Abstract
We report that the EGFR pathway plays a critical role in regulating cancer stem-like cells (CSCs) in nasopharyngeal carcinoma (NPC), one of the most common malignant tumors in Southeast Asia. Effects of EGFR on maintaining CSCs are mainly mediated by AKT signaling, and β-catenin is responsible for governing CSC properties in response to EGFR/AKT activation. Significantly, CSCs are enriched by cisplatin and decreased by gefitinib in NPC xenograft models. Upon reimplantation in secondary mice, tumor cells derived from cisplatin-treated mice grew rapidly, whereas regrowth of tumor cells from gefitinib-treated mice was severely diminished. We further demonstrate that expression of EGFR correlates with expression of β-catenin and Nanog in primary tumor specimens from NPC patients. These findings provide mechanistic and preclinical evidence supporting the use of gefitinib alone or in combination with a chemotherapeutic agent in first-line therapy for patients with NPC. In addition, our results suggest that targeting β-catenin represents a rational clinical modality for patients whose tumors harbor activated EGFR or AKT.
Keywords: Nasopharyngeal carcinoma, Cancer stem-like cells, EGFR, β-catenin, Gefitinib
Introduction
The epidermal growth factor receptor (EGFR) plays a critical role in regulating proliferation, differentiation and survival of epithelial cells and tumors of epithelial cell origin [1-3]. Previous studies have revealed that a single progenitor cell from either embryonic or adult mouse brain proliferates in response to EGF and generates undifferentiated cells with the properties of neuroepithelial stem cells [4, 5]. EGFR signaling is also required for the differentiation and maintenance of neural progenitors from Drosophila embryos [6] and for self-renewal and differentiation of rat embryonic stem cells [7]. Furthermore, EGFR modulates a side population in human head and neck carcinoma cell lines, which exhibits stem cell like properties [8]. These findings suggest that EGFR may play an important role in regulating and maintaining human cancer stem-like cells (CSCs), a rare subpopulation of self-renewal cancer cells that could initiate tumors and promote cancer progression and may account for the failure of current therapies to eradicate malignant tumors [9, 10].
Wnt/β-catenin signaling pathway has been implicated in regulation of embryonic development, cell proliferation, and self-renewal of CSCs in several types of tumors [11]. The canonical Wnt pathway consists of a series of events that eventually lead to the stabilization and translocation of β-catenin into the nucleus, where β-catenin accelerates expression of a broad range of Wnt target genes via binding to the TCF/LEF family of transcription factors. Recent studies revealed that the effect of AKT signaling on stem cells is also mediated by β-catenin [12-14]. Akt activates β-catenin and induces its nuclear translocation either by phosphorylation of the C terminus of β-catenin at Ser552 [12], or indirectly through phosphorylation and inactivation of GSK3β, resulting in hypophosphorylation of β-catenin at S33/S37/T41 [15].
Naopharyngeal carcinoma (NPC) is a rare epithelial cancer in most parts of the world. However, it is one of the most common malignant tumors in Southeast Asia and southern China, with an incidence of 25-50 per 100,000, which is 25-fold higher than that observed in western countries [16]. The 5-year survival for stage IV NPC is only 30%, and poor survival is often associated with local, regional and systemic recurrences [17]. Since surgical approaches for NPC is limited due to the tumor’s inaccessible anatomic nature and the fact that NPCs are sensitive to radiation, the primary treatment modality for NPC is radiotherapy with or without chemotherapy [18].
Recent studies showed that NPC contains a small fraction of cells with properties of CSCs; this tumor subpopulation plays a critical role in tumorigenesis and drug resistance [19-21]. In the present study, we investigated the role of EGFR in the maintenance, self-renewal and tumorigenesis of CSCs. We found that activation of EGFR increased the number of CSCs, and this effect of EGFR was mediated by PI3K/AKT/β-catenin signaling. In a NPC xenograft model using nude mice, CSCs were eradicated by treatment with gefitinib, whereas they were enriched by treatment with cisplatin. Thus, our findings reveal distinct effects of gefitinib and cisplatin on CSCs versus the general tumor cell population, which may have important clinical implications for the treatment of NPC.
Results
EGFR expression in NPC cells and inhibition effects of gefitinib
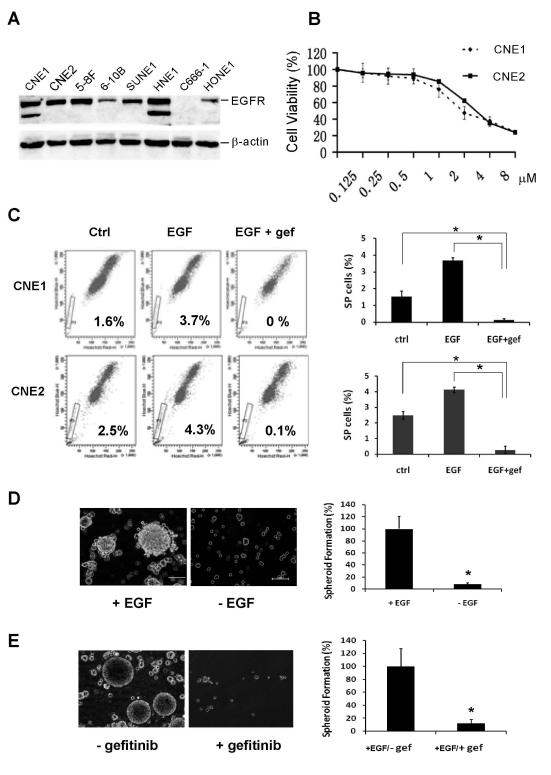
EGFR is widely expressed in a variety of human tumors, and inhibition of EGFR has been exploited as a therapeutic strategy in several solid tumor types [22, 23]. In NPC, EGFR is expressed in 50-80% of NPC specimens and represents a negative prognostic factor. Furthermore, EGFR expression was significantly linked to low overall survival and shorter time to progression [24]. To investigate the effect of EGFR on CSCs in NPC, we first examined expression levels of EGFR in NPC cell lines. Western blot analyses revealed that EGFR protein is expressed at various levels in 7 of 8 NPC cell lines analyzed (Fig. 1A). C666-1, the only cell line that did not express EGFR, exhibits mesenchyme-like morphology (data not shown). EGFR expression in primary tumor specimens from 22 NPC patients was assessed using immunohistochemical staining. Twelve samples (54.5%) showed detectable levels of EGFR expression (Fig. 6). To determine the inhibitory effects of gefitinib on NPC cell viability, two cell lines, CNE1 and CNE2, were used in this study. CNE1 cells are differentiated and CNE2 cells are poorly differentiated NPC cell lines [25]. As compared to untreated cells, treatment with gefitinib for 72 h significantly inhibited cell viability of both cell lines, with an IC50 of 2.63 μmol/L and 3.11 μmol/L for CNE1 and CNE2, respectively (Fig. 1B).
Fig. 1.
