Abstract
A number of recent reports have demonstrated that attenuated Salmonella typhimurium are capable of targeting both primary and metastatic tumors. The use of bacteria as a vehicle for the delivery of anticancer drugs requires a mechanism that precisely regulates and visualizes gene expression to ensure the appropriate timing and location of drug production. To integrate these functions into bacteria, we used a repressor-regulated tetracycline efflux system, in which the expression of a therapeutic gene and an imaging reporter gene were controlled by divergent promoters (tetAP and tetRP) in response to extracellular tetracycline. Attenuated S. typhimurium was transformed with the expression plasmids encoding cytolysin A, a therapeutic gene, and renilla luciferase variant 8, an imaging reporter gene, and administered intravenously to tumor-bearing mice. The engineered Salmonella successfully localized to tumor tissue and gene expression was dependent on the concentration of inducer, indicating the feasibility of peripheral control of bacterial gene expression. The bioluminescence signal permitted the localization of gene expression from the bacteria. The engineered bacteria significantly suppressed both primary and metastatic tumors and prolonged survival in mice. Therefore, engineered bacteria that carry a therapeutic and an imaging reporter gene for targeted anticancer therapy can be designed as a theranostic agent.
Introduction
Certain types of bacteria such as Escherichia coli,1,2,3,4,5 Salmonella,3,6,7,8 Clostridium,9,10 Bifidobacterium,11 and Listeria12 selectively colonize and grow in tumor microenvironments. A number of recent reports have demonstrated that nonpathogenic, attenuated strains of S. typhimurium are capable of targeting both primary tumors and tumor metastases, resulting in tumor suppression.1,2,3,4,5 The most renowned S. typhiumiurm strains for bacterial tumor therapy are the msbB-mutant strain with a modified lipid A moiety (VNP20009)6 and the A1-R strain, a leucine–arginine auxotroph with high antitumor virulence.8,13 VNP20009 has been used in clinical trials for patients with metastatic melanoma.14 A1-R treatment also has been reported to effectively shrink tumors in mice with primary or metastatic breast cancer,13 prostate cancer,15 osteosarcoma,16 fibrosarcoma,17 prostate cancer,18 orglioma.19
Our recent study showed that a strain of S. typhimurium with a defect in guanosine 5′-diphosphate-3′-diphosphate synthesis (ΔppGpp S. typhimurium) and carrying a cytotoxic agent such as cytolysinA (ClyA), a pore-forming bacterial toxin, was effective in regressing tumors and promoting the survival of mice with colon cancer.20 This property has been exploited in the development of tumor-selective drug delivery systems using genetically modified bacteria.4,20,21,22,23,24,25 However, a major hurdle that must be overcome in order to realize the full potential of cytotoxic drug delivery using engineered Salmonella is toxicity to nontumor tissues. Following intravenous administration, these bacteria tend to localize initially to the liver and spleen.1,3 Constitutive expression of a cytotoxic drug would therefore inevitably result in severe hepatic or splenic injury. The desired system is one in which gene expression is controlled in a way that maximizes the intratumoral effects while minimizing systemic toxicity.26 To this end, inducible systems that utilize three different external gene “triggers” have been developed:27 the L-arabinose system,20,25,28,29,30,31 the salicylate system,32 and the γ-irradiation system.33,34
Tetracycline and tetracycline analogues (i.e., doxycycline) exhibit several properties of an ideal inducer: they regulate gene expression at very low concentrations (nmol/l range); show good bioavailability in that they can penetrate both bacterial and animal cells; they are not toxic at the necessary levels; and their stability is suitable to the time courses required for a therapeutic effect.35 Repressor-regulated tetracycline efflux systems contain two divergent genes, tetA and tetR, separated by an intergenic region. This intergenic region contains two divergent promoters, one that drives the expression of tetR, which encodes the TetR repressor protein, and a second that drives the expression of tetA, which encodes the TetA efflux pump. The intergenic region also contains two tetO operators (Figure 1a). In the absence of tetracycline, TetR is bound to tetO, which prevents the expression of TetA and TetR. Upon the addition of tetracycline, TetR binds tetracycline and undergoes a conformational change that results in dissociation of TetR from the tetO operators and derepression of TetA/R expression.35,36 Because of the divergent nature of tetR and tetA transcription, replacing tetA with a ‘gene of interest' and placing ‘the second gene of interest' downstream of the repressor gene (tetR) would enable bilateral dual gene expression in a tetracycline-inducible manner. To date, this type of inducible system has yet to be fully explored in the context of a bacterial drug delivery system.
Figure 1.
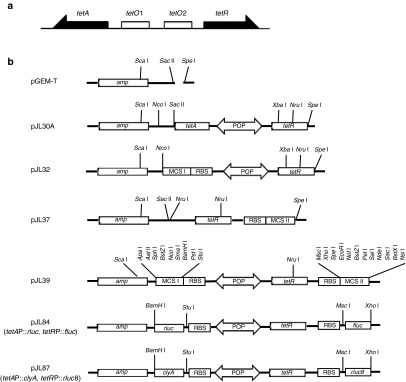
Plasmids used in this study. (a) A schematic feature of Tet system including regulatory region. The tet operators O1 and O2 overlap the divergent tetA and tetR promoters. (b) Brief depiction of the plasmids constructed in this study. See Materials and Methods for detailed description.
As with constitutive expression, a concern with inducible expression systems is the ability to achieve tight control of gene expression in the target tissue while avoiding ectopic expression. In this sense, coexpression of a reporter gene would enable monitoring of the localization, magnitude, and timing of gene expression from the bacterial delivery system, all of which are important parameters in predicting and measuring the effects of treatment.37 Here, using an attenuated strain of ΔppGpp S. typhimurium, we engineered an inducible bacterial drug delivery system, in which coexpression of a therapeutic gene and a bioluminescence reporter gene occurred only in response to the administration of exogenous doxycycline in a dose-dependent manner. This system enabled the repeated imaging of individual experimental animals in which both the therapeutic gene and reporter gene were coexpressed to achieve the desired biological effect.
Results
A doxycycline-inducible dual expression system
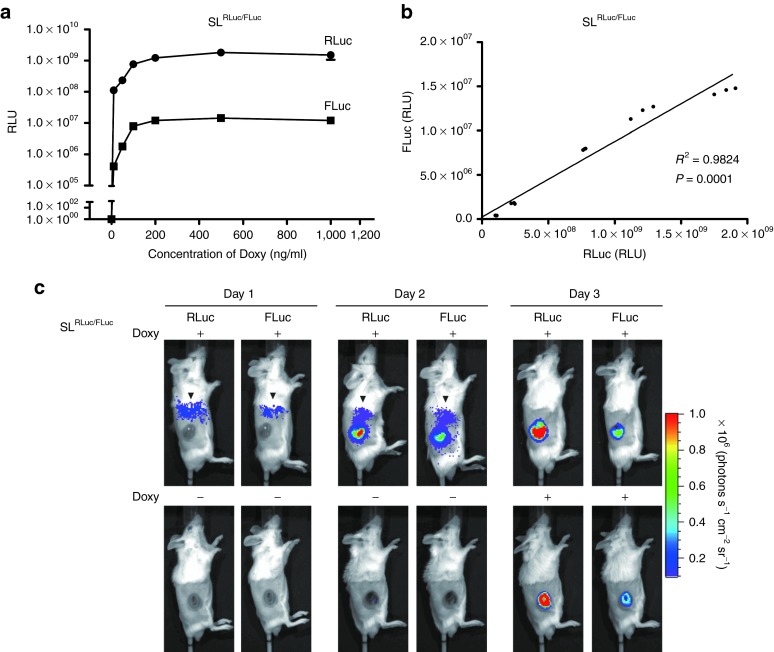
To demonstrate the feasibility of using a doxycycline (Doxy)-inducible bacterial gene expression system for antitumoral drug delivery, we first constructed a bacterial expression plasmid (pJL84), in which two reporter genes, renillaluciferase (rluc) and firefly luciferase (fluc), were placed under the control of tetA promoter (tetAP) and tetR promoter(tetRP), respectively (Figure 1). An attenuated strain of ΔppGpp S. Typhimurium was transformed with the dual-reporter gene plasmid (pJL84), and Doxy-induced rluc and fluc expression was measured in bacterial cultures in the presence of varying concentrations of Doxy (Figure 2). RLuc and FLuc activity was measured after the addition of their specific substrates. Doxy induced the expression of RLuc and Fluc in a concentration-dependent manner, with an optimal concentration of 500 ng/ml (Figure 2a). The levels of Doxy-dependent RLuc and FLuc expression were highly correlative (R2 = 0.9824, P = 0.0001) (Figure 2b). The luminescence of RLuc, under the control of tetAP, was considerably more intense (greater than 100-fold) than FLuc, under the control of tetRP (Figure 2a).
