Abstract
Adoptive transfer of virus-specific T cells can prevent and treat serious infections with Epstein-Barr virus (EBV), cytomegalovirus (CMV), and adenovirus (Adv) after allogeneic hematopoietic stem cell transplant. It has, however, proved difficult to make this approach widely available since infectious virus and viral vectors are required for T cell activation, followed by an intensive and prolonged culture period extending over several months. We now show that T cells targeting a range of viral antigens derived from EBV, CMV, and Adv can be reproducibly generated in a single culture over a 2–3-week period, using methods that exclude all viral components and employ a much-simplified culture technology. When administered to recipients of haploidentical (n = 5), matched unrelated (n = 3), mismatched unrelated (n = 1) or matched related (n = 1) transplants with active CMV (n = 3), Adv (n = 1), EBV (n = 2), EBV+Adv (n = 2) or CMV+Adv (n = 2) infections, the cells produced complete virological responses in 80%, including all patients with dual infections. In each case, a decrease in viral load correlated with an increase in the frequency of T cells directed against the infecting virus(es); both immediate and delayed toxicities were absent. This approach should increase both the feasibility and applicability of T cell therapy. The trial was registered at www.clinicaltrials.gov as NCT01070797.
Introduction
Viral infections, most commonly with Adenovirus (Adv), cytomegalovirus (CMV), or Epstein-Barr virus (EBV), remain a major cause of severe and prolonged morbidity and mortality after allogeneic hematopoietic stem cell transplant.1,2 Treatment with antiviral drugs is expensive, often ineffectual and frequently toxic.
Although the adoptive transfer of ex vivo expanded donor cytotoxic T lymphocytes (CTL) can be a safe and highly effective means of both preventing and treating viral infections including EBV, CMV, and Adv, this approach is currently impractical for widespread or urgent use due to deficiencies in the manufacturing process. For example, T cell lines directed to Adv, CMV and EBV require an 8–12-week production process that also requires repeated rounds of in vitro stimulation with adenovector-modified monocytes and EBV-transformed B lymphoblastoid cell lines (EBV-LCLs).3,4 In addition, the generated lines have unpredictable specificities and are often dominated by CMV-reactive T cells, at the expense of EBV- and Adv-reactive T cells.5 Combined with the regulatory complexities and expense of using infectious virus/vector material (EBV/Adv) in CTL generation, the result has been that this effective approach has been restricted to specialized centers.
To address the above limitations, we now report the development, clinical testing, and effectiveness of a new, rapid and simplified manufacturing strategy in which DCs nucleofected with DNA plasmids encoding a range of immunodominant and subdominant viral antigens from EBV, CMV, and Adv are used to activate T cells that were subsequently selectively expanded in culture conditions designed to decrease activation-induced cell death and increase the antigenic T cell repertoire.6,7,8
Results
Generation of rCTLs from stem cell donors
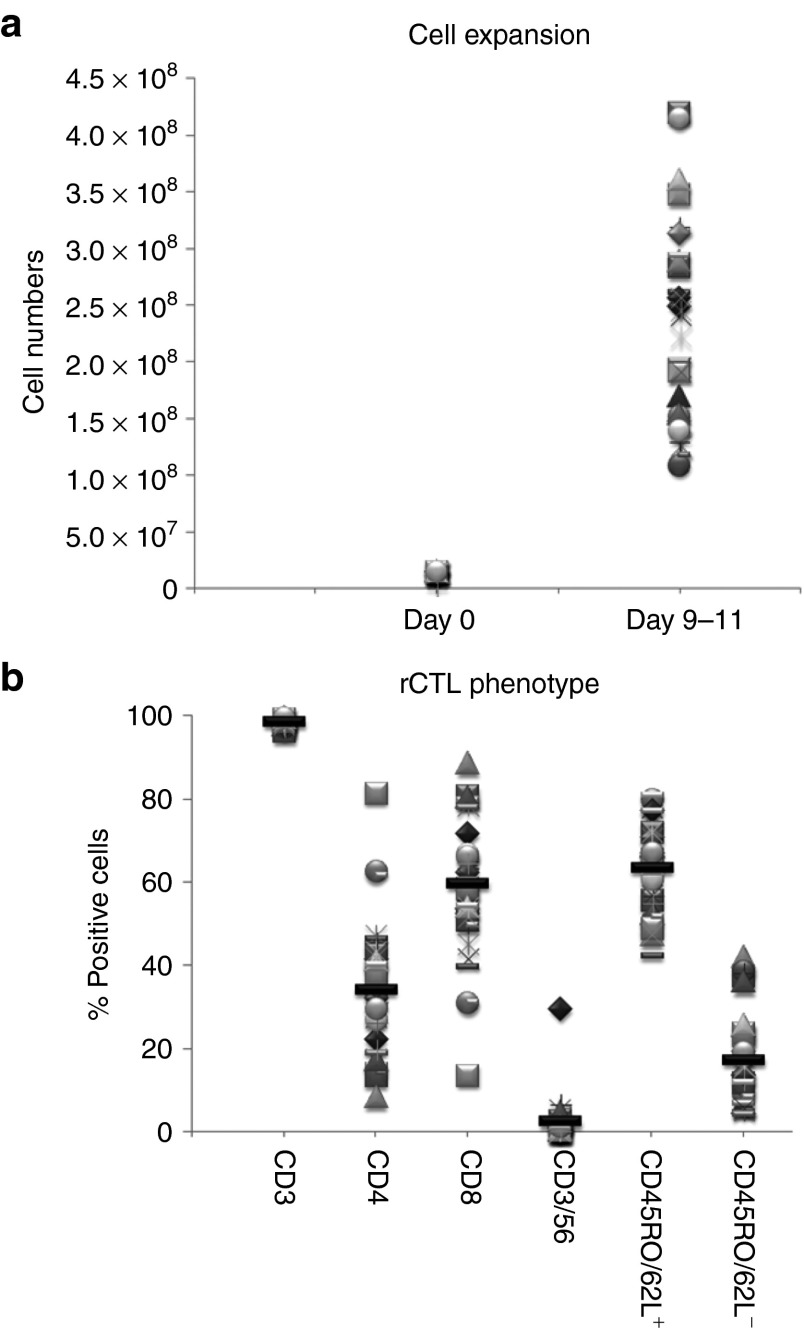
Twenty-two rCTL lines were made from normal donors who were seropositive for all three target viruses (EBV, CMV, and Adv), as well as 14 additional lines from normal donors who were CMV seronegative. The lines were manufactured as described in Materials and Methods. From 15 × 106 PBMCs, we achieved a 1.5 log expansion within 9–11 days (median 212.5 × 106 cells, range 109–420 × 106; n = 36 (Figure 1a). The lines were almost exclusively CD3+ T cells (mean 98.6 ± 0.1%), representing both cytotoxic CD8+ (59.6 ± 2.7%) and helper CD4+ (34.1 ± 2.5%) T cell subsets that expressed central memory CD45RO+/CD62L+ (63.6 ± 1.8%) or effector markers CD45RO+/CD62L- (17.1 ± 1.8%) (Figure 1b). There were few nucleofected DCs (CD83+) in the final product (mean 0.2%).
