Abstract
This chapter highlights the use of nonhuman primate models of cocaine addiction and the use of positron emission tomography (PET) imaging to study the role of individual differences in vulnerability and how environmental and pharmacological variables can impact cocaine abuse. The chapter will describe studies related to the dopamine (DA) neurotransmitter system, and focus primarily on the D2-like DA receptor, the DA transporter and the use of fluorodeoxyglucose to better understand the neuropharmacology of cocaine abuse. The use of nonhuman primates allows for within-subject, longitudinal studies that have provided insight into the human condition and serve as an ideal model of translational research. The combination of nonhuman primate behavior, pharmacology and state-of-the-art brain imaging using PET will provide the foundation for future studies aimed at developing behavioral and pharmacological treatments for drug addiction in humans.
Keywords: Animal models, PET imaging, Dopamine, D2-like receptors, Fluorodeoxyglucose (FDG), Nonhuman primates
1 Introduction
Drug dependence remains a consistent societal problem resulting in deleterious consequences on an individual’s health, work, and family that resonates throughout communities worldwide and bears with it an overwhelming financial burden (WHO 2004). In the United States alone, over 20 million people over the age of 12 met the DSM-IV criteria for drug abuse or dependence in 2008 (translating into nearly 1 in every 15 people; SAMHSA 2009) including 1.6 million cocaine users (SAMHSA 2010). Within the European Union, 56% of all countries reporting on cocaine trends documented increases (WHO 2004). Although numerous advances have been made to improve our understanding of addiction including effectively demonstrating addiction is a brain disease, treatment for numerous addictions, including psychostimulant abuse have remained elusive. Development of successful treatment relies on a strong understanding of the neurobiological etiology of addiction. The goal of the current review is to describe the influence of environmental, physiological, and pharmacological factors contributing to changes in the dopamine (DA) neurotransmitter system across various correlates of the addiction cycle including vulnerability, maintenance, abstinence, and relapse to cocaine addiction, as assessed via positron emission tomography (PET) imaging in nonhuman primate (NHP) models.
1.1 Positron Emission Tomography and Dopamine Neurotransmission
PET is an imaging technique used to visualize and quantify the interaction of a radiolabelled molecule of known structure within an organism in a noninvasive manner. The present focus will be the utility of PET neuroimaging to characterize the DA neurotransmitter system and its malleability following physiological, environmental, or pharmacological manipulations in NHPs. Briefly, the DA system is comprised of four neuronal pathways originating from midbrain nuclei with projections to various brain structures (see Beaulieu and Gainetdinov 2011 for review). The nigrostriatal pathway innervates the dorsal striatum (caudate-putamen) and is involved in motor control. The mesolimbic pathway projects to the ventral striatum (nucleus accumbens) and other limbic structures including the amygdala, hippocampus, and cingulate gyrus and mediates actions related to reward, reinforcement, emotion, and motivation. The mesocortical pathway innervates frontal cortical regions and is implicated in learning and memory. Lastly, the tuberoinfundibular pathway projects to the hypothalamus and influences anterior pituitary gland function. Dysregulation of the mesencephalic DA system through neurodegeneration or pharmacological insult can contribute to a number of disease states in addition to Parkinson’s Disease, including depression, attention-deficit/hyperactivity disorder (ADHD), schizophrenia, and addiction (for reviews see Vallone et al. 2000; Beaulieu and Gainetdinov 2011).
There are two superfamilies of DA receptors, the D1-like and D2-like G-protein coupled-receptors, originally distinguished by their ability to stimulate and inhibit adenylyl cyclase activity, respectively. D1-like receptors are primarily located postsynaptically whereas D2-like receptors are located pre- and post-synaptically functioning as autoreceptors as well as post-synaptic effectors. There are currently no D1-like PET studies in NHP models of cocaine abuse. The D2-like superfamily consists of D2, D3, and D4 receptor subtypes, which have been investigated in PET studies with [18F]N-methylspiperone (NMSP), [11C]raclopride and [18F]fluorocl-ebopride (FCP), among other tracers. Much of our research utilizes [18F]FCP, which binds with high affinity at D2-like receptors (Mach et al. 1996) to allow examination DA-rich region such as the basal ganglia (Fig. 1a). As it relates to cocaine addiction, the D3 receptor subtype has received recent attention because its expression is limited to limbic regions. However, D3 receptor-selective radioligands have only recently become available for PET studies and will not be described in this review. Also located on presynaptic DA nerve terminals are DA transporters (DAT) that function to transport synaptic DA intracellularly, where it can be repackaged in vesicles through the action of the vesicular monoamine transporters (VMAT) or degraded by catechol-O-methyltransferase (COMT; for reviews see Vallone et al. 2000; Beaulieu and Gainetdinov 2011), although the main pathway for intracellular DA catabolism is mediated by monoamine oxidase (MAO; Kopin 1985). In this review, we will describe studies using [18F](+)-N-(4-fluorobenzyl)-2β-propanoyl-3β-(4-chlorophenyl)tropane (FCT) and [18F]-[8-(2-fluoroethyl)-2β-carbomethoyx-3β-(4-chlorophenyl)nortropane] (FECNT) to label the DAT. PET studies examining VMAT and COMT will not be reviewed.
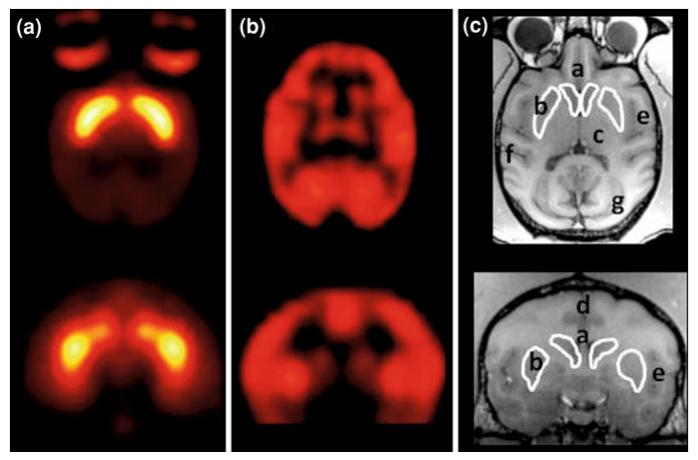
Fig. 1.
PET imaging in NHPs. FCP binding to D2-like receptors in the basal ganglia of an anesthetized monkey (a), FDG uptake occuring over 40 min of responding maintained by 190-g pellet delivery measuring cerebral glucose utilization in cortical and subcortical regions (b), and corresponding MR. c Each scan occurred in a single rhesus monkey; a caudate nucleus; b putamen; c thalamus; d anterior cingulate cortex; e insular cortex; f temporal cortex; g occipital cortex; top horizontal; bottom coronal slices
Another utility for PET imaging is the in vivo investigation of the consequences of dopaminergic activity in the central nervous system using tracers that can measure blood flow or energy use, as a means of measuring neural activity. [15O]H2O for example, is a marker of blood flow and can be used to characterize the acute effects of a pharmacological stimulus. Another tracer is [18F]-fluorodeoxyglucose (FDG) which is an analog of glucose, the sole energy substrate in the brain under normal conditions. Increased rates of uptake of FDG reflect increases in local energy use and, in turn, functional activity. These methods are intended for the evaluation of manipulations that occur over relatively short time frames (e.g., drug administration or brief behavioral task) with changes in rates of glucose utilization calculated by comparison to scans obtained during baseline conditions. For example, Fig. 1b highlights FDG uptake in cortical and subcortical regions in a rhesus monkey. FDG was administered prior to the start of a baseline motor task. Following 40 min of behavior during which each response resulted in pellet delivery, the monkey was sedated and FDG uptake was examined via PET. In addition, FDG methods can be used to assess differences in basal brain glucose metabolism associated with disease states or phenotypes. Altered basal rates of glucose utilization generally have longer time courses (hours, days, and weeks), and reflect intrinsic and ongoing changes in brain activity representing the cumulative effects of experience with the internal and external milieu (e.g., Borghammer et al. 2010). For a more comprehensive list of PET radiotracers targeting the DA and serotonin systems and their contributions to the field of addiction studies in NHPs see Howell and Murnane (2011).
