Abstract
EBV, a human herpesvirus, is commonly acquired during childhood and persists latently in B cells. EBV seropositivity has been connected to immunomodulatory effects such as altered T and NK cell functional responses as well as protection against early IgE sensitization; however, owing to the asymptomatic presentation during childhood little is known regarding the infection process in children of different ages. In this study, we used mononuclear cells from cord blood and from 2- and 5-y-old EBV-naive children for in vitro EBV infection. We show that the degree of EBV-induced B cell activation and expansion differs between age groups and in particular in relationship to IFN-γ production capacity. EBV infection induced redistribution between B cell subsets with enrichment of IgD+CD27+ cells (commonly referred to as non–switched memory) in infected cord blood cell cultures, and of IgD−CD27+ cells (switched memory) in cell cultures from older children. We also related results to serostatus to CMV, a persistent herpesvirus that can affect differentiation status of T and NK cells. As compared with CMV− children, the EBV-induced enrichment of IgD−CD27+ B cells was significantly reduced in infected cell cultures from CMV+ children. This effect was associated with high levels of IFN-γ and frequencies of highly mature CD8+CD57+ T cells in CMV+ children. Our results demonstrate that both a child’s age and serostatus to CMV will have an impact on EBV-induced B cell activation and expansion, and they point to the ability of viruses with immunomodulatory functions, such as CMV, to affect immune responses within the host system.
Introduction
Epstein–Barr virus infection is commonly contracted in early childhood and is prevalent in the majority of the human population. EBV is a B lymphotropic γ-herpesvirus that after primary infection establishes latency and persists for life. EBV latency is connected to a number of malignancies, yet it does not cause symptoms in an immunocompetent host (1). When EBV is acquired during adolescence or after, infectious mononucleosis (IM) frequently develops owing to vigorous immune activation (reviewed in Ref. 2). However, EBV infection in childhood is presumed to follow a mild or asymptomatic course and therefore goes unnoticed in most cases. This type of silent EBV acquisition early on in life has in fact been connected to positive effects regarding allergy development (3, 4), indicating that EBV may have beneficial immunomodulatory effects, at least when acquired during infancy.
The characteristics of the virus have been extensively examined using an in vitro model where resting B cells are exposed to EBV-containing supernatant, and infection in this manner yields growth-transformed B cell blasts (first demonstrated by Pope et al. (5)). Viral entry occurs through interactions between the viral envelope protein gp350 and the B cell CD21 receptor and leads to expression of virally encoded genes, including nuclear and membrane-associated proteins that have pivotal roles in driving B cell proliferation and transformation (6). In vitro–infected B cells will start to express CD23, a characteristic of EBV-driven immortalization (7) and CD27, a feature of memory B cells (8). Expression of CD27 and IgD is frequently used to subdivide B cells. IgD+CD27− cells represent the naive B cell pool whereas IgD−CD27+ cells are commonly referred to as switched memory cells. IgD+CD27+ cells have been characterized as non–switched memory cells or marginal zone–like B cells (9, 10). Healthy asymptomatic EBV carriers have low numbers of circulating EBV+ B cells that are confined mainly to the IgD−CD27+ compartment but can also be found among IgD+CD27+ cells (11, 12).
To maintain the asymptomatic carrier state, EBV needs to be under stringent control by the immune system as shown by EBV-related complications in immunocompromised individuals (1). In healthy seropositive individuals, EBV-specific T cells control the outgrowth of transformed cells by recognition of latent and lytic viral Ags (13). The generation of effector T cells has been studied in IM, where EBV-specific CD8+ T cells expand rapidly and contribute to the massive lymphocytosis observed in IM patients. EBV also stimulates both cytolytic and helper CD4+ T cells that can aid in restriction of virus replication (14, 15).
In vitro studies also demonstrate EBV-induced innate immune activation, such as accessory cell release of cytokines including type I IFNs and IL-12 (16, 17). Furthermore, dendritic cells that are activated by EBV products can prime naive T cells to become reactive against newly infected B cells (18). Other early effectors in antiviral immunity include NK cells. Conflicting data exist regarding their role in EBV infections (discussed in Hislop et al. (13)); however, they have been shown to inhibit EBV-induced transformation of B cells in vitro, partially by IFN-γ production (16, 19).
Similar to EBV, primary infection with CMV, a β-herpesvirus, generally occurs in early childhood and is followed by life-long persistence. CMV primarily resides in cells of the myeloid lineage where it can be intermittently reactivated (20). Interestingly, CMV can act as a driving force for differentiation of T and NK cells. Acutely and latently CMV-infected individuals have enriched populations of late-differentiated T cells expressing the CD57 Ag and NK cells expressing the activation receptor NKG2C, either alone or together with CD57 (21–24). The CMV-driven differentiation of T and NK cells involves acquisition of distinct functional profiles. In the case of cytotoxic T cells this includes lower activation threshold and higher degranulation and cytokine production capacity as well as bystander responses, whereas this is not always the case for NK cells (22, 25, 26). Therefore, an expansion of specific subsets of effector cells in CMV+ individuals could affect the quality of subsequent responses toward other infectious agents as previously suggested (27, 28).
Our group has found that latency with EBV and CMV influenced functional responses of T and NK cells and that these responses differed in children coinfected with both viruses compared with those with EBV alone (29, 30). Furthermore, the time point of primary EBV infection during early childhood appeared important for EBV-induced modulation of immune responses (4). Thus, we hypothesized that the maturational state of the immune system at primary EBV infection, as well as the presence of other latent infections, may affect the course of infection and possibly the long-term immunomodulation by EBV latency. Based on our results in this study we suggest that apart from degree of immune maturity (i.e., age), latent CMV infection may affect the efficacy of EBV infection, likely through IFN-γ responses from highly mature cytotoxic T cells enriched in CMV+ children.
Materials and Methods
Characterization and serostatus of children
Mononuclear cells were collected from cord blood (CBMC) of healthy neonates. PBMC were obtained from 2- or 5-y-old children who were selected from an earlier described family cohort (3, 4) on the basis of EBV seronegativity and CMV serostatus. The presence of serum IgG against EBV capsid Ag was determined according to previously published immunofluorescence assays (31) where a specific fluorescence at a dilution of 1:20 was regarded as a sign of seropositivity. IgG seropositivity to CMV was determined by ELISA using purified nuclear Ags from CMV cultivated in human fetal lung fibroblasts (32), where samples with an OD > 0.2 at a dilution of 1:100 were considered positive. The study was approved by the Human Ethics Committee at Huddinge University Hospital, Stockholm, Sweden (DNR 75/97, 113/97, 331/02), and the parents provided informed verbal consent. No written documentation of the consent was required, according to regulations at the time of initiation of the study and in agreement with the Ethics Committee.
Isolation of mononuclear cells from blood, in vitro infections, and coculture experiments
Following collection of venous blood, CBMC or PBMC were isolated by Ficoll-Paque (Pharmacia-Upjohn, Uppsala, Sweden) gradient centrifugation and cryopreserved in liquid nitrogen. Freshly isolated CBMC and frozen CBMC displayed similar EBV-induced changes in the B cell population (data not shown), and frozen cells were used throughout.
