Abstract
Mouse CD99 and its paralog CD99-like 2 (CD99L2) are surface proteins implicated in cellular adhesion and migration. Although their distributions overlap in a wide variety of cells, their physical/functional relationship is currently unknown. In this study, we show the interaction between the two molecules and its consequence for membrane trafficking of mouse (m)CD99L2. The interaction was analyzed by bimolecular fluorescence complementation, immunoprecipitation, and fluorescence resonance energy transfer assays. When coexpressed, mCD99 formed heterodimers with mCD99L2, as well as homodimers, and the heterodimers were localized more efficiently at the plasma membrane than were the homodimers. Their interaction was cytoplasmic domain–dependent and enhanced mCD99L2 trafficking to the plasma membrane regardless of whether it was transiently overexpressed or endogenously expressed. Surface levels of endogenous mCD99L2 were markedly low on thymocytes, splenic leukocytes, and CTL lines derived from CD99-deficient mice. Importantly, the surface levels of mCD99L2 on mCD99-deficient cells recovered significantly when wild-type mCD99 was exogenously introduced, but they remained low when a cytoplasmic domain mutant of mCD99 was introduced. Our results demonstrate a novel role for mCD99 in membrane trafficking of mCD99L2, providing useful insights into controlling transendothelial migration of leukocytes.
Introduction
Mouse (m)CD99 is an O-glycosylated transmembrane protein expressed on many cell types, including leukocytes and endothelial cells in various tissues. The transmembrane region of mCD99 is highly similar to that of human (h)CD99, a functional ortholog (1). hCD99 plays roles in cell adhesion (2, 3) and transcellular migration of leukocytes (4, 5). Treatment with a blocking anti-hCD99 Ab arrests the transcellular migration of leukocytes; this migration has been suggested to be mediated via homophilic interactions between hCD99 molecules on leukocytes and endothelial cells (4, 5). Similarly, the expression of mCD99 in Chinese hamster ovary cells has been reported to induce aggregation of the transfected cells (1). Additionally, anti-mCD99 Ab treatment blocked not only the cell aggregation and transendothelial migration of lymphocytes in vitro, but also the recruitment of activated T cells, monocytes, and neutrophils into inflamed tissues in vivo (1, 6), indicating the existence of functional similarities between mCD99 and hCD99.
CD99-like 2 (CD99L2) is a paralog of CD99, with moderate sequence homology in humans and mice (7). Two alternatively spliced forms (the short form, CD99L2S, and long form, CD99L2L) have been shown to be expressed on leukocytes, and their distribution patterns largely overlap with that of CD99 with few exceptions, such as bone marrow–derived neutrophils that are positive for mCD99 but negative for mCD99L2 (7, 8). mCD99L2 proteins are highly enriched at the contact regions between cultured endothelial cells and have also been implicated in cell adhesion and migration (8). Moreover, transfection of mouse L cells with mCD99L2 reportedly induced aggregation of the transfected cells, and treatment with anti-mCD99L2 Ab blocked influx of neutrophils to inflammation sites (9).
Despite these demonstrable similarities in their tissue distributions and in their suggested functions, the relationship between mCD99 and mCD99L2 has not been thoroughly investigated. mCD99 and mCD99L2 were found to function at the same site and the same stage during leukocyte transendothelial migration, separately from and independently of PECAM-1 (10). Treatment with blocking Ab against either mCD99 or mCD99L2 trapped neutrophils between endothelial cells in in vitro transmigration assays and inhibited extravasation of neutrophils into inflamed peritoneum and cremaster muscles in vivo, even in PECAM-1–deficient mice (10). Simultaneous treatment with Abs against mCD99 and mCD99L2, however, did not augment the inhibition effect on leukocyte extravasation beyond the levels exerted by treatment with each single Ab, demonstrating that the double treatment did not show an additive effect (10). These results have prompted us to explore a potential interaction between mCD99 and mCD99L2 and the physiological significance of this interaction.
hCD99 has also been implicated in molecular transport. Surface levels of several receptor proteins were shown to be closely related to the function of hCD99. For example, upon engagement with anti-hCD99 Ab, surface expression of MHC class I (MHC I) molecules was upregulated on human thymocytes but downregulated in the absence of hCD99 (11–13). Additionally, upon IFN-γ treatment of Jurkat cells, hCD99 was shown to associate with and increase the trafficking of MHC I to the cell membrane (14). The physical association of hCD99 proteins (both the long form and the alternatively spliced short isoform) with MHC molecules and tetraspanin CD81 is functionally linked to formation of an immunological synapse between B and T cells that induces T cell activation (15). hCD99 itself has been identified as a component of the lateral border recycling compartment of endothelial cells, moving to the site of transcellular diapedesis (16). The transmembrane region of hCD99, conserved among the CD99 and CD99-like molecules across species (7), was found to mediate the molecular association with MHC I and with Golgi-resident proteins, such as p230/golgin, during trafficking (14). These data regarding the involvement of hCD99 in molecular transport have suggested a possible role of mCD99 in molecular transport as well.
We and others have previously demonstrated that CD99 could form homodimers through extracellular domain–mediated interactions in mice (17) and humans (18). In this study, based on significant similarities between mCD99 and mCD99L2, we explored the possibility of interaction between the two molecules and subsequent implications. The results from this study revealed that mCD99 directly interacts with mCD99L2 to form a heterodimer via its cytoplasmic domain and subsequently enhances the surface expression of mCD99L2. Based on these findings, we propose a novel role for mCD99 as a conveyor protein carrying mCD99L2 cargo proteins via endomembrane trafficking.
Materials and Methods
Mice
C57BL/6 (B6) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). H60 congenic mice (B6.CH60) were gifts from Dr. Derry Roopenian (The Jackson Laboratory). The mCD99-deficient mouse strain B6.Cd99Gt(pU-21T)44Imeg, in which the Cd99 gene locus is trapped by the insertion of pU-21T plasmid (19), was generated at the Institute of Resource Development and Analysis, Kumamoto University (Kumamoto, Japan) and maintained through mating with B6 mice. All of the mice were maintained under specific pathogen-free conditions at the Center for Animal Resource Development of Seoul National University College of Medicine (Seoul, Korea).
Establishment of CTL lines and cell culture
The establishment of CD8 CTL lines was performed as described previously (20). In brief, wild-type (WT) B6 or mCD99-deficient B6 mice were i.p. injected with 2 × 107 splenocytes from H60 congenic mice (B6.CH60). Then, the splenic CD8 T cells were harvested from the injected mice on day 7 after injection, cultured ex vivo with irradiated H60 congenic splenocyte feeder cells in the presence of recombinant hIL-2 (50 U/ml; Sigma-Aldrich, St. Louis, MO), and maintained by periodic restimulation with irradiated feeder cells on a weekly basis. During the 7-d culture period of CTL line passage, CD8 T cells underwent activation and resting cycles. The activation (on day 5 after reactivation) and/or resting (on day 7 before reactivation) status of the CTL lines was monitored via cell counts and flow cytometric analysis of cell size and surface marker expression, such as that of CD44. Cells, including CD8 CTL lines, HEK293, and mouse L cells, were cultured in DMEM containing 5% FBS (HyClone Laboratories, Logan, UT) and antibiotics.
DNA constructs
Flag-, hemagglutinin (HA)-, or Myc-tagged mCD99L2 genes were subcloned into pBiFC-VN and pBiFC-VC vectors (provided by Dr. Chang-Deng Hu, Purdue University, West Lafayette, IN) and then the DNA fragments containing epitope-tagged mCD99L2 genes fused with VN or VC sequences were subsequently subcloned into pCI-neo (Promega, Madison, WI) or pcDNA 3.1 (Invitrogen, Carlsbad, CA) expression vectors. VN vectors carrying CD99 tagged at the N terminus with Myc and constructs for domain mutants of CD99 have been described previously (17). The mCD99 and mCD99L2 genes were also cloned to generate fusion proteins with fluorescence proteins such as yellow (YFP), cerulean (CFP), or mCherry (Clontech, Mountain View, CA). Myc-tagged mCD99-YFP, CytMutCD99-YFP, and TmMutCD99-YFP genes were subcloned into the pcDNA3.1 expression vector for coimmunoprecipitation. YFP, mCD99-YFP, and CytMutCD99-YFP genes were subcloned into pMSCV-puro (Clontech) for transduction. Plasma membrane–targeted YFP (PM-YFP) was a gift from Dr. Sunghoe Chang (Seoul National University College of Medicine, Seoul, Korea).
Transfection and transduction
HEK293 cells, which were plated onto either six-well plates or poly-l-lysine–coated glass coverslips for flow cytometry or confocal microscopic analysis, respectively, were transfected with the respective DNA constructs using the calcium phosphate transfection method. For the introduction of the mCD99-YFP fusion gene into mCD99-deficient CTL lines, the cells were incubated with filtered retroviral supernatants that were harvested from Platinum-E cells (Cell Biolabs, San Diego, CA) transiently transfected with mCD99-YFP-pMSCV-puro, CytMutCD99-YFP-pMSCV-puro, or YFP-pMSCV-puro mock vector in culture medium supplemented with Polybrene (10 μg/ml; Sigma-Aldrich) and rhIL-2 (50 U/ml; Sigma-Aldrich). After 2 more days of culture with fresh medium, transduced CTL cells were restimulated for passage in the culture medium containing 1 μg/ml puromycin (Sigma-Aldrich) for selection. After three more rounds of CTL stimulation for passage, YFP+ cells were sorted with a FACSAria (BD Biosciences, Franklin Lakes, NJ) and maintained with regular CTL passage on a weekly basis as described above.
Coimmunoprecipitation and Western blotting
Transfected HEK293 cells were lysed in RIPA buffer (150 mM NaCl, 50 mM Tris-HCl [pH 7.4], 1% Nonidet P-40, 0.1% sodium deoxycholate, 1 mM EDTA). After preclearing with protein G-agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA) for 2 h, anti-Myc epitope (Santa Cruz Biotechnology) or anti-Flag epitope (Novus Biologicals, Littleton, CO) Ab was applied. Then the Ab-bound proteins were pulled down using protein G beads (Sigma-Aldrich) and eluted by boiling in sample buffer. The coimmunoprecipitants and lysates from transfected cells or mouse splenocytes were resolved on 10% SDS-PAGE gels and subjected to immunoblotting using anti-Myc epitope (Santa Cruz Biotechnology), anti-Flag epitope (Sigma-Aldrich), and anti-HA epitope (Applied Biological Materials, Richmond, BC, Canada) primary Abs and an HRP-conjugated goat anti-mouse secondary IgG Ab (KOMA Biotechnology, Seoul, Korea). Bound proteins were visualized using ECL reagent (Intron, Seoul, Korea).
