Abstract
Germline alterations of the tumour suppressor TP53 gene are detected approximately in 25% of the families suggestive of Li-Fraumeni syndrome (LFS), characterised by a genetic predisposition to a wide tumour spectrum, including soft-tissue sarcomas, osteosarcomas, premenopausal breast cancers, brain tumours, adrenocortical tumours, plexus choroid tumours, leukaemia and lung cancer. The aim of this study was to determine the contribution of germline copy number variations (CNVs) to LFS in families without detectable TP53 mutation. Using a custom-designed high-resolution array CGH, we evaluated the presence of rare germline CNVs in 64 patients fulfilling the Chompret criteria for LFS, but without any detectable TP53 alteration. In 15 unrelated patients, we detected 20 new CNVs absent in 600 controls. Remarkably, in four patients who had developed each brain tumour, the detected CNV overlap the KDM1A, MTA3, TRRAP or SIRT3 genes encoding p53 partners involved in histone methylation or acetylation. Focused analysis of SIRT3 showed that the CNV encompassing SIRT3 leads to SIRT3 overexpression, and that in vitro SIRT3 overexpression prevents apoptosis, increases G2/M and results in a hypermethylation of numerous genes. This study supports the causal role of germline alterations of genes involved in chromatin remodelling in genetic predisposition to cancer and, in particular, to brain tumours.
Keywords: Li-Fraumeni syndrome, TP53, copy number variation, SIRT3, chromatin remodelling
INTRODUCTION
The Li-Fraumeni syndrome (LFS, OMIM no. 151623) is a Mendelian predisposition to cancer affecting children and adults, and characterised by a wide tumour spectrum.1, 2, 3, 4 The heterogeneity of the clinical presentation has led to the elaboration of the Chompret criteria in order to facilitate the clinical recognition of the syndrome.5, 6, 7 These criteria correspond to (i) a proband with a tumour belonging to the narrow LFS tumour spectrum (eg., soft tissue sarcoma, osteosarcoma, brain tumour, premenopausal breast cancer, adrenocortical carcinoma, leukaemia and lung bronchoalveolar cancer) diagnosed before age 46 years, and at least one first- or second-degree relative with an LFS tumour (except breast cancer, if proband is affected by breast cancer) before age 56 years or with multiple tumours; or (ii) a proband with multiple tumours, two of which belong to the narrow LFS tumour spectrum (except multiple primary breast cancers) and the first of which occurred before age 46 years; or (iii) a patient with adrenocortical carcinoma or a choroid plexus carcinoma, irrespective of family history.
Despite a transient controversy, which had suggested that LFS could result from other genes such as CHEK2 encoding a kinase able to phosphorylate p53 in response to DNA damage, the only gene that has been involved in the LFS is the tumour suppressor TP53 gene.4 In families fulfilling the Chompret criteria, the TP53 mutation detection rate can be estimated to 25%6 and the sensitivity and specificity of these criteria have been estimated to 82% and 58%, respectively.6, 7, 8 In the context of molecular diagnostic of LFS, our laboratory has performed in >1000 index cases of French families suggestive of LFS, mostly fulfilling the Chompret criteria, extensive analysis of the TP53 gene. This allowed us to identify a deleterious mutation in 157 unrelated families. Although some of the families fulfilling the Chompret criteria without detectable TP53 alteration can correspond to non-Mendelian aggregations of cancer, the early age of tumour onset and familial presentation strongly suggest in some families a genetic predisposition to cancer.
Copy number variations (CNVs) have been initially defined as DNA segments >1 kb, with a CNV compared with a reference genome.9, 10 Although CNVs represent one of the main forms of DNA polymorphism whose extent has been revealed by the development of array CGH, deleterious CNVs corresponding to rare CNVs present only in patients have been described in a wide range of genic and genomic diseases,11, 12 including cancer predisposition.13 The commonly used guidelines for clinical interpretation of CNVs, which were proposed by Lee et al,14 include the overlap of the CNV with a genomic imbalance listed in a database of healthy or affected individuals. The Database of Genomic Variants (DGV, http://www.projects.tcag.ca/variation/)15 is the reference database that collects and provides a genomic map of polymorphic CNVs generated from worldwide peer-reviewed studies.
The aim of this study was to determine the contribution of germline CNVs to LFS in families without detectable TP53 mutation. We identified in patients suggestive of LFS but without detectable TP53 mutation, non-polymorphic CNVs, and in particular CNVs affecting p53 partners involved in chromatin remodelling and, remarkably, these CNVs were detected in patients with brain tumours.
SUBJECTS AND METHODS
Subjects
In this study, 64 affected unrelated patients (25 men, 39 women, median age of tumour onset: 17 years) from Caucasian origin and fulfilling the Chompret criteria7 were investigated. Genomic DNA was extracted from peripheral blood lymphocytes using FlexiGene DNA kit (Qiagen, Hilden, Germany). For all patients, complete screening of TP53, based on sequencing analysis of the 11 exons, and quantitative multiplex PCR of short fluorescent fragments (QMPSF) analysis of the exons and promoter region6, 16 has detected no germline alteration. To estimate the allelic frequency of detected CNVs in the general population, DNA samples extracted from peripheral blood lymphocytes were collected from 600 Caucasian control subjects without cancer. In each case, informed consent for genetic analysis was obtained.
Custom-designed 180K oligonucleotide array CGH
We designed a custom high-resolution 180K array CGH (Agilent Technologies, Santa Clara, CA, USA), including 224 probes covering the TP53 locus, 4000 probes covering 400 kb of the TP53-surrounding regions, 24 000 probes covering 24 genes involved in the p53 pathway or Mendelian predisposition to cancer (MDM2, PTEN, CHEK2, MRE11A, RAD50, NBN, ATM, ATR, TERT, AKT, CDKN2A, CUL9, SIRT1, ALK, TP73, TP63, BRCA2, RAD51, BRCA1, BUB1, NPM1, USP7, MLH1, MSH2), 6 microRNAs of the p53 pathway (MIR34A, MIR35B, MIR125B1, MIR192, MIR145, MIR29A) and 1 50 000 probes, ensuring a coverage of the entire genome with an average probe spacing of 15 kb. The Agilent design file of the array CGH (design ID 023458) is available upon request. A non-commercial genomic DNA pool of 10 control individuals was used as a reference sample. Labelling of 700 ng genomic DNA was performed using the Enzo CGH labeling kit for oligo arrays (Enzo Life Sciences, Farmingdale, NY, USA), according to the manufacturer's instructions. Labelled DNA was purified using the Macherey-Nagel PCR clean-up Nucleospin Extract II (Macherey-Nagel, Düren, Germany). Hybridised slides were scanned at 3 μm resolution with a DNA microarray scanner (Agilent Technologies), and images were analysed using Feature Extraction software (version 10.5, Agilent Technologies). The data were graphed and analysed using Genomic Workbench software (version 6.5, Agilent Technologies). Only excellent quality data (DLRSpread<0.20) were processed using the ADM-2 algorithm, with the sensitivity threshold at 5.3. A CNV was defined as any deviation of fluorescence log2 ratio (patient/pooled controls) >0.4 for three consecutive probes. CNVs overlapping genes or microRNAs and not described as polymorphic in the DGV, http://www.projects.tcag.ca/variation/ (updated in April 2012) were selected for subsequent analyses.