For the in vitro infections, B95-8 EBV virus containing supernatant was used. The virus titer of B95-8 EBV was 2.5 × 105 Ramos infectious units. Ramos infectious units were determined by infection of the EBV− Burkitt’s lymphoma B cell line Ramos, followed by anti-complement immunofluorescent assay to detect the number of infected cells.
The experimental procedure was as follows: PBMC were thawed and then washed three times. For infections, 106 cells per 100 μl B95-8 virus–containing supernatant were incubated in a humidified incubator at 37°C and 5% CO2 for 90 min with shaking every 30 min. Thereafter, the cells were washed and resuspended in complete medium (RPMI 1640 supplemented with 10% heat-inactivated FCS [Hyclone Laboratories, Logan, UT], l-glutamine [2 mmol/l], penicillin G-sodium [100 U/ml], and streptomycin sulfate [100 μg/ml; Merck, Darmstadt, Germany]) to a concentration of 106 cells/ml and plated in 48-well plates (Sarstedt, Nümbrecht, Germany). Control cultures were plated directly after thaw/wash in complete medium as described above. The cells were analyzed on day 3, 7, or 14 and supernatants were collected and stored at −86°C. Fourteen day cultures were fed with complete medium once after 7 d. For virus titrations, cells were incubated in 2× (200 μl) 1× (100 μl), 0.5× (50 μl), or 0.1× (10 μl) virus-containing supernatant as described above with residual incubation volume (up to 100 μl) made up of complete medium.
Where indicated, human recombinant IFN-γ (PeproTech, Rocky Hill, NJ) was added to cultures either once at 10,000 pg/ml after 24 h or at 1000 or 500 pg/ml after 24 h and subsequently every 3 d. For coculture experiments PBMC were incubated either alone or with an EBV+ lymphoblastoid cell line (LCL) or EBV− Burkitt’s lymphoma B cell line (Ramos) at an E:T ratio of 1:1 or 1:2 for 3 d.
Flow cytometry
mAbs conjugated to FITC (CD3, CD4, CD14, CD56, CD69, IFN-γ), PE (CD8, CD19, NKG2C), allophycocyanin (CD27, CD57), PerCP (CD3, CD14, CD19, streptavidin), or biotin (IgD) were used for cell labeling according to standard procedures (all from BD Biosciences [San Diego, CA], except for NKG2C [eBioscience, San Diego, CA]). For detection of T and NK cell intracellular IFN-γ, GolgiStop (BD Biosciences) was added to cultures for 4 h. After surface labeling, cells were fixed, permeabilized, and stained. Data were acquired by a FACSCalibur flow cytometer (BD Biosciences) and analyzed with FlowJo software 9.4 (Tree Star, Ashland, OR). Live gate was set according to forward/side scatter properties and gating was performed on CD3+, CD19+, or CD56+ cells. Data are presented as percentage positive cells for a given variable or geometric mean fluorescence intensity.
Secreted cytokine measurements
Levels of IFN-α, IFN-γ, and IL-10 were measured by sandwich ELISA (Mabtech, Stockholm, Sweden) according to standard procedures.
Statistical analyses
Statistical differences between groups were evaluated by the Kruskal–Wallis or Mann–Whitney U nonparametric test, and correlations were made by the Spearman rank correlation test. Data in text indicate median (numbers in parentheses indicate range). All analyses were performed with Statistica (StatSoft, Tulsa, OK). Statistical significance was assumed when p < 0.05.
Results
Characteristics of transformed B cells in primary in vitro EBV infection
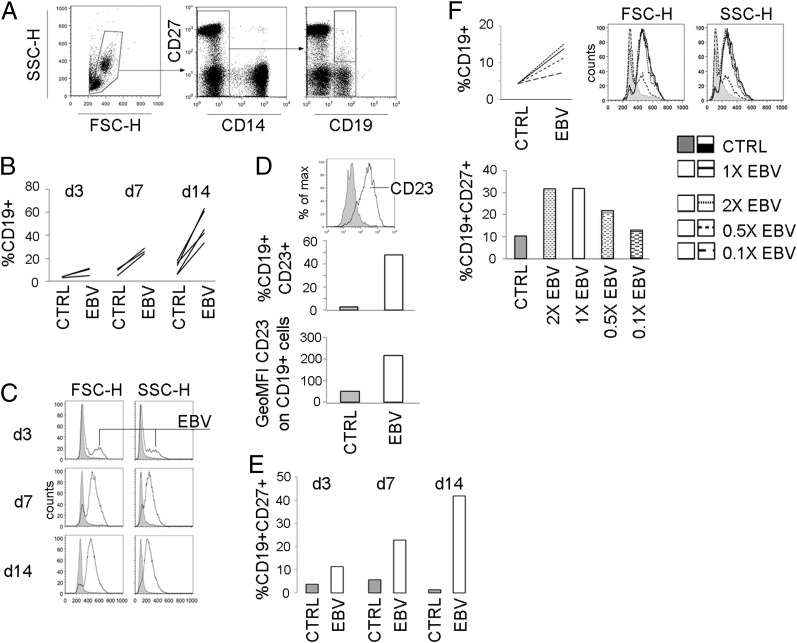
Infection of CBMC has been widely used to yield immortalized EBV-transformed LCL. We began by carefully characterizing our model system using CBMC cultures. First, the presence of EBV-infected cells was confirmed by immunofluorescence staining for latent membrane protein-1 and EBV nuclear Ag 2 (33). As well-documented for in vitro EBV infection, the proportion of B cells and B cell forward and side scatter properties increased successively over time until day 14 (Fig. 1A–C) when about half of the B cell population expressed CD23 (Fig. 1D). The B cell population also acquired the memory marker CD27 (Fig. 1E). The dose of virus used for infection was optimal in terms of increase in B cell frequency, changes in morphology, and induction of CD27 expression as shown by titration experiments (Fig. 1F).
FIGURE 1.
Frequency and characteristics of in vitro–transformed CB B cells. CBMC were subjected to in vitro EBV infection and cultured for 3, 7, or 14 d when the characteristics of the CD19+ B cell population were assessed by flow cytometry. (A) Gating strategy for CD19+ and CD19+CD27+ cells among live-gated cells. For each time point in (B) frequencies of CD19+ cells among live gated cells and in (C) CD19+ cell forward (FSC-H) and side scatter (SSC-H) properties are shown. (D) Frequency of CD19+CD23+ cells and geometric mean fluorescence intensity (GeoMFI) of CD23 on CD19+ cells on day 14. (E) For each time point, frequency of CD19+CD27+ cells is shown. (F) Characteristics of the CD19+ population as in (B), (C), and (E) on day 14 using 2×, 1×, 0.5×, or 0.1× the standard volume of EBV-containing supernatant for transformation. CB (n = 2–5) for each independent experiment.
In vitro EBV-induced B cell activation and expansion differs with age
To investigate the course of a primary infection in children of different ages we used CBMC or PBMC from 2-y-old and 5-y-old EBV-naive children. Before in vitro EBV infection we examined the baseline frequency of B cell subsets in the different age groups ex vivo based on expression of IgD and CD27. As expected, there was an age-dependent decrease in IgD+CD27− B cells and a parallel increase in IgD+CD27+ and IgD−CD27+ B cells. Furthermore, 5-y-old children had higher proportions of IgD−CD27− B cells as compared with 2-y-old children (Table I).