Flow cytometry
For surface staining, cells were incubated with anti-CD99L2 (goat anti-CD99L2; R&D Systems, Minneapolis, MN) or monoclonal anti-Flag Ab (Sigma-Aldrich) in flow cytometry buffer (1× PBS containing 0.1% bovine calf serum [w/v] and 0.05% sodium azide) at 4°C for 1 h, washed, and, stained with PE-conjugated secondary Abs at 4°C for 30 min. PE-conjugated anti-mCD99 Ab (EJ2; rat anti-mCD99) was used for staining of mCD99. For staining both surface and intracellular mCD99, cells were fixed with 4% paraformaldehyde in 1× PBS, permeabilized with 0.5% saponin in 1× PBS, and stained with Abs. Stained cells were washed and analyzed following dead cell exclusion by DAPI staining using an LSR II flow cytometer (BD Biosciences) and FlowJo software (Tree Star, Ashland, OR). Additional Abs used for flow cytometry were allophycocyanin-conjugated rat anti-CD8 (53-6.7), allophycocyanin-conjugated rat anti-mouse CD3 (145-2C11), PE-conjugated mouse anti-mouse H-2Kb (AF6-88.5; all from BD Biosciences), allophycocyanin-conjugated rat anti-CD4 (GK1.5), PE-conjugated rat anti-mouse B220 (RA3-6B2; eBioscience, San Diego, CA), and PE-conjugated donkey anti-goat IgG (R&D Systems). For inhibition of molecular transport from the Golgi to the plasma membrane or recycling at the plasma membrane, transfected HEK293 cells or transduced CD8 CTL cells were treated with 3 μg/ml brefeldin A (BFA; eBioscience) or 40 μM Dynasore (N′-(3,4-dihydroxybenzylidene)-3-hydroxy-2-naphthahydrazide; Santa Cruz Biotechnology), respectively, for 4 h prior to flow cytometry analysis. Cells treated with 0.1% DMSO (Sigma-Aldrich) were also included as a control.
Confocal microscopy assay
For assays that were analyzed by confocal microscopy, transfected HEK293 cells were washed with 1× PBS, fixed with 4% paraformaldehyde for 5 min, and, after washing, incubated with BODIPY TR ceramide (N-((4-(4,4-difluoro-5-(2-thienyl)-4-bora-3a,4a-diaza-s-indacene-3-yl)phenoxy)acetyl)sphingosine; Invitrogen) and DiI (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate; Invitrogen) to stain the Golgi and plasma membranes, respectively, at 4°C for 30 min. After washing, stained cells were counterstained with DAPI (Invitrogen) to visualize cell nuclei and then mounted with mounting medium (Dako, Glostrup, Denmark). Images were acquired at wavelengths of 488, 405, and 543 nm to detect Venus, the nucleus, and the Golgi/plasma membrane, respectively, using a TCS-SP2 scanning confocal microscope (Leica, Chicago, IL). CFP (cerulean, CN/CC fusion) and YFP (Venus/cerulean, VN/CC fusion) in the BiFC receptor fusion complexes, as well as mCherry, were detected at the 476, 527, and 559 nm emission wavelengths, respectively, using a FluoView 1000 confocal microscope (Olympus, Tokyo, Japan). Pearson’s r between two different proteins was determined using Olympus FV10-ASW 3.0 confocal microscopy software.
Measurement of fluorescence resonance energy transfer efficiency
To measure fluorescence resonance energy transfer (FRET) efficiency, HEK293 cells transfected with CFP- or YFP-fused plasmid constructs were examined with a ×100 objective lens on an Olympus FluoView 1000 confocal microscope. An excitation wavelength of 405 nm and an emission range of 450–490 nm as well as an excitation wavelength of 515 nm and an emission range of 525–565 nm were used to detect CFP and YFP, respectively. Images of CFP and YFP fluorescence after photobleaching were obtained using the respective filter set. Data were collected from at least 10 different individual cells. To measure FRET efficiency, images of the donor (CFP) and acceptor (YFP) were acquired before and after photobleaching of YFP to 25% of its initial intensity in selected regions of interest. FRET efficiency was calculated by using the following formula: [1−(CFP Intensityprebleach/CFP Intensitypostbleach)] × 100 (%). The unbleached areas were used as controls for sample movement and/or laser power fluctuations.
Statistical analysis
Statistical analysis was performed using two-tailed t tests by GraphPad Prism software (version 5; GraphPad Software, San Diego, CA). A p value < 0.05 was considered statistically significant.
Results
Formation of heterodimeric biomolecular fluorescence complementation complexes composed of mCD99 and mCD99L2
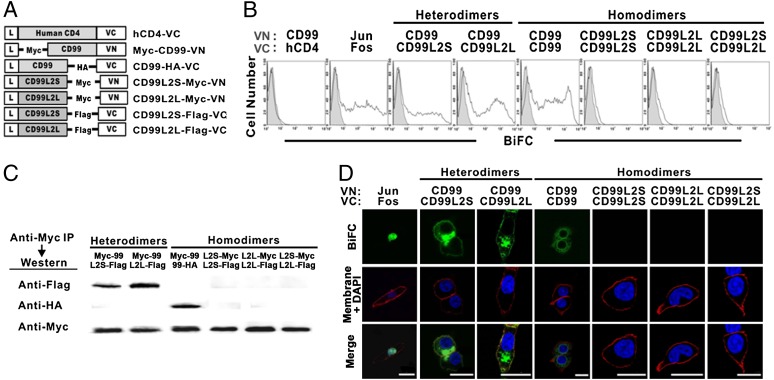
To investigate the potential interaction of mCD99 and mCD99L2, we first performed bimolecular fluorescence complementation (BiFC) analysis, which enables direct visualization of protein interactions as well as subcellular localization of protein complexes in living cells via the fluorescence caused by the association of two proteins fused to nonfluorescent fragments of a fluorescent protein (21). mCD99 and two isoforms of mCD99L2 (mCD99L2S and mCD99L2L) were tagged with Myc, Flag, or HA epitopes, not only because Abs that can differentiate the CD99L2 isoforms are not available, but also because the anti-CD99 mAb raised in our laboratory (EJ2) was not of sufficient high affinity for use in certain analyses, such as immunoprecipitation. Next, the tagged genes were fused to either a VN (N-fragment of the yellow fluorescent protein Venus) or a VC (C-fragment of Venus) fragment for BiFC analysis (Fig. 1A). The sizes and expression levels of the VN- and VC-fused forms of CD99L2S, CD99L2L, and CD99 were confirmed by immunoblotting (data not shown).
FIGURE 1.
Characterization of the interaction between mCD99 and mCD99L2. (A) Diagram of the DNA constructs for the expression of mCD99 or mCD99L2 proteins tagged with Myc, Flag, or HA epitopes and fused with the VN or VC gene. (B) Flow cytometry was used to evaluate fluorescence emission from the BiFC complex. After HEK293 cells were transfected with different combinations of VN and VC constructs for determining heterodimer formation between mCD99 and mCD99L2 proteins, cells were analyzed by flow cytometry for fluorescence emitted by BiFC complexes. Gray-filled histograms represent fluorescence emitted by cells transfected with VN construct only. (C) Physical interaction between mCD99 and mCD99L2 proteins. Proteins were pulled down from the lysates of HEK293 cells transfected with various combinations of VN and VC constructs using an anti-Myc Ab and were probed using anti-Flag, anti-Myc, or anti-HA Abs followed by HRP-conjugated goat anti-mouse IgG. (D) Localization of BiFC complexes. To localize BiFC complexes (green), transfected cells were stained with DAPI (blue) and DiI (red) to visualize nuclei and the plasma membrane, respectively, and the cells were observed under a TCS-SP2 confocal microscope (original magnification ×630). Data were processed using LCS Lite software (Leica). Scale bar, 5 μm. All the data (A–D) represent more than five independent experiments.
We next transfected HEK293 cells with mCD99-VN and mCD99L2-VC constructs in pairs and analyzed the transfected cells by flow cytometry and confocal microscopy to detect any physical interactions. Additionally, cells were cotransfected with mCD99 plus hCD4 or with Jun plus Fos (in VN and VC combinations) as negative or positive control, respectively. When the cells were subjected to flow cytometric analysis 48 h after transfection, the results revealed that the cells transfected with mCD99-VN and mCD99L2S-VC, as well as with mCD99-VN and mCD99L2L-VC, emanated Venus fluorescence, implying that mCD99 and mCD99L2 molecules formed mCD99/mCD99L2 heterodimeric complexes. The fluorescence intensities were comparable with those resulting from homodimeric interactions between mCD99-VN and mCD99-VC (Fig. 1B). The mCD99–mCD99L2 interaction was confirmed by coimmunoprecipitation assays, in which lysates from the cells cotransfected with Myc-tagged mCD99-VN plus Flag-tagged mCD99L2S-VC (or Flag-tagged mCD99L2L-VC) were precipitated using an anti-Myc Ab. Western blotting with an anti-Flag Ab yielded specific bands corresponding to mCD99L2 in both the short and long forms (Fig. 1C). Lysate from cells cotransfected with mCD99-HA-VC and Myc-mCD99-VN was used as a positive control.
By using confocal microscopic analysis that provides qualitative in-focus images of BiFC complexes, we detected the formation of mCD99/mCD99L2S and mCD99/mCD99L2L heterodimeric BiFC complexes. The heterodimeric complexes were localized to the plasma membrane and to the Golgi complex in transfected cells, as determined by costaining Venus fluorescence with membrane dye DiI (Fig. 1D) and Golgi dye BODIPY (Supplemental Fig. 1), respectively. No notable differences in heterodimer formation with mCD99 were observed between the two mCD99L2 isoforms. The subcellular localization of the heterodimeric complexes appeared to be more peripheral than that of mCD99/mCD99 homodimeric complexes; the latter showed a more concentrated pattern at the endoplasmic reticulum and Golgi regions, suggesting that mCD99-mCD99L2 heterodimers move farther along the endomembrane system than did CD99 homodimers.
We next tested whether mCD99L2 proteins themselves could form homodimers (mCD99L2S-mCD99L2S, mCD99L2L-mCD99L2L, or mCD99L2S-mCD99L2L), as does mCD99 (17). On the contrary to mCD99, none of the three CD99L2 protein combinations formed homodimers according to BiFC-based fluorescence detected via flow cytometric (Fig. 1B) or confocal microscopic (Fig. 1D) analysis. This suggests that, unlike mCD99, mCD99L2 proteins do not form homodimeric BiFC complexes, which was subsequently confirmed by coimmunoprecipitation analysis (Fig. 1C).
Taken together, the results of our BiFC analyses showed that mCD99 and mCD99L2 physically interacted with each other to form heterodimeric complexes with a subcellular distribution pattern distinct from that of mCD99-mCD99 homodimers. When we used additional pairs of VN- and VC-fused constructs, in which the CD99 and CD99L2 gene constructs used were the reciprocals of those used in the above experiments (Fig. 1B–D), we obtained similar results by BiFC analysis (data not shown).
