Abstract
Objectives
This study compared regional cerebral metabolic rates of glucose (rCMRglu) determined by [18F]-fluoro-2-deoxyglucose positron emission tomography (FDG-PET) in suicide attempters and non-attempters.
Methods
Medication-free patients with major depression (n=29) had FDG-PET after single-blind administration of placebo (day 1) and fenfluramine (day 2). Suicide attempt history was obtained before scanning and at assessments over two subsequent years. Statistical parametric mapping evaluated associations between attempt status and rCMRglu, controlling for age.
Results
The study included 13 patients with and 16 without a history of suicide attempt within 2 years before or after scanning. After placebo, rCMRglu in attempters was lower in right dorsolateral prefrontal regions and higher in ventromedial regions than in non-attempters. After fenfluramine, relatively hypometabolic areas enlarged, and no hypermetabolic areas were detected.
Conclusion
Distinct rCMRglu patterns may be serotonin-sensitive biomarkers of suicide risk.
Keywords: Suicide, Major Depressive Disorder, Bipolar Disorder, positron emission tomography, FDG
Introduction
Positron emission tomography studies using [18F]-fluoro-2-deoxyglucose (FDG-PET) find differences in relative regional cerebral metabolic rates of glucose (rCMRglu) in patients with major depression compared with healthy volunteers (Baxter et al. 1989; Biver et al. 1994; Brody et al. 2001a; Hurwitz et al. 1990; Ketter et al. 2001; Kimbrell et al. 2002; Martinot et al. 1990) (Drevets et al. 2002; Ketter et al. 2001), and rCMRglu has been found to normalize in depressed patients after successful treatment (Baxter et al. 1989; Brody et al. 2001b; Kennedy et al. 2001; Martinot et al. 1990). A blunted rCMRglu response has been observed in major depressed compared with healthy groups after administration of dl-fenfluramine (FEN), which stimulates serotonin release and inhibits serotonin transporter function (Mann et al. 1996a).
However, only one prior study has used FDG-PET to investigate correlates of suicidal behavior directly. In suicide attempters with major depression (Oquendo et al. 2003), lower rCMRglu was found in high-lethality compared with low-lethality suicide attempters in superior and inferior frontal gyri and anterior cingulate, and these areas of difference double in size after FEN administration. Suicide intent correlated with part of these areas of difference in rCMRglu, and also correlated with suicide attempt lethality. Suicide risk has been consistently associated with low serotonergic functioning, as indicated by low levels of 5-HIAA in cerebrospinal fluid in suicide attempters in vivo (Asberg 1997; Mann et al. 2006) and low midbrain levels postmortem in suicides (Arango, Underwood and Mann 1997). Two pilot studies have utilized serotonin-specific PET ligands to explore serotonergic functioning in vivo in suicide attempters compared with healthy volunteers. Leyton, et al. (Leyton et al.) studied normalized alpha-[11C]methyl-L-tryptophan trapping, a debated measure of serotonin synthesis, and found lower uptake in suicide attempters in orbital and ventromedial prefrontal cortex. Van Heeringen et al. (van Heeringen et al. 2003) found lower binding potential of prefrontal 5-HT2A receptors in depressed attempters, although some postmortem studies of suicides found higher 5-HT2A receptor binding in prefrontal cortex (Arango et al. 1990; Perroud et al. 2010).
The present FDG-PET study sought to determine whether patterns of rCMRglu in patients in a Major Depressive Episode (MDE) could differentiate depressed suicide attempters from non-attempters, and whether stimulation of serotonin release by FEN would affect rCMRglu differentially in the two groups. Additional exploratory analyses were performed to determine whether sex, diagnosis (Major Depressive Disorder [MDD] vs. Bipolar disorder [BD]), or suicidal ideation accounted for attempter/non-attempter rCMRglu differences.
Methods
Sample
Participants gave written informed consent as approved by the Review Board of the New York State Psychiatric Institute at Columbia University. Participants (N=29) met DSM-IV criteria for current major depressive episode in context of MDD (N=23) or BD (N=6) based on the Structured Clinical Interview (SCID)(First et al. 1997), and a score of greater than 16 on the 17-item Hamilton Depression Rating Scale (HDRS-17) (Hamilton 1960) at time of enrollment. Participants were free of major medical illness based on history, physical examination, and laboratory tests, and were off psychotropic medications for at least 14 days prior to the PET scan (6 weeks for fluoxetine, 4 weeks for oral antipsychotics). Lorazepam (up to 3 mg daily) was permitted until 3 days before scanning, but rarely used.
Baseline assessments included the the HDRS-17 and the Scale of Suicidal Ideation (SSI) (Beck, Kovacs and Weissman 1979). Participants were evaluated for a lifetime history of suicide attempts using the Columbia Suicide History Scale (Oquendo, Halberstam and Mann 2003); ambiguous, interrupted, or aborted attempts were not counted. Participants were re-evaluated at 3, 12, and 24 months after the PET scans, and information was gathered concerning suicide attempts in the interim. The attempter group was defined as patients who had made suicide attempts within a time window of two years before or after scanning, thus excluding patients who had made attempts in the more remote past. The non-attempter group was defined as patients who had never made a suicide attempt. We limited the sample to recent attempters in order to mitigate possible diminution over time of cerebral physiological effects associated with suicide attempts, i.e. to prevent a dilution of effect by inclusion of individuals in whom the tendency to suicidal behaviors and associated neuropathology may have resolved with time. The period of 2 years before and after FDG-PET was selected also because it created a symmetrical observation window with regard to the index scan, since we had a 2-year follow-up period. Suicide attempters and non-attempters were compared in terms of clinical and demographic characteristics, using a one-way ANOVA for continuous variables or Chi square tests for categorical variables with IBM SPSS Statistics Version 19 (Release 19.0.0) for Mac OS X.