Figure 2.
Doxy-inducible bilateral expression of rluc and fluc in ppGpp-defective S. typhimurium. Salmonellae transformed with pJL84 (tetAP::rluc, tetRP::fluc) were cultured in the presence of varying concentrations of Doxy for 4 hours. Luminescence of RLuc and FLuc was measured by luciferase assay immediately after adding the appropriate substrate, coelenterazine and D-luciferin, respectively, to the transformed bacteria (SLRLuc/FLuc). (a) Activity of RLuc and FLuc in the presence of increasing concentrations of Doxy (0, 10, 50, 100, 200, 500, and 1000 ng/ml). (b) Correlation between RLuc and FLuc activity in the presence of increasing concentrations of Doxy, P = 0.0001. (c) In vivo expression of RLuc and Fluc in tumor-colonized Salmonellae before and after (12 hours) Doxy induction. BALB/c mice inoculated subcutaneously with 1 × 106 CT26 cells were injected with engineered Salmonellae via the tail vein. Mice (n = 3 per group) were administered Doxy at 1 dpi (upper panel) or 3 dpi (lower panel). Bioluminescence imaging was performed following i.v. injection of coelenterazine (0.7 mg/kg, RLuc activity) and intraperitoneal (i.p.) injection of D-luciferin (150 mg/kg, FLuc activity) in each animal. Arrowhead indicates activity from the liver.
To evaluate whether bacterial gene expression could be controlled by oral administration of Doxy in animals, engineered Salmonellae harboring the pJL84 were injected i.v. into immunocompetent BALB/c mice bearing syngeneic tumors derived from CT26 colon carcinoma cells. Previous work from our group20 showed that bacteria are cleared from the liver and spleen and begin to proliferate in tumors 3–4 days postinoculum (dpi). Therefore, Doxy was orally administered starting a 3 dpi at a concentration of 17 mg/kg/day (see Materials and Methods).38 Bioluminescence was detected in the tumors after Doxy administration (Figure 2c). Bioluminescence gene expression was confined to the tumor tissue when induction was started at 3 dpi, whereas bioluminescence was detected in the reticuloendothelial system, including the liver, in control mice induced at 1 dpi (Figure 2c). These results strongly supported the feasibility of using a Doxy-inducible system for peripheral control of gene expression in tumor-targeting engineered Salmonellae.
Engineered S. typhimurium coexpressing therapeutic and reporter proteins
For the simultaneous expression of a therapeutic and reporter gene, S. typhimurium (ΔppGpp) was transformed with a plasmid (pJL87)-encoding clyA, under the control of tetAP, and rluc8, under the control of tetRP (Figure 1). Doxy-induced ClyA and RLuc8 expression was evaluated by immunoblot and luminescence assay. ClyA (34 kDa) expression and luciferase activity was identified specifically only in the presence of Doxy in a dose-dependent manner (Figure 3a, b). ClyA induced in response to Doxy was detected in both the bacterial cells and filtered culture supernatant (Figure 3c), which indicated that the expressed ClyA protein was secreted from S. typhimurium, as is observed in S. typhi.39
Figure 3.

Doxy-induced expression and secretion of ClyA in engineered S. typhimurium. S. typhimurium (ΔppGpp) was transformed with pJL87 (tetAP::clyA, tetRP::rluc8). (a) The indicated concentrations (~200 ng/ml) of Doxy were added to fresh bacterial cultures (1 hour) at an A600 of 0.5–0.7. After 3 hours of incubation, approximately 4 × 107 CFU were collected and analyzed by immunoblotusing an anti-ClyA antibody (lower panel). Ponceau staining indicated equal loading of protein (upper panel). (b) The indicated concentrations (~200 ng/ml) of Doxy were added to bacterial culturesatan OD600 of 0.5–0.7. After 4 hours of incubation, coelenterazine (1 μg) was added to the cultures, and bioluminescence was measured immediately using a cooled CCD camera. (c) The expression and secretion of ClyA (34 kDa) was verified by immunoblotusing an anti-ClyA antibody. Bacterial pellets and filtered culture medium (Filtrate) were collected 4 hours after induction with or without Doxy (200 ng/ml).
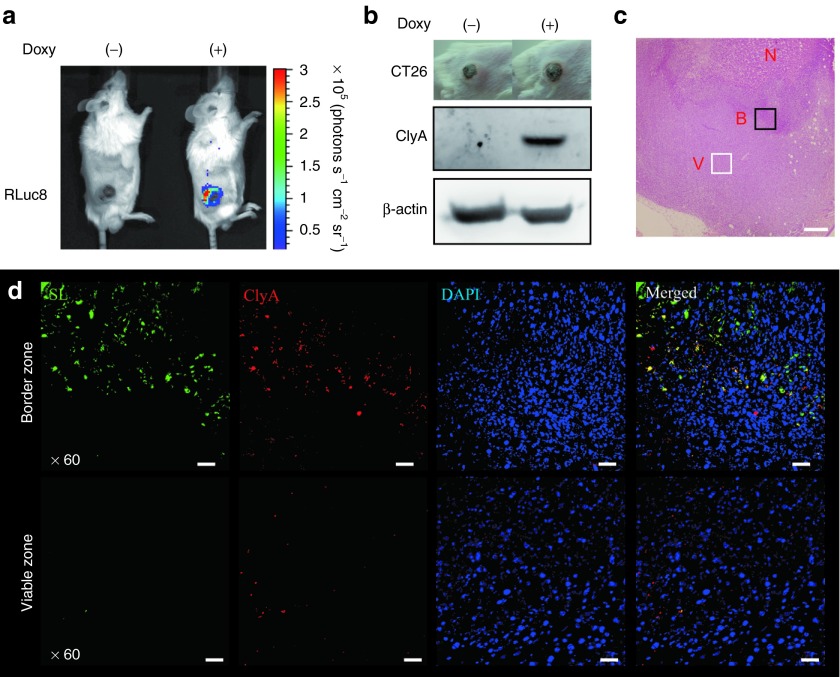
Engineered Salmonellae (3 × 107 CFU) carrying pJL87 were injected i.v. into BALB/c mice bearing CT26 tumors. Bacterial bioluminescence was detected only in the tumor tissue upon Doxy administration at 3 dpi (Figure 4a). Immunoblot analysis of excised tumor tissue demonstrated that ClyA was expressed in tumors colonized by bacteria in response to Doxy administration (Figure 4b). The expression of ClyA in tumor tissue was also assessed by immunofluorescence staining. Bacteria were present mainly at the border between viable and necrotic tumor tissue as indicated in Figure 4c, in which, ClyA spots were also found to spread wide (Figure 4d, upper panel). It should be noted that at the viable tumor area, a significantly higher number of ClyA spots were detected, but not Salmonellae (Figure 4d, lower panel). Therefore, it appeared that ClyA secreted from the bacteria at the border area diffused into the viable tumor area, suggesting a potential mechanism for the enhanced antitumor effects of this engineered bacteria.27
Figure 4.