Figure 1.
Cell expansion and immunophenotype of rCTL generated for clinical use. Plasmid-activated rCTL were expanded in the G-Rex in the presence of IL4+7 for 9–11 days. Panel a shows overall T cell expansion, based on cell counting using trypan blue exclusion. Each symbol represents an individual line, and data for 36 rCTL lines is presented. Panel b shows the phenotype of the rCTL on the day of cryopreservation. Reactivity of CTL lines (n = 36) with antibodies against the T cell surface antigens CD3, CD4, CD8, and CD56, and the activation/memory markers CD45RO and CD62L is shown. The mean for each condition is represented as a black line.
CTL lines are specific for EBV, CMV, and Adv antigens but are not alloreactive
The specificity of the rCTL lines was assessed by IFNγ enzyme-linked immunospot and 51Cr release cytotoxicity assays. All 22 lines generated from CMV, EBV, and Adv seropositive donors recognized at least one of the stimulating antigens from all three viruses (Figure 2a). For CMV, all 22 lines showed specific activity against pp65 (mean 639 ± 62 IFNγ SFC/2 × 105 (median 588)) while 20 recognized IE1 (mean 324 ± 63 IFNγ SFC/2 × 105 (median 245)). Similarly, for Adv, all 22 lines had activity directed to Hexon protein (mean 246 ± 60; median 122 IFNγ SFC/2 × 105), while 19 lines reacted against Penton protein (mean 168 ± 34; median 129 IFNγ SFC/2 × 105). Recognition of EBV antigens was more heterogeneous – all 22 donors recognized at least one target antigen – seven lines reacted against only one antigen, seven recognized two antigens and eight recognized all three targets. LMP2 was the most frequently recognized target with 20 responding donors and a mean of 219 ± 40 IFNγ SFC/2 × 105 rCTLs (median 189), followed by EBNA1, which was recognized by 13 donors (mean 72 ± 19 IFNγ SFC/2 × 105 (median 28)), while 12 recognized BZLF1 (mean 91 ± 38 IFNγ SFC/2 × 105; median 26). The CMV seronegative donors did not recognize CMV-IE1 or pp65 antigens, but all 14 of these lines were reactive against both Adv-Hexon (mean 336 ± 87; median 219 SFC/2 × 105) and Penton proteins (mean 223 ± 68; median 117 SFC/2 × 105). Similarly, all lines from EBV seropositive donors recognized at least one of the three target EBV antigens and again activity against LMP2 was most frequently detected (n = 10) with a mean of 192 ± 66 SFC/2 × 105 (median 41), followed by BZLF1 (mean 278 ± 82; median 179 SFC/2 × 105, n = 9), and EBNA1 (mean 180 ± 54; median 9, n = 5). There was no significant difference between the magnitude of the immune responses directed against EBV and Adv antigens in the bivirus or trivirus-specific T cell lines, indicating that antigenic competition between T cells did not influence the profile of specificity generated. Unstimulated rCTL and rCTL stimulated with an irrelevant pepmix (data not shown) served as negative controls and did not induce IFNγ production.
Figure 2.
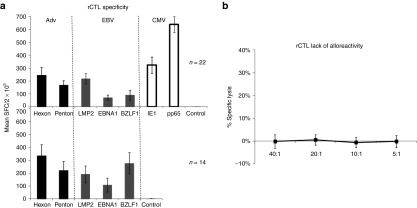
Virus-specific activity and lack of alloreactivity in rCTL. Panel a shows the frequency of Adv (Hexon and Penton), EBV (LMP2, EBNA1, BZLF1), and CMV (IE1 and pp65) reactive T cells in rCTLs using IFNγ ELIspot as readout. The top panel shows the specificity of 22 lines generated from CMV seropositive donors, while the bottom panel shows the activity against Adv (Hexon and Penton) and EBV (LMP2, EBNA1, BZLF1) in 14 lines generated from CMV seronegative donors. Results represent the mean ± SEM SFC/2 × 105 input cells. Control was IFNγ release in response to stimulation with irrelevant/no pepmix. Panel b 51Cr release at 4 hours after coincubation of rCTL lines with recipient/haploidentical PHA blasts (allogeneic targets). These data are mean percent specific lysis (± SD) of targets by 35 rCTL lines at E:T ratios of 40:1, 20:1, 10:1, and 5:1. This analysis could not be performed for one individual who was post-transplant and lacked a haploidentical donor from which PHA blasts could be generated.
We also determined whether the donor-derived rCTL lines retained residual alloreactive cells or contained virus-specific cells that crossreacted with normal donor cells.9 We used a 4-hour Cr51 release assay with recipient or haploidentical PHA blasts as T cell targets. As shown in Figure 2b, there was no evidence of alloreactivity in any line generated. Specific lysis of uninfected recipient/haploidentical PHA blasts of <10% at an E:T ratio of 20:1 was a clinical release criterion and was met by all lines (mean 1 ± 0% specific lysis, E:T 20:1, n = 35).
rCTL safety in vivo
The safety of the rCTLs was assessed in 10 recipients who received allogeneic stem cell transplantation (five Haplo, three MUD, one MMUD, and one MRD) for the treatment of malignant or nonmalignant diseases, and who had reactivation of one or more viruses (Table 1). Each patient received 0.5–2 × 107 cells/m2 between day 27 and month 52 posthematopoietic stem cell transplant. One patient (unique patient number (UPN) 2639) developed a mild and localized skin rash postinfusion that was considered as possibly stage II skin graft versus host disease but this patient also had intercurrent BK infection and had presented with a similar rash during an earlier episode of BK reactivation, which occurred prior to rCTL therapy. No other patient had infusion related toxicity.
