Abstract
Pf62-Y and Pf62-X is a pair of allelic Y chromosome-linked and X chromosome-linked markers, and have been used to identify YY super-males, XY males and XX females for commercial production of all-male populations in yellow catfish (Pelteobagrus fulvidraco). However, the SCAR primers used previously have only two nucleotide difference, which restricts the wide utility because of nucleotide polymorphism. In this study, a continuous 8102 bp Pf62-Y sequence and a 5362 bp Pf62-X sequence have been cloned by genome walking, and significant genetic differentiation has been revealed between the corresponding X and Y chromosome allele sequences. Moreover, three pairs of primers were designed to efficiently identify YY super-males, XY males and XX females in an artificial breeding population, and to distinguish XY males and XX females in various wild populations. Together, the three new sex-specific genetic markers develop a highly stable and efficient method for genetic sex identification and sex control application in sustainable aquaculture of all-male yellow catfish.
Keywords: Yellow catfish, Sex-specific marker, genome walking, YY super-males, All-male fish.
Introduction
Sex-specific or sex chromosome-linked markers have significant implications for genetic improvement of economically important traits in some aquaculture fishes 1,2 because of the uncommon growth difference between females and males 3,4,5. Previously, male- or female-specific DNA markers were isolated from several important aquaculture fishes, such as African catfish Clarias gariepinus 6, Asian arowana (Scleropages formosus) 7, Nile tilapia Oreochromis niloticus 8,9,10, salt tolerant tilapia hybrid (Oreochromis mossambicus x Oreochromis spp.)11, rainbow trout Oncorhynchus mykiss 12, half-smooth tongue sole Cynoglossus semilaevis 13,14,15, yellow catfish Pelteobagrus fulvidraco 16, common carp Cyprinus carpio 17,18, Paramisgurnus dabryanus 19, matrinchã Brycon amazonicus 20, Scophthalmus maximus 21, blunt snout bream Megalobrama amblycephala 22, and rock bream Oplegnathus fasciatus 23. In yellow catfish (Pelteobagrus fulvidraco), Wang et al. 16 identified two pairs of Y chromosome- and X chromosome-linked SCAR markers by AFLP screening from an artificial breeding population24, and thereby developed a Y- and X-specific allele marker-assisted sex control technique for mass production of all-male population. Because adult yellow catfish males are about two to three fold bigger than the females, the all-male population, as a novel variety “yellow catfish all-male No. 1”, has been widely used for commercial production throughout China since 2010 1,25.
Significant implications and application values of the all-male yellow catfish production have been evaluated 1,25,26. However, these sex-linked SCAR markers used in all-male production were only tested in one artificial breeding population as reported, and the primers of Pf62-X and Pf62-Y have only two nucleotide difference 16. In order to search for more accurate molecular markers for large-scale all-male production of yellow catfish, we performed genome walking and sequencing of the Y chromosome-linked marker Pf62-Y and X chromosome-linked marker Pf62-X, and analyzed the nucleotide divergence and genetic differentiation. Based on these studies, we developed three pairs of new markers that can efficiently identify the genetic sex of yellow catfish in breeding and wild populations.
Materials and Methods
Source of experimental samples
The artificial breeding yellow catfish including XX females, XY males and YY super-males came from Institute of Hydrobiology, Chinese Academy of Sciences and from Hubei Daming Aquatic Science-Technology Company (Jingzhou). The wild populations were collected from Liangzi Lake (Wuhan), Chang Lake (Jingzhou), Hong Lake (Honghu), South Lake (Zhongxiang) and Dongting Lake (Hunan).
Genomic DNA extraction
The genomic DNA was extracted from fin clips of the XX female, XY male and YY super-male individuals using a standard phenol-chloroform method 27, and the quality and concentration of DNA were assessed by ethidium bromide-stained 0.7% agarose gel under ultraviolet light and by spectrophotometer as described previously 28.
Genome walking
BD Genome Walker™ Universal Kit (Clontech) was used to construct the DNA fragment pools. High quality genomic DNA sample extracted from XX female and YY super-male was divided into four portions, and four kinds of restriction enzymes: Dra I, EcoR V, Pvu II, Stu I were used to digest the DNA samples for 20 h respectively. Phenol/chloroform and 80% ethanol were used to precipitate and rinse the samples, and the digested genomic DNA was dissolved in 1xTE buffer (10 mM Tris, 0.1 mM EDTA). Each batch of digested genomic DNA was then ligated separately to the adapter sequence and used as templates. The adapter primers AP1, AP2 and walking primers listed in Table 1 were designed and synthesized. The genomic walking PCR programs were referred to the manual proposal as described previously 29,30.