Inhibition of CSCs by gefitinib in NPC. (A) Expression levels of EGFR in 8 NPC cell lines. (B) Antiproliferative effects of gefitinib in CNE1 and CNE2 cell lines. Cells were treated with various concentrations of gefitinib for 72 h, and cell viability was determined by MTT assay. Error bars indicate standard deviations. The experiment was conducted three times with similar results. (C) Inhibition of side population (SP) by gefitinib (1 μM) in NPC cells. Data are presented as mean ± SD (n = 3). *, P< 0.01. (D) EGF dependence of tumor spheroid formation of CNE2 cells. (E) Inhibition of tumor spheroid formation by gefitinib. CNE2 cells derived from primary tumor spheroids were cultured in suspension growth medium containing EGF and bFGF. Gefitinib was added at 2 μM. Bar = mean ± SD (n = 3). *, P< 0.01. Scale bars: 100 μm.
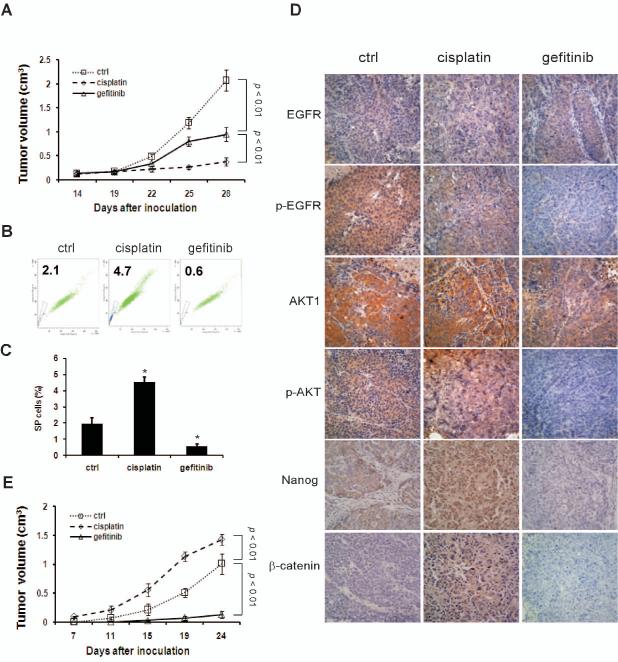
Fig. 6.
Inhibition of CSCs by gefitinib in NPC xenografts. (A) Nude mice harboring CNE2 cell xenografts were treated with saline, gefitinib, or cisplatin for 2 weeks as described in Materials and methods. Graphs represent mean volume ± SD (n = 6). P< 0.01. (B and C) Cells dissociated from primary xenografts were subjected to FACS analyses. Percentages of SP cells were significantly decreased by gefitinib and increased by cisplatin. Bar = mean ± SD (n = 3). *, P< 0.01. (D) Immunohistochemical staining of primary tumors from the mouse model. Photographs were taken at ×200 magnification. (E) Cells from primary xenografts were reimplanted into nude mice for development of secondary tumors. Gefitinib abrogated tumor regeneration in secondary mice. Graphs represent mean volume ± SD (n = 6).
Regulation of Side population (SP) cells by EGFR signaling in NPC
SP cells are a small subpopulation of tumor cells that exhibit CSC properties in a variety of neoplasms [26]. A previous study showed that SP cells in human NPC cell line CNE-2 had stem-like cell characteristics in vitro and a strong ability to form tumors in vivo [19]. These cells are characterized by their high ability to efflux the fluorescent dye Hoechst 33342 from the cytoplasm through ATP-binding cassette transporters [27, 28]. To investigate whether EGFR could be a regulatory factor for SP cells in NPC, logarithmically growing CNE1 and CNE2 cells were subjected to flow cytometric analysis. The untreated CNE1 and CNE2 cell lines contained 1.6% and 2.5% Hoechst 33342-dull SP cells, respectively. Treatment with EGF increased the SP by about 130% in CNE1 cells and 70 % in CNE2 cells, and this stimulatory effect of EGF was completely blocked by gefitinib (Fig. 1C). Similar results were observed when gefitinib was replaced with another EGFR tyrosine kinase inhibitor PD153035 in these experiments (Fig. 2A).
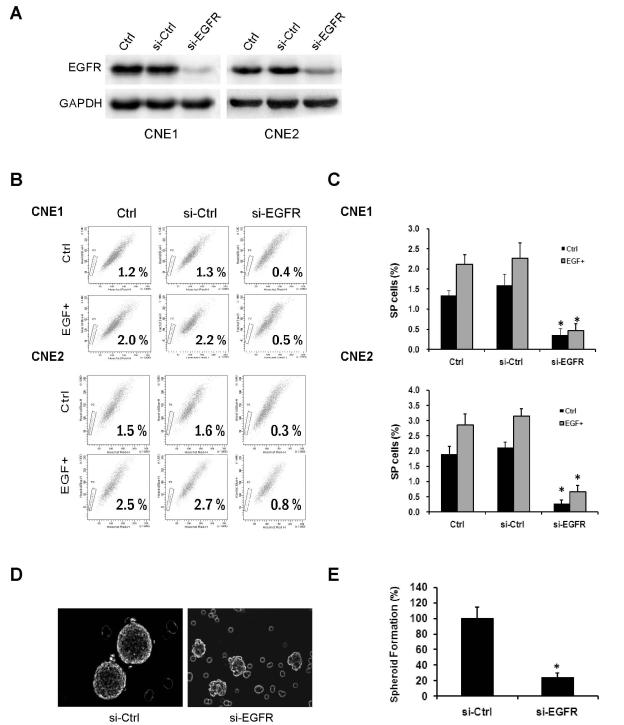
Fig. 2.
Knockdown of EGFR with siRNA diminishes CSC properties. (A) CNE1 and CNE2 cells were transfected with control siRNA or siRNA targeting EGFR followed by Western blot analysis. In both cell lines, exposure to EGFR siRNA substantially reduced EGFR expression. (B and C) After transfected with control siRNA or EGFR siRNA, CNE1 and CNE2 cells were treated with or without EGF (50 ng/mL). Knockdown of EGFR reduced the percentages of the SP of CNE1 and CNE2 at both basal levels and after EGF stimulation. Data are presented as mean ± SD (n = 3). *, P< 0.01. (D and E) Depletion of EGFR suppressed tumor spheroid formation of CNE2 cells. Bars are means ± SD (n = 3). *, P< 0.01.
EGFR signaling is essential for tumor spheroid formation and proliferation
CSCs from several types of cancer have been isolated and propagated as non-adherent three-dimensional tumor spheroids that are known to closely mimic phenotypes of in vivo tumors [29]. To examine whether EGFR signaling is essential for tumor spheroid formation in NPC, CNE2 cells were cultured in serum-free medium containing bFGF. When EGF was supplemented at 10 ng/mL, floating tumor spheroids were observed after 7 days of cultivation. In the absence of EGF, however, tumor spheroid formation was significantly inhibited (Fig. 1D). To further determine the role of EGFR signaling in the self-renewal of CSCs, tumor spheroids that formed in the presence of EGF were dissociated enzymatically to obtain a single-cell suspension and then replated in tissue culture dishes. The cells formed secondary tumor spheroids in the above growth medium supplemented with EGF. However, when gefitinib was present in the medium, tumor spheroid formation was markedly reduced, and most cells died by day 7 (Fig. 1E).