Pet Imaging
As previously described (Mann et al. 1996b), fasting participants had identical PET scans on two consecutive days, briefly described below. Participants received oral doses of placebo on the first day and approximately 0.8 mg/kg of FEN on the second day, as identical capsules, in a single-blind design. The order was not randomized because of the potential for FEN effects to persist into the next day. During each PET scan, blood was drawn prior to the scan and then hourly for five hours. Fenfluramine and norfenfluramine levels were measured by a gas-liquid chromatography method (Krebs, Cheng and Wright 1984; Myers et al. 1994). This study was conducted under an investigational drug (IND) permit prior to the removal of FEN from the US market.
At the predicted time of maximal prolactin and symptomatic response to FEN, 3 hours after drug administration, a bolus injection of approximately 5 mCi [18F]FDG was administered. Participants gazed at a uniform visual stimulus (cross hairs) in a dimmed, quiet room during the first 15 min of the 45 min [18F]FDG distribution phase, after which they were transferred to the scanner and positioned with the lowest scanning plane parallel to and approximately 1.0 cm above the canthomeatal line. Head movement was minimized with a custom-made thermoplastic mask. A Siemens ECAT EXACT 47 scanner (in plane spatial resolution 5.8 mm, axial resolution 4.3 mm FWHM at center) acquired a 60-min emission scan in 2D mode as twelve 5-min frames. Attenuation correction was based on a 15-min 68Ge/68Ga transmission scan. Images were reconstructed with a Shepp radial filter, cutoff frequency of 35 cycles per projection rays and a ramp axial filter, cutoff frequency of 0.5 S.
Image analysis
PET images were processed using automated image coregistration (AIR) (Woods, Cherry and Mazziotta 1992) to align the 12 frames within each scan, that were then summed and then transformed into Montreal Neurological Institute (MNI) atlas standard stereotaxic space. Images were smoothed by applying an isotropic 12 mm Gaussian kernel. Global normalization and proportional scaling were applied to control for individual differences in rCMRglu and other global effects.
Statistical parametric mapping analyses were performed using SPM8 (Institute of Neurology, University College of London, London, England; http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) (Friston et al. 1995) implemented in Matlab Version 7.9.0.529 (R2009b) (The Mathworks Inc, Natick, Mass). For each analysis, the adjusted mean rCMRglu and variance were computed at each voxel. For specified contrasts, whole brain parametric maps were constructed (Friston et al. 1995) based on t-values displaying all voxels above height thresholds set a priori to p<0.01 and voxel clusters found to be significant at an extent threshold of p ≤ 0.05 after family-wise error (FWE) correction for multiple comparisons. No additional statistical corrections were made for the effects of multiple testing associated with post-hoc SPM analyses. We note that subjects from this FDG-PET dataset have been included in other studies (Anderson et al. 2004; Keilp et al. 2010; Milak et al. 2005; Milak et al. 2009; Oquendo et al. 2005a; Oquendo et al. 2005b; Oquendo et al. 2003; Sublette et al. 2009) addressing different research questions.
The primary analyses compared suicide attempters with non-attempters on each of the two scan days (after placebo and after FEN), controlling for age. Secondary analyses were performed 1) to test for effects of sex by including sex as a covariate in the model comparing attempters and non-attempters with respect to rCMRglu; 2) to clarify the relevance of diagnostic differences between MDD and BD, by rerunning the primary model excluding subjects with BD; 3) to provide an alternative assessment of an association between suicide risk and rCMRglu, by independently testing the effects of suicidal ideation on rCMRglu in the entire sample; and 4) a sensitivity analysis for effects of handedness, by removing the 3 non-attempters who were left-handed and repeating the main analyses for days 1 and 2.
Clusters were identified anatomically by transforming the SPM-generated MNI coordinates into Talairach space (Brett 1999) and entering into the Talairach Client (Lancaster et al. 2000) (University of Texas Health Science Center, SanAntonio); additional detail regarding breakdown of the clusters into the proportions of voxels in specific anatomical regions was obtained via the MNI Space utility, as visualized and reported through xjview (http://www.alivelearn.net/).
Results
The sample comprised sixteen depressed subjects who had never attempted suicide, and thirteen subjects who attempted suicide within 2 years before (n=5), after (n=1), or both before and after (n=7) the PET scans. There were no significant group differences on any clinical or demographic characteristics (see Table 1).
Table 1.
Demographic and clinical characteristics of depressed participants by suicide attempt status.
| Characteristic | Attempters n=13 |
Non-attempters n=16 |
Chi2 | df | p |
|---|---|---|---|---|---|
| N (%) | |||||
| Sex: Male | 6 (46%) | 4 (25%) | 1.42 | 1 | 0.233 |
| Whitea | 12 (92%) | 13 (81%) | 0.74 | 1 | 0.390 |
| Right Handed | 13 (100%) | 13 (81%) | 2.72 | 1 | 0.099 |
| MDD (vs. BD) | 10 (77%) | 13 (91%) | 0.08 | 1 | 0.775 |
| Comorbid BPDa | 1 (8%) | 6 (37%) | 3.48 | 1 | 0.062 |
| Smoker | 5 (38%) | 5 (31%) | 0.16 | 1 | 0.684 |
| Characteristic | Attempters n=13 | Non-attempters n=16 | t-score | df | p |
| Mean (SD) | |||||
| Age | 36.0 (11.5) | 42.2 (13.0) | −1.34 | 27 | 0.192 |
| HDRS-17 | 22.5 (6.9) | 21.1 (4.8) | 0.65 | 27 | 0.523 |
| Characteristic | Attempters n=13 | Non-attempters n=16 | z-score | p | |
| Median (range) | |||||
| SSIb,c | 10.00 (32) | 7.5 (23) | −1.09 | 0.280 | |
One or more cell(s) in the Chi Square test had expected counts less than 5.