In vivo Doxy-induced gene expression in CT26 by engineered S. typhimurium. BALB/c mice (n = 5) were injected subcutaneously with CT26 (1 × 106). When the tumors reached a volume of approximately 150 mm3, tumor-bearing mice were injected with engineered S. typhimurium carrying pJL87 followed by oral administration of Doxy at 3 dpi. Bioluminescence imaging was performed following i.v. injection of coelenterazine before and after (12 hours) Doxy administration. Immunoblot analysis (n = 3) and histologic study (n = 2) was done after excision of CT26 tumor grafts 5 days after treatment with Salmonellae and 2 days after the start of Doxy administration. (a) Expression of RLuc8 in CT26 tumors of mice treated with engineered Salmonella was verified by in vivo bioluminescence imaging. (b) Immunoblot analysis of the expression of ClyA in CT26 tumor tissue from mice injected with engineered Salmonellae before (–) and after (+) Doxy induction. (c) H&E staining revealed three regions in the engrafted tumor: necrotic (N), border (B), and viable (V) zones. Scale bar = 100 μm. (d) Immunofluorescence staining of indicated H&E stained areas. Sections were stained with anti-salmonella antibody (green), anti-ClyA antibody (red) and DAPI/Antifade (blue). A merged image is shown (Merged). Scale bar= 20 μm.
Tumor suppression by engineered S. typhimurium in a mouse tumor model
To further explore the antitumor activity of engineered Salmonellae, BALB/c mice or BALB/c athymic nu−/nu− mice transplanted with CT26 or Hep3B2.1-7 tumors, respectively, were treated with engineered S. typhimurium carrying pJL87 in the presence or absence of induction with Doxy, or with Salmonellae carrying an empty vector or PBS (vehicle control). Tumor-bearing mice tolerated the treatment with all types of Salmonellae. In mice treated with the engineered Salmonellae in the presence of Doxy, tumor growth was significantly reduced compared with that of other groups (Figure 5a, b). At the endpoint of the treatment, tumors were no longer detectable in nine (64%) of 14 and seven (58%) of 12 mice bearing CT26 and Hep3B2.1-7, respectively. For example, 35 days after tumor implantation, mean CT26 tumor volume was 186 mm3 for the group treated with engineered bacteria in the presence of Doxy, whereas it was > 1,000 mm3 for the group treated with engineered bacteria in the absence of Doxy induction (P = 0.004) (Figure 5b, left panel). Eighty days after tumor implantation, mean Hep3B2.1-7 tumor volume was 148 mm3 for the group treated with engineered bacteria in the presence of Doxy, whereas it was 641 mm3 for the group treated with engineered bacteria in the absence of Doxy (P = 0.014) (Figure 5b right panel). In addition, survival was considerably longer for animals that received engineered bacteria with Doxy induction than for animals in the other groups (Figure 5c). In the absence of induction with Doxy, tumor shrinkage and survival time were significantly higher in the bacteria-treated group than in the PBS-treated group, although the tumors tended to regrow (Figure 5a, b). These results indicated that treatment with engineered Salmonellae expressing ClyA results in significant suppression of tumor growth in two different mouse tumor models: immunocompromised mice carrying human tumor xenografts, and immunocompetent mice carrying murine tumors. The effectiveness of these treatment strategies is comparable with those obtained with the strains VNP20009 and A1-R.6,13 Although discrepancies between the different approaches can be expected because of differences in the tumor model employed, further work should be done to determine whether the antitumor activity of the ΔppGpp S. typhimurium is comparable with that of the above mentioned mutant strains.
Figure 5.
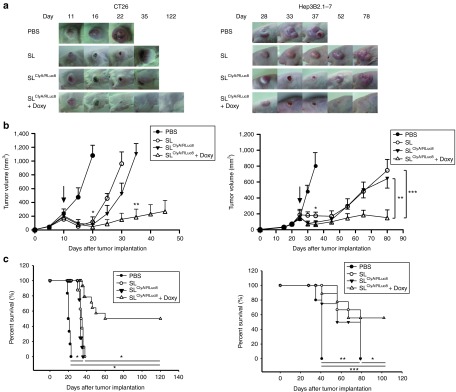
Effect of engineered Salmonellae on tumor growth and survival. BALB/c or BALB/c athymic nu−/nu− mice were injected subcutaneously with CT26 (1 × 106) or Hep3B2.1–7 cells (1 × 107), respectively. When the tumors reached a volume of approximately 150 mm3, CT26 or Hep3B2.1-7 tumor-bearing mice were treated with PBS (n = 6), S. typhimurium carrying empty vector (SL) (n = 8), or engineered S. typhimurium carrying pJL87 (SLClyA/RLuc8) (n = 8). In a separate group of tumor-bearing mice (n = 14 for CT26, n = 12 for Hep3B2.1-7), 17 mg/kg of Doxy was administered orally everyday, starting 4 days after treatment with engineered S. typhimurium (SLClyA/RLuc8 + Doxy). (a) Images of representative subcutaneous tumors. (b) Change in tumor volume. Arrows indicate time of therapeutic injection. Left panel; effect on CT26 tumor growth. *P = 0.003 (PBS versus SL, SLClyA/RLuc8, or SLClyA/RLuc8 + Doxy at day 20), **P = 0.004 (SLClyA/RLuc8 versus SLClyA/RLuc8 + Doxy at day 35), Right panel; effect on Hep3B2.1-7 tumor growth. *P = 0.001 (PBS versus SL, SLClyA/RLuc8, or SLClyA/RLuc8 + Doxy at day 35), **P = 0.002 (SL versus SLClyA/RLuc8 + Doxy at day 80), ***P = 0.014 (SLClyA/RLuc8 versus SLClyA/RLuc8 + Doxy at day 80) (c) Kaplan-Meier survival curves of mice bearing CT26 (*P < 0.0001) or Hep3B2.1–7 tumors (*P = 0.0076, **P = 0.0005, ***P = 0.0003) are shown.
Inhibition of lung metastasis by S. typhimurium expressing ClyA and RLuc8
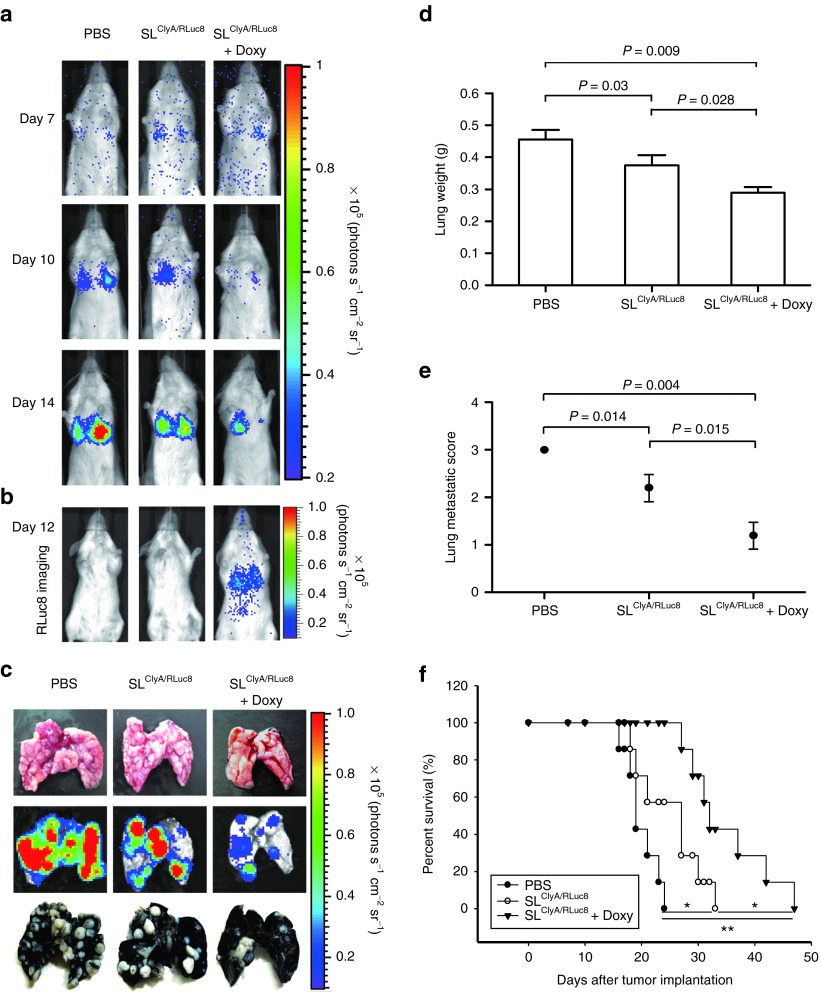
To investigate the effect of engineered Salmonellae carrying pJL87 on distant metastasis, CT26 cells (5 × 104) stably expressing Fluc were injected into mice via the tail vein. The establishment of lung metastases was monitored by bioluminescence imaging following administration of D-luciferin. Cellular bioluminescence was detected specifically in metastatic lung lesions (Figure 6a). Metastatic lesions were also observed by bioluminescence imaging of isolated organs and by India-ink staining (Figure 6c). Mice were then injected i.v. with PBS or engineered S. typhimurium carrying pJL87 7 days after the injection of CT26 cells. Successful gene expression of the engineered S. typhimurium in targeted lung metastatic lesions was confirmed by monitoring RLuc8 activity in the group with Doxy induction (Figure 6b). The antitumor effects of treatment were assessed by measuring the weights of the isolated lungs (Figure 6d), and by employing the metastasis scoring method40 (Figure 6e) and Kaplan–Meier survival curves (Figure 6f). We observed that mice treated with engineered bacteria in the presence of Doxy showed a 37 and 23% reduction in the growth of CT26 experimental metastases (as measured by lung weight) compared with the mice treated by PBS and that without Doxy administration, respectively (measured at 14 days, mean lung weights were 0.46, 0.37, and 0.29 g for the PBS, bacteria without and bacteria with Doxy induction, respectively) (Figure 6d). Treatment with engineered bacteria in the presence of Doxy markedly prolonged survival (Figure 6f). Notably, the antitumor effects of treatment with engineered bacteria in the absence of Doxy administration were greater than the effects of treatment with PBS (P = 0.03) (Figure 6d), which indicated that ΔppGpp S. typhimurium has antitumor effects on metastatic tumor growth.16,20
Figure 6.
