Abstract
Melarsoprol and pentamidine represent the two main classes of drugs, the arsenicals and diamidines, historically used to treat the diseases caused by African trypanosomes: sleeping sickness in humans and Nagana in livestock. Cross-resistance to these drugs was first observed over sixty years ago and remains the only example of cross-resistance among sleeping sickness therapies. A Trypanosoma brucei adenosine transporter is well-known for its role in the uptake of both drugs. More recently, aquaglyceroporin 2 (AQP2) loss-of-function was linked to melarsoprol-pentamidine cross-resistance. AQP2, a channel that appears to facilitate drug accumulation, may also be linked to clinical cases of resistance. Here, we review these findings and consider some new questions as well as future prospects for tackling the devastating diseases caused by these parasites.
Keywords: AT1, AQP2, MRPA, drug-resistance, MIP, Trypanosoma brucei
Chemotherapy against African trypanosomiasis
"Cellular therapy is a consequence of cellular nutrition, for only those compounds can affect the cell that are actually eaten by it." Paul Ehrlich, 1907 [1]
African trypanosomes are parasitic protists that circulate in the bloodstream and tissue fluids of their mammalian hosts. Transmitted by tsetse flies, they cause important human and animal diseases. Trypanosoma brucei gambiense and Trypanosoma brucei rhodesiense cause Human African Trypanosomiasis (HAT), also known as sleeping sickness, which is typically fatal without chemotherapy, while the closely related, but human-serum sensitive, T. b. brucei, Trypanosoma congolense and Trypanosoma vivax cause Nagana, an important veterinary disease. HAT affects 8.7 million km2 of Sub-Saharan Africa, areas where the climate and environment are suitable for the tsetse fly [2]. T. b. gambiense is endemic in many areas of West and central Africa and is currently responsible for the vast majority (>90%) of HAT cases.
For early-stage HAT cases in West Africa, pentamidine, an aromatic diamidine, is the drug of choice. Diagnosis is often late, however [3], revealing advanced infection with trypanosomes in the central nervous system (CNS). In these cases, eflornithine (in combination with nifurtimox) is the safest therapy [4], and availability of these drugs has increased in recent years [5]; nevertheless, the highly toxic, melaminophenyl arsenical melarsoprol is still used. This is explained by the lack of efficacy of eflornithine against T. b. rhodesiense [6] and the high cost and difficulty of administration for use against T. b. gambiense [5]. Thus, melarsoprol, a drug that causes an often fatal reactive encephalopathy in approximately 10% of patients [7], is currently the only drug active against both advanced T. b. rhodesiense and T. b. gambiense infections. Melarsoprol and pentamidine are also the most potent drugs used to treat HAT, both displaying low nanomolar 50% effective growth-inhibitory concentrations (EC50).
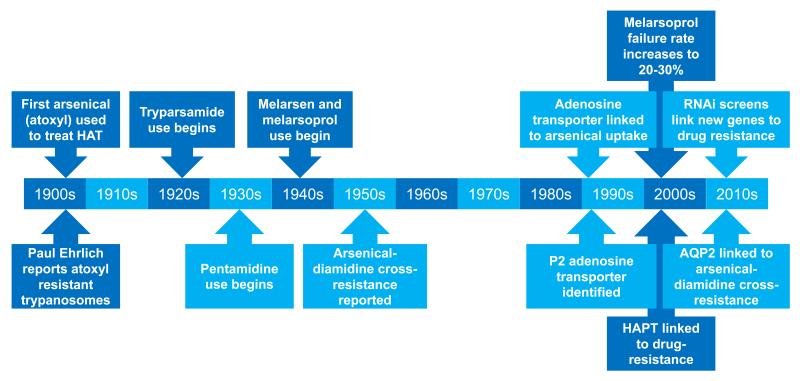
There have been three major epidemics of sleeping sickness recorded since the late 19th century. Tsetse control, the systematic screening for patients in at-risk populations followed by chemotherapy, and the introduction of nifurtimox-eflornithine combination therapy (NECT) have all contributed to the recent successful reduction in cases [8]. However, the WHO recently warned against neglect and complacency if further epidemics are to be avoided [9]. With no vaccine available and limited therapeutic alternatives, the emergence of drug resistance is a major threat in this regard [10], especially since loss of a single, non-essential transporter can result in eflornithine resistance [11]. In fact, the high cost and logistical burden of NECT might render this particular treatment unsustainable [5]. Therapies based on arsenicals and diamidines have been prominent in efforts to tackle HAT for over 100 years and selected important developments during this time are summarised in Figure 1 and detailed below.
Figure 1. Timeline of arsenical and diamidine therapies against HAT.
The timeline indicates selected key developments relating to these therapies and in our understanding of drug-uptake and resistance.
The arsenicals
The first organic arsenical, aminophenyl arsonic acid, euphemistically named atoxyl, was introduced as a treatment for HAT in the early 1900s [12]. This drug was partly replaced by its less toxic N-substituted derivative tryparsamide (arsonophenylglycineamide) [13] in the early 1920s, although it was not effective against T. b. rhodesiense or arsenic-resistant T. b. gambiense [14]. Both of these pentavalent arsenic compounds caused serious ocular lesions in many patients [15] and were eventually superseded by the trivalent melaminophenyl arsenicals melarsen (arsonophenylmelamine) [16, 17] and, ultimately, melarsoprol [18]. Combining melarsen with British anti-Lewisite (dimercaptopropanol), melarsoprol was by far the least toxic of all the arsenicals. However, it still bears unacceptable adverse effects such as reactive encephalopathy; the presence of trypanosomes in the CNS has been correlated with the incidence of this reactive encephalopathy suggesting that trypanosome lysis is the trigger for inflammation [19]. Melarsoprol is dissolved in 3.6% propylene glycol, itself an irritant at the site of injection, and administered over ten days via intravenous injection [7, 20]. This drug penetrates the blood-brain barrier, but only achieves 3-4% of the maximal levels reached in plasma [21]. Melarsen oxide is thought to be the active metabolite [22] that is taken up by trypanosomes, forming a stable adduct with trypanothione known as Mel T [23]. A combination of melarsoprol with cyclodextrins has recently been proposed as an orally administered, safer alternative arsenical therapy [24].
The diamidines
While initially the rationale for using diamidines was based on their hypoglycaemic effect, aiming to starve the trypanosomes of glucose, the diamidines were soon discovered to be directly trypanocidal [25]. Pentamidine is an aromatic diamidine which has been used in the treatment of HAT since the 1930s. The drug is administered intramuscularly once daily over a seven day treatment period [26]. Pentamidine is unsuitable for treatment of advanced disease, in part because serum binding and tissue retention reduce blood-brain barrier traversal [27]. Pentamidine that does cross the blood-brain barrier is also cleared by efflux transporters such as P-glycoprotein and multi-drug resistance-associated protein [28]. Even so, despite limited access to the CNS and efflux from the CNS, pentamidine has been reported to be effective against trypanosomiasis during the early phase of CNS involvement [29, 30].
