auvert[29] et al examined the risk associated with low-risk HPV infection in women. The latter study was excluded from the meta-analysis because the authors presented the linear trend involving the number of low-risk genotypes. In the two included studies, Averbach[24] compared infection with only low-risk genotypes (no high-risk) to no HPV, and Smith-McCune[26] compared infection with low-risk HPV irrespective of the presence of high-risk genotypes. A doubling of risk was seen with little evidence of heterogeneity between studies (Figure 2(iii) summary HR=2.01 (95%CI=1.27-3.20), I2=0%, P heterogeneity=0.29). The excluded study did not find an association between the number of low-risk HPV genotypes and HIV acquisition (adjusted hazard ratio(aHR) = 0.95 (95%CI=0.68-1.30 P linear trend=0.76)).
Smith-McCune[26] and Averbach[24] reported the risk of HIV acquisition associated with persistent and non-persistent genotype specific HPV in women (Figure 2(iv) and (v)). Both tested for HPV three monthly and defined persistent infection as two consecutive visits where genotype-specific HPV was detected. In multiple genotype infection, Averbach[24] defined persistence as all genotypes present at the following visit, and defined non-persistence as loss of any one of these. Smith-McCune[26] defined persistence as the repeat detection of any one specific genotype, and allowed non-detection between positive visits. Non-persistence was defined as HPV infection in individuals with more than one follow-up visit, which did not meet persistence criteria. Averbach[24] assessed HIV risk for any persistent or nonpersistent HPV, whereas Smith-McCune[26] disaggregated by high-risk and low-risk genotypes. All estimates of HIV risk from a persistent genotype-specific HPV infection showed no association, aHR=0.82 (95%CI=0.45-1.50) from high-risk[26], aHR=1.24 (95%CI=0.59-2.60) from low-risk[26] and aHR=0.97 (95%CI=0.51-1.85) from any HPV[24]. However in all studies there was a significantly increased risk when type-specific HPV was non-persistent aHR=1.67 (95%CI=1.03-2.74) from high-risk, aHR=2.09 (95%CI=1.27-3.44) from low-risk[26] and aHR=5.4 (95%CI=2.9-9.9) from any HPV[24]. Only one study in women assessed the risk of HIV acquisition associated with cervical cytological abnormalities. Smith-McCune[26] found no evidence that atypical squamous cells of uncertain significance (ASCUS), or any more severe cytological abnormality, diagnosed before HIV acquisition, was associated with increased risk (unadjusted HR=1.38 (95%CI=0.80-2.34)).
We found no publication reporting the association between HPV vaccine-specific genotypes and HIV acquisition in women. Authors from two of the six studies provided this on request (Table 1), although only one provided the effect estimate for the association between nonovalent vaccine genotypes HPV 6/11/16/18/31/33/45/52/58 and HIV acquisition. Averbach[24] found no association between infection with bivalent (16 or 18) or quadrivalent vaccine genotypes (6 or 11 or 16 or 18) when compared to no infection with these genotypes adjusted for the presence of other genotypes, and HIV acquisition. Smith-McCune[26], found that although infection with bivalent vaccine genotypes was not associated with HIV acquisition, infection with quadrivalent vaccine genotypes was associated with a doubling of HIV risk (aHR=2.00 (95%CI=1.00-3.99)). Further, they found that infection with nonovalent vaccine genotypes was associated with a more than 2.5 times increased risk (aHR=2.57 (95%CI=1.48-4.46)) when compared to no infection with those genotypes, even after adjustment for recent infection with other HPV genotypes and other prospectively collected confounders.
HPV infection and HIV acquisition in men
The systematic search revealed only one study in MSM and one in heterosexual men. Chin-Hong et al[23] found, in multivariable analysis, that infection with one HPV type compared with no HPV infection was not significantly associated with HIV acquisition in MSM (aHR=2.0 (95%CI=0.61-6.5)). However, the presence of infection with 2 or more HPV types compared with being HPV un-infected was associated with HIV acquisition (aHR=3.5 (95%CI=1.2-10.6)). In heterosexual men, Smith et al[30] found the presence of any HPV in the glans/coronal sulcus of the penis was associated with increased risk (aHR=1.8 (95%CI=1.1-2.9)). These authors repeated their original analysis, and compared HIV risk from infection with HPV 16/18, HPV 6/11/16/18 and HPV 6/11/16/18/31/33/45/52/58 to no infection with these genotypes adjusted for other HPV genotypes and confounding factors. Although these appeared to be associated with HIV, the associations were not statistically significant (Table 1).