Table I. B cell subset proportions ex vivo in children of different ages.
| Frequency of Cells (%) | Cord Blood (n = 7) | 2-y-Olds (n = 19) | 5-y-Olds (n = 7) | p Value |
|---|---|---|---|---|
| CD19+ | 8.5 (4.6–13.4) | 16.2 (8.5–28) | 14.6 (9.7–19.5) | °<0.001, §<0.05, ¶n.s. |
| CD19+IgD+CD27− | 90.7 (85.8–95.9) | 82.7 (72.2–89.8) | 73.5 (63.3–79.4) | °<0.001, §<0.001, ¶<0.01 |
| CD19+IgD+CD27+ | 1.9 (1.1–2.3) | 5.4 (2.7–13.6) | 7.8 (4.7–10.5) | °<0.001, §<0.001, ¶n.s. |
| CD19+IgD−CD27+ | 0.4 (0.1–0.5) | 5.7 (2.3–10.2) | 9.7 (7.2–19.8) | °<0.001, §<0.001, ¶<0.001 |
| CD19+IgD−CD27− | 6.8 (2.8–12) | 4.9 (2.1–13.5) | 8.2 (6.6–11.6) | °n.s., §n.s., ¶<0.01 |
Numbers indicate median (range).
°Comparison CB and 2-y-old children.
§Comparison CB and 5-y-old children.
¶Comparison 2- and 5-y-old children.
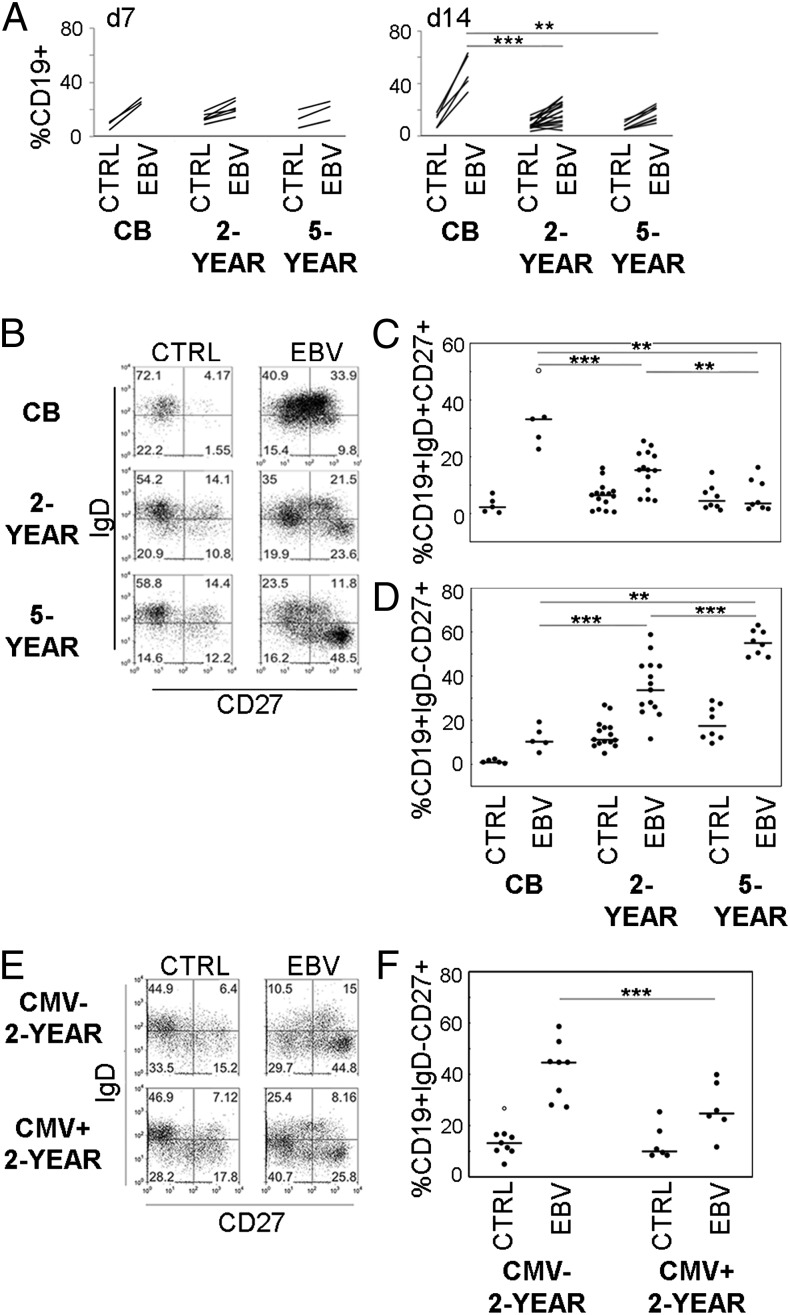
When EBV-infected CBMC cultures were compared with those of PBMC from 2- and 5-y-old children, the initial B cell expansion, measured at day 7, was similar in all groups. However, at day 14, B cells in infected PBMC cultures had expanded significantly less (Fig. 2A). In CBMC cultures the main EBV-induced change in the B cell population was a transition into IgD+CD27+ B cells, whereas in PBMC cultures IgD−CD27+ B cells increased. PBMC cultures from 2-y-olds showed less enrichment of IgD+CD27+ but more IgD−CD27+ B cells than did CBMC, and more IgD+CD27+ but less IgD−CD27+ B cells than from 5-y-olds (shown for day 14 in Fig. 2B–D; for day 7, see Supplemental Fig. 1).
FIGURE 2.
In vitro EBV-induced activation and expansion of B cells differs with age and serostatus to CMV. CBMC or PBMC derived from 2- or 5-y-old children were subjected to in vitro EBV infection and cultured for 14 d when the characteristics of the CD19+ B cell population were assessed by flow cytometry. (A) Frequencies of CD19+ cells on days 7 and 14 postinfection. (B and E) One representative example for each group of CD19+ cell subset proportions based on IgD and CD27 expression. (C, D, and F) Comparison of EBV-induced changes in the CD19+ IgD+CD27+ and IgD−CD27+ cell subsets between groups. In (E) and (F), data from the 2-y-old children are divided into CMV-seronegative (CMV−) or -seropositive (CMV+) groups. Open circles depict outlier values. CB (n = 5), 2-y-olds (n = 15, CMV−, n = 9, and CMV+, n = 6) and 5-y-olds (n = 8). **p < 0.01, ***p < 0.001.