Diamidines are nucleic acid-binding drugs [31] that typically become highly concentrated within, and destroy the mitochondrial genome, known as the kinetoplast [32], but they are also seen in the nucleus and acidocalcisomes [33, 34]. Thus, pentamidine, and other diamidines, may kill trypanosomes partly through kinetoplast disruption. However, cells lacking a kinetoplast are viable if they harbour a mutation in the γ subunit of the F1 component of the mitochondrial ATP synthase, which compensates for loss of the kinetoplast-encoded A6 subunit [35]. Thus, drugs that act via kinetoplast disruption alone might be expected to be prone to resistance due to γ subunit mutation. In the case of pentamidine, toxicity by other means is certainly likely since the drug accumulates in trypanosomes to millimolar concentrations [36]. Indeed, cells apparently lacking kinetoplast DNA remain sensitive to pentamidine [36], possibly due to disruption of mitochondrial membrane potential [37, 38]. Consequently, it is likely that the antitrypanosomal activity of pentamidine is the result of selective accumulation, leading to multiple deleterious effects, rather than effects on a specific ‘diamidine target’ [39]. Novel diamidines [40] and pentamidine-like prodrugs [41] with improved pharmacokinetic properties are under development.
Drug resistance and the P2 adenosine transporter
Drug resistance typically emerges when a genetic change, a mutation, deletion or amplification, alters uptake, drug metabolism, drug-target interaction or efflux. If there is a concomitant fitness cost, it will be less likely that resistant parasites will propagate and spread. The phenomenon of drug resistance was first described by Paul Ehrlich in the early 1900s. Ehrlich observed trypanosomes that became resistant to trypan-red and to the arsenical compound atoxyl, and noted that trypan-red resistant cells accumulated less dye. By testing different classes of compounds, Ehrlich was also able to deduce that cross-resistance was often restricted to one class and did not extend to an unrelated class, postulating that changes in specific ‘chemioreceptors’ conferred resistance [42]. These remarkable insights hold true today, but the identity of many of the molecules potentially involved in resistance have been revealed only recently.
Trypanosomes rely on uptake of essential nutrients from the host and, therefore, bear a number of transporters at the cell surface and in the flagellar pocket, an invagination of the pellicular membrane that is inaccessible to host innate immune effectors and the exclusive site for endocytosis, exocytosis and specific receptors [43]. Drugs can also enter cells via these transporters. Melarsoprol treatment failure has increased to alarming levels of 20-30% of patients in some areas [19, 44-47] and, although rarely confirmed, this is likely due to drug-resistance. Pentamidine treatment failures are rare, although massive use of pentamidine as a chemoprophylactic in The Democratic Republic of Congo may have selected for reduced sensitivity to this drug [48]. In the case of the veterinary diamidine, diminazene (Berenil®), resistance is widespread and a threat to the control of trypanosomiasis in livestock throughout the tsetse belt [39, 49].
Early research on the mechanisms of drug resistance in African trypanosomes was marked by two recurrent themes: reduced drug uptake by resistant cells and cross-resistance between arsenicals and diamidines [36, 50, 51]. Indeed, arsenical, diamidine cross-resistance was first reported over sixty years ago [52]. Reduced uptake and cross-resistance were apparently explained by the findings that melamine-based arsenicals and diamidines are imported into trypanosomes by the same transporter, and that this transporter is defective in drug-resistant cells [53-55]. The transporter was called P2 (purine transporter 2) as its physiological substrates are adenine and adenosine, both of which compete with melarsoprol for uptake and can protect trypanosomes from melarsoprol-induced lysis [54]. The gene encoding the P2 transporter, TbAT1, was cloned by taking advantage of yeast genetics; expression of TbAT1 in Saccharomyces cerevisiae enabled adenosine uptake and conferred susceptibility to arsenicals [56]. TbAT1 gene deletion and loss-of-function mutations were then described in drug resistant strains generated in the laboratory [56, 57] and the same mutations were also found in T. brucei ssp. field isolates [58] associated with melarsoprol treatment failure [58-60]. In addition, Tbat1-null trypanosomes were found to have lost the adenine-sensitive component of adenosine and melarsoprol-import [61, 62].
Other melarsoprol and pentamidine transporters
As expected, Tbat1 null mutants exhibited cross-resistance to melamine-based arsenicals and diamidines [62]. However, while a high level of resistance to diminazene was observed, the resistance factor for pentamidine and the melaminophenyl arsenicals were only two to threefold, significantly less than the arsenical-pentamidine cross-resistant strains generated in the laboratory [63-65]. Thus, it was clear that at least one additional transporter must contribute to the cross-resistance phenotype [66, 67]. The prime candidate was the high-affinity pentamidine transporter (HAPT1), an activity recorded in bloodstream-form T. brucei using low nanomolar concentrations of [3H]-pentamidine [66]. Melarsoprol also appears to be a substrate for HAPT1 [62] and selection for increased resistance to pentamidine or melarsoprol in Tbat1 null cells led to specific loss of HAPT1 activity [64].
Apart from resistance through loss of drug uptake, the other common resistance mechanism associated with changes in net drug accumulation is the energy-dependent extrusion of the compound, prodrug or active metabolite by ATP-Binding Cassette (ABC) transporters, which include P-glycoprotein and multi drug-resistance associated transporters (MRPs). These transporters perform many biological functions besides the exclusion of xenobiotics from cells [68] but have frequently been associated with antibiotic resistance in bacteria and fungi, and with treatment failure in cancer [69, 70]. Their involvement in drug resistance in protists, including Plasmodium and Leishmania spp is also well-documented [71]. In T. brucei, overexpression of the ABC transporter, MRPA, resulted in increased efflux of the melarsoprol-trypanothione adduct Mel T [72]. However, MRPA overexpression was insufficient to cause melarsoprol-resistance in vivo, and MRPA overexpression was not detected in melarsoprol-resistant trypanosomes [73]. Overexpression of an ABC transporter also confers resistance to pentamidine in Leishmania major, [74] but pentamidine appears to be effectively retained by T. brucei [75].
RNA interference library screens identify new drug action and resistance mechanisms
Experimental, sequence-specific knockdown by RNA interference (RNAi) is a powerful method for assessing the consequences of loss of gene-function. In an early RNAi library screen, tubercidin-resistant T. brucei revealed hexose transporter knockdown and inhibition of glycolysis by this drug [76]. A more recent set of high-throughput RNAi library screens linked several membrane-spanning transporters, including AT1, to melarsoprol or pentamidine sensitivity [77-79]. In one example, the plasma membrane P-type H+-ATPases, HA1-3 [80], were linked to pentamidine sensitivity [77]. Thus, HA1-3 may generate the proton motive force required for HAPT1 activity and pentamidine uptake [66]. Most notably, the screens linked just one locus, encoding a pair of closely related aquaglyceroporins (AQP2 and AQP3), to melarsoprol and pentamidine cross-resistance; it was subsequently demonstrated that knockout of the AQP2 and AQP3 locus increased the 50% effective growth-inhibitory concentration (EC50) for these drugs by >twofold and >15-fold, respectively [77].
