Abstract
Preclinical and clinical trials demonstrated that use of oncolytic viruses (OVs) is a promising new therapeutic approach to treat multiple types of cancer. To further improve their viral oncolysis, experimental strategies are now combining OVs with different cytotoxic compounds. In this study, we investigated the capacity of triptolide – a natural anticancer molecule – to enhance vesicular stomatitis virus (VSV) oncolysis in OV-resistant cancer cells. Triptolide treatment increased VSV replication in the human prostate cancer cell line PC3 and in other VSV-resistant cells in a dose- and time-dependent manner in vitro and in vivo. Mechanistically, triptolide (TPL) inhibited the innate antiviral response by blocking type I interferon (IFN) signaling, downstream of IRF3 activation. Furthermore, triptolide-enhanced VSV-induced apoptosis in a dose-dependent fashion in VSV-resistant cells, as measured by annexin-V, cleaved caspase-3, and B-cell lymphoma 2 staining. In vivo, using the TSA mammary adenocarcinoma and PC3 mouse xenograft models, combination treatment with VSV and triptolide delayed tumor growth and prolonged survival of tumor-bearing animals by enhancing viral replication. Together, these results demonstrate that triptolide inhibition of IFN production sensitizes prostate cancer cells to VSV replication and virus-mediated apoptosis.
Introduction
Oncolytic viruses (OVs) can selectively infect and kill cancer cells, while largely sparing normal tissues.1 Various OVs have been tested, with promising activity, in preclinical animal models and in phase 1/2 clinical trials.2,3 Vesicular stomatitis virus (VSV), a negative single-stranded RNA virus of the Rhabdoviridae family, is a prototypical OV that replicates preferentially in cancerous cells, resulting in virus-induced apoptosis.4,5 VSV multiplication is highly sensitive to the antiviral effects of type 1 interferon (IFN) and other innate immune effectors that strongly inhibit OVs in nonmalignant cells and tissues.4,5,6,7 However, among several defects, malignant cells acquire diminished responsiveness to the antiviral actions of IFN and thus represent a selective niche for VSV replication and virus-mediated cell killing. Nevertheless, several cancer cell lines and many primary tumors are resistant to virus-induced oncolysis by VSV; for instance, human androgen-independent prostate cancer cell line PC3 and chronic lymphocytic leukemia remain partially or completely resistant to viral oncolysis. Genetic modification of the OVs has improved their tumor specificity, such as attenuated variant-VSV-ΔM51 (deletion of methionine 51 in the matrix gene),5 which possesses increased oncolytic efficacy because of its ability to induce a strong protective antiviral response in healthy cells, while inducing apoptosis in cancer cells with a high therapeutic index and safety profile.2 However, some cancer models still remain partially or completely resistant to OV-induced oncolysis. To overcome this resistance, experimental strategies are now combining OVs with different cytotoxic molecules to enhance tumor cell killing. Indeed, VSV has been used in combination with chemotherapeutic agents such as histone deacetylase inhibitors, B-cell lymphoma 2 (Bcl-2) inhibitors, rapamycin, doxorubicin, and other compounds to enhance therapeutic activity.8,9,10,11 However, combination with chemotherapeutic agents gives rise to limitations, including nonselective toxicity in healthy tissues and the development of drug resistance.12
Triptolide (TPL) is a component extracted from the Chinese herb Tripterygium wilfordii Hook F that has been used for many centuries in traditional Chinese medicine for the treatment of inflammation and autoimmune diseases such as rheumatoid arthritis.13 This small molecule mediates a broad spectrum of biological activities, including anti-inflammatory and antineoplastic effects. TPL elicits strong therapeutic activity in animal models of various diseases, including autoimmune uveoretinitis,14 collagen-induced arthritis,15 and inflammatory bowel disease.16 TPL and its derivatives have now entered human clinical trials for the treatment of autoimmune disorders and cancer.13,17,18,19 Clinical trials on rheumatoid arthritis patients have shown that treatment with TPL significantly improved both clinical and laboratory parameters.20 The immunosuppressive action of TPL is generally ascribed to its suppression of cellular immunity. TPL impairs T-cell function both in vitro and in vivo21,22 and inhibits macrophage production of numerous proinflammatory mediators, including tumor necrosis factor-α, interleukin-6, interleukin-8, and IFN-α.23,24 Similarly, the inhibitory effects of TPL have also been validated in several animal models, such as arthritic rat models, where TPL reduced the expression of tumor necrosis factor, interleukin-6, and cyclooxygenase 2.25
Interestingly, TPL has also been shown to possess antiproliferative and proapoptotic activities in a broad spectrum of cancer cells.13,18,26,27,28,29 In vitro studies demonstrated that TPL kills cancer cells originating from different tissues, namely, prostate, blood, lung, colon, brain, breast, and kidney. Similar to its in vitro activity, TPL also demonstrated potent in vivo effects against hematological malignancies and various solid cancers in mouse xenograft models, including PC3,13,30,31,32 and in some cases, it even led to the complete disappearance of the tumor. Comparative studies reveal that the antitumor effects of TPL are comparable with or superior to those of some conventional antitumors drugs, e.g., taxol and cisplatin.13
The mechanism of action of TPL has been extensively studied; TPL has been shown to modulate a variety of genes, including those of the NF-κB and apoptotic pathways.13,29,33,34,35,36,37 Moreover, TPL has also been recently identified as a selective RNA polymerase II inhibitor.27,28,38 Given the antiproliferative and anti-inflammatory properties of TPL, we sought to examine the impact of TPL on IFN antiviral signaling and synergism with VSV in tumor cell killing. Here, we demonstrate that TPL markedly increases VSV replication and enhances VSV-induced apoptosis of OV-resistant PC3 tumor cells in vitro and in vivo through inhibition of IFN signaling.
Results
TPL dampens IFN signaling
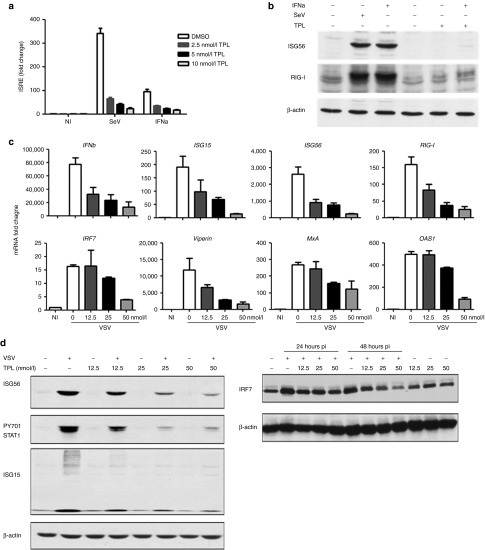
To explore the potential role of the natural molecule derived from the medicinal herb in modulating antiviral response, we performed a limited screen using a dual-luciferase reporter assay in HEK293 cells to identify either positive or negative regulators of Sendai virus (SeV)-mediated type 1 IFN signaling. TPL was identified as a negative regulator. To investigate the potential role of TPL in modulating antiviral response, HEK293 cells were transfected with the IFN-stimulated response element–luciferase reporter plasmid (ISRE-Luc) and infected with SeV or stimulated with IFN-α in the presence or absence of TPL. Results demonstrate that TPL inhibited SeV- and IFN-α–mediated induction of ISRE reporter luciferase activity in a dose-dependent manner (Figure 1a). Indeed, SeV- and IFN-α–mediated ISRE activation was inhibited by >90 and 80%, respectively, in the presence of 10 nmol/l of TPL (Figure 1a). To determine whether TPL inhibited endogenous interferon-stimulated gene (ISG) expression, immunoblot analysis was performed. As shown in Figure 1b, TPL almost completely blocked SeV- or IFN-α–induced RIG-I and ISG56 gene expression.
Figure 1.
Triptolide (TPL) inhibits vesicular stomatitis virus (VSV)-mediated ISG induction in a dose-proportional manner. (a) HEK293 cells were transfected with ISRE-Luc reporter and treated with TPL (2.5, 5.0, and 10.0 nmol/l) or vehicle. Cells were left untreated or were challenged with SeV (40 HAU/ml) or stimulated with interferon (IFN)-α (1,000 U/ml). Luciferase activity was analyzed 24 hours posttransfection and fold activation was determined compared with control. (b) HEK293 cells were treated with 50 nmol/l of TPL and left untreated or were infected with SeV (40 HAU/ml) or stimulated with IFN-α (1,000 U/ml). Western blotting was performed to assess expression of ISG-56 (top panel), RIG-I (middle panel), and β-actin (bottom panel) proteins using ISG56, RIG-I, and β-actin antibodies. (c) Human androgen-independent prostate cancer cell line PC3 cells were preincubated with TPL (12.5, 25.0, and 50.0 nmol/l) or vehicle and infected with VSV-Δ51 (0.005 MOI). The expression of ISGs was assessed using quantitative real-time PCR analysis of total RNA isolated from PC3 cells. (d) Whole-cell extracts (WCEs) were analyzed by immunoblotting with anti-ISG56 (left top panel), anti-PY701 STAT1 (left second panel), anti-ISG15 (left third panel), anti-IRF7 (right top panel), and anti-β-actin (right and left bottom panels) antibodies.
