Abstract
In a phase I study of autologous chimeric antigen receptor (CAR) anti-LeY T-cell therapy of acute myeloid leukemia (AML), we examined the safety and postinfusion persistence of adoptively transferred T cells. Following fludarabine-containing preconditioning, four patients received up to 1.3 × 109 total T cells, of which 14–38% expressed the CAR. Grade 3 or 4 toxicity was not observed. One patient achieved a cytogenetic remission whereas another with active leukemia had a reduction in peripheral blood (PB) blasts and a third showed a protracted remission. Using an aliquot of In111-labeled CAR T cells, we demonstrated trafficking to the bone marrow (BM) in those patients with the greatest clinical benefit. Furthermore, in a patient with leukemia cutis, CAR T cells infiltrated proven sites of disease. Serial PCR of PB and BM for the LeY transgene demonstrated that infused CAR T cells persisted for up to 10 months. Our study supports the feasibility and safety of CAR–T-cell therapy in high-risk AML, and demonstrates durable in vivo persistence.
Introduction
Despite improvements in chemotherapy and allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia (AML), the majority of patients with standard or high-risk AML will die of relapsed and/or progressive disease. AML shows sensitivity to T-cell–mediated control in the setting of allogeneic hematopoietic stem cell transplantation;1 however, therapeutic approaches directed at inducing autologous T-cell responses in patients with AML have shown limited efficacy.2,3,4 These limitations to antigen-specific T-cell immunotherapy can potentially be overcome by retroviral transduction of an immunogene expressing a model chimeric antigen receptor (CAR) against a known tumor-associated antigen (TAA) into autologous T cells to generate therapeutic CAR T cells.5,6,7 Immunogenes may vary in specific design; however, most contain single-chain variable (scFv) regions of TAA-specific monoclonal antibody (mAb) joined to a signal transduction domain.8,9,10,11 The first generation of CARs contained a single-signaling domain derived from the TCR-ζ chain or the FcR-γ chain.12,13 Second generation CARs, as used in this study, and third generation CARs have incorporated one and two costimulatory motifs, respectively, into their cytoplasmic domains resulting in superior cytokine and proliferative responses against tumors.14,15,16 Recent reports have demonstrated startling clinical responses using enriched CAR–T-cell infusions against CD19 in chronic and acute B-cell malignancies.17,18 However, many tumors, including AML, do not share the antigen restriction demonstrated by CD19. We wished to use a TAA with wide applicability in hematologic and solid organ malignancies. LeY is a difucosylated carbohydrate antigen and although its function is not known, it is expressed on a range of proteins including some TAA,19 often at high copy number, on a wide range of malignancies including AML20,21 but with only limited expression on normal tissue.22 Its expression has correlated with poorer prognosis in some cancers.23
We generated CAR T cells by using the single-chain variable (scFv) region of the mAb against the TAA Lewis (Le)-Y coupled to the cytoplasmic domains of CD28 and the TCR-ζ chain. We have previously shown efficacy of these cells in mouse models of LeY-expressing tumors24 in addition to functional differentiation of the human LeY CAR T cells and interferon γ (IFN-γ) and interleukin 2 (IL-2) secretion in response to LeY-expressing myeloid leukemia cells in vitro.25,26 In this study, we assessed the safety of LeY CAR T cells in patients with relapsed AML, in whom the blasts were shown to express LeY. Our findings indicate that successful T-cell transduction and expansion of CAR T cells can be undertaken in patients with relapsed AML. These autologous CAR T cells can be infused safely, show tissue specific localization, in vivo persistence, and potential antileukemic efficacy.
Results
Patient characteristics
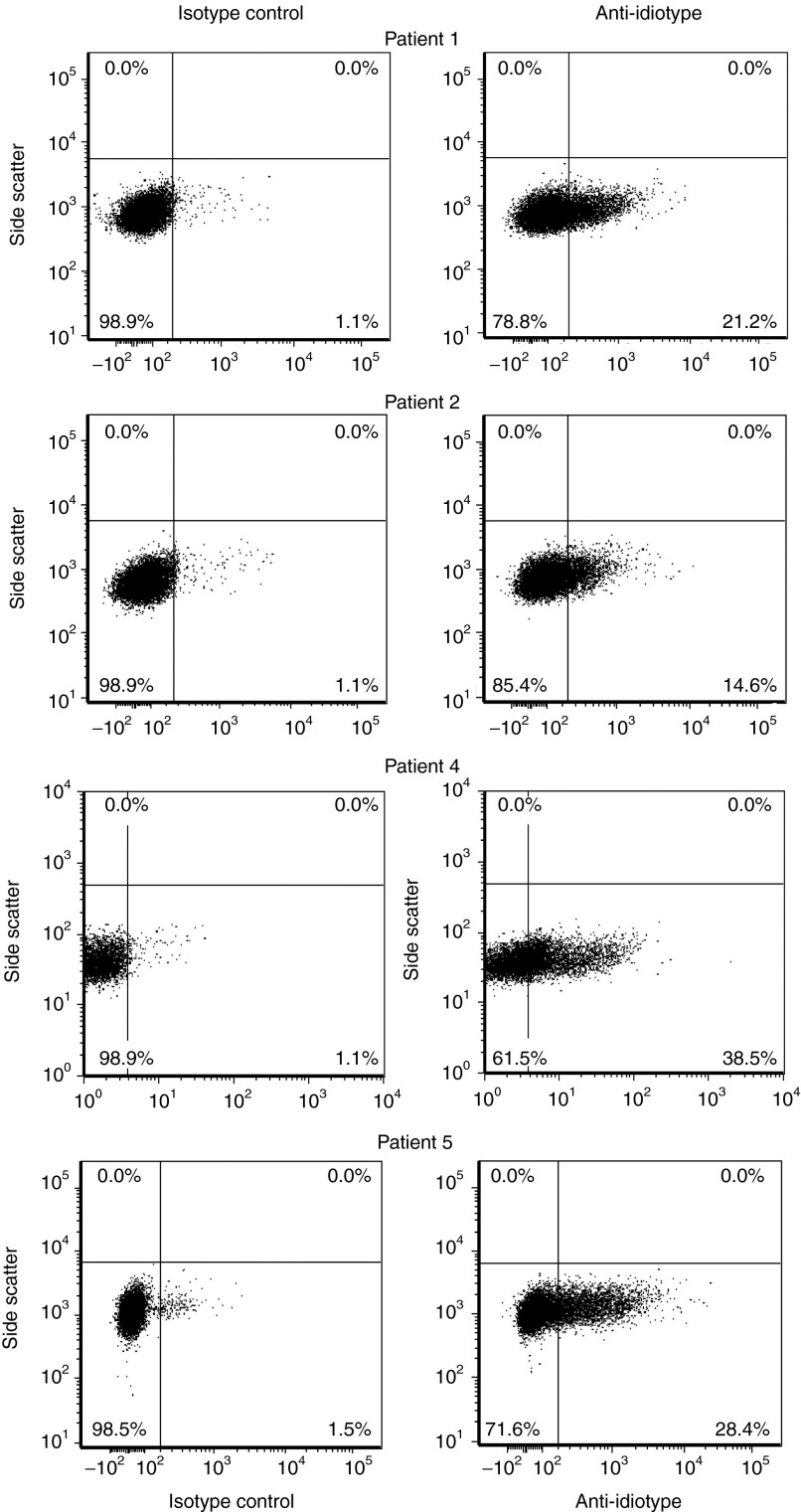
Five patients with relapsed AML were enrolled (Table 1). Adequate peripheral blood mononuclear cells (PBMC) were harvested in all cases (1.64 × 109 to 26.5 × 109). Patient 3 died from complications of sepsis related to reinduction chemotherapy. Four patients received CAR T cells. Three patients (patients 1, 2, and 5) had evidence of cytogenetic minimal residual disease at the time of CAR–T-cell infusion. Patient 4 had active leukemia in the bone marrow (BM) and peripheral blood (PB) at the time of CAR–T-cell infusion. The median dose of T cells infused was 1.1 × 109 (range 5 × 108 to 1.3 × 109). The proportion of transduced T cells was between 14 and 38% (Figure 1) and cell viability was >96% in all cases. Indium111 labeling was successful in all cases and the number of labeled cells infused was between 1 × 108 and 2.6 × 108.