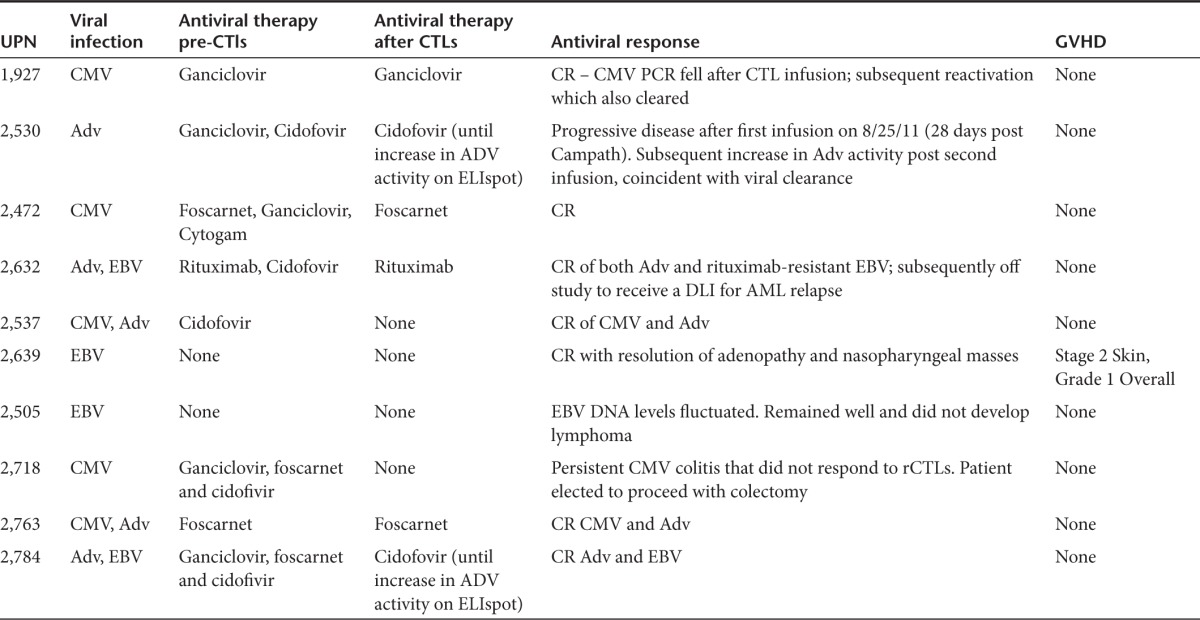
Table 1. Patient characteristics.
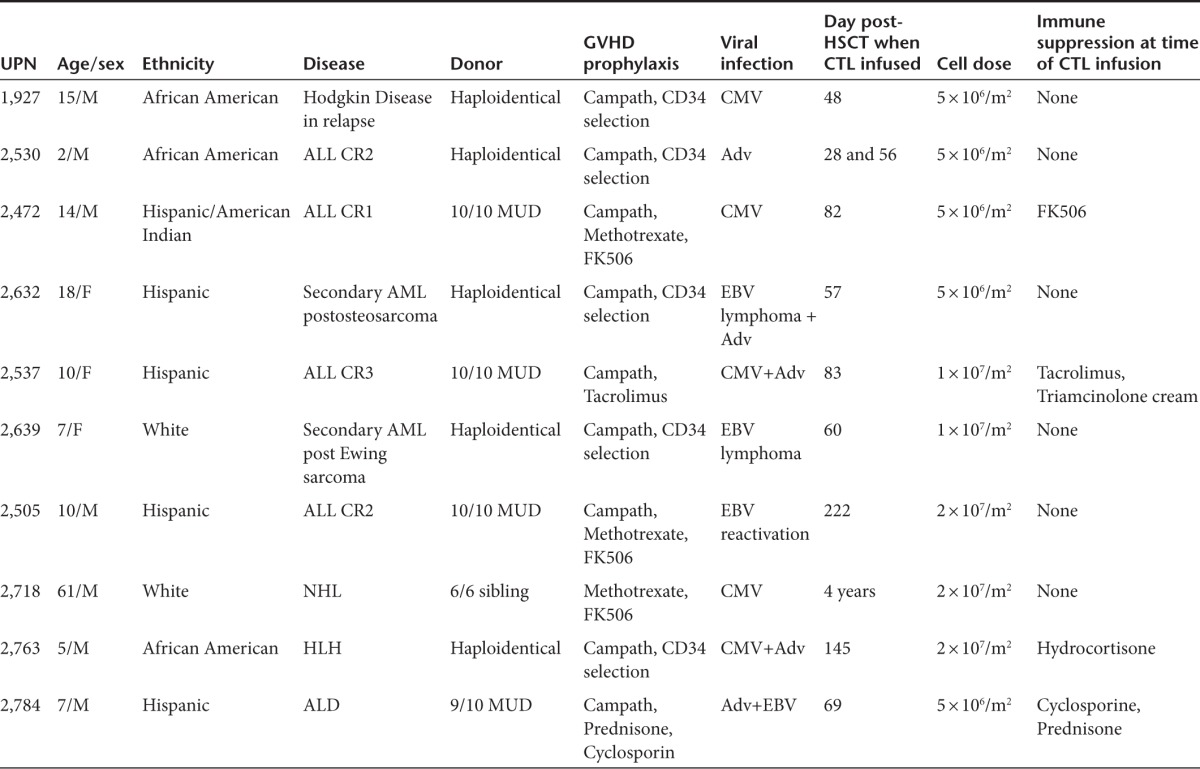
Donor-derived rCTL infusions
The in vivo expansion and antiviral activity of the rCTLs was assessed in all 10 patients infused. Three patients were treated for CMV reactivation, one for Adv infection, two for EBV reactivation, and four patients for dual infections (two for EBV+Adv and two for CMV+Adv) (Table 2).
Table 2. Antiviral responses.
Patients with single virus infections who responded to rCTLs
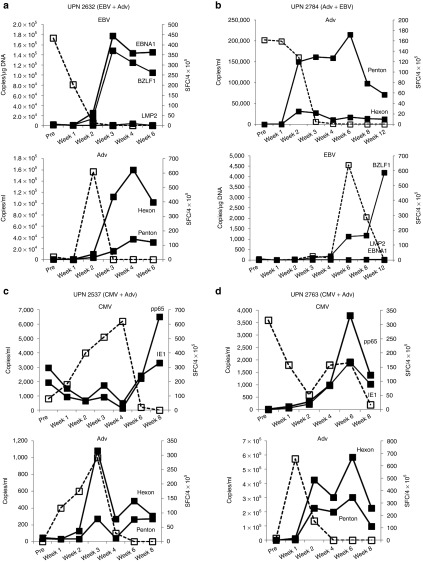
Four patients with single virus infections (UPN 1927 and UPN 2472 – CMV; UPN 2530 – Adv; UPN 2639 – EBV), receiving cell infusions ranging from 0.5–2 × 107 cells/m2 had complete responses to rCTL therapy. The viral load measurements and corresponding changes in T cell frequency pre and postinfusion are shown in Figure 3. On the date of infusion, UPN 1927 had slightly elevated levels of CMV (100 copies/ml) detected in peripheral blood, which had decreased to 0 by 2 weeks postinfusion, corresponding with a transient increase in CMV-specific T cells (30 SFC/4 × 105 PBMCs) detected at the same time point. At week 4, the CMV viral load began to increase and peaked at week 6 postinfusion. However, without additional rCTL infusions or other treatment, the viral load subsequently decreased to baseline, coinciding with a marked expansion of CMV-reactive T cell precursors in vivo (Figure 3a). Similarly in UPN 2472, within 2 weeks of receiving rCTL, the elevated CMV viral load had decreased to 0, coincident with a marked increase in CMV-directed T cells (Figure 3b). UPN 2530 received rCTL as treatment for Adv infection within 28 days of receiving the CD52-directed antibody alemtuzumab (day +27 post-transplant). Despite T cell treatment, the viral load continued to increase exponentially, with no detectable rise in Adv-specific precursors. Due to the prolonged half life of alemtuzumab, we concluded that residual antibody had eliminated the infused cells and gave a second rCTL infusion 4 weeks later. We observed initial stabilization of AdV load and then a decrease to 0, corresponding with a marked increase in the frequency of detectable Adv Hexon and Penton-specific T cells (Figure 3c). Finally, UPN 2639 had elevated EBV-DNA levels, fevers and a nasopharyngeal mass, which on biopsy showed EBV lymphoma. She was infused with rCTLs and had a rapid and sustained decrease in viral load with resolution of the nasopharyngeal mass and a corresponding increase in EBV-directed T cells (Figure 3d).