Cocaine is a psychomotor stimulant that binds with near equal affinity to the DAT, serotonin and norepinephrine transporter (SERT and NET, respectively) but its reinforcing effects are thought to be mediated mainly through DAT blockade, and subsequent elevations of extracellular DA, as first evidenced by in vivo microdialysis studies in rodents (Di Chiara and Imperato 1988). Radiolabelled cocaine ([11C]-cocaine) was tested in baboons in the late 1980s, showing sub-stantially higher binding in DAT-rich striatal regions, thus providing a noninvasive method for visualizing the binding site for cocaine within the CNS (Fowler et al.1989). Further, [11C]-cocaine receptor availability in striatum decreased following pretreatment with unlabeled cocaine and other DAT inhibitors, but not administration of NET or SERT inhibitors providing evidence of predominately DAT-specific binding (Fowler et al. 1989). Other displacement studies have involved administering compounds known to elevate DA during a PET study with a tracer that competes with DA. In one example using FCP (Mach et al. 1997), several psychomotor stimulants (including cocaine) displaced the tracer in the basal ganglia (caudate nucleus and putamen) in an orderly fashion similar to DA elevations seen in microdialysis studies. In addition, Kimmel et al. (2008) noted an inverse relationship between reinforcing effects of cocaine analogs, quantified as the peak number of injections during self-administration (SA), and the time to peak uptake of the same [11C]-labeled cocaine analogs. Together, these studies demonstrated the binding site of cocaine within the CNS, biodistribution and pharmacokinetic differences between psychostimulants, and correlations of PET with behavioral measures to provide data relevant to understanding abuse liability and pharmacotherapeutic development for potential DAT inhibitors (see Murnane and Howell 2011).
1.2 Nonhuman Primates as Research Subjects
The development of new imaging modalities has made in vivo small animal imaging using rodents an exciting line of research. For this review, however, we will focus only on PET imaging research utilizing NHPs. As it relates to translational research, NHPs are more phylogenetically related to humans and, along with baboons, Old World macaques (rhesus, Macaca mulatta, and cynomolgus, M. fascicularis) are the closest relatives of humans approved for biomedical research in the United States. Macaques have close homology to humans in terms of developmental and aging processes, neurotransmitter distribution, and complex social and cognitive behavioral repertoires (see Weerts et al. 2007 for review). For example, humans and NHPs share greater than 95% overall gene homology and greater than 98% homology in monoaminergic transporters (Hacia et al. 1998; Miller et al. 2001). Further, documented differences in DA neuron innervation (Berger et al. 1991; Joel and Weiner 2000) and affinity of DA for receptors between monkey and rodent (Weed et al. 1998) may be indicative of other differences in drug biodistribution, pharmacokinetic or pharmacodynamic interactions within the DA system (e.g., Lyons et al. 1996; Roberts et al. 1999; Lile et al. 2003). Using animal models with neurotransmitter distribution and receptor localization resembling that in humans is critical when using PET imaging to generalize preclinical results to the human condition (Nader and Czoty 2008). An additional advantage of NHP research is the ability for longitudinal study designs within a laboratory setting. Baseline behavioral, neurochemical, and hormonal measures can be correlated with changes following an experimental manipulation (e.g., chronic drug administration) while controlling for such factors as stress and nutrition over many years. As it relates to drug addiction, the longer the history of drug exposure, the stronger the face validity of the animal model.
NHPs have an extensive and complex behavioral repertoire. Macaques form a linear social hierarchy that can be quantified by measuring the number of agonistic interactions between monkeys (e.g., Kaplan et al. 1982). Typically, the most dominant monkey aggresses toward all other animals and has access to the largest allotment of resources whereas the most subordinate monkey does not aggress, submits and avoids more dominant monkeys, spends more time alone, and has less access to resources. We describe this continuum as one in which the living conditions of dominant monkeys can be considered an enriched environment while subordinate monkeys are exposed to an environment of chronic social stress. This continuum allows an examination of how social and environmental variables (i.e., acute or chronic stress and enrichment) affect neuroendocrine function (Czoty et al. 2009a; Riddick et al. 2009) or neurobiology influencing vulnerability to self-administer drugs (Morgan et al. 2002; Czoty et al. 2004; Nader and Czoty 2005). These studies provide direct translational applicability toward understanding how social stressors may influence the propensity to initiate drug use, relapse during abstinence, or conversely, positively influence sustained abstinence.
Another advantage presented by NHP models is their capacity for learning complex cognitive tasks similar to those administered to humans. Human cocaine abusers show signs of cognitive impairments on tasks measuring strategic planning, such as the ability to withhold or modify a behavioral response, working memory and measures of impulsivity (Fillmore and Rush 2002; Bolla et al. 2004; Hester and Garavan 2004; Goldstein et al. 2007, 2010); similar impairments have been shown in monkeys following cocaine SA (Liu et al. 2008, 2009; Porter et al.2011). Cognitive impairments have been hypothesized to perpetuate a cycle of compulsive drug use and increase the prevalence of relapse (Goldstein and Volkow 2002; Koob and Volkow 2011). Therefore, understanding when (duration or dose) and how (neurobiological alterations) cocaine affects executive function is integral in developing effective treatment strategies. Longitudinal studies examining cognitive performance in monkeys allows for the assessment of environmental (e.g., stressors) or pharmacological agents that can be assessed using PET (e.g., Porrino et al. 2005; Hampson et al. 2009, 2011).
Often overlooked until recently, sex differences are emerging as an important variable in preclinical (e.g., Lynch 2006; Terner and de Wit 2006) and clinical settings (Zilberman et al. 2003; O’Brien and Anthony 2005; Greenfield et al.2010). Similar to humans, female macaques have a ~28 day estrous cycle marked by similar fluctuations in hormone levels, notably those of estrogen and progesterone (e.g., Dukelow et al. 1979). Influence of hormones on various drug-related behaviors (e.g., reinforcing or discriminative stimulus effects) and effects of drugs on hormonal regulation are empirical questions beginning to be delineated (see Mello and Mendelson 2009; Evans and Foltin 2010 for reviews). For example, the relationship between menstrual cycle phase and D2-like receptor availability has been examined in women, with three different results. Using [18F]NMSP, Wong et al. (1988) observed a trend toward lower striatal uptake during the follicular versus luteal phase, indicating either lower D2-like receptor densities or higher striatal DA concentrations during the follicular phase. In a second study from this group, [11C]raclopride binding potential was lower in the putamen (but not caudate nucleus or ventral striatum) in women during the luteal versus follicular phase (Munro et al. 2006). In contrast, Nordström et al. (1998) observed no menstrualcycle dependent variation in [11C]raclopride availability in the putamen of five women. Of course, there are many factors that could influence D2-like measures, so it is not surprising that in different populations of women, the interaction between menstrual cycle phase and D2-like receptor availability is complicated. In experimentally na€ ive female macaques, though, the relationship was quite straightforward and orderly (Czoty et al. 2009b). In that study using a within-subjects design in which seven female cynomolgus monkeys were scanned in the follicular and luteal phases of the menstrual cycle, D2-like receptor availability using [18F]FCP was significantly lower during the follicular phase compared to the luteal phase. As will be described in greater detail, D2-like receptor availability is intimately related to vulnerability to self-administer drugs of abuse.