One study in men assessed the risk of HIV acquisition associated with anal cytological abnormalities. Chin-Hong[23] found that in multivariable analysis, atypical squamous cells and low grade squamous intraepithelial lesions were not significantly associated with HIV acquisition (aHR=1.8 (95%CI=0.62-5.5) and aHR=1.2 (95%CI=0.44-3.23) respectively), consistent with results for cervical cytological abnormalities in women.
Bias within and across studies
Assessment of bias within studies is summarised in Table 2. Studies by Averbach[24] and Smith McCune[26] displayed a low risk of bias in all categories. Residual confounding, however, remains a concern in all studies.
Table 2. Risk of bias within studies.
| First Author | Selection of participants1 | Time of exposure ascertainment2 | Loss to follow- up3 |
Outcome ascertainment4 |
Confounding5,6 | Selective outcome reporting7 |
|---|---|---|---|---|---|---|
| Auvert[29] | All trial participants who provided at least one HPV result (47% of trial population). Those excluded (53%) had similar baseline characteristics Low risk |
First available HPV status was used (median follow-up 2.4y/2.2y in HPV uninfected/infected). Consistent results were seen using recent HPV results. Low risk |
12% loss to follow- up at 48 weeks. Unclear risk |
Median follow-up 2.5 years. Low risk |
Adjusted for sexual behavior at baseline, no adjustment for HSV2 High risk |
No a-priori plan. No response to request for additional analyses High risk |
| Averbach | 93% of cases included, exclusions | Repeated HPV testing, median 80 days | 12% loss to follow- | Median follow-up | Adjusted for prospective | Any HPV infection was the primary |
| [24] | due to missing information. Controls selected from within same cohort study Low risk |
between exposure and outcome Low risk | up at completion. Low risk |
21.9 months Low risk |
measurement of sexual behavior and HSV2 Low risk |
exposure of interest. Responded to request for additional analyses Low risk |
| Chin-Hong [23] |
No description of how subset (30% of EXPLORE study) were selected from participants in main study Unclear risk |
HPV assessed at baseline only, median time to outcome between 6 and 36 months8 Unclear risk |
Retention rate not documented. Unclear risk |
Median follow-up 3 years Low risk |
Adjusted for prospective sexual behavior, no adjustment for HSV2 High risk |
Primary analysis assessed the association of HPV infection with HIV acquisition No additional analysis provided Unclear risk |
| Low[25] | No description of how subset (40%)of participants were selected from participants in main study Unclear risk |
HPV assessed at baseline only, median time to outcome between 4 and 24 months Unclear risk |
Retention rate not documented. Unclear risk |
Median follow-up 1.7 years Low risk |
Adjusted for HSV2, no adjustment for sexual behavior High risk |
HPV analysis was not the primary aim of the publication. Additional analysis provided Low risk |
| Myer[27] | Cohort comprised of all HPV positives in main study and HPV negatives recruited over 1 year Unclear risk |
HPV assessed at baseline only, median time to outcome between 6 and 24 months Unclear risk |
25% loss to follow- up at 12months and 68% at 24 months. High risk |
Median follow-up 14.3 months Low risk |
Adjusted for baseline sexual behavior, no adjustment for HSV2 High risk |
HPV analysis was not the primary aim of the publication. Additional analysis provided Unclear risk |
| Smith[30] | Cohort comprised of trial participants consenting to HPV testing (80%) Low risk |
HPV assessed at baseline only, median time to outcome between 3 and 42 months Unclear risk |
Retention rate not documented. Unclear risk |
Median follow-up 42 months for 71% Low risk |
Adjusted for HSV2, no adjustment for sexual behaviour High risk |
No a-priori analysis plan. Additional analysis provided Low risk |
| Smith- McCune[26] |
Cohort comprised of trial participants consenting to HPV sub-study (98%) Low risk |
HPV assessed every 3 months. Recent infection was within 6 months excluding concurrent visit Low risk |
6% loss to follow-up. Low risk | Median follow-up 21 months Low risk |
Adjusted for prospective sexual behavior and HSV2 Low risk |
A-priori analysis plan. Additional analysis provided Low risk |
| Veldhuijzen [28] |
Cohort comprised of those with HPV results from main study (92%) Low risk |
HPV assessed at baseline only, median time to outcome between 3 and 18 months Unclear risk |
12% loss to follow- up. Low risk |
Median follow-up 16.6 months Low risk |
No adjustment for confounders High risk |
No a-priori plan. No additional analysis provided Unclear risk |
In case control studies here was a high risk of bias if >90% of cases were not included (and/or exclusion was related to exposure or outcome) or controls were not selected from within the cohort study. Unclear risk when this information was not available. In cohort studies, there was low risk of bias if the cohort were representative of the average individual in the population of interest, and both the exposed and unexposed were drawn from the same population. Unclear risk if this was not the case or not documented.