We next examined the effect of TPL on VSV-mediated IFN pathway activation in PC3 cells. VSV infection induced the expression of several genes of the IFN cascade, including IFN-β, ISG15, ISG56, IRF7, MxA, OAS1, Cig5, and RIG-I, all of which are involved in the generation of the antiviral immune response.8,39 Quantitative real-time PCR analysis revealed that in PC3 cells, VSV-induced IFN-β mRNA expression was inhibited by TPL by 90% (Figure 1c). Similarly, VSV-mediated IRF7, RIG-I, ISG15, ISG56, MxA, OAS1, and viperin mRNA expression levels were inhibited by >75% in TPL-treated cells (Figure 1c). We also found that phosphorylation of STAT1 and the subsequent expression of ISG15, ISG56, and IRF7 were inhibited by 30–80% at the protein level in the presence of TPL (Figure 1d). Therefore, TPL treatment diminished the cellular IFN response triggered by VSV infection.
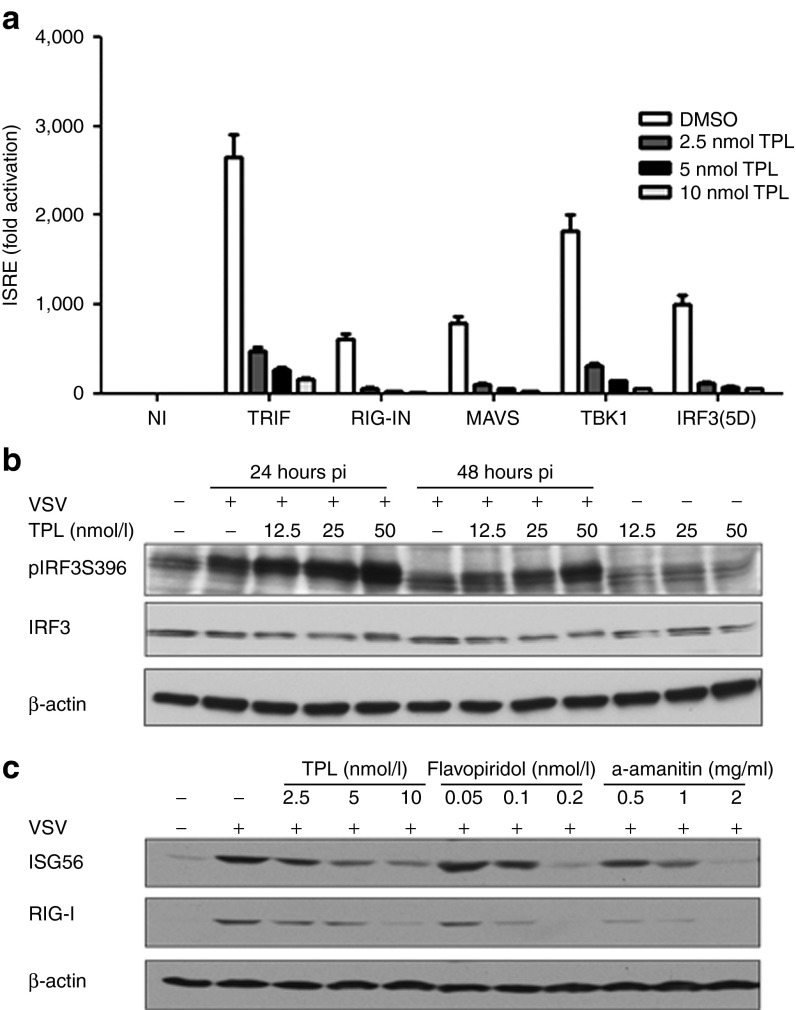
To further determine at what level in the pathway TPL blocked the IFN antiviral response, HEK293 cells were transfected with the ISRE-Luc reporter together with the plasmid encoding various IFN signaling molecules – TRIF, the active CARD domain containing the active form of RIG-I (RIG-IN), MAVS, TBK1 kinase, or the active form of IRF3 (IRF3(5D)) – in the presence or absence of TPL. Ectopic expression of the above signaling molecules resulted in a 500- to 2500-fold induction of ISRE-Luc reporter activity (Figure 2a). TPL inhibited TRIF-, RIG-IN-, MAVS-, TBK1, and IRF3(5D)-mediated ISRE activity by >80% (Figure 2a), indicating that TPL treatment blocks IFN signaling downstream of IRF3. Accordingly, phosphorylation of IRF3 was sustained in the presence of TPL (Figure 2b) due to TPL-mediated increase of VSV infectivity (Figure 3).
Figure 2.
Triptolide (TPL) inhibits IFN signaling downstream of IRF3 activation at the transcription level. (a) HEK293 cells were transfected with ISRE-Luc reporter along with vector, TRIF, RIG-IN, MAVS, TBK1, or IRF3(5D) expression plasmid. Cells were left untreated or were treated with TPL (2.5, 5.0, and 10.0 nmol/l). Luciferase activity was monitored 24 hours posttransfection. (b) PC3 cells were either treated with 12.5, 25.0, and 50.0 nmol/l of TPL or not treated and infected with VSV-Δ51 (0.005 MOI) for 24 and 48 hours. Whole-cell extracts were analyzed by immunoblotting with anti-p-S396-IRF3 (top panel), anti-IRF3 (middle panel), and anti-β-actin (bottom panel) antibodies. (c) PC3 cells were treated with TPL, flavopiridol, or α-amanitin and challenged with VSV. ISG56 (top panel), RIG-I (middle panel), and β-actin (bottom panel) expression was analyzed by western blot.
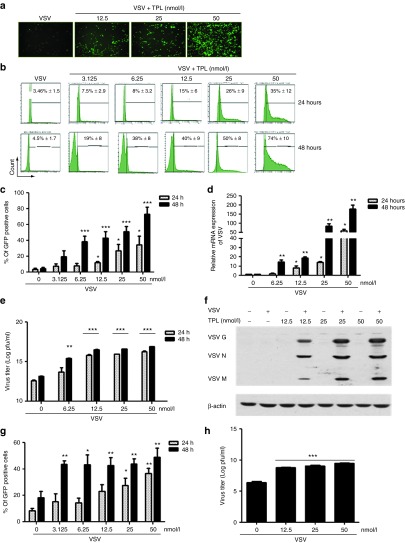
Figure 3.
Triptolide (TPL) enhances vesicular stomatitis virus (VSV) viral replication in PC3 and other resistant cell lines in a dose- and time-dependent manner. PC3 cell line was either pretreated or not with indicated doses of TPL for 30 minutes (TPL was present in the medium). Cells were infected with VSV-Δ51-GFP (0.005 MOI) for 1 hour. The images were captured at 48 hours postinfection by fluorescent microscopy (Axiovert 40 CFL, Zeiss, Thornwood, NY) at a (a) original magnification ×10. Then, 24 and 48 hours postinfection, percentage of infected cells was determined by (b and c) flow cytometry analysis of GFP expression. VSV mRNA expression was examined by quantitative real-time PCR and the VSV mRNA level is displayed (d) as fold expression relative to the untreated VSV-infected sample. Virus released from infected cells was measured by (e) plaque assay and (f) western blotting for measuring viral proteins using anti-VSV antibody was also performed. DU145 and Karpas-422 cell lines were pretreated as described above. Cells were infected with VSV-Δ51-GFP (0.005 MOI for DU145 and 1 MOI for Karpas-422) for 1 hour. Then, 24 and 48 hours postinfection, surface expression of (g) VSV on DU145 was assessed by flow cytometry. Virus released from Karpas-422 infected cells was measured by (h) plaque assay. Histograms shown are representative of five independent experiments, and the values represent the means ± SEM for three to five independent experiments. For all the bar graphs, *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001 when compared with untreated VSV-infected cells.
Given that TPL has been identified as a selective inhibitor of RNA polymerase II,28,40 we tested the effect of flavopiridol and α-amanitin, two other RNA polymerase II inhibitors,40 on the expression of ISGs. These two molecules (flavopiridol and α-amanitin) inhibited the expression of ISG56 by five and sixfold, respectively, and RIG-I by threefold in the same manner as TPL in PC3 cells (threefold (ISG56) and 2.5-fold (RIG-I), respectively; Figure 2c). Taken together, these results suggest that TPL abrogates the IFN response through inhibition of RNA polymerase function.