1.3 Animal Models
There are several advantages to using animal models, including the ability to perform experiments that are ethically or practically impossible in humans. For example, as it relates to addiction, the use of animal models allows for imaging the brain of individuals prior to any drug exposure, so as to permit the assessment of trait variables (i.e., whether that brain measure represents a pre-existing characteristic that is predictive of an outcome) and state variables (i.e., whether the independent variable was associated with a change in brain function; see Nader and Czoty 2005 for further discussion). Before discussing animal models of cocaine addiction and brain imaging studies, it is worthwhile to first describe the types of animal models (see also Katz and Higgins 2003).
At its simplest level, there are two methods for animals to receive drugs of abuse: non-contingent, i.e., administered by the experimenter, or contingent, i.e., self-administered by the animal. The choice of methods depends on the research question. If the researcher simply wants to know where in the brain a drug is binding, then non-contingent administration will suffice. This has been used with great success as part of ligand-development studies. However, if the researcher wants to understand the role of environment, the organism’s phenotype and environment × organism interactions mediating drug abuse, then contingent drug SA studies are necessary. Differences in response to contingent vs. non-contingent cocaine include the pattern and magnitude of DA efflux measured via microdialysis (Bradberry 2000; Lecca et al. 2007), HPA axis responsivity (Galici et al. 2000), and lethality (Dworkin et al. 1995), supporting the notion that addiction is more complex than just neurobiological changes resulting from drug-receptor interactions. In humans, drug abuse is a chronic condition, such that long-term SA studies in NHPs provide a homologous model. Equally important, SA paradigms allow animals to self-administer at rates and intakes that provide an index of individual differences, in contrast to fixed experimenter-administered regimens that may affect neurobiology differently.
The distinction between contingent and non-contingent drug administration can also be considered in terms of models of “formal equivalence” (Carlton 1983). As described by Katz and Higgins (2003), these are models developed to simulate a specific symptom of the human disease. In short, these types of models have established “face validity”—the endpoint in animals resembles some aspect of the human condition. Chronic drug administration impacts on receptors in the brain, resulting in (perhaps) long-term changes in brain function that can be modeled in animals and imaged using the same instruments as those used for humans. In terms of face validity, as will be described below, chronic drug administration is certainly more relevant to human addiction than, for example, acute drug treatments, but SA has higher face validity than non-contingent drug administration.
2 Models of Cocaine Addiction and DA Receptor Function
Drug SA models have been used for decades to examine the reinforcing effects of compounds (e.g., Woolverton and Nader 1990; Mello and Negus 1996), including examination of potential pharmacotherapies. Animal drug SA resembles human drug use (Griffiths et al. 1980) and produces parallel neurobiological effects (e.g., Beveridge et al. 2008; Everitt et al. 2008). For example, in monkeys, chronic cocaine SA induced long-lasting changes in the mesolimbic DAergic system (Moore et al. 1998; Letchworth et al. 2001; Nader et al. 2002, 2006; Porrino et al.2007) and altered cerebral blood flow, metabolism, and function (Lyons et al.1996; Beveridge et al. 2006) in a manner similar to what has been observed in human cocaine users (Strickland et al. 1993; Volkow et al. 1993).
2.1 Models of Vulnerability
As mentioned above, perhaps the best use of animals, including NHPs, in models of addiction involves assessing phenotypic traits that render an individual more or less vulnerable to addiction. Currently, among the best examples of the use of imaging to study trait variables have involved vulnerability to drug abuse in relation to the DA D2-like receptor. In humans, Volkow et al. (1999) reported an inverse relationship between D2-like receptor availability and subjective effects of intravenous methylphenidate (MP). In that study, 23 non-drug abusing males were scanned with [11C]raclopride in a drug-free condition, and at another time were administered MP and asked to rate its subjective effects. Volkow et al. (1999) found that men with lower D2-like binding potentials found MP “pleasant”, while men with higher D2-like binding potentials found the same dose to be “noxious”. This suggests that individuals with low D2-like receptor availability would be more vulnerable to stimulant abuse than individuals with high D2-like measures. This same inverse relationship was observed in male rhesus monkeys (Nader et al.2006). In that study, 12 experimentally na€ ive monkeys were scanned with [18F]FCP and then trained to respond on a lever maintained by banana-flavored food pellets. When responding was stable, the reinforcer was changed from banana-flavored pellets to 0.2 mg/kg intravenous cocaine. Over the first 10 weeks of exposure, cocaine-maintained responding was higher in monkeys with lower D2-like receptor measures compared to monkeys with higher baseline D2-like receptor availability. Taken together, these two findings support the hypothesis that low D2-like receptor availability makes individuals more vulnerable for stimulant abuse. The use of NHP models in the latter study controlled for many variables that exist in humans, such as past drug use, stress, etc. However, the use of NHPs can also be used to determine if these D2-like receptor measures are malleable to environmental and pharmacological variables.
If D2-like receptor availability influences vulnerability, can these levels be modified (increased or decreased) before exposure to cocaine and thereby alter vulnerability? The answer appears to be “yes”. In one study, baseline PET scans were conducted in individually housed male cynomolgus monkeys prior to random placement in social groups of four monkeys/pen (Morgan et al. 2002). As mentioned above, macaques form linear hierarchies, with the most dominant monkeys (#1) winning all fights, being groomed more, having access to the entire pen, etc., while the most subordinate monkeys (#4) being the target of most aggression, they are groomed least and are frequently used as a model of socially derived stress. Morgan et al. (2002) reported no relationship between initial D2-like receptor measures and eventual social rank, indicating that D2-like receptor availability is not a trait marker for social rank. However, D2-like receptor availability changed significantly after stable social hierarchies were formed and the change occurred in the dominant monkeys. On average, D2-like receptor availability increased by 20% after monkeys became dominant. This increase in D2-like receptor availability subsequently protected the monkeys from the initial reinforcing effects of cocaine (Morgan et al. 2002). In fact, not only did subordinate monkeys, who had the lower D2-like receptor measures, self-administer cocaine at higher rates compared to dominant animals, the dominant monkeys, with their elevated D2-like receptors, didn’t find cocaine reinforcing. These two monkey studies highlight an important role for D2-like receptors in vulnerability to cocaine abuse and suggest viable targets for pharmacotherapy development.
An important consideration that will require much future research involves the use of female subjects. The human and monkey research described above involved males. We have recently replicated the findings above using initially individually housed female cynomolgus monkeys who were then randomly assigned to social groups of four monkeys/pen. We found similar results with D2-like receptor availability as seen in males—D2-like receptors at baseline were not trait markers for eventual social rank, but these measures increased significantly in females who became dominant. However, the relationship between D2-like receptor availability and vulnerability to cocaine reinforcement was opposite. Dominant females, with higher D2-like receptor measures, acquired cocaine reinforcement at lower doses compared to subordinate females who had low D2-like receptor availability (Nader et al. under review). These findings suggest that treatment strategies that target the D2-like receptor should be opposite in males and females. For example, drugs that increase D2-like receptor availability would be hypothesized to be effective in males, but would likely fail in females. Clearly, more research involving females is required to validate this conjecture.
