Abstract
This review considers the physiological and molecular biochemical mechanisms of bile formation. The composition of bile and structure of a bile canaliculus, biosynthesis and conjugation of bile acids, bile phospholipids, formation of bile micellar structures, and enterohepatic circulation of bile acids are described. In general, the review focuses on the molecular physiology of the transporting systems of the hepatocyte sinusoidal and apical membranes. Knowledge of physiological and biochemical basis of bile formation has implications for understanding the mechanisms of development of pathological processes, associated with diseases of the liver and biliary tract.
Keywords: Bile acids, Bile phospholipids, Bile micelle structures, Bile salt transporters
Core tip: Over the past 50 years, significant progress has been made in understanding the mechanisms of bile formation and secretion. This became possible due to the advances of fundamental investigations in cell biology, molecular biology, genetics and biochemistry. Knowledge of physiological and biochemical basis of bile formation has implications for understanding the mechanisms of development of pathological processes, associated with diseases of the liver and biliary tract.
INTRODUCTION
Bile is a lipid-rich hepatic secretion that is necessary for elimination of cholesterol and xenobiotics from the body and for dispersion and efficient absorption of digested dietary lipid in the upper small intestine[1]. Biliary function is a vital function of the liver, which results from the sequential vectorial transport of endogenous and exogenous substrates through three compartments: the vascular space, cellular space and biliary space. Canalicular bile is produced by polarized hepatocytes that hold transporters in their basolateral (sinusoidal) and apical (canalicular) plasma membrane[2]. The biliary function is responsible for the homeostasis of lipid metabolism, in particular cholesterol metabolism, elimination of toxic endo- and xenobiotics such as bilirubin, lipid bacteria products (endotoxin), and several inflammatory mediators[3]. Bile coming into the canaliculi is modified by cholangiocytes through secretion and absorption. The main determinant of bile formation is an osmotic filtration process resulting from active transport of bile acids and other osmotic solutes[3]. Pavlov et al[4] established the basic mechanisms of bile secretion, its entry into the duodenum, and the role of bile in digestion. Bile is essential for the intestinal digestion and absorption of nutriments. Almost half a century had passed before the experimentally founded ideas on the mechanisms of bile formation appeared. Later, they were clarified and in the late 1970s took shape as a number of hypotheses of the mechanisms of bile formation. Recent insights into the cellular and molecular mechanisms that control the function and regulation of hepatobiliary transport have led to a greater understanding of the physiological significance of bile secretion. Individual carriers for bile acids and other organic anions in the liver and intestine have now been obtained from several species. In addition, complex networks of signals that regulate key enzymes and membrane transporters located in cells that participate in the metabolism or transport of biliary constituents are being unraveled[5]. Most of the membrane transporters ensuring bile formation have now been identified. The expression of these membrane transporters is regulated through transcriptional and post-transductional mechanisms. Transcriptional regulation is under the control of nuclear receptors activated by ligands such as bile acids, which act as endogenous steroids synthesized from cholesterol in hepatocytes[3].
Bile is an iso-osmotic electrolytic fluid that is formed in the liver and is a product of its secretory function. Bile is primarily secreted by hepatocytes (i.e., canalicular bile) and subsequently delivered to the intrahepatic bile ducts, where it is modified by cholangiocytes (i.e., ductal bile). Bile secretion by liver parenchymal cells is the result of vectorial transcellular transport of solutes and involves the coordinated action of transport proteins at the basolateral (sinusoidal) and apical (canalicular) membranes of the hepatocyte. A complex network of signals controls uptake and efflux transporters on a long- and short-term timescale, including regulation at the level of gene transcription, protein translation and maturation, covalent modification, and dynamic localization of transporter proteins, as well as substrate availability[6].