Knockdown of EGFR diminishes the CSC phenotype
To further validate the effect of EGFR signaling on the CSC phenotype, we used small-interfering RNA (siRNA) to knock down EGFR expression in CNE1 and CNE2 cells (Fig. 2A). Compared with untransfected cells, CNE1 and CNE2 cells transfected with EGFR siRNA displayed reduced percentages of SP whereas cells transfected with control siRNA had no obvious effect. When cells were grown in 50 ng/mL EGF, the percentage of SP cells was increased in untransfected cells and in cells transfected with control siRNA, but not in cells transfected with EGFR siRNA (Fig. 2B and C). Next, we measured the effect of EGFR knockdown on tumor spheroid formation. CNE2 cells were cultured in serum-free medium containing EGF and bFGF. Knockdown of EGFR significantly reduced the number of tumor spheroids (Fig. 2D and 2E).
EGFR/PI3K/AKT pathway regulates CSC phenotypes in NPC
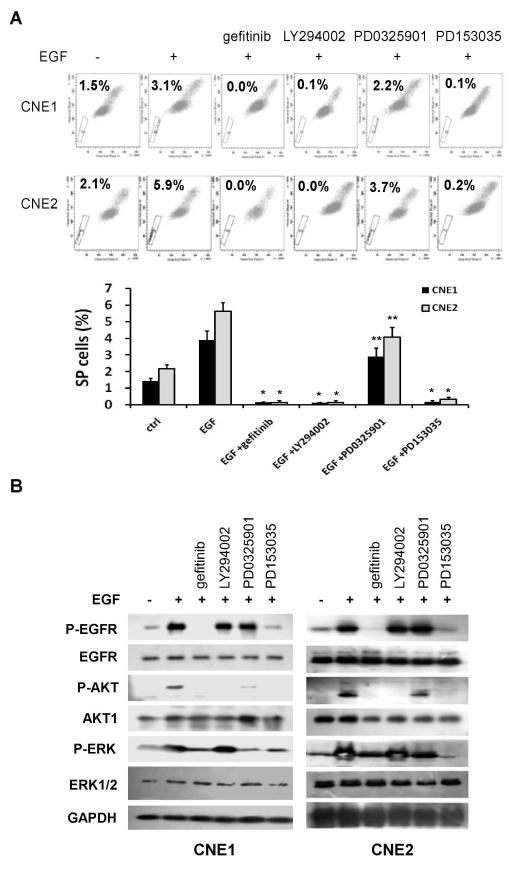
Since EGFR signaling exerts its biological functions mainly through Raf/Mek/ERK and PI3K/AKT pathways, we asked which pathway mediates the regulatory function of EGFR on CSC phenotypes. To this end, cells were treated for 24 h with EGFR inhibitor gefitinib (1 μM), PI3K inhibitor LY294002 (15 μM), or Mek inhibitor PD0325901 (10 μM). Treatment with EGF increased the percentage of SP cells, and this effect was blocked by gefitinib or PD153035. When gefitinib was replaced by LY294002, the PI3K inhibitor abolished the SP. On the other hand, the Mek inhibitor PD0325901 had only a modest effect on the percentage of SP cells (Fig. 3A).
Fig. 3.
EGFR/PI3K/AKT pathway regulates CSC phenotype in NPC. (A) CNE1 and CNE2 cells were treated with indicated inhibitors for 24 h followed by FACS SP analyses. EGF (50 ng/mL)-induced SP cells were diminished by EGFR inhibitor (either 1 μM of gefitinib or 0.5 μM PD153035) or PI3K inhibitor LY294002 (15 μM), but not by Mek inhibitor PD0325901 (10 μM). Data are presented as mean ± SD (n = 3). *, P< 0.01; **, P> 0.05. (B) Cells were treated as described in (A) followed by Western blot analysis. EGF-induced phosphorylation of EGFR, AKT and ERK1/2 was blocked by gefitinib or PD153035.
We next examined the effects of gefitinib on EGFR activity and downstream effectors using phospho-specific antibodies and Western blot analysis. As shown in Figure 3B, treatment of NPC cells with EGF for 24 h resulted in marked increases in the phosphorylation levels of EGFR, AKT and ERK1/2, indicating activation of EGFR and its downstream effectors by EGF. When gefitinib or another EGFR inhibitor PD153035 was added, the effects of EGF were diminished substantially. The molecular target specificity of LY294002 and PD0325901 was also validated: LY294002 abolished phosphorylation of AKT, but not EGFR or ERK. Likewise, PD0325901 inhibited phosphorylation of ERK, but not EGFR or AKT.
The effect of EGFR on CSCs is mediated through downstream β-catenin signaling
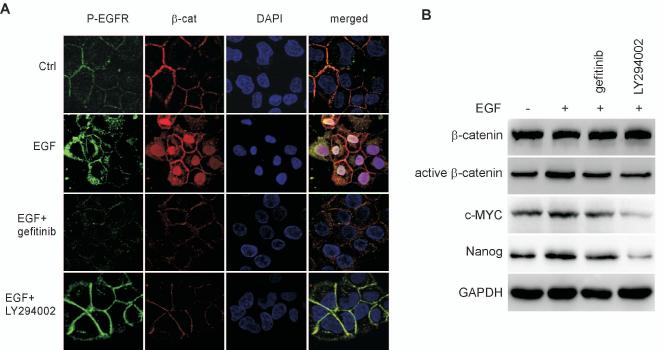
The AKT kinase family regulates a variety of cellular processes including proliferation, survival, and metabolism. AKT kinases control these processes through phosphorylation-mediated regulation of numerous substrates [30]. As shown above, we have demonstrated that the effect of EGFR signaling on the CSC phenotype is mediated by AKT, and β-catenin was reported to be responsible for mediating the effect of AKT signaling on stem cells [12, 13]. Given these observations, we next investigated whether β-catenin plays a role in the EGFR/AKT pathway governing CSCs in NPC. We first examined the effect of EGFR signaling on the subcellular localization of β-catenin in CNE2 cells (Fig. 4A). In untreated cells, β-catenin was located predominantly at the plasma membrane, with faint staining distributed in the cytoplasm. When cells were treated with EGF, β-catenin staining shifted from the cytoplasmic membrane to the nucleus. Inhibition of EGFR with gefitinib or inhibition of PI3K with LY294002 reversed the accumulation of β-catenin in the nucleus. To confirm the results of immunofluorescent staining, we performed Western blot analysis with an antibody specific for the active form of β-catenin, dephosphorylated on Ser37 or Thr41 (Fig. 4B). In response to EGF stimulation, expression level of active form of β-catenin was increased. This effect of EGF was blocked by gefitinib or LY294002. Importantly, we further found that EGFR signaling regulates expression of c-Myc oncogene that is a critical target of β-catenin [31] as well as an essential factor for reprogramming adult cells into induced pluripotent stem (iPS) [32]. Nanog, a critical stem cell maker, was also regulated by EGFR signaling, which is in agreement with a previous report demonstrating that β-catenin up-regulates Nanog expression in embryonic stem cells [33]. Thus, the above experiments reveal a functional EGFR/PI3K/AKT/β-catenin axis that regulates the CSC phenotype in NPC cells.