Current Scale for Suicidal Ideation score at the time of scanning.
Nonparametric (Wilcoxon W) testing was used due to left-skewed distribution associated with a large percentage of zeros in this measure.
Abbreviations: BD, Bipolar Disorder; BPD, Borderline Personality Disorder; HDRS-17, Hamilton Depression Rating Scale, 17-item; MDD, Major Depressive Disorder; SSI, Scale for Suicidal Ideation.
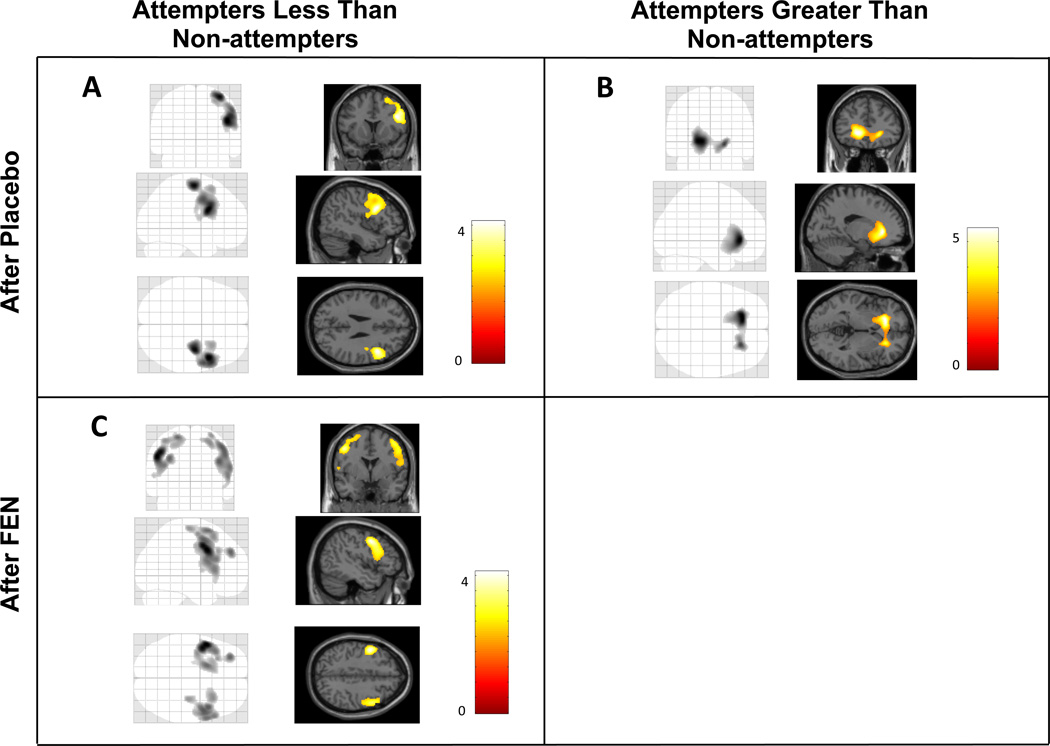
After placebo, in attempters compared with non-attempters, and controlling for age, rCMRglu was lower in a right dorsolateral prefrontal cortical region (DLPFC) where precentral gyrus and middle, superior and inferior frontal gyri comprised 92% of voxels (Table 2). rCMRglu was higher in a ventromedial region that primarily included left anterior cingulate, caudate, and putamen, with smaller contributions from inferior and medial frontal gyri and insula; this cluster extended bilaterally to a lesser extent (see Figure 1A and B and Table 2). Although significant at the cluster level by a priori standards, in this region identifiable gray matter anatomic regions accounted for only 40% of voxels (Table 2). Including sex in the model post-hoc did not reduce voxel intensity or extent of any clusters (data not shown). To explore whether suicide attempter and non-attempter differences were affected by diagnostic group, analyses were re-run without the 6 BD subjects. In this smaller sample of MDD only, essentially the same pattern of low and high rCMRglu clusters observed in the combined sample was detected at the same p value threshold for voxel intensity (p < 0.01) but did not reach significance in cluster extent (data not shown). However, post-hoc analysis leaving out the 3 left-handed non-attempters found a larger region with greater significance in which rCMRglu was higher in attempters compared with non-attempters: the total cluster extent was 1.6 times greater than in the entire sample (pFWE=0.002), comprising a larger left medial prefrontal region with a higher significance level (voxel extent=3725, pFWE=0.004) and an additional cluster in the right cerebellum (voxel extent= 2872, pFWE=0.016). The voxel cluster in which rCMRglu was lower in attempters appeared in the same right-sided brain region as in the entire sample but was 53% smaller (voxel extent 1323) and no longer significant (pFWE=0.225), likely due to a loss of statistical power.
Table 2.
Brain regions in which relative regional cerebral metabolic rate of glucose (rCMRglu) differed between depressed participants who attempted suicide within 2 years before or after scanning (attempters), compared with those who never attempted suicide (non-attempters).