Figure 3.
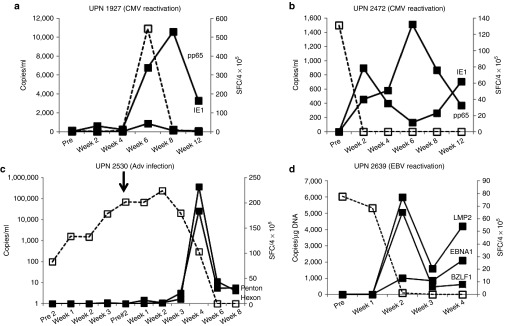
In vivo expansion and clinical benefits of rCTL in subjects infected with one virus. Panels a and b show the CMV viral load in blood (copies/ml), indicated by the dashed line (---), and frequency of T cells specific for CMV pp65 and IE1 pre and postinfusion in two subjects (UPN 1927 and UPN 2472) who were treated for a CMV reactivation. T cell frequencies are shown as a solid line. Panel c shows the Adv load in blood (copies/ml – dashed line) and frequency of T cells specific for Adv Hexon and Penton pre and postinfusion (solid lines) in subject UPN 2530 who was treated for an Adv infection. This patient received two rCTL infusions, one on day 0 and one 4 weeks after the initial infusion, which is indicated by the arrow. Panel d shows the EBV viral load (copies/µg DNA isolated from PBMCs – dashed line) and frequency of T cells specific for EBV LMP2, EBNA1, and BZLF1 (solid lines) in UPN 2639 treated for an EBV reactivation. In all cases the frequency of specific T cells was measured by IFNγ ELIspot and results are presented as average SFC/4 × 105 PBMCs.
Patients with dual virus infections who responded to rCTLs
Four patients with dual infections (UPN 2632 and UPN 2784 – Adv + EBV; UPN 2537 and UPN 2763 – Adv + CMV) also had complete responses to rCTL therapy. Figure 4 shows the viral load measurements and corresponding changes in T cell frequency pre and postinfusion for each of the treated viruses. Data for UPN 2632 are shown in Figure 4a. This subject was initially treated because of elevated EBV-DNA levels, which decreased rapidly postinfusion (Figure 4a, top panel). However, at week 2, postinfusion elevated adenoviral loads were transiently detected in peripheral blood and subsequent viral control was associated with an increase in Adv-specific precursors. Similarly, UPN 2784 initially presented with elevated adenoviral load levels and subsequently reactivated EBV. Clearance of both viruses was temporally associated with an increase in the frequency of T cells directed against the infecting viruses (Figure 4b). Similar results were seen in patients UPN 2537 and UPN 2763 (Figure 4c and 4d, respectively), both of whom controlled CMV and Adv infections post-rCTL with a corresponding increase in CMV and Adv-directed T cells.
Figure 4.
In vivo expansion and clinical benefits of rCTL in subjects with dual infections. Panel a shows the EBV and Adv load (dashed lines) detected in the peripheral blood of UPN 2632. This patient was initially infused with rCTL as treatment for an EBV reactivation and subsequently developed an Adv infection. The frequency of T cells directed to EBV (LMP2, EBNA1, BZLF1) and Adv (Hexon and Penton) (shown as solid lines) was measured over time by IFNγ ELIspot and results are presented as average SFC/4 × 105 PBMCs. Panel b shows the Adv and EBV load (dashed lines) detected in the peripheral blood of UPN 2784. This patient was initially infused with rCTL as treatment for an Adv infection and subsequently reactivated EBV. The frequency of T cells directed to Adv (Hexon and Penton) and EBV (LMP2, EBNA1, BZLF1) (solid lines) was measured over time by IFN ELIspot and results are presented as average SFC/4 × 105 PBMCs. Panels c and d show the CMV and Adv load detected in the peripheral blood of UPN 2537 and UPN 2763 (dashed lines). Both these patients were infused with rCTL as treatment for a CMV reactivation and subsequently developed an Adv infection. The frequency of T cells directed to CMV (IE1 and pp65) and Adv (Hexon and Penton) (solid lines) was measured over time by IFNγ ELIspot and results are presented as average SFC/4 × 105 PBMCs.
Patients who did not respond to rCTLs
Two patients did not respond to rCTL therapy. UPN 2505 had elevated EBV-DNA levels in peripheral blood, which we and others have found to be predictive of the development of EBV-LPD.10 The patient was infused with T cells when the viral titer was 1,126 copies/µg DNA. Thereafter, the EBV DNA levels fluctuated but never exceeded 3,000 copies/µg DNA and this patient never developed LPD (see Supplementary Figure S1 online). The second nonresponder (UPN 2718) was >4 years post-transplant with persistent CMV colitis despite high levels of circulating CMV-specific T cells directed to both IE1 and pp65 in numbers comparable with those detected in healthy seropositive individuals (969 and 202 SFC/4 × 105, respectively). Postinfusion of an rCTL line with CMV activity directed against the same two CMV antigens resulted in no significant change in the circulating frequency of detectable CMV-directed T cells (see Supplementary Figure S2 online) with no impact on the patient's clinical status and when a repeat colonoscopy showed continuing colitis, the patient elected to proceed to colectomy. Though it is unclear why neither the pre-existing endogenous CMV-specific precursors nor the infused rCTL were able to produce clinical benefit, it may relate to an inability of the cells to home or the presence of a CTL-resistant viral strain.
Discussion
In this phase I/II treatment study, we used DNA plasmids to generate donor-derived virus-directed T cell lines with specificity for Adv, EBV, and CMV, the commonest causes of viral morbidity and mortality after hematopoietic stem cell transplant. When administered to patients with active infections, these T cells expanded in vivo and produced the hoped-for clinical benefits without adverse effects. Importantly, and in contrast to previous clinical studies of virus-directed T cells, the current lines were generated without exposure to biohazards (live virus/viral vectors) and were manufactured in only 17 days from small blood volumes (maximum 60 ml).
Though adoptively-transferred virus-specific T cells have been used for almost 20 years to effectively prevent and treat viral infections after hemopoietic stem cell transplant, it has proved difficult to develop the approach as a widely distributed “standard of care”. A major limitation in the pathway to successful late-phase clinical development of the approach has been the requirement for live virus or viral vectors in the manufacturing process. For example, our previous report of trivirus-directed T cell lines used a process which required extensive in vitro culture (8–12 weeks) and repetitive stimulation with viral components (live EBV virus and adenovectors). Although such lines were clinically effective, the virus/vector components were expensive to both make and test to clinical standards, and they generated products dominated by CMV-reactive T cells at the expense of the Adv- and EBV-directed components.5 Thus, one of the major objectives of the current study was to overcome these barriers in order to make manufacturing of effective lines both robust and scalable and thereby facilitate the transition of T cell therapies to a standard of care.