Table 1. Patient characteristics.
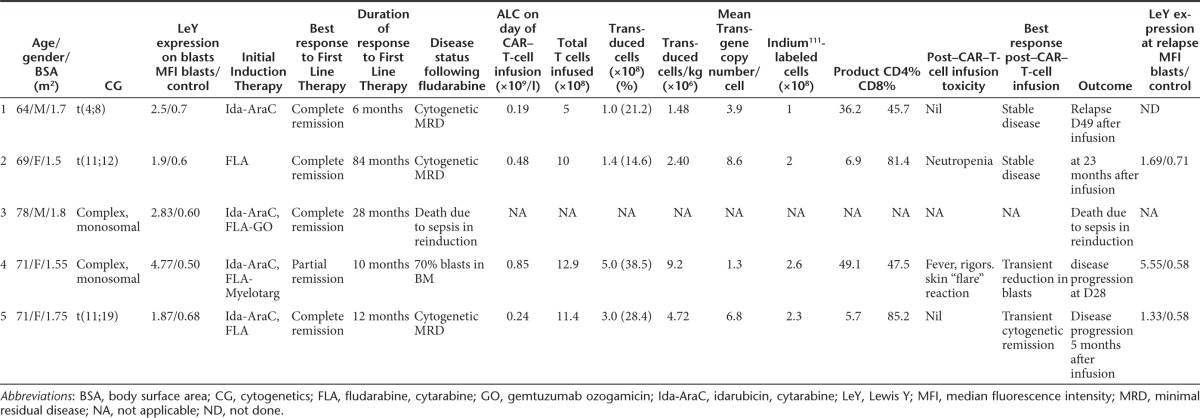
Figure 1.
AML patient PBMCs were transduced and expanded in vitro to express the LeY CAR and expanded to produce the T-cell product. Successful transduction was confirmed by T-cell expression of the LeY CAR, as detected by anti-idiotype (Id) binding and flow cytometry analysis. Data are presented as dot plots with an isotype control (left-hand panels) and anti-Id staining for each AML patient T-cell product (right-hand panels) with the percent anti-Id positive shown in each lower quadrant. AML, acute myeloid leukemia; CAR, chimeric antigen receptor; PBMC, peripheral blood mononuclear cells.
Tolerability of infusions
Infusions were well tolerated. No episodes of tumor lysis syndrome were seen. No patients experienced grade 3 or 4 toxicity. One patient (patient 2) had a transient grade 2 neutropenia 3 months after CAR–T-cell infusion, which resolved spontaneously. No autoimmune disorder or new monoclonal T-cell population was detected in the postinfusion period (data not shown).
AML response to infusion of LeY T cells
Three patients (patients 2, 4, and 5) had evidence of biological responses to CAR–T-cell infusion (Table 1). Before CAR–T-cell infusion, and despite reinduction of morphological CR with fludarabine and cytarabine, patients 1, 2, and 5 all showed evidence of persistence of a cytogenetically abnormal clone.
Following CAR–T-cell infusion in patient 2, the cytogenetic clone remained detectable on repeat BM aspirates; however, a morphologic and immunophenotypic remission was sustained for 23 months.
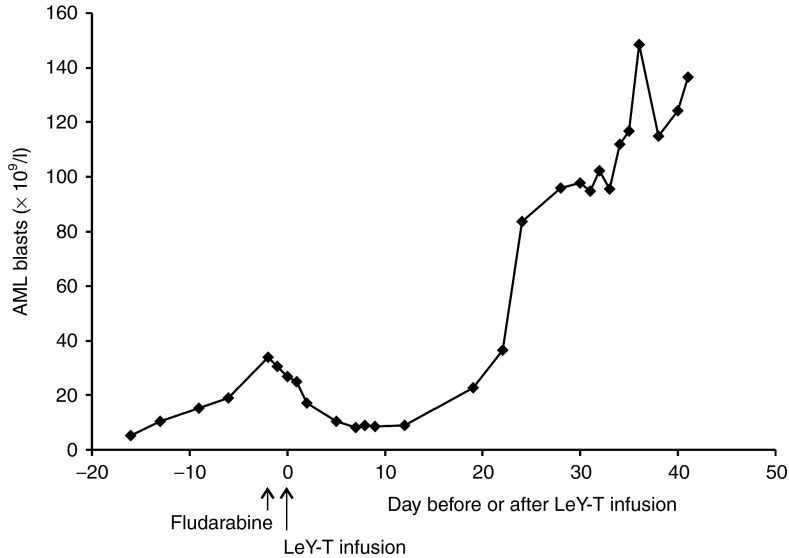
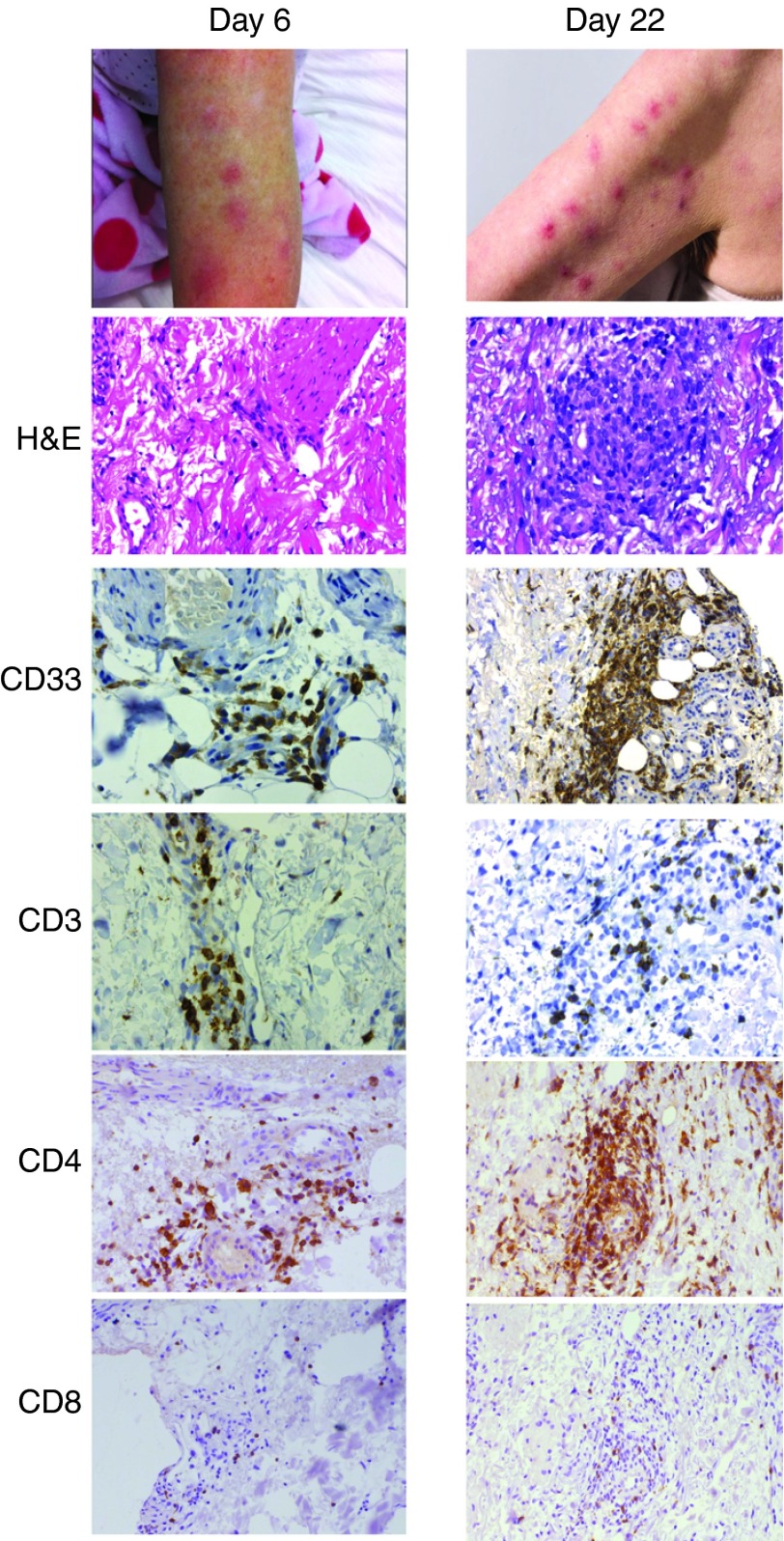
Patient 4 had no discernible benefit from reinduction chemotherapy and still had active and frank leukemia at the time of infusion of 1.2 × 108 CAR T cells. Despite this, a reduction in the PB blast count was observed from 25 × 109/l to 8 × 109/l immediately following CAR–T-cell infusion (Figure 2). In this patient, the complete absence of a previous benefit from prior Fludarabine-containing chemotherapy suggested that the infused CAR T cells contributed to the observed decrease in blast counts, although a contribution from the preconditioning fludarabine regimen cannot be fully excluded. However, this patient had also had a prior history of leukemia cutis and developed a transient skin rash 4 days after infusion, which resolved in 5 days without specific management (Figure 3). A skin biopsy from this rash on day 6 after CAR–T-cell infusion revealed a lymphocytic infiltrate (Figure 3). The patient developed a new rash on day 22 after infusion; histology and immunohistochemistry showed infiltration of the skin with AML blasts (CD33+), the skin biopsies taken on day 6 and 22 were also infiltrated with CD4+ and CD8+ T cells (Figure 3). Subsequently, disease progression occurred at 1 month after infusion.
Figure 2.