Localization and therapeutic effect of engineered Salmonellae in a mouse lung metastases model. On day 7 after i.v. injection of CT26FLuc (1 × 104), BALB/c mice were injected i.v. with engineered S. typhimurium carrying pJL87 (SLClyA/RLuc8) or with PBS. Oral administration of Doxy was started 2 days after bacterial injection (SLClyA/RLuc8 + Doxy). (a) D-luciferin bioluminescence imaging of lung metastases after the indicated treatments. (b) Coelenterazine bioluminescence imaging of bacterial gene expression on day 5 after Doxy administration. (c) Lungs were excised 7 days after treatment. Top panel: photographs of the lungs; middle: bioluminescence (FLuc) images of the same lungs; bottom: India-ink staining of lungs from separate animals. (d,e) Effects on CT26 lung metastasis. (d) Lung weights were measured 7 days after the indicated treatments (n = 5 per group). (e) Metastasis scores were measured 7 days after the indicated treatments (n = 5 per group). The fraction of lung surface covered by fused metastases was scored as follows: 0 = 0%, 1 = less than 20%, 2 = 20–50%, 3 = more than 50%. (f) Kaplan-Meier survival curves. *P = 0.042,**P = 0.0001.
Systemic toxicity of engineered Salmonellae expressing ClyA
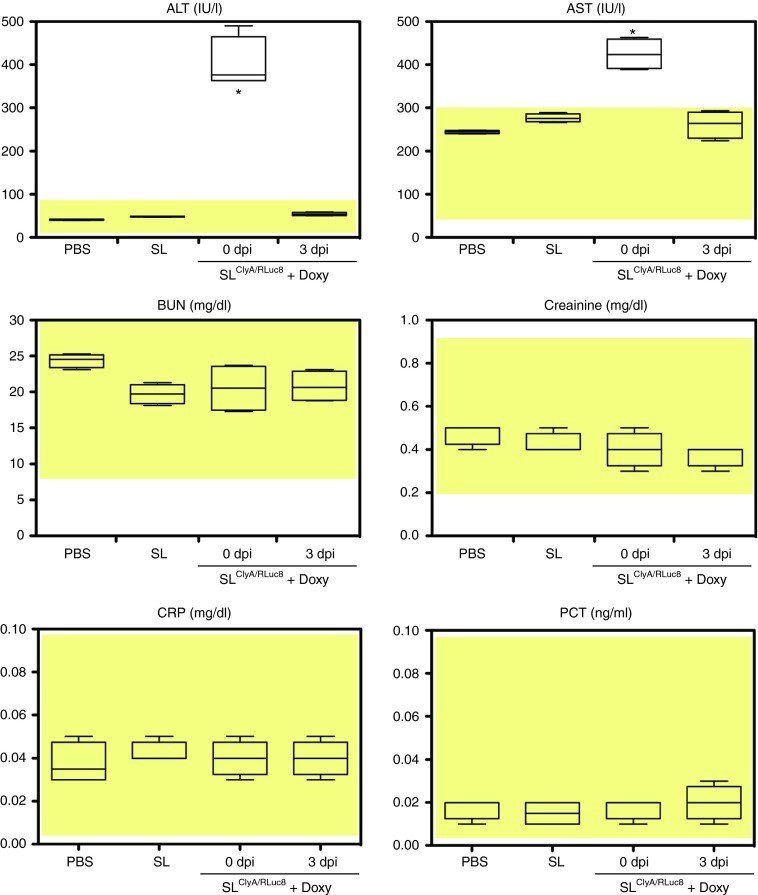
A distinct advantage of bacteria-mediated cytotoxic drug delivery systems is the ability to remotely control gene expression in the tumor-targeting bacteria.20,27,29 This feature is in stark contrast to conventional chemotherapy with cytotoxic drugs, which indiscriminately affects normal cells and cancer cells. Since bacteria administered through the tail vein initially localize to reticuloendothelial organs,2,3 cytotoxic drugs should be expressed only when the bacteria have cleared reticuloendothelial organs and accumulated in the targeted tumor tissue; this normally occurs 3 days after administration.1 We examined for potential hepatic or spleenic injury following the injection of ClyA-expressing Salmonellae by monitoring clinical chemistry markers, plasma C-reactive protein (CRP) and procalcitonin (PCT). Engineered Salmonellae carrying pJL87 were injected i.v. into BALB/c mice bearing CT26. The expression of ClyA was induced by oral administration of Doxy on the same day (0 dpi) or 3 days (3 dpi) after bacterial injection. Analysis of clinical chemistry parameters 5 days after bacterial injection revealed significant elevation of serum aspartate aminotransferase and alanine aminotransferase in the group of animals that received Doxy at 0 dpi (P < 0.01) (Figure 7). However, clinical chemistry parameters were within the normal range when the animals were treated with Doxy at 3 dpi (Figure 7). Markers of inflammation or infection, such as CRP and procalcitonin, were within the normal range in all experimental groups (Figure 7). These results strongly suggest that controlled induction of ClyA by Doxy administration at 3 dpi does not cause hepatic toxicity. The results also suggested that the bacteria-mediated cancer therapy (BCT) protocol does not induce any serious inflammatory reaction, as reported previously.25
Figure 7.
Systemic toxicity of engineered Salmonellae expressing ClyA. BALB/c mice (n = 5 in each group) were injected s.c. with CT-26 cells. After tumors reached a volume of 150 mm3, the tumor-bearing mice were injected with PBS, S. typhimurium carrying empty vector (SL) or S. typhimurium carrying pJL87 followed by oral administration of Doxy (SLClyA/RLuc8 + Doxy) at 0 dpi or 3 dpi. The level of serum aspartate aminotransferase, alanine aminotransferase, blood urea nitrogen, creatinine, plasma C-reactive protein, and procalcitonin were measured at 5 dpi. Boxes represent the quartiles and whiskers mark the 10th and 90th percentiles. *P < 0.01. Normal values: ALT 17-77 IU/L, AST 54-298 IU/L, blood urea nitrogen 8–33 mg/dl, creatinine 0.2–0.9 mg/dl, CRP < 0.5 mg/dl, procalcitonin < 0.5 ng/ml. Yellow-shaded areas = normal range of measured parameters.
