Abstract
Lactobacillus plantarum displays a substrate-inducible padA gene encoding a phenolic acid decarboxylase enzyme (PadA) that is considered a specific chemical stress response to the inducing substrate. The putative regulator of padA was located in the padA locus based on its 52% identity with PadR, the padA gene transcriptional regulator of Pediococcus pentosaceus (L. Barthelmebs, B. Lecomte, C. Diviès, and J.-F. Cavin, J. Bacteriol. 182:6724-6731, 2000). Deletion of the L. plantarum padR gene clearly demonstrates that the protein it encodes is the transcriptional repressor of divergently oriented padA. The padR gene is cotranscribed with a downstream open reading frame (ORF1), the product of which may belong to a group of universal stress proteins (Usp). The padR deletion mutant overexpressed padA constitutively, and the padA promoter appears to be tightly regulated in this bacterium. Gel mobility shift assays using the padA gene promoter region and purified PadR expressed in Escherichia coli indicated that operator DNA binding by PadR was not eliminated by addition of p-coumarate. Gel mobility shift assays using partially purified extracts of native PadR protein from both phenolic acid-induced and noninduced L. plantarum cells demonstrate that inactivation of PadR by phenolic acids requires the integrity of L. plantarum and mediation by a specific protein absent in E. coli.
Phenolic acids, also called substituted hydroxycinnamic acids, are abundant in the plant kingdom because they are involved in the structure of plant cell walls and are present in some vacuoles (35). In plant-soil ecosystems they are released as free acids by hemicellulases produced by several fungi and bacteria (11). Of these weak acids, the most abundant are p-coumaric, ferulic, and caffeic acids, considered to be natural toxins that inhibit the growth of microorganisms, especially at low pHs (30, 42). In spite of this chemical stress, some bacteria can use phenolic acids as a sole source of carbon (16, 36, 37, 40). For other microorganisms, these compounds induce a specific response by which the organism adapts to its environment. For example, ferulic acid induces virulence gene expression in the plant-associated organism Agrobacterium tumefaciens (23, 31). Chambel et al. (9) showed that H+-ATPase pumps are induced in response to inhibitory concentrations of cinnamic acid in Saccharomyces cerevisiae.
In previous work (3, 7), it has been shown that the ubiquitous lactic acid bacterium Lactobacillus plantarum exhibits an inducible phenolic acid decarboxylase (PAD) activity which converts these substrates into less-toxic vinyl phenol derivatives (called volatile phenols). These volatile phenols are valuable intermediates in the biotechnological production of new flavor and fragrance chemicals, but they are also regarded as sources of phenolic off-flavors in beers, wines, and other fermented vegetable products due to their characteristic aroma and their low detection threshold (6). The strong, rapid, and inducible Pad enzyme synthesis following exposure to phenolic acids can be considered a specific chemical stress response to overcome this phenolic acid toxicity. This was proved by disruption of this padA gene, which renders the bacterium sensitive to these substrates and makes it unable to grow at a low pH in the presence of p-coumaric acid (4). To date four bacterial pad genes have been cloned and characterized. The substrate-inducible enzymes display decarboxylase activities of about 0.5 μmol · min−1 · mg−1 (7, 8, 41). Transcriptional analyses showed that pad mRNA could be detected only in phenolic acid-induced cell extracts. The expression of pad genes is transcriptionally activated by phenolic acids (7, 8).
Recently, Barthelmebs et al. found in the lactic acid bacterium Pediococcus pentosaceus (isolated from wine) a gene, organized in an autoregulated bicistronic operon with padA, which, when heterologously coexpressed in an Escherichia coli strain, was responsible for the repression of padA gene expression (4). This gene was named padR, because these results made it the putative transcriptional repressor of the padA gene in P. pentosaceus. However, no demonstration in the original host was done due to the impossibility of interrupting the chromosomal padR gene in this bacterium. No electroporation or other transformation procedure exists, to our knowledge, for this species (4). These PadR proteins form a new class of transcriptional regulator (Pfam PF03551). Only AphA (31% identity with P. pentosaceus PadR), which activates the transcription of tcpPH and initiates the expression of the virulence cascade in Vibrio cholerae (25, 27), has been well characterized, but it does not seem to play a role in the regulation of Pad (24).
In the present work, we report the cloning of the putative padR gene from L. plantarum, using the knowledge of the sequence of padR in P. pentosaceus. We demonstrate its role as a repressor of padA gene expression in the phenolic acid stress response, by disruption, mutant characterization, and mobility shift DNA binding assays with both native protein extract and recombinant purified His-tagged PadR.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The bacterial strains and plasmids used in this study are listed in Table 1. L. plantarum NC8, kindly given by L. Axelsson, was grown in liquid MRS (14) broth at 37°C without shaking, and E. coli was grown aerobically in Luria-Bertani medium at 37°C. For selection and growth of transformants, antibiotics were used at the following concentrations in the corresponding media: erythromycin, 200 μg/ml for E. coli and 5 μg/ml for L. plantarum; lincomycin, 12 μg/ml for L. plantarum; ampicillin, 200 μg/ml; kanamycin, 50 μg/ml for E. coli.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Strains | ||
| E. coli TG1 | SupE hsdΔ5 thi Δ(lac-proAB)F′ [tra D36 pro AB+ lacIqlacZΔM15] | 20 |
| BL21 (DE3) | F−ompT hsdSB (rB− mB−) gal dcm (DE3) | Invitrogen |
| L. plantarum | ||
| NC8 (LPNC8) | Wild-type, gram-positive ubiquitous homolactic acid bacterium, plasmid-free strain | Lars Axelsson |
| NC8ΔR | L. plantarum NC8 strain with padR gene disrupted by double homologous recombination | This work |
| Plasmids | ||
| pGID023 | Shuttle vector for E. coli and L. plantarum; derivatives of pJDC9 containing the pE194 replication functions; used as an unstable integration vector; Emr | 22 |
| pJDC9 | Emr; ΔlacZ | 10 |
| pJPDC1 | Emr; pJDC9 containing the 2.3-kbp Sau3A fragment of L. plantarum corresponding to the locus with padA-padR genes | 7 |
| pET28a+ | Kanr; vector for overexpression of His-tagged proteins using the T7 bacteriophage promoter | Novagen, Darmstadt, Germany |
| pJRA | Emr; pJDC9 containing the 303-bp RA fragment amplified by PCR with primers LPFP3 and LPΔPDC4 | This work |
| pJRAB | Emr; pJDC9 containing RA and the 237-bp RB fragment obtained by EcoRI restriction of pJPDC1 | This work |
| pGΔR | Emr; pGID023 containing the 600-bp ΔR fragment amplified by PCR with the M13 universal primer and primer LPΔ16 | This work |
| pER | pET28a+ containing padR gene with a His tag fusion inserted into NcoI and XhoI sites | This work |
DNA manipulation, PCR amplification, and transformation procedures.