QMPSF and reverse transcription-QMPSF
For all the detected CNVs, we designed specific QMPSFs, as previously described,17 in order to confirm the CNV, to estimate the frequency in the general population, and to perform, when possible, segregation analysis. Briefly, short genomic fragments of the genes encompassed by the detected CNVs were simultaneously amplified from genomic DNA, using 6-FAM-labelled primer pairs (sequences and PCR conditions are available upon request). To assess the impact of the CNV on gene expression, we developed, according to the same strategy, a QMPSF assay performed on RT, as previously described.18 Total RNA was extracted from peripheral blood samples, using the PAXgene Blood RNA kit (Qiagen). Reverse transcription-QMPSF (RT-QMPSF) was performed on 100 ng of RNA, using SF3A1 and TOP1 amplicons as controls.
Cell culture
The 8MG cell line, derived from a human glioblastoma19 and expressing SIRT3 (as checked by flow cytometry), was generously provided by Professor J Honnorat (Inserm, Neurooncology and Neuroinflammation Department, Laennec Hospital, Lyon, France). The 8MG cells were cultured in DMEM (+) 4.5 g/l glucose (+) L-glutamine (+) pyruvate (Gibco-Invitrogen, Carlsbad, CA, USA) with 10% foetal bovine serum (FBS, PAA Laboratories Inc., Etobicoke, Canada). The H29/293-GPG packaging cell line20 was cultured with DMEM supplemented with 10% FBS (Hyclone Laboratories, Logan, UT, USA), 0.3 mg/ml G418, 2 μg/ml puromycin and 1 μg/ml tetracycline (Sigma-Aldrich, St Louis, MO, USA). Cells were cultured at 37 °C in humidified 5% CO2 incubator.
Generation of SIRT3-overexpressing cells
SIRT3 or GFP cDNAs were cloned downstream of an internal ribosome entry site (IRES) in a γ-retrovirus-derived SFG dicistronic vector, containing upstream of the IRES element the puromycin-N-acetyltransferase open reading frame.21 All constructs were verified by DNA sequencing. H29/293-GPG packaging cells were transfected with each vector using calcium chloride precipitation method, and cell lines were infected with cell-free retroviral supernatant in the presence of 8 μg/ml of polybrene (Sigma-Aldrich) for 16 h. Puromycin (Sigma-Aldrich) was then added at 5–20 μg/ml to the medium for 1 week to select the cells expressing the vector-encoded puromycin-N-acetyltransferase.21 Overexpression of SIRT3 in transduced cells was checked by RT-QMPSF and western blot analysis, using the HA026809 anti-SIRT3 antibody from Sigma-Aldrich.
Downregulation of SIRT3 expression
The 8MG cell line was transfected with Mission esiRNA (600 ng) targeting human SIRT3 mRNA (EHU093591, Sigma-Aldrich), using oligofectamine (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's protocols. Universal Negative Control no. 1 (SIC001, Sigma-Aldrich) was used as control. Flow cytometry experiments were performed 72 h after transfection.
Cell cycle and apoptosis analyses
Cell cycle analysis of cells at 80% of confluence was performed by DNA staining with propidium iodide and flow cytometry. Briefly, after treatment, cells were harvested in PBS, and fixed in ice-cold 70% ethanol for 2 h. Staining was done with 0.6 mg/ml RNase (30 min, RT, Sigma-Aldrich) and 50 μg/ml propidium iodide (30 min, RT, Sigma-Aldrich). Cell cycle phases were quantified by FacsCalibur flow cytometer (BD Biosciences, Woburn, MA, USA), using CellQuest analysis software (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA). For apoptosis analysis, cells were labelled using TetraMethyl Rhodamine Methyl ester (TMRM). Briefly, cells were harvested, resuspended in a buffer containing 10 mM HEPES, 135 mM NaCl, 5 mM CaCl2 and incubated 15 min in a fresh TMRM solution (200 nM, 37 °C into the dark, Molecular Probes-Invitrogen). Apoptosis was quantified using flow cytometer and CellQuest analysis software (Becton Dickinson Immunocytometry Systems).
Methylation analysis
Genomic DNA (500 ng) from GFP or SIRT3-overexpressing 8MG cells were subjected to bisulphite modification using the EZ DNA Methylation-Gold Kit (Zymo Research, Irvine, CA, USA). Quality of modification was then checked by pyrosequencing (PSQTM 96MA, Qiagen), as previously described.22 Methylation profiles of treated and untreated samples were analysed using the 450K Infinium methylation bead arrays (Illumina, San Diego, CA, USA), following the recommended protocols for amplification, labelling, hybridisation and scanning. Each methylation analysis was performed in duplicate. GenomeStudio Methylation Module software (version 2010.3, Illumina) was used to obtain raw data and display beta values. Differential methylation comparing GFP with SIRT3-overexpressing cells were obtained using Illumina custom algorithm. We then filtered probes with a differential score (DiffScore) >200 and Delta Beta >0.14, in order to retain only probes exhibiting a significant difference of methylation of at least 14% between GFP and SIRT3-overexpressing cells (P-value<0.001). Using Infinium annotation data, Infinium sites (cytosines) were classified according to their relation to CpG islands and to the closest annotated gene. Sites unrelated to any annotated gene were classified as intergenic.