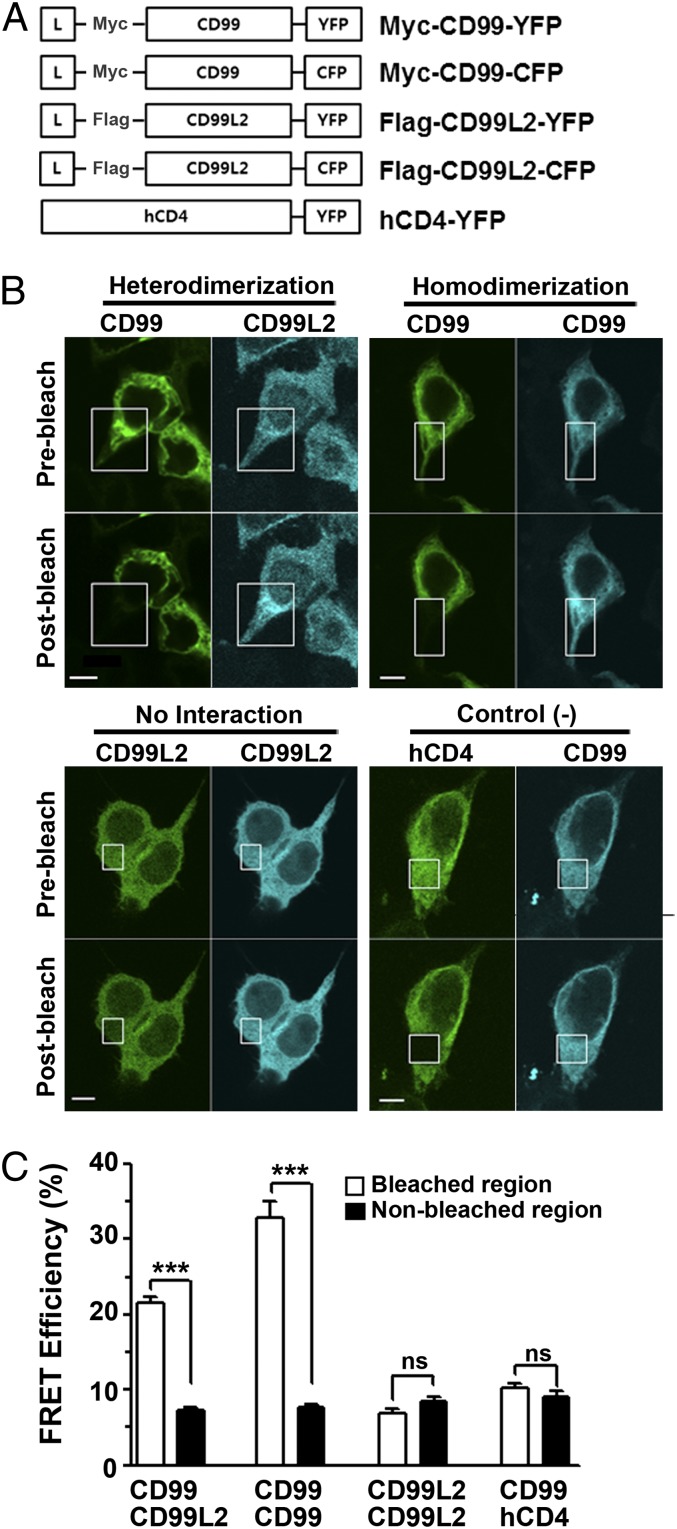
Specific interaction of mCD99 and mCD99L2 in an acceptor photobleaching FRET assay
Although the BiFC assay has been reported to be so sensitive as to detect not only transient but also very weak interactions (22), we tried to confirm the interaction between mCD99 and mCD99L2 using another method, the FRET assay, which can detect protein–protein interactions within a 1–10 nm range (23–25). We cotransfected HEK293 cells with constructs to express mCD99 and mCD99L2 proteins tagged with YFP and CFP at the C terminus, respectively (Fig. 2A). Additionally, cells were transfected to coexpress other combinations of YFP- or CFP-fused mCD99 and mCD99L2 proteins to check for homodimerization between mCD99 proteins or between mCD99L2 proteins; coexpressed mCD99 and hCD4 fusion proteins were used as a no interaction (negative) control. We examined whether the emission of CFP donor signals became more intense after photobleaching of the YFP acceptor within the intracellular and plasma membrane regions of the cotransfected cells. The signal quench of the YFP acceptor by photobleaching enables the CFP donor signal to be directly detected without being diverted (i.e., absorbed) for YFP acceptor activation (24). Comparison of the cell images taken before and after the localized photobleaching of the YFP acceptor, which were then processed into spectrally unmixed images, revealed that in the cells cotransfected with mCD99-YFP and mCD99L2-CFP DNA constructs, the intensity of the mCD99L2-CFP donor signal increased after photobleaching, but only within the photobleached regions and not in nonphotobleached regions (Fig. 2B). The FRET efficiency values observed in the bleached areas were significant (mean, 21.52 ± 0.7 arbitrary units [AU]; n = 26), indicating the occurrence of FRET in the cells and thereby confirming the interaction of mCD99 and mCD99L2 (Fig. 2C). FRET was also observed in the photobleached regions of cells coexpressing YFP- and CFP-fused mCD99 proteins, with significantly high efficiency (mean, 32.90 ± 2.0 AU; n = 14), but not in those coexpressing hCD4 and mCD99 fusion proteins or in those coexpressing CFP- and YFP-tagged mCD99L2, verifying the lack of interaction between mCD99L2 proteins themselves. Altogether, the results from the FRET assays proved the existence of molecular interactions between mCD99 and mCD99L in the cells where they were coexpressed. Furthermore, the molecular specificity for dimerization was validated to occur between mCD99L2 and mCD99 molecules as well as between mCD99 molecules, consistent with the results of the BiFC analysis.
FIGURE 2.
Specific interaction between mCD99 and mCD99L2 in FRET analysis. (A) Diagram of the DNA constructs used in FRET assays. hCD4-YFP DNA was used as a negative control. (B) Confocal microscopic FRET analysis of the interaction between mCD99 and mCD99L2 proteins. HEK293 cells, which were cotransfected with mCD99-YFP/mCD99L2-CFP, mCD99-YFP/mCD99-CFP, mCD99L2-YFP/mCD99L2-CFP, or hCD4-YFP/mCD99-CFP pairs, were subjected to the FRET assay 48 h after transfection. The YFP fluorescence expressed in the cells was partially photobleached with a 488-nm laser (white boxed area). Scale bar, 5 μm. (C) FRET efficiency was calculated using CFP intensity, as described in Materials and Methods. ***p < 0.001. Data (A–C) are representative of three independent experiments.
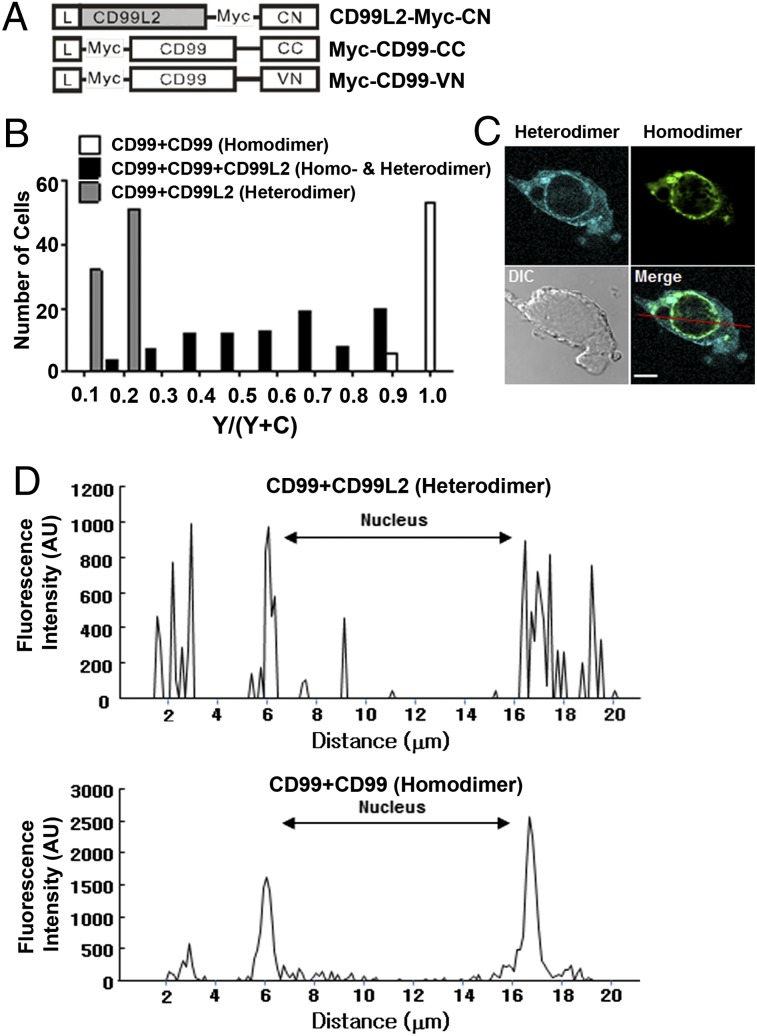
Coexistence of mCD99-mCD99 and mCD99-mCD99L2 dimers within a cell
Because mCD99 interacts not only with itself but also with mCD99L2 to form dimers, we tested which partner it preferred for dimer formation. Using BiFC and FRET analyses, we detected a slight difference in the pattern of subcellular localization between homodimeric and heterodimeric complexes; heterodimers appeared more frequently at the plasma membrane than did homodimers (Figs. 1D, 2B). To directly compare the subcellular localizations of the two different dimers, we performed multicolor BiFC analysis, because two different fluorescence colors (YFP and CFP) can be simultaneously expressed in a cell through interaction of VN- and CC-tagged proteins and of CN- and CC-tagged proteins, respectively (26). HEK293 cells were cotransfected with three DNA constructs for the expression of mCD99-VN, mCD99-CC, and mCD99L2-CN proteins (Fig. 3A). Our speculation was that if mCD99 preferred one partner over the other, only a single fluorescence signal, YFP or CFP, would be expressed in the transfected cells. As single-color controls, cells were cotransfected with two of the DNA constructs to express VN and CC fusion proteins (for emitting YFP only) or CN and CC fusion proteins (for emitting CFP only). Upon examining the three-construct transfected cells under confocal microscopy, we observed that, although most cells emanated both fluorescent colors, the signal intensities of YFP and CFP varied, indicating the formation of different levels of the two dimers depending on the status of individual cells. To quantify each signal, we converted the absolute values of YFP and CFP intensities produced by a single cell into a relative YFP value (YFP/[YFP + CFP]) and were thus able to evaluate >90 different cells according to their relative YFP values. When we plotted cell numbers versus relative YFP values, we found that whereas the distribution of single-color control cells skewed toward 0.1–0.2 (i.e., heterodimers producing CFP) or 1 (i.e., homodimers producing YFP), that of the three-construct transfected cells varied widely from 0.1 to 0.9 of the relative YFP values, indicating expression of both colors (i.e., the coexistence of heterodimers and homodimers) at varying ratios in cells coexpressing mCD99-VN, mCD99-CC, and mCD99L2-CN proteins (Fig. 3B, Supplemental Fig. 2A).
FIGURE 3.
Coexistence of mCD99-mCD99L2 hetero- and mCD99-mCD99 homodimers. To verify whether mCD99 and mCD99L2 compete to form dimers, two-color BiFC assays were performed. (A) Diagram of the DNA constructs used for the expression of mCD99 or mCD99L2 proteins fused with VN, CC, or CN. (B) Plot of cell numbers versus relative YFP values. HEK293 cells were transfected with different combinations of VN, CC, and CN constructs for testing hetero- and homodimer formation between mCD99 and mCD99L2 proteins. CFP and YFP intensities were quantified using a 436/470 nm “C” filter and a 500/535 nm “Y” filter of a TCS-SP2 scanning confocal microscope, and the YFP/(YFP + CFP) ratios versus cell numbers were plotted in the histogram. Data were processed with LCS Lite software. (C) Representative confocal image of homodimeric YFP and heterodimeric CFP localization within a transfected cell 48 h after transfection. CFP and YFP from the BiFC complexes were detected at the 476 and 527 nm emission wavelengths, respectively, using a FluoView 1000 confocal microscope (original magnification ×1000). The red line on the merged image indicates the line of measurement for the fluorescence intensity of homo- and heterodimers. The gamma setting did not need to be changed to adjust the images. Scale bar, 5 μm. (D) Fluorescence intensity of each dimer. Fluorescence intensity from the merged cell image (C) was measured as the area under the curve representing the nucleus or other regions using FV10-ASW 3.0 Viewer software (Olympus). Representative data from more than five independent experiments are shown (A–D).