COMPOSITION OF BILE AND STRUCTURE OF A BILE CANALICULUS
Bile contains almost all body components: proteins, lipids, carbohydrates, vitamins, mineral salts, and trace elements. The greater part of the bile proteins consists of globulins, and the lesser part comprises albumins. Phospholipids, cholesterol and its esters, neutral fats, and fatty acids rank high among bile lipids. Lecithins (phosphatidylcholines, PCs) are the major representatives of bile phospholipids. They are synthesized in the liver from the same components as plasma PCs; however, they differ from the latter in the higher content of palmitic acid[7]. The human bile concentration of free fatty acids and α-monoglycerides is small. Human cystic bile shows small quantities of diglycerides and is virtually free of triglycerides.
The electrolyte content of bile is similar to that of plasma. The major cations are sodium, potassium, and calcium; the anions are chloride and bicarbonate. The bile content of sodium is about 10 times higher than that of potassium. Excretion of sodium, potassium, and calcium into the bile is closely related to the rate of metabolic processes in the liver and depends on its functional state and on the content of salts in the body. The bile concentration of anions is 5-15 times smaller than that of cations.
A deficit of anions is compensated for by taurocholate (Figure 1). Bile contains a considerable quantity of phosphorus, magnesium, iodine, iron, and copper. The relative proportions of the major bile components are distributed in the following order: bile acids (67%), phospholipids (22%), proteins (4.5%), cholesterol (4%), and bilirubin (0.3%). Among the bile acids, the primary bile acids, cholic and chenodeoxycholic acids in a ratio of 1:1, account for about 50%. These are followed by the secondary bile acids, deoxycholic and lithocholic acids, as well as ursodeoxycholic and sulfolithocholic acids in the decreasing order.
Figure 1.
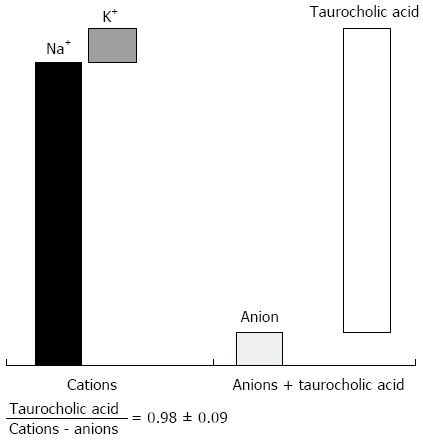
The bile content of anions, cations and taurocholic acid.
The use of color cathode-luminescence scanning electron microscopy (CCL SEM) has made it possible to study the qualitative composition of dehydrated human bile and its components in the native state. CCL SEM provides video information about the structural pattern of bile and its chemical composition. Loginov et al[8] were the first to show the earlier unknown phenomenon of cathode autoluminescence of non-conjugated bilirubin, unesterified cholesterol, high-molecular-weight protein, and other organic compounds. The pictures (Figure 2) show that the amorphous powder mass of bilirubin is red-emitting (Figure 2A), crystal cholesterol luminesces blue (Figure 2B), and the green coloration tingled with red is predominant in the sample of protein (Figure 2C). Graphically, all three samples have chromaticity histograms in the form close to the Gaussian curve.
Figure 2.