Preclinical testing showed that the plasmid-activated T cell lines used here were comparable with “conventional” CTLs with respect to composition (broad-spectrum specificity, polyclonal CD4+ and CD8+ T cells expressing central memory/activation markers) and function (proliferative capacity, effector cytokine production, ability to kill virus-infected targets).6,8 It was, however, unclear whether they would have equivalent performance in vivo. Plasmid-mediated stimulation of T cells, unlike stimulation with virus or viral vectors, occurs in the absence of viral-derived “danger signals”, which may adversely effect the in vivo persistence and performance of the infused cells. Secondly, we did not know whether plasmids inducing responses against a limited array of Adv, CMV and EBV antigens would be sufficient to provide effective immunity while preventing the immune escape that is a risk when limited viral epitopes are recognized by effector T cells.11,12 Although our own and other studies suggested that T cells targeting hexon and penton (Adv) and IE1 and pp65 from (CMV) would be sufficient for in vivo protection,3,13,14,15,16,17,18,19,20,21 data showing that a product directed against the three EBV antigens expressed in the plasmid-stimulated cultures (as opposed to the multiplicity of EBV antigens expressed by virus-infected B cells in conventional CTL cultures)22,23,24 would effectively reconstitute sustained EBV immunity was limited.25,26 To prevent immune escape of EBV-infected target cells in vivo, we used the combination of two latent (EBNA1 and LMP2) and one lytic (BZLF1) antigen, which are broadly immunogenic across multiple HLA types; stimulate both CD4+ and CD8+ T cells; and should allow both latent and lytic cycle infected-cells to be recognized.27 Further, EBNA1 and LMP2 are expressed in the majority of EBV+ tumors. Although these are not the classical immunodominant latency-associated antigens (EBNAs 3A, 3B and 3C),27 these antigen choices appear appropriate since we could generate lines with activity against at least two of the three targets in 68% of seropositive donors, and adoptive transfer of donor rCTLs to four patients who reactivated EBV produced complete responses in three. In all four cases, the lines infused recognized all three target antigens but nevertheless in UPN 2505, this was insufficient to reduce the virus load, which remained elevated for 3 months after receiving the cells, but neither did it ever reach 4,000 copies/µg DNA – a threshold we previously established as being predictive of early PTLD.10 Thus, the T cell precursors specific for all three EBV target antigens that were detectable postinfusion in this subject may also have played a role in controlling the extent of the reactivation (see Supplementary Figure S1 online).
Overall, our clinical response rate was equivalent to that achieved in our previous trials of trivirus-directed T cells generated using EBV and Adv vectors for manufacturing, with 80% of the subjects with active Adv, CMV, and/or EBV infections having a measurable increase in the frequency of T cells directed against the infecting virus(es), and clearance of episodes of both single and dual viral reactivation/disease. Thus, DNA plasmids can be substituted for the viruses previously used, without evident loss of efficacy, and when administered in the donor-specific setting should have the potential to persist long-term in vivo.22
Donor-derived virus-specific T cells may cause direct toxicity by crossreacting with normal host cells or because of the presence of residual T cells with primary alloreactivity.28,29,30,31,32,33,34 Crossreactivity by virus-specific T cells occurs if individual clones crossreact with allogeneic HLA molecules expressed on normal recipient cells. Fortunately, this effect has been little seen in extensive human studies after the adoptive transfer of donor-derived or third party virus-specific T cells to allogeneic stem cell transplant recipients.35,36,37,38 Further, in a lung transplant recipient with an HLA-B4402 allograft and detectable circulating CD8+ T cells directed against the HLA-B8-FLRGRAYGL peptide (derived from EBNA3A) with known alloreactivity against B4402, Mifsud and colleagues reported no evident clinical consequences.39
A greater potential concern for the current study was the persistence of residual T cells with primary alloreactivity, since in conventional, longer-term cultures, these are diluted through repetitive rounds of in vitro virus-specific stimulation. Although we have not seen significant de novo graft versus host disease associated with the infusion of conventionally-generated CTLs in >150 subjects,9 the substantial abbreviation of the production process to just a single in vitro stimulation with a short (10-day) culture could, in principle, have allowed alloreactive T cells to persist in numbers sufficient to cause in vivo toxicity. However, coculture of 36 donor-derived virus-specific rCTLs with normal recipient/haploidentical cells showed no evidence of specific lysis and no toxicity attributable to rCTLs was observed in the 10 patients infused. We attribute this rapid loss of residual alloreactive T to the use of the cytokines IL4 and IL7, which we have shown can selectively support the expansion and survival of polyclonal (CD4+ and CD8+) virus-specific T cells, resulting in a rapid enrichment (>10-fold) of antigen-specific populations and a corresponding reduction in alloreactive cells.7
Thus, our rapid and apparently effective trivirus CTLs can be produced more cheaply, quickly, and easily than has previously been possible, facilitating introduction of the approach into broader clinical practice.
Materials and Methods
Patients. The investigation was approved by the Food and Drug Administration, the hospitals' Institutional Review Boards and the National Marrow Donor Program's Institutional Review Boards. The protocol was open to all patients at Texas Children's Hospital and The Methodist Hospital who were candidates for stem cell transplant from an HLA-matched or mismatched related or unrelated donor. All rapid CTL lines (rCTL) were generated from healthy donors who were donating marrow or peripheral blood stem cells to matched related, matched or mismatched unrelated or haploidentical recipients (n = 36). Patients received their SCT under our routine institutional conditioning and supportive care protocols. Patients who were at least 30 days post-transplant, had a life expectancy >30 days, with no severe kidney or liver disease, were without graft versus host disease of >grade 2 and who had reactivations of CMV, EBV or Adv were eligible to receive infusions of rCTLs. Clinical details of the 10 patients who received rCTL lines are provided in Table 1.
Plasmids. The NTC8385-Hexon-IRES-Penton (Adv), NTC8385-IE1-IRES-pp65 (CMV), and NTC8385E1dGALMP2-IRES-BZLF1 plasmids, which are minimalized vectors that contain a short 140 bp RNA-based selection marker rather than an antibiotic resistance marker, were generated by Nature Technology Corporation.40,41 These were produced to cGMP standards by Puresyn, Inc. (Malvern, PA). The DNA homogeneity of all three plasmids was >95%, based on scanning densitometry. Additional release criteria included sterility, endotoxin testing <5 EU/ml, and identity confirmed by restriction digest and complete plasmid sequencing (SeqWright, Houston, TX).