2.2 Models of Maintenance
Drug addiction is characterized as a chronic, relapsing condition. Thus, it is imperative for our animal models to incorporate long-term chronic drug administration. The use of NHPs is particularly advantageous in this regard, because monkeys can be used in intravenous drug SA studies for many years, and can self-administer extremely high doses of drug that better model the human condition compared to rodent models. In male monkeys, we found that long-term cocaine SA produced robust decreases in D2-like receptor availability as assessed with [18F]FCP and PET (Nader et al. 2006). The mechanism(s) for this reduction in D2-like receptor availability may be (1) receptor down-regulation, (2) elevations in extracellular DA concentrations or (3) both. Without additional studies, it is impossible to determine which mechanism mediates the effects we are observing. There have been, however, several studies by multiple groups that can provide important information related to the mechanisms involved in the reductions in D2-like availability. Our group has conducted terminal in vitro receptor autoradiography studies confirming that level of protein (i.e., D2-like receptor density) is significantly lower in monkeys with a cocaine SA history compared to control monkeys (Moore et al. 1998; Nader et al. 2002). Concerning the second possible mechanism, acute cocaine elevates extracellular DA (Czoty et al. 2000), and acute methamphetamine reduced the apparent affinity of [11C]-raclopride (Doudet and Holden 2003) consistent with increased interstitial DA levels, but it is unlikely that chronic cocaine SA produces long-lasting elevations since Bradberry (2000) reported within-session tolerance to cocaine-induced elevations in DA. Another method used in conjunction with PET imaging is the co-administration of a “cold” compound that can reduce extracellular DA concentrations, thereby increasing the likelihood that the assessment of receptor availability will occur under conditions of low competition with DA. Using baboons, Dewey et al. (1992) conducted two studies in the same animal on the same day to investigate GABA-mediated inhibition of DA release. The first PET study of the day was a baseline measure of D2-like receptor availability using [11C]raclopride. The second PET study was preceded by administration of either the benzodiazepine lorazepam or the indirectacting GABA agonist gamma-vinyl-GABA (GVG); both compounds should decrease extracellular DA concentrations. Consistent with their hypothesis, administration of lorazepam and GVG increased D2-like receptor availability by nearly 50 and 30%, respectively. The PET studies showing reductions in D2-like availability following cocaine SA included the use of lorazepam in order to decrease DA concentrations (Nader et al. 2006).
Up to this point, we could summarize our findings as follows: in males, low D2-like receptor availability appears to increase vulnerability to cocaine abuse and that continued cocaine use results in further decreases in D2-like receptor availability, perhaps developing a spiral cycle toward addiction (Koob and Le Moal 2000). Another question that can be explored with PET involves “drug seeking” vs. “drug taking”. Volkow et al. (1993) noted that there was no relationship between D2-like receptor function and her subjects’ typical cocaine dose, but there was a significant correlation between D2-like binding potential and duration of cocaine use. To better explore this relationship, we conducted a study in 12 experimentally na€ ive male rhesus monkeys in which baseline D2-like receptor availability and DAT availability was determined in all monkeys prior to cocaine access (Czoty et al.2007). Monkeys were then trained to self-administer cocaine under a very lean schedule of reinforcement—a fixed-interval 30 min schedule of 0.03 mg/kg cocaine. The cocaine dose was low and the contingency was such that the first response after 30 min resulted in a cocaine injection; sessions ended after 2 injections. Thus, monkeys were “drug seeking” every day, but were not self-administering very high doses. Monkeys self-administered cocaine under these conditions for approximately 9 weeks and were then rescanned. The mean cocaine intake over that time was approximately 5 mg/kg. There were no effects of cocaine SA on D2-like or DAT availability in the caudate nucleus and putamen—two regions that have been shown to be sensitive to environmental and pharmacological manipulations (e.g., Morgan et al. 2002; Czoty et al. 2005; Nader et al. 2006). Such findings suggest that during acquisition, the pharmacological effect of cocaine, and not drug seeking per se, is the critical determinant of decreases in D2-like receptor availability.
Howell and colleagues have extended the use of PET to examine relationships between receptor occupancy and the ability of drugs to decrease cocaine SA (e.g., Lindsey et al. 2004; Howell et al. 2007). In one study (Lindsey et al. 2004), several drugs that bind to the DAT were substituted for cocaine in rhesus monkeys trained to SA drugs under a second-order schedule of reinforcement. These same drugs were also tested for their ability to decrease cocaine SA. In a final experiment, DAT occupancy was measured by administering behaviorally active doses during a PET study with the DAT ligand [18F]FECNT. These investigators found that selective DAT inhibitors required high DAT occupancy (between 50% and 90%) to reduce cocaine SA and to function as reinforcers; such information will be critical for the development of long-acting compounds that reduce cocaine abuse and that have low abuse liability.
2.3 Models of Abstinence and Relapse
The previous sections have documented a relationship between D2-like receptor availability and vulnerability and changes during chronic cocaine exposure. Of critical relevance is whether these changes in D2-like receptor availability persist during abstinence. Volkow et al. (1993), using [18F]NMSP, reported significantly lower D2-like binding potentials in abstinent cocaine abusers compared to control subjects. These investigators were only able to examine abstinence periods up to 4 months; all subjects had relapsed by then. Although it would be several years later before a potential inverse relationship between D2-like receptor availability and vulnerability would be identified, the authors speculated that it was possible the lower D2-like receptor measures during abstinence were due to a predisposition of individuals with low D2-like receptor availability to use cocaine, rather than a consequence of chronic cocaine exposure. The studies described in the previous two sections indicate that low D2-like receptor availability increases vulnerability to use drugs and that chronic cocaine use further decreases D2-like receptor function (in males). The use of NHPs has allowed for long-term study of SA and permits the study of abstinence beyond the 4 months reported in human cocaine abusers.
Nader et al. (2006) examined recovery of D2-like receptor availability in male rhesus monkeys that had been self-administering cocaine. In one study, short access to cocaine reduced D2-like receptor availability, but recovery occurred in all monkeys within 1–3 weeks. Five animals were studied in abstinence after 1 year of cocaine SA; D2-like receptor availability was reduced by ~20% in all monkeys. For three monkeys, there was complete recovery of [18F]FCP signal within 3 months of abstinence. However, in two monkeys, there was no recovery of D2-like receptor availability after 1 year of abstinence. There were no differences in the amount of cocaine self-administered over the 1 year or in the rate of cocaine SA between monkeys that showed D2-like receptor recovery and those that did not. However, there were subtle behavioral differences that may have predicted a lack of recovery (see Nader et al. 2006). The main point is that the combination of behavioral pharmacology and PET imaging provided novel and important information that may guide future research into environmental and/or pharmacological modifications of D2-like receptor availability.
3 Functional Sequelae of Cocaine Administration
3.1 Blood Flow and Metabolic Responses to Cocaine-Related Stimuli
Physiological and functional changes can be measured via PET in response to environmental (e.g., conditioned stimuli) or pharmacological (e.g., cocaine) challenges that may contribute to repeated drug use, cognitive decline and relapse. Howell et al. (2001) trained drug-na€ ive rhesus monkeys to lie still in a head and body restraint system to allow examination of cerebral blood flow (CBF) in the awake, unstressed condition. An acute non-contingent cocaine injection increased cerebral blood flow primarily in the dorsolateral prefrontal cortex (PFC), in a dosedependent manner, measured with [15O]H2O-PET (Howell et al. 2002). Further, pretreatment with the SERT inhibitor alaproclate blocked the cocaine-induced elevation in CBF (Howell et al. 2002).