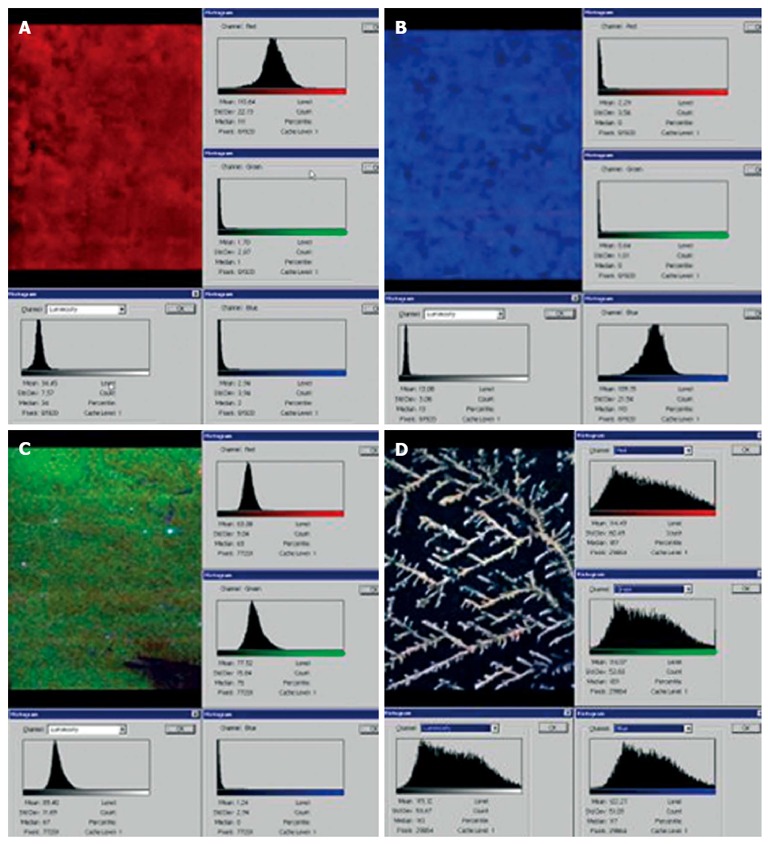
Color cathode-luminescence scanning electron microscopy images. Color cathode-luminescence scanning electron microscopy (CCL SEM) micro images of unconjugated bilirubin (A), unesterified cholesterol (B), high-molecular-weight protein (C), and dehydrated sample of normal human cystic bile (D).
Examination of the dehydrated samples of normal bile by CCL SEM has ascertained the tree-type structure of precipitates (Figure 2D). The qualitative assessment of the samples suggests their balanced content of three major bile components: bilirubin, cholesterol, and protein. Hepatocytes are the major epithelial cells in the liver, and are polarized. The polarized surfaces of hepatocytes consist of a basolateral domain facing the circulation and an apical domain that forms the bile canaliculus; the smallest branch of the biliary tree[9]. A major function of the liver is biliary secretion, which requires hepatocyte polarization. The mechanisms controlling hepatocyte polarization are only partially understood[10]. Structurally, they include cytoskeletal, tight junction and intracellular trafficking components. Recently, it was discovered that the major mammalian bile acid, taurocholate, accelerated polarity in primary rat hepatocytes. Taurocholate increases cellular cAMP and signals through an exchange protein activated by cAMP (EPAC)-Rap1-MEK-liver kinase B1 (LKB1)-AMP-activated protein kinase (AMPK) pathway for its polarity effect. Fu et al[9] have discussed possible mechanisms for how taurocholate affects different cell polarity factors, particularly AMPK, and thereby regulates events that generate polarity.
Bile production starts in the intercellular bile canaliculi. The bile canalicular lumen is formed by the external hemileaflet of the apical part of the plasma membrane of the adjacent hepatocytes and the tight junctions located at the point of contact of the hepatocytes. The hepatocyte tight junctions separate the bile canalicular lumen from the hepatic blood system[11]. Tight junctions contain proteins, including occludin, claudin and zona occludens (ZO)-1 protein. The integrity of tight junctions depends on the presence of protein ZO-1 on the inner surface of the plasma membrane. The impaired integrity of the tight junctions is accompanied by regurgitation of canalicular bile into the sinusoids. Three individual domains, such as sinusoidal, lateral, and canalicular parts of the membrane, are arbitrarily identified in the hepatocyte plasma membrane. The differences in the lipid and protein composition of the three domains of the hepatocyte plasma membrane determine its functional polarity. The sinusoidal membrane is enriched with receptors, enzymes, and transport proteins. The lateral membrane of the hepatocyte is involved in intercellular interaction, but it contains no transport systems. Transport systems for bile acids, organic anions and cations, as well as the enzymes γ-glutamyltransferase, Mg2+-ATPase, and alkaline phosphatase are located on the canalicular membrane. Cl-/HCO3- exchangers and selective Сl- channels are also identified[12]. Whether there are transport systems for glutathione and its conjugates in the apical membrane is still debated[13]. The release of glutathione S-conjugates from cells is an ATP-dependent process mediated by integral membrane glycoproteins[14].
