Abstract
Magnetic resonance imaging (MRI) techniques for assessment of morphology and function of the pancreas have been improved dramatically the recent years and MRI is very often used in diagnosing and follow-up of chronic pancreatitis (CP) patients. Standard MRI including fat-suppressed T1-weighted and T2-weighted imaging techniques reveal decreased signal and glandular atrophy of the pancreas in CP. In contrast-enhanced MRI of the pancreas in CP the pancreatic signal is usually reduced and delayed due to decreased perfusion as a result of chronic inflammation and fibrosis. Thus, morphological changes of the ductal system can be assessed by magnetic resonance cholangiopancreatography (MRCP). Furthermore, secretin-stimulated MRCP is a valuable technique to evaluate side branch pathology and the exocrine function of the pancreas and diffusion weighted imaging can be used to quantify both parenchymal fibrotic changes and the exocrine function of the pancreas. These standard and advanced MRI techniques are supplementary techniques to reveal morphological and functional changes of the pancreas in CP. Recently, spectroscopy has been used for assessment of metabolite concentrations in-vivo in different tissues and may have the potential to offer better tissue characterization of the pancreas. Hence, the purpose of the present review is to provide an update on standard and advanced MRI techniques of the pancreas in CP.
Keywords: Magnetic resonance, Chronic pancreatitis, Secretin, Diffusion weighted imaging, Exocrine pancreatic function
Core tip: Magnetic resonance imaging (MRI) techniques for assessment of morphology and function of the pancreas are often used in diagnosing and follow-up of chronic pancreatitis patients. The purpose of the present review is to provide an update on standard and advanced MRI techniques of the pancreas in chronic pancreatitis. In addition to standard MRI techniques, advanced MRI techniques including magnetic resonance cholangiopancreatography (MRCP), secretin-stimulated MRCP and diffusion weighted imaging can also provide important microstructural and functional information.
INTRODUCTION
The diagnosis and follow-up of chronic pancreatitis (CP) patients rely on both clinical and imaging information such as the Mayo Clinic diagnostic criteria[1]. Especially, the diagnosis of CP at an early stage is a clinical challenge. The development in imaging techniques, and especially magnetic resonance imaging (MRI), has dramatically improved the information on both morphology and function of the pancreas. In the Mayo diagnostic criteria for CP, MRI is now an accepted method for assessing ductal pathology and concrements[1].
A few decades ago, before the clinical introduction of cross-sectional imaging techniques, the imaging evaluation of CP was limited to plain radiography depicting calcifications. Traditionally, the ductal morphology has been assessed with endoscopic retrograde cholangiopancreatography (ERCP), which is based on intraductal contrast-enhancement with severity assessed using the Cambridge classification[2]. Today routine imaging modalities in the evaluation of CP typically include: Computed tomography (CT) with one or more contrast-enhancement phases, MRI with or without magnetic resonance cholangiopancreatography (MRCP) and ultrasound with a transabdominal or endoscopic approach. The advantage of MRI is the superior soft tissue visualization without radiation exposure. The main focus of imaging is typically to describe glandular atrophy and calcifications, duct pathology, pseudocysts and complications such as abscess formation and concomitant acute inflammation.
Recently, more advanced MRI methods have emerged which also provide important information on both tissue characteristics and pancreatic function.
The aim of this review is to provide an update on standard and advanced MRI techniques of the pancreas in CP.
STANDARD MRI
Parenchymal changes can be assessed in the early stage of CP by standard MRI typically including fat-suppressed T1-weighted images, T2-weighted images and gadolinium-enhanced imaging[3-6].
Fat-suppressed T1-weighted images show high signal intensity in normal pancreas and decreased signal intensity in CP due to loss of aqueous protein in the acini within the glandular elements of the pancreas caused by chronic inflammation and fibrosis[7-9]. Fat-suppression of T1-weighted images enhance the signal of the pancreas in relation to the surrounding retroperitoneal fat and the signal intensity can be compared to surrounding organs and tissue such as the spleen or muscles[9]. The biliary system and pancreatic ducts are enhanced on fat-suppressed T2-weighted images (Figure 1). The size of pancreas normally decreases with age but as acinar atrophy occurs more rapidly in CP, the pancreas diminishes segmentally or diffusely in the anteroposterior dimensions. The diameter can typically be assessed at the head, body and tail of the pancreas and compared with age-related normal values of the anteroposterior diameter[10].