DNA manipulation, purification, ligation, restriction analysis, and gel electrophoresis were carried out as described by Sambrook et al. (39). L. plantarum chromosomal DNA was prepared by the method described by Posno et al. (38). PCR was performed with the primers (Eurogentec, Seraing, Belgium) listed in Table 2 by using 0.1 U of Taq (Qbiogene, Montréal, Canada) or PWO (Eurogentec) DNA polymerase according to the manufacturers' recommendations in an automatic thermocycler (Eppendorf, Hamburg, Germany). PCR products and restriction products were purified by using a Qiaquick PCR purification kit or a Qiagel agarose gel extraction kit (Qiagen, Hilden, Germany). E. coli and L. plantarum strains were transformed by electroporation as described by Dower et al. (17) and Aukrust and Nes (1), respectively. E. coli BL21(DE3) was transformed by the CaCl2 procedure (12).
TABLE 2.
Primers
| Primer | 5′ → 3′ sequencea | Site created |
|---|---|---|
| LPD3 | CACTTGATGACTTTCTCGGCAC | |
| LPD6 | CACCGATCTCGTCATCAAACG | |
| LPD8 | TCTTCAACCCACTTTGGGAAG | |
| LPD9 | CACCGATCTCGTCATCAAAGG | |
| LPD16 | CTCGTATTCCCAGCCGTTATC | |
| LPINV2 | ATTCGCCGATAGACAAGTGG | |
| LPREP2 | CTCCGGTAAGGCTTTCAACG | |
| LPΔPDC1 | AGCCTGCAGACCGACACTGATCCACTCAT | PstI |
| LPΔPDC4 | GGAAAGCTTGCAGAGCAAGGTAAG | HindIII |
| LPFP3 | CCAGAATTCACCGATCTCGTCATCAAAGG | EcoRI |
| LPYFMUT1 | CGAAGCTTCGCTTTCCAGCACCGAAG | HindIII |
| LPPADR1 | CCCATGGCGCAAAAAAATAAGTTACAA | NcoI |
| LPPADR2 | CCCCTCGAGCTCCGGTAAGGCTTTCAAC | XbaI |
| LPGS1 | CCCTGCAGTGGAAATTCCTCCTTGACG | PstI |
| LPGS2 | CCGAATTCTAGAATTACCTTCCTTATG | EcoRI |
| LPLDH1 | CCGTGACCGACGGCAGCCGC | |
| LPLDH2 | CCGTCGCCCACTAACACAAC | |
| M13 universal | TGTAAAACGACGGCCAGT |
Underlined nucleotides correspond to restriction sites named in last column.
Whole-RNA extraction.
Cells from 20 ml of a mid-exponential-growth-phase (optical density at 600 nm [OD600], 0.8) culture of L. plantarum, either induced or not with 2.4 mM p-coumaric acid, were harvested and washed with ultrapure deionized water by centrifugation and then resuspended in 1 ml of Tri-reagent (Sigma) with 100 mg of 50- to 70-μm-diameter glass beads. Cells were disrupted by using a FastPrep system (Qbiogene). Then the procedure for total-RNA purification was carried out according to the guidelines of the Tri-Reagent manufacturer.
Northern blot, slot blot, and primer extension analysis.
Total RNA was separated by denaturing formaldehyde agarose gel electrophoresis and transferred to nylon membranes (NytranN; Schleicher and Schuell, Dassel, Germany) by using the Pharmacia vacuum system. The formaldehyde-denatured RNA was also spotted onto a nylon membrane by using a Hybrid-Slot manifold. Putative padR and padA gene-specific probes were generated by PCR amplification using primer pairs LPΔPDC1-LPD6 and LPD3-LPD8, respectively, designed from L. plantarum NC8 chromosomal DNA. The probes were labeled using a random-priming kit (Invitrogen, Paisley, United Kingdom) and [α-32P]dATP (3,000 Ci/mmol; Perkin-Elmer, Boston, Mass.). Transcript size was determined by comparison with an RNA ladder (0.24 to 9.5 kb; Invitrogen). Primer extension analysis was performed with two antisense primers, LPD9 and LPΔPDC1. Reverse transcription was performed with 10 U of Superscript II reverse transcriptase (Invitrogen) at 42°C as previously described (8). The mixture was loaded onto a 6% polyacrylamide manual sequencing gel in parallel with sequencing reactions with the padR DNA as the template and the same primers.
RT-PCR analysis.
Equal amounts of RNA were treated with 2 U of RNase-free DNase I (Invitrogen), and cDNA was synthesized by using 10 U of Superscript II reverse transcriptase and 2 ng of hexamers (Invitrogen)/μl. Five percent of the reverse transcription (RT) product was used as the substrate for PCR amplification with primers LPYFMUT1 and LPD6. PCR products were analyzed via standard agarose gel electrophoresis.
Deletion of the putative padR gene.
The pJRAB plasmid, which carries the putative padR gene with a deletion between the EcoRI site 106 bp downstream from the start codon and another EcoRI site 131 bp after the stop codon, was constructed by simultaneously cloning two separate fragments between the EcoRI and SmaI sites of pJPDC9. Primer LPFP3, which includes an EcoRI site, and primer LPD16 were used to amplify the 303 bp of the RA region encompassing the first 81 bp of the padA gene 5′ end, the putative promoter of the putative padR gene, and the first 106 bp of the putative open reading frame 1 (ORF1) downstream of padR. The 237-bp DNA fragment of the RB region was obtained by EcoRI restriction of pJPDC1. The ΔR fragment, which corresponds to the RB region and the 303-bp RA region, was generated by amplification with the M13 universal primer and primer LPD16 from the pJRAB plasmid and was cloned into the SmaI site of vector pGID023 to yield the pGΔR plasmid.
Preparation of cell extracts and assays for PAD activity.
L. plantarum cultures and cell disruption were performed as previously described (3). PAD activity in whole resting cells and in cell extracts was measured by the procedure of Barthelmebs et al. (3). This procedure consists of monitoring by UV spectrophotometry the disappearance of absorption peaks of the substrates (phenolic acids) and the simultaneous appearance of new peaks corresponding to vinyl derivatives. Protein concentrations in cell extracts were determined by using a protein assay kit (Bio-Rad, Richmond, Calif.) with bovine serum albumin as the standard.
Cloning of the padR gene in plasmid pET28a and purification of PadR protein.