IFN responses following in vitro EBV infection varies with age
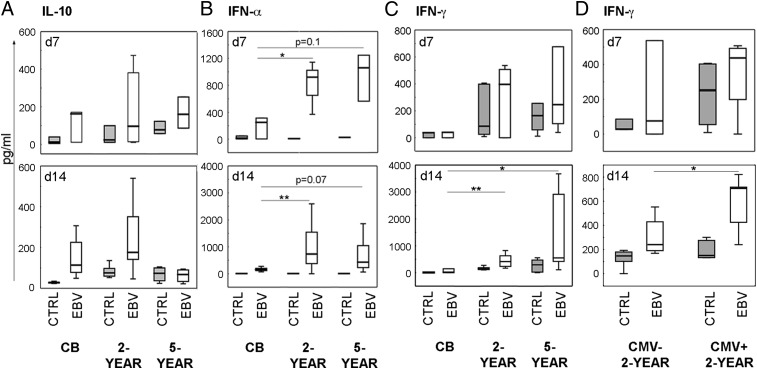
We measured cytokines linked to viral infection in the 7 and 14 d cultures to assess the quality of immune effector responses and their relationship to different rates of B cell activation and proliferation. EBV infection stimulated IL-10 production to similar levels in cultures from both CBMC and PBMC from older children (Fig. 3A), but we observed significant differences in IFN release. IFN-α levels peaked early and IFN-γ later, but production was higher in EBV-infected PBMC than in CBMC cultures at both time points (Fig. 3B, 3C). To relate the levels of cytokine release to the expansion of EBV-infected B cells we performed correlations with data from all children and found a negative correlation between released IFN-γ, but not IFN-α or IL-10, and the percentage of B cells in EBV-infected cultures (rs = −0.46, p = 0.03 for IFN-γ; rs = −0.29, p = 0.16 for IFN-α; rs = −0.04, p = 0.82 for IL-10).
FIGURE 3.
IFN levels following in vitro EBV infection vary with age and serostatus to CMV. CBMC or PBMC derived from 2- or 5-y-old children were subjected to in vitro EBV infection, and cell culture supernatants were collected and examined for cytokines. Secreted levels of (A) IL-10, (B) IFN-α and (C, D) IFN-γ. Shaded histograms represent control cultures and unfilled EBV-infected cultures. In (D), data from the 2-y-old children are divided into CMV-seronegative (CMV−) or -seropositive (CMV+). CB (day 7, n = 3; day 14, n = 6–7), 2-y-olds (day 7, n = 6; day 14, n = 12–16; CMV− day 7, n = 3, day 14, n = 7–9; CMV+ day 7, n = 3, day 14, n = 5–6) and 5-y-olds (day 7, n = 3; day 14, n = 7–8). *p < 0.05, **p < 0.01.
Because IFN-γ appeared important for control of EBV infection, we wanted to determine which cells were responsible for the IFN-γ production in infected cultures and stained for intracellular IFN-γ in CD4+ and CD8+ T cells and CD3−CD56+ NK cells on days 7 and 14. At both time points T and NK cells were activated and produced IFN-γ, albeit at low frequencies (CD3+CD4+ cells, 3.8% [0.5–11.5%]; CD3+CD8+ cells, 3.7% [1.4–14.7%]; and CD56+ cells, 2.1% (0–12%) positive for IFN-γ) at day 14. No differences were detected between age groups (data not shown).
EBV-induced enrichment of IgD−CD27+ B cells is inhibited in CMV-seropositive children and associated with high IFN-γ production
We observed a large spread in data in B cell transition into IgD−CD27+ in the 2-y-old children (Fig 2D). To address whether this was dependent on CMV serostatus, we stratified our group of 2-y-old children into CMV− and CMV+. In infected PBMC cultures from CMV+ children the EBV-induced enrichment of IgD−CD27+ B cells was significantly smaller than in cultures of CMV− children (Fig. 2E, 2F). This was not explained by baseline differences in the frequency and subset composition of the B cell population between CMV− and CMV+ children (Supplemental Table I). We then compared the cytokine production on days 7 and 14. IFN-α or IL-10 levels did not differ between groups (data not shown) but IFN-γ levels were higher in EBV-infected PBMC cultures from CMV+ children at day 14 (Fig. 3D). Moreover, in infected cultures from our 2-y-olds we found a strong negative correlation between released IFN-γ, but not IFN-α or IL-10, and the frequency of IgD−CD27+ B cells (rs = −0.85, p = 0.001 for IFN-γ; rs = −0.05, p = 0.85 for IFN-α; rs = −0.42, p = 0.12 for IL-10).
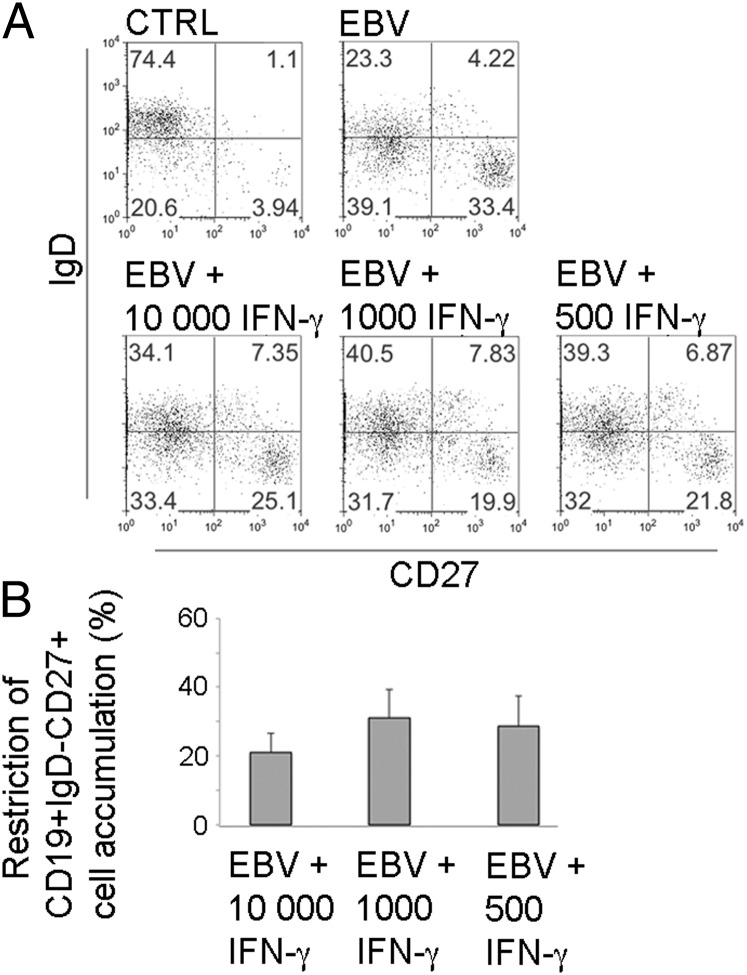
To directly test the effect of IFN-γ on enrichment of IgD−CD27+ B cells, we added recombinant human IFN-γ in different doses and intervals to EBV-infected PBMC cultures of 2-y-old CMV− children who normally displayed large memory populations after infection. From all treatments, the EBV-induced increase in IgD−CD27+ B cell frequency was reduced by on average 26% (17–40%) in three independent experiments, where continuous addition every 3 d with levels of IFN-γ corresponding to those found in cultures from CMV+ children (500–1000 pg/ml) was the most effective treatment (Fig. 4).
FIGURE 4.
IFN-γ can reduce EBV-induced enrichment of IgD−CD27+ B cells. PBMC from 2-y-old CMV− children (n = 3) were subjected to in vitro EBV infection and cultured for 14 d. Recombinant human IFN-γ was added either once at 10,000 pg/ml after 24 h, or at 1,000 or 500 pg/ml after 24 h and subsequently every 72 h. (A) One representative example of CD19+ B cell subset frequencies in control and EBV-infected PBMC cultures after addition of rIFN-γ. (B) Combined data from all three independent experiments shown as percentage IFN-γ–mediated restriction (EBV only cultures represent 0% restriction) on frequencies of CD19+IgD−CD27+ cells in EBV-infected cultures.