Discussion
Here, we describe a Doxy-inducible expression system that enables systemic control of gene expression in tumor-targeting bacteria. Taking advantage of the bidirectional transcription of the tet system, we engineered an attenuated strain of S. typhimurium carrying a dual gene expression cassette encoding an antitumor protein (ClyA) and a bioluminescence reporter gene. The therapeutic efficacy of the system was tested in both primary and metastatic mouse tumor models. The antitumor activity of the engineered bacteria significantly suppressed not only primary tumor growth, but also the growth of lung metastases, as is consistent with previous reports.4,16,17,20,21,40
Many therapeutic gene products are cytotoxic proteins that cannot be constitutively expressed without significant damage to nontumor tissue. We demonstrated here that the timing of Doxy induction was a critical factor in achieving tumor-specific production of ClyA while at the same time sparing nontumor tissue from any nonspecific toxicity associated with the drug. This should result in a considerable reduction in systemic toxicity (Figure 7). ClyA is a hemolytic protein that is produced by E. coli and Salmonella enterica serova Typhi and Paratyphi A.39 The pore-forming activity of ClyA is cytotoxic to mammalian cells; therefore, it is crucial that any inducible gene expression strategy results in the production of ClyA only at the appropriate sites. In the same sense, the use of other gene products such as cytokines and antiangiogenic agents would also require tight control of gene expression. Our results clearly showed the potential of tumor-specific gene expression using a Doxy-inducible system encoding both therapeutic and reporter genes.
Histological analysis indicated that ClyA was secreted by engineered S. typhimurium and localized to areas of viable tumor tissue. Since tumors are immune-privileged environments, bacteria are able to proliferate in the absence of the typical macrophage and neutrophil clearance mechanisms that normally serve as the primary immune function against pathogens.41 Tumor-targeting bacteria localized to the border region (hypoxic area) between necrotic and proliferating (viable) tumor tissue, and spared a peripheral proliferating tumor tissue.1,5,41 Typically, the bacteria-containing region of the tumor is separated from viable tumor cells by a barrier of host neutrophils that have migrated into the tumor, which suggests that host phagocytes are responsible for containing the microorganisms in this area and keeping them from spreading. Such restriction of bacterial colonization in the hypoxic region of the tumor may explain why the efficacy of bacterial tumor therapy is limited against rapidly proliferating tumors. In the current study, ClyA was detected in the viable region of the tumor tissue, which suggests that it was secreted by the engineered bacteria and was able to penetrate the neutrophil barrier and permeate the proliferating area of the tumor. This may explain the improved efficacy of engineered bacteria carrying therapeutic proteins over bacteria alone.27 In this model, the destruction of viable tumor cells would depend on the amount of secreted ClyA in the peripheral tumor tissue. Additional studies are underway to investigate whether there is a correlation between efficacy and the amount of ClyA that localizes to the proliferating region of the tumor, and to optimize the bacterial system for the delivery of therapeutic proteins to viable tumor tissue.
A single vector system for coexpression can be achieved either by using multiple expression cassettes or a single expression cassette (polycistronic or monocistronic). In the latter case, the expression cassette consists of a single transcriptional unit with “promoter-ORF(s)-terminator” sequence elements. With the multicassette approach, individual genes/ORFs are expressed separately, although the cassettes are carried on a single vector.42 With the single-cassette-polycistronic approach, the distal ORFs are expressed often less efficiently than the proximal ones, and there can also be considerable variation depending on the bacterial strain and the growth conditions. With the multicassette approach, differences in the rate of transcription, translation, and the stability of RNA and protein products can result in imbalances in the amounts of the protein products.42 The present bidirectional tet promoter system resulted in a 100-fold difference in the expression from tetAP and tetRP. However, the correlation in expression between the bipartite tet promoters was excellent, which allowed a reliable estimation of therapeutic gene expression from the estimated levels of reporter gene expression. Further study is underway to modify tetRP to increase transcriptional activity further.
The tet regulatory system has several properties that make it amenable to use in an inducible bacterial drug delivery system.43 The tet system is not toxic in mammalian cells. The MIC of Doxy was approximately 4 μg/ml (Supplementary Figure S1a, b), and the concentration used for induction in the current study was at least eightfold lower than the MIC both in vitro and in vivo. There was no inhibitory effect of Doxy on bacterial growth over the induction period on the range of Doxy concentrations tested (Supplementary Figure S1c). Finally, Doxy and tetracycline are antibiotics with both a long history of use and extensive documentation on their safety in humans; therefore, they are safe and effective induction agents for this strategy.
Tumor metastasis is the primary cause of mortality in cancer patients. BCT might be clinically more acceptable in cancer patients with distant metastases than in patients with only primary tumors. Two mouse model systems have been established for lung metastasis: the spontaneous metastasis model and the experimental metastasis model.44 In the spontaneous metastasis model of the lung, tumor cells are subcutaneously injected into syngeneic mice. This model is the most relevant as it includes the whole natural process of cancer metastasis: malignant cells invade the tissue (invasion), migrate from the tumor to blood vessels (intravasation), attach to the vessel wall of the colonized organ, migrate to the organ parenchyma (extravasation) and develop new tumors there. In the present experimental metastasis model, luciferase-labeled cancer cells were injected into the tail vein of syngeneic mice and the extent of lung metastasis was scored primarily by noninvasive bioluminescence imaging.20 Although this model may not produce human-like metastasis, it is easy and quick to establish models in this way in a controlled manner; therefore, it is widely accepted in preclinical experiment.16,20,44 We observed that treatment with ClyA-expressing Salmonellae resulted in a considerable reduction in CT26 experimental metastases, demonstrating the effectiveness of this antitumor strategy.16,17,20
In the present study, we employed bioluminescence to visualize bacterial gene expression. The light source of bioluminescence was the luciferin–luciferase reaction, in which an enzyme catalyzes the oxidation of a specific substrate molecule. By contrast to fluorescence, no external excitation source is required for luminescence and therefore, the background signal is negligible.1,2,20,25 This feature allowed us to assess dual-gene expression in bacteria where the activities of FLuc and RLuc were individually measured after administration of the specific substrates D-luciferin and coelenterazine, respectively (Figure 2). An alternative way of imaging bacteria in intact animals would be to use bacteria expressing green fluorescent protein. Using this technique, the spatiotemporal dynamics of bacterial infection have been reported in great detail.45,46
Although many experimental reports have demonstrated that BCT can regress tumors in mouse tumor models, several key issues remain to be solved before BCT can be applied in the clinic, including intrinsic bacterial toxicity, targeting efficiency, genetic instability, and the antitumor mechanism.27 Bacterial toxicity is the most important issue for safety and regulatory approval. Although many reports have shown that attenuated Salmonellae are nonpathogenic in animals,6,25,27 residual virulence could be problematic for immunocompromised, late-stage cancer patients. Variations in targeting efficiency and genetic instability are additional problems that could lead to poor therapeutic efficacy or harmful mutations. These challenges can be addressed using synthetic biology techniques that manipulate multiple factors. Determining the correct combination of BCT with other cancer therapies will be crucial for creating strategies that lead to synergistic effects on primary and metastatic tumors. A complete understanding of host immunity in bacteria-mediated tumor regression could lead to increases in the safety and efficacy of this therapy.
In conclusion, the results suggest that bacteria engineered to express therapeutic genes under the control of the tet system have potential as a novel form of treatment for cancer. The bacterially expressed cytotoxic proteins penetrated the immune cell barrier and localized to regions of viable tumor tissue, resulting in therapeutic effects. Additional studies would be needed to employ PET reporter gene system that would enable tomographic imaging of the therapeutic gene expression in larger subjects including humans.