Low risk if HPV was tested prospectively and/or the time between exposure and outcome was <1year. High risk if this time was ≥ 1 year and unclear risk if insufficient information was available.
Low risk if retention was ≥80% at the end of the study. Unclear risk if information not available.
High risk of bias if follow-up was not long enough for HIV to be acquired (less than 1 year). Low risk if median follow-up was at least 1 year and unclear risk if information not available.
See table 1 for full list of confounders adjusted for.
Low risk of bias if adjustment for prospective measurements of sexual behavior AND HSV2. Unclear risk if adjustment for sexual behavior OR HSV2 serology at baseline only. High risk if HSV2 OR sexual behavior not adjusted for.
If (i)authors stated there was an a priori plan or stated which outcomes were of primary interest or (ii) authors responded to a request for further information and analysis then there was a low risk of bias. Of one of these was met this was unclear risk, if none were met this was high risk
Where median time was not available in studies which tested baseline HPV only it is assumed to be between minimum follow-up HIV test frequency and total study follow-up.
Low[25], Veldhuijzen[28] and Smith[30] et al did not adjust for sexual behaviour, which is associated with both HIV and HPV acquisition[31-33]. Chin-Hong[23], Smith-McCune[26] and Averbach[24] et al were the only authors who repeated the collection of sexual behaviour data prospectively. Even with the best sexual behaviour measure, prospectively collected, it is reasonable to assume that residual confounding will persist. Most studies included the parameters of condom use[23-24, 26-27, 29], multiple recent partners [23-24, 26-27, 29] and high-risk sex partners[23-24, 26], but only some studies recorded other important confounders such as transactional sex[24, 26, 29].
The panel of STIs tested, and whether they were measured prospectively, varied by study (Table 1). In some studies, HSV2[27-29] and bacterial vaginosis (BV)[26-27, 29-30] were not tested for, although both are associated with HPV and HIV acquisition[3, 34-36]. One study did not test for low-risk HPV[27], and others did not adjust for the presence of low-risk or other HPV types, although high and low-risk HPV were identified as independent risk factors for HIV[24, 26]. Reassuringly, contacted authors presented the HIV risk associated with vaccine genotypes adjusted for the presence of other (potentially confounding) HPV genotypes, and still identified a positive association.
Weak evidence of publication bias was seen using Begg’s test (P=0.06) (see supplementary Figure 1s for funnel plot).
Population attributable fractions
Three studies provided sufficient data to allow calculation of the proportion of HIV infections attributable to prevalent HPV infection (Table 3). 21 and 37% of HIV infections in women in studies in Zimbabwe[24] and South Africa[29] were attributable to infection with prevalent HPV of any genotype at the visit prior to HIV acquisition. 28% of HIV infections in Kenyan heterosexual men[30] were attributable to infection with HPV at baseline.