Aquaglyceroporin 2 controls melarsoprol-pentamidine cross-resistance
AQPs are major intrinsic proteins (MIPs) that constitute a super-family of aquaporins and aquaglyceroporins, membrane proteins that facilitate the transport of water and small neutral solutes across membranes in organisms from bacteria to humans (AQPs in parasitic protists are reviewed in [81]). The T. brucei genome encodes three aquaglyceroporins, AQP1, AQP2 and AQP3, and all three transport water, glycerol, urea, dihydroxyacetone [82] and ammonia [83]. It seems likely that they are naturally involved in osmoregulation and metabolism, efflux of glycerol from the bloodstream form, for example [84]. AQP1 and AQP3 are flagellar membrane and plasma membrane proteins, respectively [85]; likely shielded from host antibodies by the dense variant surface glycoprotein coat in bloodstream-form cells. Although the discovery of an AQP locus linked to melarsoprol and pentamidine cross-resistance was an important development, it remained unclear whether AQP2, AQP3 or both contributed to the phenotype [77].
A first suggestion that T. brucei AQP2 and AQP3 have distinct functions came from simple sequence comparison. Some key residues that determine the selectivity and gating of AQP channels have been identified and these residues from more than 1000 MIPs from 340 organisms are documented in the MIPModDB database [86]. Although AQP2 and AQP3 are otherwise closely related, AQP2 lacks the usual motifs of the selectivity filter. Indeed, AQP2 is the only MIP with NSA/NPS or IVLL motifs; AQP1 and AQP3 harbour the conventional ‘NPA/NPA’ motifs, seen in 78% of these proteins, and a WGYR selectivity filter shared with 118 other proteins. An arginine residue is seen in 89% of aromatic/arginine (ar/R) motifs, and the absence of this residue in AQP2 may be of particular significance, since it is thought to repel protons [87-89]. Thus, this feature of AQP2 could be important for the uptake of di-cationic pentamidine.
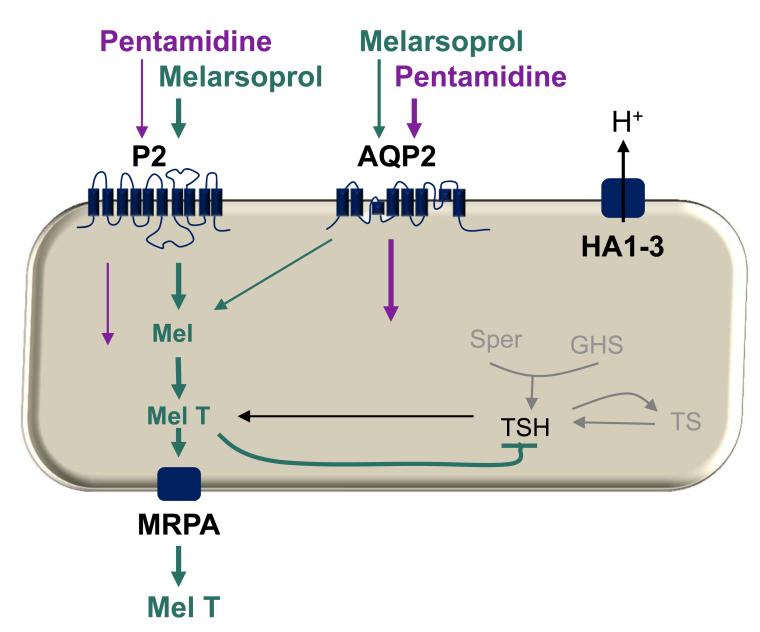
Since no fitness cost was observed in aqp2-aqp3 null strains [77], an aqp2 null strain was constructed, and this revealed that AQP2 expression was specifically required for drug sensitivity [90]. Restoring AQP2 or AQP3 function in the aqp2-aqp3 null strains demonstrated that AQP2 restored drug sensitivity to wild-type levels, even in the absence of AQP3. Similar results were obtained in both the developmentally distinct bloodstream-stage and insect-stage cells, indicating that AQP2 is functional in both stages; yet, neither AQP is required for viability in these stages or for the developmental transition between them [90]. Interestingly, and distinct from AQP1 or AQP3, AQP2 was shown to be restricted to the flagellar pocket. It was concluded from these results that AQP2 likely corresponds to the long-sought high-affinity pentamidine transporter (HAPT1) [45]. Our current understanding of melarsoprol and pentamidine transport in T. brucei is summarised in Figure 2.
Figure 2. Schematic model of melarsoprol and pentamidine transport in T. brucei.
The schematic shows known and putative mechanisms. Both drugs are thought to enter trypanosomes via the P2 and AQP2 transporters; the weight of the arrows reflects relative contribution to uptake. The presence of the AQP2 gene appears to correlate with HAPT1 activity but it is as yet not certain that AQP2 codes for this activity. The P-type H+ ATPases (HA1-3) may provide a proton motive force that drives pentamidine uptake. Melarsen oxide forms a toxic adduct with trypanothione (TSH), known as Mel T, which inhibits TSH synthesis and is also removed from the cell via the ABC transporter, MRPA. An additional low affinity pentamidine transport activity (not shown) may contribute to transport at pentamidine at concentrations above 0.1 μM [29].
An obvious question that arises from these recent findings is whether AQP2 loss-of-function explains clinical drug-resistance. One step towards addressing this question has already been taken using a laboratory-selected drug-resistant strain. The advantage of using this approach is that closely related sensitive and resistant strains are readily available, in contrast to the situation when examining clinical isolates. Strikingly, the AQP2 gene is chimeric [90] in a clonal line long-known to be resistant to both melarsoprol and pentamidine [64], whereas the drug-sensitive parental clone was homozygous for wild-type AQP2. The AQP2/AQP3 chimera contains a 272-bp in-frame AQP3-segment, demonstrating that the deleted region of AQP2 determines the drug sensitivity phenotype of the channel. Taken together, the new findings establish a central role for T. brucei AQP2 in drug-sensitivity and it is now plausible that mutations affecting AQP2 are also linked to clinical resistance.
An old question and four more new questions
How do arsenicals and diamidines kill trypanosomes?
This is an old question but it is worth reconsidering here. Part of the answer will clearly derive from understanding how these drugs are delivered to their intracellular targets. The other part of the answer lies in the identity of those intracellular targets. Drugs identified through phenotypic screening are selected on the basis of killing capacity rather than inhibition of a specific enzyme, and there may be no single, specific enzymatic target for these drugs. Melarsoprol generates a major, likely toxic, adduct with trypanothione, Mel T, and depletion of trypanothione likely compromises defence against oxidative damage [91]. Pentamidine may destroy kinetoplast DNA and also disrupt the mitochondrial membrane potential [37]. Thus, these drugs possibly have multiple non-enzymatic targets, and as the same or similar targets are present in host cells it follows that drug accumulation is the primary determinant of selective anti-parasite selectivity. This scenario presents challenges in terms of defining intracellular targets but, on the other hand, explains why drug-sensitivity and resistance are highly dependent upon drug-uptake. In this respect, the new data for AQP2 represent an important new development which, nevertheless, raises several new questions, four of which are considered below.
Do drugs transit directly via AQP2?