Materials and Methods
Cell lines. Murine CT26 colon carcinoma cells, CT26 cells stably expressing firefly luciferase (CT26FLuc), and Hep3B2.1-7 human hepatocellular carcinoma cells were obtained from the American Type Culture Collection (CRL-2638 and HB-8064, respectively; Rockville, MD). The cell lines were authenticated by the Waterborne Virus Bank (Seoul, Korea). Genetic background, including expression and function of proteins was tested most recently in July 2012 using western blot analysis and luciferase assay. CT26 and Hep3B2.1-7 cells were cultured in high-glucose Dulbecco's Modified Eagles Medium containing 10% fetal bovine serum and 1% penicillin–streptomycin.
Construction of the tetracycline (Tet)-responsive plasmid system. The primers, plasmids, and bacterial strains used in this study are summarized in Figure 1 and Supplementary Table S1. A rudimentary Tet plasmid, pJL30A, was constructed as follows. The tetRA element was amplified by PCR using the primers, RItetR and RItetA, and genomic DNA from Salmonella enterica serova Typhimurium (TH9952) containing the tetRA element as a template.47 The amplified product was cloned into pGEM-T-Easy (Promega) to generate pJL30A. To delete the tetA gene and introduce a ribosome-binding site (RBS) and multiple cloning site I (MCS I), an RBS-MCS I DNA fragment containing the divergent tet regulatory region (POP) and tetR regions was generated by PCR using the primers TetPXbaIF and TetPXbaIrev, and pJL30A as the template. The amplified product was digested with NcoI-XbaI, and inserted into the corresponding sites of pJL30A to generate pJL32. An RBS and multiple cloning site II (MCS II) were subsequently introduced downstream of tetR as follows. An RBS-MCS II DNA fragment containing tetR was generated by PCR using the TetRNruIF and TerRNruIrev primers, and pJL30A as the template. The amplified product was cloned into pGEM-T-Easy to generate pJL37. The SacI-NruI restriction fragment of pJL32 was replaced with the SacI-NruI restriction fragment of pJL37 to generate pJL39. Finally, the imaging reporter genes, renilla luciferase (rluc) and firefly luciferase (fluc) or a therapeutic gene (clyA) were cloned into the two MCSs of pJL39 to generate pJL84 and pJL87, respectively. pJL84 carried rluc and fluc under the control of the tetA promoter (tetAP) and the tetR promoter (tetRP), respectively (tetAP::rluc, tetRP::fluc). Note that fluc was placed downstream of tetR, such that the induction system remained intact. For the expression of the therapeutic transgene and imaging reporter gene, cytolysin A (clyA) was placed under the control of tetAP, and rluc8, a variant of rluc with enhanced enzymatic activity and stability,48 was placed under the control of the tetRP (pJL87; tetAP::clyA, tetRP::rluc8). The related primers are shown in Supplementary Table S1.
Preparation of Salmonellae for animal experiment. ΔppGpp S. typhimurium strain SHJ2037 (relA::cat, spot::kan) has been previously described.49 The strain was streaked on an LB plate containing appropriate antibiotics. A single colony was picked out, dipped into LB/antibiotic medium containing 0.2% glucose, and grown overnight in a shaking incubator (37°C, 200 rpm). The next day, the overnight culture was diluted 50-fold into fresh medium and grown to early stationary phase (A600 = 2–2.5), and the cells were harvested by centrifugation (5000 rpm, 10 minutes), washed with PBS, quantified by spectrophotometry, and diluted in PBS to obtain the desired concentration of bacteria in an appropriate volume for animal experiments. The bacterial number was calculated by considering 1.0 A600 = 0.8 × 109 CFU.
Luciferase assay. S. typhimurium (2 × 108 CFU/100 µl) carrying pJL84 (tetAP::rluc, tetRP::fluc) grown to early stationary phase (4 hours) under aerobic condition in LB medium containing different concentrations of Doxy (0, 10, 50, 100, 200, 500, and 1000 ng/ml) were used for the assays. Luminescence was measured immediately after adding substrate, either D-luciferin (Caliper, Hopkinton, MA) for FLuc activity (50 μg) or coelenterazine (Biotium, Hayward, CA) for Rluc activity (1 μg), using a luminometer (Microlumat Plus LB96V; Berthold Technologies, Badwildbad, Germany) every 1 second (for FLuc) or 10 seconds (for RLuc). Results were expressed as relative light units (RLU).
Western blot analysis. Total proteins (40 μg) were separated by electrophoresis and blotted as described previously.49 For the analysis of ClyA in tumor tissues, homogenized tissue samples were standardized according to protein content, and samples of 100 μg protein were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 12% linear gradient gels. Proteins were transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA), and the membranes were first probed using a mouse anti-ClyA monoclonal antibody (1:250 dilution), and then a horseradish peroxidase-conjugated antirabbit secondary antibody (1: 2,000 dilution) (Amersham, UK). Immunoreactive proteins were detected using luminal reagent (Santa Cruz Biotechnology, Santa Cruz, CA) and visualized by a Fuji Film image reader LAS-3000 machine.
Animal models. Male BALB/c and BALB/c athymic nu−/nu− mice (age: 5–6 weeks; body weight: 20–30 g) were purchased from the Orient Company (Seongnam, Korea). Animal care, all experiments, and the euthanasia procedure were performed in accordance with the protocols approved by the Chonnam National University Animal Research Committee (Gwangju, Korea). Anesthesia was performed using 2% isoflurane (for imaging) or a mixture of ketamine (200 mg/kg) and xylasine (10 mg/kg) (for surgery). Subcutaneous tumor volume was assessed with calipers every 5 days from days 5 to 45 for CT 26 and from days 15 to 80 for Hep3B2.1-7. Tumor volume (mm3) was calculated using the following formula: (L × H × W)/2, where L is the length, W is the width, and H is the height of the tumor in millimeters.3 Mice with tumor volume ≥ 1,000 mm3 were scheduled to be euthanized. For the lung metastases model, BALB/c mice were injected i.v. with 5 × 104 CT26 cells stably expressing firefly luciferase (CT26 FLuc).
Doxycycline administration. Doxycycline hyclate (Doxy; Sigma, Deisenhofen, Germany) was dissolved to a concentration of 5 mg/ml in water. The concentrations of Doxy used for induction were less than the minimum inhibitory concentration (see Supplementary Figure S1). Doxy was administered by gavage once daily following a 1-hour period of fasting during the entire course of the experiment. On the basis of the data previously generated in mice, in which the serum concentration of Doxy reached a peak of 8.7, 6, and 4.8 μg/ml, 1, 2, and 3 hours after a single peroral dose of 300 mg/kg, respectively, we assumed that the serum concentration of Doxy in our model would reach approximately 500 ng/ml 1 hour after oral administration of 17 mg/kg.38
Histopathological study. The tumor was removed, fixed in the 3.7% paraformaldehyde overnight at room temperature. The tumors were blocked in longitudinal section and processed for paraffin embedding. Representative sections were sliced into 4-μm thickness sections and stained with hematoxylin–eosin (H&E). Briefly, for immunofluorescent staining, 4-μm thick paraffin-embedded tissue sections were collected on aminopropyltriethoxysilane-coated slides and immunostained with the avidin–biotin conjugation method using Sequenza Rack (Shandon, UK). Pretreatment of tissues with heat-induced epitope retrieval was carried out for 30 minutes at 125 °C pressure cooker in 10 mmol/l of citrate buffer, pH 6.0. Endogenous peroxidase activity was blocked by incubating samples in PBS (pH 7.4) containing 1.5% H2O2. The slides were incubated with primary antibody for overnight at 4 °C degree, anti-ClyA (1:80) and antisalmonella (1:100, Abcam, Cambridge, UK). After washing three times with PBS-T, secondary antibodies were used with donkey antirabbit 555 (1:100, Invitrogen, Eugene, OR) and donkey anti-rat 488 (1:100, Invitrogen) for 2 hours at room temperature. The sample was stained with DAPI/Antifade (1:10,000, Invitrogen). Images of H&E stain labeled were acquired using a Nikon 80i microscope (Nikon). For fluorescence staining, stained tissue were subsequently washed with PBS and mounted on glass slides for analysis by FV1000D confocal laser scanning microscope (Olympus, Tokyo, Japan).