The padR gene was cloned into plasmid pET28a by inserting a 549-bp NcoI/XhoI DNA fragment corresponding to the padR coding sequence between the NcoI and XhoI sites of pET28a. The DNA fragment corresponding to the padR sequence was generated by PCR using primers LPPADR1 and LPPADR2, thus replacing the TAA stop codon by the XhoI restriction site. This allowed the creation of a translational fusion adding six histidine residues to the carboxyl terminus of the protein and placed expression of the padR gene under the control of a T7 promoter. The resulting plasmid, pER, was used to transform E. coli BL21(DE3), in which the T7 RNA polymerase gene is under the control of the lacUV5 promoter, which is inducible with isopropyl-β-d-thiogalactoside (IPTG; 1 mM).
IPTG was added at the mid-exponential-growth phase to a 500-ml culture of E. coli BL21 pER (0.7 OD600 unit), and incubation was continued overnight at 37°C. After centrifugation, cells were suspended in 2% of the initial culture volume in a 50 mM sodium phosphate-300 mM NaCl-10 mM imidazole buffer at pH 8 and were then disrupted with a French press as previously described (2). The crude protein extracts were loaded onto a 0.5-ml nickel-nitrilotriacetate (Ni-NTA) agarose column (Qiagen). PadR protein was eluted with an imidazole gradient (20 to 100 mM) and resolved by denaturing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (with a 12.5% polyacrylamide resolving gel) (29).
Partial purification of the native PadR protein from L. plantarum cells.
Four liters of LPNC8 culture in MRS medium in the mid-logarithmic-growth phase (0.8 OD600 unit) was divided into two individual cultures. The first was incubated under the same conditions without any change, while the second was induced by addition of 2.4 mM p-coumaric acid. After incubation for 30 min, cells were harvested, washed with saline, and disrupted with a French press as previously described. The two crude protein extracts obtained by centrifugation were fractionated by adding (NH4)2SO4 (30, 40, and 50% [wt/vol] [near saturation] at 0°C) as previously described (6). The crude extracts and these fractions, dialyzed against 25 mM phosphate buffer at pH 6, were tested in binding assays with the padA gene promoter. Fractions presenting interactions with the padA DNA probe (see Results) were subjected to an additional purification step. Size exclusion chromatography with a Sephacryl S 200 HR (Amersham Biosciences Europe, Orsay, France) column (10 by 400 mm), eluted with 25 mM sodium phosphate buffer and 0.15 M NaCl at pH 6, was applied to these fractions in order to obtain, after elution of the dead volume, eight 2-ml fractions containing the purified proteins. This was done also to enrich the native PadR in one of these fractions. Fractions were dialyzed to eliminate NaCl and were concentrated by spreading flakes of polyethylene glycol 20000 (Sigma-Aldrich, Steinheim, Germany) on the dialysis tubes. Fractions were characterized by SDS-PAGE, and their protein concentrations were determined by using the Bio-Rad kit for protein analysis. Only four significantly different SDS-PAGE profiles were obtained (data not shown), and some fractions were pooled to obtain four purified protein samples at the same protein concentration (5 mg/ml). These fractions were kept at −20°C until use for binding assays.
Gel mobility shift assays.
The 115-bp DNA probe corresponding to the promoter region of the padA gene was generated by PCR with primers LPGS1 and LPGS2. This fragment was labeled by 10 cycles of PCR with [α-32P]dATP and was used as the probe for binding assays. Binding of PadR to DNA was carried out in a 20-μl reaction mixture containing 3 × 10−7 M α-32P-labeled padA promoter region DNA, 25 μg of salmon sperm DNA/μl, 10 mM Tris-HCl, 10 mM HEPES-NaOH, 50 mM KCl, 2 mM MgCl2, 2 mM dithiotreitol, 0.2 mM Na2 EDTA, and 5 g of glycerol/liter at pH 7.8. The DNA binding reaction was initiated by the addition of either purified His-tagged PadR produced in a recombinant E. coli strain or native PadR protein produced in L. plantarum. The mixture was incubated at room temperature for 30 min, and 10-μl samples were then loaded directly onto a 5% polyacrylamide gel that was run in a 20 mM Tris-HCl-400 mM glycine-1 mM EDTA (pH 7.8) buffer at 12 V · cm−1 for 1 h at room temperature. Then the gel was dried and analyzed by autoradiography. Competitor DNA consisted of the 273-bp DNA from the l-lactate dehydrogenase constitutive promoter region (19) (ldhL gene sequence, TrEMBL accession no. X70926), which was obtained by PCR amplification using the two primers LPLDH1 and LPLDH2 (Table 2). The PCR product was resolved by agarose gel electrophoresis, purified with the Qiagel extraction kit (Qiagen), and added to the mixture at 3 × 10−7 M in some binding assays.
Glutaraldehyde cross-linking of PadR.
The procedure of Derré et al. (15) was modified as follows. Twenty microliters of purified PadR (0.5 μg/μl) was incubated with 20 μl of the glutaraldehyde cross-linking reagent containing 20 mM glutaraldehyde, 100 mM NaH2PO4 (pH 7.5), 100 mM NaCl, and 200 g of glycerol/liter. After incubation for 1 h at 37°C, the reaction was stopped by the addition of SDS loading buffer. The samples were boiled and analyzed by SDS-PAGE on a 12% polyacrylamide gel. The gel was stained with Coomassie blue.
RESULTS
Nucleotide and protein sequences.
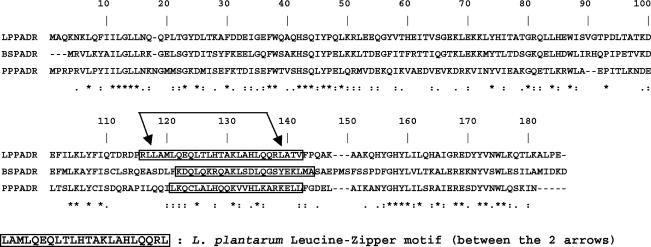
Based on the recent discovery of the padR genes in P. pentosaceus (4) (TrEMBL accession no. AJ276891) and Bacillus subtilis (unpublished data; TrEMBL accession no. P94443), a putative padR gene was identified in the L. plantarum clone pJPDC1 (6). This putative padR encodes a protein with 52 and 50% amino acid identities to PadR of P. pentosaceus and B. subtilis, respectively (Fig. 1) and with 31% identity to AphA of V. cholerae (TrEMBL accession no. Q9X399). The putative padR gene of 549 bp, located 115 bp upstream of the ATG start codon of the padA gene (7), is divergently oriented (Fig. 2A and C) and encodes a 183-amino-acid protein of 21 kDa. This padR gene is preceded by a putative ribosome binding site (5′-AAGGAGG-3′) complementary to the 3′ end of the 16S rRNA from L. plantarum (3′-UUCCUCC-5′) (TrEMBL accession no. M58827). No dyad symmetry that could act as a transcriptional terminator was found downstream of the TAA stop codon, but the start of an ORF (ORF1) was found downstream of this putative padR gene; these two genes may form an operon.