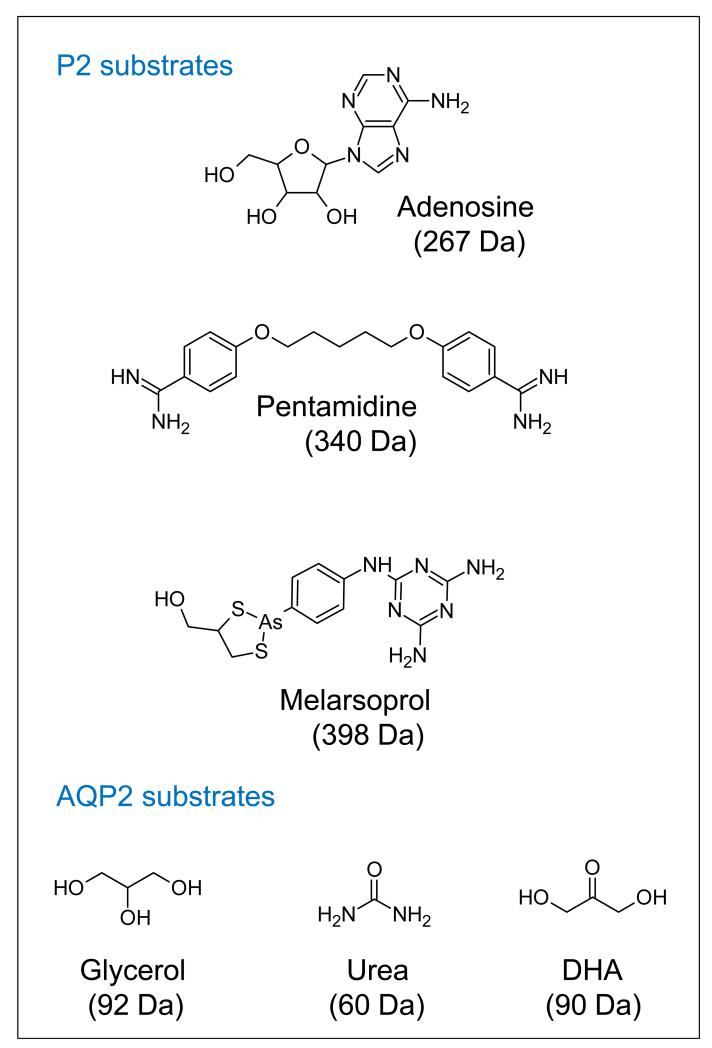
Although melarsoprol and pentamidine do not have closely related structures (Figure 3), current results are consistent with transit of both drugs via AQP2; sensitivity to a lipophilic arsenical is unaffected by AQP2 knock-out [90] and the HAPT1-deficient strain expressing chimeric AQP2 is deficient in accumulation of pentamidine at low concentration [64, 90]. However, with molecular masses of 398 Da and 340 Da, respectively, these drugs are substantially larger than glycerol at 92 Da, which could argue against drug transit via AQP2; no AQP has been shown to transport molecules of this mass [81, 92]. Other aquaglyceroporins, such as Leishmania major AQP1 [93], transport trivalent arsenic and antimony but these inorganic compounds have far lower masses. Both of the larger compounds do appear to enter trypanosomes intact [23, 75], so AQP2 may indeed accommodate these relatively bulky drugs. An alternative possibility that cannot be ruled out at present is that AQP2 expression regulates HAPT1 expression or function, and thus controls pentamidine uptake indirectly. Heterologous expression systems may provide an answer to this question.
Figure 3. P2 and AQP2 substrates.
Substrates known to transit through each transporter are shown. Inorganic arsenite and antimonite are known to be transported via aquaglyceroporins, but an outstanding question is how AQP2 facilitates the transit of the much larger pentamidine and melarsoprol structures. In the case of pentamidine, competition studies suggest that a single benzene ring with the amidine in the fourth position is sufficient for recognition [75].
What is the natural function of the trypanosomal AQPs?
T. brucei AQPs likely have roles in osmoregulation and glycerol transit, but these functions remain to be tested. Such an analysis, and some of the other analyses suggested below, would be facilitated by the availability of an aqp null T. brucei strain. AQP2 and AQP3 are dispensable in vitro, but it remains to be seen whether AQP1 is also dispensable. Dispensability will facilitate an assessment of the roles of these channels both in vitro and in vivo.
Which AQP residues control drug-sensitivity phenotypes?
It will be important to dissect the key AQP2 residues that are specifically required for the drug sensitivity phenotype. These likely include residues that line the channel, and are possibly among the six residues of the selectivity-filter that differ between AQP2 and AQP3. Related studies may also reveal the residues that determine the distinct sub-cellular localisation for each T. brucei AQP. A recent study revealed a phosphorylated residue within LmAQP1 that is associated with relocalisation from the flagellum to the entire cell surface for example [94].
Extending this analysis to the diversity of AQP sequences from the various trypanosomatid genome sequences will reveal the other trypanosomal AQPs that play roles in drug action and resistance. For example, AQPs from the T. b. gambiense, T. congolense, T. vivax, T. cruzi and Leishmania spp genome reference strains could be assessed in drug sensitivity assays, and this may reveal residues linked to drug transport and allow prediction of whether each (sub)species and strain is drug sensitive or resistant.
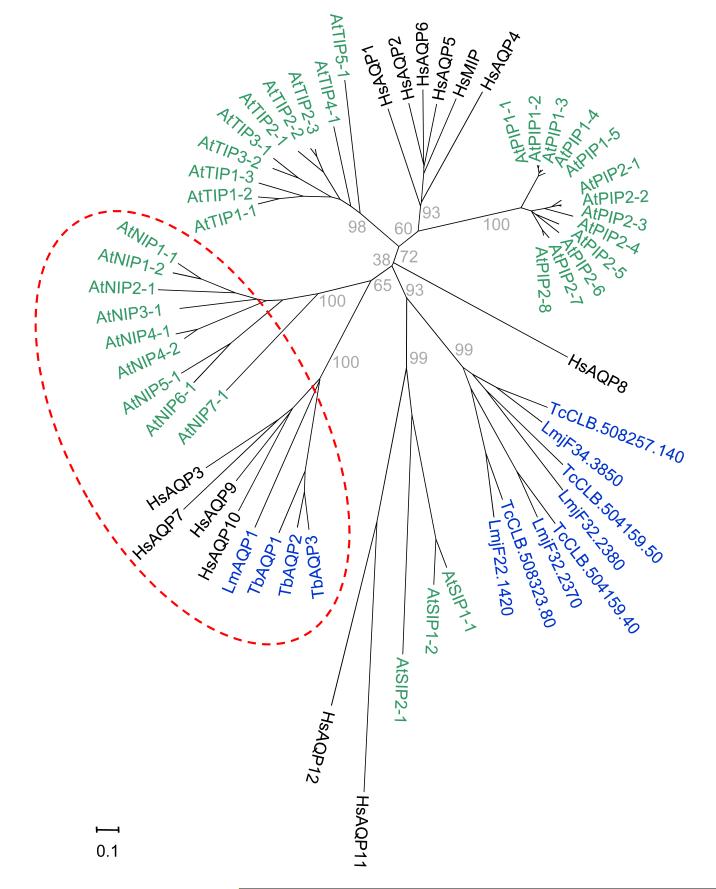
Notably, TbAQP2 and LmAQP1, both linked to drug uptake and resistance, cluster with human AQP7 and AQP9 and plant NIP1-1, NIP5-1, NIP6-1 and NIP7-1 (Figure 4), all shown to transport arsenite and/or antimonite [95-98]. Notably, T. cruzi does not appear to encode an AQP on this branch. Conversely, T. brucei lacks members of the major clade of trypanosomatid AQPs that have not been linked to drug transport.