Monocyte isolation and DC generation. DCs were generated as previously described.6,8 Briefly, monocytes were isolated from PBMC by plastic adherence and cultured in complete DC media (CellGenix supplemented with 2 mmol/l L-glutamax) (CellGenix, Antioch, IL; GlutaMAX, Invitrogen, Carlsbad, CA) with 800 U/ml GM-CSF (Sargramostim Leukine, Immunex, Seattle, WA) and 400 U/ml IL4 (R&D Systems, Minneapolis, MN) for 5 days. IL4 and GM-CSF were replenished on day 3 or 4. On day 5 or 6, DCs were matured in DC media using a cytokine cocktail containing 10 ng/ml IL1β, 10 ng/ml TNFα (R&D Systems), 100 ng/ml IL6 (CellGenix), 0.1 µg/ml PGE1 (Sicor Pharmaceuticals, Irvine, CA), 800 U/ml GM-CSF and 400 U/ml IL4.
DC nucleofection. DCs were nucleofected 16–18 hours after maturation using the Lonza Nucleofector Kit for Human Dendritic Cells (Lonza, Allendale, NJ). The cells were divided into three aliquots of 0.5–2 × 106, as per manufacturers' instructions, and each aliquot was nucleofected with 5 µg of the EBV, CMV or Adv plasmids using the program U2 on the Lonza Nucleofector 2b device. After nucleofection, DCs were resuspended in complete DC media supplemented with the cytokine maturation cocktail, and were matured for a further 18–24 hours before being used to stimulate rCTL.
Generation of rCTL lines. Twenty-two trivirus (from Adv, CMV, and EBV seropositive donors) and 14 bivirus (from CMV seronegative donors) were produced. To generate rCTLs, 15 × 106 nonadherent PBMCs were used as responder cells and stimulated with nucleofected DCs, which had been irradiated at 30Gy, using a minimum stimulator:responder (S:R) ratio of 1:10. The cells were then transferred to a G-Rex10 device (Wilson Wolf, Minneapolis, MN) and cultured in 30 ml CTL media (RPMI 1640 supplemented with 45% Click's medium (Irvine Scientific, Santa Ana, CA), 2 mM GlutaMAX, and 10% FBS (Hyclone, Logan, Utah)) supplemented with 400 U/ml IL4 and 10 ng/ml IL7 (R&D Systems).42 On day 6–8, cells were harvested, counted, expanded to a second G-Rex10 if >5 × 107 cells were present, and fed with CTL media supplemented with IL4+7. On day 9–11 of culture rCTL were harvested, counted to assess viability using trypan blue exclusion. Appropriate samples were sent for quality assurance/quality control testing as well as for phenotypic and functional studies and the remainder was cryopreserved for clinical use. Release criteria for rCTL included viability >70%, negative culture for bacteria and fungi after 7 days, endotoxin testing <5EU/ml, negative result for mycoplasma, <10% killing of recipient/haploidentical PHA (Sigma-Aldrich, St Louis, MO)-activated lymphoblasts at a 20:1 ratio, <2% CD83 positive DCs and HLA identity. For this phase I/II treatment study, 36 rCTL lines were produced and characterized, 10 of which were infused. The main reason that lines were not infused was that the patient did not have a reactivation of one of the three viruses (n = 21) but some patients were not eligible due to graft versus host disease (n = 1), severe intercurrent infection (n = 2), or failure to engraft (n = 2).
Immunophenotyping. CTL lines were stained with CD3, CD4, CD8, CD14, CD16, CD56, CD62L, CD45RA, CD83, CCR7, TCR αβ, TCR γδ, CD19 (Becton Dickinson, San Jose, CA). For each sample, at least 10,000 cells were analyzed by FACS Calibur with the Cell Quest Software (Becton Dickinson).
Cytotoxicity assay. The alloreactive potential of each rCTL line was analyzed in a standard 4-hour chromium (Cr51) release assay with patient-derived or haploidentical PHA blasts as targets. Specific lysis was analyzed at effector:target (E:T) ratios ranging from 40:1 to 5:1.
ELIspot assay. Enzyme-linked immunospot analysis was used to quantitate the frequency of T cells that secreted IFNγ in response to Adv, EBV, and CMV antigen exposure. Enzyme-linked immunospots were performed on the donor rCTL lines and on patient PBMCs pre and post-rCTL infusion. As a positive control, PBMC were stimulated with Staphylococcal Enterotoxin B (1 µg/ml) (Sigma-Aldrich). Hexon and penton (Adv), IE1 and pp65 (CMV), and EBNA1, LMP2, BZLF1 (EBV) pepmixes, diluted to 200 ng/peptide/ml, were used to stimulate T cells. For analyzing the specificity of rCTL, the T cell lines were resuspended at 2 × 106/ml in CTL media. Pre and postinfusion PBMCs were resuspended at 4–5 × 106/ml in CTL media. All pepmixes, which are overlapping peptide libraries (15mers overlapping by 11 amino acids), were purchased from JPT Technologies (Berlin, Germany). Each condition was run in duplicate or triplicate. After 20 hours of incubation, plates were developed as previously described,13 dried overnight at room temperature in the dark, and then sent to Zellnet Consulting (New York, NY) for quantification. Spot-forming cells (SFC) and input cell numbers were plotted, and the frequency of T cells specific to each antigen was expressed as specific SFC per input cell numbers.
Detection of EBV and adenovirus-DNA in PBMCs by quantitative real-time PCR. To quantitate EBV load in patient blood, DNA was isolated from 3–5 × 106 PBMCs using an anion exchange column (Qiagen, Valencia, CA). 500 ng of DNA was analyzed by Q-PCR as previously described.10 Adenovirus and CMV DNA was quantified by Viracor IBT Laboratories (Lee's Summit, MO).
Statistical analysis. All in vitro data were presented graphically and summarized by mean ± 1 standard error of mean (SEM) or median with range. Smoothing splines were fitted to describe the trend over time for T cell frequency data. Differences between means were analyzed by Student's t-tests after appropriate log transformation, and differences between medians were analyzed by the nonparametric Wilcoxon rank sum test. P value less than or equal to 0.05 was considered statistically significant.
SUPPLEMENTARY MATERIAL Figure S1. The EBV viral load (copies/µg DNA isolated from PBMCs) and frequency of T cells specific for EBV LMP2, EBNA1, and BZLF1 in UPN 2505 treated for an EBV reactivation. The frequency of specific T cells was measured by IFN ELIspot and results are presented as average SFC/4x10–5 PBMCs. Figure S2. The frequency of T cells specific for CMV IE1 and pp65 in UPN 2718 treated for persistent CMV colitis that was present >4 years post-transplant. The frequency of specific T cells was measured by IFN ELIspot and results are presented as average SFC/4 × 10–5 PBMCs.