| rCMRglu Analysis |
Cluster- level PFWE |
Cluster Extent (voxels) |
Left/ Right |
Anatomic Locations |
Number of Voxels |
Brodmann Areas |
|---|---|---|---|---|---|---|
| Attempters greater than non-attempters after placebo | 0.032 | 2,489 | Left > Right | Anterior Cingulate | 423 | 24 |
| Caudate | 227 | |||||
| Putamen | 221 | |||||
| Inferior Frontal | 84 | 47 | ||||
| Insula | 53 | |||||
| Medial Frontal | 48 | 10 | ||||
| Attempters greater than non-attempters after fenfluramine | No Significant Findings | |||||
| Attempters less than non-attempters after placebo | 0.019 | 2,814 | Right | Middle Frontal | 1171 | 6 |
| Inferior Frontal | 684 | 9 | ||||
| Precentral | 529 | 6 | ||||
| Superior Frontal | 163 | 8 | ||||
| Attempters less than non-attempters after fenfluramine | 0.013 | 2,956 | Right | Inferior Frontal | 1093 | 9, 45 |
| Middle Frontal | 916 | 6 | ||||
| Precentral | 605 | 6 | ||||
| Superior Frontal | 156 | 8 | ||||
| 0.020 | 2,693 | Left | Middle Frontal | 1140 | 6, 9, 46 | |
| Superior Frontal | 558 | 6, 8 | ||||
| Inferior Frontal | 476 | 9 | ||||
| Precentral | 183 | 6 | ||||
Voxel clusters with local maxima more than 8 mm apart identified by statistical parametric analyses as correlating significantly with rCMRglu (extent threshold, pFWE= p ≤ 0.05 after FWE correction; height threshold, p ≤ 0.01) were transformed into Tailarach space and labelled anatomically to the nearest gray matter location by Brodmann areas using the Tailarach Client. Breakdown of clusters into the numbers of voxels in specific anatomical regions was performed using xjview.
Abbreviations: rCMRglu, relative regional cerebral metabolic rate of glucose; FWE, familywise error rate; MNI, Montreal Neurological Institute standard brain.
Figure 1. Statistical parametric maps of relative regional cerebral metabolic rates of glucose uptake (rCMRglu) in depressed participants reporting attempted suicide within 2 years before or after PET scanning, compared with depressed non-attempters, after placebo and after dl-fenfluramine hydrochloride (FEN).
A: rCMRglu less in depressed attempters than non-attempters after placebo. B: rCMRglu greater in depressed attempters than non-attempters after placebo. C: rCMRglu less in depressed attempters than non-attempters after fenfluramine. Left panels show statistical parametric maps as maximum intensity projections on ‘glass brains’. Right panels illustrate the same maps superimposed on standardized MRI brain slices.
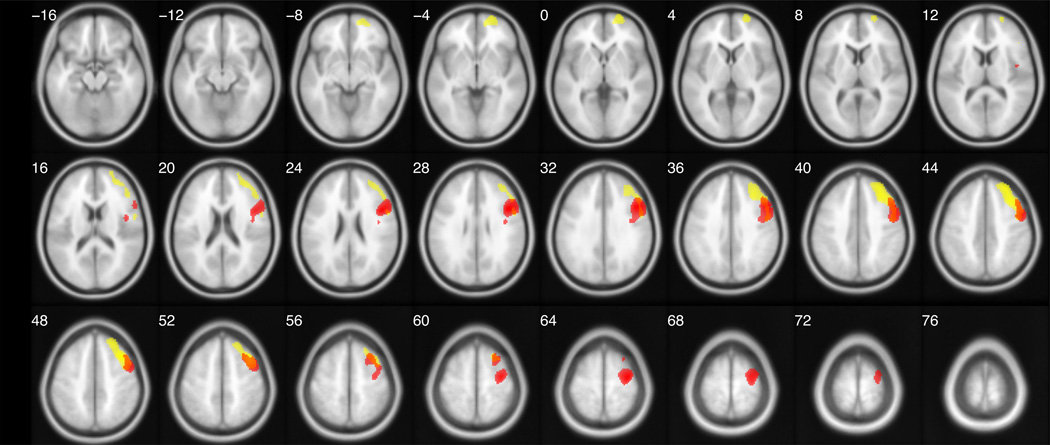
Suicidal ideation (SSI) scores at the time of enrollment in the study correlated negatively with rCMRglu (pFWE = 0.011) in a region that overlapped the DLPFC region in which attempters were relatively hypometabolic, by 30% (see Figure 2). The SSI-correlated region was greater in size than the attempter-specific region (cluster extent [voxels]= 3174 vs. 2814, respectively), and comprised a larger segment of both the middle and superior frontal gyri. Middle, superior, and inferior frontal and precentral gyri accounted for 96% of the voxels in this cluster.
Figure 2. Statistical parametric maps of relative regional cerebral metabolic rates of glucose uptake (rCMRglu) after placebo: correlation with suicidal ideation (SSI) scores (yellow) superimposed on the comparison of rCMRglu in suicide attempters less than non-attempters (red).
Using the toolbox utility, xjview, SPM-derived t-score maps are overlaid on a series of transaxial slices [4 mm apart] of a coregistered magnetic resonance image template.
After FEN administration, areas of relative hypometabolism approximately doubled in extent by essentially becoming totally bilateral (see Figure 1C and Table 2), while areas of relative hypermetabolism were decreased in size by about half, becoming nonsignificant. In the post-hoc analysis after removing 3 left-handed non-attempters, the same pattern was seen: areas of relative hypometabolism were more than doubled in size and bilateral after FEN, in this case altering from not statistically significant to a larger and more significant area than in the total sample (voxel extent=30590 for right-sided cluster, pFWE=0.010; voxel extent=32470 for left-sided cluster, pFWE<0.001). Areas of relative hypermetabolism, left medial prefrontal and cerebellar regions, likewise decreased by about half, becoming nonsignificant.
Summed plasma levels of FEN and norfenfluramine at 3 and 4 hour time points did not differ significantly between groups (mean summed levels for attempters, in ng/ml (SD): 47.0 (14.7); for non-attempters: 59.9 (27.1); t-score = −1.36, df = 22, p = 0.188).
Discussion
This study identified two brain regions in which relative differences in rCMRglu distinguished MDE patients who made suicide attempt(s) within two years of the index FDG-PET scan from those with MDE but no lifetime history of suicide attempt. The major findings were relative hypometabolism in a right DLPFC region and hypermetabolism in a left ventromedial region.