Using a within-subject design and FDG-PET, Henry et al. (2010) examined the effects of a single non-contingent cocaine dose compared to saline, on glucose metabolism prior to and following a history of cocaine SA in rhesus monkeys. In this study, cocaine-induced elevations in glucose metabolism were restricted to the PFC in cocaine-na€ ive monkeys. Following 60 sessions of limited access (1 h) cocaine SA, the same non-contingent cocaine injection increased glucose metabolism in the PFC and also striatum (primarily dorsal striatum). Following an additional 60 sessions of extended access (4 h) cocaine SA, cocaine increased glucose metabolism throughout the PFC and striatum. This pattern of activation was diminished following 1 month of cocaine abstinence, compared to the response in the cocaine-na€ ive state. This study showed a progressive expansion of cocaine’s effects from cortical to mesolimbic regions with extended access to cocaine in a pattern similar to studies using the [14C]-deoxyglucose autoradiographic technique (2DG method; see Porrino et al.2007 for review). However, FDG-PET revealed progressive increases in glucose metabolism following non-contingent cocaine exposure, whereas results from 2DG studies showed progressive decreases in these dopaminergic-rich brain regions. The differences in directional effects may be due to differences in reinforcement schedule, dose, or timing of drug administration and data collection between studies.
While the previous studies examined non-contingent administration of cocaine, similar activation patterns were shown when rhesus monkeys were allowed to self-administer cocaine during PET image acquisition (Howell et al. 2010). In addition to the PFC and striatum the anterior cingulate cortex (ACC) was metabolically more active following cocaine SA, a region widely shown to be hypoactive during executive function tasks in recent cocaine users (e.g., Bolla et al. 2004; Hester and Garavan 2004; Kubler et al. 2005; Tomasi et al. 2007a, b). Further, when saline was substituted for cocaine during a subsequent PET scan such that only the conditioned stimuli (e.g., lights) were present, activation of the dorsomedial PFC was still present. Brain activation patterns in these NHP studies utilizing awake PET imaging during cocaine SA or extinction conditions are similar to those induced by acute cocaine injections (Breiter et al. 1997) or cocaine-related visual cues (e.g., Wilcox et al. 2011) in humans measured with functional MRI. Such functional responses to drug-related cues are in part attributed to increased DA release determined via displacement of [11C]-raclopride and PET imaging in humans (Volkow et al. 2006). These studies present a neurobiological mechanism underlying drug stimuli-induced craving that may contribute to compulsive drug use and relapse in humans. PET studies in NHPs may be utilized to assess novel, putative pharmacotherapies to block the CNS response to cocaine-related stimuli during abstinence to aid in relapse prevention.
FDG-PET can also be used to examine the effects of various environmental influences on drug-related behavior. For example, preliminary FDG-PET data from our laboratory have shown that glucose metabolism is differentially altered following a stressful encounter with an unknown monkey based on social rank. Dominant monkeys showed increased glucose metabolism in mesocorticolimbic regions and chose fewer cocaine injections and more food pellets in a food-drug choice procedure following this encounter, whereas their subordinate counterparts showed reduced metabolism in mesolimbic regions and an increased preference for cocaine. This model can be used to assess neurobiological differences influencing subsequent behavior based on different environmental histories.
3.2 Models of Cognitive Decline
One of the hallmark consequences of cocaine addiction is a disruption of cognitive behavior. In human cocaine users, fMRI studies complement PET studies and have provided new opportunities to examine specific brain function underlying cognitive tasks. Recent fMRI studies have shown differences associated with impaired executive function compared to control groups across cognitive domains such as behavioral inhibition, cognitive flexibility, updating or working memory, and measures of impulsivity (Fillmore and Rush 2002; Bolla et al. 2004; Hester and Garavan 2004; Goldstein et al. 2007, 2010; Woicik et al. 2011). Despite strong evidence supporting cocaine-induced cognitive impairments, studies cannot discount cognitive predispositions such as heightened impulsivity or risk-taking behavior that may lead to drug use.
Recent studies have used FDG-PET to identify the substrates underlying cognitive behavior. For example, Porrino et al. (2005) used a delay match-to-sample (DMS) task as a measure of working memory in NHPs. In this task monkeys are shown a ‘sample’ image followed by a delay of varying length (1–30 s). The monkeys are then shown an array of 2–8 images from which they must ‘match’ the one from the sample phase of the task for a juice reward. The task ranges in difficulty with short intervals and a small number of images during the match phase (low cognitive load) to long intervals with a high number of images (high cognitive load). Performance was associated with increases in glucose utilization in the hippocampal region, dorsal striatum, and the dorsolateral prefrontal cortex. When cocaine was substituted for juice as the reward, there was a dose-dependent decline in performance particularly in high-load trials that was associated with increased activation in the dorsolateral PFC. The basis for the increased activation was a disruption in the firing patterns of dorsolateral PFC neurons recorded in the same animals under the same conditions (Hampson et al. 2011).
NHPs can be trained to perform tasks probing specific cognitive domains known to be impaired in human cocaine users. Following establishment of a stable cognitive baseline, cocaine SA can be initiated. The effects of acute and chronic drug exposure, or abstinence from cocaine on cognitive performance can be systematically examined, controlling for both intake and duration. Cocaine SA in monkeys brought about impairments in stimulus discrimination and reversal learning, response inhibition, measures of impulsivity, and working memory (Liu et al. 2008, 2009; Porter et al. 2011). In another paradigm of similar design, sleep deprivation impaired percent accuracy on a DMS task and was accompanied by reductions in glucose metabolism in working memory-related cortical areas (Porrino et al. 2005). Administration of the ampakine, CX717 or orexin-A attenuated the deleterious effects of sleep deprivation on both percent accuracy and glucose metabolism (Porrino et al. 2005; Deadwyler et al. 2007; Hampson et al. 2009). Putative cognitive enhancers aimed to attenuate cocaine-induced hypoactive brain function are also being tested in monkeys and humans. For example, recent cocaine users showed deficits in an attention task and showed lower activation within the ACC compared to controls (Goldstein et al. 2010). Following administration of methylphenidate, ACC activity and percent accuracy improved, demonstrating the attenuation of functional alterations underlying cognitive deficits via a pharmacological treatment. The generalizability of methylphenidate to enhance cocaine-associated neural and cognitive deficits has yet to be examined across other cognitive domains.
4 Summary
This review has attempted to briefly highlight the use of NHP models of cocaine addiction and in vivo brain imaging using PET to better understand the neuropharmacology of drug addiction. While we describe key PET studies using DAT tracers and FDG, the focus on DA D2-like receptors has allowed for an assessment of a potential trait marker, which was also influenced by environmental, social and pharmacological variables. D2-like receptor availability influenced vulnerability to cocaine abuse in a manner that was orderly and quite malleable to environmental or pharmacological manipulations. Certainly future studies must examine how other neurotransmitter and neurohormone systems change in concert with brain DA systems in these NHP models.
Acknowledgments
We would like to thank Susan Nader, Tonya Calhoun, Mack Miller, and Josh Long for technical assistance and the long-standing collaborations with Paul Czoty, Ph.D., Don Gage, Ph.D., Pradeep Garg, Ph.D. and Sam Deadwyler, Ph.D. The research described in this review was supported by National Institute on Drug Abuse grants DA10584, DA14637, DA09085, DA017763 and P50 DA06634.