Reduction in peripheral blood blast count in patient 4 following adoptive cell transfer of CAR T cell (indicated at day 0 by arrow). CAR, chimeric antigen receptor.
Figure 3.
Localization of CAR T cells to skin in patient 4. In skin biopsies (taken days 6 and 22 after CAR T infusion), sections were stained by H&E, and IHC for CD33 and CD3, CD4, and CD8; staining shows infiltration of the skin by CD4+ and CD8+ T cells and CD33+ blasts. CAR, chimeric antigen receptor.
Patient 5 showed post–CAR-T infusion clearance of the cytogenetically abnormal clone. This cytogenetic response was maintained until disease progression at 5 months.
In patients 2, 4, and 5 the AML blasts present at relapse continued to express LeY antigen at levels comparable with those seen in enrolment samples (Table 1) indicating that progression was not due to antigenic shift in the AML population.
In summary, patient 1 relapsed 49 days after T-cell infusion (see Table 1) and subsequently died with AML disease restricted to the marrow. Patient 2 remained with cytogenetic disease out to 23 months, before enrolment in another trial. A transient reduction in circulating blasts was observed in patient 4, but disease progressed at day 28 with AML blasts present in the circulation. Patient 5 achieved a transient cytogenetic remission, but relapsed 5 months after T-cell transfer and subsequently died with AML disease restricted to the marrow.
LeY T cells traffic to the BM and persist after adoptive transfer
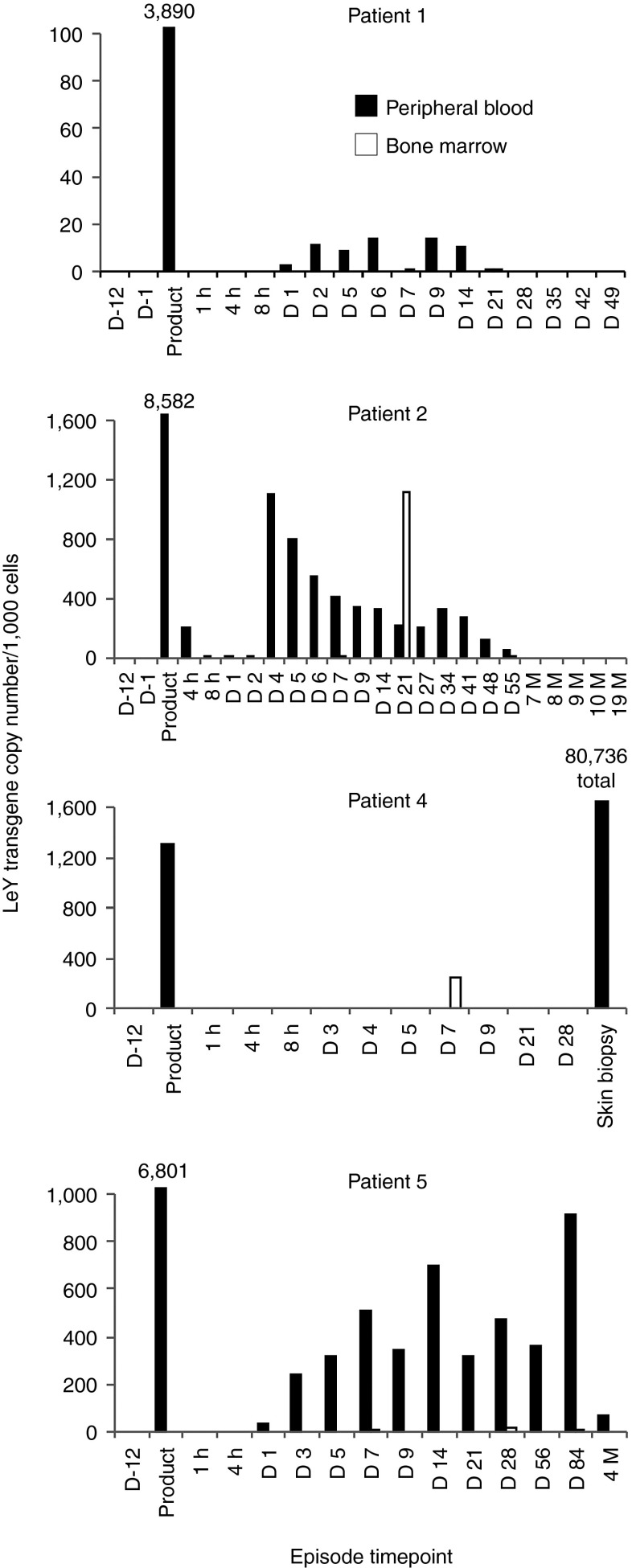
Due to the limited sensitivity of the anti-idiotype antibody to detect functional CAR expression on T cells ex vivo, a quantitative PCR (qPCR)-based assay was used to determine trafficking and persistence of T cells following transfer. Trafficking and persistence of CAR T cells was detected by PCR after infusion in the PB and BM of all patients. Patient 1 was infused with 0.9 × 108 transduced T cells. Qualitative PCR for the LeY transgene demonstrated persistence of the infused CAR T cells in the PB and BM until disease progression at day 49 (Supplementary Figure S1). However, qPCR demonstrated a copy number of 3,890 per 1,000 cells in the T-cell product and low-level persistence of CAR T cells at a level of ~10 per 1,000 PBMC only until day 14 after infusion (4). Patient 2 was infused with 1.3 × 108 LeY T cells, which remained detectable in the PB and BM by PCR until 10 months after adoptive transfer (Supplementary Figure S1) and by qPCR analyses until day 55 (Figure 4). The T-cell product for this patient showed by LeY transgene qPCR 8,600 transgene copies per 1,000 cells and showed a spike in LeY transgene copy number on day 21 in the BM of 1,100 copies per 1,000 cells, indicating increased trafficking or expansion of the CAR T cells in the BM (Figure 4). This evidence of CAR–T-cell persistence and likely expansion in patient 2 was associated with the longest time to progression (23 months) of any of the patients treated.