Figure 1.
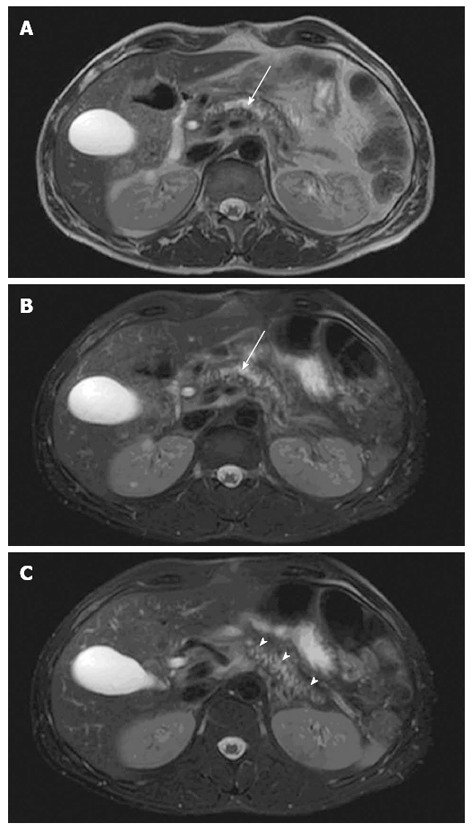
Pancreatic morphology. Axial T2-weighted magnetic resonance imaging views showing glandular atrophy (A), dilated irregular duct (B, arrow) and irregular side-branches (C, arrow heads) in a patient with chronic pancreatitis.
Gadolinium-enhanced imaging is used to investigate the perfusion of the pancreas during a series of contrast-enhanced images with repeated sequential scans. During the arterial phase of gadolinium infusion the normal pancreas shows pronounced enhancement due to high vascular perfusion and decreased signal during the venous phase[11]. The perfusion of the pancreas is decreased in patients with CP due to chronic inflammation and fibrosis resulting in reduced and delayed enhancement of the pancreatic signal[7,12].
Standard MRI is often used together with MRCP to assess both parenchymal and ductal changes of the pancreas. One important limitation of MRI lies in its inability to depict small parenchymal calcifications, which can easily be evaluated with CT.
MRI also has a role in diagnosing autoimmune pancreatitis (AIP) which can be a differential diagnosis to CP[13,14]. In diffuse AIP, MRCP may show a decreased diameter of the main pancreatic duct and can be accompanied by strictures and an irregular wall. However, in AIP the pancreas is typically with diffuse or localized enlargement with reduced signal on T1 and increased signal on T2-weighted images. A surrounding capsule with reduced signal in T2-weighted images can be seen[13,14].
MRCP
MRCP was first described in 1991, providing a non-invasive alternative to ERCP, which relies on the endoscopic injection of contrast fluid into the common bile duct[15]. By applying either a single-shot breath-hold technique or a free-breathing technique with respiratory triggering, MRCP can provide both 2D and 3D images[16,17]. Advances in scanner technology in the recent years allow faster image acquisition and better quality with more detailed images including 3D reconstructions. These advances benefit from a high signal-to-noise ratio. Additionally, the 3D free-breathing protocol makes it superior to 2D imaging in patients who are unable or unwilling to hold their breath for the duration of the scan[18].
MRCP relies on heavily T2-weighted pulse sequences, benefiting from the T2-weighted differences in relaxation time between fluid-filled compartments and adjacent soft tissue. Hence, the pancreato-biliary tree is displayed as high signal intensity and the pancreatic duct is clearly visualized in the normal pancreas. Accordingly, the MRCP technique is relevant in detecting pancreatic ductal dilation, small filling defects (stones and protein plugs), strictures, irregularities of the main pancreatic duct, and irregularity (sacculation and/or ectasia) of side branches[6] (Figure 2). Furthermore, pseudocysts and other ductal congenital abnormalities and normal variants (such as pancreas divisum) can be visualized[19,20].
Figure 2.