Acknowledgments
We are grateful to the study co-ordinators Tiffany Dean, Janet Salinas and Brooke Straub, Deborah Lyon for QC testing, April Durett for phenotypic analyses, Rong Cai and Yijiu Tong for PHA blast generation and Oumar Diouf for assistance with GMP production. AML was supported by the National Marrow Donor Program through funding from the Amy Strelzer Manasevit Research Program. UG was funded by a Leukemia and Lymphoma Society Special Fellow in Clinical Research Award, an ASBMT Young Investigator Award and the HHV-6 Foundation. HEH is supported by a Dan L Duncan Chair and MKB by a Fayez Sarofim Chair. This clinical trial was supported in part by the Production Assistance for Cellular Therapies (PACT) program [NHLBI contract #HHSN268201000007C], the Clinical Research Center at Texas Children's Hospital, the Dan L. Duncan Institute for Clinical and Translational Research at Baylor College of Medicine, and Alex's Lemonade Stand Foundation. We also appreciate the support of shared resources by Dan L Duncan Cancer Center support grant P30CA125123.
Supplementary Material
References
- Schonberger S, Meisel R, Adams O, Pufal Y, Laws HJ, Enczmann J, et al. Prospective, comprehensive and effective viral monitoring in children undergoing allogeneic hematopoietic stem cell transplantation. Biol.Blood Marrow Transplant. 2010;16:1428–1435. doi: 10.1016/j.bbmt.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Verdeguer A, de Heredia CD, González M, Martínez AM, Fernández-Navarro JM, Pérez-Hurtado JM, GETMON: Spanish Working Party for Blood and Marrow Transplantation in Children et al. Observational prospective study of viral infections in children undergoing allogeneic hematopoietic cell transplantation: a 3-year GETMON experience. Bone Marrow Transplant. 2011;46:119–124. doi: 10.1038/bmt.2010.52. [DOI] [PubMed] [Google Scholar]
- Leen AM, Myers GD, Sili U, Huls MH, Weiss H, Leung KS, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med. 2006;12:1160–1166. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]
- Sili U, Leen AM, Vera JF, Gee AP, Huls H, Heslop HE, et al. Production of good manufacturing practice-grade cytotoxic T lymphocytes specific for Epstein-Barr virus, cytomegalovirus and adenovirus to prevent or treat viral infections post-allogeneic hematopoietic stem cell transplant. Cytotherapy. 2012;14:7–11. doi: 10.3109/14653249.2011.636963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leen AM, Christin A, Khalil M, Weiss H, Gee AP, Brenner MK, et al. Identification of hexon-specific CD4 and CD8 T-cell epitopes for vaccine and immunotherapy. J Virol. 2008;82:546–554. doi: 10.1128/JVI.01689-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdemann U, Christin AS, Vera JF, Ramos CA, Fujita Y, Liu H, et al. Nucleofection of DCs to generate Multivirus-specific T cells for prevention or treatment of viral infections in the immunocompromised host. Mol Ther. 2009;17:1616–1625. doi: 10.1038/mt.2009.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdemann U, Keirnan JM, Katari UL, Yanagisawa R, Christin AS, Huye LE, et al. Rapidly generated multivirus-specific cytotoxic T lymphocytes for the prophylaxis and treatment of viral infections. Mol Ther. 2012;20:1622–1632. doi: 10.1038/mt.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdemann U, Vera JF, Rooney CM, Leen AM. Generation of multivirus-specific T cells to prevent/treat viral infections after allogeneic hematopoietic stem cell transplant. J Vis Exp. 2011. [DOI] [PMC free article] [PubMed]
- Melenhorst JJ, Leen AM, Bollard CM, Quigley MF, Price DA, Rooney CM, et al. Allogeneic virus-specific T cells with HLA alloreactivity do not produce GVHD in human subjects. Blood. 2010;116:4700–4702. doi: 10.1182/blood-2010-06-289991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner HJ, Cheng YC, Huls MH, Gee AP, Kuehnle I, Krance RA, et al. Prompt versus preemptive intervention for EBV lymphoproliferative disease. Blood. 2004;103:3979–3981. doi: 10.1182/blood-2003-12-4287. [DOI] [PubMed] [Google Scholar]
- Gottschalk S, Ng CY, Perez M, Smith CA, Sample C, Brenner MK, et al. An Epstein-Barr virus deletion mutant associated with fatal lymphoproliferative disease unresponsive to therapy with virus-specific CTLs. Blood. 2001;97:835–843. doi: 10.1182/blood.v97.4.835. [DOI] [PubMed] [Google Scholar]
- Riker A, Cormier J, Panelli M, Kammula U, Wang E, Abati A, et al. Immune selection after antigen-specific immunotherapy of melanoma. Surgery. 1999;126:112–120. [PubMed] [Google Scholar]
- Leen AM, Christin A, Myers GD, Liu H, Cruz CR, Hanley PJ, et al. Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein-Barr virus infections after haploidentical and matched unrelated stem cell transplantation. Blood. 2009;114:4283–4292. doi: 10.1182/blood-2009-07-232454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuchtinger T, Opherk K, Bethge WA, Topp MS, Schuster FR, Weissinger EM, et al. Adoptive transfer of pp65-specific T cells for the treatment of chemorefractory cytomegalovirus disease or reactivation after haploidentical and matched unrelated stem cell transplantation. Blood. 2010;116:4360–4367. doi: 10.1182/blood-2010-01-262089. [DOI] [PubMed] [Google Scholar]
- Feuchtinger T, Matthes-Martin S, Richard C, Lion T, Fuhrer M, Hamprecht K, et al. Safe adoptive transfer of virus-specific T-cell immunity for the treatment of systemic adenovirus infection after allogeneic stem cell transplantation. Br J Haematol. 2006;134:64–76. doi: 10.1111/j.1365-2141.2006.06108.x. [DOI] [PubMed] [Google Scholar]
- Micklethwaite K, Hansen A, Foster A, Snape E, Antonenas V, Sartor M, et al. Ex vivo expansion and prophylactic infusion of CMV-pp65 peptide-specific cytotoxic T-lymphocytes following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:707–714. doi: 10.1016/j.bbmt.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Bunde T, Kirchner A, Hoffmeister B, Habedank D, Hetzer R, Cherepnev G, et al. Protection from cytomegalovirus after transplantation is correlated with immediate early 1-specific CD8 T cells. J Exp Med. 2005;201:1031–1036. doi: 10.1084/jem.20042384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter EA, Greenberg PD, Gilbert MJ, Finch RJ, Watanabe KS, Thomas ED, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333:1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- Cobbold M, Khan N, Pourgheysari B, Tauro S, McDonald D, Osman H, et al. Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA-peptide tetramers. J Exp Med. 2005;202:379–386. doi: 10.1084/jem.20040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peggs KS, Verfuerth S, Pizzey A, Khan N, Guiver M, Moss PA, et al. Adoptive cellular therapy for early cytomegalovirus infection after allogeneic stem-cell transplantation with virus-specific T-cell lines. Lancet. 2003;362:1375–1377. doi: 10.1016/S0140-6736(03)14634-X. [DOI] [PubMed] [Google Scholar]