Next, we compared images of cells emitting equal levels of YFP and CFP signals to examine the subcellular localizations of the two types of dimers. Individual nonoverlapping cell images showed that mCD99-mCD99L2 heterodimers broadly distributed from the perinuclear region to the plasma membrane, whereas mCD99 homodimers were more restricted near perinuclear vesicles (Fig. 3C, Supplemental Fig. 2B). Thus, major heterodimeric CFP peaks with >500 intensity AU were measured at the perinuclear regions (5.9, 6.1, 6.3, 16.6, 17.1, 17.2, and 17.6 μm from one end of a cell along an arbitrary line crossing the nucleus) and at more peripheral regions (2.1, 2.9, and 19.3 μm from the end of a cell). Additionally, major homodimeric YFP peaks were detected at 5.9, 6.1, 6.2, 16.9, 17.1, 17.2, and 17.3 μm from the end of a cell (in the perinuclear region) with strong intensities (>1000 AU). These data provide clear evidence for different localization patterns of the two types of dimers: more focused perinuclear localization of homodimers versus more diffuse, peripheral, but distinct periplasmic localization of heterodimers (Fig. 3D, Supplemental Fig. 2C). Altogether, the results from the multiple BiFC and subsequent analyses demonstrated that mCD99-mCD99L2 heterodimers and mCD99-mCD99 homodimers coexist within the same cell with differing localization patterns.
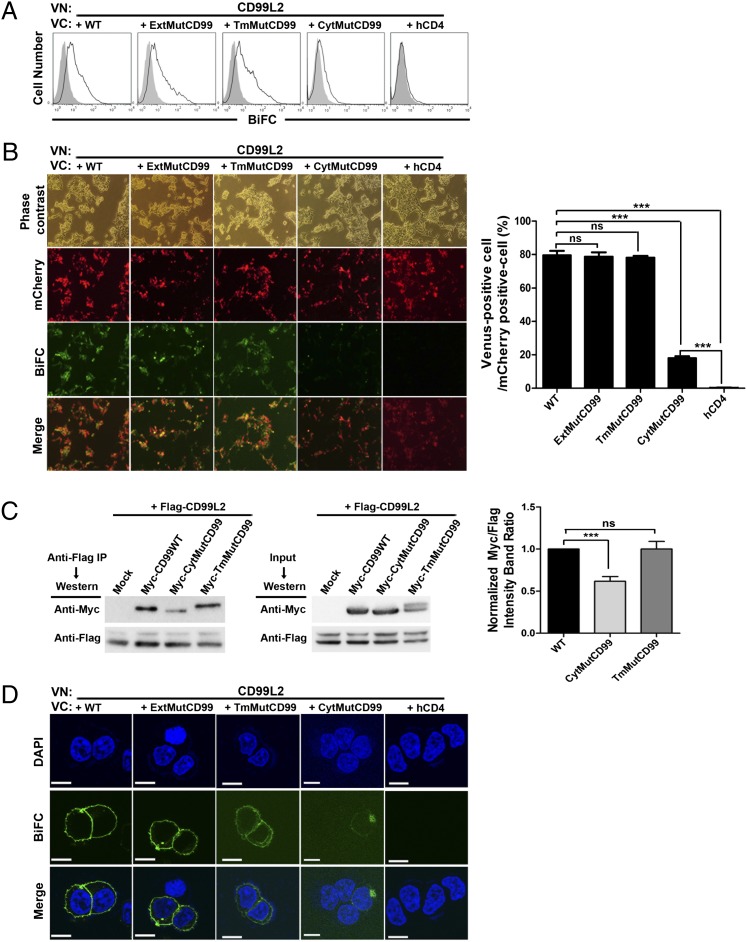
Significance of the cytoplasmic domain of mCD99 in dimerization with mCD99L2
The ability of mCD99 to form two different types of dimers within an individual cell led us to question which domain of mCD99 was responsible for interaction with mCD99L2 because its extracellular domain has been found to mediate homodimer formation in domain-mutant analysis using BiFC assays (17). To answer this question, we performed BiFC analyses with VN- and VC-fused combinations of mCD99L2 and mCD99 domain mutants, in which the extracellular, transmembrane, or cytoplasmic domain (ExtMutCD99, TmMutCD99, or CytMutCD99, respectively) was replaced with the corresponding domain of hCD4 (17). Flow cytometric analysis of HEK293 cells cotransfected with mutant mCD99 and mCD99L2 constructs revealed a marked reduction in BiFC-based fluorescence in cells expressing CytMutCD99 together with mCD99L2. However, other BiFC combinations of mCD99 mutants yielded strong fluorescent signals (Fig. 4A).
FIGURE 4.
Requirement of cytoplasmic domain of mCD99 for interaction with mCD99L2. (A and B) Flow cytometric (A) and fluorescence microscopic (B) analyses were performed to evaluate the interaction between mCD99L2 and mCD99 mutants based on the BiFC assay. (A) HEK293 cells, cotransfected with mCD99L2 and mCD99 mutant VN-VC pairs, were subjected to flow cytometric analysis 48 h after transfection. Gray-filled histograms show fluorescence emission by cells transfected with VN constructs only. (B) After cotransfection of HEK293 cells with three DNA constructs for the expression of VN- and VC-fused proteins and fluorescence protein mCherry, cells emitting BiFC-based Venus fluorescence among mCherry+ cells was counted in five random microscopic fields under fluorescence microscopy (original magnification ×100; Nikon Eclipse Ti fluorescence microscope, Nikon, Tokyo, Japan) using Nikon NIS-Elements F3.0 software (Nikon). Next, the number of Venus+ cells per 100 mCherry+ cells in each field were plotted. (C) Coimmunoprecipitation analysis was performed using cell lysates from HEK293 cells transiently cotransfected with the indicated constructs. mCD99L2 proteins were precipitated from the lysates with anti-Flag Ab, and the precipitants were subjected to immunoblotting with anti-Flag and anti-Myc mAbs to detect mCD99L2 proteins and each mCD99 mutant protein, respectively. Band intensities were measured with LAS 4000 Mini (Fujifilm, Tokyo, Japan), equipped with Fujifilm Multi Gauge 3.0 software (Fujifilm); those for Myc-tagged proteins were normalized to those for Flag-tagged mCD99L2 in each lane. Then, normalized values of each different type of mCD99 mutant relative to those for WT mCD99 were plotted. (D) Confocal images were obtained using a FluoView 1000 confocal microscope (original magnification ×1000). Scale bar, 10 μm. *p < 0.05, ***p < 0.001. Representative data (A–D) from at least five independent experiments are shown.
Next, to quantify the difference in the degrees of interaction with mCD99L2 between different types of mCD99, we cotransfected HEK293 cells with three DNA constructs for the expression of mCherry fluorescence protein to mark transfected cells in addition to VN- and VC-fused mCD99L2 and mCD99 expression, and counted cells emitting Venus fluorescence among the mCherry+ transfected cells in five different fields under a fluorescence microscope. When numbers of the Venus-expressing cells per 100 mCherry+ cells in each field were processed to a plot, the formation of BiFC complexes occurred in ∼80% of the transfected cells in which mCD99L2-VN was coexpressed with WT, ExtMut, or TmMut mCD99-VC. However, the complex occurred in <20% of the cells transfected to express CytMutCD99-VC and mCD99L2-VN pairs (Fig. 4B), a lower frequency than those obtained from other combinations but still significant compared with the negative control where hCD4 was expressed as VC partner to mCD99L2-VN. Therefore, these results suggest that the efficiency or affinity of the interaction between mCD99 and mCD99L2 was significantly reduced by the absence of original cytoplasmic domain of mCD99.
In coimmunoprecipitation assays using lysates from cells transfected to coexpress Flag-tagged mCD99L2-YFP and each type of the Myc-tagged mCD99-YFP proteins, immunoblots of precipitants of anti-Flag Ab with anti-Myc Ab showed that intensities of the bands corresponding to CytMutCD99 were relatively low, on average 0.6-fold of those corresponding to WT or TmMut CD99 (Fig. 4C). Results from the coimmunoprecipitation assays confirmed the occurrence of a strong interaction between mCD99 and mCD99L2 and suggested the involvement of the cytoplasmic domain of mCD99 in mediating the interaction with mCD99L2, which differs from the extracellular domain–mediated homodimeric interaction between mCD99 molecules.
Additionally, confocal microscopy of the cells expressing BiFC-based Venus fluorescence revealed that, despite the infrequent formation, BiFC complexes once formed by mCD99L2 with the cytoplasmic domain mutant of mCD99 were localized mostly at intracellular vesicles (Fig. 4D), whereas those with the other types of mCD99 were localized to the plasma membrane as well as intracellular vesicles. This observation implied the significance of the cytoplasmic domain of mCD99 in the localization of the complex formed between mCD99 and mCD99L2.
Enhanced localization of mCD99L2 at the plasma membrane via coexpressed mCD99
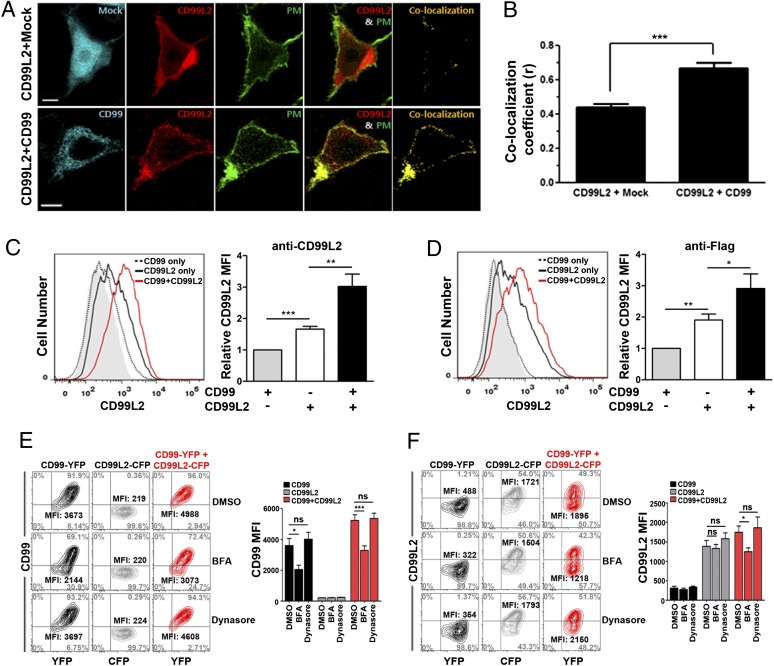
The functional significance of the interaction of mCD99 and mCD99L2 was next addressed. Based on previous reports on the role of hCD99 in controlling MHC I trafficking (12, 14, 15) and our results showing the more peripheral localization of the heterodimer (Fig. 3), we investigated a role for mCD99 in trafficking of mCD99L2. To explore the influence of the interaction with mCD99 on mCD99L2 trafficking, we compared the level of mCD99L2 localized at the plasma membrane between cells expressing mCD99L2 alone and those coexpressing mCD99L2 and mCD99. HEK293 cells were transiently transfected to express an mCD99L2-mCherry fusion protein in combination with a native unfused CFP control protein or with an mCD99-CFP fusion protein, respectively, and analyzed for localization of mCD99L2 at the cell membrane via confocal microscopy. To visualize the plasma membrane region of transfected cells, a chimeric construct of the 10 N-terminal amino acids of Lyn with YFP (PM-YFP), which is targeted to the inner monolayer of the plasma membrane (27, 28), was included. Then, colocalization of mCherry (mCD99L2) and YFP (PM) was considered indicative of mCD99L2 localization at the plasma membrane. Confocal images taken 48 h after transfection demonstrated that expression of mCD99L2 at the plasma membrane, as denoted by colocalization with PM-YFP, was not significantly high when coexpressed with native CFP, but it was substantially enhanced when coexpressed with mCD99-CFP (Fig. 5A). According to a quantitative analysis (29), the colocalization of mCD99L2-mCherry with PM-YFP increased 1.5-fold, on average, by the presence of mCD99 (mean colocalization r value, 0.67) compared with that in the absence of mCD99 coexpression (mean r value, 0.44; Fig. 5B). These results indicated a positive impact of mCD99 coexpression on the localization of mCD99L2 to the plasma membrane.