Figure 4. The two branches of trypanosomatid aquaporins.
The AQPs from T. brucei, T. cruzi and L. major (n=12, blue) are shown in the context of AQP diversity of Arabidopsis thaliana (n=35, green) and Homo sapiens (n=13, black). The Neighbour-Joining tree was built from a MUSCLE alignment of the amino acid sequences [101] and drawn with MEGA5 [102]. Evolutionary distances were computed using the Poisson correction and are in number of amino acid substitutions per site (scale bar). The grey numbers on the main branches indicate percent positives of 2000 rounds of bootstrapping. Aquaglyceroporins linked to drug transport are within the red dashed oval: NIP, PIP, SIP and TIP; Nodulin-26 like, Plasma-membrane, Small basic and Tonoplast Intrinsic Proteins. Trypanosomatid accession numbers: LmAQP1, LmjF.31.0020; TbAQP1, Tb927.6.1520; TbAQP2, Tb927.10.14170, TbAQP3, Tb927.10.14160.
Does AQP2 disruption explain clinical cases of resistance?
AQP2 disruption in a laboratory-selected drug-resistant strain [90] raises the likelihood that a similar mechanism operates in a clinical setting. Thus, absence or loss of AQP2 function could explain innate or acquired resistance. Obtaining T. b. gambiense isolates from patients is challenging, but a first step towards translating the new findings to application will be the assessment and sequencing of AQP genes from such drug-resistant clinical isolates. The T. b gambiense reference sequence reveals two tandem copies of AQP2 and an absence of AQP3 so it will be of particular interest to identify mutations or deletions in the AQP2 gene sequences associated with drug-resistance at this locus.
Concluding remarks
Few would have predicted an AQP as a candidate regulator of bulky drug transport. It was the power of forward genetic screening that suggested this possibility. With only the AT1 gene in hand since the 1990s, inevitably, studies on the genetic basis of melarsoprol and pentamidine cross-resistance have focussed on this transporter. However, the discovery of additional pentamidine [66, 67] and arsenical [64] transporter activities highlighted major gaps in our understanding of the genetic basis of drug transport and limitations in terms of fully understanding drug-resistance. The new link established with AQP2 should reinvigorate research in this area and lead to insights that clarify the emergence of drug-resistance and, ultimately, to benefits for the patients that fall victim to these drug-resistant infections.
Affordable, safe, easily administered and effective HAT therapies are needed, particularly for the advanced stage of the disease; this would decrease the dire need for an alternative to lumbar puncture for the diagnosis of late-stage disease [3]. Novel arsenical formulations, such as cyclodextrin complexes, could be used as oral therapies in the future [24], and a programme to develop novel diamidines that efficiently penetrate the CNS, some suitable for oral administration, also aims to address this need [40, 41, 99]. Notably, AQP2 does not affect sensitivity to the trivalent arsenical, phenyl arsine oxide, or diminazene [90] so some of these new drugs could circumvent this resistance mechanism. Ideally, novel drugs would not display cross-resistance with pentamidine and melarsoprol. If some did, an appropriate test might predict a high frequency of treatment failure in some areas, thereby aiding the selection of a different treatment option. A genetic test could be used to assess whether AQP2, or residues specifically involved in drug-sensitivity, are absent, rendering certain arsenicals and diamidines ineffective. Alternatively, a simple fluorescence test, as reported for P2-transporter defective cells [100], could be developed. Any such test could also find utility as a surveillance tool for the presence of drug-resistant parasites in reservoir hosts, since East African trypanosomiasis is a zoonotic disease.
Paul Ehrlich’s observations, over 100 years ago, on cellular nutrition and drug uptake, remain as relevant today as they were then. Thus, an improved understanding of active drug uptake by parasites should facilitate the development of improved and selective therapies. These therapies could exploit known delivery mechanisms or killing mechanisms or could rely upon synergy within a drug combination; one drug may increase uptake, enhance the production of toxic metabolites or reduce efflux of a second drug, for example. This improved understanding should also facilitate the development of predictive tools that help to combat drug resistance.
Acknowledgements
Work in the authors’ laboratories is funded by grants from The Wellcome Trust (093010/Z/10/Z) at the LSHTM, from the Medical Research Council (84733) at the University of Glasgow and from the Swiss National Science Foundation at the Swiss Tropical and Public Health Institute. N.B. was supported by a Bloomsbury colleges Ph.D. studentship.
References
- 1.Ehrlich P. Chemotherapeutische Trypanosomen-Studien. Berliner klinische Wochenschrift. 1907;9:233–236. [Google Scholar]
- 2.Simarro PP, et al. Eliminating human African trypanosomiasis: where do we stand and what comes next? PLoS Med. 2008;5:e55. doi: 10.1371/journal.pmed.0050055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wastling SL, Welburn SC. Diagnosis of human sleeping sickness: sense and sensitivity. Trends Parasitol. 2011;27:394–402. doi: 10.1016/j.pt.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Priotto G, et al. Nifurtimox-eflornithine combination therapy for second-stage African Trypanosoma brucei gambiense trypanosomiasis: a multicentre, randomised, phase III, non-inferiority trial. Lancet. 2009;374:56–64. doi: 10.1016/S0140-6736(09)61117-X. [DOI] [PubMed] [Google Scholar]
- 5.Simarro PP, et al. Update on field use of the available drugs for the chemotherapy of human African trypanosomiasis. Parasitology. 2012;139:842–846. doi: 10.1017/S0031182012000169. [DOI] [PubMed] [Google Scholar]
- 6.Iten M, et al. Alterations in ornithine decarboxylase characteristics account for tolerance of Trypanosoma brucei rhodesiense to D,L-α-difluoromethylornithine. Antimicrob Agents Chemother. 1997;41:1922–1925. doi: 10.1128/aac.41.9.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuepfer I, et al. Safety and efficacy of the 10-day melarsoprol schedule for the treatment of second stage rhodesiense sleeping sickness. PLoS Negl Trop Dis. 2012;6:e1695. doi: 10.1371/journal.pntd.0001695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nimmo C. Time to put out the lights on sleeping sickness? Travel Med Infect Dis. 2010;8:263–268. doi: 10.1016/j.tmaid.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 9.WHO. Neglected tropical diseases, hidden successes, emerging opportunities. WHO; Geneva, Switzerland: 2009. pp. 38–39. (WHO report). [Google Scholar]