FIG. 1.
Alignment of amino acid sequences of PadR. LPPADR, PadR of L. plantarum (accession no. AJ289188); BSPADR, PadR of B. subtilis (accession no. P94443) (unpublished data); PPPADR, PadR of P. pentosaceus (accession no. AJ276891). Asterisks designate identical residues, colons designate conserved substitutions, and periods designate semiconserved substitutions. Predicted coiled-coil domains, obtained by using the COILS computer program with a 21-amino-acid window (32-34), are boxed. Gaps in the alignment (dashes) are indicated.
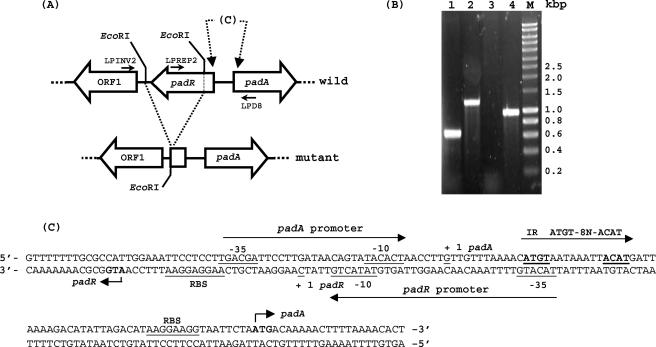
FIG. 2.
(A) Physical map of the padA locus in a wild-type strain of L. plantarum and characterization of physical deletion in an LPNC8ΔR strain. Large open arrows represent the different genes and ORF1 and their orientation. Small bars indicate the restriction sites. Small horizontal arrows indicate primers for PCR. (B) PCR of the padR region with two primer pairs on chromosomal DNAs of a mutant strain and a wild-type strain, respectively. Lanes 1 and 2, LPMINV2-LPD8 amplification. Lanes 3 and 4, LPREP2-LPD8 amplification. Smart ladder (Eurogentec). (C) Nucleotide sequence of the overlapping diverging promoter region of the padA gene and the padR-ORF1 operonic structure (accession no. AJ289188). Putative promoters are indicated with their putative −10 and −35 boxes. ATG start codons of padA and padR genes are boldfaced. The two putative ribosome binding site (RBS) regions are underlined. The transcription starting points (+1) of padA (6) and padR (see Fig. 4A) are underlined. The inverted-repeat (IR) sequence of the PadR putative DNA binding site is boldfaced and underlined.
Deletion of the putative padR gene in L. plantarum NC8.
The first aim of this work was to study the role of the putative padR gene and its product in the regulation of L. plantarum pad gene expression. A deleted copy of the putative padR gene was cloned into the pGID023 shuttle vector and transformed into L. plantarum NC8 (for details, see Materials and Methods). After recombination events, PCR analysis on total DNA of antibiotic-sensitive candidates (Ems) was carried out to screen clones bearing the deleted padR gene copy. Three primers located on each side and in the deleted region were used; this should result in amplification of 1.2- or 0.63-kbp fragments with primers LPINV2 and LPD8 and in amplification of a 957-bp fragment or no signal with primers LPREP2 and LPD8 (Fig. 2B). Among several Ems colonies tested, one clone yielded the shorter PCR fragment of 0.63 kbp, and the lack of signal corresponding to the deleted fraction was confirmed by sequencing the 0.63-kbp PCR fragment (data not shown). This mutant strain presenting a 569-bp deletion in the 3′ region of padR was named LPNC8ΔR and used in this work.
The LPNC8ΔR mutant constitutively overexpresses PAD activity.
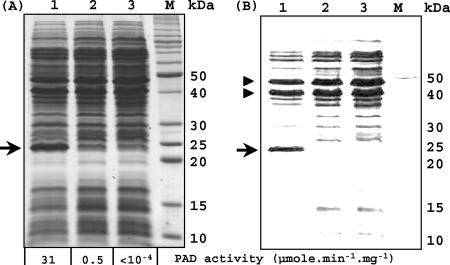
In order to test the PAD activity of the mutant strain, noninduced and induced (by addition of 2.4 mM p-coumaric acid) cultures of LPNC8 and noninduced LPNC8ΔR mutant culture were performed. Corresponding crude cell extracts were analyzed for PAD activity and were subjected to SDS-PAGE (Fig. 3A). No PAD activity was detected in the extract from noninduced LPNC8 cells (detection threshold, 10−4 μmol · min−1 · mg−1), while an activity of about 0.5 μmol · min−1 · mg−1 was observed in the p-coumaric acid-induced LPNC8 cell extract. The noninduced extract from the LPNC8ΔR mutant displayed a very high PAD specific activity of 31 μmol · min−1 · mg−1 on p-coumaric acid, indicating constitutive overexpression of the padA gene. SDS-PAGE of these crude extracts showed the presence of a strong protein band of about 25 kDa, corresponding to the PadA enzyme, that is not detectable as a protein band in the crude extract from the noninduced culture of the wild-type strain LPNC8 and is masked by other proteins in the crude extract of the induced wild-type strain. Increasing the brightness and contrast of the corresponding digital photograph (Fig. 3B) allows proportional subtraction of less-intense protein bands and reveals that the PadA enzyme is one of three main proteins in the LPNC8ΔR mutant. These results indicate that the disruption of padR results in a strong and constitutive overexpression of the PadA protein without p-coumaric acid induction. Slot blot analysis with whole RNA from noninduced LPNC8ΔR cells allowed the detection of the padA transcript, which was not detectable in noninduced cells of the wild-type strain LPNC8 (data not shown). These results demonstrate that the putative padR gene encodes the transcriptional repressor of padA gene expression and that the padA gene is under the control of a very tightly inducible promoter.
FIG. 3.
(A) SDS-PAGE of crude protein extracts from noninduced (lane 3) and 2.4 mM p-coumaric acid-induced (lane 2) LPNC8 cells and from the noninduced LPNC8ΔR mutant (lane 1). M, SDS-PAGE molecular mass standards (Invitrogen). Arrow indicates the protein band corresponding to the constitutively overexpressed PadA enzyme in the LPNC8ΔR mutant. (B) Identical to panel A, with a simple increase in brightness and contrast to enable evaluation of the relative concentration of PadA in the LPNC8ΔR mutant compared to the concentrations of the other two main protein bands (indicated by arrowheads) that are constitutively expressed in the wild-type and mutant strains.
Transcriptional analysis of the padR gene.