Table 1.
Genomic walking primers for cloning Pf62 markers in yellow catfish
| left fragment primers(5'-3') | Right fragment primers(5'-3') | |
|---|---|---|
| GSP-1a: CTCTGTGCGACACGGGGAACGGTGAAACCT | GSPr-1a: CATAAGCCCTGGGGTAAGATGTCTCTCGGA | |
| GSP-1b: CCGAGAGACATCTTACCCCAGGGCTTATGG | GSPr-1b: AGGTTTCACCGTTCCCCGTGTCGCACAGA | |
| GSP-2a: TCAAGATGTAAAGATGCCTCCCTTCTCGCT | GSPr-2a: TCTCCCTCAAGCTGTTGTCCTTCAAGATG | |
| GSP-2b: GCTTTGATCAGGTTGCTGGATTGCGATGT | GSPr-2b: AACGTGTCGGTGATTTGCATGCGCTCTC | |
| GSPr-3a: GAGCTGTATGCTGGTCTAATAAACACAT | ||
| GSPr-3b: CTCCTTGCTCAGTGGCGAAGACATCA | ||
| GSPr-4a: CCCTTTTTTCCTTGGGGCTCAAATGAGTC | ||
| GSPr-4b: CCTTACAAATGAAGGTTGATGTTGAAGTTG | ||
| Adaptor primers: | GSPr-5a: AGATGTAAAGGAGGCAGGTTAGCTGCTG | |
| AP1: GTAATACGACTCACTATAGGGC | GSPr-5b: AAAAAGGAGGGGTGGATAAGAAGAGGAC | |
| AP2: ACTATAGGGCACGCGTGGTC | ||
The amplified products were examined by 1.0% agarose gel electrophoresis. Single and clear major bands (>500bp long bands) were extracted and purified from gel, and directly sequenced in both directions 31,32. Sequence fragments were assembled by DNAMAN software.
To confirm the assembled sequence, a pair of primers, 62F (5'-GATCTGGTGACCTACAAAATTGACA-3') and 62R (5'-AAGCACGACTGGGCCAATGTTTCCATC-3') was designed according to both ends of the entire sequence. L-A Taq polymerase (Cat.No.A0901A, Takara) was used for amplification reaction through the following PCR program: denaturation in 94℃ for 30 seconds, annealing and extension at 68℃ for 9 minutes, 36 cycles. The PCR products were detected and purified by 0.8% agarose gel electrophoresis, and sequenced as described previously 33.
Genetic sex identification in yellow catfish
Genomic DNA was respectively extracted from fin clips of the artificial breeding and wild groups yellow catfish. Then, the genomic DNA of each sample was used for PCR amplification to detect male/female genotype by specific primers designed from the genomic walking sequence. Further, the physical sex of yellow catfish was determined by anatomical dissection.