References
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63(1):182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Berger B, Gaspar P, Verney C. Dopaminergic innervation of the cerebral cortex: unexpected differences between rodents and primates. Trends Neurosci. 1991;14(1):21–27. doi: 10.1016/0166-2236(91)90179-x. [DOI] [PubMed] [Google Scholar]
- Beveridge TJR, Smith HR, Daunais JB, Nader MA, Porrino LJ. Chronic cocaine self-administration is associated with altered functional activity in the temporal lobes of nonhuman primates. Eur J Neurosci. 2006;23:3109–3118. doi: 10.1111/j.1460-9568.2006.04788.x. [DOI] [PubMed] [Google Scholar]
- Beveridge TJ, Gill KE, Hanlon CA, Porrino LJ. Review: parallel studies of cocaine-related neural and cognitive impairment in humans and monkeys. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3257–3266. doi: 10.1098/rstb.2008.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla K, Ernst M, Kiehl K, Mouratidis M, Eldreth D, Contoreggi C, et al. Prefrontal cortical dysfunction in abstinent cocaine abusers. J Neuropsychiatry Clin Neurosci. 2004;16(4):456–464. doi: 10.1176/appi.neuropsych.16.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghammer P, Chakravarty M, Jonsdottir KY, Sato N, Matsuda H, Ito K, Arahata Y, Kato T, Gjedde A. Cortical hypometabolism and hypoperfusion in Parkinson’s disease is extensive: probably even at early disease stages. Brain Struct Funct. 2010;214(4):303–317. doi: 10.1007/s00429-010-0246-0. [DOI] [PubMed] [Google Scholar]
- Bradberry CW. Acute and chronic dopamine dynamics in a nonhuman primate model of recreational cocaine use. J Neurosci. 2000;20:7109–7115. doi: 10.1523/JNEUROSCI.20-18-07109.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, et al. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19(3):591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Carlton PL. A primer of behavioral pharmacology: concepts and principles in the behavioral analysis of drug action. W.H. Freeman; New York: 1983. [Google Scholar]
- Czoty PW, Justice JB, Jr, Howell LL. Cocaine-induced changes in extracellular dopamine determined by microdialysis in awake squirrel monkeys. Psychopharmacology (Berl) 2000;148(3):299–306. doi: 10.1007/s002130050054. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Morgan D, Shannon EE, Gage HD, Nader MA. Characterization of dopamine D1 and D2 receptor function in socially housed cynomolgus monkeys self-administering cocaine. Psychopharmacology (Berl) 2004;174(3):381–388. doi: 10.1007/s00213-003-1752-z. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Gage HD, Nader MA. PET imaging of striatal dopamine D2 receptors in nonhuman primates: increases in availability produced by chronic raclopride treatment. Synapse. 2005;58(4):215–219. doi: 10.1002/syn.20200. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Gage DG, Nader SH, Reboussin BA, Bounds M, Nader MA. PET imaging of dopamine D2 receptors and transporter availability during acquisition of cocaine self-administration in rhesus monkeys. J Addict Med. 2007;1(1):33–39. doi: 10.1097/ADM.0b013e318045c038. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Gould RW, Nader MA. Relationship between social rank and cortisol and testosterone concentrations in male cynomolgus monkeys (Macaca fascicularis) J Neuroen-docrinol. 2009a;21(1):68–76. doi: 10.1111/j.1365-2826.2008.01800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Riddick NV, Gage HD, Sandridge M, Nader SH, Garg S, et al. Effect of menstrual cycle phase on dopamine D2 receptor availability in female cynomolgus monkeys. Neuropsychopharmacology. 2009b;34(3):548–554. doi: 10.1038/npp.2008.3. [DOI] [PubMed] [Google Scholar]
- Deadwyler SA, Porrino L, Siegel JM, Hampson RE. Systemic and nasal delivery of orexin-A (Hypocretin-1) reduces the effects of sleep deprivation on cognitive performance in nonhuman primates. J Neurosci. 2007;27(52):14239–14247. doi: 10.1523/JNEUROSCI.3878-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey SL, Smith GS, Logan J, Brodie JD, Yu DW, Ferrieri RA, King PT, MacGregor RR, Martin TP, Wolf AP, et al. GABAergic inhibition of endogenous dopamine release measured in vivo with 11C-raclopride and positron emission tomography. J Neurosci. 1992;12:3773–3780. doi: 10.1523/JNEUROSCI.12-10-03773.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doudet DJ, Holden JE. Sequential versus nonsequential measurement of density and affinity of dopamine D2 receptors with [11C]raclopride: effect of methamphetamine. J Cereb Blood Flow Metab. 2003;23(12):1489–1494. doi: 10.1097/01.WCB.0000093325.88757.92. [DOI] [PubMed] [Google Scholar]
- Dukelow WR, Grauwiler J, Bruggemann S. Characteristics of the menstrual cycle in nonhuman primates: I. similarities and dissimilarities between Macaca fascicularis and Macaca arctoides. J Med Primatol. 1979;8(1):39–47. doi: 10.1159/000460174. [DOI] [PubMed] [Google Scholar]
- Dworkin SI, Mirkis S, Smith JE. Response-dependent versus response-independent presentation of cocaine: differences in the lethal effects of the drug. Psychopharmacology (Berl) 1995;117(3):262–266. doi: 10.1007/BF02246100. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Does the response to cocaine differ as a function of sex or hormonal status in human and non-human primates? Horm Behav. 2010;58(1):13–21. doi: 10.1016/j.yhbeh.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Peloux Y, Dalley JW, Robbins TW. Review: neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Phil Trans R Soc B. 2008;363(1507):3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR. Impaired inhibitory control of behavior in chronic cocaine users. Drug Alcohol Depend. 2002;66(3):265–273. doi: 10.1016/s0376-8716(01)00206-x. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, MacGregor RR, et al. Mapping cocaine binding sites in human and baboon brain in vivo. Synapse. 1989;4(4):371–377. doi: 10.1002/syn.890040412. [DOI] [PubMed] [Google Scholar]
- Galici R, Pechnick RN, Poland RE, France CP. Comparison of noncontingent versus contingent cocaine administration on plasma corticosterone levels in rats. Eur J Pharmacol. 2000;387(1):59–62. doi: 10.1016/s0014-2999(99)00780-3. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159(10):1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Rajaram S, Cottone LA, Zhang L, Maloney T, et al. Role of anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neuroscience. 2007;144(4):1153–1159. doi: 10.1016/j.neuroscience.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Woicik PA, Maloney T, Tomasi D, Alia-Klein N, Shan J, et al. Oral methylphenidate normalizes cingulate activity in cocaine addiction during a salient cognitive task. Proc Nat Acad Sci. 2010;107(38):16667–16672. doi: 10.1073/pnas.1011455107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield SF, Back SE, Lawson K, Brady KT. Substance abuse in women. Psychiatr Clin North Am. 2010;33(2):339–355. doi: 10.1016/j.psc.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Bigelow GE, Henningfield JE. Similarities in animal and human drug-taking behavior. In: Mello NK, editor. Advances in substance abuse. JAI Press; Greenwich: 1980. pp. 1–90. [Google Scholar]
- Hacia JG, Makalowski W, Edgemon K, Erdos MR, Robbins CM, Fodor SP, et al. Evolutionary sequence comparisons using high-density oligonucleotide arrays. Nat Genet. 1998;18(2):155–158. doi: 10.1038/ng0298-155. [DOI] [PubMed] [Google Scholar]