TPL increases VSV replication in resistant cancer cell lines
Given that VSV is very sensitive to IFN,5 we tested the effect of TPL on VSV replication in OV-resistant cancer cell lines. Cell lines PC3, DU145 (human prostate cancer), and Karpas-422 (human B-lymphoma) do not support significant VSV replication.8,9,12,41 Initially, the effect of TPL on the replication of a recombinant VSV expressing the green fluorescent protein (VSV-Δ51-GFP) in these cells was determined. PC3 and DU145 cells were pretreated with increasing concentrations of TPL (3–50 nmol/l) and then infected with VSV-Δ51-GFP. The expression of VSV in PC3 cells treated with the VSV-and-TPL combination was examined using Axiovert 40 CFL inverted microscope. We observed that TPL potentiated VSV replication (Figure 3a). Accordingly, TPL also enhanced VSV replication in these cells in a dose-dependent manner as measured by flow cytometry analysis of VSV-GFP-positive cells (Figure 3b,c,g). At 24-hour postinfection, VSV-infected cells increased 2- to 10-fold among the PC3 cells and two to fourfold among the DU145 cells following TPL treatment (Figure 3b,c,g). Indeed, viral replication increased from 4.5% with VSV alone to 74% with 50 nmol/l TPL 48 hours postinfection in PC3 cells (Figure 3b,c). Lower concentrations of TPL (<5 nmol/l) were ineffective in stimulating VSV replication (Figure 3b,c). Real-time PCR analysis showed that VSV-infected PC3 cells treated with TPL expressed 170-fold more VSV mRNA compared with the control cells (Figure 3c). Furthermore, the titer of virus released from infected cells increased by three logs when PC3 and Karpas-422 cells were pretreated with 50 nmol/l TPL (Figure 3e,h, respectively). In addition, VSV viral proteins, measured by western blot, increased in PC3 cells with combination treatment compared with the same in cells infected with VSV alone (Figure 3f). These results demonstrate that TPL enhances VSV replication in resistant cell lines.
TPL sensitizes VSV-mediated oncolysis in resistant cancer cell lines
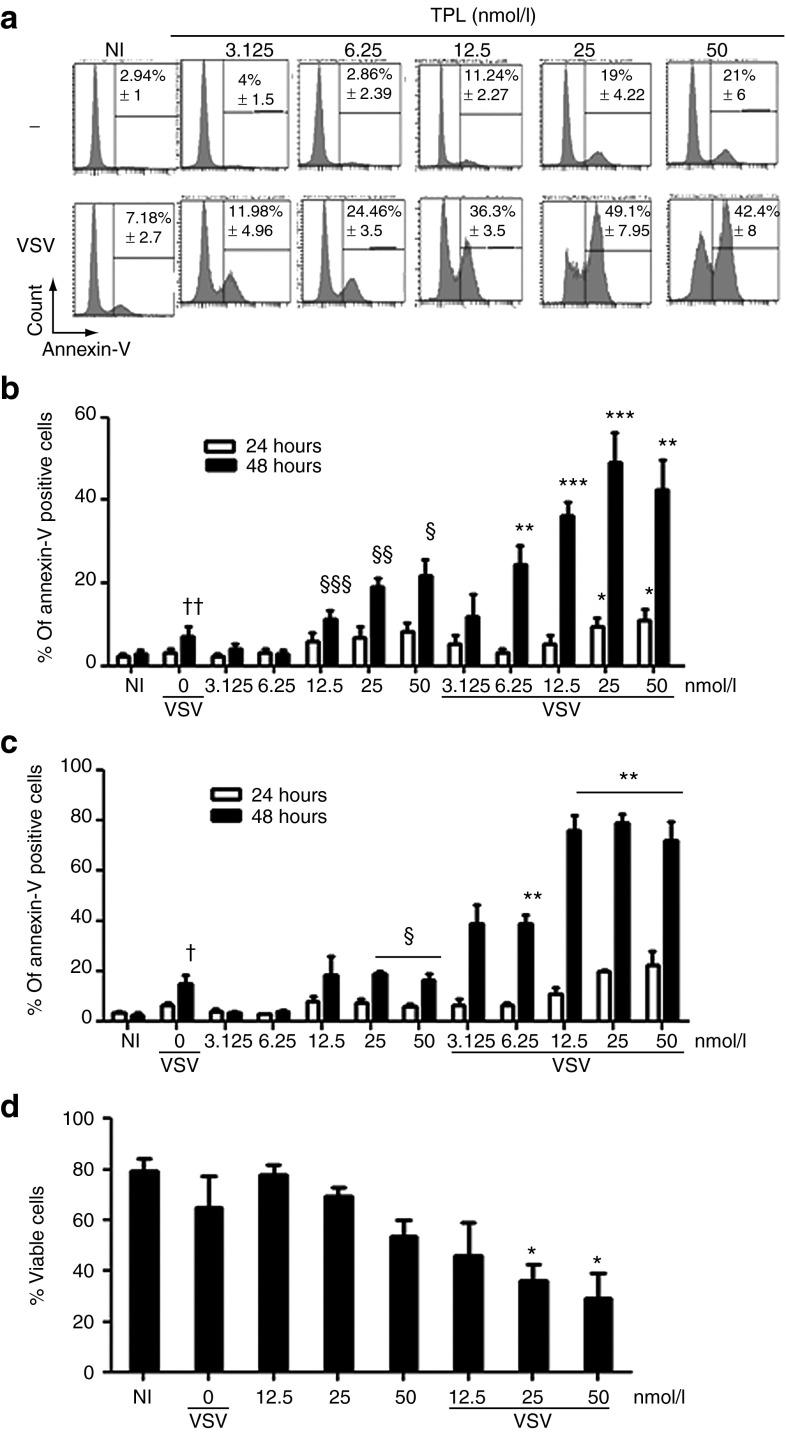
Because dampening of cellular IFN responses correlated with an augmentation of VSV-induced apoptosis and increase of oncolytic activity,8 we determined the cytotoxic effect of TPL on these tumor cells (PC3, DU145, and Karpas 422 cells) using annexin-V staining.
TPL has been shown to inhibit the proliferation of all 60 US National Cancer Institute cancer cell lines and to induce apoptosis in a number of cancer cell lines.28 Accordingly, we observed that VSV infection or TPL treatment alone slightly triggered apoptosis in PC3 cells (7 and 20%, respectively, at 48 hours; Figure 4a,b)13, whereas the combination of TPL and VSV significantly enhanced VSV-mediated apoptosis (~50% with 25 nmol/l TPL at 48 hours; Figure 4a,b). Similarly, in DU145 and Karpas-422 cells, a significant increase in apoptosis was observed when cells were treated with VSV plus TPL combination compared with VSV or TPL treatment alone (Figure 4c,d). Notably, TPL-enhanced VSV-mediated apoptosis was observed at 48 hours postinfection, and TPL-enhanced VSV replication occurred within 24 hours postinfection (Supplementary Figure S1). Therefore, our data indicated that TPL-mediated increase in VSV replication preceded the induction of apoptosis in this in vitro model.
Figure 4.
Triptolide (TPL) enhances vesicular stomatitis virus (VSV) oncolytic activity in VSV-resistant cell lines. PC3 were preincubated with or without TPL (3.125–50 nmol/l) for 30 minutes and then infected with VSV-Δ51-GFP. Induction of apoptosis was monitored 24 and 48 hours postinfection by (a and b) flow cytometry using annexin-V-allophycocyanin. DU145 and Karpas-422 cell lines were treated as described above. Induction of apoptosis in DU145 was monitored 24 and 48 hours postinfection by (c) flow cytometry using annexin-V-allophycocyanin. The levels of annexin-V binding and propidium iodide (PI) were also measured on (d) VSV-infected Karpas-422. Histograms shown are representative of five independent experiments, and the graphs represent the mean ± SEM of at least three independent experiments. Each value for TPL plus VSV was compared with vehicle control. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; TPL alone was compared with NI. NI denotes non–TPL-treated and non–VSV-infected samples. §P ≤ 0.05; §§P ≤ 0.01; §§§P ≤ 0.001; VSV-infected cells were compared with NI. †P ≤ 0.05; ††P ≤ 0.01.