The right DLPFC region hypometabolic in attempters compared with non-attempters (BA 6,8,9) overlapped with regions in which hypometabolism was also found to be associated with greater suicidal ideation (see Figure 2). This DLPFC region may therefore represent a brain location for cognitive processes related to suicide risk, an idea that has face validity since the DLPFC, including BA 6 and 9, is involved in complex planning (Elliott et al. 1997), and the right DLPFC in particular (Cho et al. 2010) is involved in inhibitory control of impulsive decision making.
Since this study was limited to a depressed sample, the differences between attempters and nonattempters are not due to the effects of depression. However, no direct conclusions can be drawn concerning differences between depressed and healthy groups, nor concerning characteristics of non-depressed suicide attempters. Comparison of our current findings with those of FDG-PET studies in depression suggests a broad hypothesis in which rCMRglu patterns may be a biomarker of suicide risk, as follows: We here observe that lower rCMRglu in certain DLPFC regions (BA 6,8,9) differentiated attempters from non-attempters, and previously reported that it differentiated high-lethality from low-lethality attempters (Oquendo et al. 2003). Moreover, the depression literature finds lower rCMRglu in depressed patients compared with healthy volunteers in similar prefrontal cortical areas (Baxter et al. 1989; Biver et al. 1994; Hurwitz et al. 1990; Ketter et al. 2001; Kimbrell et al. 2002; Martinot et al. 1990) (although findings are not always consistent with regard to laterality). Finally, we have also reported (Milak et al. 2010) lower rCMRglu in a region that included BA 6,8,9 associated with higher factor scores for subjective depression as assessed by the Beck Depression Inventory (BDI) (Beck et al. 1961). BDI depression scores have been significantly associated with suicide risk (Mann et al. 2008; Mann et al. 1999). We therefore hypothesize that relative levels of rCMRglu in the BA 6,8,9 regions of the superior, middle, inferior, and precentral gyri might serve as an indicator of suicide risk in which rCMRglu deficit increases along a continuum from virtually no risk (non-depressed individuals) to very low risk (depressed non-attempters) to some risk (low-lethality depressed attempters) to highest risk (high-lethality depressed attempters). This hypothesis ideally should be tested in an independent sample.
Since rCMRglu is measured while the participant is lying quietly, unengaged in specific cognitive tasks, FDG-PET provides information about the default mode network, a group of brain regions that are activated during self-referential mentation. Relative dominance of default-mode network activity on BOLD fMRI in depressed compared to healthy adults in the resting state is associated with increased maladaptive ruminative thinking (Hamilton et al. 2011). As ruminative thinking is closely associated with suicidal ideation and behavior (Morrison and O'Connor 2008), this ties in conceptually with our observations of relatively elevated rCMRglu in default mode network region BA 24 in the anterior cingulate, in suicide attempters over non-attempters. Elevated activation of anterior cingulate and prefrontal cortex was also seen on BOLD fMRI in combat-exposed veterans with a history of suicidal ideation compared with non-ideators in association with error processing, a form of self-monitoring, during a stop task (Matthews et al. 2012). In contrast to these findings of increased rCMRglu or BOLD activity during resting state or self-monitoring, decreased anterior cingulate activation in suicide attempters is seen during cognitively challenging tasks: compared with depressed non-attempters during response inhibition of a go no-go task (Pan et al. 2011), and compared with healthy volunteers on a category verbal fluency task (Audenaert et al. 2002).
The combination we observed in suicide attempters, of relatively decreased rCMRglu in DLPFC and increased rCMRglu in anterior medial regions, is also consistent with BOLD fMRI activity in suicide attempters in whom recall of mental pain during suicidal episodes was associated with lower prefrontal cortical activity (BA 6,10,46) while recall of suicide action was associated with higher activity in the medial PFC, the anterior cingulate cortex, and the hippocampus. (Reisch et al. 2010). In parallel with these findings, in right-handed men with a past history of MDD, suicide attempters compared with non-attempters showed decreased BOLD fMRI activity in right superior frontal cortex (BA 6) and increased activity in right lateral orbitofrontal cortex (BA 47), in response to angry vs neutral face stimuli (Jollant et al. 2008).
The latter study (Jollant et al. 2008) additionally found increased BOLD fMRI activity in right cerebellum, which we did not find in our main analysis. However, our post-hoc analysis excluding left-handed participants likewise found increased rCMRglu in right cerebellum in attempters over non-attempters, while our previous report on lethality (Oquendo et al. 2003) also found increased rCMRglu in cerebellum bilaterally in right-handed high-lethality compared with low-lethality attempters. It has been suggested that the cerebellum, which has reciprocal connections with various limbic structures, may play a role in the regulation of emotion (Schutter and van Honk 2009).
Blunted rCMRglu responses to FEN are thought to be due to lower available serotonin and thus an impaired ability to respond to an acute serotonergic stimulus (Mann et al. 1996a). This model is consistent with evidence of reduced prolactin levels in response to FEN, another proxy for serotonergic functioning, in suicide attempters with major depression (Correa et al. 2000; Keilp et al. 2010) or personality disorders (Coccaro, Lee and Kavoussi 2010) relative to psychiatric control non-attempters (Coccaro, Lee and Kavoussi 2010; Correa et al. 2000) or healthy controls (Correa et al. 2000; Keilp et al. 2010), and in high-lethality attempters compared with low-lethality attempters with MDD (Malone et al. 1996; Oquendo et al. 2003).
We observed that after FEN administration, brain areas where attempters were relatively hypometabolic were enlarged, and previously hypermetabolic brain regions shrank in extent below the level of significance. Since these results are all relative, a parsimonious explanation is that non-attempters responded to FEN with globally increased rCMRglu and attempters exhibited more blunted responses. Thus, in regions where rCMRglu is lower in attempters at baseline, if FEN selectively increases rCMRglu in non-attempters, the disparity between the groups becomes even greater. In contrast, in regions where baseline rCMRglu is higher in attempters, after FEN the rCMRglu increase in non-attempters would cause the difference to disappear. This is consistent with our results.