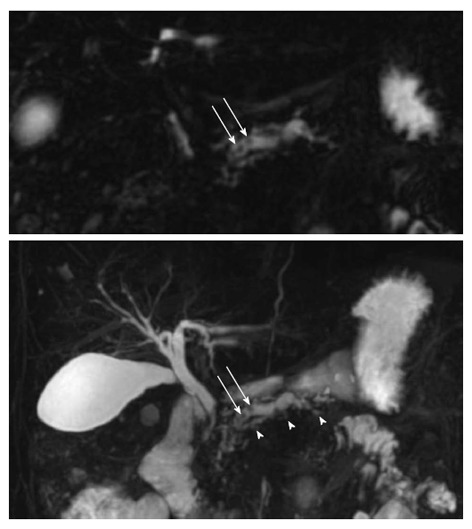
Magnetic resonance cholangiopancreatography. Upper figure displays a single coronal image and the lower figure a 3D view of the pancreato-biliary tree. In this chronic pancreatitis patient the dilated irregular main duct has irregular side branches (arrow heads) and contains multiple rounded filling defects (arrows).
The Cambridge classification system of ductal dimensional changes has been modified for the MRCP technique in the following fashion: Cambridge 1 (normal pancreas): pancreatic ducts are normal; Cambridge 2 (equivocal pancreas): 1-2 side branches and main duct 2-4 mm; Cambridge 3 (mild disease): ≥ 3 side branches and main duct 2-4 mm; Cambridge 4 (moderate disease): ≥ 3 side branches and main duct > 4 mm; Cambridge 5 (marked disease): as traditional Cambridge classification system[2,6].
The acquisition of MRCP sequences following intravenous administration of secretin hormone allows a better visualization of subtle ductal changes especially in the early stage of CP[21].
SECRETIN-STIMULATED MRCP
The secretin hormone stimulates pancreatic duct cells to produce a large volume of watery bicarbonate-rich pancreatic juice, which is secreted into the ducts and duodenum. Typically, images are obtained before and frequently after secretin stimulation for a period. In the normal pancreas the effect starts almost immediately and peaks between 2-5 min and by 10 min the caliber of the duct should return to baseline[21]. In secretin-stimulated MRCP (s-MRCP) the ductal system including side branch pathology and filling defects are better visualized compared to traditional MRCP, and s-MRCP provides images comparable to ERCP with Cambridge classification[21-23]. Furthermore, the exocrine function can be evaluated with assessment of duodenal filling, changes in pancreatic duct caliber, change in anteroposterior diameter of the pancreas, and change in signal intensity ratio between pancreas and spleen on T1-weighted and arterial-venous enhancement ratios[10,23,24]. The s-MRCP findings in CP (reduced duodenal filling grade and reduced increase in pancreatic duct caliber) are comparable to the results of endoscopic pancreatic function testing (ePFT)[23]. Studies in CP by Manfredi et al[25] and Schneider et al[26] showed correlation between assessment of the pancreatic exocrine reserve by dynamic s-MRCP and exocrine function assessed by fecal elastase test and 13C-mixed chain triglyceride breath test. Wathle et al[27] found correlation between endoscopic aspiration-based bicarbonate test and s-MRCP findings in healthy controls. However, the use of s-MRCP has not yet been integrated as a part of the Mayo diagnostic criteria for CP and further evaluation of s-MRCP as a test for exocrine function in comparison to traditional tests is needed.
Furthermore, s-MRCP can be combined with diffusion weighted imaging (DWI) of the pancreas.
DWI
DWI is an emerging technology to assess early parenchymal changes associated with CP and has shown results comparable to other existing methods such as MRCP and ePFT[9,28]. DWI assesses the random microscopic motion of water protons to obtain the apparent diffusion coefficient (ADC). The flow of water is less restricted in fluid-rich tissues, which will then be represented by a high ADC value. Water molecules interacting with cell membranes and macromolecules will be restricted thereby causing a reduction in the ADC value. A reduced amount of diffusible water is present in fibrotic tissue and in reduced pancreatic exocrine function[28]. Hence, presence of parenchymal fibrosis in CP causes diffusion restriction and results in lower ADC values[29,30] (Figure 3).
Figure 3.
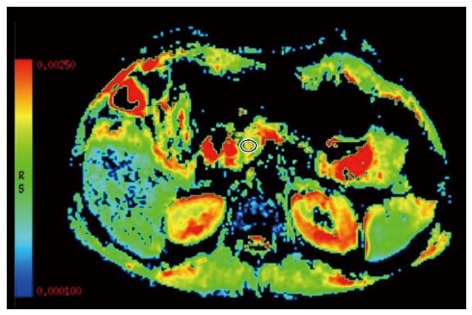
Diffusion weighted imaging. The apparent diffusion coefficient map of a chronic pancreatitis patient is shown with a measuring region of interest positioned in the pancreatic head to assess the degree of parenchymal fibrosis (red is high and blue low water diffusion).