FIGURE 5.
Enhanced surface expression of mCD99L2 via interaction with mCD99. (A) Changes in surface expression of mCD99L2 after cotransfection of HEK293 cells with or without mCD99. mCD99-CFP, PM-YFP, and mCD99L2-mCherry were detected at 417, 515, and 559 nm emission wavelengths, respectively, using a FluoView 1000 confocal microscope. PM-YFP DNA was used as a plasma membrane marker control. Scale bar, 5 μm. (B) Colocalization (r) analysis comparing PM and mCD99L2 proteins. The r values reflect the proportion of mCD99L2 surface expression. An r of 1 indicates perfect colocalization of PM and mCD99L2 proteins. ***p < 0.001. (C and D) Flow cytometric analysis of the surface expression of mCD99L2 after cotransfection of HEK293 cells with or without mCD99. Flag-mCD99L2-pEYFP construct with pCerulean-N1 (CD99L2 only) or with mCD99-CFP construct (CD99 plus CD99L2) was cotransfected into HEK293 cells. Cotransfection with mCD99-CFP and pEYFP-N1 (CD99 only) was included as negative control. Transfected cells were stained with anti-CD99L2 (C) or anti-Flag (D) primary Ab and PE-conjugated secondary Abs 48 h after transfection. Then, CD99L2 levels were compared after gating on CFP+- and YFP+-transfected cells. Relative MFI values of mCD99L2 in relationship to those obtained from the cells transfected with CD99 only were formulated. (E and F) Flow cytometric analysis for surface levels of mCD99 (E) and mCD99L2 (F) after treatment of cells with DMSO (0.1%), BFA (3 μg/ml), or Dynasore (40 μM). HEK293 cells expressing mCD99-YFP, mCD99L2-CFP, or both mCD99-YFP and mCD99L2-CFP were treated for 4 h prior to cell staining with anti-CD99 or anti-CD99L2 Ab followed by flow cytometric analysis. Next, the MFI values of mCD99 (E) and mCD99L2 (F) were plotted. *p < 0.05, **p < 0.01, ***p < 0.001. Data shown (A–F) represent at least five independent experiments.
To verify the results obtained from confocal microscopic analysis, we compared the level of mCD99L2 expressed on the cell surface under the two conditions (the presence and absence of mCD99 coexpression) by flow cytometry after staining the transfected cells with anti-mCD99L2 Ab. Results from the flow cytometric analysis, shown as overlaid single histograms (Fig. 5C), demonstrated a shift to the right of the histograms corresponding to CD99L2 expression (Fig. 5C). This indicated enhanced localization of mCD99L2 at the surface of cells coexpressing mCD99 and mCD99L2 (average relative mean fluorescence intensity [MFI], 3.0), with a 1.8-fold increase in MFI compared with cells expressing mCD99L2 alone (average relative MFI, 1.7). A similar increase in mCD99L2 surface expression (average relative MFI, 2.9 versus 1.9; 1.5-fold) was obtained when the cells were stained with anti-Flag Ab, which was used to detect the Flag epitope-tagged mCD99L2 (Fig. 5D). This result ruled out the possibility of false positives due to cross-reactivity of the anti-CD99L2 Ab to the mCD99 protein expressed on the cotransfected cells. Taken together, the results from the confocal microscopic and flow cytometric analyses using cotransfected HEK293 cells demonstrated that localization of mCD99L2 to the plasma membrane was enhanced by coexpression of mCD99, suggesting a positive role for mCD99 in molecular trafficking of mCD99L2 to the plasma membrane.
Next, to determine the implication of mCD99 in molecular trafficking of interacting mCD99L2 from the Golgi to the plasma membrane, HEK293 cells were transfected to express mCD99 and mCD99L2 together or each molecule alone with BFA (a blocker of Golgi to membrane trafficking), Dynasore (a blocker of membrane recycling), or DMSO control solution for 4 h and then stained with anti-mCD99 or anti-mCD99L2 Ab for flow cytometric analysis. Results from flow cytometry analysis showed that, compared with levels expressed on cells treated with DMSO or Dynasore, the surface level of mCD99 itself was significantly reduced by BFA treatment whether it was expressed alone or together with mCD99L2 (Fig. 5E). In the case of mCD99L2, BFA treatment did not influence surface levels of mCD99L2 when the molecule was expressed alone, but it did reduce the levels when it was coexpressed with mCD99. The MFI values of mCD99L2 on the BFA-treated cells that coexpressed mCD99 and mCD99L2 were low compared with those of cells treated with DMSO or Dynasore (Fig. 5F). Taken together, these data suggest that surface level of mCD99 is dependent on the trafficking activity from the Golgi to the plasma membrane on its own, and surface expression of mCD99L2 is enhanced via the activity of mCD99 upon interaction.
Increased transport of endogenously expressed mCD99L2 to the plasma membrane via interaction with mCD99
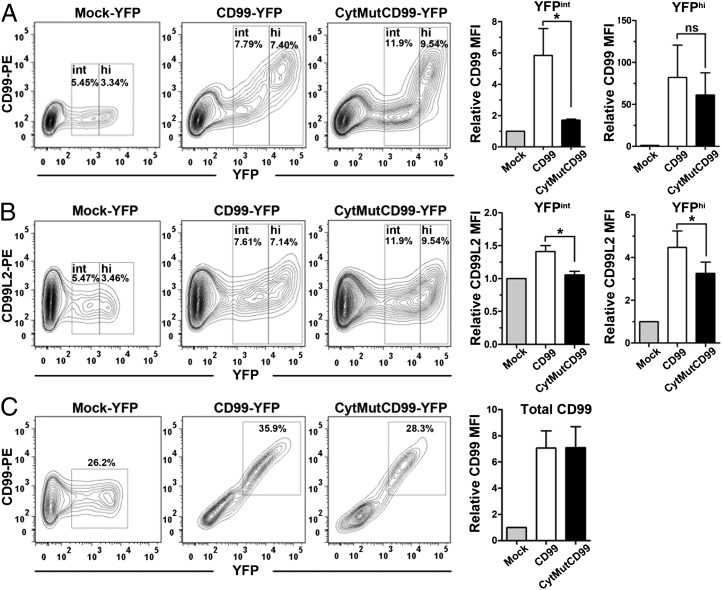
The positive effect of coexpressed mCD99 on the peripheral localization of mCD99L2 (Fig. 5) was determined using transiently transfected HEK293 cells. Thus, we next sought to confirm those results in cells that endogenously express mCD99L2. The mouse L cell line, which expresses mCD99L2 but lacks mCD99 expression (Fig. 6A), was transfected with mCD99-YFP and analyzed for endogenous mCD99L2 expression on the cell surface by flow cytometry after staining with anti-mCD99L2 Ab. Data from the flow cytometric analysis demonstrated that compared with cells mock-transfected with native YFP and untransfected YFP− cells, the YFP+ mCD99-transfected cells expressed more mCD99L2 on their surface; mCD99L2 surface expression was 1.3-fold greater, on average, in the presence of mCD99-YFP (Fig. 6B). Even though the increase was not dramatic, possibly due to a limit on endogenously expressed mCD99L2 protein, the phenomenon was consistently and significantly reproducible.
FIGURE 6.
Changes in surface expression of endogenous mCD99L2 protein due to mCD99 overexpression. (A) Surface levels of mCD99 were measured after transfection with mCD99-YFP or CytMutCD99-YFP plasmid DNA by staining with PE-conjugated anti-CD99 Ab and flow cytometric analysis. Relative MFI values of mCD99 on the YFPint or YFPhi population in comparison with those transfected with Mock-YFP were plotted. (B) Surface expression of mCD99L2 was measured by flow cytometric analysis after staining with anti-CD99L2 Ab and PE-conjugated donkey anti-goat IgG secondary Ab. The relative MFI values of mCD99L2 were formulated as described above. (C) Total expression of mCD99 or cytoplasmic mutant mCD99 proteins after transfection with mCD99-YFP or CytMutCD99-YFP plasmid DNA, respectively. Staining with PE-conjugated anti-mCD99 Ab was performed following membrane permeabilization with 0.5% Triton X-100 buffer. Relative MFI values of mCD99 in relationship to those obtained from Mock-YFP–transfected cells were plotted. pEYFP-N1 DNA (Clontech) was used as a negative control (Mock-YFP). *p < 0.05. Data shown (A–C) represent three independent experiments.
To examine whether this effect of mCD99 coexpression was due to the interaction of mCD99 with mCD99L2, we also transfected L cells to express CytMutCD99-YFP, which has a limited ability to interact with mCD99L2 (Fig. 4), followed by staining with anti-mCD99 Ab or anti-mCD99L2 Ab for flow cytometric analysis. To confirm the expression of CytMutCD99 on the surface of the transfected (YFP+) cells, we used flow cytomtery analysis after staining with anti-mCD99 Ab and revealed that trafficking of CytMutCD99 itself to the plasma membrane was inefficient (Fig. 6A); the level of CytMutCD99 protein on the surface of YFP+ cells was low when the mutant proteins were present in a low to intermediate concentration (YFPint), and was high, comparable to those of the WT mCD99, when the mutant protein was at a high concentration (YFPhi). This surface expression pattern for CytMutCD99 was different from that of WT mCD99, which showed a proportional increase relative to YFP intensity, suggesting a differential molecular trafficking pattern of the mutant protein, in addition to inefficient interaction with mCD99L2.
Additionally, flow cytometric analysis after staining with anti-mCD99L2 Ab showed that CytMutCD99-expressing cells displayed mCD99L2 on their surface at lower levels in the YFPhi population as well as in the YFPint population than WT mCD99-expressing cells (Fig. 6B).
Next, we stained cells with anti-mCD99 Ab after membrane permeabilization to detect proteins present inside and outside of the cell to determine whether CytMutCD99 and WT mCD99 proteins were produced at similar levels in the transfected cells. After gating on YFP+ cells, flow cytometric analysis confirmed that the transfected cells produced CytMutCD99 and mCD99 proteins in similar amounts, indicating that the cytoplasmic domain mutant mCD99 indeed could not reach the cell surface as efficiently as did the WT mCD99 protein (Fig. 6C). These data were in agreement with the aforementioned results from confocal microscopic analysis (Fig. 4D), suggesting that the low level of mCD99L2 expressed on the surface of CytMutCD99-transfected cells was due to the inefficient trafficking of CytMutCD99 itself and/or insufficient interaction between the CytMutCD99 and mCD99L2.
Taken together, these results demonstrated that forced expression of mCD99 enhanced the trafficking to the plasma membrane of endogenous mCD99L2 and this positive effect was dependent on the cytoplasmic domain of mCD99.