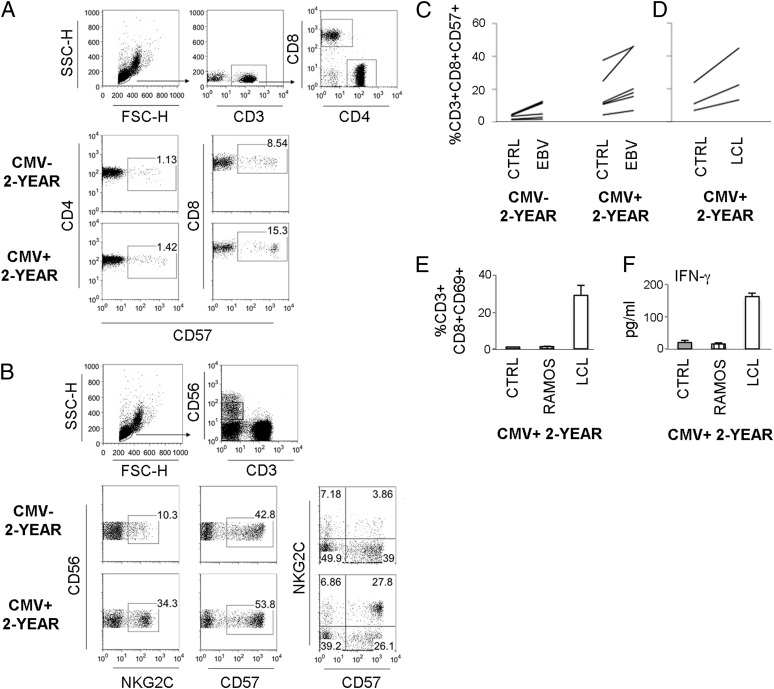
High frequencies of CD8+CD57+ T cells in CMV+ 2-y-old children correlate to IFN-γ levels in EBV infections
CMV-infected individuals have enriched populations of CD57+ T cells and NKG2C+CD57+ NK cells (21–24). In accordance with these studies we found expanded subsets of CD8+CD57+ T cells and NKG2C+ and NKG2C+CD57+ NK cells prior to EBV infection among PBMC of our CMV+ 2-y-old children (Fig. 5A, 5B, Supplemental Table II). Interestingly, upon EBV infection the frequency of CD8+CD57+ T cells doubled in CMV+ children. Also, in the CMV− group the CD8+CD57+ T cells increased in frequency in some, but not all, children (Fig. 5C). To further assess the effect on the CD57-expressing T cell population upon interactions with infected B cell blasts we incubated PBMC of CMV+ children together with EBV+ LCL at an E:T ratio of 1:1 or 1:2 for 3 d. Coculture with LCL led to a doubling of CD8+CD57+ T cell frequencies in this time period regardless of the size of the starting population for both ratios (shown in Fig. 5D for 1:1 ratio). In LCL cocultures CD8+ T cells were activated in terms of CD69 expression, and IFN-γ was detected in supernatants, which was not observed in cocultures with the EBV− B cell line Ramos (Fig. 5E, 5F).
FIGURE 5.
High frequencies of CD8+CD57+ T cells in CMV+ 2-y-old children in the presence of EBV-infected cells. PBMC derived from 2-y-old children that were either CMV-seronegative (CMV−) or -seropositive (CMV+) were phenotyped by flow cytometry ex vivo. (A) Gating strategy for CD3+CD4+ and CD3+CD8+ T cells and typical staining pattern of CD57 expression. (B) Gating strategy for CD56dim NK cells and typical staining pattern of NKG2C and CD57 expression. (C) PBMC were subjected to in vitro EBV infection and cultured for 14 d or (D) were cocultured with EBV+ LCL at a ratio of 1:1 for 3 d and the percentage of CD8+CD57+ T cells was evaluated by flow cytometry. (E and F) PBMC were cocultured with LCL or the EBV− B cell line Ramos at an E:T ratio of 1:1 for 3 d. The percentage of CD8+CD69+ T cells (E) and IFN-γ levels in supernatants (F) was evaluated. For infections: CMV− (n = 5–9) and CMV+ (n = 6–10) 2-y-olds. For coculture experiments: CMV+ 2-y-olds (n = 2–3).
CD8+CD57+ T cells are known to be potent IFN-γ producers (25, 34) and thus their high frequencies in EBV-infected cultures may contribute to the high IFN-γ levels seen therein. We tried to assess IFN-γ production in CD8+CD57+ versus CD8+CD57− T cells in response to the in vitro infection. As mentioned above, intracellular detection of IFN-γ was low, and we found no differences between CD57− and CD57+ CD8+ T cells (CD3+CD8+CD57−, 3.8% [1.1–7.3]; CD3+CD8+CD57+, 4.5% [1.2-5.7%] positive for IFN-γ) at day 14. However, IFN-γ levels in supernatants on day 14 in the 2-y-old group correlated strongly with the proportions of CD8+CD57+ T cells (rs = 0.83, p = 0.01), indicating that they contributed to IFN-γ production in infected cultures. Additionally, there was a strong inverse correlation between the percentages of CD8+CD57+ T cells and CD19+ B cells (rs = −0.73, p = 0.02).
There was no correlation between NKG2C+ or CD57+ NK cells to IFN-γ levels (rs = −0.15, p = 0.67 and rs = −0.42, p = 0.29), and overall effects of in vitro EBV infection on the NK cell population were inconsistent. In EBV-infected cultures the frequency of CD57+ NK cells increased, and the NKG2C+ NK cell subset decreased in most, but not all, children regardless of CMV serostatus (Supplemental Fig. 2).
Discussion
The quality of immune responses develops gradually from birth into adulthood and in the context of the host microbial environment (35). Herpesviruses represent a significant part of our “virome” whose role in shaping immunity is becoming more and more appreciated (36, 37). Owing to the asymptomatic presentation of EBV during infancy, little is known regarding the course of infection in children of different ages. Our previous studies indicated that the time point of EBV acquisition during early childhood, and the occurrence of coinfection with CMV, might affect immunomodulation by the virus (4, 29). In this study, we show in an in vitro–based infection system that a child’s age and serostatus to CMV, and subsequent ability to mount IFN-γ responses, affect EBV-induced activation and expansion of B cells.