Results
Genomic walking and sequence analysis of Y-linked marker Pf62-Y
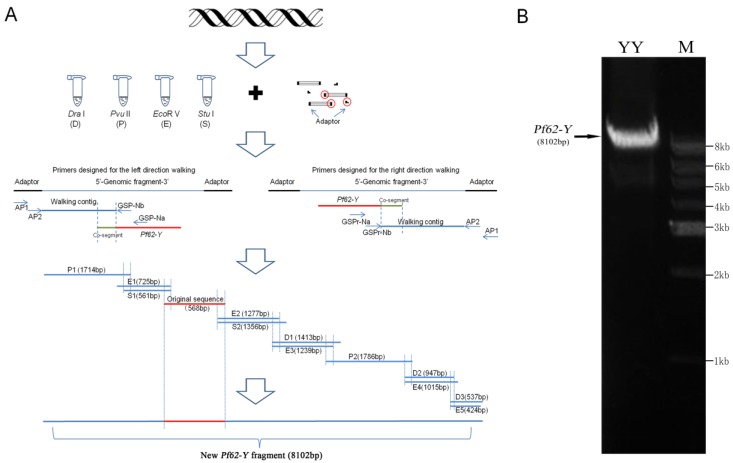
Fig.1A summarized the genomic walking process for Pf62-Y sequence. Firstly, the genomic DNA extracted from YY super-male was digested with 4 different restrict enzymes such as Dra I, Pvu II, EcoR V and Stu I, and the digested fragments were ligated to adaptor respectively to construct four fragment pools. Then, the designed walking primers and adapter primers were used to amplify the corresponding genomic fragments from the constructed four DNA fragment pools, and the amplified fragments were sequenced. A total of 12 fragments were distinguished. As shown in Fig. 1A, three fragments (D1, D2 and D3) were amplified from the Dra I digested DNA fragment pool, two (P1 and P2) from the Pvu II pool, five (E1, E2, E3, E4 and E5) from EcoR V pool, and two (S1 and S2) from Stu I pool. The longest fragment (P2) contained 1786 bp, and the shortest (E5) was only 424 bp. These walking fragment sequences and the original Pf62-Y sequence were analyzed by DNAMAN software and assembled into a continuous 8102 bp Pf62-Y fragment (GenBank accession number: KF593811).
Fig 1.
Genomic walking process and sequence confirmation of Y-linked marker Pf62-Y. (A) The schematic diagram of genomic walking process for Pf62-Y sequence. (B) Detection of the 8102 bp Pf62-Y fragment amplified from an YY super-male in agarose gel.
Moreover, an 8102 bp fragment was amplified from an YY super-male by PCR using L-A Taq polymerase (Fig.1B), and its sequence was identical to the assembled sequence from the walking fragments, which confirmed the genomic walking correction.
Genetic differentiation between Pf62-Y and Pf62-X allele sequences
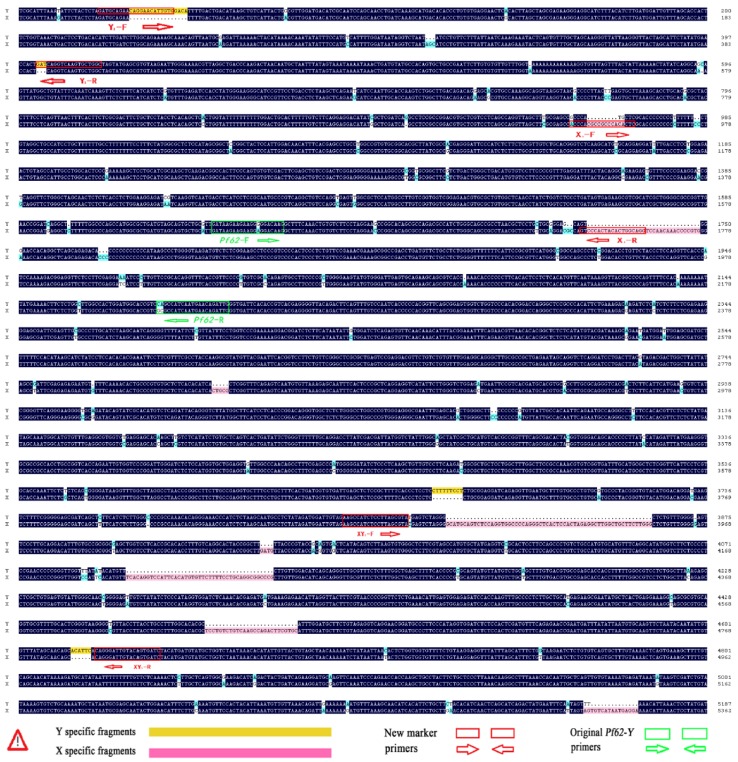
To reveal genetic difference between Pf62-Y and Pf62-X alleles, we further obtained a 5362 bp Pf62-X allele sequence(GenBank accession number: KF593812) from a XX female individual by genomic walking and PCR amplification using degenerate primers. Sequence alignment of the 5362 bp Pf62-X allele and the corresponding Pf62-Y allele shows that the nucleotide identity between them is 87.36%, implicating that there exists substantial genetic differentiation between the Pf62-X and Pf62-Y alleles (Fig.2). Interestingly, besides a large number of single nucleotide differences, Pf62-Y allele has some small segment deletion in comparison with the corresponding Pf62-X allele sequence. It seems to imply that the yellow catfish Y chromosome might be highly differentiated during the sex chromosome evolution process.