RESULTS
Detection of CNVs affecting the TP53 locus
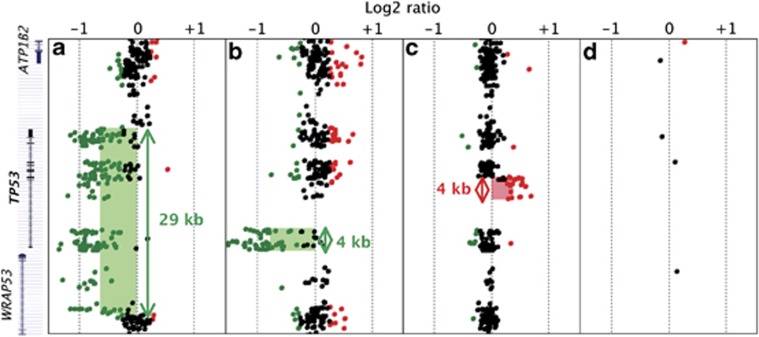
In order to validate our custom-designed 180K oligonucleotide array CGH dedicated to LFS, we first analysed a series of six DNA samples from LFS patients, in whom QMPSF analysis had previously revealed different types of CNVs, encompassing the TP53 locus. As illustrated by Figure 1, the TP53 CNVs, which could not be detected by a catalogue 180K array CGH, were successfully detected by our custom-designed array CGH enriched in probes at the TP53 locus. In particular, this array allowed us to confirm the first case of a 4-kb TP53 partial duplication, involving exons 2–4 (Figure 1c). When we analysed the 64 patients without detectable TP53 mutation or genomic rearrangement, we detected no additional CNV affecting either the TP53 locus or surrounding regions.
Figure 1.
Detection in LFS patients of CNVs targeting the TP53 locus, using a custom-designed 180K array CGH. Detection in three LFS patients, using custom-designed 180K array CGH of a complete deletion (a), a partial deletion removing the promoter region and exon 1 (b) and a duplication of exons 2–4 (c). (d) Probe coverage of the TP53 locus in the human catalogue 180K array CGH. CNVs detected by the ADM-2 algorithm are indicated by shaded area (green for deletion; red for duplication). Sizes of rearrangements are represented by double-headed arrows. Mapping of the corresponding genomic regions with the name of the genes (ATP1B2, TP53, WRAP53) are indicated on left.
Detection of new CNVs in LFS patients
Among the 64 patients without any detectable TP53 alteration, we identified in 17 unrelated patients (26%) 21 new CNVs, not previously described in the DGV, http://projects.tcag.ca/variation/ (Table 1). Only one CNV was detected in two families; thanks to the probe-enriched part of the array CGH. This CNV corresponded to a small deletion (4 kb), encompassing the microRNAs 194-1/215 cluster, a known p53 target.23 This deletion was confirmed by a QMPSF-based targeted analysis, and sequencing analysis of the rearranged fragment revealed that the CNV boundaries were identical in the two families (data not shown). However, QMPSF screening of genomic DNA from 600 controls revealed that this CNV had in fact an allelic frequency of 2% in the general population, and therefore corresponds to a polymorphic CNV. In contrast, the 20 other CNVs, detected in the 15 other unrelated patients (Supplementary Table S1) and also confirmed by QMPSF, were not found in 600 controls, indicating that these CNVs are non-polymorphic. These CNVs corresponded to eight heterozygous deletions and 12 duplications with a size extending from 82–642 kb, and involved a total of 49 genes or microRNAs (Table 1).
Table 1. Detection in 17 patients suggestive of LFS of 21 germline CNVs not recorded in the DGV.
| Cytoband | CNV coordinates (hg18)a | CNV type | Protein-coding genes and microRNAs involvedb | CNV size (kb) | Patient ID | |
|---|---|---|---|---|---|---|
| 1 | p36.12 | chr1:23244421–23562102 | del | KDM1A (part), LUZP1, HTR1D, HNRNPR, ZNF436 (part) | 318 | 53 |
| 1 | q41 | chr1:218356562–218359749 | del | IARS2 (part), MIR215, MIR194-1 | 4 | 16, 57 |
| 2 | p21 | chr2:42591803–42763199 | dup | MTA3 (part) | 172 | 33 |
| 2 | p15 | chr2:61978338–62094667 | del | COMMD1 (part) | 116 | 14 |
| 3 | p14.1 | chr3:65642102–65889226 | del | MAGI1 (part) | 248 | 53 |
| 3 | q13.13 | chr3:110094154–110495138 | dup | GUCA1C, MORC1, DPPA2 (part) | 401 | 53 |
| 3 | q29 | chr3:198768821–198969885 | dup | BDH1 (part), KIAA0226, MIR922, FYTTD1 (part) | 201 | 6 |
| 4 | q32.2 | chr4:162313725–162948651 | dup | FSTL5 (part) | 635 | 17 |
| 5 | p13.3 | chr5:34024123–34126697 | del | AMACR, C1QTNF3 | 103 | 2 |
| 5 | q13.2 | chr5:68405282–68519258 | del | SLC30A5, CCNB1 | 114 | 38 |
| 5 | q14.1 | chr5:79525024–79740886 | dup | SERINC5 (part), SPZ1, ZFYVE16 (part) | 216 | 38 |
| 6 | p21.31 | chr6:34977196–35058894 | del | ANKS1A (part) | 82 | 60 |
| 6 | p12.3 | chr6:47509689–47664601 | del | CD2AP (part) | 155 | 27 |
| 6 | q14.1 | chr6:80910016–81553302 | dup | BCKDHB (part) | 642 | 5 |
| 7 | q22.1 | chr7:98286465–98454581 | dup | TMEM130 (part), TRRAP | 169 | 63 |
| 9 | q22.32 | chr9:96591859–96705414 | del | C9orf3 (part) | 114 | 55 |
| 11 | p15.5 | chr11:200300–332720 c | dup | RIC8A, SIRT3, PSMD13, NLRP6, ATHL1, IFITM5, IFITM2, IFITM1, IFITM3 | 133 | 17 |
| 11 | p13 | chr11:32989228–33445279 | dup | DEPDC7, TCP11L1, CSTF3, HIPK3 | 456 | 24 |
| 11 | q13.2 | chr11:68358122–68452839 | dup | CPT1A (part), MRPL21, IGHMBP2 (part) | 95 | 33 |
| 21 | q22.3 | chr21:42945519–43196797 | dup | PDE9A, WDR4, NDUFV3 (part) | 252 | 54 |
| X | q13.1 | chrX:71771657–71876745 | dup | PHKA1 (part) | 105 | 19 |
Abbreviations: CNV, copy number variation; DGV, Database of Genomic Variants; del, deletion; dup, duplication; LFS, Li-Fraumeni syndrome; part, partial rearrangement of the gene.
Defined according to the genomic positions of the first oligonucleotides of the first and last deviated probes, respectively.