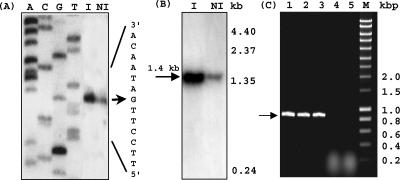
Primer extension experiments performed with the two primers LPD9 (Fig. 4A) and LPΔPDC1 (same results) (data not shown) and using the RNA samples from induced or noninduced cultures as templates allowed the identification of a single guanine as a putative transcription starting point, located 27 nucleotides upstream from the putative initiation codon ATG, and of putative −10 and −35 boxes (Fig. 2C). Evaluation of relative concentrations of primer extension products by using a Bio-Rad digital gel documentation system indicates that the level of mRNA from the padR gene in the induced cell extract was about 20-fold higher than that in noninduced cells. Northern blot hybridization using the same templates was performed to determine the size of the corresponding mRNA with the padR probe (Fig. 4B). Signals were detected in RNA extracts from both p-coumarate-induced and noninduced L. plantarum cultures. As observed with the 5′ mapping results, the Northern blot band corresponding to the padR transcript for the induced condition was about 20-fold more intense than that for the noninduced condition. A single transcript of approximately 1.4 kb was detected in both RNA extracts. This size is about twice the expected size of the padR gene transcript, indicating that the padR gene is probably cotranscribed with another putative gene. A putative ORF1 was observed 181 bp downstream from the TAA stop codon of padR. Since the analysis was done on a pJPDC1 library sequence (6), only 180 bp of this putative ORF1 was observed. Screening of the L. plantarum genomic DNA library (7) with a probe consisting of the partial DNA sequence of this putative ORF1 failed to produce a signal. PCR gene walking experiments were recently successful at cloning the 0.7-kbp 3′ downstream region of ORF1 (data not shown) (accession no. AJ289188). To confirm this hypothetical cotranscription of padR and ORF1, RT-PCR amplification experiments were carried out with mRNAs prepared from noninduced L. plantarum cells and from L. plantarum cells induced by addition of 2.4 mM p-coumaric acid; cDNA synthesis was performed with random hexamers as primers and by PCR using primers LPYFMUT1 and LPD6, located in ORF1 and the padR gene, respectively. A PCR product of the expected size (812 bp) was obtained by use of either of the mRNA extracts as a template (Fig. 4C), supporting the hypothesis of an operon arrangement of padR and ORF1. Forty-four nucleotides downstream from ORF1, a putative rho-independent transcriptional terminator with a ΔG of −26.9 kcal/mol was found. ORF1 displays a significant identity with Listeria monocytogenes Lmo1580 protein (accession no. AL591979), a putative Usp (universal stress protein) of unknown function.
FIG. 4.
Transcriptional analysis of padR gene. (A) Mapping of the 5′ end of padR mRNA by extension analysis using primer LPD9 with total L. plantarum RNA from noninduced (NI) and 2.4 mM p-coumaric acid-induced (I) cells. The products of the reverse transcriptase reactions were analyzed by 6% sequencing gel reactions (ACGT) with the same primer. Arrow indicates the 5′ end of padR gene mRNA (C for the coding sequence). (B) Northern blot analysis of total RNAs purified from NI and I cultures of L. plantarum. A padR-specific probe was used. (C) RT-PCR of the padR gene and the 5′-most 180 bp of putative ORF1 by using total RNAs purified from NI and I cells of L. plantarum as the matrix. This region of interest was amplified by PCR using LPYFMUT1 and LPD6. Lane 1, positive control from chromosomal DNA; lanes 2 and 3, RT-PCR with total RNAs from NI and I cells, respectively; lanes 4 and 5, negative controls with no RT step; M, DNA Smart ladder (Eurogentec).
Purified PadR binds specifically to the padA promoter region.
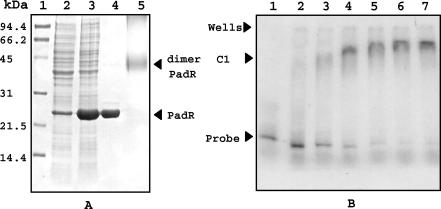
To determine how PadR controls padA expression, mobility shift DNA binding assays were performed with purified PadR and the padA promoter region. The PadR protein was overproduced by using the pET28a vector and was purified by using an Ni-NTA agarose column. Analysis by SDS-PAGE of crude extracts from E. coli cells containing the pER plasmid showed an overproduced band of the expected molecular mass for PadR (approximately 22 kDa) under IPTG induction; this band was also produced at a low level by basal expression in E. coli BL21 without induction (Fig. 5A). Its purity was checked by SDS-PAGE analysis and was shown to be >95%. The recombinant PadR was used in binding assays with radiolabeled PCR-generated probe DNA corresponding to the padA promoter region (Fig. 5B). Detection of a C1 complex confirms that PadR binds specifically to the padA promoter region. This binding is strong and specific, as evidenced by the fact that addition of increasing concentrations of unlabeled DNA fragments corresponding to the ldhL promoter regions of L. plantarum, a strong and constitutive promoter for L. plantarum (19), did not prevent the formation of the C1 complex (data not shown). Half binding was obtained at about 2 × 10−6 M PadR (Fig. 5B, lane 3), which corresponds to a protein/DNA probe molar ratio of about 6. In contrast, for the interaction of E. coli LacI with the lacZ promoter, half binding under optimized conditions was obtained at a protein/DNA molar ratio of 50 (18). Our present result indicates the particularly high affinity of PadR for the padA promoter region and explains the lack of padA basal expression and detectable PAD activity in noninduced cells.
FIG. 5.
Overexpression, purification, and mobility shift assays with purified PadR. (A) SDS-PAGE analysis of crude extracts and purified His-tagged PadR from E. coli BL21(DE3) carrying the pER plasmid. Lane 1, molecular mass standards; lane 2, crude protein extract from noninduced E. coli; lane 3, crude protein extract from 1 mM IPTG-induced E. coli; lane 4, PadR purified by Ni-NTA affinity chromatography; lane 5, purified PadR treated with 10 mM glutaraldehyde. Dimer PadR, protein band corresponding to the putative dimerized purified PadR with a molecular mass of about 45 kDa. (B) Mobility shift assays of the DNA probe corresponding to the padA promoter region, generated and labeled by PCR amplification with [α-32P]dATP, with or without purified PadR. Lane 1, probe (3 × 10−7 M pad promoter DNA) without protein; lanes 2 to 7, probes with increasing concentrations of purified PadR (lane 2, 10−6 M; lane 3, 2 × 10−6 M; lane 4, 3 × 10−6 M; lane 5, 4 × 10−6 M; lane 6, 5 × 10−6 M; lane 7, 6 × 10−6 M).
To see if PadR was able to multimerize in a solution, purified PadR was incubated with the cross-linking reagent glutaraldehyde and subjected to SDS-PAGE analysis (Fig. 5A). PadR was present as a monomer in the absence of glutaraldehyde, with a molecular mass of about 22 kDa, and appeared as a protein band of approximately 45 kDa after treatment with glutaraldehyde. These results indicate that the PadR repressor dimerizes in vitro and that it could be the active form in vivo.