Figure 4.
LeY T cells are present in PB BM and skin and persist up to 10 months postadoptive transfer. Genomic DNA was extracted from the T-cell product or PB and BM samples of patient 2. Subsequently, LeY transgene qPCR was performed as per the methods. All episodes are PB unless designated as BM. Time points before infusion are designated as D-12, D-1; all other time points are postadoptive transfer and designated in hours, days, or months. LeY transgene qPCR data are expressed as the number of transgene copies/1,000 cells (from which genomic DNA was harvested). Numbers above bars represent gene copies per 1,000 cells, except for the skin biopsy of patient 4 where total gene copy number is presented although lacking information on the number of cells in the sample. Data are shown for the PB (black bars) and BM samples (white bars) from each episode. BM, bone marrow; D, day; h, hour; M, month; PB, peripheral blood.
Patient 4 was infused with 1.2 × 108 transduced T cells, containing 1,300 copies per 1,000 cells. qPCR showed that despite low-level persistence in BM (Figure 4) high-level persistence of transduced T cells were found within the biopsies of affected skin using histology (Figure 3) and qPCR (Figure 4), indicating focal trafficking to areas of AML involvement.
Patient 5 was infused with 3.4 × 108 transduced T cells containing 6,801 copies per 1,000 cells. qPCR showed persistence of infused cells until 4 months in PB (Figure 4), a result which was confirmed by qualitative PCR (Supplementary Figure S1).
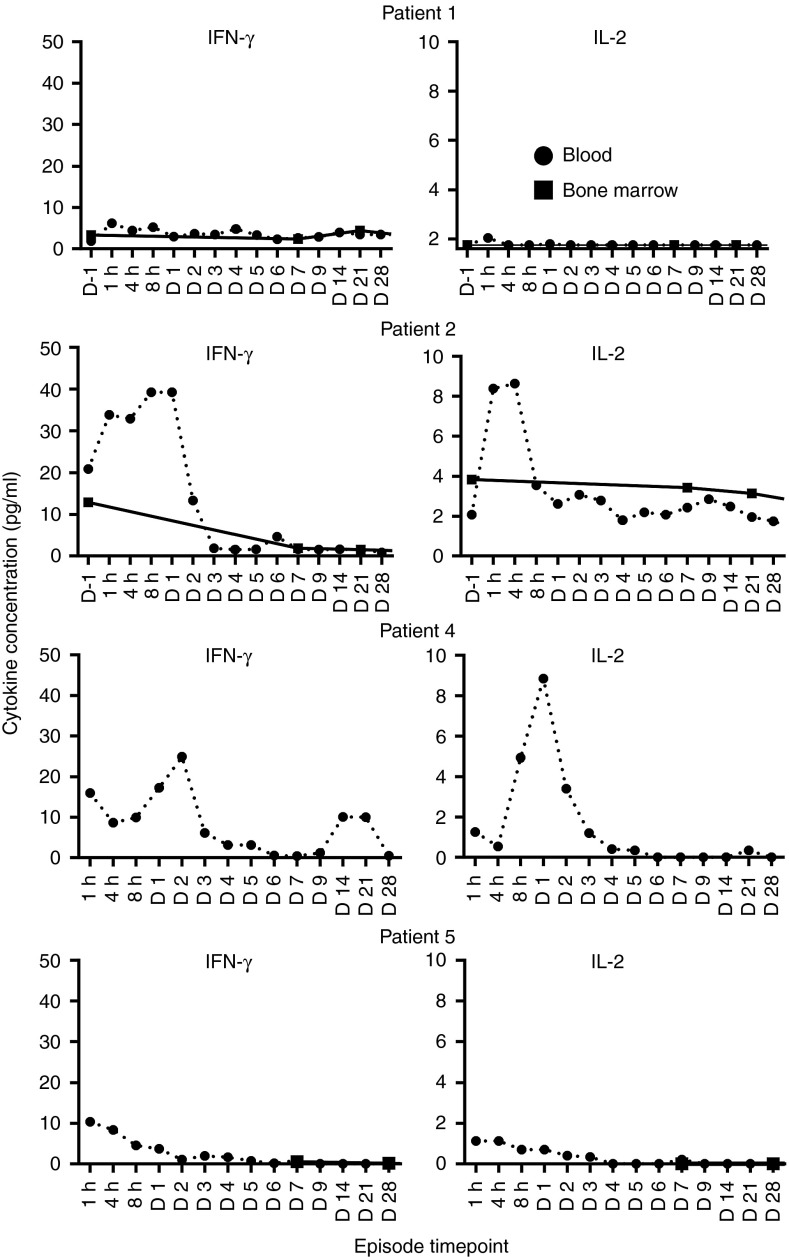
Commensurate with the increase in copy number in patient 2, a spike in serum interferon (IFN)-γ and IL-2 was also seen in the first 48 hours after CAR–T-cell infusion (Figure 5). A similar IFN-γ and IL-2 spikes were seen in patient 4 (Figure 5). Serum IL-4, IL-5, IL-10, and TNF were also assessed but showed no change after infusion of CAR T cells.
Figure 5.
Increase in systemic IFN-γ and IL-2 occurred after adoptive transfer of LeY T cells. Cytokines were assessed in the serum of patients before infusion or after infusion of LeY CAR T cells at the indicated times. Cytokine levels were measured in duplicate wells by Luminex assay as per the methods. CAR, chimeric antigen receptor. D, day; h, hour.