Genes in bold are involved in chromatin remodelling.
On chromosome 11, the first telomeric probe starts on genomic position 2 00 300, consequently no information before this position is available.
CNVs target genes involved in chromatin remodelling
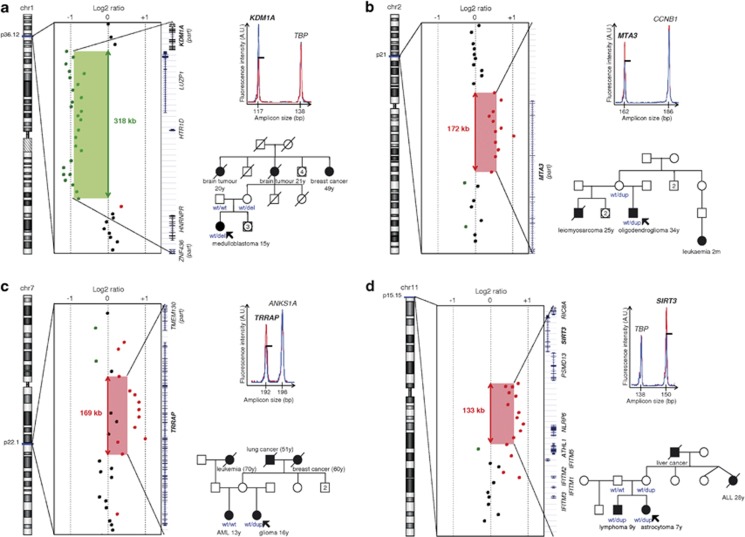
Gene ontology analysis of the 49 genes and microRNAs covered by the detected CNVs was performed using the functional annotation tool of the Database for Annotation, Visualization and Integrated Discovery (DAVID, http://www.david.abcc.ncifcrf.gov/).24, 25 This analysis revealed a significant enrichment (P-value=0.0093) in genes (KDM1A, MTA3, TRRAP and SIRT3) encoding p53 partners involved in chromatin packaging and remodelling (Panther Biological Pathway BP00273), each gene being subjected to one CNV in a single patient. As shown in Figure 2a, heterozygous 318 kb deletion removing exons 3–19 of the KDM1A/LSD1 (lysine (K)-specific demethylase 1A) gene, encoding a histone demethylase, was found in a proband who had developed a medulloblastoma at 15 years of age. A 172-kb duplication encompassing the promoter region and exons 1–9 of the metastasis-associated 1 family, member 3 (MTA3) gene, composed of 14 exons and encoding a deacetylase belonging to the nucleosome remodelling and deacetylase (NuRD) complex, was detected in a proband who developed an oligodendroglioma at 34 years of age. A 169-kb duplication affecting entirely the transformation/transcription domain-associated protein (TRAPP) gene encoding a histone acetyltransferase (HAT) was identified in a proband who developed a high-grade glioma at 16 years of age. The last CNV, detected in a patient who developed an astrocytoma at 7 years of age, was a 133-kb duplication, affecting entirely the SIRT3 gene encoding the Sirtuin 3, a member of the Sirtuin family, known to deacetylate both histone and non-histone proteins including p5326.
Figure 2.
Germline CNVs encompassing the KDM1A, MTA3, TRRAP or SIRT3 genes involved in chromatin remodelling and detected in four unrelated patients suggestive of LFS. Each panel (a–d) presents the detection of CNV by array CGH; the validation of the detected CNV by QMPSF (fluorescence profile obtained from the patient (red) was superimposed to that obtained from a control (blue) and adjusted using the height of a control amplicon); the partial pedigree of the family in whom the CNV was detected (analysed genotypes are indicated). (a) A 318-kb deletion partially removing the KDM1A gene (exons 3–19) in patient ID 53. (b) A 172-kb duplication of the MTA3 gene (promoter and exons 1–9) in patient ID 33. (c) A 169-kb duplication covering the TRRAP gene in patient ID 63. (d) A 133-kb duplication including the SIRT3 gene in patient ID 17.
SIRT3 duplication induces SIRT3 overexpression
The established importance of sirtuins in cell survival and p53 regulation led us then to focus our work on the SIRT3 duplication detected in a LFS patient without any detectable TP53 alteration. Within this family, this SIRT3 duplication was also found in the patient's brother who developed a lymphoma at 9 years of age and in the mother whose sister had developed an acute lymphoblastic leukaemia at the age of 28 years (Figure 2d). In order to assess the impact of this CNV on SIRT3 expression, we performed a semiquantitative measurement of SIRT3 mRNA extracted from peripheral blood of the different relatives. The three SIRT3 duplication carrier relatives exhibited a 1.92-fold significant increase of SIRT3 mRNA expression, as compared with a non-carrier relative (the father) and to 25 additional controls (Mann-Whitney test, P-value=0.0059; Supplementary Figure S1).