- 10.Fairlamb AH. Chemotherapy of human African trypanosomiasis: current and future prospects. Trends Parasitol. 2003;19:488–494. doi: 10.1016/j.pt.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Vincent IM, et al. A molecular mechanism for eflornithine resistance in African trypanosomes. PLoS Pathog. 2010;6:e1001204. doi: 10.1371/journal.ppat.1001204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas HW. Some Experiments in the Treatment of Trypanosomiasis. Br Med J. 1905;1:1140–1143. doi: 10.1136/bmj.1.2317.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobs WA, Heidelberger M. Chemotherapy of trypanosome and spirochete infections: Chemical series I. N-phenylglycineamide-p-arsonic acid. J Exp Med. 1919;30:411–415. doi: 10.1084/jem.30.5.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williamson J. Review of Chemotrerapeutic and chemoprophylactic agents. In: Mulligan HW, editor. The African Trypanosomiases. George Allen and Unwin; London: 1970. pp. 125–221. [Google Scholar]
- 15.Ridley H. Ocular lesions in trypanosomiasis. Ann Trop Med Parasitol. 1945;39:66–82. [Google Scholar]
- 16.Friedheim EA. Melarsen oxide in the treatment of human trypanosomiasis. Ann Trop Med Parasitol. 1948;42:357–363. doi: 10.1080/00034983.1948.11685383. [DOI] [PubMed] [Google Scholar]
- 17.Steverding D. The development of drugs for treatment of sleeping sickness: a historical review. Parasit Vectors. 2010;3:15. doi: 10.1186/1756-3305-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedheim EA. Mel B in the treatment of human trypanosomiasis. Am J Trop Med Hyg. 1949;29:173–180. doi: 10.4269/ajtmh.1949.s1-29.173. [DOI] [PubMed] [Google Scholar]
- 19.Pepin J, Milord F. The treatment of human African trypanosomiasis. Adv Parasitol. 1994;33:1–47. doi: 10.1016/s0065-308x(08)60410-8. [DOI] [PubMed] [Google Scholar]
- 20.Burri C, et al. Efficacy of new, concise schedule for melarsoprol in treatment of sleeping sickness caused by Trypanosoma brucei gambiense: a randomised trial. Lancet. 2000;355:1419–1425. doi: 10.1016/S0140-6736(00)02141-3. [DOI] [PubMed] [Google Scholar]
- 21.Maes L, et al. ELISA assay for melarsoprol. Bull Soc Pathol Exot Filiales. 1988;81:557–560. [PubMed] [Google Scholar]
- 22.Keiser J, et al. Investigations of the metabolites of the trypanocidal drug melarsoprol. Clin Pharmacol Ther. 2000;67:478–488. doi: 10.1067/mcp.2000.105990. [DOI] [PubMed] [Google Scholar]
- 23.Fairlamb AH, et al. Trypanothione is the primary target for arsenical drugs against African trypanosomes. Proc Natl Acad Sci U S A. 1989;86:2607–2611. doi: 10.1073/pnas.86.8.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodgers J, et al. Melarsoprol cyclodextrin inclusion complexes as promising oral candidates for the treatment of human African trypanosomiasis. PLoS Negl Trop Dis. 2011;5:e1308. doi: 10.1371/journal.pntd.0001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lourie E, Yorke W. Studies in chemotherapy. XVI. The trypanocidal action of synthalin. Ann. Trop. Med. Parasitol. 1937;31:435–445. [Google Scholar]
- 26.Jannin J, Cattand P. Treatment and control of human African trypanosomiasis. Curr Opin Infect Dis. 2004;17:565–571. doi: 10.1097/00001432-200412000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Barrett MP, et al. Drug resistance in human African trypanosomiasis. Future Microbiol. 2011;6:1037–1047. doi: 10.2217/fmb.11.88. [DOI] [PubMed] [Google Scholar]
- 28.Sanderson L, et al. Pentamidine movement across the murine blood-brain and blood-cerebrospinal fluid barriers: effect of trypanosome infection, combination therapy, P-glycoprotein, and multidrug resistance-associated protein. J Pharmacol Exp Ther. 2009;329:967–977. doi: 10.1124/jpet.108.149872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bray PG, et al. Pentamidine uptake and resistance in pathogenic protozoa: past, present and future. Trends Parasitol. 2003;19:232–239. doi: 10.1016/s1471-4922(03)00069-2. [DOI] [PubMed] [Google Scholar]
- 30.Doua F, et al. The efficacy of pentamidine in the treatment of early-late stage Trypanosoma brucei gambiense trypanosomiasis. Am J Trop Med Hyg. 1996;55:586–588. doi: 10.4269/ajtmh.1996.55.586. [DOI] [PubMed] [Google Scholar]
- 31.Edwards KJ, et al. Crystal structure of a pentamidine-oligonucleotide complex: implications for DNA-binding properties. Biochemistry. 1992;31:7104–7109. doi: 10.1021/bi00146a011. [DOI] [PubMed] [Google Scholar]
- 32.Shapiro TA, Englund PT. Selective cleavage of kinetoplast DNA minicircles promoted by antitrypanosomal drugs. Proc Natl Acad Sci U S A. 1990;87:950–954. doi: 10.1073/pnas.87.3.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathis AM, et al. Accumulation and intracellular distribution of antitrypanosomal diamidine compounds DB75 and DB820 in African trypanosomes. Antimicrob Agents Chemother. 2006;50:2185–2191. doi: 10.1128/AAC.00192-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson WD, et al. Antiparasitic compounds that target DNA. Biochimie. 2008;90:999–1014. doi: 10.1016/j.biochi.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schnaufer A. Evolution of dyskinetoplastic trypanosomes: how, and how often? Trends Parasitol. 2010;26:557–558. doi: 10.1016/j.pt.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Damper D, Patton CL. Pentamidine transport and sensitivity in brucei-group trypanosomes. J Protozool. 1976;23:349–356. doi: 10.1111/j.1550-7408.1976.tb03787.x. [DOI] [PubMed] [Google Scholar]
- 37.Lanteri CA, et al. The mitochondrion is a site of trypanocidal action of the aromatic diamidine DB75 in bloodstream forms of Trypanosoma brucei. Antimicrob Agents Chemother. 2008;52:875–882. doi: 10.1128/AAC.00642-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moreno SN. Pentamidine is an uncoupler of oxidative phosphorylation in rat liver mitochondria. Arch Biochem Biophys. 1996;326:15–20. doi: 10.1006/abbi.1996.0041. [DOI] [PubMed] [Google Scholar]
- 39.Delespaux V, de Koning HP. Drugs and drug resistance in African trypanosomiasis. Drug Resist Updat. 2007;10:30–50. doi: 10.1016/j.drup.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Wenzler T, et al. New treatment option for second-stage African sleeping sickness: in vitro and in vivo efficacy of aza analogs of DB289. Antimicrob Agents Chemother. 2009;53:4185–4192. doi: 10.1128/AAC.00225-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kotthaus J, et al. New prodrugs of the antiprotozoal drug pentamidine. ChemMedChem. 2011;6:2233–2242. doi: 10.1002/cmdc.201100422. [DOI] [PubMed] [Google Scholar]