Fig. 4.
The effect of EGFR on the CSC phenotype is mediated through β-catenin signaling. (A) In untreated CNE2 cells, β-catenin was located in cytoplasmic membranes and cytoplasm. Treatment with EGF (50 ng/mL) resulted in increased β-catenin nuclear staining, which was reversed by either gefitinib (1 μM) or LY294002 (15 μM). (B) Western blot analysis showed that elevated expression of active β-catenin, c-myc, and Nanog in response to EGF (50 ng/ml) can be reversed by either gefitinib (1 μM) or LY294002 (15 μM).
Subsequently, we investigated the requirement of β-catenin in the maintenance of CSCs in NPC. To this end, β-catenin was knocked down with lentivirus-mediated shRNAs (Fig. 5A). Compared with cells infected with the control shRNA (sh-GFP) lentivirus, cells infected with sh-β-catenin lentivirus exhibited decreased percentage of SP cells at both basal levels and after EGF stimulation (Fig. 5B). Likewise, knockdown of β-catenin also markedly reduced tumor spheroid formation in the absence or presence of gefitinib (Fig. 5C). Taken together, these results provide mechanistic evidence for a role of β-catenin in maintaining the CSC population and indicate that β-catenin is a crucial mediator of a signal transduction pathway downstream of EGFR/PI3K/AKT.
Fig. 5.
Knockdown of β-catenin inhibits CSC properties. (A) CNE1 and CNE2 cells were infected with control shRNA lentivirus or β-catenin shRNA. In both cell lines, β-catenin protein was markedly reduced following expression of β-catenin shRNA. (B) Infection with β-catenin shRNA lentivirus reduced the percentage of SP cells at the basal level and after EGF (50 ng/mL) stimulation. Bar = mean ± SD (n = 3). *, P< 0.01. (C) Compared with cells infected with the control shRNA (sh-GFP) lentivirus, cells infected with sh-β-catenin lentivirus exhibited reduced tumor spheroid formationin the absence or presence of gefitinib. Bar = mean ± SD (n = 3). *, P< 0.01. Scale bars: 100 μm.
Gefitinib inhibits CSCs in NPC xenografts
To further evaluate the importance of EGFR signaling in the maintenance of CSC self-renewal and evaluate the anti-CSC efficacy of gefitinib in vivo, we performed experiments in xenograft models with nude mice. CNE2 cells were subcutaneously implanted into the flanks of mice. After two weeks, mice were treated with saline, gefitinib or cisplatin, the latter being the most commonly prescribed chemotherapeutic drug for patients with NPC. Mice were euthanized 2 weeks later, and tumor size was measured. Treatment with gefitinib resulted in a 53% decrease in the mean tumor size, while cisplatin dramatically reduced the tumor size by 82% when compared to the control treatment (Fig. 6A). Drug toxicity was evaluated by measuring body weight of the mice. Gefitinib-treated mice showed only a modest (7%) weight loss. In contrast, cisplatin treatment resulted in a 30% reduction in body weight (data not shown), underscoring the differential toxicities of the two drugs.
To determine if CSCs are affected by these treatments, tumor tissues were isolated from animals and disaggregated into single cells. CSCs were evaluated by SP analyses (Fig. 6B). Compared with the control, gefitinib reduced the percentage of SP cells by 71%, whereas cisplatin increased the SP-positive populations by 123%. To further determine the effects of gefitinib and cisplatin on EGFR/AKT/β-catenin signaling, primary tumor sections were stained immunohistochemically with antibodies against different components of this pathway (Fig. 6D). While neither cisplatin nor gefitinib had an effect on the expression levels of EGFR or AKT1/2, gefitinib, but not cisplatin, markedly inhibited phosphorylation of EGFR and AKT. Importantly, while tumors from control mice exhibited faint and diffused staining of β-catenin, treatment with cisplatin resulted in strong nuclear accumulation of the protein that was barely noticeable in tumors treated with gefitinib. Furthermore, the stem cell maker Nanog was reduced by gefitinib and moderately increased by cisplatin.
To determine tumorigenic potential of the residual cancer cells spared by drug treatments, we performed a more definitive assay to evaluate their self-renewal ability in nude mice [34]. For this purpose, 1×105 living cells dissociated from primary xenografts were reimplanted into nude mice to assess the development of secondary tumors. Analysis of the resulting tumors from each group revealed that cancer cells from cisplatin-treated mice grew rapidly and formed visible tumors by day 7, while cells from the control group produced tumors by day 11. In contrast, the regrowth capability of cells from gefitinib-treated mice was severely diminished in the recipient animals. At 24 days, tumors derived from xenografts of cisplatin-treated mice reached an average size of 1.42 ± 0.09 cm3, 1.4 times larger than those from the control mice (1.01 ± 0.18 cm3) (Fig. 6E). The above xenograft experiments were performed in duplicate, with similar results obtained. In summary, our in vitro and in vivo experiments indicate that gefitinib preferentially targets CSCs and eliminates tumor cell regrowth, whereas cisplatin predominantly kills the bulk population of the tumor, leading to enrichment of CSC and fostering tumor regrowth.
Expression of EGFR, β-catenin, and Nanog is correlated in human NPC samples
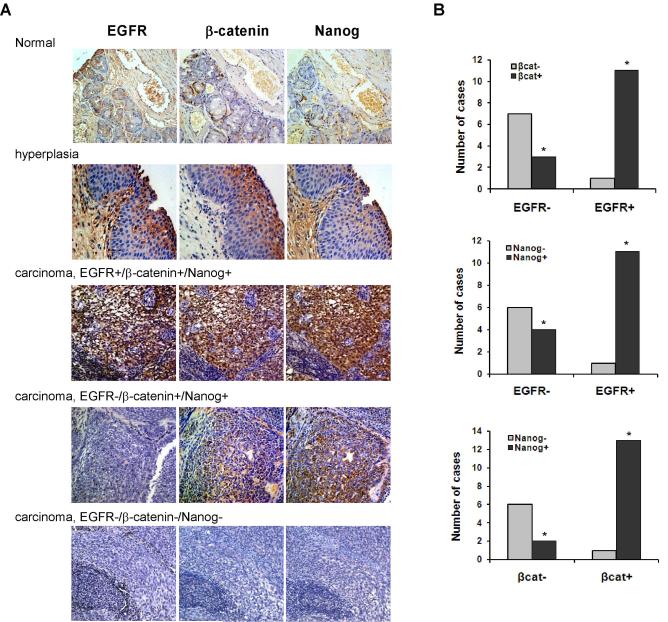
Our in vitro and in vivo experiments demonstrate that CSCs are regulated by EGFR signaling. To determine whether the same effect of EGFR occurs in primary tumors from NPC patients, we performed an immunohistochemical analysis using a tumor tissue microarray consisting of paraffin-embedded NPC samples derived from 22 patients using antibodies against EGFR, β-catenin, and Nanog. In addition, the microarray slides also contain cores from normal nasopharyngeal epithelial tissues, used as negative controls (Fig. 7A). Positive immunostaining for EGFR was found in 12 of 22 tumors (54.5%). Eleven of the 12 (92%) specimens that stained positively for EGFR also displayed both β-catenin and Nanog immunoreactivity. Among the 10-EGFR negative specimens, 3 displayed both β-catenin and Nanog staining, whereas 6 showed neither β-catenin nor Nanog staining, and 1 stained only for Nanog (Fig. 7B, upper and middle graphs). Importantly, both β-catenin and Nanog displayed nuclear staining patterns in cancer tissues, while β-catenin showed cell membrane localization and Nanog exhibited cytoplasmic distribution in hyperplasia specimens. Statistical analysis of these results revealed that EGFR positivity is significantly associated with that of β-catenin and Nanog (P< 0.01 and P< 0.05, respectively). Intriguingly, immunoreactivity of β-catenin also correlated significantly with that of Nanog (P< 0.01), with 12 of 22 tumors (58%) tumors displaying both β-catenin and Nanog staining, and 7 of 22 (34%) tumors displaying neither β-catenin nor Nanog staining (Fig. 7B, lower graph).