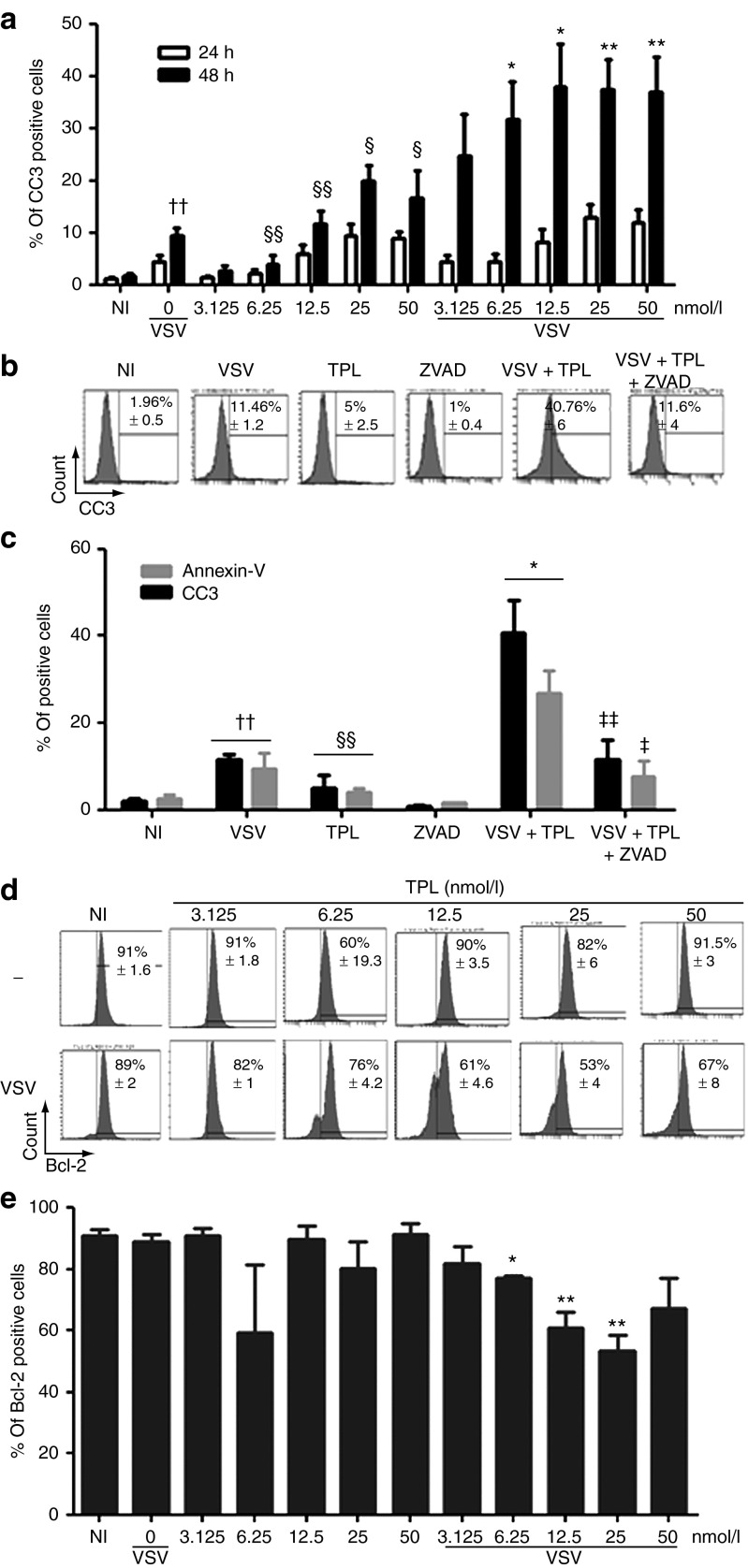
We next measured caspase-3 activation as an indicator of apoptosis induction because different upstream pathways that lead to apoptosis converge on caspase-3 for apoptotic execution. Figure 5a shows the effect of TPL and VSV combination treatment on the level of caspase-3 activation as measured by flow cytometry analysis. There was a significant increase (two to fourfold) of caspase-3-activated cell numbers after 48 hours with the combination treatment compared with either agent alone (Figure 5a). We further confirmed this result using a general caspase inhibitor, Z-VAD-FMK, and observed a 70% decrease in activated caspase-3 in VSV plus TPL-treated PC3 cells in the presence of Z-VAD-FMK (Figure 5b,c). Interestingly, annexin-V-positive cells, an early marker of apoptosis, decreased by 65% in the presence of Z-VAD-FMK (Figure 5c), whereas VSV replication was not affected by the caspase inhibitor (data not shown). Therefore, TPL and VSV when used alone or in combination were able to trigger apoptosis by activating caspase-3. Furthermore, a 45% decrease in Bcl-2 expression, an antiapoptotic protein that regulates the cleavage of caspase-3,42 was observed in PC3 cells treated with TPL and VSV combination (Figure 5d,e). All together, these results demonstrate for the first time that TPL enhances VSV replication and VSV-induced apoptosis in resistant cell lines by activating caspase-3 and inhibiting Bcl-2 expression.
Figure 5.
Triptolide (TPL) enhanced vesicular stomatitis virus (VSV) apoptosis in PC3 cell line through caspase-3 activation and a decrease in Bcl-2 expression. PC3 cells were pretreated with or without TPL and then infected with VSV-Δ51-GFP as described above. The level of apoptosis was measured by flow cytometry using (a) cleaved caspase-3-PE (CC3) and (d and e) Bcl-2-V450 antibodies. The levels of annexin-V binding and cleaved caspase-3 were also measured at day 1 and day 2 postinfection in VSV-infected PC3 pretreated or untreated with TPL (6.25 nmol/l) and/or with ZVAD (100 μmol/l; b and c). Histograms shown are representative of at least three independent experiments and the values represent the means ± SEM. P values were calculated to compare the following: Each value for TPL plus VSV was compared with vehicle control. *P ≤ 0.05; **P ≤ 0.01. TPL alone was compared with NI. NI denotes non–TPL-treated and non–VSV-infected samples. §P ≤ 0.05; §§P ≤ 0.01. VSV-infected cells were compared with NI. ††P ≤ 0.01. VSV plus TPL value was compared with VSV plus TPL plus ZVAD. ‡P ≤ 0.05; ‡‡P ≤ 0.01.
Coadministration of TPL with VSV increases VSV replication at the tumor site
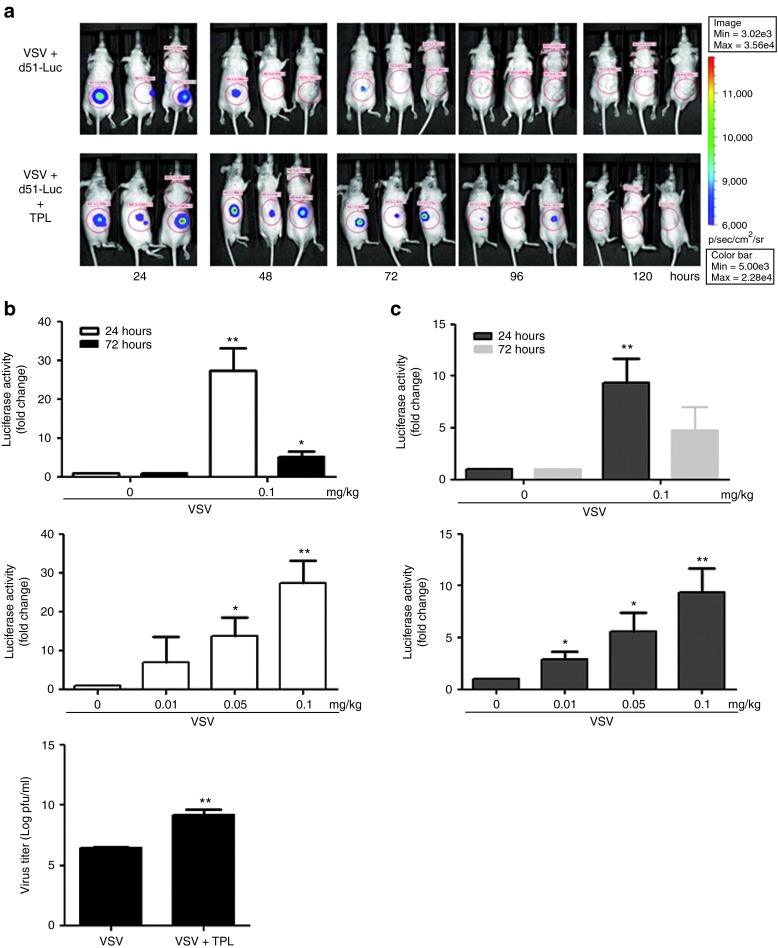
To investigate whether TPL can enhance VSV replication in in vivo cancer models, athymic nude mice bearing PC3 subcutaneous tumors were administered TPL intraperitoneally (IP) daily, and VSV-Δ51-Luc was inoculated intratumorally at day 0. Virus replication was monitored daily using an in vivo imaging system; the bioluminescent signal corresponding to virus replication was restricted to the site of the tumor and no evidence of off-site infection of normal tissues was detected in animals receiving combination treatment (data not shown). TPL treatment was able to promote and sustain VSV replication at the tumor site (Figure 6a). These results were confirmed in independent experiments by measuring firefly luciferase activity 24 and 72 hours postinjection (Figure 6b for PC3 and Figure 6c for TSA xenograft models, top panel). Indeed, a 30- and 10-fold increase in luciferase activity, which corresponds to viral replication, was observed in PC3 and TSA tumor homogenates, respectively (Figure 6b,c, top panel). Concomitantly, virus production in tumor homogenates was also measured by plaque assay; TPL strongly enhanced VSV replication at 24 hours by three logs in the PC3 xenograft model (Figure 6b, bottom panel). Similar to the in vitro data (Figure 3), TPL also significantly augmented VSV replication at lower doses in vivo (Figure 6b, middle panel; and Figure 6c, bottom panel). Therefore, coadministration of TPL with VSV enhances viral replication in vivo.
Figure 6.