- 42.Ehrlich P. Address in Pathology, ON CHEMIOTHERAPY: Delivered before the Seventeenth International Congress of Medicine. Br Med J. 1913;2:353–359. doi: 10.1136/bmj.2.2746.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Field MC, Carrington M. The trypanosome flagellar pocket. Nat Rev Microbiol. 2009;7:775–786. doi: 10.1038/nrmicro2221. [DOI] [PubMed] [Google Scholar]
- 44.Burri C, Keiser J. Pharmacokinetic investigations in patients from northern Angola refractory to melarsoprol treatment. Trop Med Int Health. 2001;6:412–420. doi: 10.1046/j.1365-3156.2001.00725.x. [DOI] [PubMed] [Google Scholar]
- 45.de Koning HP. Ever-increasing complexities of diamidine and arsenical crossresistance in African trypanosomes. Trends Parasitol. 2008;24:345–349. doi: 10.1016/j.pt.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 46.Legros D, et al. Treatment of human African trypanosomiasis - present situation and needs for research and development. Lancet Infect Dis. 2002;2:437–440. doi: 10.1016/s1473-3099(02)00321-3. [DOI] [PubMed] [Google Scholar]
- 47.Robays J, et al. High failure rates of melarsoprol for sleeping sickness, Democratic Republic of Congo. Emerg Infect Dis. 2008;14:966–967. doi: 10.3201/eid1406.71266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kayembe D, Wery M. Observations on the diamidine sensitivity of strains of Trypanosoma gambiense recently isolated in the Republic of Zaire. Ann Soc Belg Med Trop. 1972;52:1–8. [PubMed] [Google Scholar]
- 49.Geerts S, et al. African bovine trypanosomiasis: the problem of drug resistance. Trends Parasitol. 2001;17:25–28. doi: 10.1016/s1471-4922(00)01827-4. [DOI] [PubMed] [Google Scholar]
- 50.Frommel TO, Balber AE. Flow cytofluorimetric analysis of drug accumulation by multidrug-resistant Trypanosoma brucei brucei and T. b. rhodesiense. Mol Biochem Parasitol. 1987;26:183–191. doi: 10.1016/0166-6851(87)90142-3. [DOI] [PubMed] [Google Scholar]
- 51.Maser P, et al. Drug transport and drug resistance in African trypanosomes. Drug Resist Updat. 2003;6:281–290. doi: 10.1016/j.drup.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 52.Rollo IM, Williamson J. Acquired resistance to ‘Melarsen’, tryparsamide and amidines in pathogenic trypanosomes after treatment with ‘Melarsen’ alone. Nature. 1951;167:147–148. doi: 10.1038/167147a0. [DOI] [PubMed] [Google Scholar]
- 53.Carter NS, et al. Uptake of diamidine drugs by the P2 nucleoside transporter in melarsen-sensitive and -resistant Trypanosoma brucei brucei. J Biol Chem. 1995;270:28153–28157. doi: 10.1074/jbc.270.47.28153. [DOI] [PubMed] [Google Scholar]
- 54.Carter NS, Fairlamb AH. Arsenical-resistant trypanosomes lack an unusual adenosine transporter. Nature. 1993;361:173–176. doi: 10.1038/361173a0. [DOI] [PubMed] [Google Scholar]
- 55.de Koning HP, et al. Further evidence for a link between melarsoprol resistance and P2 transporter function in African trypanosomes. Mol Biochem Parasitol. 2000;106:181–185. doi: 10.1016/s0166-6851(99)00206-6. [DOI] [PubMed] [Google Scholar]
- 56.Maser P, et al. A nucleoside transporter from Trypanosoma brucei involved in drug resistance. Science. 1999;285:242–244. doi: 10.1126/science.285.5425.242. [DOI] [PubMed] [Google Scholar]
- 57.Stewart ML, et al. Multiple genetic mechanisms lead to loss of functional TbAT1 expression in drug-resistant trypanosomes. Eukaryot Cell. 2010;9:336–343. doi: 10.1128/EC.00200-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nerima B, et al. Detection of mutant P2 adenosine transporter (TbAT1) gene in Trypanosoma brucei gambiense isolates from northwest Uganda using allele-specific polymerase chain reaction. Trop Med Int Health. 2007;12:1361–1368. doi: 10.1111/j.1365-3156.2007.01918.x. [DOI] [PubMed] [Google Scholar]
- 59.Kazibwe AJ, et al. Genotypic status of the TbAT1/P2 adenosine transporter of Trypanosoma brucei gambiense isolates from Northwestern Uganda following melarsoprol withdrawal. PLoS Negl Trop Dis. 2009;3:e523. doi: 10.1371/journal.pntd.0000523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matovu E, et al. Genetic variants of the TbAT1 adenosine transporter from African trypanosomes in relapse infections following melarsoprol therapy. Mol Biochem Parasitol. 2001;117:73–81. doi: 10.1016/s0166-6851(01)00332-2. [DOI] [PubMed] [Google Scholar]
- 61.Geiser F, et al. Molecular pharmacology of adenosine transport in Trypanosoma brucei: P1/P2 revisited. Mol Pharmacol. 2005;68:589–595. doi: 10.1124/mol.104.010298. [DOI] [PubMed] [Google Scholar]
- 62.Matovu E, et al. Mechanisms of arsenical and diamidine uptake and resistance in Trypanosoma brucei. Eukaryot Cell. 2003;2:1003–1008. doi: 10.1128/EC.2.5.1003-1008.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bernhard SC, et al. Melarsoprol- and pentamidine-resistant Trypanosoma brucei rhodesiense populations and their cross-resistance. Int J Parasitol. 2007;37:1443–1448. doi: 10.1016/j.ijpara.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 64.Bridges DJ, et al. Loss of the high-affinity pentamidine transporter is responsible for high levels of cross-resistance between arsenical and diamidine drugs in African trypanosomes. Mol Pharmacol. 2007;71:1098–1108. doi: 10.1124/mol.106.031351. [DOI] [PubMed] [Google Scholar]
- 65.Teka IA, et al. The diamidine diminazene aceturate is a substrate for the high-affinity pentamidine transporter: implications for the development of high resistance levels in trypanosomes. Mol Pharmacol. 2011;80:110–116. doi: 10.1124/mol.111.071555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Koning HP. Uptake of pentamidine in Trypanosoma brucei brucei is mediated by three distinct transporters: implications for cross-resistance with arsenicals. Mol Pharmacol. 2001;59:586–592. doi: 10.1124/mol.59.3.586. [DOI] [PubMed] [Google Scholar]
- 67.de Koning HP, Jarvis SM. Uptake of pentamidine in Trypanosoma brucei brucei is mediated by the P2 adenosine transporter and at least one novel, unrelated transporter. Acta Trop. 2001;80:245–250. doi: 10.1016/s0001-706x(01)00177-2. [DOI] [PubMed] [Google Scholar]
- 68.Sauvage V, et al. The role of ATP-binding cassette (ABC) proteins in protozoan parasites. Mol Biochem Parasitol. 2009;167:81–94. doi: 10.1016/j.molbiopara.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 69.Borst P, Elferink RO. Mammalian ABC transporters in health and disease. Annu Rev Biochem. 2002;71:537–592. doi: 10.1146/annurev.biochem.71.102301.093055. [DOI] [PubMed] [Google Scholar]
- 70.Nikaido H. Multidrug resistance in bacteria. Annu Rev Biochem. 2009;78:119–146. doi: 10.1146/annurev.biochem.78.082907.145923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Klokouzas A, et al. ABC transporters and drug resistance in parasitic protozoa. Int J Antimicrob Agents. 2003;22:301–317. doi: 10.1016/s0924-8579(03)00210-3. [DOI] [PubMed] [Google Scholar]