These results indicate that the L. plantarum PadR protein that is produced by recombinant E. coli is in the active form. In order to see if inactivation of the PadR repressor could be possible in vitro or in recombinant E. coli strains expressing His-tagged PadR, p-coumaric acid was added either to the purified PadR protein or, before extraction, to the recombinant E. coli strain in the mid-logarithmic-growth phase. No change in binding was observed either with the recombinant PadR protein preincubated with 2.4 mM p-coumaric acid or with purified L. plantarum PadR protein from recombinant E. coli cells induced with 2.4 mM p-coumaric acid (data not shown). It has already been demonstrated that p-coumaric acid enters E. coli cells very easily and is not metabolized by this bacterium (2, 4). Burlingame and Chapman (5) have previously shown that E. coli is not able to grow on cinnamic acid or 2- or 4-hydroxycinnamic acid (p-coumarate). The lack of inactivation of PadR in E. coli could not result from a p-coumaric acid uptake problem in this species. Moreover, previous studies have shown that padA mRNA levels decrease rapidly after complete degradation of p-coumarate (7), indicating that products of p-coumarate metabolism which accumulated in the culture medium were not able to induce the Pad system in L. plantarum. These results indicate that p-coumaric acid cannot directly inactivate the PadR transcriptional repressor in vitro or in recombinant living E. coli cells, in contrast to what was observed in the living L. plantarum host. These results suggest the existence of a mechanism or a gene present in this host and absent in E. coli that mediates the transduction of the p-coumaric acid signal responsible for the inactivation of PadR.
The native PadR repressor is indirectly inactivated in L. plantarum cells by addition of p-coumaric acid.
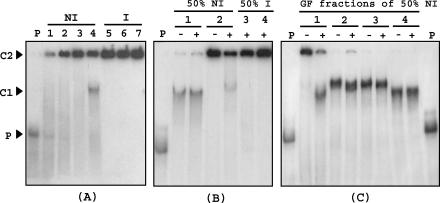
To confirm the hypothesis for a biological mechanism through which p-coumaric acid inactivates the PadR repressor in growing cells of L. plantarum, binding assays with the same DNA probe were performed by using partially purified [with (NH4)2SO4] protein extracts from noninduced and induced LPNC8 cells (Fig. 6A). Specific binding (C1 complex) with the padA promoter DNA probe was observed only for the 50% (NH4)2SO4 protein extract fraction from the noninduced culture. Binding assays with the three corresponding protein fractions from p-coumaric acid-induced cells revealed the absence of a C1 complex and the presence of a second complex, designated C2, which did not migrate in PAGE. This complex corresponds to the DNA probe and a high-molecular-mass protein present in crude cell extracts. One candidate for this protein should be the RNA polymerase, a protein of about 400 kDa (13), which is able to form a complex with the DNA probe, as previously described, in gel mobility shift assays with crude cell extracts of Staphylococcus aureus (21). Addition of nonlabeled ldhL promoter competitor DNA, which displays a strong affinity for the RNA polymerase, reinforced this hypothesis. As demonstrated in Fig. 6B, lane 2+, this competitor was able to mobilize the protein responsible for C2 formation and liberate the probe for PadR binding.
FIG. 6.
Mobility shift assay of a DNA probe corresponding to the padA promoter region (2 × 10−8 M), generated and labeled by PCR with [α-32P]dATP, with crude protein extracts, partially purified with (NH4)2SO4 and by gel filtration, from noninduced (NI) or p-coumaric acid-induced (I) L. plantarum cells. (A) Binding assay with (NH4)2SO4 fractions from NI or I cells. P, DNA probe without protein extract; lane 1, crude extract from NI cells; lanes 2 and 5, 30% (NH4)2SO4 fraction; lanes 3 and 6, 40% (NH4)2SO4 fraction; lanes 4 and 7, 50% (NH4)2SO4 fraction. (B) Binding assays with 50% (NH4)2SO4 fractions from NI and I cells preincubated with (+) or without (−) 3 × 10−7 M unlabeled ldhL promoter DNA as a competitor (see Materials and Methods). P, DNA probe without protein extract; lanes 1 and 3, 0.025 μg of protein/μl; lanes 2 and 4, 0.075 μg of protein/μl. (C) Binding assays with the four pools of protein obtained by gel filtration (GF) of the 50% (NH4)2SO4 protein fraction from the NI protein extract with (+) or without (−) ldhL promoter DNA as a competitor. P, probe without protein extract; lanes 1, 2, 3, and 4, respectively, the first, second, third, and fourth pools of proteins collected by gel filtration elution. The protein concentration of the pools was the same as that in the binding assays (0.025 μg/μl). C1, band corresponding to a specific binding of the probe with a protein in extracts or protein fractions exclusively from NI cells; C2, band corresponding to the binding of the probe with a high-molecular-mass protein.
In order to reduce the C2 complex, the 50% (NH4)2SO4 extract from noninduced cells which displayed specific binding (C1) was subjected to gel filtration with the aim of separating the high-molecular-mass protein from the PadR repressor, whose native molecular mass was previously estimated at 45 kDa. The four protein pools obtained were tested in binding assays with or without ldhL promoter DNA (Fig. 6C). Binding assays with the first protein pool, corresponding to the exclusion volume, in which the RNA polymerase complex was eluted according to its ∼400-kDa molecular mass with other high-molecular-mass proteins, revealed a C2 binding complex, which was less intense in the presence of competitor DNA than the C1 binding complex for reasons previously given. The other fractions (fractions 2, 3, and 4), corresponding to the elution of progressively lower molecular mass proteins, displayed the specific and intense C1 binding complex, without the C2 binding complex.
DISCUSSION
Recently, a gene in P. pentosaceus that encodes the potential repressor of the pad system was described by heterologous expression in E. coli due to the impossibility of interrupting the chromosomal padR gene in this bacterium. In the present study, we have cloned, characterized, and deleted the putative padR gene in L. plantarum and demonstrated the function of the padA gene repressor, PadR. While in P. pentosaceus the padA and padR genes reside in an operon (4), in L. plantarum NC8, as well as in strain LPCHL2 (6), the padR gene is divergently oriented in relation to the padA gene, with only 115 bp between the two genes. This divergent orientation is also observed for the putative padR gene of Bacillus pumilus (4). The padC and padR genes of B. subtilis (8) are not located in each other’s vicinity, as for the pad (TrEMBL accession no. Q9KPX2) and aphA genes from V. cholerae. AphA has been reported to repress the pva gene, encoding a penicillin V amidase, and does not seem to play a role in regulation of PadA (24). For P. pentosaceus, a strong transcriptional terminator with a ΔG of about −20 kcal/mol was identified downstream of the stop codon, whereas the L. plantarum padR gene is cotranscribed with another putative gene, ORF1, a 462-bp region encoding a protein of 153 amino acid residues whose function is unknown.