Table 3. Study-specific population attributable fractions.
| First Author | Study population | HPV genotype | Prevalence of HPV genotypes in HIV cases1 (%) |
Adjusted effect estimate (95% CI) |
Population attributable fraction (%) (95% CI2) |
|---|---|---|---|---|---|
| Averbach[24] | Women, Zimbabwe | Any HPV | 63.4 | 2.4 (1.5-4.0) | 37.0 (21.1-47.6) |
| Smith-McCune[26] | Women, Zimbabwe | Any HPV | 50.0 | 1.7 (1.0-2.9) | 20.8 (0-32.9) |
| Smith[30] | Heterosexual men, Kenya | Any HPV3 | 61.9 | 1.8 (1.1-2.9) | 27.5 (5.6-40.6) |
Before HIV acquisition
the C-I applies only to the proportion of HIV infections attributable to HPV in the individuals in these studies, and not in the wider population.
HPV in glans/coronal sulcus of penis
Discussion
This systematic review of the literature provides the first summary of published evidence of the association between prevalent HPV infection and HIV acquisition. Seven of eight studies showed evidence of an association between these infections and, where it was possible to calculate a PAF, a high proportion of HIV infections are attributable to infection with any HPV genotype. Combining the studies in women revealed a near doubling of risk when an HPV genotype was identified prior to HIV acquisition, with similar associations seen in the two studies in men. Although these results appear similar to associations between other STIs, such as HSV2, and HIV acquisition[3], significant concerns are raised in the assessment of quality: only two of the eight studies had a low risk of bias in all domains. Further, studies were performed in populations with a high prevalence of STIs and high-risk sexual behaviour (three of the studies in women were in commercial sex workers for example), and results may not be generalisable to women outside these groups.
Study quality was assessed by a components approach. In this assessment, two studies were identified as having a high risk of bias in more than one category. Prospective testing for HSV2 and sexual behaviour, two potentially strong confounders of the association between HPV infection and HIV acquisition, was only performed in two studies leaving the remaining six with a high risk of residual confounding. It is particularly difficult to collect sufficiently detailed, rigorous, sexual behaviour data and for that reason, residual confounding may affect all studies. These concerns serve to illustrate the limitation of observational research in determining causation. Additionally, all studies included in this analysis were secondary analyses of data, and only one stated an a-priori analysis plan; in-spite of this however, only weak evidence of publication bias was seen.
It was not possible to perform meta-analyses of the association between HPV and HIV in MSM or heterosexual men because the systematic search only revealed one of each of these studies. Combining these two studies, or all eight, was considered inappropriate because of the implicit heterogeneity in HIV acquisition between MSM, heterosexual men and women. The findings in men were of a positive association, with similar effect estimates seen for the risk of HIV acquisition from prevalent HPV infection, adding plausibility to the findings in women. A specific limitation of the study in MSM was the lack of testing for penile HPV[23]. Although HIV is frequently acquired through receptive anal intercourse in MSM, penile acquisition by the insertive male partner is possible.
HPV may display viral latency, with persistence in tissue below the limit of detection[37]. If this theory is correct, detection of HPV at the cervix may not necessarily indicate infection and likewise, lack of detection may not indicate absence of infection. HIV infection leads to a 5-fold increase in multiple new HPV infections within 6 weeks of HIV seroconversion[14]. The association between prevalent HPV infection and HIV acquisition may therefore be due to reverse causality if a recent, undiagnosed HIV infection had led to a rapid increased HPV prior to HIV confirmation in these studies. However, this would occur in a minority of cases. To minimise the risk of associations due to reverse causality, we excluded one study which described a strong association between HPV infection and HIV acquisition but included some HPV samples taken after HIV seroconversion[38]. Genital tract exposure to HIV prior to establishment of infection induces TLR-7. Since TLR-7 agonists (imiquimod) are used to treat genital warts, the observation seen in two studies that HPV clearance is association with HIV acquisition could in fact be attributed to HPV clearance being a marker of HIV exposure prior to infection rather than a risk-factor[24].