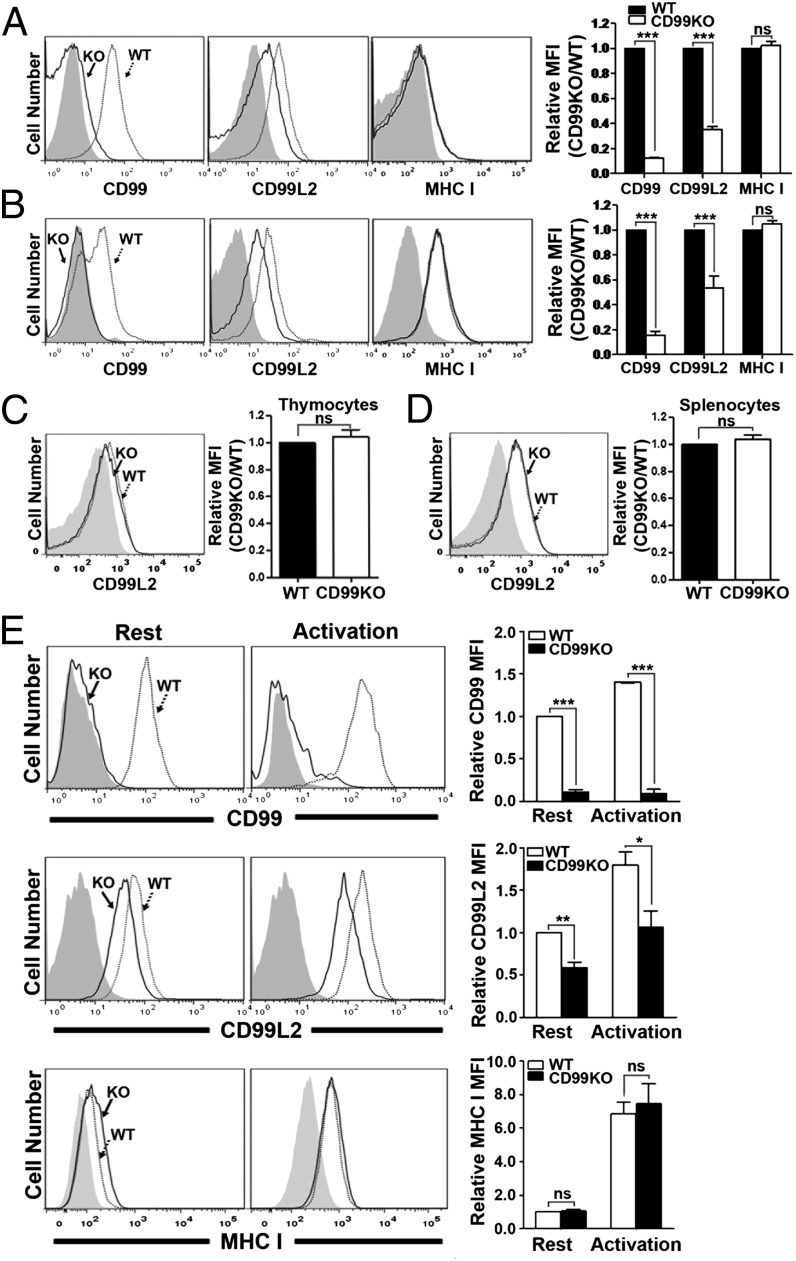
Reduced surface expression of mCD99L2 on cells from CD99-deficient mice
Because the above results demonstrated the role of mCD99 in terms of gain-of-function analysis, we next questioned whether this function of mCD99 would be revealed via loss-of-function experiments. To address this question, we obtained mice in which the Cd99 gene was trapped by a random pUT-21 plasmid insertion and was consequently not translated (19). Using these mice, we performed flow cytometry to detect the surface expression of mCD99L2. Cells from the thymi and the spleens of CD99-deficient mice displayed no abnormal phenotypic features under normal conditions (without antigenic or pathologic stimulation; data not shown). However, flow cytometric analysis revealed that total thymocytes and splenocytes from the CD99-deficient mice displayed reduced surface levels of mCD99L2 (Fig. 7A, 7B). The levels of mCD99L2 on CD99-deficient thymocytes and splenocytes were 0.3- and 0.5-fold reduced, respectively, compared with those on WT cells. When CD4 and CD8 single-positive thymocytes (minor populations in the thymocytes) and CD3+ T cells or B220+ B cells (two major populations in the splenocytes) were analyzed for the mCD99L2 surface levels via flow cytometry, we observed the same phenotype that mCD99L2 levels were comparatively lower on the CD99-deficient cells (Supplemental Fig. 3). The reduction in mCD99L2 surface expression was in contrast to the consistent surface levels of MHC I (H-2Kb), a target molecule related to the transport activity of hCD99 (12, 14, 15), regardless of the presence of mCD99 expression (Fig. 7A, 7B). When flow cytometric analysis was performed after staining the membrane-permeabilized cells with anti-mCD99L2 Ab, overall mCD99L2 protein levels were found to be comparable between mCD99-deficient and WT cells (Fig. 7C, 7D), implying that the difference in surface levels of mCD99L2 between the two types of cells was not due to differences in production of the mCD99L2 protein. Based on immunoblot and RT-PCR analyses using the lysates of WT or CD99-deficient splenocytes to detect mCD99L2 proteins and transcripts, respectively, the amounts of those produced by CD99-deficient cells were comparable to those produced by WT cells (Supplemental Fig. 4), supporting the results obtained from flow cytometric analysis after intracellular staining.
FIGURE 7.
Downregulation of surface mCD99L2 in mCD99-deficient mouse cells. (A and B) Surface expression of mCD99, mCD99L2, and MHC I proteins on thymocytes (A) or splenocytes (B) isolated from B6 WT or mCD99-deficient (KO) mice. The cells were stained with PE-conjugated anti-CD99, anti-CD99L2 plus PE–conjugated donkey anti-goat IgG, or PE-conjugated anti–H-2Kb Abs. Gray-filled histograms represent isotype or secondary Ab staining control. (C and D) Staining of mCD99L2 after membrane permeabilization of thymocytes (C) and splenocytes (D). (A–D) Relative MFI values of mCD99, mCD99L2, and MHC I in mCD99 KO compared with WT cells were plotted. (E) Surface expression of mCD99, mCD99L2, and MHC I on H60-specific CTL lines originated from WT or CD99 KO mice. The resting (on day 7 prior to next round of restimulation) or activated (on day 5 after restimulation) state of CTL lines was determined based on the fluctuation of surface Ags (e.g., CD44 and CD11a). Relative MFI values of mCD99, mCD99L2, and MHC I were calculated in relationship to the MFI values obtained with WT cells under resting status and were plotted. Data shown (A–E) represent the results obtained from more than three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001.
The levels of both mCD99 and mCD99L2 were not sufficiently high on the naive ex vivo cells and, therefore, the difference in surface mCD99L2 expression between the WT and mCD99-deficient cells was not dramatic (Fig. 7A, 7B). Because expression of mCD99 is upregulated on activated T cells (17), we postulated that the difference in surface mCD99L2 expression between mCD99-deficient and WT cells could be more clearly distinguished when considered alongside T cell activation status. To address this issue, we established CD8 CTL lines from both the CD99-deficient and WT mice and analyzed them for surface expression of mCD99L2 by flow cytometry after staining with anti-mCD99L2 Ab. Both mCD99L2 and mCD99 protein levels were upregulated when the cells were activated (Fig. 7E), with mCD99L2 levels remaining higher on WT CTLs than on CD99-deficient CTLs. As before, the surface MHC I expression levels on the two types CTL lines did not vary.
Collectively, these results demonstrated the negative influence of mCD99 deficiency on surface expression of mCD99L2 specifically and confirmed the involvement of mCD99 in the molecular transport of mCD99L2 to the plasma membrane.
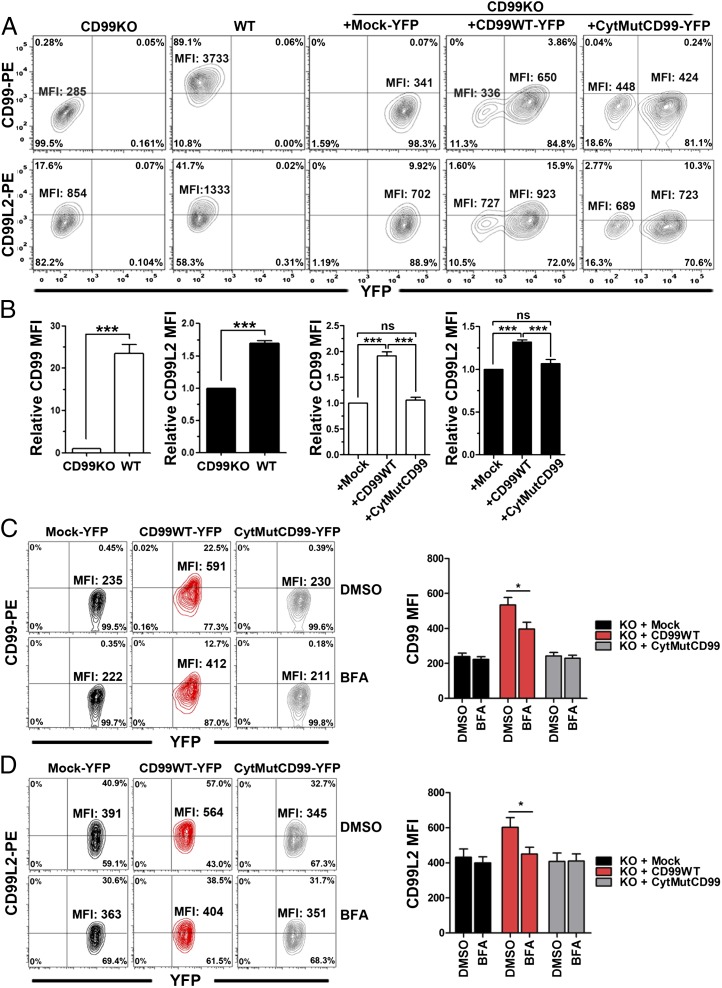
Increased mCD99L2 expression on mCD99-deficient cells after mCD99 gene transduction
Finally, we tested whether the level of mCD99L2 expressed on the surface of mCD99-deficient cells could be reestablished when mCD99 expression was reinstated. To this end, mCD99-deficient CTL cells were retrovirally transduced to express mCD99-YFP and then stained with anti-mCD99 and anti-mCD99L2 Abs to check their expression levels on the surface of YFP+ cells by flow cytometry. The flow cytometry data showed that surface mCD99 expression increased 1.9-fold, on average, after the transduction of mCD99-YFP compared with nontransduced or mock-transduced mCD99-deficient cells, even though the increased expression did not reach the level expressed by WT cells (Fig. 8A, 8B). The surface level of endogenous mCD99L2 on mCD99-deficient cells increased 1.3-fold, on average, after the mCD99-YFP transduction (Fig. 8A, 8B), but it was still lower than on WT CTLs. Such a significant increase in the surface expression of endogenous mCD99L2 on the mCD99-deficient cells was not observed after the transduction with YFP-fused cytoplasmic domain mutant of mCD99 (Fig. 8A, 8B), which was considered to be due to the low capability of the mutant to interact with mCD99L2 and its inefficient trafficking to the plasma membrane. In support of this notion, when we treated CTLs transduced to express YFP-fused WT or the cytoplasmic domain mutant type of mCD99 with BFA, we found that the BFA treatment reduced the levels of endogenous mCD99L2 on the cells transduced with mCD99-YFP, as well as surface levels of the exogenous mCD99-YFP (Fig. 8C, 8D). However, such a reduction in mCD99L2 surface levels was not observed when the cells reconstituted with CytMutCD99-YFP were treated with BFA.