Deletion of the padR gene leads to the constitutive overexpression of the PadA enzyme, and the specific activity of the LPNC8ΔR crude extract is about 60-fold higher than the highest specific activity previously measured in the p-coumaric acid-induced wild-type strain of L. plantarum. The PadA protein is one of the three main proteins of the LPNC8ΔR mutant (Fig. 3B), indicating that the padA gene promoter is one of the three most tightly regulated gene promoters so far observed in L. plantarum. This food-grade mutant obtained by double crossing over could be useful for biotechnology applications to constitutively overproduce any protein of interest. The wild-type L. plantarum strain is suitable for inducible overexpression by food-grade natural inducers (phenolic acids) of any gene inserted downstream of the padA promoter. The interest of this expression system is reinforced by the absence of detectable basal expression in noninduced bacteria.
The padR gene of L. plantarum is regulated at a transcriptional level with about a 20-fold increase in cells induced by p-coumaric acid. The PadR repressor could interact with its own promoter due to the padR −35 sequence included in the putative PadR binding site (Fig. 2C). When PadR binds to the padA gene promoter, it might mask, in part, the promoter of its coding gene by simple steric inhibition due to the overlapping promoters, thus reducing its own transcription. When p-coumaric acid is added to the medium, the inactivation of PadR releases it from the padA promoter region, as shown in binding assays, and allows increased transcription of the padR gene (Fig. 4B). Results obtained in binding assays indicate that recombinant PadR binds specifically and strongly to the padA gene promoter and that contact with the PadR protein cannot be inactivated by preincubation with phenolic acids before binding assays (Fig. 5B). Binding assays performed with native protein extracts from noninduced and p-coumaric acid-induced L. plantarum cells indicate that the p-coumaric acid response may need a specific mediator which is responsible for PadR inactivation and which is absent in E. coli. The regulation may be as complex as that of tcpPH expression, which needs both AphA and AphB (25) and which is transcriptionally regulated by quorum sensing through the repressive action of HapR (26). Nevertheless, no significant difference was observed in induction with p-coumarate at low or high cell densities in L. plantarum. Even if the AphA and PadR amino acid sequences are quite homologous, PadR does not follow the same regulation as AphA. The mechanism of PadR derepression by p-coumarate is currently under study.
The analysis of the PadR amino acid sequence reveals a coiled-coil structure with a putative leucine zipper-like dimerization motif between leucine 117 and leucine 138 (Fig. 1) (32-34). This hypothesis is reinforced by the results of in vitro dimerization (Fig. 5A), generating a coiled-coil structure for binding to the padA DNA promoter in homodimeric form. No “helix-turn-helix” DNA binding type motif was found in PadR. A structure-function analysis of PadR should allow determination of the regions involved in inactivation and DNA binding. The role of the putative Usp ORF1 gene, which forms a putative operon with padR and whose expression is increased in the presence of phenolic acids, is currently under study, because it may be a component of the phenolic acid response. The recent bioinformatics data from whole-genome sequencing projects make the Usp's a growing family of proteins. In most cases these Usp's are produced in response to a large number of environmental stresses, but their exact role remains enigmatic today (28).
Acknowledgments
We are very grateful to Torey Arvik for critical review of the manuscript and to Christine Rojas for laboratory work.
J. Gury, a Ph.D student, is supported by a grant from INRA and the Conseil Régional de Bourgogne.
REFERENCES
- 1.Aukrust, T., and I. F. Nes. 1988. Transformation of Lactobacillus plantarum with the plasmid pTV1 by electroporation. FEMS Microbiol. Lett. 52:127-132. [Google Scholar]
- 2.Barthelmebs, L., C. Diviès, and J.-F. Cavin. 2001. Expression in Escherichia coli of native and chimeric phenolic acid decarboxylases with modified enzymatic activities and method for screening recombinant E. coli strains expressing these enzymes. Appl. Environ. Microbiol. 67:1063-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barthelmebs, L., C. Diviès, and J.-F. Cavin. 2000. Knockout of the p-coumarate decarboxylase gene from Lactobacillus plantarum reveals the existence of two other inducible enzymatic activities involved in phenolic acid metabolism. Appl. Environ. Microbiol. 66:3368-3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barthelmebs, L., B. Lecomte, C. Diviès, and J.-F. Cavin. 2000. Inducible metabolism of phenolic acids in Pediococcus pentosaceus is encoded by an autoregulated operon which involves a new class of negative transcriptional regulator. J. Bacteriol. 182:6724-6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burlingame, R. P., and P. J. Chapman. 1983. Catabolism of phenylpropionic acid and its 3-hydroxy derivative in Escherichia coli. J. Bacteriol. 155:113-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavin, J. F., V. Andioc, P. X. Etiévant, and C. Diviès. 1993. Ability of wine lactic acid bacteria to metabolize phenol carboxylic acids. Am. J. Enol. Vitic. 44:76-80. [Google Scholar]
- 7.Cavin, J.-F., L. Barthelmebs, and C. Diviès. 1997. Molecular characterization of an inducible p-coumaric acid decarboxylase from Lactobacillus plantarum: gene cloning, transcriptional analysis, overexpression in Escherichia coli, purification, and characterization. Appl. Environ. Microbiol. 63:1939-1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavin, J.-F., V. Dartois, and C. Diviès. 1998. Gene cloning, transcriptional analysis, purification, and characterization of phenolic acid decarboxylase from Bacillus subtilis. Appl. Environ. Microbiol. 64:1466-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chambel, A., C. A. Viegas, and I. Sa-Corriea. 1999. Effect of cinnamic acid on growth and on plasma membrane H+-ATPase activity of Saccharomyces cerevisiae. Int. J. Food Microbiol. 50:173-176. [Google Scholar]
- 10.Chen, J. D., and D. A. Morrison. 1988. Construction and properties of a new insertion vector, pJDC9, that is protected by transcriptional terminators and useful for cloning of DNA from Streptococcus pneumoniae. Gene 64:155-164. [DOI] [PubMed] [Google Scholar]
- 11.Christov, L. P., and B. A. Prior. 1993. Esterases of xylan-degrading microorganisms: production, properties, and significance. Enzyme Microb. Technol. 15:460-475. [DOI] [PubMed] [Google Scholar]
- 12.Cohen, S. N., A. C. Chang, and L. Hsu. 1972. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc. Natl. Acad. Sci. USA 69:2110-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darst, S. A. 2001. Bacterial RNA polymerase. Curr. Opin. Struct. Biol. 11:155-162. [DOI] [PubMed] [Google Scholar]
- 14.De Man, P. J., M. Rogosa, and M. Sharpe. 1960. A medium for the cultivation of Lactobacilli. J. Appl. Bacteriol. 23:130-135. [Google Scholar]
- 15.Derré, I., G. Rapoport, and T. Msadek. 1999. CtsR, a novel regulator of stress and heat shock response, controls clp and molecular chaperone gene expression in gram-positive bacteria. Mol. Microbiol. 31:117-131. [DOI] [PubMed] [Google Scholar]
- 16.Diaz, E., A. Ferrandez, M. A. Prieto, and J. L. Garcia. 2001. Biodegradation of aromatic compounds by Escherichia coli. Microbiol. Mol. Biol. Rev. 65:523-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dower, W. J., J. F. Miller, and C. W. Ragsdale. 1988. High efficiency transformation of Escherichia coli by high voltage electroporation. Nucleic Acids Res. 16:6127-6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falcon, C. M., and K. S. Matthews. 2000. Operator DNA sequence variation enhances high affinity binding by hinge helix mutants of lactose repressor protein. Biochemistry 39:11074-11083. [DOI] [PubMed] [Google Scholar]
- 19.Ferain, T., D. Garmyn, N. Bernard, P. Hols, and J. Delcour. 1994. Lactobacillus plantarum ldhL gene: overexpression and deletion. J. Bacteriol. 176:596-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gibson, T. J. 1984. Studies on the Epstein-Barr virus genome. Ph.D. thesis. Cambridge University, Cambridge, United Kingdom.