Furthermore, when used in combination with secretin stimulation, the ADC value increases both in normal pancreas and in CP as the secretion stimulation facilitates an increased mobility of water molecules and increased circulation in the pancreatic capillaries. Following secretin stimulation, the diffusion coefficients have either delayed or lower peak values in CP patients, indicating reduced exocrine function[29,31]. Also, patients in risk of CP (such as alcohol consumption, nicotine consumption, nutritional factors, hereditary factors, efferent duct obstructions, immunological factors and rare miscellaneous factors) generally exhibit a delayed peak in diffusion coefficients compared to controls[31].
This technique can be particularly useful in patients with early stage CP where atrophy and ductal pathology are subtle. Furthermore, the technique is useful in differentiating between pancreatic cysts, inflammatory cysts and cystic neoplasms and between pancreatic adenocarcinoma and normal pancreas due to different content of cellular elements[32]. Inan et al[33] calculated ADC values and ADC cyst-to-pancreas ratios and found significant lower values for abscesses, hydatid cysts and neoplastic cysts compared to values of simple cysts and pseudocysts. This technique may be useful in the diagnosis of intraductal papillary mucinous neoplasms (IPMN), which is often difficult since CP and IPMN may have overlapping imaging findings[13,34]. Patients with main duct IPMN can present with ductal dilatation and associated parenchymal atrophy, and patients with side-branch IPMN can present with cystic lesions often confused with pseudocysts. ADC values of the cystic lesions may be helpful in deciding the malignant potential of IPMN[34,35].
NEW TECHNIQUES
Recently, other advanced MRI techniques have become more interesting as the development within the MR hardware and software is expanding and allows increased signal-to-noise ratios, shorter scan time and breath-hold imaging.
MR spectroscopy with non-invasive in-vivo assessment of metabolite concentrations has been applied in a variety of different tissues (e.g., brain, prostate, breast and liver). Hence, spectroscopy of the pancreas has the potential to offer a more accurate tissue characterization. Due to methodological challenges, the pancreas has only been studied to a very limited degree with spectroscopy. Despite of this, Su et al[36] characterized the normal pancreas at 3T and identified metabolites such as lipid, choline and cholesterol. Cho et al[37] used MR spectroscopy to distinguish between patients with chronic focal pancreatitis and patients with pancreatic carcinoma and found less lipid in pancreatitis than in pancreatic carcinoma. Furthermore, other studies also detected differences between normal pancreatic tissue and carcinoma tissue with alterations in lipid, choline and fatty acids[38,39]. However, to the best of our knowledge, this technique has not yet been applied in the characterization of CP patients.
CONCLUSION
This review provides an update on standard and advanced MRI of the pancreas in CP. Table 1 summarizes the capability of the different MRI techniques to display the different aspects of pancreatic changes and dysfunction in CP. Depending on local practice, scanner configuration and radiological experience, it should be possible to construct or customize individual MR protocols including the (or some of the) techniques reviewed in this paper to get the best possible morphological and functional information in CP.
Table 1.
Advantages of magnetic resonance imaging techniques
| MRI | MRCP | s-MRCP | DWI | |
| Loss of aqueous protein | Yes | No | No | No |
| Glandular atrophy | Yes | No | No | No |
| Perfusion | Yes | No | No | No |
| Calcification1 | No | No | No | No |
| Pancreatic ductal dilation | No | Yes | No | No |
| Filling defects | No | Yes | Yes | No |
| Strictures | No | Yes | Yes | No |
| Irregularities | No | Yes | Yes | No |
| Pseudocysts | Yes | Yes | Yes | No |
| Side branch pathology | No | Yes | Yes | No |
| Exocrine function | No | No | Yes | Yes |
| Parenchymal fibrosis | No | No | No | Yes |
Advantages of the different magnetic resonance imaging (MRI) techniques in diagnosing chronic pancreatitis.
A disadvantage of MRI is the inability to detect calcifications. This can be achieved through computed tomography scans. DWI: Diffusion weighted imaging; MRCP: Magnetic resonance cholangiopancreatography; s-MRCP: Secretin-stimulated MRCP.