SIRT3 overexpression alters cell cycle and prevents apoptosis
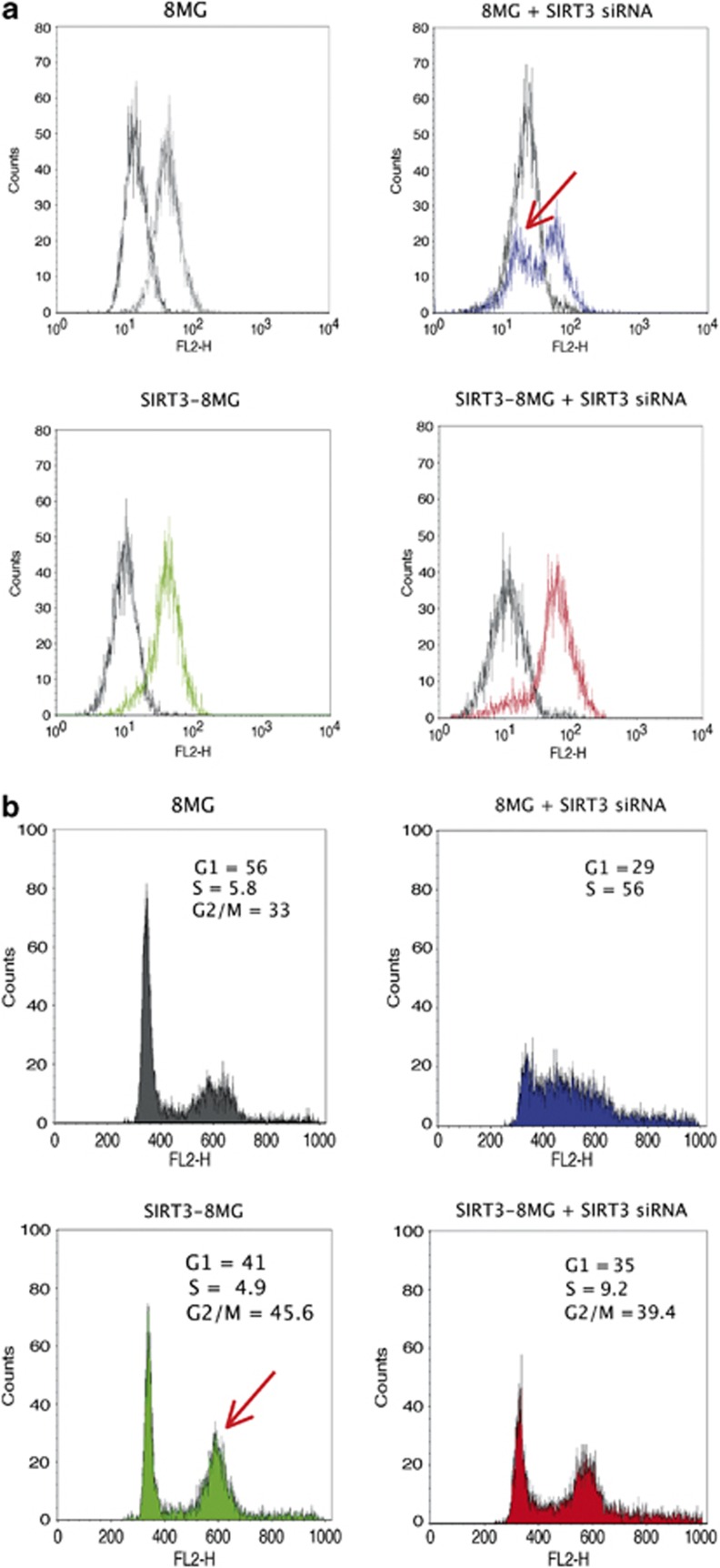
Considering the involvement of SIRT3 in apoptosis,26 we evaluated the impact of SIRT3 deregulation on apoptosis in the 8MG glioma cell line. Knock-down regulation of SIRT3, using siRNA, induced a decrease of the mitochondrial membrane potential, as measured by the TMRM labelling assay, reflecting induction of apoptosis (Figure 3a). In contrast, cells transfected by scramble siRNA or overexpressing SIRT3 exhibited no apoptotic features.
Figure 3.
SIRT3 overexpression prevents apoptosis and increases G2/M phase in the 8MG glioma-derived cell line. (a) Apoptosis analysis using TMRM analysis. (b) Cell cycle analysis by flow cytometer. The arrows indicate the increase of apoptotic cell number and of G2/M phase, resulting from SIRT3 knockdown or overexpression, respectively. Abbreviations: 8MG, parental 8MG cell line; SIRT3-8MG, SIRT3-overexpressing 8MG cells; 8MG+SIRT3 siRNA, parental 8MG cell line transfected with siRNA-targeting SIRT3; SIRT3-8MG+SIRT3 siRNA, SIRT3-overexpressing 8MG cells transfected with siRNA-targeting SIRT3.
We then analysed the impact of SIRT3 deregulation on cell cycle, using flow cytometry. As shown on Figure 3b, SIRT3 overexpression led to an increase of the cell proportion in G2/M phase. Knockdown of SIRT3 in the parental 8MG cell line resulted in S phase accumulation, but this effect was not observed in cells transduced with SIRT3 (Figure 3b).
SIRT3 overexpression alters methylation
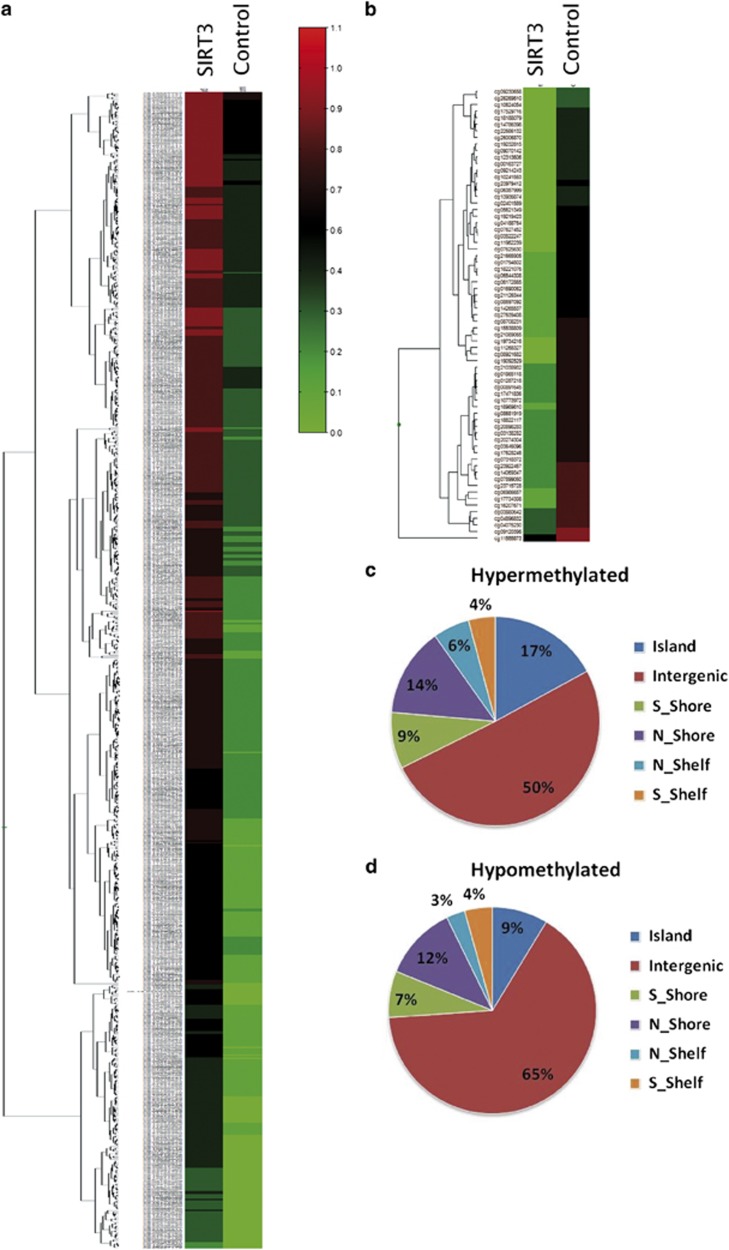
Considering the role of the sirtuin family in histone deacetylation and epigenetic gene silencing, the ability of SIRT3 to deacetylate H4K16 and to repress transcription27 and the demonstration that activating mutations of mitochondrial enzymes, such as IDH2, are associated with methylation alteration,28 we evaluated the impact of SIRT3 overexpression on methylation, using the 8MG glioma-derived cell line. As indicated in Figure 4, we found that 814 probes, corresponding to 315 genes, exhibited a significant different methylation in SIRT3-overexpressing cells. The majority of the probes (n=745; 92%) were hypermethylated in SIRT3-overexpressing cells (Figures 4a and b). Analysis of the distribution of the differentially methylated sites showed that the majority of hypermethylated sites were localised in CpG islands (Figure 4c), whereas a high proportion of hypomethylated probes were not associated with genes (Figure 4d).