- 21.Gregory, P. D., R. A. Lewis, S. P. Curnock, and K. G. H. Dyke. 1997. Studies of the repressor (BlaI) of β-lactamase synthesis in Staphylococcus aureus. Mol. Microbiol. 24:1025-1037. [DOI] [PubMed] [Google Scholar]
- 22.Hols, P., T. Ferain, D. Garmyn, N. Bernard, and J. Delcour. 1994. Use of homologous expression-secretion signals and vector-free stable chromosomal integration in engineering of Lactobacillus plantarum for α-amylase and levanase expression. Appl. Environ. Microbiol. 60:1401-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalogeraki, V. S., J. Zhu, A. Eberhard, E. L. Madsen, and S. C. Winans. 1999. The phenolic vir gene inducer ferulic acid is O-demethylated by the VirH2 protein of an Agrobacterium tumefaciens Ti plasmid. Mol. Microbiol. 34:512-522. [DOI] [PubMed] [Google Scholar]
- 24.Kovacikova, G., W. Lin, and K. Skorupski. 2003. The virulence activator AphA links quorum sensing to pathogenesis and physiology in Vibrio cholerae by repressing the expression of a penicillin amidase gene on the small chromosome. J. Bacteriol. 185:4825-4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kovacikova, G., and K. Skorupski. 2001. Overlapping binding sites for the virulence gene regulators AphA, AphB and cAMP-CRP at the Vibrio cholerae tcpPH promoter. Mol. Microbiol. 41:393-407. [DOI] [PubMed] [Google Scholar]
- 26.Kovacikova, G., and K. Skorupski. 2002. Regulation of virulence gene expression in Vibrio cholerae by quorum sensing: HapR functions at the aphA promoter. Mol. Microbiol. 46:1135-1147. [DOI] [PubMed] [Google Scholar]
- 27.Kovacikova, G., and K. Skorupski. 1999. A Vibrio cholerae LysR homolog, AphB, cooperates with AphA at the tcpPH promoter to activate expression of the ToxR virulence cascade. J. Bacteriol. 181:4250-4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kvint, K., L. Nachin, A. Diez, and T. Nyström. 2003. The bacterial universal stress protein: function and regulation. Curr. Opin. Microbiol. 6:140-145. [DOI] [PubMed] [Google Scholar]
- 29.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 30.Lambert, L. A., K. Abshire, D. Blankenhorn, and J. L. Slonczewski. 1997. Proteins induced in Escherichia coli by benzoic acid. J. Bacteriol. 179:7595-7599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee, Y. W., S. Jin, W. S. Sim, and E. W. Nester. 1995. Genetic evidence for direct sensing of phenolic compounds by the VirA protein of Agrobacterium tumefaciens. Proc. Natl. Acad. Sci. USA 92:12245-12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lupas, A. February 1996, posting date. COILS, version 2.2. [Online.] Max-Planck-Institut für Biochemie, Martinsried, Germany. http://www.ch.embnet.org/software/COILS_form.html.
- 33.Lupas, A. 1996. Prediction and analysis of coiled-coil structures. Methods Enzymol. 266:513-525. [DOI] [PubMed] [Google Scholar]
- 34.Lupas, A., M. Van Dyke, and J. Stock. 1991. Predicting coiled coils from protein sequences. Science 252:1162-1164. [DOI] [PubMed] [Google Scholar]
- 35.Lynd, L. R., P. J. Weimer, W. H. van Zyl, and I. S. Pretorius. 2002. Microbial cellulose utilization: fundamentals and biotechnology. Microbiol. Mol. Biol. Rev. 66:506-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narbad, A., and M. J. Gasson. 1998. Metabolism of ferulic acid via vanillin using a novel CoA-dependent pathway in a newly-isolated strain of Pseudomonas fluorescens. Microbiology 144:1397-1405. [DOI] [PubMed] [Google Scholar]
- 37.Overhage, J., H. Priefert, and A. Steinbuchel. 1999. Biochemical and genetic analyses of ferulic acid catabolism in Pseudomonas sp. strain HR199. Appl. Environ. Microbiol. 65:4837-4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Posno, M., R. J. Leer, N. Van Luik, M. J. F. Van Giezen, P. T. H. M. Heuvelmans, B. C. Lockman, and P. H. Pouwels. 1991. Incompatibility of Lactobacillus vectors with replicons derived from small cryptic Lactobacillus plasmids and segregational instability of the introduced vectors. Appl. Environ. Microbiol. 57:1822-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 40.Segura, A., P. V. Bunz, D. A. D'Argenio, and L. N. Ornston. 1999. Genetic analysis of a chromosomal region containing vanA and vanB, genes required for conversion of either ferulate or vanillate to protocatechuate in Acinetobacter. J. Bacteriol. 181:3494-3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zago, A., G. Degrassi, and C. V. Bruschi. 1995. Cloning, sequencing, and expression in Escherichia coli of the Bacillus pumilus gene for ferulic acid decarboxylase. Appl. Environ. Microbiol. 61:4484-4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zaldivar, J., and L. O. Ingram. 1999. Effect of organic acids on the growth and fermentation of ethanologenic Escherichia coli LY01. Biotechnol. Bioeng. 66:203-210. [DOI] [PubMed] [Google Scholar]