The generation of EBV-transformed B cell blasts was most obvious in cultures of CBMC where the B cell population increased rapidly. EBV infection induced enrichment of CD27+ B cells, but CBMC B cells remained largely IgD+ whereas older children had large populations of IgD−CD27+ B cells with a switched memory phenotype. The populations of IgD−CD27+ B cells may have arisen through downregulation of IgD and/or class-switching of infected naive cells. Conversely, memory cells already present in cultures of older children could have been infected to a higher extent or may have proliferated faster than other subsets. EBV is known to induce factors involved in the somatic hypermutation process, including the enzyme AID (8, 38), although additional T cell signals are needed to alter the Ig phenotype of the parental population (naive or non-switched memory) (39). Furthermore, previous studies found that all B cell subsets irrespective of Ig class and memory status have the same ability to be infected in vitro and that the proliferation rate of infected switched memory B cells was equal to that of naive cells (39, 40). Thus, the emergence of a large IgD−CD27+ B cell population in EBV-infected cultures of older children likely represents acquisition of a memory phenotype by infected naive cells along with class-switching/downregulation of IgD, rather than preferential expansion of pre-existing CD27+ B cells. Upon measuring T cell–related cytokines that may have aided isotype switching, IL-21 and TGF-β were detected in control and EBV-infected culture supernatants, but no large differences in levels were found between age groups, indicating that other factors also played a role in the propensity of B cells to switch/downregulate IgD following EBV infection. IL-2, IL-4, and IL-5 were not detected (data not shown).
There is a possibility that inherent B cell–related factors, other than B cell subset distribution, in older children gave rise to differences in infection rate as compared with CBMC. However, on day 7 the B cell population appeared to be similarly infected in all age groups, but as effector responses developed in cultures of older children, B cell proliferation was slowed. Early studies have shown that IFN-γ can limit, but not abolish, transformation of B cells (41, 42), and recently this was proposed to occur through delayed upregulation of latent membrane protein-1 (16). IFN-γ levels in cultures on day 14 were negatively correlated to the percentage of B cells after infection, suggesting that this cytokine (along with other aspects of T cell activation) (43) was involved in the inhibition of EBV expansion observed in the older age groups. In accordance with previous studies (13–16, 19), both T and NK cells were active and produced IFN-γ, although intracellular staining revealed little in terms of which cell type was the main contributor to the secreted IFN-γ. This may be due to the difficulty in capturing the development of effector cell responses without more regular kinetic studies throughout this time period, which were impossible due to restrictions in cell availability.
Latency with EBV and CMV can affect cell differentiation and the quality of immune responses (22–26, 29, 30). A key observation was that EBV-induced enrichment of IgD−CD27+–switched memory B cells was restricted in PBMC cultures of CMV+ 2-y-old children. These cultures also contained more IFN-γ, both in the initial stages of the infection that may be important (16) and, later on, as compared with those of CMV− children. Furthermore, IFN-γ levels negatively correlated to frequencies of IgD−CD27+ B cells, and addition of rIFN-γ reduced IgD−CD27+ B cells in cultures from CMV− children. This strongly suggests that potent IFN-γ responses activated in cell cultures of CMV+ children contributed to restriction of IgD−CD27+ B cell formation, although the mechanism whereby this occurs needs further investigation.
CMV-infected individuals have large populations of CD8+CD57+ T cells (21, 22). These cells are potent effectors owing to high expression of cytolytic molecules and IFN-γ (25, 44). Although we did not find preferential activation of CD8+CD57+ T cells in our in vitro EBV infections, this population, already at high frequencies in CMV+ children, increased further in EBV-infected cultures and upon coculture with EBV+ LCL (but not with the EBV− cell line Ramos). The increase in frequency could result from preferential survival/maintenance of this population as opposed to CD8+CD57− T cells, upregulation of CD57 on CD57− cells, something that normally occurs upon persistent antigenic stimulation (45), or through division of pre-existing CD57+ cells. Although CD57 has been associated with replicative senescence (46), these cells are able to divide under conditions where costimulatory factors are present (34). Regardless, high frequencies of CD8+CD57+ cells were present in EBV-infected cultures from CMV+ children, and their proportions correlated strongly to secreted IFN-γ levels and thus to restriction of B cell expansion and memory formation.
Activation of specific memory T cells can occur in response to secondary related or unrelated infections. This has been shown with herpesvirus infections, either due to cross-recognition of epitopes or through bystander activation mediated by the cytokine environment (28, 47, 48). Specific memory T cells could also be activated as a result of reactivating latent pathogen. Although CMV may reactivate as a consequence of cytokine-driven differentiation of myeloid cells (20), reactivation is not necessary to activate CMV-specific T cells in acute EBV, HIV, or hepatitis B virus infection (28, 48, 49). Collectively, these studies suggest that the activation of pre-existing memory cells could be important for the course of a secondary infection and for T cell homeostasis (discussed in Ref. 50).
In this study, infections were performed with mononuclear cells that originated from EBV-naive children, as determined by antiviral serology. This type of serology in children is complicated. In those children undergoing IM, the development of in particular IgM responses is highly variable, and PCR may be a more definitive test for EBV status (51). That the children in this study were indeed negative for EBV was however strengthened by the fact that their serostatus was determined at several time points during childhood. Three to 5 y after the initial analysis >60% of the children were still EBV−, indicating strongly that at the time of the present study they had not recently converted to EBV with ongoing T cell responses.
Importantly, note that although we employed a well-accepted in vitro model of EBV infection of B cells within PBMC, the natural infection occurs in the oropharynx where EBV is spread via saliva (1). The occurrence of antiviral effector cells and their IFN-γ production in response to EBV infection within this anatomical compartment could differ from that of blood. For example, NK cells produce higher levels of IFN-γ in tonsillar sites (16), and T cells that have homed to tonsils during latent EBV infection have increased cytolytic activity, which correlates with immune control of primary EBV infection (52).
In summary, data in this study illuminate the EBV infection process in children, an understudied population due to asymptomatic presentation in childhood. Our study material originated from children between birth and 5 y of age, the time period when the greatest changes in the B cell population occur (53), where effector cell responses mature, and when EBV is normally encountered. We show that in vitro, EBV activation and expansion of B cells are most evident in CBMC with low antiviral responses and that the subset of B cells amplified/generated by EBV infection is dependent on the child’s age, where older children enrich large switched memory B cell populations. Furthermore, we suggest that effector T cell populations in CMV+ children increase in frequency, produce IFN-γ upon encounter with EBV-infected cells, and that this may restrict EBV establishment through limited formation of switched memory B cells. Thus, immune maturity at primary EBV infection and the presence, or absence, of viruses with immunomodulatory functions within the host system may affect the outcome and course of EBV and other related or unrelated infections.
Acknowledgments
We are grateful to Mats Bemark (Department of Microbiology and Immunology, Institute of Biomedicine, Gothenburg University) for assistance in B cell analysis and to Claudia Carvalho-Queiroz (Department of Molecular Bioscience, Wenner-Gren Institute, Stockholm University) for assistance with ELISAs.
This work was supported by Swedish Research Council Grants 57X-15160-07-03 and 57X-15160-10-4, the Swedish Association for Allergology, the Ragnar Söderberg Foundation, the Ellen, Walter and Lennart Hesselman Foundation, the Konsul Th. C. Bergh Foundation, the Golden Jubilee Memorial Foundation, the Petrus and Augusta Hedlund Foundation, the Crown Princess Lovisa’s/Axel Tielman’s Foundation, the Swedish Cancer Society, and the Karolinska Institute. E.R. and N.N. are recipients of cancer research fellowships from the Cancer Research Institute (New York)/Concern Foundation (Los Angeles).
The online version of this article contains supplemental material.