Figure 4.
SIRT3 overexpression induces hypermethylation in the 8MG glioma-derived cell line. Methylation profiles of bisulphite-treated and -untreated samples from SIRT3-overexpressing cells (SIRT3) and GFP-overexpressing cells (Control) were analysed using the 450K Infinium methylation bead arrays (see Subjects and methods). The figure presents the heatmap of significant probes using beta values; the scale indicates the level of methylation, from 0 (no methylation, green) to 1 (100% methylation, red). (a) CpG sites hypermethylated in SIRT3-overexpressing cells. (b) CpG sites hypomethylated in SIRT3-overexpressing cells. (c) Distribution of CpG sites hypermethylated in SIRT3-overexpressing cells. (d) Distribution of CpG sites hypomethylated in SIRT3-overexpressing cells.
DISCUSSION
In this study, we evaluated the contribution of germline non-polymorphic CNVs in families suggestive of LFS without detectable TP53 alteration, using a custom-designed 180K array CGH. This CGH analysis of 64 LFS patients did not reveal additional alterations within the TP53 locus, neither in the 400-kb genomic region surrounding the TP53 locus. This validates the efficiency of the QMPSF assay for the routine detection of TP53 genomic rearrangements and shows that rearrangements of the TP53 surrounding regulatory regions do not constitute a major cause of LFS. We did not find any genomic alteration within the candidate genes, selected on the basis of their role in the p53 pathway or in Mendelian predisposition to cancer, despite the high resolution of the analysis, suggesting that CNVs affecting these genes are not involved in LFS.
The main result of this study is the detection of 20 new private CNVs detected in 15 patients. Like in other studies reporting germline CNVs exclusively found in a genetic disease, the challenge is the interpretation. Unfortunately, the size of the LFS families included in this study and the difficulty to obtain samples from relatives hampered a systematic segregation analysis. Therefore, we cannot claim that all the 20 non-polymorphic CNVs are deleterious and contribute to genetic predisposition to cancer, and it is possible that some of them correspond to private non-pathogenic CNVs. We also cannot exclude, as suggested by the identification in several patients of more than one non polymorphic CNV, that at least some CNVs are involved in an oligogenic determinism to cancer. Nevertheless, the identification in four patients with brain tumour of four CNVs, affecting four genes coding p53 partners involved in transcriptional regulation and chromatin remodelling, strongly suggests that these CNVs are pathogenic and contribute to the genetic determinism of cancers observed within the corresponding families:
(1) The KDM1A/LSD1 gene encodes the histone H3K4/K9 lysine-specific demethylase 1A, which participates to different corepressor complexes, such as the NuRD complex.29 LSD1 has been shown to interact in vitro with p53 and to repress p53-mediated transcriptional activation.30 In fact, the different LSD1 complexes target numerous genes involved in signalling pathways, such as TGFβ, MAPK and cell cycle, and LSD1 inhibits cancer cell invasion in vitro.29 Therefore, the partial deletion of KDM1A/LSD1, which we detected in a patient with medulloblastoma, might contribute to oncogenesis. As indicated in Table 1, this patient also harboured a duplication involving the MORC1 gene, whose homologue in C. elegans has recently been shown to have a key role in heterochromatin condensation and gene silencing.31
(2) The MTA3 gene, which we found partially duplicated in another patient with an oligodendroglioma at 34 years of age, encodes a deacetylase participating, like LSD1, to the NuRD complex. MTA3 was initially characterised as an estrogen-dependent component of the NuRD complex in breast epithelial cells.32 Interestingly, both MTA3 paralogues, MTA1 and MTA2, components of the NuRD complex, interact directly with p53, and MTA2 has been shown to deacetylate p53 and to repress p53-dependent transcriptional activation.33 The biological consequences of the MTA3 duplication that we observed is difficult to predict, as the duplication encompassed the promoter region and exons 1–9, but not the 3' exons and different MTA3 mRNA have been described. Therefore, this partial duplication could result either in loss or gain of function.
(3) The TRAPP gene, also duplicated in another patient with brain tumour, encodes a subunit of many HAT complexes. Interestingly, p53 mediates the recruitment of TRRAP/HAT complexes at the MDM2 promoter.34 Remarkably, a kinome-wide RNA interference screen revealed that TRRAP knockdown increases differentiation of brain tumour-initiating cells derived from patients with glioblastoma, sensitises cells to apoptotic stimuli and inhibits cycle progression.35 Exome sequencing analysis of melanoma revealed a recurrent somatic missense TRRAP mutation, which has been shown to be oncogenic in vitro, providing an additional argument indicating that TRRAP functions as an oncogene.36 Therefore, the gain of copy number of the TRAPP gene detected in our patient could also have an oncogenic effect.