It is biologically plausible that prevalent HPV may increase the risk of HIV acquisition. It has been demonstrated that the E7 protein of HPV type-16 down-regulates an epithelial adhesion molecule called E-Cadherin[39], potentially increasing permeability of the genital lining to HIV. The lining of the genital tract contains Langerhans’ cells (LC), which can internalise HIV, preventing onward infection[40]. In HPV-infected tissue, a reduced density and altered morphology of LCs has been demonstrated[41-43]. The host immune response to HPV is mediated by T-lymphocytes[44], and this response may increase HIV risk since T-lymphocytes are primary target cells for HIV. An increased presence of these cells has been seen in HPV-infected cervical tissue[45]. Further, HPV non-persistence, which is likely to be associated with a T-lymphocyte influx, was associated with HIV acquisition in 2 studies in this review[24, 26], when persistent infection was not. Elevated levels of cytokine IL-Iβ, which activates a promoter region in the HIV genome[46], have also been demonstrated in women with HPV-associated abnormal cervical cytology[47] and defensins and thrombospondins, anti-HIV proteins, are also lower in precancerous cervical lesions[48] (although in this review cytological abnormalities were not associated with HIV acquisition[23, 26]).
In one Zimbabwean study[24], 37% of HIV infections in women have been attributed to infection with any HPV genotype. This is due to the high prevalence of HPV in women who later became infected with HIV (63%) and a large effect measure (aHR=2.4). Lower proportions (21% and 28%) were identified in studies with smaller effect measures and HPV prevalence. Since the PAF assumes a fully causal relationship these results must be interpreted with caution. Although vaccines protecting against infection with increasing number of HPV genotypes are being developed, and cross-protection has been demonstrated with current vaccines[12-13], a pan-valent vaccine is not currently available and prevention of infection from all HPV genotypes is not currently possible. Despite their limitations, the PAF estimates are presented to give some indication of the overall effect of HPV on HIV acquisition in endemic settings, and suggest that effective HPV control measures might have a significant impact on the HIV epidemic.
The proportion of HIV infections attributable to infection with high-risk, low-risk, and vaccine-specific HPV genotypes are not presented. There were insufficient data from existing studies to provide accurate estimates for PAFs for vaccine-specific HPV genotypes since studies were not powered to detect this specific association. Assuming a causal effect from a small number of genotypes and attributing HIV to those genotypes could be misleading. Further robust observational studies are needed to address this issue.
In conclusion, meta-analysis of studies in women showed a strong association between prevalent HPV infection and HIV acquisition, although studies were at risk of residual confounding. In heterosexual men and MSM the findings were consistent with those in women, although there were insufficient studies to perform meta-analysis. Of the three studies which evaluated the association between detection of HPV genotypes available in vaccines and HIV acquisition, one found a strong independent association.
The HPV vaccine is highly effective in the primary prevention of HPV-associated cervical cancers and genital warts. Clarification of the findings presented in this study through well-conducted research is needed in high HPV/HIV settings, in order to assess whether HPV vaccination might have an effect on HIV incidence. Surveillance of HIV incidence rates over time in counties implementing HVP vaccination of girls, and nested case control studies examining HPV vaccination status in HIV positive cases versus HIV negative controls, are necessary.
Supplementary Material
Acknowledgments
We thank Rebecca Nowak of Johns Hopkins University for additional analyses from the dataset provided by Averbach et al[24].
Conflicts of interest and sources of funding: DWJ has received research grants from GSK Biologicals for HPV vaccine-related research. CH was supported through an MRC Masters Award (grant no. MRC002630) and a Wellcome Trust Clinical Fellowship (grant no. ITCRBE30). LK, KSM and SS receive funding from the Bill and Melinda Gates Foundation, JSS is funded by the National Cancer Institute and PEG and KSM by the National Institute for Health (NIH)
Footnotes
Data presented previously at XIX International AIDS Conference 22-27 July, abstract number WEPE258.