In studies of depressed patients compared with healthy volunteers, there is a blunted response to FEN in the depressed group that also involves increased left-sided hypometabolism in superior frontal, middle frontal, and inferior frontal gyri (BA 4,8,9,10,44,45,46) compared with healthy subjects (Anderson et al. 2004). In a similar design, in higher-lethality compared with lower-lethality suicide attempters (Oquendo et al. 2003), FEN administration also caused increased hypometabolic regions, bilaterally, and disappearance of hypermetabolic areas. However, in the lethality study the regional pattern of increase involved extension to the anterior cingulate along with loss of medial and lateral PFC components, not observed in the current analyses; and the hypermetabolic area which disappeared was in the cerebellum, observed in the present study only after exclusion of left-handed participants. In concept, however, these combined findings suggest blunting of response to FEN-stimulated serotonin release along an illness severity gradient.
Taken together, our current findings and previous literature support a hypothesis that alterations in glucose metabolism in specific brain regions associated with suicide attempter status in depression are related to altered serotonergic function and vary across a continuum from lowest to highest suicide risk. The rCMRglu patterns we observed were consistent with findings using other neuroimaging modalities assessing brain regional association with suicide attempter status (Jollant et al. 2008; Reisch et al. 2010).
Limitations
Since all but one subsequent suicide attempter had prior attempt history, indicating a trait susceptibility to suicidal behavior, it was not possible to determine whether rCMRglu prior to the first suicide attempt could predict future suicide attempt. Although subjects from this study overlap with our previous study of suicide lethality (Oquendo et al. 2003), for the present study we added a non-attempter group on the basis of depressed individuals who had data from a 2-year follow-up after their FDG-PET scan. Also, to increase the chance of detecting neurobiologic differences between attempters and non-attempters, we only included those suicide attempters whose attempt had occurred within 2 years before or after the FDG-PET. Too few bipolar subjects were included to evaluate BD separately. The sample size also did not permit correction for additional covariates such as age at onset of depression or length of current major depressive episode. The findings are not likely to be due to the presence of comorbid BPD among suicide attempters since the rates did not differ in the two groups, and in fact there was a trend in toward fewer BPD among the suicide attempters in this sample. Moreover, the brain regions that distinguished attempters from non-attempters are not consistent with those previously reported by us in MDD subjects with and without BPD (Oquendo et al. 2005b). Findings are not likely due to differences in FEN pharmacokinetics between suicide attempters and non-attempters, because no between-group differences in FEN metabolite levels were seen on quantitative metabolite analysis. As this study included only patients with a major depressive episode, it is not known whether these findings reflect brain pathology in non-depressed suicide attempters.
Conclusions
This pilot study finds a link between specific brain regions and suicidal behavior/ideation in MDD that may be related to altered serotonergic functioning. Future studies must determine whether these regional brain differences in glucose uptake may be a predictive biomarker for suicide attempt risk.
Acknowledgements
This study was supported in part by grants MH079033, MH40695, and MH62185 from the National Institutes of Health, Bethesda, MD, and by the National Alliance for Research on Schizophrenia and Depression, Great Neck, New York. We thank Ramin V. Parsey, M.D.Ph.D. for his critical reading of the manuscript.
Footnotes
Conflicts of Interest: Dr. Oquendo has received an unrestricted educational grant from Eli Lilly and has served as a consultant to Pfizer Inc. Dr. Mann received unrelated past imaging grants from GSK and Novartis. Dr. Sublette has received a donation of nutritional supplements from Unicity, International, for an unrelated study. All other authors report no competing interests to disclose.
References
- Anderson AD, Oquendo MA, Parsey RV, et al. Regional brain responses to serotonin in major depressive disorder. Journal of Affective Disorders. 2004;82(3):411–417. doi: 10.1016/j.jad.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Arango V, Ernsberger P, Marzuk PM, et al. Autoradiographic demonstration of increased serotonin 5-HT2 and beta-adrenergic receptor binding sites in the brain of suicide victims. Archives of General Psychiatry. 1990;47(11):1038–1047. doi: 10.1001/archpsyc.1990.01810230054009. [DOI] [PubMed] [Google Scholar]
- Arango V, Underwood MD, Mann JJ. Postmortem findings in suicide victims. Implications for in vivo imaging studies. Annals of the New York Academy of Sciences. 1997;836:269–287. doi: 10.1111/j.1749-6632.1997.tb52365.x. [DOI] [PubMed] [Google Scholar]
- Asberg M. Neurotransmitters and suicidal behavior. The evidence from cerebrospinal fluid studies. Annals of the New York Academy of Sciences. 1997;836:158–181. doi: 10.1111/j.1749-6632.1997.tb52359.x. [DOI] [PubMed] [Google Scholar]
- Audenaert K, Goethals I, Van Laere K, et al. SPECT neuropsychological activation procedure with the Verbal Fluency Test in attempted suicide patients. Nuclear Medicine Communications. 2002;23(9):907–916. doi: 10.1097/00006231-200209000-00015. [DOI] [PubMed] [Google Scholar]
- Baxter LR, Jr, Schwartz JM, Phelps ME, et al. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Archives of General Psychiatry. 1989;46(3):243–250. doi: 10.1001/archpsyc.1989.01810030049007. [DOI] [PubMed] [Google Scholar]