Fig 2.
Sequence alignment between Pf62-Y and Pf62-X alleles. There are numerous single nucleotide differences and some small fragment deletion differences between Pf62-Y and Pf62-X.
Design of three pairs of new markers and genetic sex identification in XX females, XY males and YY super-males
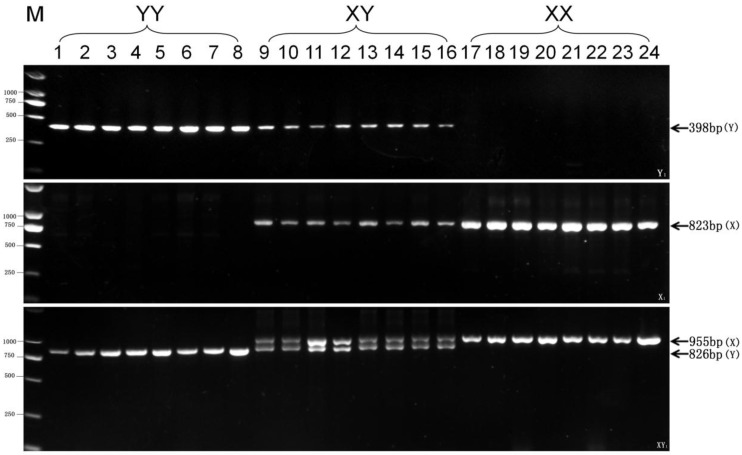
Based on the significant genetic difference between Pf62-Y and Pf62-X allele sequences, we further designed three pairs of new sequence-characterized primers, such as Y chromosome-specific primer pair Y1-F and Y1-R, X chromosome-specific primer pair X1-F and X1-R, as well as Y and X chromosomes-shared primer pair XY1-F and XY1-R (Fig. 2), to identify YY super-males, XY males, and XX females. As shown in Fig. 3, a Y chromosome-specific 398 bp fragment is amplified by the primer pair of Y1-F (GATGCAGAACAGGAACATTGTG) and Y1-R (GCCACCACTTGACCTGATC) from all the YY super-males and XY males, whereas no any product is observed from the XX females. An X chromosome-specific 823 bp fragment is produced by the X1-F (ACCCACGCCGCCCACACTC) and X1-R (ACCTGCCAGTGTAGTGGGAC) primer pair from XX females and XY males, while no any amplified sequence is obtained from the YY super-males. Owing to the existence of 4 fragment deletions on the Pf62-Y allele, two different size fragments (X-fragment: 955bp, Y-fragment: 826bp) are amplified by the primer pair of XY1-F (GATTGTAGAAGCCATCTCCTTAGCGTA) and XY1-R (CATGTAGATCACTGTACAATCCCTG) from the XY males, while only 955bp X-fragment is observed in XX females, and only 826bp Y-fragment in YY super-males. Therefore, three pairs of new genetic markers (Y1, X1 and XY1) were developed to efficiently identify YY super-males, XY males and XX females, and the sex determination mechanism (XX♀/XY♂) of yellow catfish was further proofed by the X- and Y-specific markers.
Fig 3.
Test results of three pairs of new markers (Y1, X1 and XY1) in 8 YY super-males, 8 XY males, and 8 XX females respectively. The 398 bp Y-specific fragment amplified by Y1 primer pair, the 823bp X-specific fragment by X1 primer pair, as well as 955 bp X-specific fragment and 826 bp Y-specific fragment by XY1, are indicated by arrows on the right. The DL2000 DNA marker sizes are shown on the left.