The hepatocyte cytoplasm around the bile canaliculus contains cytoskeletal structures: microtubules, microfilaments, and intermediate filaments[15]. The microtubules take part in the mediated vesicular transport, secretion of lipids. The microfilaments determine canalicular contractility and motor activity. The intermediate filaments form a network between the plasma membranes, nucleus, intracellular organelles, and other cytoskeletal structures. The canaliculus is the least branch of a biliary tree and has a diameter of approximately 1 μm.
BIOSYNTHESIS OF BILE ACIDS
In 1848 Strecker discovered bile acids[16,17]. In chemical structure, they belong to a group of steroids and are cholanic acid derivatives. Bile acids are the end metabolic product of cholesterol and one of the most important routes of its elimination from the body[18-20]. Bile acid synthesis occurs through two pathways: the classic (neutral) pathway or the alternative (acidic) pathway[21,22]. The liver is the only organ that has all 14 enzymes required for de novo synthesis of two primary bile acids in humans, cholic acid (CA; 3α, 7α, 12α-trihydroxy-cholanic acid) and chenodeoxycholic acid (CDCA; 3α, 7α-dihydroxy-cholanic acid)[23].
The biosynthetic pathway includes a number of successive enzymatic conversions associated with the oxidation of cholesterol in the smooth endoplasmic reticulum and the shortening of its side chain in mitochondria. The production of bile acids involves restoration of a double bond in cholesterol, С-3 inversion to give rise to a 3α-ОН-group, further α-hydroxylation of either only a 7-carbon atom or 7- and 12-carbon atoms, as well as β-oxidation of the side chain of cholesterol. Cytochrome Р450 is involved in all oxidative reactions. The classic bile acid biosynthetic pathway is initiated by enzyme cholesterol-7α-hydroxylase (CYP7A1) (Figure 3)[20]. The enzyme CYP7A1 of smooth endoplasmic reticulum is a limiting link in the synthesis of bile acids[20]. The activity of this enzyme is regulated by the quantity of intestinally absorbed bile acids, other than cholesterol[20,24]. Numerous studies have demonstrated that bile acids, steroid hormones, inflammatory cytokines, insulin, and growth factors inhibit CYP7A1 transcription through the 5′-upstream region of the promoter[25-30]. Sterol 12 α-hydroxylase (CYP8B1) is required for synthesis of CA. Mitochondrial sterol 27 hydroxylase (CYP27A1) catalyzes sterol side chain oxidation, after which cleavage of a three-carbon unit in the peroxisomes leads to formation of a C24 bile acid. Cholesterol is converted to two primary bile acids in human liver, CA and CDCA. Key regulated enzymes, CYP7A1, CYP8B1, CYP27A1, and 7α-hydroxylase (CYP7B1) expressed, in the pathways are indicated. CYP7A1 initiates the classic (neutral) bile acid biosynthetic pathway in the liver. CYP27A1 initiates the alternative (acidic) pathway in the liver and macrophages. CA and CDCA are conjugated to glycine (G) and taurine (T). Bile acid: CoA synthase (BACS) and bile acid: amino acid transferase (BAT) are two key enzymes involved in amino conjugation of bile acids[20].
Figure 3.