Footnotes
Supported by The Danish Council for Strategic Research
P- Reviewers: Abraham P, Hard PD, Kamisawa T, Pezzilli R S- Editor: Gou SX L- Editor: A E- Editor: Zhang DN
References
- 1.Layer P, Yamamoto H, Kalthoff L, Clain JE, Bakken LJ, DiMagno EP. The different courses of early- and late-onset idiopathic and alcoholic chronic pancreatitis. Gastroenterology. 1994;107:1481–1487. doi: 10.1016/0016-5085(94)90553-3. [DOI] [PubMed] [Google Scholar]
- 2.Sarner M, Cotton PB. Classification of pancreatitis. Gut. 1984;25:756–759. doi: 10.1136/gut.25.7.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Semelka RC, Kroeker MA, Shoenut JP, Kroeker R, Yaffe CS, Micflikier AB. Pancreatic disease: prospective comparison of CT, ERCP, and 1.5-T MR imaging with dynamic gadolinium enhancement and fat suppression. Radiology. 1991;181:785–791. doi: 10.1148/radiology.181.3.1947098. [DOI] [PubMed] [Google Scholar]
- 4.Semelka RC, Shoenut JP, Kroeker MA, Micflikier AB. Chronic pancreatitis: MR imaging features before and after administration of gadopentetate dimeglumine. J Magn Reson Imaging. 1993;3:79–82. doi: 10.1002/jmri.1880030114. [DOI] [PubMed] [Google Scholar]
- 5.Sica GT, Miller FH, Rodriguez G, McTavish J, Banks PA. Magnetic resonance imaging in patients with pancreatitis: evaluation of signal intensity and enhancement changes. J Magn Reson Imaging. 2002;15:275–284. doi: 10.1002/jmri.10066. [DOI] [PubMed] [Google Scholar]
- 6.Choueiri NE, Balci NC, Alkaade S, Burton FR. Advanced imaging of chronic pancreatitis. Curr Gastroenterol Rep. 2010;12:114–120. doi: 10.1007/s11894-010-0093-4. [DOI] [PubMed] [Google Scholar]
- 7.Ly JN, Miller FH. MR imaging of the pancreas: a practical approach. Radiol Clin North Am. 2002;40:1289–1306. doi: 10.1016/s0033-8389(02)00056-8. [DOI] [PubMed] [Google Scholar]
- 8.Ito K, Koike S, Matsunaga N. MR imaging of pancreatic diseases. Eur J Radiol. 2001;38:78–93. doi: 10.1016/s0720-048x(01)00293-5. [DOI] [PubMed] [Google Scholar]
- 9.Balcı C. MRI assessment of chronic pancreatitis. Diagn Interv Radiol. 2011;17:249–254. doi: 10.4261/1305-3825.DIR.3889-10.0. [DOI] [PubMed] [Google Scholar]
- 10.Balci NC, Alkaade S, Magas L, Momtahen AJ, Burton FR. Suspected chronic pancreatitis with normal MRCP: findings on MRI in correlation with secretin MRCP. J Magn Reson Imaging. 2008;27:125–131. doi: 10.1002/jmri.21241. [DOI] [PubMed] [Google Scholar]
- 11.Balci NC, Alkaade S, Akduman IE, Bilgin M, Murdock CP, Burton FR. Serial contrast-enhanced MRI of the pancreas: correlation with secretin-stimulated endoscopic pancreatic function test. Acad Radiol. 2006;13:1367–1372. doi: 10.1016/j.acra.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Coenegrachts K, Van Steenbergen W, De Keyzer F, Vanbeckevoort D, Bielen D, Chen F, Dockx S, Maes F, Bosmans H. Dynamic contrast-enhanced MRI of the pancreas: initial results in healthy volunteers and patients with chronic pancreatitis. J Magn Reson Imaging. 2004;20:990–997. doi: 10.1002/jmri.20212. [DOI] [PubMed] [Google Scholar]
- 13.Perez-Johnston R, Sainani NI, Sahani DV. Imaging of chronic pancreatitis (including groove and autoimmune pancreatitis) Radiol Clin North Am. 2012;50:447–466. doi: 10.1016/j.rcl.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Detlefsen S, Löhr JM, Drewes AM, Frøkjær JB, Klöppel G. Current concepts in the diagnosis and treatment of type 1 and type 2 autoimmune pancreatitis. Recent Pat Inflamm Allergy Drug Discov. 2011;5:136–149. doi: 10.2174/187221311795399228. [DOI] [PubMed] [Google Scholar]