- CB
- cord blood
- CBMC
- cord blood mononuclear cell
- IM
- infectious mononucleosis
- LCL
- lymphoblastoid cell line.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Rickinson A. B., Kieff E. 2007. Epstein-Barr Virus and its replication. In Fields Virology, Vol. 2, 5th Ed Fields B. N., Knipe D. M., Howley P. M. Lippincott Williams & Wilkins, Philadelphia, p. 2603–2654 [Google Scholar]
- 2.Vetsika E. K., Callan M. 2004. Infectious mononucleosis and Epstein-Barr virus. Expert Rev. Mol. Med. 6: 1–16 [DOI] [PubMed] [Google Scholar]
- 3.Nilsson C., Linde A., Montgomery S. M., Gustafsson L., Näsman P., Blomberg M. T., Lilja G. 2005. Does early EBV infection protect against IgE sensitization? J. Allergy Clin. Immunol. 116: 438–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saghafian-Hedengren S., Sverremark-Ekström E., Linde A., Lilja G., Nilsson C. 2010. Early-life EBV infection protects against persistent IgE sensitization. J. Allergy Clin. Immunol. 125: 433–438 [DOI] [PubMed] [Google Scholar]
- 5.Pope J. H., Horne M. K., Scott W. 1968. Transformation of foetal human keukocytes in vitro by filtrates of a human leukaemic cell line containing herpes-like virus. Int. J. Cancer 3: 857–866 [DOI] [PubMed] [Google Scholar]
- 6.Klein G., Klein E., Kashuba E. 2010. Interaction of Epstein-Barr virus (EBV) with human B-lymphocytes. Biochem. Biophys. Res. Commun. 396: 67–73 [DOI] [PubMed] [Google Scholar]
- 7.Thorley-Lawson D. A., Mann K. P. 1985. Early events in Epstein-Barr virus infection provide a model for B cell activation. J. Exp. Med. 162: 45–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siemer D., Kurth J., Lang S., Lehnerdt G., Stanelle J., Küppers R. 2008. EBV transformation overrides gene expression patterns of B cell differentiation stages. Mol. Immunol. 45: 3133–3141 [DOI] [PubMed] [Google Scholar]
- 9.Klein U., Rajewsky K., Küppers R. 1998. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J. Exp. Med. 188: 1679–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanz I., Wei C., Lee F. E., Anolik J. 2008. Phenotypic and functional heterogeneity of human memory B cells. Semin. Immunol. 20: 67–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joseph A. M., Babcock G. J., Thorley-Lawson D. A. 2000. EBV persistence involves strict selection of latently infected B cells. J. Immunol. 165: 2975–2981 [DOI] [PubMed] [Google Scholar]
- 12.Chaganti S., Heath E. M., Bergler W., Kuo M., Buettner M., Niedobitek G., Rickinson A. B., Bell A. I. 2009. Epstein-Barr virus colonization of tonsillar and peripheral blood B-cell subsets in primary infection and persistence. Blood 113: 6372–6381 [DOI] [PubMed] [Google Scholar]
- 13.Hislop A. D., Taylor G. S., Sauce D., Rickinson A. B. 2007. Cellular responses to viral infection in humans: lessons from Epstein-Barr virus. Annu. Rev. Immunol. 25: 587–617 [DOI] [PubMed] [Google Scholar]
- 14.Nikiforow S., Bottomly K., Miller G. 2001. CD4+ T-cell effectors inhibit Epstein-Barr virus-induced B-cell proliferation. J. Virol. 75: 3740–3752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heller K. N., Gurer C., Münz C. 2006. Virus-specific CD4+ T cells: ready for direct attack. J. Exp. Med. 203: 805–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strowig T., Brilot F., Arrey F., Bougras G., Thomas D., Muller W. A., Münz C. 2008. Tonsilar NK cells restrict B cell transformation by the Epstein-Barr virus via IFN-γ. PLoS Pathog. 4: e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiola S., Gosselin D., Takada K., Gosselin J. 2010. TLR9 contributes to the recognition of EBV by primary monocytes and plasmacytoid dendritic cells. J. Immunol. 185: 3620–3631 [DOI] [PubMed] [Google Scholar]
- 18.Bickham K., Goodman K., Paludan C., Nikiforow S., Tsang M. L., Steinman R. M., Münz C. 2003. Dendritic cells initiate immune control of Epstein-Barr virus transformation of B lymphocytes in vitro. J. Exp. Med. 198: 1653–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masucci M. G., Bejarano M. T., Masucci G., Klein E. 1983. Large granular lymphocytes inhibit the in vitro growth of autologous Epstein-Barr virus-infected B cells. Cell. Immunol. 76: 311–321 [DOI] [PubMed] [Google Scholar]
- 20.Sinclair J. 2008. Human cytomegalovirus: latency and reactivation in the myeloid lineage. J. Clin. Virol. 41: 180–185 [DOI] [PubMed] [Google Scholar]
- 21.Gratama J. W., Kluin-Nelemans H. C., Langelaar R. A., den Ottolander G. J., Stijnen T., D’Amaro J., Torensma R., Tanke H. J. 1988. Flow cytometric and morphologic studies of HNK1+ (Leu 7+) lymphocytes in relation to cytomegalovirus carrier status. Clin. Exp. Immunol. 74: 190–195 [PMC free article] [PubMed] [Google Scholar]
- 22.Wang E. C., Taylor-Wiedeman J., Perera P., Fisher J., Borysiewicz L. K. 1993. Subsets of CD8+, CD57+ cells in normal, healthy individuals: correlations with human cytomegalovirus (HCMV) carrier status, phenotypic and functional analyses. Clin. Exp. Immunol. 94: 297–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gumá M., Angulo A., Vilches C., Gómez-Lozano N., Malats N., López-Botet M. 2004. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood 104: 3664–3671 [DOI] [PubMed] [Google Scholar]
- 24.Lopez-Vergès S., Milush J. M., Schwartz B. S., Pando M. J., Jarjoura J., York V. A., Houchins J. P., Miller S., Kang S. M., Norris P. J., et al. 2011. Expansion of a unique CD57+ NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc. Natl. Acad. Sci. USA 108: 14725–14732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kern F., Khatamzas E., Surel I., Frömmel C., Reinke P., Waldrop S. L., Picker L. J., Volk H. D. 1999. Distribution of human CMV-specific memory T cells among the CD8pos. subsets defined by CD57, CD27, and CD45 isoforms. Eur. J. Immunol. 29: 2908–2915 [DOI] [PubMed] [Google Scholar]
- 26.Béziat V., Dalgard O., Asselah T., Halfon P., Bedossa P., Boudifa A., Hervier B., Theodorou I., Martinot M., Debré P., et al. 2012. CMV drives clonal expansion of NKG2C+ NK cells expressing self-specific KIRs in chronic hepatitis patients. Eur. J. Immunol. 42: 447–457 [DOI] [PubMed] [Google Scholar]
- 27.Barton E. S., White D. W., Cathelyn J. S., Brett-McClellan K. A., Engle M., Diamond M. S., Miller V. L., Virgin H. W., IV 2007. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature 447: 326–329 [DOI] [PubMed] [Google Scholar]