- 72.Shahi SK, et al. Overexpression of the putative thiol conjugate transporter TbMRPA causes melarsoprol resistance in Trypanosoma brucei. Mol Microbiol. 2002;43:1129–1138. doi: 10.1046/j.1365-2958.2002.02831.x. [DOI] [PubMed] [Google Scholar]
- 73.Alibu VP, et al. The role of Trypanosoma brucei MRPA in melarsoprol susceptibility. Mol Biochem Parasitol. 2006;146:38–44. doi: 10.1016/j.molbiopara.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 74.Coelho AC, et al. Functional genetic identification of PRP1, an ABC transporter superfamily member conferring pentamidine resistance in Leishmania major. Mol Biochem Parasitol. 2003;130:83–90. doi: 10.1016/s0166-6851(03)00162-2. [DOI] [PubMed] [Google Scholar]
- 75.Damper D, Patton CL. Pentamidine transport in Trypanosoma brucei-kinetics and specificity. Biochem Pharmacol. 1976;25:271–276. doi: 10.1016/0006-2952(76)90213-6. [DOI] [PubMed] [Google Scholar]
- 76.Drew ME, et al. The adenosine analog tubercidin inhibits glycolysis in Trypanosoma brucei as revealed by an RNA interference library. J Biol Chem. 2003;278:46596–46600. doi: 10.1074/jbc.M309320200. [DOI] [PubMed] [Google Scholar]
- 77.Alsford S, et al. High-throughput decoding of antitrypanosomal drug efficacy and resistance. Nature. 2012;482:232–236. doi: 10.1038/nature10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baker N, et al. Genome-wide RNAi screens in African trypanosomes identify the nifurtimox activator NTR and the eflornithine transporter AAT6. Mol Biochem Parasitol. 2011;176:55–57. doi: 10.1016/j.molbiopara.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schumann Burkard G, et al. Genome-wide RNAi screens in bloodstream form trypanosomes identify drug transporters. Mol Biochem Parasitol. 2011;175:91–94. doi: 10.1016/j.molbiopara.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 80.Luo S, et al. Molecular characterization of Trypanosoma brucei P-type H+-ATPases. J Biol Chem. 2006;281:21963–21973. doi: 10.1074/jbc.M601057200. [DOI] [PubMed] [Google Scholar]
- 81.Beitz E. Aquaporins from pathogenic protozoan parasites: structure, function and potential for chemotherapy. Biol Cell. 2005;97:373–383. doi: 10.1042/BC20040095. [DOI] [PubMed] [Google Scholar]
- 82.Uzcategui NL, et al. Cloning, heterologous expression, and characterization of three aquaglyceroporins from Trypanosoma brucei. J Biol Chem. 2004;279:42669–42676. doi: 10.1074/jbc.M404518200. [DOI] [PubMed] [Google Scholar]
- 83.Zeuthen T, et al. Ammonia permeability of the aquaglyceroporins from Plasmodium falciparum, Toxoplasma gondii and Trypansoma brucei. Mol Microbiol. 2006;61:1598–1608. doi: 10.1111/j.1365-2958.2006.05325.x. [DOI] [PubMed] [Google Scholar]
- 84.Gruenberg J, et al. Role of glycerol permeation in the bloodstream form of Trypanosoma brucei. J Protozool. 1980;27:484–491. doi: 10.1111/j.1550-7408.1980.tb05404.x. [DOI] [PubMed] [Google Scholar]
- 85.Bassarak B, et al. Functional characterization of three aquaglyceroporins from Trypanosoma brucei in osmoregulation and glycerol transport. Cell Physiol Biochem. 2011;27:411–420. doi: 10.1159/000327968. [DOI] [PubMed] [Google Scholar]
- 86.Gupta AB, et al. MIPModDB: a central resource for the superfamily of major intrinsic proteins. Nucleic Acids Res. 2012;40:D362–369. doi: 10.1093/nar/gkr914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Beitz E, et al. Point mutations in the aromatic/arginine region in aquaporin 1 allow passage of urea, glycerol, ammonia, and protons. Proc Natl Acad Sci U S A. 2006;103:269–274. doi: 10.1073/pnas.0507225103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li H, et al. Enhancement of proton conductance by mutations of the selectivity filter of aquaporin-1. J Mol Biol. 2011;407:607–620. doi: 10.1016/j.jmb.2011.01.036. [DOI] [PubMed] [Google Scholar]
- 89.Sui H, et al. Structural basis of water-specific transport through the AQP1 water channel. Nature. 2001;414:872–878. doi: 10.1038/414872a. [DOI] [PubMed] [Google Scholar]
- 90.Baker N, et al. Aquaglyceroporin 2 controls susceptibility to melarsoprol and pentamidine in African trypanosomes. Proc Natl Acad Sci U S A. 2012;109:10996–11001. doi: 10.1073/pnas.1202885109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fairlamb AH, Cerami A. Metabolism and functions of trypanothione in the Kinetoplastida. Annu Rev Microbiol. 1992;46:695–729. doi: 10.1146/annurev.mi.46.100192.003403. [DOI] [PubMed] [Google Scholar]
- 92.Wu B, Beitz E. Aquaporins with selectivity for unconventional permeants. Cell Mol Life Sci. 2007;64:2413–2421. doi: 10.1007/s00018-007-7163-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Figarella K, et al. Biochemical characterization of Leishmania major aquaglyceroporin LmAQP1: possible role in volume regulation and osmotaxis. Mol Microbiol. 2007;65:1006–1017. doi: 10.1111/j.1365-2958.2007.05845.x. [DOI] [PubMed] [Google Scholar]
- 94.Mandal G, et al. Modulation of Leishmania major aquaglyceroporin activity by a mitogen-activated protein kinase. Mol Microbiol. 2012;85:1204–1218. doi: 10.1111/j.1365-2958.2012.08169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bienert GP, et al. A subgroup of plant aquaporins facilitate the bi-directional diffusion of As(OH)3 and Sb(OH)3 across membranes. BMC Biol. 2008;6:26. doi: 10.1186/1741-7007-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Isayenkov SV, Maathuis FJ. The Arabidopsis thaliana aquaglyceroporin AtNIP7;1 is a pathway for arsenite uptake. FEBS Lett. 2008;582:1625–1628. doi: 10.1016/j.febslet.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 97.Kamiya T, et al. NIP1;1, an aquaporin homolog, determines the arsenite sensitivity of Arabidopsis thaliana. J Biol Chem. 2009;284:2114–2120. doi: 10.1074/jbc.M806881200. [DOI] [PubMed] [Google Scholar]
- 98.Liu Z, et al. Arsenite transport by mammalian aquaglyceroporins AQP7 and AQP9. Proc Natl Acad Sci U S A. 2002;99:6053–6058. doi: 10.1073/pnas.092131899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Thuita JK, et al. Pharmacology of DB844, an orally active aza analogue of pafuramidine, in a monkey model of second stage human African trypanosomiasis. PLoS Negl Trop Dis. 2012;6:e1734. doi: 10.1371/journal.pntd.0001734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stewart ML, et al. Detection of arsenical drug resistance in Trypanosoma brucei with a simple fluorescence test. Lancet. 2005;366:486–487. doi: 10.1016/S0140-6736(05)66793-1. [DOI] [PubMed] [Google Scholar]
- 101.Edgar RC. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics. 2004;5:113. doi: 10.1186/1471-2105-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tamura K, et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]