Tracking of In111-labeled cells with SPECT scan
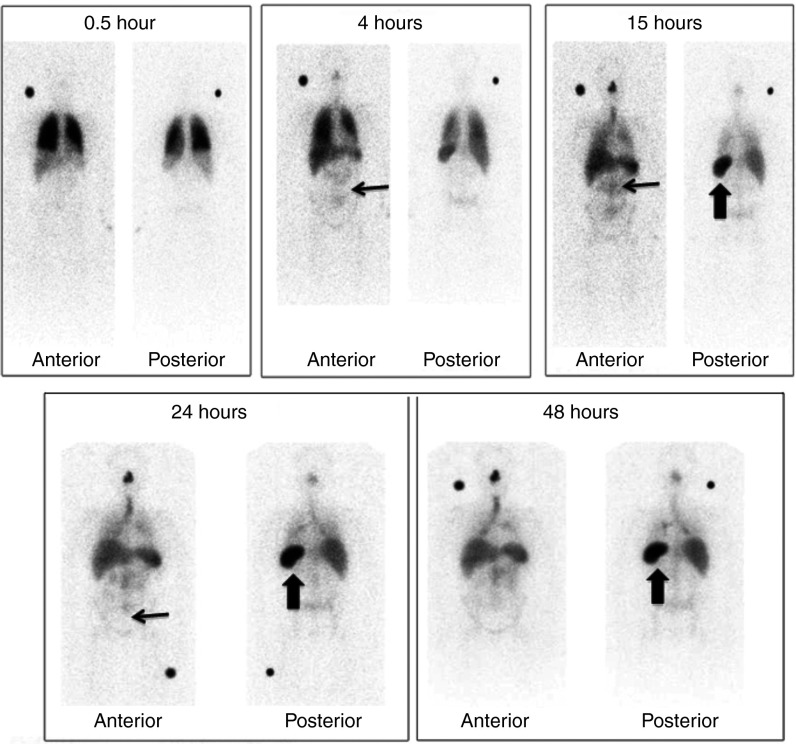
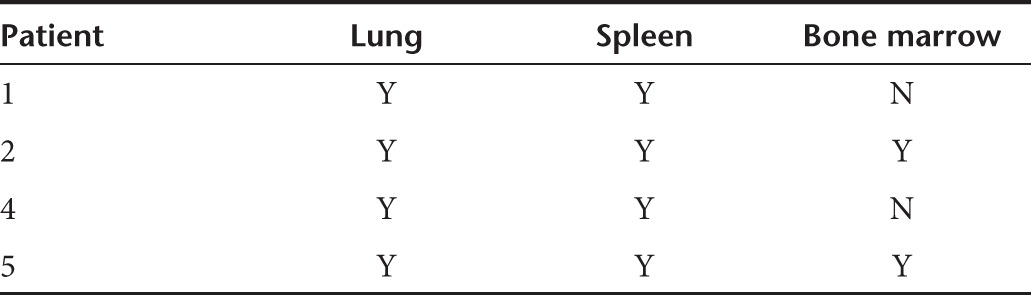
In111-labeled cells were serially imaged with SPECT scanning at five time points after infusion. A similar pattern was demonstrated in all patients. A representative series of images is shown for patient 2; initial pulmonary localization at 0.5 hours was observed followed by redistribution and accumulation in the BM and spleen from 4 hours (Figure 6). A summary of distribution of tracking in the remaining patients is outlined in Table 2.
Figure 6.
Tracking of In111-labeled cells with SPECT scan. In111-labeled cells were serially imaged with SPECT scanning at five time points after infusion in patient 2. Initial pulmonary localization at 0.5 hours is seen followed by redistribution and accumulation in the bone marrow (thin arrow) and spleen from 4 hours (thick arrow). In addition, increased trafficking can be seen in the nasal mucosa, due to a coexisting viral upper respiratory tract infection. A summary of distribution of In111-labeled cells in the other three patients after infusion is shown in Table 2.
Table 2. Distribution of radiolabeled cells.
Discussion
Adoptive immunotherapy, using autologous CAR T cells is an emerging therapy in solid tumors and hematologic cancers,2,18,27,28 which merges the targeted specificity of monoclonal antibodies with the potent cytotoxicity, potential for expansion, and long-term persistence of cytotoxic T cells.29 We found that the program of treatment including the generation and infusion of CAR T cells could be safely delivered to patients with relapsed/refractory AML. Other investigators have observed symptoms such as fever, malaise, and myalgias after CAR–T-cell infusion coinciding with increases in IFN-γ and IL-6 production.30 These transient acute toxicities were not seen in our patients even though we were able to demonstrate production of IFN-γ by the infused T cells in the early postinfusion period in two patients. We did not encounter the serious immune activation syndrome previously reported with adoptive transfer of T cells.31
We also identified, by both qualitative and quantitative PCR, that transduced CAR T cells persisted in patients for, in some instances, many months. Our qPCR data on the preinfusion product suggested that between 8 and 17 DNA copies per cell was being achieved. This is more that the rather modest transduction rates suggested by anti-idiotype staining. We have previously shown that there is a relationship between DNA copy number and transduction efficiency with this system (Neeson et al.29). In the non–Good Manufacturing Practice (GMP) research laboratory setting, we have previously found that we achieved between 1.9 and 14.7 LeY transgene copies per cell with a transduction efficiency demonstrated by anti-Id staining of between 21 and 69%. Furthermore, we also showed a correlation between high transgene copy number by PCR and higher CAR expression by flow cytometry. The apparent discrepancy seen in the GMP-grade product between the copy number detected in or qPCR assay and the percentage transduced cells in the infused product likely reflects an underestimate of the transduction achieved in the large-scale GMP-grade production runs used in this clinical trial. However, we acknowledge that further optimization of vector and transduction procedures are necessary to optimize clinical efficacy. Nevertheless, our infused absolute T-cell doses of between 5 × 108 and 1.3 × 109 T cells equates to an infused LeY cell dose of 1.48–4.72 × 106/kg similar to the T-cell dose/kg anti-CD19 CAR T cells/kg which have resulted in clinical responses in chronic lymphocytic leukemia.18
We demonstrated by serial SPECT scanning that the infused T cells localize to the BM, a finding confirmed by PCR analysis of PB and marrow samples. LeY T cells were detectable in all patients by PCR until relapse, which ranged from 28 days to 23 months. The average copy number of transgene within the cells in the T-cell product was highly variable ranging from 1.3 to 8.6. However, it is of interest that patient 2 with the highest transgene copy number in the T-cell product (8.6) had the highest transgene content detectable in the marrow at day 21 (1.1 transgene copies per cell) and also had the most durable clinical response. This patient's demonstrable increase in transgene copy number in the BM sample 21 days after CAR–T-cell infusion, suggests increased trafficking and/or proliferation of the cells in the BM. We have previously shown that, in preinfusion CAR–T-cell products, a high LeY transgene copy number is associated with increased expression of the CAR and was associated with increase antigen-specific CAR–T-cell cytotoxicity and cytokine release.25 Unfortunately, as our pilot studies indicated the anti-Id antibody had a low affinity and could only detect LeY T cells when they were present in a spiked sample at a ratio of >1:10, this was unlikely to be useful as a detection assay. Therefore, due to the limited sensitivity of the anti-idiotype antibody to detect functional CAR expression on T cells ex vivo we did not incorporate anti-idiotype assessment of ex vivo samples.
Using the PCR to detect the presence of transgene, we also demonstrated localization of the infused T cells to the skin of patient 4, who developed a skin rash shortly after the infusion. In this patient, CAR T cells showed the ability to traffic to the BM and the skin, both were sites of disease involvement. This demonstrable migration of effector CAR T cells to the skin and BM was accompanied by a transient drop in leukemic blast count. Interestingly, a higher percentage of CD4+ T cells compared with CD8+ T cells was found in skin biopsies using histological analyses, despite an approximately equal CD4:CD8 ratio (49.1%:47.9%) of these cells in the T-cell–infusion product. The reasons for this are not clear, but could include differential expression of skin-homing chemokine receptors on the two T-cell subsets. It is worth noting that although the CAR construct contains the hinge region from CD8, it is only a short segment of the entire CD8 molecule and unlikely to be detected using the antibodies in our histological analyses.