- 15.Wallner BK, Schumacher KA, Weidenmaier W, Friedrich JM. Dilated biliary tract: evaluation with MR cholangiography with a T2-weighted contrast-enhanced fast sequence. Radiology. 1991;181:805–808. doi: 10.1148/radiology.181.3.1947101. [DOI] [PubMed] [Google Scholar]
- 16.Takehara Y, Ichijo K, Tooyama N, Kodaira N, Yamamoto H, Tatami M, Saito M, Watahiki H, Takahashi M. Breath-hold MR cholangiopancreatography with a long-echo-train fast spin-echo sequence and a surface coil in chronic pancreatitis. Radiology. 1994;192:73–78. doi: 10.1148/radiology.192.1.8208969. [DOI] [PubMed] [Google Scholar]
- 17.Barish MA, Yucel EK, Soto JA, Chuttani R, Ferrucci JT. MR cholangiopancreatography: efficacy of three-dimensional turbo spin-echo technique. AJR Am J Roentgenol. 1995;165:295–300. doi: 10.2214/ajr.165.2.7618543. [DOI] [PubMed] [Google Scholar]
- 18.Kim JH, Hong SS, Eun HW, Han JK, Choi BI. Clinical usefulness of free-breathing navigator-triggered 3D MRCP in non-cooperative patients: comparison with conventional breath-hold 2D MRCP. Eur J Radiol. 2012;81:e513–e518. doi: 10.1016/j.ejrad.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Kamisawa T, Tu Y, Egawa N, Tsuruta K, Okamoto A, Kamata N. MRCP of congenital pancreaticobiliary malformation. Abdom Imaging. 2007;32:129–133. doi: 10.1007/s00261-006-9005-3. [DOI] [PubMed] [Google Scholar]
- 20.Yu J, Turner MA, Fulcher AS, Halvorsen RA. Congenital anomalies and normal variants of the pancreaticobiliary tract and the pancreas in adults: part 2, Pancreatic duct and pancreas. AJR Am J Roentgenol. 2006;187:1544–1553. doi: 10.2214/AJR.05.0774. [DOI] [PubMed] [Google Scholar]
- 21.Griffin N, Charles-Edwards G, Grant LA. Magnetic resonance cholangiopancreatography: the ABC of MRCP. Insights Imaging. 2012;3:11–21. doi: 10.1007/s13244-011-0129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee NJ, Kim KW, Kim TK, Kim MH, Kim SY, Park MS, Kim AY, Ha HK, Kim PN, Lee MG. Secretin-stimulated MRCP. Abdom Imaging. 2006;31:575–581. doi: 10.1007/s00261-005-0118-x. [DOI] [PubMed] [Google Scholar]
- 23.Balci NC, Smith A, Momtahen AJ, Alkaade S, Fattahi R, Tariq S, Burton F. MRI and S-MRCP findings in patients with suspected chronic pancreatitis: correlation with endoscopic pancreatic function testing (ePFT) J Magn Reson Imaging. 2010;31:601–606. doi: 10.1002/jmri.22085. [DOI] [PubMed] [Google Scholar]
- 24.Nøjgaard C, Olesen SS, Frøkjaer JB, Drewes AM. Update of exocrine functional diagnostics in chronic pancreatitis. Clin Physiol Funct Imaging. 2013;33:167–172. doi: 10.1111/cpf.12011. [DOI] [PubMed] [Google Scholar]
- 25.Manfredi R, Perandini S, Mantovani W, Frulloni L, Faccioli N, Pozzi Mucelli R. Quantitative MRCP assessment of pancreatic exocrine reserve and its correlation with faecal elastase-1 in patients with chronic pancreatitis. Radiol Med. 2012;117:282–292. doi: 10.1007/s11547-011-0774-6. [DOI] [PubMed] [Google Scholar]
- 26.Schneider AR, Hammerstingl R, Heller M, Povse N, Murzynski L, Vogl TJ, Caspary WF, Stein J. Does secretin-stimulated MRCP predict exocrine pancreatic insufficiency?: A comparison with noninvasive exocrine pancreatic function tests. J Clin Gastroenterol. 2006;40:851–855. doi: 10.1097/01.mcg.0000225652.00308.a2. [DOI] [PubMed] [Google Scholar]
- 27.Wathle GK, Tjora E, Ersland L, Dimcevski G, Salvesen OO, Molven A, Njølstad PR, Haldorsen IS. Assessment of exocrine pancreatic function by secretin-stimulated magnetic resonance cholangiopancreaticography and diffusion-weighted imaging in healthy controls. J Magn Reson Imaging. 2013:Epub ahead of print. doi: 10.1002/jmri.24167. [DOI] [PubMed] [Google Scholar]