- Hampson RE, España RA, Rogers GA, Porrino LJ, Deadwyler SA. Mechanisms underlying cognitive enhancement and reversal of cognitive deficits in nonhuman primates by the ampakine CX717. Psychopharmacology (Berl) 2009;202(1-3):355–369. doi: 10.1007/s00213-008-1360-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson RE, Porrino LJ, Opris I, Stanford T, Deadwyler SA. Effects of cocaine rewards on neural representations of cognitive demand in nonhuman primates. Psychopharmacology (Berl) 2011;213(1):105–118. doi: 10.1007/s00213-010-2017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry PK, Murnane KS, Votaw JR, Howell LL. Acute brain metabolic effects of cocaine in rhesus monkeys with a history of cocaine use. Brain Imaging and Behav. 2010;4(3-4):212–219. doi: 10.1007/s11682-010-9100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci. 2004;24(49):11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell LL, Murnane KS. Nonhuman primate positron emission tomography neuroimaging in drug abuse research. J Pharmacol Exp Ther. 2011;337(2):324–334. doi: 10.1124/jpet.108.136689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell LL, Hoffman JM, Votaw JR, Landrum AM, Jordan JF. An apparatus and behavioral training protocol to conduct positron emission tomography (PET) neuroimaging in conscious rhesus monkeys. J Neurosci Methods. 2001;106(2):161–169. doi: 10.1016/s0165-0270(01)00345-4. [DOI] [PubMed] [Google Scholar]
- Howell LL, Hoffman JM, Votaw JR, Landrum AM, Wilcox KM, Lindsey KP. Cocaine-induced brain activation determined by positron emission tomography neuroimaging in conscious rhesus monkeys. Psychopharmacology. 2002;159(2):154–160. doi: 10.1007/s002130100911. [DOI] [PubMed] [Google Scholar]
- Howell LL, Carroll FI, Votaw JR, Goodman MM, Kimmel HL. Effects of combined dopamine and serotonin transporter inhibitors on cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther. 2007;320(2):757–765. doi: 10.1124/jpet.106.108324. [DOI] [PubMed] [Google Scholar]
- Howell LL, Votaw JR, Goodman MM, Lindsey KP. Cortical activation during cocaine use and extinction in rhesus monkeys. Psychopharmacology (Berl) 2010;208(2):191–199. doi: 10.1007/s00213-009-1720-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel D, Weiner I. The connections of dopaminergic system with the striatum in rats and primates: an analysis with respect to the functional and compartmental organization of the striatum. Neuroscience. 2000;96(3):451–474. doi: 10.1016/s0306-4522(99)00575-8. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB, Clarkson TB, Lusso FM, Taub DM. Social status, environment, and atherosclerosis in cynomolgus monkeys. Arterosclerosis. 1982;2:359–368. doi: 10.1161/01.atv.2.5.359. [DOI] [PubMed] [Google Scholar]
- Katz JL, Higgins ST. The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology. 2003;168:21–30. doi: 10.1007/s00213-003-1441-y. [DOI] [PubMed] [Google Scholar]
- Kimmel HL, Negus SS, Wilcox KM, Ewing SB, Stehouwer J, Goodman MM, et al. Relationship between rate of drug uptake in brain and behavioral pharmacology of monoamine transporter inhibitors in rhesus monkeys. Pharmacol Biochem Behav. 2008;90(3):453–462. doi: 10.1016/j.pbb.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2000;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2011;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopin IJ. Catecholamine metabolism: basic aspects and clinical significance. Pharmacol Rev. 1985;37(4):333–364. [PubMed] [Google Scholar]
- Kubler A, Murphy K, Garavan H. Cocaine dependence and attention switching within and between verbal and visuospatial working memory. Eur J Neurosci. 2005;21(7):1984–1992. doi: 10.1111/j.1460-9568.2005.04027.x. [DOI] [PubMed] [Google Scholar]
- Lecca D, Cacciapaglia F, Valentini V, Acquas E, Di Chiara G. Differential neurochemical and behavioral adaptation to cocaine after response contingent and noncontingent exposure in rat. Psychopharmacology (Berl) 2007;191(3):653–667. doi: 10.1007/s00213-006-0496-y. [DOI] [PubMed] [Google Scholar]
- Letchworth SR, Nader MA, Smith HR, Friedman DP, Porrino LJ. Progression of changes in dopamine transporter binding site density as a result of cocaine self-administration in rhesus monkeys. J Neurosci. 2001;21(8):2799–2807. doi: 10.1523/JNEUROSCI.21-08-02799.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA, Wang Z, Woolverton WL, France JE, Gregg TC, Davies HM, et al. The reinforcing efficacy of psychostimulants in rhesus monkeys: the role of pharmacokinetics and pharmacodynamics. J Pharmacol Exp Ther. 2003;307(1):356–366. doi: 10.1124/jpet.103.049825. [DOI] [PubMed] [Google Scholar]
- Lindsey KP, Wilcox KM, Votaw JR, Goodman MM, Plisson C, Carroll FI, et al. Effects of dopamine transporter inhibitors on cocaine self-administration in rhesus monkeys: relationship to transporter occupancy determined by positron emission tomography neuroimaging. J Pharmacol Exp Ther. 2004;309(3):959–969. doi: 10.1124/jpet.103.060293. [DOI] [PubMed] [Google Scholar]
- Liu S, Heitz RP, Sampson AR, Zhang W, Bradberry CW. Evidence of temporal cortical dysfunction in rhesus monkeys following chronic cocaine self-administration. Cerebral Cortex. 2008;18:2109–2116. doi: 10.1093/cercor/bhm236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Heitz RP, Bradberry CW. A touch screen based stop signal response task in rhesus monkeys for studying impulsivity associated with chronic cocaine self-administration. J Neurosci Methods. 2009;177(1):67–72. doi: 10.1016/j.jneumeth.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ. Sex differences in vulnerability to drug self-administration. Exp Clin Psychopharmacol. 2006;14(1):34–41. doi: 10.1037/1064-1297.14.1.34. [DOI] [PubMed] [Google Scholar]
- Lyons D, Friedman DP, Nader MA, Porrino LJ. Cocaine alters cerebral metabolism within the ventral striatum and limbic cortex of monkeys. J Neurosci. 1996;16(3):1230–1238. doi: 10.1523/JNEUROSCI.16-03-01230.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach RH, Nader MA, Ehrenkaufer RLE, Line SW, Smith CR, Luedtke RR, Kung MP, Kung HF, Lyons D, Morton TE. Comparison of two fluorine-18 labeled benzamide derivatives that bind reversibly to dopamine D2 receptors: in vitro binding studies and positron emission tomography. Synapse. 1996;24:322–333. doi: 10.1002/(SICI)1098-2396(199612)24:4<322::AID-SYN2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Mach RH, Nader MA, Ehrenkaufer RL, Line SW, Smith CR, Gage HD, et al. Use of positron emission tomography to study the dynamics of psychostimulant-induced dopamine release. Pharmacol Biochem Behav. 1997;57(3):477–486. doi: 10.1016/s0091-3057(96)00449-2. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. Cocaine, hormones, and behavior: clinical and preclinical studies. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, brain and behavior. 2nd edn. Academic Press; San Deigo: 2009. pp. 3081–3139. [Google Scholar]
- Mello NK, Negus SS. Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology. 1996;14(6):375–424. doi: 10.1016/0893-133X(95)00274-H. [DOI] [PubMed] [Google Scholar]
- Miller GM, Yatin SM, De La Garza R, II, Goulet M, Madras BK. Cloning of dopamine, norepinephrine and serotonin transporters from monkey brain: relevance to cocaine sensitivity. Brain Res Mol Brain Res. 2001;87(1):124–143. doi: 10.1016/s0169-328x(00)00288-6. [DOI] [PubMed] [Google Scholar]