FIGURE 8.
Restoration of mCD99 and mCD99L2 on the surface of CD99-deficient CTL lines by overexpression of mCD99. (A) Flow cytometric analysis of the restoration of mCD99 and mCD99L2 expression on CD99-deficient CTL cells by retrovirus-mediated mCD99-YFP expression. CD99-deficient (CD99KO) CTL cells that were transduced with retrovirus expressing Mock-YFP (CD99KO + Mock-YFP), mCD99-YFP (CD99KO + CD99-YFP), or CytMutCD99-YFP (CD99cyt-YFP) were selected in culture medium containing puromycin and subsequently sorted for YFP+ cells using a FACSAria. Sorted cells were stained with PE-conjugated anti-CD99 or anti-CD99L2 Ab plus donkey anti-goat IgG secondary Ab and analyzed by flow cytometry using an LSR II flow cytometer. (B) Comparison of relative mCD99 and mCD99L2 MFI values. Relative MFI values of mCD99 and mCD99L2 on WT or on mCD99-YFP-transduced (+CD99) or CytMutCD99-YFP-transduced (+CD99cyt) CD99KO cells were obtained in comparison with MFI values on CD99KO or on CD99KO plus Mock-YFP (+Mock) cells, respectively, and were plotted. Data (A, B) represent the results obtained from three independent experiments. ***p < 0.001. (C and D) Flow cytometric analysis was performed to detect surface levels of mCD99 and mCD99L2 expressed in CD99-deficient CTL cells transduced to express Mock-YFP, mCD99-YFP, or CytMutCD99-YFP after treatment with DMSO or BFA. DMSO (0.1%) or BFA (3 μg/ml) was added to transduced CTL cells on regular stimulation for cell passage for 4 h. Then, the cells were harvested, washed, and stained with anti-CD99 Ab (C) or anti-CD99L2 Ab (D), in addition to anti-CD8 Ab. Cells were analyzed via flow cytometry after gating YFP+ and CD8+ cells, and the MFI values for mCD99 (C) or mCD99L2 (D) were plotted. Data shown (C, D) represent three independent experiments. *p < 0.05.
Altogether, the results, which demonstrated fluctuation in the surface level of mCD99L2 according to expression of WT of mCD99, confirmed the supportive role of coexpression of and the interaction with mCD99 in the membrane localization of endogenous mCD99L2.
Discussion
In this study, we demonstrated that mCD99 and mCD99L2 interact to form heterodimers and that this interaction enhances the trafficking of mCD99L2 to the plasma membrane. This is the first demonstration of involvement of mCD99 in intracellular mCD99L2 transport.
As initially postulated by the fact that hCD99 participates in the molecular transport of several surface molecules via physical association, we confirmed physical association of mCD99 with mCD99L2 using BiFC, FRET, and immunoprecipitation assays. We also observed that mCD99L2 transport to the plasma membranes is increased by interaction with mCD99 regardless of whether the former is expressed exogenously or endogenously. The significance of mCD99 involvement in the molecular transport of mCD99L2 was verified by modulation of mCD99L2 surface levels depending on the presence or absence of mCD99. However, unlike hCD99, the positive effect of mCD99 coexpression was not extended to membrane trafficking of MHC I, the most intensely investigated target protein in terms of the trafficking-related function of hCD99 (12, 14, 15, 30). Rather, the surface levels of the mouse counterpart (H-2Kb) were steadily maintained on thymocytes, splenocytes, and CTLs regardless of mCD99 expression. Moreover, the transmembrane domain of CD99, which mediates the association of hCD99 with HLA and Golgi-resident p230/golgin-245 proteins (14, 31–33), was dispensable for the interaction of mCD99 with mCD99L2. These discordant features of hCD99 and mCD99 suggest that CD99 may have diverged in its function with regard to MHC I trafficking. It is also possible that the function of mCD99 in regulating the trafficking of CD99L2 may be unique to mouse cells. Thus, further investigation on the relationship between hCD99 and hCD99L2 and detailed characterization of mCD99 at the molecular level are necessary to clarify these discrepancies.
In our study, the intact cytoplasmic domain of mCD99 was required not only for sufficient interaction with mCD99L2, but also for efficient membrane trafficking of both mCD99 itself and mCD99L2. Because the cytoplasmic domain of mCD99 is too short to contain any signaling motif, recruitment of a signaling molecule may be necessary for further events such as facilitating cytoskeletal reorganization for the transport of cargo proteins. In contrast to mCD99, mCD99L2 proteins (both the short and long forms) have a longer cytoplasmic domain that contains a proline-rich (EPPPPEPPR) sequence (7). Because this motif is recognized by SH3 domains, mCD99L2 itself may play a certain role in the signaling pathways that control cytoskeletal reorganization. Therefore, the cytoplasmic domain–mediated interaction with mCD99 may stabilize or enhance signaling through the cytoplasmic motif of mCD99L2, consequently upregulating the membrane trafficking of mCD99L2. Currently, we are generating various mutants with serial deletions or amino acid substitutions at the cytoplasmic regions of mCD99 and mCD99L2 to pinpoint amino acids critical for their interaction and membrane trafficking of both molecules.
While screening various types of peripheral blood leukocytes from normal B6 mice via flow cytometry, we found that most of the cells were dual-positive cells that express both CD99 and CD99L2 (data not shown). However, single-positive cells were also identified, such as mCD99+mCD99L2− bone marrow–derived neutrophils (8) and mCD99−mCD99L2+ L cells (Fig. 6), suggesting the presence of mCD99 (homodimer and heterodimer) and mCD99L2 (monomer and heterodimer) in various forms at the plasma membrane. Molecular existence and trafficking to plasma membrane in homo- and heterodimeric forms have been reported for some chemokines. Dynamic interplay between these complexes at the cell membrane is considered as adaptation to changes in milieu. CXCR1 and CXCR2, for instance, can form homo- and heterodimers with similar efficiency (34, 35). Homo- and heterodimers of CXCR1 and CXCR2 coexist at the cell surface in the absence of ligand binding. However, ligand CXCL8 disturbs the heterodimers and stabilizes both homodimeric forms. Then, the CXCL8-mediated activation triggers CXCR2 internalization more rapidly owing to its high affinity for CXCR2, leading to rapid reduction of CXCR2 and, relatively, persistence of CXCR1 at the cell membrane (36). CCR5 is mainly maintained within the intracellular compartment of Jurkat cells when it forms homodimers, but it is exported to the plasma membrane by its association with CD4 (37). Membrane trafficking of CCR5 homodimers and CCR5-CXCR4 heterodimers relies on different Rab GTPases, depending on the presence or absence of CD4 (38). Likewise, a shift in molecular forms of mCD99 and mCD99L2 may occur dynamically according to the functional needs or depending on availability of other partner molecules.
An additional role of mCD99, implication in cell death, has been reported; mCD99+ cells were killed through the recognition of mCD99 by PILRβ-expressing NK cells (39), and the ligation of mCD99 to the polymeric fusion protein PILR-FC induced apoptosis in immature thymocytes (40), as is implicated in apoptosis of thymocytes by hCD99 (41), Ewing sarcoma cells (42, 43), and lymphoblast leukemia cells (44). Given the physiologic heterodimeric and homodimeric forms of mCD99, therefore, the identity of CD99 family dimers dominant on the cell surface under normal physiological conditions, during inflammation-induced diapedesis, or during apoptosis would be necessary to be defined to understand the biological relevance of CD99 oligomerization and, ultimately, the role of CD99 protein, and this is currently under investigation.
To date, conflicting reports exist on the role of mCD99L2 in homophilic cell adhesion. Transient mCD99L2 gene transfection has been reported to induce homotypic adhesion of mouse L cells (9), but not that of Chinese hamster ovary cells (8). mCD99L2-transfected HEK293 cells also failed to show significant aggregation in our experiments (data not shown). This discrepant role of mCD99L2 in homophilic cell aggregation might be attributable to the use of different cell lines by the different studies, including concerns of animal origin: mouse versus hamster versus human. With the unique roles of mCD99L2 still unidentified, evaluation of the influence of mCD99 coexpression on the function of mCD99L2 was limited. The overlap in known functions of mCD99 and mCD99L2 (which are both implicated in cell adhesion and migration) hampered the design of an experiment that could discriminate between functional enhancement of mCD99L2 by mCD99 coexpression and an additive functional effect. We elucidated the positive influence of mCD99 coexpression on membrane trafficking of mCD99L2. This finding, combined with the fact that mCD99 and mCD99L2 function at the same site and stage during leukocyte diapedesis (10), suggests another possible role of mCD99: in addition to the simple conveyor role of carrying mCD99L2 to the plasma membrane, mCD99 may also act as a partner to mCD99L2 function at the plasma membrane of leukocyte during transendothelial migration. More data regarding the functions of mCD99 and mCD99L2 need to be accumulated to understand the mechanisms by which they perform their various functions and to address the possibilities raised by this study.
In summary, we demonstrated that a physical interaction between mCD99 and mCD99L2 that influences the molecular trafficking of mCD99. The findings from this study may be of direct relevance to human CD99. Together with previous demonstrations of the roles of mCD99 and mCD99L2 during leukocyte diapedesis, our results provide useful insights into the possible mechanism(s) of controlling transendothelial migration of leukocytes.
This work was supported by grants from the National Research Foundation of Korea (BioDiscovery Research Program Grant 2007-2003619, Basic Science Research Program Grant 2009-0079925, and Science Research Center Program Grant 2010-0029399).
The online version of this article contains supplemental material.