Triptolide (TPL) increases vesicular stomatitis virus (VSV) virus replication in dose-dependent manner in xenograft models. (a) A PC3 subcutaneous xenograft tumor model was established in nude mice. Each group received VSV-Luc (1 × 107 pfu) intratumorally on day 0 and was either treated (with TPL (0.1 mg/Kg; day 0–6), intraperitoneally (IP)) or left untreated. (A) Viral replication at the tumor site was imaged using an in vivo imaging system. (b) Mice were injected with VSV-Luc (day 0) and treated (IP) with TPL or vehicle for 1 and 3 days, respectively. Five mice were killed at each time and tumors were collected to determine virus replication using luciferase (top panel) and plaque (bottom panel) assays. Mice were also injected with VSV-Luc (day 0) and treated (IP) with TPL at 0.1, 0.05, and 0.01 mg/kg. Tumors were collected 24 hours posttreatment and virus production was analyzed by luciferase assay (middle panel). (c) A murine TSA mammary adenocarcinoma cell (3 × 105 cells) subcutaneous graft model was established in immunocompetent mice. One week later (palpable tumors), animals were treated by intratumoral injection with VSV (2 × 107 pfu/dose) on day 0 and treated (IP) with TPL at 0.1, 0.05, and 0.01 mg/kg. Luciferase assay was performed to monitor virus replication 1 day after infection initiation. *P < 0.05; **P < 0.01 when compared with vehicle.
TPL–VSV combination therapy delays tumor growth and prolongs survival in mouse xenograft models
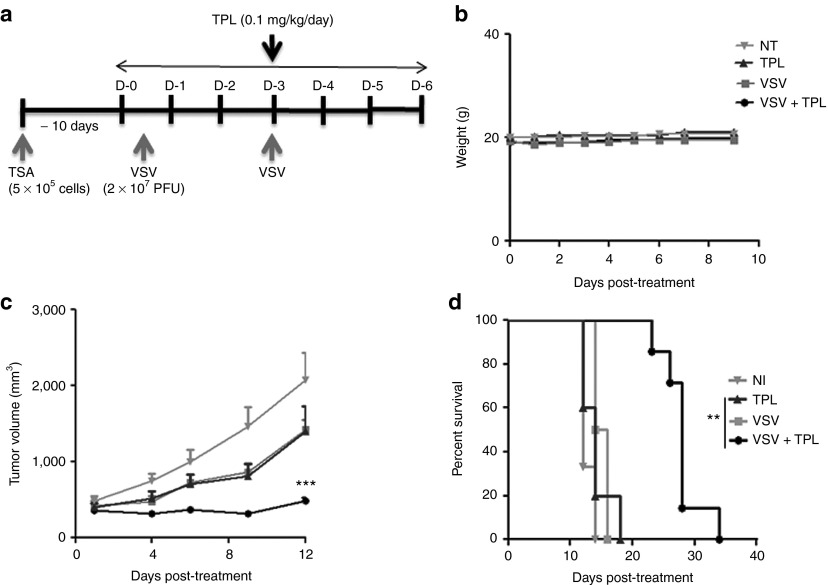
The above results demonstrated that TPL enhanced VSV replication and apoptosis in vitro and increased VSV replication in vivo in athymic nude mouse and syngeneic models (Figures 3 and 6). To determine the potency of TPL therapy in enhancing VSV-induced oncolysis in vivo, TSA cells were implanted subcutaneously in immunocompetent BALB/c mice. When tumors were palpable, mice were treated with TPL or vehicle daily at 0.1 mg/kg (Figure 7a); tumor size was measured every 2 days and body weight every day. As shown in Figure 7c, tumors treated with VSV plus TPL were significantly reduced in size (~80%), compared with animals receiving single treatment (VSV alone, TPL alone, and vehicle). The tumor size was also decreased (>40% reduction) in animals receiving lower dose of TPL (0.05 mg/kg) in combination with VSV (data not shown). Furthermore, survival was significantly improved in combination-treated mice (34 days) compared with vehicle-treated mice (14 days; Figure 7d). Indeed, 85% of VSV plus TPL-treated mice survived, whereas 100% of the control animals died by day 23.
Figure 7.
Vesicular stomatitis virus (VSV) plus triptolide (TPL) combination treatment reduces tumor progression in mouse xenograft model and prolongs survival. (a) Schematic representation of the treatment schedule for TSA xenograft model. Murine TSA (3 × 105 cells) subcutaneous graft model was established in BALB/c. Animals were treated by intratumoral injection with VSV (2 × 107 pfu/dose) on days 0 and 3 and treated (intraperitoneally) with TPL (0.1 mg/kg) or vehicle for a period of 7 days. (b) The cytotoxicity of TPL was assessed by monitoring mouse body weight. (c) The tumor growth was monitored using caliper measurements three times per week and the average tumor volumes (n = 8) are shown. (d) Cumulative survival rate was also evaluated. **P ≤ 0.01; ***P ≤ 0.001; when comparing VSV plus TPL combination treatment with single treatments and NI. NI denotes non–TPL-treated and non–VSV-infected samples.
Similarly, TPL–VSV combination therapy significantly reduced the tumor volume by ~90% compared with the vehicle groups (VSV or phosphate-buffered saline (PBS)) and improved the survival in the PC3 xenograft model (Supplementary Figure S2b,c). Consistent with previous reports,13 TPL treatment alone decreased the tumor size in these mice. Importantly, the tumor volume was also significantly decreased in TPL–VSV-treated mice compared with TPL-treated mice. Concomitantly, there was no significant difference in body weight between the TPL-treated and the control groups (Figure 7b and Supplementary Figure S2d), indicating that this dose of TPL caused no significant toxicity to mice. These data are consistent with previous in vivo studies that have demonstrated no toxicity of TPL at doses ≥0.25–0.4 mg/kg.13 Altogether, VSV–TPL combination has a potent therapeutic effect in vivo.
Discussion
OVs, including VSV, have been designed to specifically infect cancers sparing normal cells and tissue. Indeed, several studies have shown that oncolytic vectors based on VSV are promising agents for antitumor therapy.43 Various cancer cell types, including both primary and immortalized tumor cell lines, have defective IFN signaling and are selectively killed by VSV. However, some cancer cells retain IFN responsiveness. Predictably, these cancers are relatively resistant to oncolytic VSV. In an analysis of VSV infection in the NCI-60 cell panel, 81% of cell lines were found to be unresponsive to type I IFN, whereas the remaining 19% retained type I IFN responsiveness.5 PC3 prostate cancer cells are an example of a cell line that has retained type I IFN responsiveness, and these cells were observed to be relatively resistant to VSV infection.44 To overcome this resistance, several groups have recently engineered or selected more potent viruses by incorporating virulence or suicide genes into attenuated strains with the hope of making more clinically efficacious strains12 or by combining two distinct OVs.45 A concern with this approach is the possibility of creating a virus that on its own can overcome the antiviral programs that normal cells have in place to control virus spread. As an alternative to the creation of a single more virulent virus and the associated safety concerns that would go with it, we propose the possibility to combine an OV with a cytotoxic agent to improve the antitumoral/oncolytic activity of the OV, particularly in poorly permissive cancer types such as human prostate cancer. We chose the oncolytic candidate VSV from the Rhabdoviridae family for its rapid replication and spread in cancer cells with impaired innate antiviral immune responses. Because TPL, a natural compound, has been shown to possess several virtues, including tumor cell killing and inhibition of IFN expression in lipopolysaccharide-stimulated murine macrophages,23,24 we hypothesized that TPL could enhance OVs by facilitating viral infection and inducing tumor killing. Indeed, one striking observation in these studies was the ability of TPL to enhance VSV replication in VSV-resistant cells and in xenograft models when both TPL and VSV were used at lower doses (Figures 3 and 6). Importantly, we demonstrated that, at least in the animal model tested herein, the combination of VSV and TPL enhanced virus replication, which was only restricted to the tumor bed with minimal impact on normal tissues (data not shown). Although the exact mechanism of this selectivity remains unclear at this time, these results are consistent with the concept that TPL dampened the cellular IFN response in these cells as shown in our cellular model (Figures 1 and 2). Indeed, our data demonstrated that in TPL and VSV combination-treated PC3 cells, TPL inhibits some key antiviral genes, including the expression of IFN and phosphorylation of STAT1 and the subsequent induction of ISGs (i.e., IRF7, ISG56, and MxA), thus blunting IFN cellular response at the transcription level (Figure 2c). Although our result is consistent with reports that TPL inhibits IFN expression23,24 and that TPL is a selective inhibitor of RNA polymerase II,28 this study reports for the first time the effect of TPL on IFN signaling following virus infection. Although we attributed a key role to TPL in dampening IFN signaling in cancer cells, the involvement of additional TPL-modulated immune responses that affect virus replication cannot be excluded, i.e., TPL can affect VSV replication at the level of virus penetration, primary transcription, or virus assembly. Future experiments will be needed to understand how TPL can affect virus infectivity.
Another plausible mechanism that may be involved in the potentiating effect of TPL on VSV replication and hence enhancement of tumor oncolysis is the participation of the antitumoral immune response. Indeed, several attempts to modulate the immune response have involved increasing the efficacy of OVs, such as the use of some immunosuppressive agents (i.e., cyclophosphamide) that benefit virotherapy.46
Given that TPL has multiple and pleiotropic effects, including anti-inflammatory and immunosuppressive actions (NF-Kβ inhibitor), we believe that TPL may enhance viral replication through reduction of neutralizing antibodies, T cell response, or innate immune cell infiltration and function by inhibiting release of cytokines and chemokines. Further studies are required to address this point.