Genetic sex identification of new markers in 5 different wild populations of yellow catfish
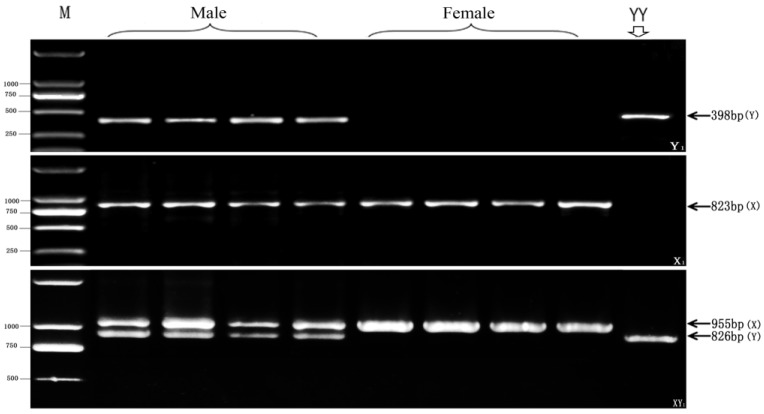
In order to test detection efficiency and applicable scope of the three new genetic markers, we collected different wild population samples of yellow catfish from 5 segregate lakes, such as Liangzi Lake in Wuhan of Hubei, Chang Lake in Jingzhou of Hubei, Hong Lake in Honghu of Hubei, South Lake in Zhongxiang of Hubei, and Dongting Lake in Changde of Hunan. The extracted genome DNAs were respectively used to identify genetic sex and their female or male phenotypes were affirmed by anatomizing ovary or testis. Fig. 4 shows the test results of three new genetic markers in Dongting Lake population samples. Consistent with the detection data in artificial breeding population, a Y chromosome-specific 398 bp fragment is amplified by the primer pair of Y1-F and Y1-R only in male individuals, whereas an X chromosome-specific 823 bp fragment is detected by the X1-F and X1-R primer pair in both of females and males. And, two different size fragments (X-fragment: 955bp, Y-fragment: 826bp) are amplified by the primer pair of XY1-F and XY1-R from the XY males, while only 955bp X-fragment is observed in XX females.
Fig 4.
Test results of three pairs of new markers (Y1, X1 and XY1) in 4 phenotypic male and 4 female individuals from Dongting Lake wild population. The 398 bp Y-specific fragment amplified by Y1 primer pair, the 823bp X-specific fragment by X1 primer pair, as well as 955 bp X-specific fragment and 826 bp Y-specific fragment by XY1, are indicated by arrows on the right. The DL2000 DNA marker sizes are shown on the left. YY individual is the control for the YY super-male.
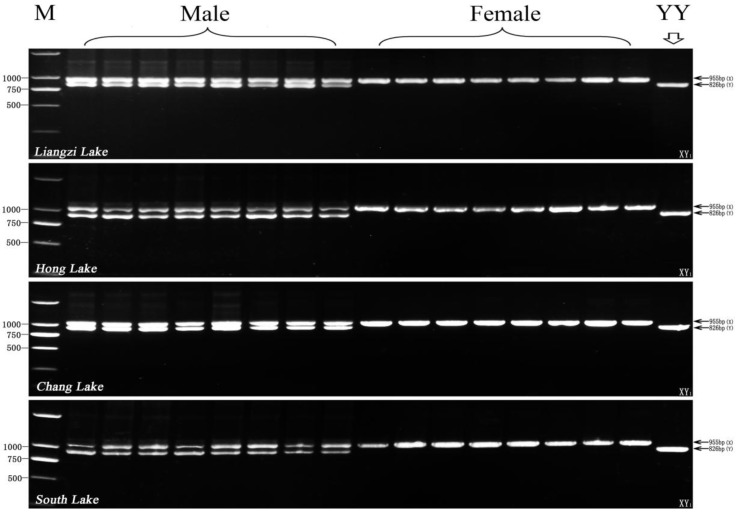
Furthermore, we used XY1-F and XY1-R primer pair to test four other wild populations sampled from Liangzi Lake, Hong Lake, Chang Lake, and South Lake. As shown in Fig. 5, two different size fragments (X-fragment: 955bp, Y-fragment: 826bp) are amplified by the primer pair of XY1-F and XY1-R from all of the male individuals in 4 wild populations, and only 955bp X-fragment is detected from all of the four wild population female samples. Altogether, the three pairs of new genetic markers (Y1, X1 and XY1) can efficiently use genetic sex identification of yellow catfish in all sampled populations, and their detection efficiency reaches to 100%.
Fig 5.
Test results of the XY1 marker in other four wild populations (8 male and 8 female individuals from each population) from Liangzi Lake (Wuhan), Hong Lake (Honghu) Chang Lake (Jingzhou), and South Lake (Zhongxiang). The 955 bp X-specific fragment and 826 bp Y-specific fragment amplified by XY1 primer pair are indicated by arrows on the right. The DL2000 DNA marker sizes are shown on the left. YY individual is the control for the YY super-male.