- AU
- arbitrary unit
- B6
- C57BL/6
- BFA
- brefeldin A
- BiFC
- bimolecular fluorescence complementation
- CD99L2
- CD99-like 2
- CD99L2L
- CD99-like 2 (long form)
- CD99L2S
- CD99-like 2 (short form)
- CFP
- cerulean fluorescent protein
- FRET
- fluorescence resonance energy transfer
- h
- human
- HA
- hemagglutinin
- KO
- knockout
- m
- mouse
- MFI
- mean fluorescence intensity
- MHC I
- MHC class I
- PM-YFP
- plasma membrane–targeted yellow fluorescent protein
- WT
- wild-type
- YFP
- yellow fluorescent protein.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Bixel G., Kloep S., Butz S., Petri B., Engelhardt B., Vestweber D. 2004. Mouse CD99 participates in T-cell recruitment into inflamed skin. Blood 104: 3205–3213 [DOI] [PubMed] [Google Scholar]
- 2.Bernard G., Zoccola D., Deckert M., Breittmayer J. P., Aussel C., Bernard A. 1995. The E2 molecule (CD99) specifically triggers homotypic aggregation of CD4+ CD8+ thymocytes. J. Immunol. 154: 26–32 [PubMed] [Google Scholar]
- 3.Bernard G., Raimondi V., Alberti I., Pourtein M., Widjenes J., Ticchioni M., Bernard A. 2000. CD99 (E2) up-regulates α4β1-dependent T cell adhesion to inflamed vascular endothelium under flow conditions. Eur. J. Immunol. 30: 3061–3065 [DOI] [PubMed] [Google Scholar]
- 4.Schenkel A. R., Mamdouh Z., Chen X., Liebman R. M., Muller W. A. 2002. CD99 plays a major role in the migration of monocytes through endothelial junctions. Nat. Immunol. 3: 143–150 [DOI] [PubMed] [Google Scholar]
- 5.Lou O., Alcaide P., Luscinskas F. W., Muller W. A. 2007. CD99 is a key mediator of the transendothelial migration of neutrophils. J. Immunol. 178: 1136–1143 [DOI] [PubMed] [Google Scholar]
- 6.Dufour E. M., Deroche A., Bae Y., Muller W. A. 2008. CD99 is essential for leukocyte diapedesis in vivo. Cell Commun. Adhes. 15: 351–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suh Y. H., Shin Y. K., Kook M. C., Oh K. I., Park W. S., Kim S. H., Lee I. S., Park H. J., Huh T. L., Park S. H. 2003. Cloning, genomic organization, alternative transcripts and expression analysis of CD99L2, a novel paralog of human CD99, and identification of evolutionary conserved motifs. Gene 307: 63–76 [DOI] [PubMed] [Google Scholar]
- 8.Bixel M. G., Petri B., Khandoga A. G., Khandoga A., Wolburg-Buchholz K., Wolburg H., März S., Krombach F., Vestweber D. 2007. A CD99-related antigen on endothelial cells mediates neutrophil but not lymphocyte extravasation in vivo. Blood 109: 5327–5336 [DOI] [PubMed] [Google Scholar]
- 9.Schenkel A. R., Dufour E. M., Chew T. W., Sorg E., Muller W. A. 2007. The murine CD99-related molecule CD99-like 2 (CD99L2) is an adhesion molecule involved in the inflammatory response. Cell Commun. Adhes. 14: 227–237 [DOI] [PubMed] [Google Scholar]
- 10.Bixel M. G., Li H., Petri B., Khandoga A. G., Khandoga A., Zarbock A., Wolburg-Buchholz K., Wolburg H., Sorokin L., Zeuschner D., et al. 2010. CD99 and CD99L2 act at the same site as, but independently of, PECAM-1 during leukocyte diapedesis. Blood 116: 1172–1184 [DOI] [PubMed] [Google Scholar]
- 11.Kim S. H., Shin Y. K., Lee I. S., Bae Y. M., Sohn H. W., Suh Y. H., Ree H. J., Rowe M., Park S. H. 2000. Viral latent membrane protein 1 (LMP-1)-induced CD99 down-regulation in B cells leads to the generation of cells with Hodgkin’s and Reed-Sternberg phenotype. Blood 95: 294–300 [PubMed] [Google Scholar]
- 12.Sohn H. W., Shin Y. K., Lee I. S., Bae Y. M., Suh Y. H., Kim M. K., Kim T. J., Jung K. C., Park W. S., Park C. S., et al. 2001. CD99 regulates the transport of MHC class I molecules from the Golgi complex to the cell surface. J. Immunol. 166: 787–794 [DOI] [PubMed] [Google Scholar]
- 13.Gil M. C., Lee M. H., Seo J. I., Choi Y. L., Kim M. K., Jung K. C., Park S. H., Kim T. J. 2002. Characterization and epitope mapping of two monoclonal antibodies against human CD99. Exp. Mol. Med. 34: 411–418 [DOI] [PubMed] [Google Scholar]
- 14.Brémond A., Meynet O., Mahiddine K., Coito S., Tichet M., Scotlandi K., Breittmayer J. P., Gounon P., Gleeson P. A., Bernard A., Bernard G. 2009. Regulation of HLA class I surface expression requires CD99 and p230/golgin-245 interaction. Blood 113: 347–357 [DOI] [PubMed] [Google Scholar]
- 15.Pata S., Otáhal P., Brdička T., Laopajon W., Mahasongkram K., Kasinrerk W. 2011. Association of CD99 short and long forms with MHC class I, MHC class II and tetraspanin CD81 and recruitment into immunological synapses. BMC Res. Notes 4: 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mamdouh Z., Mikhailov A., Muller W. A. 2009. Transcellular migration of leukocytes is mediated by the endothelial lateral border recycling compartment. J. Exp. Med. 206: 2795–2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi G., Lee S. W., Jung K. C., Choi E. Y. 2007. Detection of homodimer formation of CD99 through extracelluar domain using bimolecular fluorescence complementation analysis. Exp. Mol. Med. 39: 746–755 [DOI] [PubMed] [Google Scholar]
- 18.Lee M. K., Kim H. S., Kim S. S., Cho M. H., Lee I. S. 2008. Analysis of the dimerization of human CD99 using bimolecular fluorescence complementation technique. J. Microbiol. Biotechnol. 18: 472–476 [PubMed] [Google Scholar]
- 19.Araki K., Araki M., Yamamura K. 1997. Targeted integration of DNA using mutant lox sites in embryonic stem cells. Nucleic Acids Res. 25: 868–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi E. Y., Yoshimura Y., Christianson G. J., Sproule T. J., Malarkannan S., Shastri N., Joyce S., Roopenian D. C. 2001. Quantitative analysis of the immune response to mouse non-MHC transplantation antigens in vivo: the H60 histocompatibility antigen dominates over all others. J. Immunol. 166: 4370–4379 [DOI] [PubMed] [Google Scholar]
- 21.Kerppola T. K. 2006. Visualization of molecular interactions by fluorescence complementation. Nat. Rev. Mol. Cell Biol. 7: 449–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong K. A., O’Bryan J. P. 2011. Bimolecular fluorescence complementation. J. Vis. Exp. (50). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masi A., Cicchi R., Carloni A., Pavone F. S., Arcangeli A. 2010. Optical methods in the study of protein-protein interactions. Adv. Exp. Med. Biol. 674: 33–42 [DOI] [PubMed] [Google Scholar]
- 24.Liu J., Ernst S. A., Gladycheva S. E., Lee Y. Y., Lentz S. I., Ho C. S., Li Q., Stuenkel E. L. 2004. Fluorescence resonance energy transfer reports properties of syntaxin1a interaction with Munc18-1 in vivo. J. Biol. Chem. 279: 55924–55936 [DOI] [PubMed] [Google Scholar]
- 25.Karpova T., McNally J. G. 2006. Detecting protein-protein interactions with CFP-YFP FRET by acceptor photobleaching. Curr Protoc. Cytom. Chapter 12: Unit 12.17. [DOI] [PubMed] [Google Scholar]
- 26.Shyu Y. J., Hu C. D. 2008. Fluorescence complementation: an emerging tool for biological research. Trends Biotechnol. 26: 622–630 [DOI] [PubMed] [Google Scholar]
- 27.Várnai P., Balla T. 1998. Visualization of phosphoinositides that bind pleckstrin homology domains: calcium- and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. J. Cell Biol. 143: 501–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teruel M. N., Blanpied T. A., Shen K., Augustine G. J., Meyer T. 1999. A versatile microporation technique for the transfection of cultured CNS neurons. J. Neurosci. Methods 93: 37–48 [DOI] [PubMed] [Google Scholar]
- 29.Zinchuk V., Grossenbacher-Zinchuk O. 2009. Recent advances in quantitative colocalization analysis: focus on neuroscience. Prog. Histochem. Cytochem. 44: 125–172 [DOI] [PubMed] [Google Scholar]
- 30.Kim S. H., Choi E. Y., Shin Y. K., Kim T. J., Chung D. H., Chang S. I., Kim N. K., Park S. H. 1998. Generation of cells with Hodgkin’s and Reed-Sternberg phenotype through downregulation of CD99 (Mic2). Blood 92: 4287–4295 [PubMed] [Google Scholar]
- 31.Barrett S. P., Gleeson P. A., de Silva H., Toh B. H., van Driel I. R. 1996. Interferon-γ is required during the initiation of an organ-specific autoimmune disease. Eur. J. Immunol. 26: 1652–1655 [DOI] [PubMed] [Google Scholar]
- 32.Kakinuma T., Ichikawa H., Tsukada Y., Nakamura T., Toh B. H. 2004. Interaction between p230 and MACF1 is associated with transport of a glycosyl phosphatidyl inositol-anchored protein from the Golgi to the cell periphery. Exp. Cell Res. 298: 388–398 [DOI] [PubMed] [Google Scholar]
- 33.Barth H., Cerino R., Arcuri M., Hoffmann M., Schürmann P., Adah M. I., Gissler B., Zhao X., Ghisetti V., Lavezzo B., et al. 2005. Scavenger receptor class B type I and hepatitis C virus infection of primary tupaia hepatocytes. J. Virol. 79: 5774–5785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richardson R. M., Marjoram R. J., Barak L. S., Snyderman R. 2003. Role of the cytoplasmic tails of CXCR1 and CXCR2 in mediating leukocyte migration, activation, and regulation. J. Immunol. 170: 2904–2911 [DOI] [PubMed] [Google Scholar]
- 35.Trettel F., Di Bartolomeo S., Lauro C., Catalano M., Ciotti M. T., Limatola C. 2003. Ligand-independent CXCR2 dimerization. J. Biol. Chem. 278: 40980–40988 [DOI] [PubMed] [Google Scholar]
- 36.Martínez Muñoz L., Lucas P., Navarro G., Checa A. I., Franco R., Martínez-A C., Rodríguez-Frade J. M., Mellado M. 2009. Dynamic regulation of CXCR1 and CXCR2 homo- and heterodimers. J. Immunol. 183: 7337–7346 [DOI] [PubMed] [Google Scholar]
- 37.Achour L., Scott M. G., Shirvani H., Thuret A., Bismuth G., Labbé-Jullié C., Marullo S. 2009. CD4-CCR5 interaction in intracellular compartments contributes to receptor expression at the cell surface. Blood 113: 1938–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Charette N., Holland P., Frazer J., Allen H., Dupré D. J. 2011. Dependence on different Rab GTPases for the trafficking of CXCR4 and CCR5 homo or heterodimers between the endoplasmic reticulum and plasma membrane in Jurkat cells. Cell. Signal. 23: 1738–1749 [DOI] [PubMed] [Google Scholar]
- 39.Shiratori I., Ogasawara K., Saito T., Lanier L. L., Arase H. 2004. Activation of natural killer cells and dendritic cells upon recognition of a novel CD99-like ligand by paired immunoglobulin-like type 2 receptor. J. Exp. Med. 199: 525–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park H. J., Ban Y. L., Byun D., Park S. H., Jung K. C. 2010. Interaction between the mouse homologue of CD99 and its ligand PILR as a mechanism of T cell receptor-independent thymocyte apoptosis. Exp. Mol. Med. 42: 353–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bernard G., Breittmayer J. P., de Matteis M., Trampont P., Hofman P., Senik A., Bernard A. 1997. Apoptosis of immature thymocytes mediated by E2/CD99. J. Immunol. 158: 2543–2550 [PubMed] [Google Scholar]
- 42.Sohn H. W., Choi E. Y., Kim S. H., Lee I. S., Chung D. H., Sung U. A., Hwang D. H., Cho S. S., Jun B. H., Jang J. J., et al. 1998. Engagement of CD99 induces apoptosis through a calcineurin-independent pathway in Ewing’s sarcoma cells. Am. J. Pathol. 153: 1937–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scotlandi K., Baldini N., Cerisano V., Manara M. C., Benini S., Serra M., Lollini P. L., Nanni P., Nicoletti G., Bernard G., et al. 2000. CD99 engagement: an effective therapeutic strategy for Ewing tumors. Cancer Res. 60: 5134–5142 [PubMed] [Google Scholar]
- 44.Husak Z., Printz D., Schumich A., Potschger U., Dworzak M. N. 2010. Death induction by CD99 ligation in TEL/AML1-positive acute lymphoblastic leukemia and normal B cell precursors. J. Leukoc. Biol. 88: 405–412 [DOI] [PubMed] [Google Scholar]