- Zandvliet ML, Falkenburg JH, van Liempt E, Veltrop-Duits LA, Lankester AC, Kalpoe JS, et al. Combined CD8+ and CD4+ adenovirus hexon-specific T cells associated with viral clearance after stem cell transplantation as treatment for adenovirus infection. Haematologica. 2010;95:1943–1951. doi: 10.3324/haematol.2010.022947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslop HE, Slobod KS, Pule MA, Hale GA, Rousseau A, Smith CA, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115:925–935. doi: 10.1182/blood-2009-08-239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslop HE, Ng CY, Li C, Smith CA, Loftin SK, Krance RA, et al. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat Med. 1996;2:551–555. doi: 10.1038/nm0596-551. [DOI] [PubMed] [Google Scholar]
- Rooney CM, Smith CA, Ng CY, Loftin S, Li C, Krance RA, et al. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet. 1995;345:9–13. doi: 10.1016/s0140-6736(95)91150-2. [DOI] [PubMed] [Google Scholar]
- Icheva V, Kayser S, Wolff D, Tuve S, Kyzirakos C, Bethge W, et al. Adoptive transfer of epstein-barr virus (EBV) nuclear antigen 1-specific t cells as treatment for EBV reactivation and lymphoproliferative disorders after allogeneic stem-cell transplantation. J Clin Oncol. 2013;31:39–48. doi: 10.1200/JCO.2011.39.8495. [DOI] [PubMed] [Google Scholar]
- Moosmann A, Bigalke I, Tischer J, Schirrmann L, Kasten J, Tippmer S, et al. Effective and long-term control of EBV PTLD after transfer of peptide-selected T cells. Blood. 2010;115:2960–2970. doi: 10.1182/blood-2009-08-236356. [DOI] [PubMed] [Google Scholar]
- Hislop AD, Taylor GS, Sauce D, Rickinson AB. Cellular responses to viral infection in humans: lessons from Epstein-Barr virus. Annu Rev Immunol. 2007;25:587–617. doi: 10.1146/annurev.immunol.25.022106.141553. [DOI] [PubMed] [Google Scholar]
- Amir AL, D'Orsogna LJ, Roelen DL, van Loenen MM, Hagedoorn RS, de Boer R, et al. Allo-HLA reactivity of virus-specific memory T cells is common. Blood. 2010;115:3146–3157. doi: 10.1182/blood-2009-07-234906. [DOI] [PubMed] [Google Scholar]
- Amir AL, Hagedoorn RS, van Luxemburg-Heijs SA, Marijt EW, Kruisselbrink AB, Frederik Falkenburg JH, et al. Identification of a coordinated CD8 and CD4 T cell response directed against mismatched HLA Class I causing severe acute graft-versus-host disease. Biol Blood Marrow Transplant. 2012;18:210–219. doi: 10.1016/j.bbmt.2011.10.018. [DOI] [PubMed] [Google Scholar]
- D'Orsogna LJ, Amir AL, Zoet YM, van der Meer-Prins PM, van der Slik AR, Kester MG, et al. New tools to monitor the impact of viral infection on the alloreactive T-cell repertoire. Tissue Antigens. 2009;74:290–297. doi: 10.1111/j.1399-0039.2009.01311.x. [DOI] [PubMed] [Google Scholar]
- Rist M, Smith C, Bell MJ, Burrows SR, Khanna R. Cross-recognition of HLA DR4 alloantigen by virus-specific CD8+ T cells: a new paradigm for self-/nonself-recognition. Blood. 2009;114:2244–2253. doi: 10.1182/blood-2009-05-222596. [DOI] [PubMed] [Google Scholar]
- Burrows SR, Silins SL, Moss DJ, Khanna R, Misko IS, Argaet VP. T cell receptor repertoire for a viral epitope in humans is diversified by tolerance to a background major histocompatibility complex antigen. J Exp Med. 1995;182:1703–1715. doi: 10.1084/jem.182.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows SR, Silins SL, Khanna R, Burrows JM, Rischmueller M, McCluskey J, et al. Cross-reactive memory T cells for Epstein-Barr virus augment the alloresponse to common human leukocyte antigens: degenerate recognition of major histocompatibility complex-bound peptide by T cells and its role in alloreactivity. Eur J Immunol. 1997;27:1726–1736. doi: 10.1002/eji.1830270720. [DOI] [PubMed] [Google Scholar]
- Burrows SR, Khanna R, Burrows JM, Moss DJ. An alloresponse in humans is dominated by cytotoxic T lymphocytes (CTL) cross-reactive with a single Epstein-Barr virus CTL epitope: implications for graft-versus-host disease. J Exp Med. 1994;179:1155–1161. doi: 10.1084/jem.179.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doubrovina E, Oflaz-Sozmen B, Prockop SE, Kernan NA, Abramson S, Teruya-Feldstein J, et al. Adoptive immunotherapy with unselected or EBV-specific T cells for biopsy-proven EBV+ lymphomas after allogeneic hematopoietic cell transplantation. Blood. 2012;119:2644–2656. doi: 10.1182/blood-2011-08-371971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JN, Doubrovina E, Sauter C, Jaroscak JJ, Perales MA, Doubrovin M, et al. Successful treatment of EBV-associated posttransplantation lymphoma after cord blood transplantation using third-party EBV-specific cytotoxic T lymphocytes. Blood. 2010;116:5045–5049. doi: 10.1182/blood-2010-04-281873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque T, Wilkie GM, Taylor C, Amlot PL, Murad P, Iley A, et al. Treatment of Epstein-Barr-virus-positive post-transplantation lymphoproliferative disease with partly HLA-matched allogeneic cytotoxic T cells. Lancet. 2002;360:436–442. doi: 10.1016/S0140-6736(02)09672-1. [DOI] [PubMed] [Google Scholar]
- Haque T, Wilkie GM, Jones MM, Higgins CD, Urquhart G, Wingate P, et al. Allogeneic cytotoxic T-cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a phase 2 multicenter clinical trial. Blood. 2007;110:1123–1131. doi: 10.1182/blood-2006-12-063008. [DOI] [PubMed] [Google Scholar]
- Mifsud NA, Nguyen TH, Tait BD, Kotsimbos TC. Quantitative and functional diversity of cross-reactive EBV-specific CD8+ T cells in a longitudinal study cohort of lung transplant recipients. Transplantation. 2010;90:1439–1449. doi: 10.1097/TP.0b013e3181ff4ff3. [DOI] [PubMed] [Google Scholar]
- Luke J, Carnes AE, Hodgson CP, Williams JA. Improved antibiotic-free DNA vaccine vectors utilizing a novel RNA based plasmid selection system. Vaccine. 2009;27:6454–6459. doi: 10.1016/j.vaccine.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke JM, Vincent JM, Du SX, Gerdemann U, Leen AM, Whalen RG, et al. Improved antibiotic-free plasmid vector design by incorporation of transient expression enhancers. Gene Ther. 2011;18:334–343. doi: 10.1038/gt.2010.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera JF, Brenner LJ, Gerdemann U, Ngo MC, Sili U, Liu H, et al. Accelerated production of antigen-specific T cells for preclinical and clinical applications using gas-permeable rapid expansion cultureware (G-Rex). J Immunother. 2010;33:305–315. doi: 10.1097/CJI.0b013e3181c0c3cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.