Fig. 7.
Correlation of expression of EGFR, β-catenin, and Nanog in human NPC samples. (A) Immunohistochemical studies of representative NPC specimens showing staining of EGFR, β-catenin, and Nanog. Normal and hyperplastic nasopharyngeal tissues were included as controls. Photographs were taken at ×200 magnification. (B) Expression of EGFR is associated with that of both β-catenin (P< 0.01) and Nanog (P< 0.05), and immunoreactivity of β-catenin is associated with that of Nanog (P< 0.01).
Discussion
In this report, we have shown that the EGFR pathway plays an important role in regulation of CSC properties in NPC. We found that CSC numbers are increased by EGF and suppressed not only by the EGFR specific inhibitors gefitinib or PD153035, but also by siRNA-mediated knockdown of EGFR. It has been reported previously that gefitinib inhibits ABC transporters involved in the generation of the SP phenotype [35, 36]. Our experiments with siRNA-mediated knockdown of EGFR suggested that EGFR signaling is essential for the CSC phenotype and inhibition of EGFR by gefitinib contributes significantly to suppression of SP cells. We further demonstrated that regulation of the CSC phenotype by EGFR is dependent mainly on the PI3K/AKT pathway. β-catenin is a key effector of Wnt signaling and is also a substrate of AKT. AKT stimulates β-catenin nuclear translocation and activation either directly through phosphorylation of β-catenin at S552 [12], or indirectly through phosphorylation and inactivation of GSK3β, resulting in hypophosphorylation of β-catenin at S33/S37/T41 [15]. By immunofluorescent staining and western blot analysis, we have elucidated a functional EGFR/PI3K/AKT/β-catenin axis that regulates the CSC phenotype in NPC cells. In response to EGF, β-catenin is translocated from the plasma membrane/cytoplasm to the nucleus. Treating cells with either gefitinib or LY294002, however, prevented EGF-induced β-catenin nuclear localization. We also showed that genetic inhibition of β-catenin expression via lentiviral shRNA blocks the effect of EGF stimulation. In NPC xenograft models, we found that residual CSCs spared by drug treatment were reduced in gefitinib-treated mice and enriched in cisplatin-treated animals. These results reveal profound differences between the effects of gefitinib and cisplatin on CSCs versus the bulk tumor cell population of a NPC. Importantly, our study also provides clinical evidence that expression of EGFR correlates significantly with that of β-catenin and Nanog in primary tumor specimens from NPC patients. Thus, our results for the first time demonstrate a critical role of β-catenin in mediating the effect of EGFR/PI3K/AKT signaling on CSCs in NPC.
The generation of a continuously growing tumor is a fundamental property of CSCs. In NPC xenograft models, we determined that CSCs among the residual tumor cells spared by drug treatment in “first generation” mice were reduced by gefitinib but not by cisplatin. In fact, treatment with cisplatin actually increased the proportion of residual cell with CSC properties. In the secondarily-transplanted mice, cells transplanted from the cisplatin-treated first generation mice grew more rapidly and formed larger tumors when compared to the cells from a control group. In contrast, cells transplanted from the gefitinib-treated mice developed tumors that were barely noticeable in the second generation animals. These results reveal distinct effects of gefitinib and cisplatin on CSCs versus the bulk tumor cell population.
Our findings have potential clinical implications. Gefitinib was the first targeted drug to enter clinical use for the treatment of non-small cell lung cancer (NSCLC) [37]. Subsequently, it has been evaluated in patients with different epithelial cancers with variable responses [37]. Several multicenter phase III clinical trials in patients with NSCLC demonstrated that patients with activating EGFR mutations have significantly longer progression-free survival if they are treated with gefitinib as a first-line therapy than if treated with cisplatin [38-40]. In a phase II study of NPC patients who had recurrent or metastatic tumors after prior platinum-based chemotherapy, gefitinib had little activity as a monotherapy [41]. However, the failure of this clinical trial may be explained by the inability of gefitinib to target CSCs that had accumulated more mutations under the selection pressure of the first-line chemotherapeutic agents such as cisplatin. In accord with our assumption, a recent phase II study of NPC employing the EGFR inhibitor cetuximab, in combination with other therapies, showed significant and encouraging improvement over previous modalities. The trial, consisting of concurrent cetuximab-cisplatin and radiotherapy in NPC, resulted in a 2-year distant-metastases-free survival of 92.8% and a loco-regional failure-free rate of 93% [42]. In the present study, we establish potent anti-CSC activity of gefitinib in xenograft models of NPC. Our results provide not only strong preclinical evidence, but also a mechanism, supporting the use of gefitinib in combination with a chemotherapeutic agent in first-line therapy for patients with NPC.
Resistance to gefitinib or other EGFR tyrosine kinase inhibitors represents a major therapeutic challenge. Several clinical studies have shown that despite initial responses to gefitinib, all patients will eventually develop resistance to this agent. The EGFR T790M point mutation, c-Met oncogene amplification, and PI3K/AKT activation account for most of the acquired resistance seen in the clinic [43, 44] . In line with these observations, we have provided evidence that the EGFR/AKT/β-catenin axis regulates CSCs that are preferentially targeted by gefitinib, and modulation of various components of the pathway was able to alter the effects of gefitinib on CSC properties. This suggests that resistance to gefitinib or other EGFR inhibitors in NPC may result from aberrant activation of any component of the EGFR/AKT/β-catenin pathway. Since we showed that AKT, but not ERK, is the major mediator of EGFR in regulating the CSC phenotype, our results provide the rationale for targeting AKT to overcome gefitinib resistance conferred by either EGFR mutations or by EGFR-independent activation of AKT such as c-Met amplification, ErbB3 overexpression, or loss of PTEN. In addition, our findings also suggest that activation of β-catenin is sufficient to confer resistance to gefitinib and that targeting β-catenin may represent an effective modality for patients whose tumors harbor activated EGFR or AKT.