Given the progression of AML in all patients, despite demonstrable CAR–T-cell persistence, we explored the possibility that residual AML blasts had lost LeY expression. In all patients, LeY expression on myeloblasts analyzed at progression remained comparable with that expressed at enrolment. Alternatively, the failure of adoptively transferred T cells to control disease may have been due to downregulation of transgene expression as has been observed previously.32 In the present study, we were unable to definitively determine transgene expression on T cells following transfer due to their low frequency and low sensitivity of the anti-idiotype antibody. Another possible explanation for AML progression despite continued antigen expression and CAR–T-cell persistence is that the BM of patients with AML is an immune suppressive environment rendering the CAR T cells unresponsive. Support for this concept can be found in a mouse model of AML,33 where PD-1/PDL-1 and TIM-3/Galectin-9 interactions were shown to suppress tumor-resident T-cell responses in vivo. Inhibitory molecules (TIM-3, CTA-4, PD-1, BTLA, LAG3, and CD200R) play a role in immune suppression induced by hematological cancers including AML.34 Thus, to circumvent such phenomena, adoptive therapy with CAR T cells could be enhanced by immune checkpoint blockade combination therapy; blocking antibodies to CTLA-4 (ipilimumab)35,36,37 and PD-138 have already shown efficacy in clinical trials.
We have demonstrated that CAR T cells against the novel antigen LeY can be successfully generated and safely infused in patients with high-risk AML and show appropriate in vivo trafficking, appropriate persistence and functional activity. These characteristics are all likely to be critical to induce and maintain clinical responses and underscore the feasibility of this approach in a heavily pretreated population of patients with high-risk hematologic malignancy.
Our demonstration of long-term persistence of the CAR T cells indicates that this strategy provides a highly suitable platform for combinational immunotherapies that promote ongoing CAR expression by T cells.
This may be best achieved by the development banks of preproduced CAR T cells which are frozen for later, potentially multiple, infusions. In our study, we used a single injection of freshly transduced autologous T cells. However, T-cell products typically retain good viability following cryopreservation, and this procedure may provide an opportunity for repeated administration of gene modified T cells. Similarly, the development of third generation CAR T cells, which have recently shown potent clinical effect and the ability to both persist and expand in vivo, may be necessary for optimal tumor control. However, equally likely as contributors to the success of the anti-CD19 CAR T cells is the density of antigenic target on the surface of tumor cells. LeY, although expressed on a proportion of AML blasts, is expressed more strongly and more uniformly on epithelial tumors. We propose to now extend our initial findings in this study to a study in advanced lung cancer.
Materials and Methods
Patients and study design. This study is a single centre phase 1 clinical trial approved by the institutional ethics committee (PMCLeYPh1-01) and the Therapeutic Goods Administration of Australia; trial registration number: CTX 08-0002. All patients provided written informed consent. We evaluated the safety and tolerability of an intravenous infusion of autologous PB T lymphocytes transduced with the anti-LeY-scFv-CD28-ζ vector in patients with LeY-expressing AML. In addition, we assessed clinical responses, immunologic activities, in vivo tracking pattern, and persistence of the LeY CAR T cells.
Eligibility criteria. Patients were eligible for enrolment if their diagnostic or relapsed BM sample showed positive expression of LeY by flow cytometry defined as having
LeY expression on at least 20% of myeloid blasts and
the median fluorescence intensity of LeY expression on myeloblasts of at least twice that of lymphocytes contained within the same sample
and were >18 years of age and had a confirmed diagnosis of high-risk AML, defined as >65 years or 56–65 years with any of monosomy 7, monosomy 5, trisomy 8, abnormal 3q, t(6;9), t(9;22), or t(9;11), normal karyotype with FLT3-ITD or with a complex aberrant karyotype defined as >4 cytogenetic abnormalities or if they had relapsed or refractory disease, had an ECOG performance status of 0–1, a lymphocyte count of ≥0.5 × 109/l at enrolment and adequate organ function. Patients were excluded if the white cell count exceeded 30 × 109/l, or if they had received immunotherapy, chemotherapy, or G-CSF within 4 weeks of enrolment.
CAR–T-cell production and infusion. All cell production occurred under GMP conditions in the Centre for Blood Cell Therapies at the Peter MacCallum Cancer Centre (www.celltherapies.com.au) and satisfied strict release quality criteria (Supplementary Tables S1 and S2).
PBMC were collected from eligible patients by steady state apheresis (2 hour collection procedure on Cobe Spectra; Terumo BCT, Sydney, Australia) and cryopreserved for potential manufacture of CAR T cells.
The CAR gene construct used in this study used the extracellular membrane-proximal 60 amino acids from the immunoglobulin superfamily member, CD8, which are thought to function as a hinge/spacer region. Hinge regions from other immunoglobulin superfamily members can also be used in CAR constructs, including those from IgG1 and IgG4, but we used the CD8 hinge in our developmental work and this was determined to be adequate to enable antigen-specific function from transduced T cells.
The chimeric receptor was engineered as previously reported using the extracellular humanized scFv, recognizing the LeY Ag, linked to an extracellular CD8 hinge region, a transmembrane and cytoplasmic CD28 signaling domain, and CD3 ζ-signaling chain.24 The humanized anti-LeY scFv-CD28-ζ receptor was cloned into the pSAMEN retroviral vector (lacking the neomycin phosphotransferase gene) and transfected into the packaging line PG13.24 In pSAMEN, expression of the construct is driven by the long terminal repeat promoter region of the Moloney murine leukemia virus. A retroviral vector was used in this study because, at the time of protocol development, this vector system was seen as more established and readily acceptable to our regulatory body, the Therapeutic Goods Administration.
Following successful collection of PBMC, and within 24 months of collection, each eligible patient was treated with one cycle of a fludarabine 30 mg/m2 D1-5 and, cytarabine 2 gm/m2 D1-5 to achieve both reduction of AML disease burden and to promote homeostatic proliferation of the subsequently infused CAR T cells. Following chemotherapy and after BM recovery (or 4 weeks which ever occurred first), patients with proven residual disease were eligible to receive autologous LeY CAR T cells, which were infused within 6 weeks from the end of the chemotherapy.
CAR T cells were generated with a 12 day manufacturing process. On day 12, before planned infusion, patient's frozen MNC were rapidly thawed then cultured in vitro for 3 days in the presence of the T-cell–activating antibody anti-CD3, (OKT-3 monoclonal antibody 30 ng/ml; Janssen-Cilag Pharmaceuticals, North Ryde, Australia) and recombinant human IL-2 (PROLEUKIN 600 IU/ml; Chiron Therapeutics, Emeryville, CA) in AIM-V CTS medium (Therapeutic Grade; GIBCO, Life Technologies, Mulgrave, Australia) supplemented with 2% human pooled AB serum (Valley Biomedical, Winchester, VA) in cell culture vessels at 37 °C in a humidified 5% CO2 incubator. On day 9, activated T cells (1.75 × 108 to 2.61 × 108) were transduced with retroviral supernatant (EUFETS AG Aktiengesellschaft, Idar-Oberstein, Germany) containing retroviral vector constructs. The transduction procedure was performed in the cell culture vessels which are precoated with clinical grade RetroNectin, (Takara Bio, Shiga, Japan). Transduction was performed twice (on consecutive days 9 and 8).
CAR T cells were expanded for a further 7 days in AIM-V CTS medium supplemented with 2% human pooled AB serum and IL-2 at 600 IU/ml. Viable cell concentrations were generally maintained between 0.5 × 106 and 0.75 × 106 per milliliter by the addition of fresh medium.