- Beck AT, Kovacs M, Weissman A. Assessment of suicidal intention: the Scale for Suicide Ideation. Journal of Consulting and Clinical Psychology. 1979;47(2):343–352. doi: 10.1037//0022-006x.47.2.343. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Biver F, Goldman S, Delvenne V, et al. Frontal and parietal metabolic disturbances in unipolar depression. Biological Psychiatry. 1994;36(6):381–388. doi: 10.1016/0006-3223(94)91213-0. [DOI] [PubMed] [Google Scholar]
- Brett M. The MNI brain and the Talairach atlas. 1999 [Google Scholar]
- Brody AL, Barsom MW, Bota RG, et al. Prefrontal-subcortical and limbic circuit mediation of major depressive disorder. Seminars in Clinical Neuropsychiatry. 2001a;6(2):102–112. doi: 10.1053/scnp.2001.21837. [DOI] [PubMed] [Google Scholar]
- Brody AL, Saxena S, Stoessel P, et al. Regional brain metabolic changes in patients with major depression treated with either paroxetine or interpersonal therapy: preliminary findings. Archives of General Psychiatry. 2001b;58(7):631–640. doi: 10.1001/archpsyc.58.7.631. [DOI] [PubMed] [Google Scholar]
- Cho SS, Ko JH, Pellecchia G, et al. Continuous theta burst stimulation of right dorsolateral prefrontal cortex induces changes in impulsivity level. Brain Stimul. 2010;3(3):170–176. doi: 10.1016/j.brs.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccaro EF, Lee R, Kavoussi RJ. Aggression, suicidality, and intermittent explosive disorder: serotonergic correlates in personality disorder and healthy control subjects. Neuropsychopharmacology. 2010;35(2):435–444. doi: 10.1038/npp.2009.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa H, Duval F, Mokrani M, et al. Prolactin response to D-fenfluramine and suicidal behavior in depressed patients. Psychiatry Research. 2000;93(3):189–199. doi: 10.1016/s0165-1781(00)00114-1. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Bardgett ME, et al. Glucose metabolism in the amygdala in depression: relationship to diagnostic subtype and plasma cortisol levels. Pharmacology, Biochemistry and Behavior. 2002;71(3):431–447. doi: 10.1016/s0091-3057(01)00687-6. [DOI] [PubMed] [Google Scholar]
- Elliott R, Baker SC, Rogers RD, et al. Prefrontal dysfunction in depressed patients performing a complex planning task: a study using positron emission tomography. Psychological Medicine. 1997;27(4):931–942. doi: 10.1017/s0033291797005187. [DOI] [PubMed] [Google Scholar]
- First M, Williams J, Spitzer R, et al. Structured Clinical Interview for DSM-IV Axis I Disorders. Washington D.C.: American Psychiatric Publishing, Inc.; 1997. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, et al. Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapping. 1995:189–210. [Google Scholar]
- Hamilton JP, Furman DJ, Chang C, et al. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biological Psychiatry. 2011;70(4):327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz TA, Clark C, Murphy E, et al. Regional cerebral glucose metabolism in major depressive disorder. Canadian Journal of Psychiatry. Revue Canadienne de Psychiatrie. 1990;35(8):684–688. doi: 10.1177/070674379003500807. [DOI] [PubMed] [Google Scholar]
- Jollant F, Lawrence NS, Giampietro V, et al. Orbitofrontal cortex response to angry faces in men with histories of suicide attempts. American Journal of Psychiatry. 2008;165(6):740–748. doi: 10.1176/appi.ajp.2008.07081239. [DOI] [PubMed] [Google Scholar]
- Keilp JG, Oquendo MA, Stanley BH, et al. Future suicide attempt and responses to serotonergic challenge. Neuropsychopharmacology. 2010;35(5):1063–1072. doi: 10.1038/npp.2008.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SH, Evans KR, Kruger S, et al. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. American Journal of Psychiatry. 2001;158(6):899–905. doi: 10.1176/appi.ajp.158.6.899. [DOI] [PubMed] [Google Scholar]
- Ketter TA, Kimbrell TA, George MS, et al. Effects of mood and subtype on cerebral glucose metabolism in treatment-resistant bipolar disorder. Biological Psychiatry. 2001;49(2):97–109. doi: 10.1016/s0006-3223(00)00975-6. [DOI] [PubMed] [Google Scholar]
- Kimbrell TA, Ketter TA, George MS, et al. Regional cerebral glucose utilization in patients with a range of severities of unipolar depression. Biological Psychiatry. 2002;51(3):237–252. doi: 10.1016/s0006-3223(01)01216-1. [DOI] [PubMed] [Google Scholar]
- Krebs HA, Cheng LK, Wright GJ. Determination of fenfluramine and norfenfluramine in plasma using a nitrogen-sensitive detector. Journal of Chromatography. 1984;310(2):412–417. doi: 10.1016/0378-4347(84)80109-7. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, et al. Automated Talairach atlas labels for functional brain mapping. Human Brain Mapping. 2000;10(3):120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyton M, Paquette V, Gravel P, et al. alpha-[11C]Methyl-L-tryptophan trapping in the orbital and ventral medial prefrontal cortex of suicide attempters. European Neuropsychopharmacology. 2006;16(3):220–223. doi: 10.1016/j.euroneuro.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Malone KM, Corbitt EM, Li S, et al. Prolactin response to fenfluramine and suicide attempt lethality in major depression. British Journal of Psychiatry. 1996;168(3):324–329. doi: 10.1192/bjp.168.3.324. [DOI] [PubMed] [Google Scholar]