EGFR and Notch pathway interaction have fundamental roles in regulating stem and progenitor cell signaling in Drosophila, C. elegans, and mammals [45]. Recently it was found that β-catenin activates Notch signaling through inducing Hes1 and down-regulating Atoh1[46]. Interestingly, a more recent study with NPC cell lines found that Notch signaling was highly activated in SP cells and Notch inhibition resulted in depletion of SP [47]. Here we showed that effects of EGFR signaling on SP andtumor spheroids are mediated by β-catenin. SP cells possess CSC properties and are regulated by drug transporters ABCG2, ABCB1 and ABCC1-5[26]. Our results suggest that β-catenin may represent a missing link connecting EGFR and Notch pathways. Thus, Notch signaling could be activated in response to EGF stimulation in our system and this possibility is currently under investigation.
In conclusion, our results show that EGFR signaling plays a crucial role in the regulation of stem cells of nasopharyngeal cancer. EGFR exerts these effects through the downstream effectors AKT/β-catenin. Importantly, the EGFR tyrosine kinase inhibitor gefitinib preferentially targets the CSC population. In contrast, the chemotherapeutic agent cisplatin predominantly eliminates the general tumor cell population. These findings provide preclinical evidence supporting the use of gefitinib alone or in combination with a chemotherapeutic agent in first-line therapy for patients with NPC. In addition, our results suggest that targeting β-catenin represents a rational clinical modality for patients whose tumors harbor activated EGFR or AKT.
Materials and methods
Cell lines and reagents
All cell lines were preserved in our laboratory. The NPC cell lines CNE1, CNE2, HNE1, HONE1, SUNE1, C666-1 and 5-8F were maintained in RPMI-1640 medium supplemented with 10% newborn calf serum. A normal human nasopharyngeal epithelial cell line, NP69, was maintained in keratinocyte-SFM supplemented with bovine pituitary extract and recombinant EGF (Invitrogen, Carlsbad, CA, USA). Gefitinib (AstraZeneca, Wilmington, DE, USA) was purchased from the Pharmacy of Nanfang Hospital of South Medical University. Cisplatin, Hoechst 33342, propidium iodide and LY294002 were from Sigma-Aldrich (St. Louis, MO, USA). PD0325901 and PD153035 were from Calbiochem (Billerica, MA, USA). Antibodies against the following proteins were used: EGFR, phospho-EGFR and phospho-AKT (Cell-signaling Technology, Danvers, MA, USA); AKT, ERK1/2, phospho-ERK1/2, β-catenin, Nanog and GAPDH (Santa-Cruz Biotechnology, Santa Cruz, CA, USA); Active β-Catenin (Millipore, Temecula, CA).
MTT cell viability assay
The inhibitory effect of gefitinib on NPC cell viability was evaluated by using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT; Sigma-Aldrich). CNE1 or CNE2 cells were seeded on a 96-well culture plates at a density of 1,500 cells per well and treated with increasing concentrations of gefitinib as indicated in the figure. MTT reagent was added to the plate 72 hours after treatment and absorbance was measured at 590 nm.
Tumor spheroid formation assay
Tumor spheroid culture was performed as described previously [29]. Single cells were plated in Ultra Low Attachment plates (Corning, Acton, MA, USA) in serum-free DMEM-F12 supplemented with 10 ng/mL bFGF, 10 ng/mL EGF and B27 (all from Invitrogen). Under these conditions, the cells grew in suspension as spherical clusters. To assess whether spheroid formation is EGF-dependent, cells were cultured in the above medium with or without EGF. Primary spheroids were dissociated enzymatically with trypsin to obtain a single-cell suspension and then replated to evaluate self-renewal by formation of secondary tumor spheroids.
Side population analysis
CNE1 and CNE2 cells in logarithmic growth phase were serum-starved for 24 hours followed by 16 hours treatment with EGF (50 ng/mL) in the absence or presence of gefitinib (1 μM). Subsequently, cells were suspended in DMEM plus 2% fetal bovine serum at 1×106cells/mL and stained with Hoechst 33342 dye (5 μg/mL) for 90 min at 37°C with continuous mixing. Following incubation, cells were washed with ice-cold PBS, stained with propidium iodide (1 μg/mL), and maintained at 4°C for flow cytometric analyses and for sorting of side population (SP) fraction using a FACSAria Flow cytometer (Beckton Dickson, Franklin Lakes, NJ, USA). The Hoechst dye was excited with an UV laser at 351 to 364 nm, and its fluorescence was measured with a 515-nm side population filter (Hoechst blue) and a 608 EFLP optical filter (Hoechst red). A 540 DSP filter was used to separate the emission wavelengths.
Western blot analysis
Cells were lysed, and then equal amount of protein were subjected to electrophoresis on a SDS-PAGE gel. The separated proteins were transferred to PVDF membranes (Millipore, Billerica, MA, USA) and probed with appropriate primary antibodies. Protein bands were detected by using enhanced chemiluminescence reagents (Pierce Biotechnology, Rockford, IL, USA).
Immunohistochemistry
For immunohistochemistry, paraffin-embedded sections were deparaffinized in xylene and rehydrated in a graded alcohol series. Antigen retrieval was performed by boiling the slide preparations in 10 mM sodium citrate buffer, pH 6.0. Staining was carried out using an EliVision Plus Kit (Maixin Bio, Beijing, China), according to the manufacturer’s protocol. DAB was used as a substrate for peroxidase, and expression levels were evaluated following criteria reported elsewhere [48].
Immunofluorescent staining
For immunofluorescent staining, cells were grown on the surface of cover slides and fixed with 4% paraformaldehyde. After rehydration in PBS, the fixed cells were incubated with respective primary antibodies at room temperature for 1 h or at 4°C overnight. FITC-conjugated secondary antibodies were incubated for 30 min at room temperature. The nuclei were stained with DAPI. Sections were examined with a Nikon Eclipse 80i fluorescent microscope (Nikon Instruments, Inc., Melville, NY, USA).
Lentiviral constructs and infection of NPC cells
Both pLKO.1 lentiviral shRNA vector and control shRNA targeting GFP were from Aldrich -Sigma. The β-catenin targeting sequence, GCTTGGAATGAGACTGCTGAT, was previously described [49]. The sense and antisense oligonucleotides were annealed and ligated into pLKO.1 lentiviral vector. The viruses were then packaged in 293T cells according to standard protocols. Viral production and infection of target cells were previously described [50]. Infected cells were selected using 2 μg/mL puromycin.
In vivo tumorigenicity assay
Animal studies were conducted in strict accordance with the principles and procedures approved by the Committee on the Ethics of Animal Experiments of Southern Medical University. Nude mice (BALB/C nu/nu) were fed autoclaved water and laboratory rodent chow. A volume of 100 μl of culture medium mixed with Matrigel (BD Biosciences, San Jose, CA, USA) containing 3×106 CNE2 cells was transplanted into the flanks of mice by subcutaneous injection. Two weeks after implantation, the mice were randomly separated into different treatment groups (6 mice per arm) and subjected to one of the following treatments: Group 1) control mice receiving either i.p.-injected or orally-administrated control solvent; Group 2) mice treated with gefitinib administrated orally at 150 mg/kg each every day; and Group 3) mice treated with cisplatin injected i.p. twice weekly at 3 mg/kg. The animals were monitored daily, and tumor volumes were measured every 3 d using a caliper slide rule. Tumor volume was calculated as follows: V = 1/2 (width2 × length).