On day 0, expanded CAR T cells were washed with phosphate buffered saline and concentrated using the CytoMate Cell Washer (Nexell, Zug, Switzerland). The final T-cell product was formulated into 100 ml of sterile normal saline supplemented with 5% Albumex20 (CSL, Melbourne, Australia).
Phenotypic characterization of day 2 culture was evaluated by using flow cytometry of the surface markers CD3, CD4, and CD8 (BD Biosciences) expression. The percentage of LeY-positive T cells was determined by staining with an anti-idiotype (custom conjugated) antibody.
All cryopreserved MNC products and T-cell products (unlabeled and Indium111 labeled) met the acceptance criteria defined in the protocol. Our data demonstrated the feasibility and efficiency of the manufacturing process and associated quality control testing for the CAR T cells. Samples were collected at day 12 and day 0 of the manufacturing procedure for subsequent functional analyses.
CAR–T-cell labeling for SPECT scanning. Based on T-cell count in the product, a defined aliquot was calculated and removed for labeling with Indium-111 Oxine (GE Healthcare, Arlington Heights, IL). Labeled cells were washed, assessed for activity, and then resuspended in 50 ml of sterile normal saline supplemented with 5% Albumex20 (CSL), and a minimum of 1 × 108 T-labeled cells were coinfused with the remainder of the CAR T cells. Indium-labeled CAR T cells were tracked by serial SPECT scanning at 0.5, 4, 15, 24, and 48 hours after infusion.39
T-cell product infusion and monitoring schedule. Following preconditioning with fludarabine 30 mg/m2 D1-5 and cytarabine 2 gm/m2 D1-5 and after BM recovery (or 4 weeks whichever occurred first), patients with proven residual disease were eligible to receive autologous LeY CAR T cells at a minimum absolute cell dose of 5 × 108 T cells including a minimum of 1 × 108 anti-LeY–positive T cells. The maximum number of reinfused T cells could not exceed 4 × 1010 as required by the IRB and Therapeutic Goods Administration in keeping with the first-time-in-man nature of the CAR construct. Adverse events were recorded according to NCI CTCAE version 3.
Monitoring of LeY T cells by qualitative and quantitative transgene PCR and functional assays. Anticoagulated PB was collected at 1, 2, 4, and 8 hours, days 1–7, 9, 14, 21, and monthly until progression. BM samples were collected at day 7 and 21, and 3 monthly until disease progression. Lymphocytes and serum were stored for immunological analysis.
In vivo persistence of infused LeY CAR T cells was assessed by qualitative and quantitative PCR for the LeY transgene.
Patient PBMC or BM genomic DNA were prepared using lysis buffer (1 × Promega Colorless Go Taq Flexi Buffer, 2 mmol/l MgCl2, 100 ng/ml proteinase K, and 0.5% Tween-20 in MilliQ water; Promega, Madison, WI). DNA was extracted from cryopreserved BM or PBMC pellets by lysing 1 × 106 cells in 100 µl of lysis buffer at 55 °C for 1 hour, heat inactivating at 95 °C for 15 minutes and stored at −20 °C. The primers and probes for both PCR and qPCR are listed below (5′ to 3′ in each case) (Supplementary Table S3); primers were obtained from Sigma-Aldrich (St Louis, MO) and the TaqMan TAMRA probes were purchased from Applied Biosystems, Life Technologies (Mulgrave, Australia).
PCR was performed using the MJ Research PTC-200 Peltier Thermal Cycler (MJ Research, St Bruno, Quebec, Canada) on 10 µl of DNA extracts with 250 nmol/l LeY forward and reverse primers, 0.5 mmol/l MgCl2, 0.2 mmol/l dNTPs, and 0.025 U/µl Go Flexi DNA polymerase (Promega) for 35 cycles of 30 seconds denaturation at 95 °C, 30 seconds annealing at 62 °C and 30 seconds extension at 72 °C. pSAMEN-LeY and pSAMEN vectors of 10 ng were added as positive and negative controls in all PCR runs.
qPCR was performed on a ABI Prism 7000 Sequence Detection System (Applied Biosystems, Life Technologies) using TaqMan (Applied Biosystems, Life Technologies) chemistry according to manufacturer's instructions with 10 µl DNA solution and primers LeY (forward) LeY (reverse) and am (reverse) at 300 nmol/l, am (forward) at 100 nmol/l and probes at 200 nmol/l. All samples were run in triplicate. Standard curves were prepared using serial dilutions of pSAMEN-LeY plasmid (LeY) or DNA prepared from normal PBMC (am). The copy number of unknown samples was interpolated from the standard curves using GraphPad Prism Software (La Jolla, CA). The am (housekeeping) gene was assumed to be single copy and diploid and used to estimate copy number per cell for the LeY gene. The sensitivity of the PCR and qPCR assays was assessed by titrating transduced normal donor cells into autologous mock-transduced cells. This study showed LeY transgene could be reliably detected at 1:100,000 cells (PCR) or 1:5,000 (qPCR) (data not shown).
Plasma cytokine assays. Plasma separated from PB and BM aspirate were analyzed in batches on the Luminex 200 (Luminex xMAP Technology; Luminex, Austin, TX) using the Millipore Milliplex Human Cytokine/Chemokine kit (Luminex) for IFN-γ, IL-2, IL-4, IL-6, IL-10, and TNF, and the Milliplex MAP kit (Luminex) for TGF-β Single Plex according to manufacturer's instructions for plasma samples.
SUPPLEMENTARY MATERIAL Figure S1. LeY T cells are present in the peripheral blood (PB) and bone marrow (BM) for extended periods after infusion. Table S1. Release criteria for radiolabeled T-cell product for T-cell–tracking studies. Table S2. Release criteria for manufactured T-cell product for therapeutic administration. Table S3. Primer and probe set used to detect LeY transcripts in CAR–T-cell product and patient samples.
Acknowledgments
We acknowledge the research funding from the Leukemia Lymphoma Society (USA) Translational Research Programme and the National Health and Medical Research Council and the generous philanthropic support of Maree and Chris Morris. M.J.S. was supported by a NH&MRC Australia Fellowship. M.H.K. and P.K.D. were supported by NH&MRC Senior Research Fellowships. We also acknowledge the expert contribution of Stephen Lade in the completion of the immunohistochemistry studies.