Discussion
In this study, we have cloned and sequenced the Y chromosome-linked marker Pf62-Y 8102 bp fragment by genome walking, and also obtained its corresponding 5362 bp Pf62-X allele sequence. Their corresponding sequence alignment reveal significant genetic difference between them, implicating that significant genetic differentiation has occurred between the corresponding X and Y chromosome regions. It should be a significant finding for this study. In theory, sex chromosome pair has been proposed to evolve from recombination suppression of a pair of autosomes, and the recombination suppression leads to further genetic differentiation and even hetromorphic sex chromosomes 34. Recently, high degree of sex chromosome differentiation has been also revealed in stickleback fishes by using a set of microsatellite markers 35.
Our previous study has demonstrated that the markers Pf62-Y and Pf62-X are respectively linked to Y chromosome and X chromosome, and they can be used to identify XX females, XY males and YY super-males 16, but the used SCAR primers have only two nucleotide difference, which restricts the wide utility because of nucleotide polymorphism. The genetic difference between Pf62-Y and Pf62-X allele sequences directly reflects genetic differentiation between Y chromosome and X chromosome. According to the significant genetic difference between Pf62-Y and Pf62-X allele sequences revealed by the current study, we have designed three pairs of sex-specific primers, and thereby developed three pairs of new genetic markers, such as Y1, X1 and XY1. It has been demonstrated that the three pairs of new genetic markers can efficiently identify YY super-males, XY males and XX females in artificial breeding populations and can efficiently distinguish XY males and XX females in different wild populations of yellow catfish. In summary, our molecular markers highlight a method for sustainable aquaculture of all-male yellow catfish in China.
Acknowledgments
This work was supported by grants from the special Fund for Agro-scientific Research in the Public Interest from the Ministry of Agriculture of China (2009030406), the National Key Basic Research Program (2010CB126301), the Innovation Project of Chinese Academy of Sciences (KSCX2-EW-N-04), and the Autonomous Project of State Key Laboratory of Freshwater Ecology and Biotechnology (2011FBZ17).
References
- 1.Gui JF, Zhu ZY. Molecular basis and genetic improvement of economically important traits in aquaculture animals. Chin Sci Bull. 2012;57:1751–1760. [Google Scholar]
- 2.Zheng XH, Kuang YY, Lv WH, Cao DC, Zhang XF, Li C, Lu CY, Sun XW. A consensus linkage map of common carp (Cyprinus carpio L.) to compare the distribution and variation of QTLs associated with growth traits. Sci China Life Sci. 2013;56:351–359. doi: 10.1007/s11427-012-4427-3. [DOI] [PubMed] [Google Scholar]
- 3.Gui JF. Genetic basis and artificial control of sexuality and reproduction in fish. Beijing: Science Press; 2007. [Google Scholar]
- 4.Gui JF, Zhou L. Genetic basis and breeding application of clonal diversity and dual reproduction modes in polyploid Carassius auratus gibelio. Sci China Life Sci. 2010;53:409–415. doi: 10.1007/s11427-010-0092-6. [DOI] [PubMed] [Google Scholar]
- 5.Piferrer F, Ribas L, Díaz N. Genomic approaches to study genetic and environmental influences on fish sex determination and differentiation. Mar Biotechnol. 2012;14:591–604. doi: 10.1007/s10126-012-9445-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kovacs B, Egedi S, Bartfai R, Orban L. Male-specific DNA markers from African catfish (Clarias gariepinus) Genetica. 2000;110:267–276. doi: 10.1023/a:1012739318941. [DOI] [PubMed] [Google Scholar]