- 28.Sandalova E., Laccabue D., Boni C., Tan A. T., Fink K., Ooi E. E., Chua R., Shafaeddin Schreve B., Ferrari C., Bertoletti A. 2010. Contribution of herpesvirus specific CD8 T cells to anti-viral T cell response in humans. PLoS Pathog. 6: e1001051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saghafian-Hedengren S., Sundström Y., Sohlberg E., Nilsson C., Linde A., Troye-Blomberg M., Berg L., Sverremark-Ekström E. 2009. Herpesvirus seropositivity in childhood associates with decreased monocyte-induced NK cell IFN-γ production. J. Immunol. 182: 2511–2517 [DOI] [PubMed] [Google Scholar]
- 30.Nilsson C., Larsson Sigfrinius A. K., Montgomery S. M., Sverremark-Ekström E., Linde A., Lilja G., Blomberg M. T. 2009. Epstein-Barr virus and cytomegalovirus are differentially associated with numbers of cytokine-producing cells and early atopy. Clin. Exp. Allergy 39: 509–517 [DOI] [PubMed] [Google Scholar]
- 31.Linde A., Andersson J., Lundgren G., Wahren B. 1987. Subclass reactivity to Epstein-Barr virus capsid antigen in primary and reactivated EBV infections. J. Med. Virol. 21: 109–121 [DOI] [PubMed] [Google Scholar]
- 32.Sundqvist V. A., Wahren B. 1981. An interchangeable ELISA for cytomegalovirus antigen and antibody. J. Virol. Methods 2: 301–312 [DOI] [PubMed] [Google Scholar]
- 33.Rasul A. E., Nagy N., Sohlberg E., Ádori M., Claesson H. E., Klein G., Klein E. 2012. Simultaneous detection of the two main proliferation driving EBV encoded proteins, EBNA-2 and LMP-1 in single B cells. J. Immunol. Methods 385: 60–70 [DOI] [PubMed] [Google Scholar]
- 34.Chong L. K., Aicheler R. J., Llewellyn-Lacey S., Tomasec P., Brennan P., Wang E. C. 2008. Proliferation and interleukin 5 production by CD8hi CD57+ T cells. Eur. J. Immunol. 38: 995–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Renz H., Brandtzaeg P., Hornef M. 2012. The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nat. Rev. Immunol. 12: 9–23 [DOI] [PubMed] [Google Scholar]
- 36.Virgin H. W., Wherry E. J., Ahmed R. 2009. Redefining chronic viral infection. Cell 138: 30–50 [DOI] [PubMed] [Google Scholar]
- 37.White D. W., Suzanne Beard R., Barton E. S. 2012. Immune modulation during latent herpesvirus infection. Immunol. Rev. 245: 189–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He B., Raab-Traub N., Casali P., Cerutti A. 2003. EBV-encoded latent membrane protein 1 cooperates with BAFF/BLyS and APRIL to induce T cell-independent Ig heavy chain class switching. J. Immunol. 171: 5215–5224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heath E., Begue-Pastor N., Chaganti S., Croom-Carter D., Shannon-Lowe C., Kube D., Feederle R., Delecluse H. J., Rickinson A. B., Bell A. I. 2012. Epstein-Barr virus infection of naïve B cells in vitro frequently selects clones with mutated immunoglobulin genotypes: implications for virus biology. PLoS Pathog. 8: e1002697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ehlin-Henriksson B., Gordon J., Klein G. 2003. B-lymphocyte subpopulations are equally susceptible to Epstein-Barr virus infection, irrespective of immunoglobulin isotype expression. Immunology 108: 427–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thorley-Lawson D. A. 1981. The transformation of adult but not newborn human lymphocytes by Epstein Barr virus and phytohemagglutinin is inhibited by interferon: the early suppression by T cells of Epstein Barr infection is mediated by interferon. J. Immunol. 126: 829–833 [PubMed] [Google Scholar]
- 42.Lotz M., Tsoukas C. D., Fong S., Carson D. A., Vaughan J. H. 1985. Regulation of Epstein-Barr virus infection by recombinant interferons: selected sensitivity to interferon-γ. Eur. J. Immunol. 15: 520–525 [DOI] [PubMed] [Google Scholar]
- 43.Nagy N., Adori M., Rasul A., Heuts F., Salamon D., Ujvári D., Madapura H. S., Leveau B., Klein G., Klein E. 2012. Soluble factors produced by activated CD4+ T cells modulate EBV latency. Proc. Natl. Acad. Sci. USA 109: 1512–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chattopadhyay P. K., Betts M. R., Price D. A., Gostick E., Horton H., Roederer M., De Rosa S. C. 2009. The cytolytic enzymes granyzme A, granzyme B, and perforin: expression patterns, cell distribution, and their relationship to cell maturity and bright CD57 expression. J. Leukoc. Biol. 85: 88–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strioga M., Pasukoniene V., Characiejus D. 2011. CD8+ CD28− and CD8+ CD57+ T cells and their role in health and disease. Immunology 134: 17–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brenchley J. M., Karandikar N. J., Betts M. R., Ambrozak D. R., Hill B. J., Crotty L. E., Casazza J. P., Kuruppu J., Migueles S. A., Connors M., et al. 2003. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood 101: 2711–2720 [DOI] [PubMed] [Google Scholar]
- 47.Clute S. C., Watkin L. B., Cornberg M., Naumov Y. N., Sullivan J. L., Luzuriaga K., Welsh R. M., Selin L. K. 2005. Cross-reactive influenza virus-specific CD8+ T cells contribute to lymphoproliferation in Epstein-Barr virus-associated infectious mononucleosis. J. Clin. Invest. 115: 3602–3612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doisne J. M., Urrutia A., Lacabaratz-Porret C., Goujard C., Meyer L., Chaix M. L., Sinet M., Venet A. 2004. CD8+ T cells specific for EBV, cytomegalovirus, and influenza virus are activated during primary HIV infection. J. Immunol. 173: 2410–2418 [DOI] [PubMed] [Google Scholar]
- 49.Odumade O. A., Knight J. A., Schmeling D. O., Masopust D., Balfour H. H., Jr., Hogquist K. A. 2012. Primary Epstein-Barr virus infection does not erode preexisting CD8+ T cell memory in humans. J. Exp. Med. 209: 471–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Welsh R. M., Che J. W., Brehm M. A., Selin L. K. 2010. Heterologous immunity between viruses. Immunol. Rev. 235: 244–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dohno S., Maeda A., Ishiura Y., Sato T., Fujieda M., Wakiguchi H. 2010. Diagnosis of infectious mononucleosis caused by Epstein-Barr virus in infants. Pediatr. Int. 52: 536–540 [DOI] [PubMed] [Google Scholar]
- 52.Hislop A. D., Kuo M., Drake-Lee A. B., Akbar A. N., Bergler W., Hammerschmitt N., Khan N., Palendira U., Leese A. M., Timms J. M., et al. 2005. Tonsillar homing of Epstein-Barr virus-specific CD8+ T cells and the virus-host balance. J. Clin. Invest. 115: 2546–2555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morbach H., Eichhorn E. M., Liese J. G., Girschick H. J. 2010. Reference values for B cell subpopulations from infancy to adulthood. Clin. Exp. Immunol. 162: 271–279 [DOI] [PMC free article] [PubMed] [Google Scholar]