Supplementary Material
References
- Gupta V, Tallman MS, Weisdorf DJ. Allogeneic hematopoietic cell transplantation for adults with acute myeloid leukemia: myths, controversies, and unknowns. Blood. 2011;117:2307–2318. doi: 10.1182/blood-2010-10-265603. [DOI] [PubMed] [Google Scholar]
- Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12 20 Pt 1:6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen YJ, Barlogie B, Yi Q. Idiotype-specific cytotoxic T lymphocytes in multiple myeloma: evidence for their capacity to lyse autologous primary tumor cells. Blood. 2001;97:1750–1755. doi: 10.1182/blood.v97.6.1750. [DOI] [PubMed] [Google Scholar]
- Wen YJ, Min R, Tricot G, Barlogie B, Yi Q. Tumor lysate-specific cytotoxic T lymphocytes in multiple myeloma: promising effector cells for immunotherapy. Blood. 2002;99:3280–3285. doi: 10.1182/blood.v99.9.3280. [DOI] [PubMed] [Google Scholar]
- Parker LL, Do MT, Westwood JA, Wunderlich JR, Dudley ME, Rosenberg SA, et al. Expansion and characterization of T cells transduced with a chimeric receptor against ovarian cancer. Hum Gene Ther. 2000;11:2377–2387. doi: 10.1089/104303400750038480. [DOI] [PubMed] [Google Scholar]
- Cooper LJ, Ausubel L, Gutierrez M, Stephan S, Shakeley R, Olivares S, et al. Manufacturing of gene-modified cytotoxic T lymphocytes for autologous cellular therapy for lymphoma. Cytotherapy. 2006;8:105–117. doi: 10.1080/14653240600620176. [DOI] [PubMed] [Google Scholar]
- Lamers CH, Willemsen RA, Luider BA, Debets R, Bolhuis RL. Protocol for gene transduction and expansion of human T lymphocytes for clinical immunogene therapy of cancer. Cancer Gene Ther. 2002;9:613–623. doi: 10.1038/sj.cgt.7700477. [DOI] [PubMed] [Google Scholar]
- Stancovski I, Schindler DG, Waks T, Yarden Y, Sela M, Eshhar Z. Targeting of T lymphocytes to Neu/HER2-expressing cells using chimeric single chain Fv receptors. J Immunol. 1993;151:6577–6582. [PubMed] [Google Scholar]
- Hwu P, Shafer GE, Treisman J, Schindler DG, Gross G, Cowherd R, et al. Lysis of ovarian cancer cells by human lymphocytes redirected with a chimeric gene composed of an antibody variable region and the Fc receptor gamma chain. J Exp Med. 1993;178:361–366. doi: 10.1084/jem.178.1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadelain M, Rivière I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer. 2003;3:35–45. doi: 10.1038/nrc971. [DOI] [PubMed] [Google Scholar]
- Kershaw MH, Westwood JA, Hwu P. Dual-specific T cells combine proliferation and antitumor activity. Nat Biotechnol. 2002;20:1221–1227. doi: 10.1038/nbt756. [DOI] [PubMed] [Google Scholar]
- Moritz D, Wels W, Mattern J, Groner B. Cytotoxic T lymphocytes with a grafted recognition specificity for ERBB2-expressing tumor cells. Proc Natl Acad Sci USA. 1994;91:4318–4322. doi: 10.1073/pnas.91.10.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci USA. 1993;90:720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes NM, Trapani JA, Teng MW, Jackson JT, Cerruti L, Jane SM, et al. Single-chain antigen recognition receptors that costimulate potent rejection of established experimental tumors. Blood. 2002;100:3155–3163. doi: 10.1182/blood-2002-04-1041. [DOI] [PubMed] [Google Scholar]
- Maher J, Brentjens RJ, Gunset G, Rivière I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta/CD28 receptor. Nat Biotechnol. 2002;20:70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- Pulè MA, Straathof KC, Dotti G, Heslop HE, Rooney CM, Brenner MK. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol Ther. 2005;12:933–941. doi: 10.1016/j.ymthe.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin BW, Finstad CL, Kitamura K, Federici MG, Welshinger M, Kudryashov V, et al. Serological and immunochemical analysis of Lewis y (Ley) blood group antigen expression in epithelial ovarian cancer. Int J Cancer. 1996;65:406–412. doi: 10.1002/(SICI)1097-0215(19960208)65:4<406::AID-IJC2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Zhang S, Zhang HS, Cordon-Cardo C, Reuter VE, Singhal AK, Lloyd KO, et al. Selection of tumor antigens as targets for immune attack using immunohistochemistry: II. Blood group-related antigens. Int J Cancer. 1997;73:50–56. doi: 10.1002/(sici)1097-0215(19970926)73:1<50::aid-ijc9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Sakamoto J, Furukawa K, Cordon-Cardo C, Yin BW, Rettig WJ, Oettgen HF, et al. Expression of Lewisa, Lewisb, X, and Y blood group antigens in human colonic tumors and normal tissue and in human tumor-derived cell lines. Cancer Res. 1986;46:1553–1561. [PubMed] [Google Scholar]
- Kobayashi K, Sakamoto J, Kito T, Yamamura Y, Koshikawa T, Fujita M, et al. Lewis blood group-related antigen expression in normal gastric epithelium, intestinal metaplasia, gastric adenoma, and gastric carcinoma. Am J Gastroenterol. 1993;88:919–924. [PubMed] [Google Scholar]
- Miyake M, Taki T, Hitomi S, Hakomori S. Correlation of expression of H/Le(y)/Le(b) antigens with survival in patients with carcinoma of the lung. N Engl J Med. 1992;327:14–18. doi: 10.1056/NEJM199207023270103. [DOI] [PubMed] [Google Scholar]
- Westwood JA, Smyth MJ, Teng MW, Moeller M, Trapani JA, Scott AM, et al. Adoptive transfer of T cells modified with a humanized chimeric receptor gene inhibits growth of Lewis-Y-expressing tumors in mice. Proc Natl Acad Sci USA. 2005;102:19051–19056. doi: 10.1073/pnas.0504312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeson P, Shin A, Tainton KM, Guru P, Prince HM, Harrison SJ, et al. Ex vivo culture of chimeric antigen receptor T cells generates functional CD8+ T cells with effector and central memory-like phenotype. Gene Ther. 2010;17:1105–1116. doi: 10.1038/gt.2010.59. [DOI] [PubMed] [Google Scholar]
- Peinert S, Prince HM, Guru PM, Kershaw MH, Smyth MJ, Trapani JA, et al. Gene-modified T cells as immunotherapy for multiple myeloma and acute myeloid leukemia expressing the Lewis Y antigen. Gene Ther. 2010;17:678–686. doi: 10.1038/gt.2010.21. [DOI] [PubMed] [Google Scholar]
- Brentjens RJ, Rivière I, Park JH, Davila ML, Wang X, Stefanski J, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–4828. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till BG, Jensen MC, Wang J, Qian X, Gopal AK, Maloney DG, et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood. 2012;119:3940–3950. doi: 10.1182/blood-2011-10-387969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DW, Barrett DM, Mackall C, Orentas R, Grupp SA. The future is now: chimeric antigen receptors as new targeted therapies for childhood cancer. Clin Cancer Res. 2012;18:2780–2790. doi: 10.1158/1078-0432.CCR-11-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns WR, Zheng Z, Rosenberg SA, Morgan RA. Lack of specific gamma-retroviral vector long terminal repeat promoter silencing in patients receiving genetically engineered lymphocytes and activation upon lymphocyte restimulation. Blood. 2009;114:2888–2899. doi: 10.1182/blood-2009-01-199216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Munger ME, Veenstra RG, Weigel BJ, Hirashima M, Munn DH, et al. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood. 2011;117:4501–4510. doi: 10.1182/blood-2010-10-310425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norde WJ, Hobo W, van der Voort R, Dolstra H. Coinhibitory molecules in hematologic malignancies: targets for therapeutic intervention. Blood. 2012;120:728–736. doi: 10.1182/blood-2012-02-412510. [DOI] [PubMed] [Google Scholar]
- Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenger YM, Wolchok JD. The heterogeneity of the kinetics of response to ipilimumab in metastatic melanoma: patient cases. Cancer Immun. 2008;8:1. [PMC free article] [PubMed] [Google Scholar]
- Small EJ, Tchekmedyian NS, Rini BI, Fong L, Lowy I, Allison JP. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin Cancer Res. 2007;13:1810–1815. doi: 10.1158/1078-0432.CCR-06-2318. [DOI] [PubMed] [Google Scholar]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince HM, Wall DM, Ritchie D, Honemann D, Harrrison S, Quach H, et al. In vivo tracking of dendritic cells in patients with multiple myeloma. J Immunother. 2008;31:166–179. doi: 10.1097/CJI.0b013e31815c5153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.