After treatment for 2 weeks, animals were humanely euthanized and tumors harvested. To obtain a single-cell suspension, tumors were minced using scalpels and incubated in RPMI-1640 medium containing collagenase/hyaluronidase at 37°C for 60 min. The tissues were further dissociated by pipet trituration and then passed through a 40-μm nylon mesh to produce a single-cell suspension used for subsequent experiments. In the secondary tumor experiment, 1×105 cells dissociated from first-generation tumors were implanted into the flanks of recipient mouse (6 mice per group).
Statistical analysis
Data were expressed as means ± SD. Significant differences between groups were determined by analysis of variance and by Student’s t test. Graphs summarizing immunohistochemical staining results were analyzed using the Fisher’s exact test. Differences were considered significant when the P value was less than 0.05.
Acknowledgements
We thank Dr. Craig Menges for helpful discussion. This study was supported by the National Basic Research Program of China (973 Program, No 2010CB529401) and a Natural Science Foundation of China grant (81072205). J.R.T.’s work on AKT is supported, in part, by NCI Grant CA77429.
Abbreviations
- EGFR
epidermal growth factor receptor
- NPC
nasopharyngeal carcinoma
- CSC
cancer stem-like cell
- SP
side population
- bFGF
basic fibroblast growth factor
- shRNA
short hairpin RNA
References
- 1.Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F, Salomon DS. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene (Amsterdam) 2006;366:2–16. doi: 10.1016/j.gene.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 2.Mangelberger D, Kern D, Loipetzberger A, Eberl M, Aberger F. Cooperative Hedgehog-EGFR signaling. Front Biosci. 2012;17:90–9. doi: 10.2741/3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eccles SA. The epidermal growth factor receptor/Erb-B/HER family in normal and malignant breast biology. Int J Dev Biol. 2011;55:685–96. doi: 10.1387/ijdb.113396se. [DOI] [PubMed] [Google Scholar]
- 4.Reynolds BA, Weiss S. Generation of Neurons and Astrocytes from Isolated Cells of the Adult Mammalian Central-Nervous-System. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 5.Reynolds BA, Tetzlaff W, Weiss S. A Multipotent Egf-Responsive Striatal Embryonic Progenitor-Cell Produces Neurons and Astrocytes. Journal of Neuroscience. 1992;12:4565–4574. doi: 10.1523/JNEUROSCI.12-11-04565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dumstrei K, Nassif C, Abboud G, Aryai A, Aryai A, Hartenstein V. EGFR signaling is required for the differentiation and maintenance of neural progenitors along the dorsal midline of the Drosophila embryonic head. Development. 1998;125:3417–3426. doi: 10.1242/dev.125.17.3417. [DOI] [PubMed] [Google Scholar]
- 7.Maric D, Maric I, Chang YH, Barker JL. Prospective cell sorting of embryonic rat neural stem cells and neuronal and glial progenitors reveals selective effects of basic fibroblast growth factor and epidermal growth factor on self-renewal and differentiation. Journal of Neuroscience. 2003;23:240–251. doi: 10.1523/JNEUROSCI.23-01-00240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen JS, Pardo FS, Wang-Rodriguez J, Chu TS, Lopez JP, Aguilera J, Altuna X, Weisman RA, Ongkeko WM. EGFR regulates the side population in head and neck squamous cell carcinoma. Laryngoscope. 2006;116:401–6. doi: 10.1097/01.mlg.0000195075.14093.fb. [DOI] [PubMed] [Google Scholar]
- 9.Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CHM, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer research. 2006;66:9339–44. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 10.Garvalov BK, Acker T. Cancer stem cells: a new framework for the design of tumor therapies. J Mol Med (Berl) 2011;89:95–107. doi: 10.1007/s00109-010-0685-3. [DOI] [PubMed] [Google Scholar]
- 11.Fodde R, Brabletz T. Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Curr Opin Cell Biol. 2007;19:150–8. doi: 10.1016/j.ceb.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 12.He XC, Yin T, Grindley JC, Tian Q, Sato T, Tao WA, Dirisina R, Porter-Westpfahl KS, Hembree M, Johnson T, Wiedemann LM, Barrett TA, Hood L, Wu H, Li LH. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nature Genetics. 2007;39:189–198. doi: 10.1038/ng1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee G, Goretsky T, Managlia E, Dirisina R, Singh AP, Brown JB, May R, Yang GY, Ragheb JW, Evers BM, Weber CR, Turner JR, He XC, Katzman RB, Li L, Barrett TA. Phosphoinositide 3-kinase signaling mediates beta-catenin activation in intestinal epithelial stem and progenitor cells in colitis. Gastroenterology. 2010;139:869–81. 881, e1–9. doi: 10.1053/j.gastro.2010.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korkaya H, Paulson A, Charafe-Jauffret E, Ginestier C, Brown M, Dutcher J, Clouthier SG, Wicha MS. Regulation of Mammary Stem/Progenitor Cells by PTEN/Akt/beta-Catenin Signaling. PLoS Biology. 2009;7 doi: 10.1371/journal.pbio.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yost C, Torres M, Miller RR, Huang E, Kimelman D, Moon RT. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes & Development. 1996;10:1443–1454. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- 16.Yu MC, Yuan JM. Epidemiology of nasopharyngeal carcinoma. Semin Cancer Biol. 2002;12:421–9. doi: 10.1016/s1044579x02000858. [DOI] [PubMed] [Google Scholar]
- 17.Chen WZ, Zhou DL, Luo KS. Long-Term Observation after Radiotherapy for Nasopharyngeal Carcinoma (Npc) International Journal of Radiation Oncology Biology Physics. 1989;16:311–314. [PubMed] [Google Scholar]
- 18.Wei WI, Kwong DL. Current management strategy of nasopharyngeal carcinoma. Clin Exp Otorhinolaryngol. 2010;3:1–12. doi: 10.3342/ceo.2010.3.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J, Guo UP, Chen LZ, Zeng YX, Lu SH. Identification of cancer stem cell-like side population cells in human nasopharyngeal carcinoma cell line. Cancer research. 2007;67:3716–3724. doi: 10.1158/0008-5472.CAN-06-4343. [DOI] [PubMed] [Google Scholar]