- Mann J, Currier D, Stanley B, et al. Can biological tests assist prediction of suicide in mood disorders? International Journal of Neuropsychopharmacology. 2006;9(4):465–474. doi: 10.1017/S1461145705005687. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Ellis SP, Waternaux CM, et al. Classification trees distinguish suicide attempters in major psychiatric disorders: a model of clinical decision making. Journal of Clinical Psychiatry. 2008;69(1):23–31. doi: 10.4088/jcp.v69n0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann JJ, Malone KM, Diehl DJ, et al. Demonstration in vivo of reduced serotonin responsivity in the brain of untreated depressed patients. American Journal of Psychiatry. 1996a;153(2):174–182. doi: 10.1176/ajp.153.2.174. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Malone KM, Diehl DJ, et al. Positron emission tomographic imaging of serotonin activation effects on prefrontal cortex in healthy volunteers. Journal of Cerebral Blood Flow and Metabolism. 1996b;16(3):418–426. doi: 10.1097/00004647-199605000-00008. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Waternaux C, Haas GL, et al. Toward a clinical model of suicidal behavior in psychiatric patients. American Journal of Psychiatry. 1999;156(2):181–189. doi: 10.1176/ajp.156.2.181. [DOI] [PubMed] [Google Scholar]
- Martinot JL, Hardy P, Feline A, et al. Left prefrontal glucose hypometabolism in the depressed state: a confirmation. American Journal of Psychiatry. 1990;147(10):1313–1317. doi: 10.1176/ajp.147.10.1313. [DOI] [PubMed] [Google Scholar]
- Matthews S, Spadoni A, Knox K, et al. Combat-exposed war veterans at risk for suicide show hyperactivation of prefrontal cortex and anterior cingulate during error processing. Psychosomatic Medicine. 2012;74(5):471–475. doi: 10.1097/PSY.0b013e31824f888f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milak MS, Keilp J, Parsey RV, et al. Regional brain metabolic correlates of self-reported depression severity contrasted with clinician ratings. Journal of Affective Disorders. 2010;126(1–2):113–124. doi: 10.1016/j.jad.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milak MS, Parsey RV, Keilp J, et al. Neuroanatomic correlates of psychopathologic components of major depressive disorder. Archives of General Psychiatry. 2005;62(4):397–408. doi: 10.1001/archpsyc.62.4.397. [DOI] [PubMed] [Google Scholar]
- Milak MS, Parsey RV, Lee L, et al. Pretreatment regional brain glucose uptake in the midbrain on PET may predict remission from a major depressive episode after three months of treatment. Psychiatry Research. 2009;173(1):63–70. doi: 10.1016/j.pscychresns.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison R, O'Connor RC. A systematic review of the relationship between rumination and suicidality. Suicide and Life-Threatening Behavior. 2008;38(5):523–538. doi: 10.1521/suli.2008.38.5.523. [DOI] [PubMed] [Google Scholar]
- Myers JE, Mieczkowski T, Perel J, et al. Abnormal behavioral responses to fenfluramine in patients with affective and personality disorders. Correlation with increased serotonergic responsivity. Biological Psychiatry. 1994;35(2):112–120. doi: 10.1016/0006-3223(94)91200-9. [DOI] [PubMed] [Google Scholar]
- Oquendo M, Krunic A, Parsey R, et al. Positron Emission Tomography of Regional Brain Metabolic Responses to a Serotonergic Challenge in Major Depressive Disorder with and without Borderline Personality Disorder. Neuropsychopharmacology. 2005a;30(6):1163–1172. doi: 10.1038/sj.npp.1300689. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Halberstam B, Mann JJ. Risk factors for suicidal behavior: utility and limitations of research instruments. In: First MB, editor. Standardized Evaluation in Clinical Practice. Washington, DC: APPI Press; 2003. pp. 103–30. [Google Scholar]
- Oquendo MA, Krunic A, Parsey RV, et al. Positron emission tomography of regional brain metabolic responses to a serotonergic challenge in major depressive disorder with and without borderline personality disorder. Neuropsychopharmacology. 2005b;30(6):1163–1172. doi: 10.1038/sj.npp.1300689. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Placidi GP, Malone KM, et al. Positron emission tomography of regional brain metabolic responses to a serotonergic challenge and lethality of suicide attempts in major depression. Archives of General Psychiatry. 2003;60(1):14–22. doi: 10.1001/archpsyc.60.1.14. [DOI] [PubMed] [Google Scholar]
- Pan LA, Batezati-Alves SC, Almeida JR, et al. Dissociable patterns of neural activity during response inhibition in depressed adolescents with and without suicidal behavior. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50(6):602–611. doi: 10.1016/j.jaac.2011.03.018. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perroud N, Neidhart E, Petit B, et al. Simultaneous analysis of serotonin transporter, tryptophan hydroxylase 1 and 2 gene expression in the ventral prefrontal cortex of suicide victims. American Journal of Medical Genetics Part B Neuropsychiatric Genetics. 2010;153B(4):909–918. doi: 10.1002/ajmg.b.31059. [DOI] [PubMed] [Google Scholar]
- Reisch T, Seifritz E, Esposito F, et al. An fMRI study on mental pain and suicidal behavior. Journal of Affective Disorders. 2010;126(1–2):321–325. doi: 10.1016/j.jad.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Schutter DJ, van Honk J. The cerebellum in emotion regulation: a repetitive transcranial magnetic stimulation study. Cerebellum. 2009;8(1):28–34. doi: 10.1007/s12311-008-0056-6. [DOI] [PubMed] [Google Scholar]
- Sublette ME, Milak MS, Hibbeln JR, et al. Plasma polyunsaturated fatty acids and regional cerebral glucose metabolism in major depression. Prostaglandins Leukotrienes and Essential Fatty Acids. 2009;80(1):57–64. doi: 10.1016/j.plefa.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heeringen C, Audenaert K, Van Laere K, et al. Prefrontal 5-HT2a receptor binding index, hopelessness and personality characteristics in attempted suicide. Journal of Affective Disorders. 2003;74(2):149–158. doi: 10.1016/s0165-0327(01)00482-7. [DOI] [PubMed] [Google Scholar]
- Woods RP, Cherry SR, Mazziotta JC. Rapid automated algorithm for aligning and reslicing PET images. Journal of Computer Assisted Tomography. 1992;16(4):620–633. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]