(4) The SIRT3 gene found duplicated and overexpressed in a family with brain tumour encodes a mitochondrial deacetylase controlling reactive oxygen species levels.37 SIRT3 belongs to the sirtuin family (SIRT1-7), acting as molecular sensors of cellular energy balance and involved in the regulation of metabolism, stress responses, DNA repair, genomic stability and aging.38 SIRT3 is able to deacetylate H4K16 and to repress transcription,27 and a recent report demonstrated that this transcriptional repressor is rapidly degraded in response to DNA damage stress to derepress nuclear genes.39 If SIRT3 has been considered as a tumour suppressor gene,40 several arguments, as recently highlighted, support the role of SIRT3 as a tumour promoter.26 Indeed, SIRT3 overexpression has been reported in breast and oral squamous cell carcinoma.41 Remarkably, SIRT3 has been shown to rescue cancer cells from p53-mediated growth arrest and, like SIRT1, to deacetylate p53 in vitro.42 Therefore, SIRT3 could enhance tumourigenesis, at least in certain cells, by promoting survival signals and suppressing apoptotic signals. These data could support the oncogenic role of SIRT3 gene dosage and expression increase, which we report in a patient with brain tumour. In this study, first, we confirmed that patients with SIRT3 duplication constitutively overexpress SIRT3 mRNA, as compared with controls; second, we found that in vitro overexpression of SIRT3 in a glioma cell line protects cells from apoptosis and drastically modifies cell cycle progression with an increase of G2/M, suggesting that SIRT3 overexpression favours cell survival (Figure 3); third, we found that SIRT3 overexpression results in a drastic modification of the methylation profile in a glioma-derived cell line, with a hypermethylation of numerous genes at CpG islands (Figure 4).
Two major articles have recently highlighted in glioma the oncogenic role of somatic mutations, altering directly or indirectly chromatin remodelling. In paediatric glioblastoma multiforme, somatic recurrent mutations in the H3F3A gene encoding the replication-independent histone 3 variant H3.3 have been frequently detected, and these mutations are predicted to directly disturb the methylation of H3.3 K27(ref. 43). Recurrent somatic IDH1 mutations, detected in up to 90% of low-grade glioma with a CpG island mutator phenotype44, 45 and resulting into high amounts of the oncometabolite 2-hydroxyglutarate, have been shown to remodel the global methylome and epigenome pattern, which will finally lead to aberrant cell differentiation and proliferation.45
In conclusion, we think that the CNVs that we report here are likely to be pathogenic, considering their absence in the control population, the role of the targeted genes in chromatin remodelling and their link with p53, and the potential effect of gene dosage alteration predicted according to the previously published studies and shown by our biological analyses concerning SIRT3. Therefore, this study yields new arguments supporting the oncogenic role of histone alterations in cancer, and shows that a fraction of families suggestive of genetic predisposition to cancer might be explained by rare/private deleterious CNV. Further studies will be required to determine whether or not the germline alterations of the genes targeted by these CNVs are sufficient to induce a high tumour risk, or whether they are involved in an oligogenic determinism to cancer.
Acknowledgments
We thank Florence Le-Calvez Kelm and Geoffroy Durand, from IARC's Genetic Cancer Susceptibility Group, for their technical assistance with the Illumina bead array assay. This work was supported by the French National Cancer Institute (INCa), the Cancéropôle Nord-Ouest (CNO), and l′Association pour la Recherche sur le Cancer (ARC), France. Juliette Aury-Landas was supported by a fellowship from the Région Haute-Normandie. The work of the IARC Epigenetics Group is supported by grants from l′Association pour la Recherche sur le Cancer (ARC), France; the French National Cancer Institute (INCa), la Ligue Nationale Contre le Cancer, France; and European Union FP7.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on European Journal of Human Genetics website (http://www.nature.com/ejhg)
Supplementary Material
References
- Li FP, Fraumeni JF. Rhabdomyosarcoma in children: epidemiologic study and identification of a familial cancer syndrome. J Natl Cancer Inst. 1969;43:1365–1373. [PubMed] [Google Scholar]
- Li FP, Fraumeni JF. Soft-tissue sarcomas, breast cancer, and other neoplasms a familial syndrome. Ann Intern Med. 1969;71:747–752. doi: 10.7326/0003-4819-71-4-747. [DOI] [PubMed] [Google Scholar]
- Li FP, Fraumeni JF, Mulvihill JJ, et al. A cancer family syndrome in twenty-four kindreds. Cancer Res. 1988;48:5358–5362. [PubMed] [Google Scholar]
- Malkin D. Li-Fraumeni syndrome. Genes Cancer. 2011;2:475–484. doi: 10.1177/1947601911413466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chompret A, Abel A, Stoppa-Lyonnet D, et al. Sensitivity and predictive value of criteria for p53 germline mutation screening. J Med Genet. 2001;38:43–47. doi: 10.1136/jmg.38.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougeard G, Sesboüé R, Baert-Desurmont S, et al. Molecular basis of the Li–Fraumeni syndrome: an update from the French LFS families. J Med Genet. 2008;45:535–538. doi: 10.1136/jmg.2008.057570. [DOI] [PubMed] [Google Scholar]
- Tinat J, Bougeard G, Baert-Desurmont S, et al. Version of the Chompret Criteria for Li Fraumeni syndrome. J Clin Oncol. 2009;2009;27:e108–e109. doi: 10.1200/JCO.2009.22.7967. [DOI] [PubMed] [Google Scholar]
- Ruijs MWG, Verhoef S, Rookus MA, et al. TP53 germline mutation testing in 180 families suspected of Li–Fraumeni syndrome: mutation detection rate and relative frequency of cancers in different familial phenotypes. J Med Genet. 2010;47:421–428. doi: 10.1136/jmg.2009.073429. [DOI] [PubMed] [Google Scholar]
- Scherer SW, Lee C, Birney E, et al. Challenges and standards in integrating surveys of structural variation. Nat Genet. 2007;39:S7–15. doi: 10.1038/ng2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad DF, Pinto D, Redon R, et al. Origins and functional impact of copy number variation in the human genome. Nature. 2010;464:704–712. doi: 10.1038/nature08516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Gu W, Hurles ME, Lupski JR. Copy number variation in human health, disease, and evolution. Annu Rev Genomics Hum Genet. 2009;10:451–481. doi: 10.1146/annurev.genom.9.081307.164217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankiewicz P, Lupski JR. Structural variation in the human genome and its role in disease. Annu Rev Med. 2010;61:437–455. doi: 10.1146/annurev-med-100708-204735. [DOI] [PubMed] [Google Scholar]