- 28.Balci NC, Momtahen AJ, Akduman EI, Alkaade S, Bilgin M, Burton FR. Diffusion-weighted MRI of the pancreas: correlation with secretin endoscopic pancreatic function test (ePFT) Acad Radiol. 2008;15:1264–1268. doi: 10.1016/j.acra.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Akisik MF, Aisen AM, Sandrasegaran K, Jennings SG, Lin C, Sherman S, Lin JA, Rydberg M. Assessment of chronic pancreatitis: utility of diffusion-weighted MR imaging with secretin enhancement. Radiology. 2009;250:103–109. doi: 10.1148/radiol.2493080160. [DOI] [PubMed] [Google Scholar]
- 30.Frøkjær JB, Olesen SS, Drewes AM. Fibrosis, atrophy, and ductal pathology in chronic pancreatitis are associated with pancreatic function but independent of symptoms. Pancreas. 2013;42:1182–1187. doi: 10.1097/MPA.0b013e31829628f4. [DOI] [PubMed] [Google Scholar]
- 31.Erturk SM, Ichikawa T, Motosugi U, Sou H, Araki T. Diffusion-weighted MR imaging in the evaluation of pancreatic exocrine function before and after secretin stimulation. Am J Gastroenterol. 2006;101:133–136. doi: 10.1111/j.1572-0241.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- 32.Balci NC, Perman WH, Saglam S, Akisik F, Fattahi R, Bilgin M. Diffusion-weighted magnetic resonance imaging of the pancreas. Top Magn Reson Imaging. 2009;20:43–47. doi: 10.1097/RMR.0b013e3181b48667. [DOI] [PubMed] [Google Scholar]
- 33.Inan N, Arslan A, Akansel G, Anik Y, Demirci A. Diffusion-weighted imaging in the differential diagnosis of cystic lesions of the pancreas. AJR Am J Roentgenol. 2008;191:1115–1121. doi: 10.2214/AJR.07.3754. [DOI] [PubMed] [Google Scholar]
- 34.Sandrasegaran K, Akisik FM, Patel AA, Rydberg M, Cramer HM, Agaram NP, Schmidt CM. Diffusion-weighted imaging in characterization of cystic pancreatic lesions. Clin Radiol. 2011;66:808–814. doi: 10.1016/j.crad.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 35.Kang KM, Lee JM, Shin CI, Baek JH, Kim SH, Yoon JH, Han JK, Choi BI. Added value of diffusion-weighted imaging to MR cholangiopancreatography with unenhanced MR imaging for predicting malignancy or invasiveness of intraductal papillary mucinous neoplasm of the pancreas. J Magn Reson Imaging. 2013:Epub ahead of print. doi: 10.1002/jmri.24022. [DOI] [PubMed] [Google Scholar]
- 36.Su TH, Jin EH, Shen H, Zhang Y, He W. In vivo proton MRS of normal pancreas metabolites during breath-holding and free-breathing. Clin Radiol. 2012;67:633–637. doi: 10.1016/j.crad.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 37.Cho SG, Lee DH, Lee KY, Ji H, Lee KH, Ros PR, Suh CH. Differentiation of chronic focal pancreatitis from pancreatic carcinoma by in vivo proton magnetic resonance spectroscopy. J Comput Assist Tomogr. 2005;29:163–169. doi: 10.1097/01.rct.0000153956.33296.b5. [DOI] [PubMed] [Google Scholar]
- 38.Yao X, Zeng M, Wang H, Fei S, Rao S, Ji Y. Metabolite detection of pancreatic carcinoma by in vivo proton MR spectroscopy at 3T: initial results. Radiol Med. 2012;117:780–788. doi: 10.1007/s11547-011-0757-7. [DOI] [PubMed] [Google Scholar]
- 39.Ma X, Zhao X, Ouyang H, Sun F, Zhang H, Zhou C, Shen H. The metabolic features of normal pancreas and pancreatic adenocarcinoma: preliminary result of in vivo proton magnetic resonance spectroscopy at 3.0 T. J Comput Assist Tomogr. 2011;35:539–543. doi: 10.1097/RCT.0b013e318227a545. [DOI] [PubMed] [Google Scholar]