Based on these observations and the known oncolytic specificity of VSV, an anticipated finding from this study was the effect of TPL on the apoptotic pathway of infected cells; we found that TPL interacts synergistically with VSV, leading to better cell killing. VSV plus TPL combination therapy appears to target preferentially the intrinsic mitochondrial pathway, as demonstrated by the cleavage of caspase-3 and decreased expression of Bcl-2 following treatment (Figure 5). In addition to a direct virus-mediated oncolysis, cell death may also be mediated by indirect mechanisms that trigger apoptosis through the release of danger-associated molecular patterns, such as ATP47,48 and/or inflammatory mediators from infected dying tumor cells. Other mechanisms such as autophagy or endoplasmic reticulum stress responses could be also implicated in TPL-mediated apoptosis. Interestingly, we observed that expression of the glucose-regulated protein 78-kDa (GRP78), an antiapoptotic protein that is induced by a wide variety of physiologic and pathophysiologic stresses and is upregulated in many types of cancer,49 was decreased in TPL plus VSV combination samples (data not shown). However, future investigations will be needed to address this issue.
In conclusion, this study demonstrates for the first time that TPL is a potent enhancer of VSV in vitro and in vivo, most probably acting by dampening the antiviral immune response. Moreover, TPL was able to inhibit tumor growth and increase animal survival. Therefore, we believe that the safety and potential application of this small OV enhancer could be used to ameliorate the effectiveness of OV therapy, and further clinical evaluation of this possibility is warranted.
Materials and Methods
Cancer cell cultures. Human androgen-independent prostate cancer cell line PC3 was purchased from the American Type Culture Collection (ATCC; Manassas, VA) and human B-cell non-Hodgkin lymphoma cell line Karpas-422 from the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany). TSA cells (murine mammary adenocarcinoma) were a kind gift from Dr Barber (University of Miami, Miami, FL) and DU145 (human prostate carcinoma) from Dr Hiscott (Vaccines & Gene Therapy Institute of Florida, Port St Lucie, FL). PC3, TSA, Karpas-422, and DU145 cells were grown in one of the following media: Roswell Park Memorial Institute (RPMI 1640), Dulbecco's modified Eagle's medium (DMEM), or Eagle's minimal essential medium (EMEM) (Wisent, St Bruno, Québec, Canada), respectively, supplemented with 10% fetal bovine serum (Wisent). Cells were maintained at 37 °C and 5% CO2.
Virus production, quantification, and infection. VSV-Δ51 expressing GFP and GFP–firefly luciferase fusion are recombinant derivatives of VSV-Δ51, a naturally occurring IFN-inducing mutant of VSV Indiana serotype.8 They were kindly provided by Dr Bell and Dr Stojdl (Ottawa Health Research Institute, Ottawa, Ontario, Canada). Virus stocks were propagated and purified as described8,41 in Vero cells (ATCC). Briefly, virus was grown in Vero cells, concentrated from cell-free supernatants by centrifugation (15,000 rpm, 4 °C, 90 minutes), and titrated in duplicate by standard plaque assay. PC3, DU145, and Karpas-422 cells were preincubated with or without TPL (Sigma-Aldrich, St Louis, MO) at different concentrations and infected with VSV-Δ51 at a multiplicity of infection of 0.005 (for PC3 and DU145 cells) and 1 plaque-forming unit (pfu) per cell (for Karpas-422) in a small volume of medium for 1 hour at 37 °C. Cells were then incubated in complete medium for the indicated period of time before analysis.
Flow cytometry. As described above, PC3, DU145, and Karpas-422 cells were pretreated with or without TPL and then infected in the presence or absence of GFP-VSV for 24 and 48 hours. To evaluate infectivity, GFP fluorescence intensity was measured by flow cytometry using BD Fortessa flow cytometer (Becton Dickinson, Franklin Lakes, NJ); data were analyzed using the DIVA software. To measure the level of apoptosis, cells were first washed and labeled with annexin-V-allophycocyanin (BD Biosciences, Franklin Lakes, NJ) in 1× calcium buffer (0.01 mol/l HEPES (pH7.4), 0.14 mol/l NaCl, 2.5 mmol/l CaCl2) (Becton Dickinson) for 10 minutes at 4 °C. After two washes with calcium buffer, the cells were then fixed in BD FACS lysing buffer (prediluted 10 times in calcium buffer; Becton Dickinson) at room temperature and incubated with mouse antihuman Bcl-2-V450 and rabbit antiactive caspase-3-PE (BD Biosciences), diluted in the presence of 0.25% saponin, for 20 minutes at room temperature. After three washes in calcium buffer, cells were studied using a BD Fortessa flow cytometer (20,000 gated events/measurement) and analyzed as described above.
Protein extraction and immunoblot analysis. Cells were washed twice with ice-cold PBS, and proteins were extracted as follows. Briefly, cell pellets were lysed in ice-cold buffer containing PBS, 0.05% NP40, 0.1% glycerol, 30 mmol/l NaF, 40 mmol/l β-glycerophosphate, 10 mmol/l Na3VO4, and protease inhibitor cocktail (Sigma-Aldrich) in 1:1,000 dilution. Extracts were kept on ice for 30 minutes and centrifuged at 10,000g for 25 minutes (4 °C); supernatants were stored at −80 °C. Protein concentration was determined with Bio-Rad protein assay reagent (BioRad, Hercules, CA). Whole-cell lysates (30–40 μg) were resolved using 7.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane (0.45 μm, Bio-Rad, Mississauga, Ontario, Canada) at 4 °C for 1 hour at 100 V in a buffer containing 30 mmol/l Tris, 200 mmol/l glycine, and 20% (vol/vol) ethanol. Membranes were blocked for 1 hour in 5% nonfat dried milk in PBST (phosphate-buffered saline + 0.1% Tween-20) and then incubated with any of the following primary antibodies: VSV whole-virus antisera, anti-IRF3 (IBL, Tokyo, Japan), anti-ISG56 (Thermo Fischer Scientific, Waltham, MA), anti-pSTAT1 at Tyr701 and anti-ISG15 (Cell Signaling Technology, Boston, MA), anti-RIG-I, anti-pIRF3 at Ser396, anti-IRF7, and anti-β-actin (EMD Millipore, Bedford, MA). After three 10-minute washes with PBST, membranes were incubated for 1 hour with horseradish peroxidase-conjugated goat antirabbit or antimouse antibodies (Amersham, Piscataway, NJ) at a dilution of 1:3,000 in blocking solution. The reaction was then visualized with an enhanced chemiluminescence detection system as recommended by the manufacturer (PerkinElmer, Waltham, MA). Densitometry analysis was performed using ImageJ software (NIH Windows version).
RNA extraction and quantitative real-time PCR. DNase-treated total RNA from PC3 cells was prepared using the RNeasy kit (QIAGEN, Valencia, CA). RNA concentration was determined by its absorption at 260 nm, and RNA quality was ensured by a 260/280 absorption ratio ≥2.0. One microgram of RNA was reverse transcribed using high-capacity cDNA reverse transcription kits from Applied Biosystems (Burlington, Ontario, Canada) according to manufacturer's instructions. Parallel reactions without reverse transcriptase were included as negative controls. Quantitative real-time PCR assays were performed using the SYBR Green I on a Light Cycler apparatus (Roche Diagnostics, Indianapolis, IN). Human primer sequences used in this study are summarized in Supplementary Table S1. PCR efficiency results were obtained from triplicate measurements of individual cDNA samples.
Plasmid construction. Plasmids encoding GFP-MAVS, GFP-ΔRIG-I, GFP-TBK1, GFP-IRF3(5D), GFP-TRIF, ISRE-luciferase, and pRLTK have been previously described.50
Transfection and luciferase assay. HEK293 cells were grown in DMEM supplemented with 10% (vol/vol) fetal bovine serum, l-glutamine (Wisent), and antibiotics. For luciferase assays, HEK293 cells were transfected with 50 ng of PRLTK reporter, 100 ng of ISRE-Luc reporter, expression plasmid encoding ΔRIG-I (200 ng), MAVS, TRIF, TBK1, or IRF3 (5D) (50 ng each), together with 400 ng of empty vector, by the calcium phosphate transfection method.50 At 24 hours after transfection, luciferase activity was measured with a dual-luciferase reporter assay system according to the manufacturer's instructions (Promega, Madison, WI). Some cells were treated with SeV (40 hemagglutination units per ml, Charles River Laboratories, Pointe Claire, Québec, Canada) or IFN-α (1,000 U/ml, Intron A, Schering Plough, Kenilworth, NJ).
Mice and tumor models. All mice used were obtained from Charles River Laboratories. All procedures involving animals were reviewed and approved by the McGill University Animal Care Committee.
Imaging studies. PC3 (5 × 106) tumors were established subcutaneously in 6- to 8-week-old male nude mice (n = 4). VSV-Luc (1 × 107 pfu) was administered intratumorally on the first day and TPL (0.1 mg/kg) was given daily by IP injection. Mice were injected IP with D-luciferin (200 ml at 10 mg/ml in PBS, Molecular Imaging Products, Bend, OR) for firefly luciferase imaging. Animals were anesthetized under 3% isoflurane (Baxter, Mississauga, Ontario, Canada) and imaged with the in vivo imaging system 200 Series (Xenogen, Alameda, CA). Data acquisition and analysis were performed using Living Image v2.5 software. For each experiment, images were captured under identical exposures, and bioluminescence was plotted on identical color scales.