- Krepischi ACV, Pearson PL, Rosenberg C. Germline copy number variations and cancer predisposition. Future Oncol. 2012;8:441–450. doi: 10.2217/fon.12.34. [DOI] [PubMed] [Google Scholar]
- Lee C, Iafrate AJ, Brothman AR. Copy number variations and clinical cytogenetic diagnosis of constitutional disorders. Nat Genet. 2007;39:S48–S54. doi: 10.1038/ng2092. [DOI] [PubMed] [Google Scholar]
- Iafrate AJ, Feuk L, Rivera MN, et al. Detection of large-scale variation in the human genome. Nat Genet. 2004;36:949–951. doi: 10.1038/ng1416. [DOI] [PubMed] [Google Scholar]
- Bougeard G, Brugières L, Chompret A, et al. Screening for TP53 rearrangements in families with the Li-Fraumeni syndrome reveals a complete deletion of the TP53 gene. Oncogene. 2003;22:840–846. doi: 10.1038/sj.onc.1206155. [DOI] [PubMed] [Google Scholar]
- Charbonnier F, Raux G, Wang Q, et al. Detection of exon deletions and duplications of the mismatch repair genes in hereditary nonpolyposis colorectal cancer families using multiplex polymerase chain reaction of short fluorescent fragments. Cancer Res. 2000;60:2760–2763. [PubMed] [Google Scholar]
- Vezain M, Saugier-Veber P, Goina E, et al. A rare SMN2 variant in a previously unrecognized composite splicing regulatory element induces exon 7 inclusion and reduces the clinical severity of spinal muscular atrophy. Hum Mutat. 2010;31:E1110–E1125. doi: 10.1002/humu.21173. [DOI] [PubMed] [Google Scholar]
- Perzelová A, Máciková I, Mráz P, Bízik I, Steno J. Characterization of two new permanent glioma cell lines 8-MG-BA and 42-MG-BA. Neoplasma. 1998;45:25–29. [PubMed] [Google Scholar]
- Ory DS, Neugeboren BA, Mulligan RC. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc Natl Acad Sci USA. 1996;93:11400–11406. doi: 10.1073/pnas.93.21.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latouche JB, Sadelain M. Induction of human cytotoxic T lymphocytes by artificial antigen-presenting cells. Nat Biotechnol. 2000;18:405–409. doi: 10.1038/74455. [DOI] [PubMed] [Google Scholar]
- Hernandez-Vargas H, Lambert M-P, Le Calvez-Kelm F, et al. Hepatocellular carcinoma displays distinct DNA methylation signatures with potential as clinical predictors. PLoS ONE. 2010;5:e9749. doi: 10.1371/journal.pone.0009749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun CJ, Zhang X, Savelyeva I, et al. p53-Responsive micrornas 192 and 215 are capable of inducing cell cycle arrest. Cancer Res. 2008;68:10094–10104. doi: 10.1158/0008-5472.CAN-08-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhazzazi TY, Kamarajan P, Verdin E, Kapila YL. SIRT3 and cancer: tumor promoter or suppressor. Biochim Biophys Acta. 2011;1816:80–88. doi: 10.1016/j.bbcan.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher MB, Vaquero A, Reinberg D. SirT3 is a nuclear NAD+-dependent histone deacetylase that translocates to the mitochondria upon cellular stress. Genes Dev. 2007;21:920–928. doi: 10.1101/gad.1527307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noushmehr H, Weisenberger DJ, Diefes K, et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell. 2010;17:510–522. doi: 10.1016/j.ccr.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang H, Chen Y, et al. LSD1 is a subunit of the NuRD complex and targets the metastasis programs in breast cancer. Cell. 2009;138:660–672. doi: 10.1016/j.cell.2009.05.050. [DOI] [PubMed] [Google Scholar]
- Huang J, Sengupta R, Espejo AB, et al. p53 is regulated by the lysine demethylase LSD1. Nature. 2007;449:105–108. doi: 10.1038/nature06092. [DOI] [PubMed] [Google Scholar]
- Moissiard G, Cokus SJ, Cary J, et al. MORC family ATPases required for heterochromatin condensation and gene silencing. Science. 2012;336:1448–1451. doi: 10.1126/science.1221472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita N, Jaye DL, Kajita M, et al. MTA3, a Mi-2/NuRD complex subunit, regulates an invasive growth pathway in breast cancer. Cell. 2003;113:207–219. doi: 10.1016/s0092-8674(03)00234-4. [DOI] [PubMed] [Google Scholar]
- Luo J, Su F, Chen D, Shiloh A, Gu W. Deacetylation of p53 modulates its effect on cell growth and apoptosis. Nature. 2000;408:377–381. doi: 10.1038/35042612. [DOI] [PubMed] [Google Scholar]
- Ard PG, Chatterjee C, Kunjibettu S, Adside LR, Gralinski LE, McMahon SB. Transcriptional regulation of the mdm2 oncogene by p53 requires TRRAP acetyltransferase complexes. Mol Cell Biol. 2002;22:5650–5661. doi: 10.1128/MCB.22.16.5650-5661.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurdak H, Zhu S, Romero A, et al. An RNAi screen identifies TRRAP as a regulator of brain tumor-initiating cell differentiation. Cell Stem Cell. 2010;6:37–47. doi: 10.1016/j.stem.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Wei X, Walia V, Lin JC, et al. Exome sequencing identifies GRIN2A as frequently mutated in melanoma. Nat Genet. 2011;43:442–446. doi: 10.1038/ng.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigis MC, Deng C-X, Finley LWS, Kim H-S, Gius D. SIRT3 is a mitochondrial tumor suppressor: a scientific tale that connects aberrant cellular ROS, the Warburg effect, and carcinogenesis. Cancer Res. 2012;72:2468–2472. doi: 10.1158/0008-5472.CAN-11-3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Newman JC, Wang MZ, Ho L, Verdin E. Mitochondrial sirtuins: regulators of protein acylation and metabolism. Trends Endocrinol Metab. 2012;23:467–476. doi: 10.1016/j.tem.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Iwahara T, Bonasio R, Narendra V, Reinberg D. SIRT3 functions in the nucleus in the control of stress-related gene expression. Mol Cell Biol. 2012;32:5022–5034. doi: 10.1128/MCB.00822-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley LWS, Carracedo A, Lee J, et al. SIRT3 opposes reprogramming of cancer cell metabolism through HIF1α destabilization. Cancer Cell. 2011;19:416–428. doi: 10.1016/j.ccr.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf N, Zino S, Macintyre A, et al. Altered sirtuin expression is associated with node-positive breast cancer. Br J Cancer. 2006;95:1056–1061. doi: 10.1038/sj.bjc.6603384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Banck M, Mujtaba S, Zhou M-M, Sugrue MM, Walsh MJ. p53-induced growth arrest is regulated by the mitochondrial SirT3 deacetylase. PLoS One. 2010;5:e10486. doi: 10.1371/journal.pone.0010486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzentruber J, Korshunov A, Liu X-Y, et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482:226–231. doi: 10.1038/nature10833. [DOI] [PubMed] [Google Scholar]
- Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcan S, Rohle D, Goenka A, et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature. 2012;483:479–483. doi: 10.1038/nature10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.