References
- 1.UNAIDS . Global Report: UNAIDS Report on the Global AIDS Epidemic. 2010. [Google Scholar]
- 2.Rerks-Ngarm S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361(23):2209–20. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 3.Freeman EE, et al. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20(1):73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 4.Winer RL, et al. Risk of female human papillomavirus acquisition associated with first male sex partner. J Infect Dis. 2008;197(2):279–82. doi: 10.1086/524875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munoz N, et al. Chapter 1: HPV in the etiology of human cancer. Vaccine. 2006;24(Suppl 3):1–10. doi: 10.1016/j.vaccine.2006.05.115. [DOI] [PubMed] [Google Scholar]
- 6.Dunne EF, et al. Prevalence of HPV infection among men: A systematic review of the literature. J Infect Dis. 2006;194(8):1044–57. doi: 10.1086/507432. [DOI] [PubMed] [Google Scholar]
- 7.Smith JS, et al. Age-specific prevalence of infection with human papillomavirus in females: a global review. J Adolesc Health. 2008;43(4 Suppl):S5–25. S25 e1–41. doi: 10.1016/j.jadohealth.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Monk BJ, Tewari KS. The spectrum and clinical sequelae of human papillomavirus infection. Gynecol Oncol. 2007;107(2 Suppl 1):S6–13. doi: 10.1016/j.ygyno.2007.07.076. [DOI] [PubMed] [Google Scholar]
- 9.Romanowski B, et al. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet. 2009;374(9706):1975–85. doi: 10.1016/S0140-6736(09)61567-1. [DOI] [PubMed] [Google Scholar]
- 10.Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356(19):1915–27. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 11.Paavonen J, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374(9686):301–14. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 12.Bonanni P, Boccalini S, Bechini A. Efficacy, duration of immunity and cross protection after HPV vaccination: a review of the evidence. Vaccine. 2009;27(Suppl 1):A46–53. doi: 10.1016/j.vaccine.2008.10.085. [DOI] [PubMed] [Google Scholar]
- 13.Wheeler CM, et al. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13(1):100–10. doi: 10.1016/S1470-2045(11)70287-X. [DOI] [PubMed] [Google Scholar]
- 14.Wang C, et al. Rapid rise in detection of human papillomavirus (HPV) infection soon after incident HIV infection among South African women. J Infect Dis. 2011;203(4):479–86. doi: 10.1093/infdis/jiq083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molijn A, et al. Molecular diagnosis of human papillomavirus (HPV) infections. J Clin Virol. 2005;32(Suppl 1):S43–51. doi: 10.1016/j.jcv.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Carter JJ, et al. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J Infect Dis. 2000;181(6):1911–9. doi: 10.1086/315498. [DOI] [PubMed] [Google Scholar]
- 17.Ogilvie GS, et al. Diagnostic accuracy of self collected vaginal specimens for human papillomavirus compared to clinician collected human papillomavirus specimens: a meta-analysis. Sex Transm Infect. 2005;81(3):207–12. doi: 10.1136/sti.2004.011858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Cochrane Collaboration . In: Cochrane handbook for systematic reviews of interventions. Higgins Julian PT, Green Sally., editors. 2009. [Google Scholar]
- 19.Liberati A, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DerSimonian R, N Laird, Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002;21(11):1559–73. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
- 22.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 23.Chin-Hong PV, et al. Anal human papillomavirus infection is associated with HIV acquisition in men who have sex with men. AIDS. 2009;23(9):1135–42. doi: 10.1097/QAD.0b013e32832b4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Averbach SH, et al. The association between cervical human papillomavirus infection and HIV acquisition among women in Zimbabwe. AIDS. 2010;24(7):1035–42. doi: 10.1097/qad.0b013e3283377973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Low AJ, et al. Genital warts and infection with human immunodeficiency virus in high-risk women in Burkina Faso: a longitudinal study. BMC Infect Dis. 2011;11:20. doi: 10.1186/1471-2334-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith-McCune KK, et al. Type-specific cervico-vaginal human papillomavirus infection increases risk of HIV acquisition independent of other sexually transmitted infections. PLoS One. 2010;5(4):e10094. doi: 10.1371/journal.pone.0010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myer L, et al. Prospective study of hormonal contraception and women’s risk of HIV infection in South Africa. Int J Epidemiol. 2007;36(1):166–74. doi: 10.1093/ije/dyl251. [DOI] [PubMed] [Google Scholar]