- 7.Ezaz MT, Harvey SC, Boonphakdee C, Teale AJ, McAndrew BJ, Penman DJ. Isolation and physical mapping of sex-linked AFLP markers in nile tilapia (Oreochromis niloticus L.) Mar Biotechnol. 2004;6:435–445. doi: 10.1007/s10126-004-3004-6. [DOI] [PubMed] [Google Scholar]
- 8.Lee BY, Penman DJ, Kocher TD. Identification of a sex-determining region in Nile tilapia (Oreochromis niloticus) using bulked segregant analysis. Anim Genet. 2003;34:379–383. doi: 10.1046/j.1365-2052.2003.01035.x. [DOI] [PubMed] [Google Scholar]
- 9.Lee BY, Coutanceau JP, Ozouf-Costaz C, D'Cotta H, Baroiller JF, Kocher TD. Genetic and physical mapping of sex-linked AFLP markers in Nile tilapia (Oreochromis niloticus) Mar Biotechnol. 2011;13:557–562. doi: 10.1007/s10126-010-9326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu F, Sun F, Li J, Xia JH, Lin G, Tu RJ, Yue GH. A microsatellite-based linkage map of salt tolerant tilapia (Oreochromis mossambicus x Oreochromis spp.) and mapping of sex-determining loci. BMC Genomics. 2013;14:58. doi: 10.1186/1471-2164-14-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yue GH, Ong D, Wong CC, Lim LC, Orban L. A strain-specific and a sex-associated STS marker for Asian arowana (Scleropages formosus, Osteoglossidae) Aqua Res. 2003;34:951–957. [Google Scholar]
- 12.Felip A, Young WP, Wheeler PA, Thorgaard GH. An AFLP-based approach for the identification of sex-linked markers in rainbow trout (Oncorhynchus mykiss) Aquaculture. 2005;247:35–43. [Google Scholar]
- 13.Chen SL, Li J, Deng SP, Tian YS, Wang QY, Zhuang ZM, Sha ZX, Xu JY. Isolation of female-specific AFLP markers and molecular identification of genetic sex in half-smooth tongue sole (Cynoglossus semilaevis) Mar Biotechnol. 2007;9:273–280. doi: 10.1007/s10126-006-6081-x. [DOI] [PubMed] [Google Scholar]
- 14.Chen SL, Deng SP, Ma HY, Tian YS, Xu JY, Yang JF, Wang QY, Ji XS, Shao CW, Wang XL, Wu PF, Deng H, Zhai JM. Molecular marker-assisted sex control in half-smooth tongue sole (Cynoglossus semilaevis) Aquaculture. 2008;283:7–12. [Google Scholar]
- 15.Ma Q, Liu S, Zhuang Z, Lin L, Sun Z, Liu C, Ma H, Su Y, Tang Q. Genomic structure, polymorphism and expression analysis of the growth hormone (GH) gene in female and male Half-smooth tongue sole (Cynoglossus semilaevis) Gene. 2012;493:92–104. doi: 10.1016/j.gene.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Wang D, Mao HL, Chen HX, Liu HQ, Gui JF. Isolation of Y- and X-linked SCAR markers in yellow catfish and application in the production of all-male populations. Anim Genet. 2009;40:978–981. doi: 10.1111/j.1365-2052.2009.01941.x. [DOI] [PubMed] [Google Scholar]
- 17.Chen J, Wang Y, Yue Y, Xia X, Du Q, Chang Z. A novel male-specific DNA sequence in the common carp, Cyprinus carpio. Mol Cell Probes. 2009;23:235–239. doi: 10.1016/j.mcp.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Chen JJ, Du QY, Yue YY, Dang BJ, Chang ZJ. Screening and identification of male-specific DNA fragments in common carps Cyprinus carpio using suppression subtractive hybridization. J Fish Biol. 2010;77:403–413. doi: 10.1111/j.1095-8649.2010.02700.x. [DOI] [PubMed] [Google Scholar]
- 19.Xia XH, Zhao J, Du QY, Zhi JH, Chang ZJ. Cloning and identification of a female-specific DNA marker in Paramisgurnus dabryanus. Fish Physiol Biochem. 2011;37:53–59. doi: 10.1007/s10695-010-9415-6. [DOI] [PubMed] [Google Scholar]
- 20.da Silva EM, Wong MS, Martins C, Wasko AP. Screening and characterization of sex-specific DNA fragments in the freshwater fish matrincha, Brycon amazonicus (Teleostei: Characiformes: Characidae) Fish Physiol Biochem. 2012;38:1487–96. doi: 10.1007/s10695-012-9638-9. [DOI] [PubMed] [Google Scholar]