- 20.Liang Y, Zhong Z, Huang Y, Deng W, Cao J, Tsao G, Liu Q, Pei D, Kang T, Zeng YX. Stem-like cancer cells are inducible by increasing genomic instability in cancer cells. J Biol Chem. 2010;285:4931–40. doi: 10.1074/jbc.M109.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xia H, Cheung WK, Sze J, Lu G, Jiang S, Yao H, Bian XW, Poon WS, Kung HF, Lin MC. miR-200a regulates epithelial-mesenchymal to stem-like transition via ZEB2 and beta-catenin signaling. J Biol Chem. 2010;285:36995–7004. doi: 10.1074/jbc.M110.133744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F, Salomon DS. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366:2–16. doi: 10.1016/j.gene.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 23.Ratushny V, Astsaturov I, Burtness BA, Golemis EA, Silverman JS. Targeting EGFR resistance networks in head and neck cancer. Cell Signal. 2009;21:1255–68. doi: 10.1016/j.cellsig.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma BBT, Poon TCW, To KF, Zee B, Mo FKF, Chan CML, Ho S, Teo PML, Johnson PJ, Chan ATC. Prognostic significance of tumor angiogenesis, Ki 67, p53 oncoprotein, epidermal growth factor receptor and HER2 receptor protein expression in undifferentiated nasopharyngeal carcinoma - A prospective study. Head and Neck-Journal for the Sciences and Specialties of the Head and Neck. 2003;25:864–872. doi: 10.1002/hed.10307. [DOI] [PubMed] [Google Scholar]
- 25.Sizhong Z, Xiukung G, Yi Z. Cytogenetic studies on an epithelial cell line derived from poorly differentiated nasopharyngeal carcinoma. Int J Cancer. 1983;31:587–90. doi: 10.1002/ijc.2910310509. [DOI] [PubMed] [Google Scholar]
- 26.Golebiewska A, Brons NH, Bjerkvig R, Niclou SP. Critical appraisal of the side population assay in stem cell and cancer stem cell research. Cell Stem Cell. 2011;8:136–47. doi: 10.1016/j.stem.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 27.Challen GA, Little MH. A side order of stem cells: The SP phenotype. Stem Cells. 2006;24:3–12. doi: 10.1634/stemcells.2005-0116. [DOI] [PubMed] [Google Scholar]
- 28.Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, Sorrentino BP. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nature Medicine. 2001;7:1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 29.Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–70. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bellacosa A, Kumar CC, Di Cristofano A, Testa JR. Activation of AKT kinases in cancer: Implications for therapeutic targeting. Advances in Cancer Research. 2005;Vol 94:29. doi: 10.1016/S0065-230X(05)94002-5. 94. + [DOI] [PubMed] [Google Scholar]
- 31.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–12. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 33.Takao Y, Yokota T, Koide H. Beta-catenin up-regulates Nanog expression through interaction with Oct-3/4 in embryonic stem cells. Biochem Biophys Res Commun. 2007;353:699–705. doi: 10.1016/j.bbrc.2006.12.072. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Zhang T, Korkaya H, Liu S, Lee HF, Newman B, Yu Y, Clouthier SG, Schwartz SJ, Wicha MS, Sun D. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin Cancer Res. 2010;16:2580–90. doi: 10.1158/1078-0432.CCR-09-2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yanase K, Tsukahara S, Asada S, Ishikawa E, Imai Y, Sugimoto Y. Gefitinib reverses breast cancer resistance protein-mediated drug resistance. Molecular cancer therapeutics. 2004;3:1119–25. [PubMed] [Google Scholar]
- 36.Leggas M, Panetta JC, Zhuang Y, Schuetz JD, Johnston B, Bai F, Sorrentino B, Zhou S, Houghton PJ, Stewart CF. Gefitinib modulates the function of multiple ATP-binding cassette transporters in vivo. Cancer research. 2006;66:4802–7. doi: 10.1158/0008-5472.CAN-05-2915. [DOI] [PubMed] [Google Scholar]
- 37.Herbst RS, Fukuoka M, Baselga J. Gefitinib--a novel targeted approach to treating cancer. Nat Rev Cancer. 2004;4:956–65. doi: 10.1038/nrc1506. [DOI] [PubMed] [Google Scholar]
- 38.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Okinaga S, Hirano H, Yoshimori K, Harada T, Ogura T, Ando M, Miyazawa H, Tanaka T, Saijo Y, Hagiwara K, Morita S, Nukiwa T. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 39.Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, Asami K, Katakami N, Takada M, Yoshioka H, Shibata K, Kudoh S, Shimizu E, Saito H, Toyooka S, Nakagawa K, Fukuoka M. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–8. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 40.Tiseo M, Bartolotti M, Gelsomino F, Bordi P. Emerging role of gefitinib in the treatment of non-small-cell lung cancer (NSCLC) Drug Des Devel Ther. 2010;4:81–98. doi: 10.2147/dddt.s6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma B, Hui EP, King A, To KF, Mo F, Leung SF, Kam M, Lo YM, Zee B, Mok T, Ahuja A, Chan AT. A phase II study of patients with metastatic or locoregionally recurrent nasopharyngeal carcinoma and evaluation of plasma Epstein-Barr virus DNA as a biomarker of efficacy. Cancer Chemother Pharmacol. 2008;62:59–64. doi: 10.1007/s00280-007-0575-8. [DOI] [PubMed] [Google Scholar]
- 42.Ma BB, Kam MK, Leung SF, Hui EP, King AD, Chan SL, Mo F, Loong H, Yu BK, Ahuja A, Chan AT. A phase II study of concurrent cetuximab-cisplatin and intensity-modulated radiotherapy in locoregionally advanced nasopharyngeal carcinoma. Ann Oncol. 2011 doi: 10.1093/annonc/mdr401. [DOI] [PubMed] [Google Scholar]
- 43.Nguyen KS, Kobayashi S, Costa DB. Acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancers dependent on the epidermal growth factor receptor pathway. Clin Lung Cancer. 2009;10:281–9. doi: 10.3816/CLC.2009.n.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wheeler DL, Dunn EF, Harari PM. Understanding resistance to EGFR inhibitors-impact on future treatment strategies. Nat Rev Clin Oncol. 2010;7:493–507. doi: 10.1038/nrclinonc.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aguirre A, Rubio ME, Gallo V. Notch and EGFR pathway interaction regulates neural stem cell number and self-renewal. Nature. 2010;467:323–7. doi: 10.1038/nature09347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peignon G, Durand A, Cacheux W, Ayrault O, Terris B, Laurent-Puig P, Shroyer NF, Van Seuningen I, Honjo T, Perret C, Romagnolo B. Complex interplay between beta-catenin signalling and Notch effectors in intestinal tumorigenesis. Gut. 2011;60:166–76. doi: 10.1136/gut.2009.204719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu S, Zhang R, Liu F, Wang H, Wu J, Wang Y. Notch inhibition suppresses nasopharyngeal carcinoma by depleting cancer stem-like side population cells. Oncol Rep. 2012;28:561–6. doi: 10.3892/or.2012.1830. [DOI] [PubMed] [Google Scholar]
- 48.Altomare DA, You H, Xiao GH, Ramos-Nino ME, Skele KL, De Rienzo A, Jhanwar SC, Mossman BT, Kane AB, Testa JR. Human and mouse mesotheliomas exhibit elevated AKT/PKB activity, which can be targeted pharmacologically to inhibit tumor cell growth. Oncogene. 2005;24:6080–9. doi: 10.1038/sj.onc.1208744. [DOI] [PubMed] [Google Scholar]
- 49.Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer research. 2008;68:3645–3654. doi: 10.1158/0008-5472.CAN-07-2938. [DOI] [PubMed] [Google Scholar]
- 50.Wiznerowicz M, Trono D. Conditional suppression of cellular genes: lentivirus vector-mediated drug-inducible RNA interference. J Virol. 2003;77:8957–61. doi: 10.1128/JVI.77.16.8957-8961.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]