- 28.Veldhuijzen NJ, Vyankandondera J, van de Wijgert JH. HIV acquisition is associated with prior high-risk human papillomavirus infection among high-risk women in Rwanda. AIDS. 2010;24(14):2289–92. doi: 10.1097/QAD.0b013e32833cbb71. [DOI] [PubMed] [Google Scholar]
- 29.Auvert B, et al. High-Risk Human Papillomavirus Is Associated with HIV Acquisition among South African Female Sex Workers. Infect Dis Obstet Gynecol. 2011:692012. doi: 10.1155/2011/692012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith JS, et al. Increased risk of HIV acquisition among Kenyan men with human papillomavirus infection. J Infect Dis. 2010;201(11):1677–85. doi: 10.1086/652408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giuliano AR, Anic G, Nyitray AG. Epidemiology and pathology of HPV disease in males. Gynecol Oncol. 2010;117(2 Suppl):S15–9. doi: 10.1016/j.ygyno.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Auvert B, et al. Ecological and individual level analysis of risk factors for HIV infection in four urban populations in sub-Saharan Africa with different levels of HIV infection. AIDS. 2001;15(Suppl 4):S15–30. doi: 10.1097/00002030-200108004-00003. [DOI] [PubMed] [Google Scholar]
- 33.Stanley M. Pathology and epidemiology of HPV infection in females. Gynecol Oncol. 2010;117(2 Suppl):S5–10. doi: 10.1016/j.ygyno.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 34.van de Wijgert JH, et al. Bacterial vaginosis and vaginal yeast, but not vaginal cleansing, increase HIV-1 acquisition in African women. J Acquir Immune Defic Syndr. 2008;48(2):203–10. doi: 10.1097/QAI.0b013e3181743936. [DOI] [PubMed] [Google Scholar]
- 35.Veldhuijzen NJ, et al. Factors affecting transmission of mucosal human papillomavirus. Lancet Infect Dis. 2010;10(12):862–74. doi: 10.1016/S1473-3099(10)70190-0. [DOI] [PubMed] [Google Scholar]
- 36.Gillet E, et al. Bacterial vaginosis is associated with uterine cervical human papillomavirus infection: a meta-analysis. BMC Infect Dis. 2011;11:10. doi: 10.1186/1471-2334-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gravitt PE. The known unknowns of HPV natural history. J Clin Invest. 2011;121(12):4593–9. doi: 10.1172/JCI57149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Auvert B, et al. Association of oncogenic and nononcogenic human papillomavirus with HIV incidence. J Acquir Immune Defic Syndr. 2010;53(1):111–6. doi: 10.1097/QAI.0b013e3181b327e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laurson J, et al. Epigenetic repression of E-cadherin by human papillomavirus 16 E7 protein. Carcinogenesis. 31(5):918–26. doi: 10.1093/carcin/bgq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Witte L, et al. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat Med. 2007;13(3):367–71. doi: 10.1038/nm1541. [DOI] [PubMed] [Google Scholar]
- 41.Jimenez-Flores R, et al. High-risk human papilloma virus infection decreases the frequency of dendritic Langerhans’ cells in the human female genital tract. Immunology. 2006;117(2):220–8. doi: 10.1111/j.1365-2567.2005.02282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caberg JH, et al. Increased migration of Langerhans cells in response to HPV16 E6 and E7 oncogene silencing: role of CCL20. Cancer Immunol Immunother. 2009;58(1):39–47. doi: 10.1007/s00262-008-0522-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uchimura NS, et al. Evaluation of Langerhans’ cells in human papillomavirus-associated squamous intraepithelial lesions of the uterine cervix. Clin Exp Obstet Gynecol. 2004;31(4):260–2. [PubMed] [Google Scholar]
- 44.Stanley MA. Immunobiology of papillomavirus infections. J Reprod Immunol. 2001;52(1-2):45–59. doi: 10.1016/s0165-0378(01)00113-9. [DOI] [PubMed] [Google Scholar]
- 45.Scott M, Nakagawa M, Moscicki AB. Cell-mediated immune response to human papillomavirus infection. Clin Diagn Lab Immunol. 2001;8(2):209–20. doi: 10.1128/CDLI.8.2.209-220.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Osborn L, Kunkel S, Nabel GJ. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci U S A. 1989;86(7):2336–40. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Behbakht K, et al. Role of the vaginal microbiological ecosystem and cytokine profile in the promotion of cervical dysplasia: a case-control study. Infect Dis Obstet Gynecol. 2002;10(4):181–6. doi: 10.1155/S1064744902000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herfs M, et al. Mucosal junctions: open doors to HPV and HIV infections? Trends Microbiol. 2010;19(3):114–120. doi: 10.1016/j.tim.2010.12.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.