Virus titration. PC3 and TSA tumors were established subcutaneously in 6- to 8-week-old male nude mice as described above and in 6- to 8-week-old female BALB/c mice as described previously.12 To monitor VSV replication in the presence of TPL at the tumor site, 1 × 107 pfu (athymic nude mice) and 2 × 107 pfu (immunocompetent mice) of VSV-Luc was administered intratumorally on the first day and various doses of TPL (0.1, 0.05, and 0.01 mg/kg) were given daily by IP injection. Four to five mice in each group were killed 24 and 72 hours after treatment, and tumors were weighed. Tumors were homogenized in 20% (w/v) of serum-free media using a Polytron PT1200 homogenizer (Kinematica, Bohemia, NY). The supernatants were clarified by centrifugation and 10-fold serial dilutions of samples were prepared in serum-free media. Viral titers were quantified by standard plaque assay on Vero monolayers. To measure luciferase activity, tumor homogenates were also analyzed by luciferase reporter assay (Promega) according to the manufacturer's instructions using a GLIOMAX 20/20 luminometer (Promega). Luciferase activity was expressed as relative light units/g of tumor, and the data represent fold increase.
Efficacy studies. TSA (n = 8/group) and PC3 (n = 3–4/group) xenograft models were established in the hind flanks of 6- to 8-week-old female BALB/c and male athymic nude mice, respectively. After tumors became palpable (100–150 mm3), the double-treated group received TPL (IP) at a concentration of 0.1 mg/kg/day for a period of 7 days (for TSA model) or 2 weeks (for PC3 xenograft model). Four hours after administering TPL, all mice were injected intratumorally with 2 × 107 pfu of VSV-Luc on day 0 and day 3 (Figure 7a) and 1 × 107 pfu of VSV-Luc on day 0, day 3, day 6, and day 9 (Supplementary Figure S2a). To assess the safety of TPL plus VSV combination treatment, mice were weighed every day for the first 10 days and then every 2 days until the end of the treatment. Tumor sizes were measured every 2 days using an electronic caliper, and tumor volume was calculated using the formula length × (width)2/2. The average tumor size in each treatment group was calculated for each time point, and standard error was calculated to determine statistical significance (two-way ANOVA, Graph Pad, San Diego, CA). Mice were killed when tumor volume reached 2,000 mm3 and log-rank analyses were performed on Kaplan–Meier survival graphs using Prism 4 (Graph Pad, San Diego, CA).
Statistical analysis. Values are expressed as the mean ± SEM. The significance of the difference between the control and each experimental test condition was analyzed using unpaired Student's t-test and P value < 0.05 was considered significant. For the in vivo experiments, analysis of the effect of TPL on tumor size was conducted using two-way ANOVA, and P < 0.05 was considered statistically significant.
SUPPLEMENTARY MATERIAL Figure S1. Kinetics of TPL-mediated VSV replication and apoptosis in PC3 cells. Figure S2. VSV plus TPL combination treatment reduces tumor progression in PC3 mouse xenograft model and prolongs survival. Table S1. Primer sequences for real-time RT-PCR.
Acknowledgments
The authors thank John Bell (Ottawa Health Research Institute, University of Ottawa, Ottawa, Ontario, Canada) for kindly providing the VSV-GFP virus. The authors also thank Sara Samuel and Zhengyun Xu (Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Quebec, Canada) for the help with experiments and Sara Samuel for help with the writing of this paper. This research was supported by grants from the Canadian Institutes of Health Research (MOP 42562 to RL) and the Terry Fox Foundation to JH. There are no conflicts of interest to disclose.
Supplementary Material
Kinetics of TPL-mediated VSV replication and apoptosis in PC3 cells.
VSV plus TPL combination treatment reduces tumor progression in PC3 mouse xenograft model and prolongs survival.
Primer sequences for real-time RT-PCR.
References
- Kaur B, Cripe TP, Chiocca EA. “Buy one get one free”: armed viruses for the treatment of cancer cells and their microenvironment. Curr Gene Ther. 2009;9:341–355. doi: 10.2174/156652309789753329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerani S. Oncolytic viruses in cancer therapy. Eur J Sci Res. 2010;40:156–171. [Google Scholar]
- Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30:658–670. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichty BD, Power AT, Stojdl DF, Bell JC. Vesicular stomatitis virus: re-inventing the bullet. Trends Mol Med. 2004;10:210–216. doi: 10.1016/j.molmed.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Stojdl DF, Lichty BD, tenOever BR, Paterson JM, Power AT, Knowles S, et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4:263–275. doi: 10.1016/s1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- Stojdl DF, Lichty B, Knowles S, Marius R, Atkins H, Sonenberg N, et al. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat Med. 2000;6:821–825. doi: 10.1038/77558. [DOI] [PubMed] [Google Scholar]
- Barber GN. VSV-tumor selective replication and protein translation. Oncogene. 2005;24:7710–7719. doi: 10.1038/sj.onc.1209042. [DOI] [PubMed] [Google Scholar]
- Nguyên TL, Abdelbary H, Arguello M, Breitbach C, Leveille S, Diallo JS, et al. Chemical targeting of the innate antiviral response by histone deacetylase inhibitors renders refractory cancers sensitive to viral oncolysis. Proc Natl Acad Sci USA. 2008;105:14981–14986. doi: 10.1073/pnas.0803988105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumilasci VF, Olière S, Nguyên TL, Shamy A, Bell J, Hiscott J. Targeting the apoptotic pathway with BCL-2 inhibitors sensitizes primary chronic lymphocytic leukemia cells to vesicular stomatitis virus-induced oncolysis. J Virol. 2008;82:8487–8499. doi: 10.1128/JVI.00851-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alain T, Lun X, Martineau Y, Sean P, Pulendran B, Petroulakis E, et al. Vesicular stomatitis virus oncolysis is potentiated by impairing mTORC1-dependent type I IFN production. Proc Natl Acad Sci USA. 2010;107:1576–1581. doi: 10.1073/pnas.0912344107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schache P, Gürlevik E, Strüver N, Woller N, Malek N, Zender L, et al. VSV virotherapy improves chemotherapy by triggering apoptosis due to proteasomal degradation of Mcl-1. Gene Ther. 2009;16:849–861. doi: 10.1038/gt.2009.39. [DOI] [PubMed] [Google Scholar]
- Leveille S, Samuel S, Goulet ML, Hiscott J. Enhancing VSV oncolytic activity with an improved cytosine deaminase suicide gene strategy. Cancer Gene Ther. 2011;18:435–443. doi: 10.1038/cgt.2011.14. [DOI] [PubMed] [Google Scholar]
- Zhou ZL, Yang YX, Ding J, Li YC, Miao ZH. Triptolide: structural modifications, structure-activity relationships, bioactivities, clinical development and mechanisms. Nat Prod Rep. 2012;29:457–475. doi: 10.1039/c2np00088a. [DOI] [PubMed] [Google Scholar]
- Wu Y, Wang Y, Zhong C, Li Y, Li X, Sun B. The suppressive effect of triptolide on experimental autoimmune uveoretinitis by down-regulating Th1-type response. Int Immunopharmacol. 2003;3:1457–1465. doi: 10.1016/S1567-5769(03)00144-9. [DOI] [PubMed] [Google Scholar]
- Wang Y, Jia L, Wu CY. Triptolide inhibits the differentiation of Th17 cells and suppresses collagen-induced arthritis. Scand J Immunol. 2008;68:383–390. doi: 10.1111/j.1365-3083.2008.02147.x. [DOI] [PubMed] [Google Scholar]
- Wei X, Gong J, Zhu J, Wang P, Li N, Zhu W, et al. The suppressive effect of triptolide on chronic colitis and TNF-alpha/TNFR2 signal pathway in interleukin-10 deficient mice. Clin Immunol. 2008;129:211–218. doi: 10.1016/j.clim.2008.07.018. [DOI] [PubMed] [Google Scholar]
- Kitzen JJ, de Jonge MJ, Lamers CH, Eskens FA, van der Biessen D, van Doorn L, et al. Phase I dose-escalation study of F60008, a novel apoptosis inducer, in patients with advanced solid tumours. Eur J Cancer. 2009;45:1764–1772. doi: 10.1016/j.ejca.2009.01.026. [DOI] [PubMed] [Google Scholar]
- Kiviharju TM, Lecane PS, Sellers RG, Peehl DM. Antiproliferative and proapoptotic activities of triptolide (PG490), a natural product entering clinical trials, on primary cultures of human prostatic epithelial cells. Clin Cancer Res. 2002;8:2666–2674. [PubMed] [Google Scholar]