The presence of pulmonary metastases was evaluated in mice after euthanasia followed by an intratracheal injection of 1.5 ml of 15% India-ink solution via a blunt-ended needle. The stained lungs were carefully resected and rinsed in Fekete's solution (300 ml 70% ethanol, 30 ml 37% formaldehyde, 5 ml glacial acetic acid) and then placed in fresh Fekete's solution overnight in a 60 × 15 mm tissue culture dish.
Optical bioluminescence imaging. Bioluminescence imaging was performed as previously described using an IVIS 100 system (Caliper).2,25 The establishment of lung metastases was assessed after i.p. injection of D-luciferin (150 mg/kg). Bacterial gene expression was also monitored after i.v.injection of coelenterazine (0.7 mg/kg).
Clinical chemistry parameters. Blood samples were always collected during the same time interval in the afternoon (15:00 to 16:00). The samples were obtained by heart puncture using heparinized syringes. Blood samples were deposited in serum separator gel tubes (Microtainer, Becton-Dickinson, Franklin Park, NJ) and centrifuged (9300g, 30 minutes 4 °C) for serum separation. After centrifugation, the serums immediately applied for biochemistry parameters. Serum activity of aspartate aminotransferase and alanine aminotransferase and concentrations of blood urea nitrogen, creatinine, C-reactive protein and procalcitonin were determined by using an automated analyzer (Hitachi instruments, Tokyo, Japan) according to the manufacturers' instructions. Standard controls were run before each determination, and the values obtained for the different biochemical parameters were always within the expected ranges.
Statistical analysis. Statistical analysis was performed using the SPSS 18.0 statistical packages (SPSS, Chicago, IL). The two-tailed Student's t-test was used to determine the statistical significance of tumor growth between control and treatment groups. Bivariate correlations were analyzed using Spearman's ρ test. Kruskal–Wallis test was used to determine the statistical significance in clinical chemistry parameters. A P value of <0.05 was considered statistically significant. Survival analysis was performed using a Kaplan–Meier curve and log–rank test. All data are expressed as means ± SD.
SUPPLEMENTARY MATERIAL Figure S1. Minimal inhibitory concentration of doxycycline for bacterial growth. Table S1. Primers used in this study.
Acknowledgments
We thank Su Woong Yoo (Department of Nuclear Medicine) for advise on statistical analysis. This work was supported by the National Research Foundation of Korea (NRF) (No. 2012-0006072). H.E.C. and S.C.K. were supported by the Intelligent Synthetic Biology Center of Global Frontier Project funded by MEST (2011-0031958). Y.H. was supported by the Pioneer Research Center Program “Bacteriobot” (2012-0001031). M.S. was supported by the Leading Foreign Research Institute Recruitment Program (2011-0030034). These authors have no conflict of interest.
Supplementary Material
References
- Min JJ, Kim HJ, Park JH, Moon S, Jeong JH, Hong YJ, et al. Noninvasive real-time imaging of tumors and metastases using tumor-targeting light-emitting Escherichia coli. Mol Imaging Biol. 2008;10:54–61. doi: 10.1007/s11307-007-0120-5. [DOI] [PubMed] [Google Scholar]
- Min JJ, Nguyen VH, Kim HJ, Hong Y, Choy HE. Quantitative bioluminescence imaging of tumor-targeting bacteria in living animals. Nat Protoc. 2008;3:629–636. doi: 10.1038/nprot.2008.32. [DOI] [PubMed] [Google Scholar]
- Yu YA, Shabahang S, Timiryasova TM, Zhang Q, Beltz R, Gentschev I, et al. Visualization of tumors and metastases in live animals with bacteria and vaccinia virus encoding light-emitting proteins. Nat Biotechnol. 2004;22:313–320. doi: 10.1038/nbt937. [DOI] [PubMed] [Google Scholar]
- Jiang SN, Phan TX, Nam TK, Nguyen VH, Kim HS, Bom HS, et al. Inhibition of tumor growth and metastasis by a combination of Escherichia coli-mediated cytolytic therapy and radiotherapy. Mol Ther. 2010;18:635–642. doi: 10.1038/mt.2009.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel S, Stritzker J, Eck M, Goebel W, Szalay AA. Colonization of experimental murine breast tumours by Escherichia coli K-12 significantly alters the tumour microenvironment. Cell Microbiol. 2008;10:1235–1248. doi: 10.1111/j.1462-5822.2008.01122.x. [DOI] [PubMed] [Google Scholar]
- Low KB, Ittensohn M, Le T, Platt J, Sodi S, Amoss M, et al. Lipid A mutant Salmonella with suppressed virulence and TNFalpha induction retain tumor-targeting in vivo. Nat Biotechnol. 1999;17:37–41. doi: 10.1038/5205. [DOI] [PubMed] [Google Scholar]
- Pawelek JM, Low KB, Bermudes D. Bacteria as tumour-targeting vectors. Lancet Oncol. 2003;4:548–556. doi: 10.1016/s1470-2045(03)01194-x. [DOI] [PubMed] [Google Scholar]
- Zhao M, Yang M, Li XM, Jiang P, Baranov E, Li S, et al. Tumor-targeting bacterial therapy with amino acid auxotrophs of GFP-expressing Salmonella typhimurium. Proc Natl Acad Sci USA. 2005;102:755–760. doi: 10.1073/pnas.0408422102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal N, Bettegowda C, Cheong I, Geschwind JF, Drake CG, Hipkiss EL, et al. Bacteriolytic therapy can generate a potent immune response against experimental tumors. Proc Natl Acad Sci USA. 2004;101:15172–15177. doi: 10.1073/pnas.0406242101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang LH, Bettegowda C, Huso DL, Kinzler KW, Vogelstein B. Combination bacteriolytic therapy for the treatment of experimental tumors. Proc Natl Acad Sci USA. 2001;98:15155–15160. doi: 10.1073/pnas.251543698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohwi Y, Imai K, Tamura Z, Hashimoto Y. Antitumor effect of Bifidobacterium infantis in mice. Gann. 1978;69:613–618. [PubMed] [Google Scholar]
- Akin D, Sturgis J, Ragheb K, Sherman D, Burkholder K, Robinson JP, et al. Bacteria-mediated delivery of nanoparticles and cargo into cells. Nat Nanotechnol. 2007;2:441–449. doi: 10.1038/nnano.2007.149. [DOI] [PubMed] [Google Scholar]
- Zhao M, Yang M, Ma H, Li X, Tan X, Li S, et al. Targeted therapy with a Salmonella typhimurium leucine-arginine auxotroph cures orthotopic human breast tumors in nude mice. Cancer Res. 2006;66:7647–7652. doi: 10.1158/0008-5472.CAN-06-0716. [DOI] [PubMed] [Google Scholar]
- Toso JF, Gill VJ, Hwu P, Marincola FM, Restifo NP, Schwartzentruber DJ, et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J Clin Oncol. 2002;20:142–152. doi: 10.1200/JCO.2002.20.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Geller J, Ma H, Yang M, Penman S, Hoffman RM. Monotherapy with a tumor-targeting mutant of Salmonella typhimurium cures orthotopic metastatic mouse models of human prostate cancer. Proc Natl Acad Sci USA. 2007;104:10170–10174. doi: 10.1073/pnas.0703867104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Zhao M, Yamauchi K, Yamamoto N, Tsuchiya H, Tomita K, et al. Systemic targeting of primary bone tumor and lung metastasis of high-grade osteosarcoma in nude mice with a tumor-selective strain of Salmonella typhimurium. Cell Cycle. 2009;8:870–875. doi: 10.4161/cc.8.6.7891. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Zhao M, Yamauchi K, Yamamoto N, Tsuchiya H, Tomita K, et al. Cancer metastasis directly eradicated by targeted therapy with a modified Salmonella typhimurium. J Cell Biochem. 2009;106:992–998. doi: 10.1002/jcb.22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam C, Zhao M, Hayashi K, Ma H, Kishimoto H, McElroy M, et al. Monotherapy with a tumor-targeting mutant of S. typhimurium inhibits liver metastasis in a mouse model of pancreatic cancer. J Surg Res. 2010;164:248–255. doi: 10.1016/j.jss.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momiyama M, Zhao M, Kimura H, Tran B, Chishima T, Bouvet M, et al. Inhibition and eradication of human glioma with tumor-targeting Salmonella typhimurium in an orthotopic nude-mouse model. Cell Cycle. 2012;11:628–632. doi: 10.4161/cc.11.3.19116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen VH, Kim HS, Ha JM, Hong Y, Choy HE, Min JJ. Genetically engineered Salmonella typhimurium as an imageable therapeutic probe for cancer. Cancer Res. 2010;70:18–23. doi: 10.1158/0008-5472.CAN-09-3453. [DOI] [PubMed] [Google Scholar]