- Moore RJ, Vinsant SL, Nader MA, Porrino LJ, Friedman DP. Effect of cocaine self-administration on dopamine D2 receptors in rhesus monkeys. Synapse. 1998;30(1):88–96. doi: 10.1002/(SICI)1098-2396(199809)30:1<88::AID-SYN11>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, et al. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5(2):169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Munro CA, McCaul ME, Wong DF, Oswald LM, Zhou Y, Brasic J, et al. Sex differences in striatal dopamine release in healthy adults. Biol Psychiatry. 2006;59(10):966–974. doi: 10.1016/j.biopsych.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Murnane KS, Howell LL. Neuroimaging and drug taking in primates. Psychopharmacology (Berl) 2011;206:153–171. doi: 10.1007/s00213-011-2222-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader MA, Czoty PW. PET imaging of dopamine D2 receptors in monkey models of cocaine abuse: genetic predisposition versus environmental modulation. Am J Psychiatry. 2005;162(8):1473–1482. doi: 10.1176/appi.ajp.162.8.1473. [DOI] [PubMed] [Google Scholar]
- Nader MA, Czoty PW. Brain imaging in nonhuman primates: insights into drug addiction. ILAR J. 2008;49(1):89–102. doi: 10.1093/ilar.49.1.89. [DOI] [PubMed] [Google Scholar]
- Nader MA, Daunais JB, Moore T, Nader SH, Moore RJ, Smith HR, et al. Effects of cocaine self-administration on striatal dopamine systems in rhesus monkeys: initial and chronic exposure. Neuropsychopharmacology. 2002;27(1):3–46. doi: 10.1016/S0893-133X(01)00427-4. [DOI] [PubMed] [Google Scholar]
- Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, et al. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci. 2006;9(8):1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- Nordström AL, Olsson H, Halldin C. A PET study of D2 dopamine receptor density at different phases of the menstrual cycle. Psychiatry Res. 1998;83:1–6. doi: 10.1016/s0925-4927(98)00021-3. [DOI] [PubMed] [Google Scholar]
- O’Brien MS, Anthony JC. Risk of becoming cocaine dependent: epidemiological estimates for the United States, 2000-2001. Neuropsychopharmacology. 2005;30(5):1006–1018. doi: 10.1038/sj.npp.1300681. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Daunais JB, Rogers GA, Hampson RE, Deadwyler SA. Facilitation of task performance and removal of the effects of sleep deprivation by an ampakine (CX717) in nonhuman primates. PLoS Biol. 2005;3(9):299. doi: 10.1371/journal.pbio.0030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Smith HR, Nader MA, Beveridge TJ. The effects of cocaine: a shifting target over the course of addiction. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(8):1593–1600. doi: 10.1016/j.pnpbp.2007.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JN, Olsen AS, Gurnsey K, Dugan BP, Jedema HP, Bradberry CW. Chronic cocaine self-administration in rhesus monkeys: impact on associative learning, cognitive control, and working memory. J Neurosci. 2011;31(13):4926–4934. doi: 10.1523/JNEUROSCI.5426-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddick NV, Czoty PW, Gage HD, Kaplan JR, Nader SH, Icenhower M, et al. Behavioral and neurobiological characteristics influencing social hierarchy formation in female cynomolgus monkeys. Neurosci. 2009;158(4):1257–1265. doi: 10.1016/j.neuroscience.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DCS, Phelan R, Hodges LM, Hodges MM, Bennett BA, Childers SR, et al. Self-administration of cocaine analogs by rats. Psychopharmacology. 1999;144(4):389–397. doi: 10.1007/s002130051022. [DOI] [PubMed] [Google Scholar]
- SAMHSA . Substance Abuse and Mental Health Services Administration: Results from the 2008 national survey on drug use and health: national findings. Office of Applied Studies; Rockville: 2009. NSDUH series H-36, HHS publication number SMA 09-4434. [Google Scholar]
- SAMHSA . Substance Abuse and Mental Health Services Administration: Reliability of key measures in the national survey on drug use and health. Substance Abuse and Mental Health Services Administration, US Department of Health and Human Services; Rockville: 2010. [PubMed] [Google Scholar]
- Strickland TL, Mena I, Villanueva-Meyer J, Miller BL, Cummings J, Mehringer CM, et al. Cerebral perfusion and neuropsychological consequences of chronic cocaine abuse. J Neuropsychiatry Clin Neurosci. 1993;5(4):419–427. doi: 10.1176/jnp.5.4.419. [DOI] [PubMed] [Google Scholar]
- Terner JM, de Wit H. Menstrual cycle phase and responses to drugs of abuse in humans. Drug Alcohol Depend. 2006;84(1):1–13. doi: 10.1016/j.drugalcdep.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Goldstein RZ, Telang F, Maloney T, Alia-Klein N, Caparelli EC, Volkow ND. Thalamo-cortical dysfunction in cocaine abusers: implications in attention and perception. Psychiatry Res. 2007a;155(3):189–201. doi: 10.1016/j.pscychresns.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Goldstein RZ, Telang F, Maloney T, Alia-Klein N, Caparelli EC, Volkow ND. Widespread disruption in brain activation patterns to a working memory task during cocaine abstinence. Brain Res. 2007b;1171:83–92. doi: 10.1016/j.brainres.2007.06.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallone D, Picetti R, Borrelli E. Structure and function of dopamine receptors. Neurosci Biobehav Rev. 2000;24(1):125–132. doi: 10.1016/s0149-7634(99)00063-9. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, et al. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14(2):169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Gifford A, Hitzemann R, Ding YS, Pappas N. Prediction of reinforcing responses to psychostimulants in humans by brain D2 dopamine receptors. Am J Psychiatry. 1999;156:1440–1443. doi: 10.1176/ajp.156.9.1440. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26(24):6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed MR, Woolverton WL, Paul IA. Dopamine D1 and D2 receptor selectivities of phenylbenzazepines in rhesus monkey striata. Eur J Pharmacol. 1998;361(1):129–142. doi: 10.1016/s0014-2999(98)00669-4. [DOI] [PubMed] [Google Scholar]
- Weerts EM, Fantegrossi WE, Goodwin AK. The value of nonhuman primates in drug abuse research. Exp Clin Psychopharmacol. 2007;15(4):309–327. doi: 10.1037/1064-1297.15.4.309. [DOI] [PubMed] [Google Scholar]
- WHO . Neuroscience of psychoactive substance use and dependence. World Health Organization; Geneva: 2004. [Google Scholar]
- Wilcox CE, Teshiba TM, Merideth F, Ling J, Mayer AR. Enhanced cue reactivity and fronto-striatal functional connectivity in cocaine use disorders. Drug Alcohol Depend. 2011;115(1-2):137–144. doi: 10.1016/j.drugalcdep.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woicik PA, Urban C, Alia-Klein N, Henry A, Maloney T, Telang F, et al. A pattern of perseveration in cocaine addiction may reveal neurocognitive processes implicit in the Wisconsin card sorting task. Neuropsychologia. 2011;49(7):1660–1669. doi: 10.1016/j.neuropsychologia.2011.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DF, Broussolle EP, Wand G, Villemagne V, Dannals RF, Links JM, et al. In vivo measurement of dopamine receptors in human brain by positron emission tomography: age and sex differences. Ann N Y Acad Sci. 1988;515:203–214. doi: 10.1111/j.1749-6632.1988.tb32986.x. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Nader MA. Experimental evaluation of the reinforcing effects of drugs. In: Adler MW, Cowans A, editors. Modern methods in pharmacology: testing and evaluation of drugs of abuse. Wiley-Liss; New York: 1990. pp. 165–192. [Google Scholar]
- Zilberman M, Tavares H, el-Guebaly N. Gender similarities and differences: the prevalence and course of alcohol- and other substance-related disorders. J Addict Dis. 2003;22(4):61–74. doi: 10.1300/j069v22n04_06. [DOI] [PubMed] [Google Scholar]