- Wong KF, Yuan Y, Luk JM. Tripterygium wilfordii bioactive compounds as anticancer and anti-inflammatory agents. Clin Exp Pharmacol Physiol. 2012;39:311–320. doi: 10.1111/j.1440-1681.2011.05586.x. [DOI] [PubMed] [Google Scholar]
- Lipsky PE, Tao XL. A potential new treatment for rheumatoid arthritis: thunder god vine. Semin Arthritis Rheum. 1997;26:713–723. doi: 10.1016/s0049-0172(97)80040-6. [DOI] [PubMed] [Google Scholar]
- Qiu D, Kao PN. Immunosuppressive and anti-inflammatory mechanisms of triptolide, the principal active diterpenoid from the Chinese medicinal herb Tripterygium wilfordii Hook. f. Drugs R D. 2003;4:1–18. doi: 10.2165/00126839-200304010-00001. [DOI] [PubMed] [Google Scholar]
- Chen BJ. Triptolide, a novel immunosuppressive and anti-inflammatory agent purified from a Chinese herb Tripterygium wilfordii Hook F. Leuk Lymphoma. 2001;42:253–265. doi: 10.3109/10428190109064582. [DOI] [PubMed] [Google Scholar]
- Wu Y, Cui J, Bao X, Chan S, Young DO, Liu D, et al. Triptolide attenuates oxidative stress, NF-kappaB activation and multiple cytokine gene expression in murine peritoneal macrophage. Int J Mol Med. 2006;17:141–150. [PubMed] [Google Scholar]
- Premkumar V, Dey M, Dorn R, Raskin I. MyD88-dependent and independent pathways of Toll-Like Receptors are engaged in biological activity of Triptolide in ligand-stimulated macrophages. BMC Chem Biol. 2010;10:3. doi: 10.1186/1472-6769-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Zhou J, He Y, Jia H, Zhao L, Zhao N, et al. Effects of triptolide from Radix Tripterygium wilfordii (Leigongteng) on cartilage cytokines and transcription factor NF-kappaB: a study on induced arthritis in rats. Chin Med. 2009;4:13. doi: 10.1186/1749-8546-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamon LA, Pezzuto JM, Graves JM, Mehta RR, Wangcharoentrakul S, Sangsuwan R, et al. Evaluation of the mutagenic, cytotoxic, and antitumor potential of triptolide, a highly oxygenated diterpene isolated from Tripterygium wilfordii. Cancer Lett. 1997;112:113–117. doi: 10.1016/S0304-3835(96)04554-5. [DOI] [PubMed] [Google Scholar]
- Pan J. RNA polymerase - an important molecular target of triptolide in cancer cells. Cancer Lett. 2010;292:149–152. doi: 10.1016/j.canlet.2009.11.018. [DOI] [PubMed] [Google Scholar]
- Titov DV, Gilman B, He QL, Bhat S, Low WK, Dang Y, et al. XPB, a subunit of TFIIH, is a target of the natural product triptolide. Nat Chem Biol. 2011;7:182–188. doi: 10.1038/nchembio.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips PA, Dudeja V, McCarroll JA, Borja-Cacho D, Dawra RK, Grizzle WE, et al. Triptolide induces pancreatic cancer cell death via inhibition of heat shock protein 70. Cancer Res. 2007;67:9407–9416. doi: 10.1158/0008-5472.CAN-07-1077. [DOI] [PubMed] [Google Scholar]
- Yang S, Chen J, Guo Z, Xu XM, Wang L, Pei XF, et al. Triptolide inhibits the growth and metastasis of solid tumors. Mol Cancer Ther. 2003;2:65–72. [PubMed] [Google Scholar]
- Carter BZ, Mak DH, Shi Y, Fidler JM, Chen R, Ling X, et al. MRx102, a triptolide derivative, has potent antileukemic activity in vitro and in a murine model of AML. Leukemia. 2012;26:443–450. doi: 10.1038/leu.2011.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q. Triptolide and its expanding multiple pharmacological functions. Int Immunopharmacol. 2011;11:377–383. doi: 10.1016/j.intimp.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Carter BZ, Mak DH, Schober WD, McQueen T, Harris D, Estrov Z, et al. Triptolide induces caspase-dependent cell death mediated via the mitochondrial pathway in leukemic cells. Blood. 2006;108:630–637. doi: 10.1182/blood-2005-09-3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Hu H, Qiu P, Yan G. Triptolide induces apoptosis in human anaplastic thyroid carcinoma cells by a p53-independent but NF-kappaB-related mechanism. Oncol Rep. 2009;22:1397–1401. doi: 10.3892/or_00000580. [DOI] [PubMed] [Google Scholar]
- Yinjun L, Jie J, Yungui W. Triptolide inhibits transcription factor NF-kappaB and induces apoptosis of multiple myeloma cells. Leuk Res. 2005;29:99–105. doi: 10.1016/j.leukres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Jiang XH, Wong BC, Lin MC, Zhu GH, Kung HF, Jiang SH, et al. Functional p53 is required for triptolide-induced apoptosis and AP-1 and nuclear factor-kappaB activation in gastric cancer cells. Oncogene. 2001;20:8009–8018. doi: 10.1038/sj.onc.1204981. [DOI] [PubMed] [Google Scholar]
- Chen Q, Lu Z, Jin Y, Wu Y, Pan J. Triptolide inhibits Jak2 transcription and induces apoptosis in human myeloproliferative disorder cells bearing Jak2V617F through caspase-3-mediated cleavage of Mcl-1. Cancer Lett. 2010;291:246–255. doi: 10.1016/j.canlet.2009.10.019. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lu JJ, He L, Yu Q. Triptolide (TPL) inhibits global transcription by inducing proteasome-dependent degradation of RNA polymerase II (Pol II). PLoS ONE. 2011;6:e23993. doi: 10.1371/journal.pone.0023993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noser JA, Mael AA, Sakuma R, Ohmine S, Marcato P, Lee PW, et al. The RAS/Raf1/MEK/ERK signaling pathway facilitates VSV-mediated oncolysis: implication for the defective interferon response in cancer cells. Mol Ther. 2007;15:1531–1536. doi: 10.1038/sj.mt.6300193. [DOI] [PubMed] [Google Scholar]
- Bensaude O. Inhibiting eukaryotic transcription: Which compound to choose? How to evaluate its activity. Transcription. 2011;2:103–108. doi: 10.4161/trns.2.3.16172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel S, Tumilasci VF, Oliere S, Nguyên TL, Shamy A, Bell J, et al. VSV oncolysis in combination with the BCL-2 inhibitor obatoclax overcomes apoptosis resistance in chronic lymphocytic leukemia. Mol Ther. 2010;18:2094–2103. doi: 10.1038/mt.2010.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanton E, Savory P, Cosulich S, Clarke P, Woodman P. Bcl-2 regulates a caspase-3/caspase-2 apoptotic cascade in cytosolic extracts. Oncogene. 1999;18:1781–1787. doi: 10.1038/sj.onc.1202490. [DOI] [PubMed] [Google Scholar]
- Carey BL, Ahmed M, Puckett S, Lyles DS. Early steps of the virus replication cycle are inhibited in prostate cancer cells resistant to oncolytic vesicular stomatitis virus. J Virol. 2008;82:12104–12115. doi: 10.1128/JVI.01508-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed M, Cramer SD, Lyles DS. Sensitivity of prostate tumors to wild type and M protein mutant vesicular stomatitis viruses. Virology. 2004;330:34–49. doi: 10.1016/j.virol.2004.08.039. [DOI] [PubMed] [Google Scholar]
- Le Boeuf F, Diallo JS, McCart JA, Thorne S, Falls T, Stanford M, et al. Synergistic interaction between oncolytic viruses augments tumor killing. Mol Ther. 2010;18:888–895. doi: 10.1038/mt.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestwich RJ, Errington F, Diaz RM, Pandha HS, Harrington KJ, Melcher AA, et al. The case of oncolytic viruses versus the immune system: waiting on the judgment of Solomon. Hum Gene Ther. 2009;20:1119–1132. doi: 10.1089/hum.2009.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon MJ, Lee HJ, Kim JH, Kim DK. Extracellular ATP induces apoptotic signaling in human monocyte leukemic cells, HL-60 and F-36P. Arch Pharm Res. 2006;29:1032–1041. doi: 10.1007/BF02969288. [DOI] [PubMed] [Google Scholar]
- Deli T, Csernoch L. Extracellular ATP and cancer: an overview with special reference to P2 purinergic receptors. Pathol Oncol Res. 2008;14:219–231. doi: 10.1007/s12253-008-9071-7. [DOI] [PubMed] [Google Scholar]
- Zhou H, Zhang Y, Fu Y, Chan L, Lee AS. Novel mechanism of anti-apoptotic function of 78-kDa glucose-regulated protein (GRP78): endocrine resistance factor in breast cancer, through release of B-cell lymphoma 2 (BCL-2) from BCL-2-interacting killer (BIK). J Biol Chem. 2011;286:25687–25696. doi: 10.1074/jbc.M110.212944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Yang L, Sun Q, Arguello M, Ballard DW, Hiscott J, et al. The NEMO adaptor bridges the nuclear factor-kappaB and interferon regulatory factor signaling pathways. Nat Immunol. 2007;8:592–600. doi: 10.1038/ni1465. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Kinetics of TPL-mediated VSV replication and apoptosis in PC3 cells.
VSV plus TPL combination treatment reduces tumor progression in PC3 mouse xenograft model and prolongs survival.
Primer sequences for real-time RT-PCR.