- 21.Viñas A, Taboada X, Vale L, Robledo D, Hermida M, Vera M, Martínez P. Mapping of DNA sex-specific markers and genes related to sex differentiation in turbot (Scophthalmus maximus) Mar Biotechnol. 2012;14:655–663. doi: 10.1007/s10126-012-9451-6. [DOI] [PubMed] [Google Scholar]
- 22.Rao HO, Deng JC, Wang WM, Gao ZX. An AFLP-based approach for the identification of sex-linked markers in blunt snout bream, Megalobrama amblycephala (Cyprinidae) Genet Mol Res. 2012;11:1027–1031. doi: 10.4238/2012.April.19.7. [DOI] [PubMed] [Google Scholar]
- 23.Xu D, Lou B, Xu H, Li S, Geng Z. Isolation and characterization of male-specific DNA markers in the rock bream Oplegnathus fasciatus. Mar Biotechnol. 2013;15:221–229. doi: 10.1007/s10126-012-9480-1. [DOI] [PubMed] [Google Scholar]
- 24.Liu HQ, Cui SQ, Hou CC, Xu J, Chen HX. YY super-male generated gynogenetically from XY female in Pelteobagrus fulvidraco (Richardson) Acta Hydrobiologica Sinica. 2007;31:718–725. [Google Scholar]
- 25.Liu HQ, Guan B, Xu J, Hou CC, Tian H, Chen HX. Genetic manipulation of sex ratio for the large-scale breeding of YY super-male and XY all-male yellow catfish (Pelteobagrus fulvidraco Richardson) Mar Biotechnol. 2013;15:321–328. doi: 10.1007/s10126-012-9487-7. [DOI] [PubMed] [Google Scholar]
- 26.Zhong L, Chen J, Cai Y, Pan J, Xia A. Research progress in DNA molecular markers of yellow catfish Pseudobagrus fulvidraco. Chinese Agriculture Science Bulletin. 2012;28:67–75. [Google Scholar]
- 27.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: A laboratory manual, 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. pp. 463–468. [Google Scholar]
- 28.Zhou L, Wang Y, Gui JF. Genetic evidence for gonochoristic reproduction in gynogenetic silver crucian carp (Carassius auratus gibelio bloch) as revealed by RAPD assays. J Mol Evol. 2000;51:498–50. doi: 10.1007/s002390010113. [DOI] [PubMed] [Google Scholar]
- 29.Xia W, Zhou L, Yao B, Li CJ, Gui JF. Differential and spermatogenic cell-specific expression of DMRT1 during sex reversal in protogynous hermaphroditic groupers. Mol Cell Endocrinol. 2007;263:156–172. doi: 10.1016/j.mce.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 30.Huang W, Zhou L, Li Z, Gui JF. Expression pattern, cellular localization and promoter activity analysis of ovarian aromatase (Cyp19a1a) in protogynous hermaphrodite red-spotted grouper. Mol Cell Endocrinol. 2009;307:224–236. doi: 10.1016/j.mce.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Li FB, Gui JF. Clonal diversity and genealogical relationships of gibel carp in four hatcheries. Anim Genet. 2008;39:28–33. doi: 10.1111/j.1365-2052.2007.01671.x. [DOI] [PubMed] [Google Scholar]
- 32.Xu S, Xia W, Zohar Y, Gui JF. Zebrafish dmrta2 regulates the expression of cdkn2c in spermatogenesis in the adult testis. Biol. Reprod. 2013;88(14):1–12. doi: 10.1095/biolreprod.112.105130. [DOI] [PubMed] [Google Scholar]
- 33.Jiang FF, Wang ZW, Zhou L, Jiang L, Zhang XJ, Apalikova OV, Brykov VA, Gui JF. High male incidence and evolutionary implications of triploid form in northeast Asia Carassius auratus complex. Mol Phylogenet Evol. 2013;66:350–359. doi: 10.1016/j.ympev.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 34.Charlesworth D, Charlesworth B, Marais G. Steps in the evolution of heteromorphic sex chromosomes. Heredity. 2005;95:118–128. doi: 10.1038/sj.hdy.6800697. [DOI] [PubMed] [Google Scholar]
- 35.Shikano T, Natri HM, Shimada Y, Merila J. High degree of sex chromosome differentiation in stickleback fishes. BMC Genomics. 2011;12:474. doi: 10.1186/1471-2164-12-474. [DOI] [PMC free article] [PubMed] [Google Scholar]