- Loeffler M, Le'Negrate G, Krajewska M, Reed JC. Attenuated Salmonella engineered to produce human cytokine LIGHT inhibit tumor growth. Proc Natl Acad Sci USA. 2007;104:12879–12883. doi: 10.1073/pnas.0701959104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seow Y, Wood MJ. Biological gene delivery vehicles: beyond viral vectors. Mol Ther. 2009;17:767–777. doi: 10.1038/mt.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa H, Sato E, Briones G, Chen LM, Matsuo M, Nagata Y, et al. In vivo antigen delivery by a Salmonella typhimurium type III secretion system for therapeutic cancer vaccines. J Clin Invest. 2006;116:1946–1954. doi: 10.1172/JCI28045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelek JM, Low KB, Bermudes D. Tumor-targeted Salmonella as a novel anticancer vector. Cancer Res. 1997;57:4537–4544. [PubMed] [Google Scholar]
- Le UN, Kim HS, Kwon JS, Kim MY, Nguyen VH, Jiang SN, et al. Engineering and visualization of bacteria for targeting infarcted myocardium. Mol Ther. 2011;19:951–959. doi: 10.1038/mt.2011.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes NS, Munn LL, Fukumura D, Jain RK. Sparse initial entrapment of systemically injected Salmonella typhimurium leads to heterogeneous accumulation within tumors. Cancer Res. 2003;63:5188–5193. [PubMed] [Google Scholar]
- Forbes NS. Engineering the perfect (bacterial) cancer therapy. Nat Rev Cancer. 2010;10:785–794. doi: 10.1038/nrc2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loessner H, Leschner S, Endmann A, Westphal K, Wolf K, Kochruebe K, et al. Drug-inducible remote control of gene expression by probiotic Escherichia coli Nissle 1917 in intestine, tumor and gall bladder of mice. Microbes Infect. 2009;11:1097–1105. doi: 10.1016/j.micinf.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Loessner H, Endmann A, Leschner S, Westphal K, Rohde M, Miloud T, et al. Remote control of tumour-targeted Salmonella enterica serovar Typhimurium by the use of L-arabinose as inducer of bacterial gene expression in vivo. Cell Microbiol. 2007;9:1529–1537. doi: 10.1111/j.1462-5822.2007.00890.x. [DOI] [PubMed] [Google Scholar]
- Stritzker J, Weibel S, Hill PJ, Oelschlaeger TA, Goebel W, Szalay AA. Tumor-specific colonization, tissue distribution, and gene induction by probiotic Escherichia coli Nissle 1917 in live mice. Int J Med Microbiol. 2007;297:151–162. doi: 10.1016/j.ijmm.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Guzman LM, Belin D, Carson MJ, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royo JL, Becker PD, Camacho EM, Cebolla A, Link C, Santero E, et al. In vivo gene regulation in Salmonella spp. by a salicylate-dependent control circuit. Nat Methods. 2007;4:937–942. doi: 10.1038/nmeth1107. [DOI] [PubMed] [Google Scholar]
- Nuyts S, Van Mellaert L, Theys J, Landuyt W, Bosmans E, Anné J, et al. Radio-responsive recA promoter significantly increases TNFalpha production in recombinant clostridia after 2 Gy irradiation. Gene Ther. 2001;8:1197–1201. doi: 10.1038/sj.gt.3301499. [DOI] [PubMed] [Google Scholar]
- Nuyts S, Van Mellaert L, Barbé S, Lammertyn E, Theys J, Landuyt W, et al. Insertion or deletion of the Cheo box modifies radiation inducibility of Clostridium promoters. Appl Environ Microbiol. 2001;67:4464–4470. doi: 10.1128/AEM.67.10.4464-4470.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KJ, Joyce G, Robertson BD. Improved mycobacterial tetracycline inducible vectors. Plasmid. 2010;64:69–73. doi: 10.1016/j.plasmid.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grkovic S, Brown MH, Skurray RA. Regulation of bacterial drug export systems. Microbiol Mol Biol Rev. 2002;66:671–701, table of contents. doi: 10.1128/MMBR.66.4.671-701.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambhir SS. Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer. 2002;2:683–693. doi: 10.1038/nrc882. [DOI] [PubMed] [Google Scholar]
- Chang HR, Comte R, Pechère JC. In vitro and in vivo effects of doxycycline on Toxoplasma gondii. Antimicrob Agents Chemother. 1990;34:775–780. doi: 10.1128/aac.34.5.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wai SN, Lindmark B, Söderblom T, Takade A, Westermark M, Oscarsson J, et al. Vesicle-mediated export and assembly of pore-forming oligomers of the enterobacterial ClyA cytotoxin. Cell. 2003;115:25–35. doi: 10.1016/s0092-8674(03)00754-2. [DOI] [PubMed] [Google Scholar]
- Loeffler M, Le'Negrate G, Krajewska M, Reed JC. Inhibition of tumor growth using salmonella expressing Fas ligand. J Natl Cancer Inst. 2008;100:1113–1116. doi: 10.1093/jnci/djn205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal K, Leschner S, Jablonska J, Loessner H, Weiss S. Containment of tumor-colonizing bacteria by host neutrophils. Cancer Res. 2008;68:2952–2960. doi: 10.1158/0008-5472.CAN-07-2984. [DOI] [PubMed] [Google Scholar]
- Kerrigan JJ, Xie Q, Ames RS, Lu Q. Production of protein complexes via co-expression. Protein Expr Purif. 2011;75:1–14. doi: 10.1016/j.pep.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Blau HM, Rossi FM. Tet B or not tet B: advances in tetracycline-inducible gene expression. Proc Natl Acad Sci USA. 1999;96:797–799. doi: 10.1073/pnas.96.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Zhang JJ, Huang XY. Mouse models for tumor metastasis. Methods Mol Biol. 2012;928:221–228. doi: 10.1007/978-1-62703-008-3_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Yang M, Baranov E, Wang X, Penman S, Moossa AR, et al. Spatial-temporal imaging of bacterial infection and antibiotic response in intact animals. Proc Natl Acad Sci USA. 2001;98:9814–9818. doi: 10.1073/pnas.161275798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman RM, Zhao M. Whole-body imaging of bacterial infection and antibiotic response. Nat Protoc. 2006;1:2988–2994. doi: 10.1038/nprot.2006.376. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Hughes KT. Posttranscriptional control of the Salmonella enterica flagellar hook protein FlgE. J Bacteriol. 2006;188:3308–3316. doi: 10.1128/JB.188.9.3308-3316.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loening AM, Fenn TD, Wu AM, Gambhir SS. Consensus guided mutagenesis of Renilla luciferase yields enhanced stability and light output. Protein Eng Des Sel. 2006;19:391–400. doi: 10.1093/protein/gzl023. [DOI] [PubMed] [Google Scholar]
- Song M, Kim HJ, Kim EY, Shin M, Lee HC, Hong Y, et al. ppGpp-dependent stationary phase induction of genes on Salmonella pathogenicity island 1. J Biol Chem. 2004;279:34183–34190. doi: 10.1074/jbc.M313491200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.