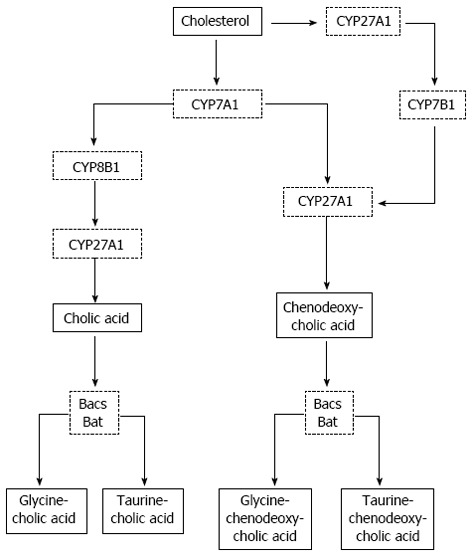
Bile acid synthesis. CYP7B1: 7α-hydroxylase; CYP7A1: Enzyme cholesterol-7α-hydroxylase; CYP8B1: Sterol 12 α-hydroxylase; CYP27A1: Mitochondrial sterol 27 hydroxylase.
An alternative (acidic) pathway is initiated by CYP27A1, which in addition to the liver is expressed in macrophages and most other tissues, and may contribute significantly to total bile acid synthesis (Figure 3). Other minor pathways initiated by 25-hydroxylase in the liver and 24-hydroxylase in the brain also may contribute to bile acid synthesis. A nonspecific CYP7B1 expressed in all tissues is involved in the generation of oxidized metabolites (oxysterols), which may be transported to the liver and converted to CDCA[20]. Farnesoid X receptor (FXR) plays a central role in the regulation of bile acid synthesis, excretion, and transport[31,32]. FXR inhibits CYP7A1[33], CYP8B1[34], and CYP27A1[35] transcription. It has been reported that peroxisome proliferator activated receptor (PPAR)α plays a role in the regulation of bile acid synthesis[36]. Bile acids induce PPARα transcription via induction of FXR[37].
Di- and tri-hydroxycholanic primary bile acids are produced from cholesterol during biosynthesis of bile acids in humans. Under normal conditions, 200-600 mg primary bile acids is formed daily[20,38]. Bile acid synthesis increases in the morning regardless of food intake[39]. In humans, bile acid synthesis exhibits a diurnal rhythm with two peaks around 15:00 and 21:00 h[20]. Bile acids lost in the feces (0.2-0.6 g/d) are replenished by de novo synthesis in the liver to maintain a constant bile acid pool. Hepatic conversion of cholesterol to bile acid balances fecal bile acid excretion and this process represents a major route for elimination of cholesterol from the body[40,41].
Bile acids are physiological detergents that generate bile flow and facilitate intestinal absorption and transport of lipids, nutrients and vitamins[20]. Bile acids, end products of the pathway for cholesterol elimination, are required for dietary lipid and fat-soluble vitamin absorption and maintain the balance between cholesterol synthesis in the liver and cholesterol excretion[21].
CONJUGATION OF BILE ACIDS
Under physiological conditions, free bile acids are not frequently found in bile. Newly synthesized bile acids conjugate with glycine or taurine, by giving rise to bile salts: (chenodeoxy-)cholylglycine or (chenodeoxy-)cholyltaurine (Figure 3). The physiological significance of bile acids conjugation is that their salts are more polar compounds than free bile acids. Conjugation of bile acids to glycine or taurine decreases bile acid toxicity and increases their solubility that benefits secretion into bile. Conjugated bile acids have a smaller critical concentration for micellar formation[42]. The taurine conjugates of bile acids are more polar compounds than their glycine conjugates[43]. Taurine (2-aminoethanesulfonic acid) is the most abundant free amino acid in humans and plays an important role in several essential biological processes such as bile acid conjugation, maintenance of calcium homeostasis, osmoregulation and membrane stabilization[44]. Taurine is efficient at reducing plasma and liver cholesterol concentrations. The cholesterol-lowering effect of taurine is involved in the regulatory mechanism of cholesterol and bile acid homeostasis that is mediated by CYP7A1, which has become a biomarker for cholesterol metabolism and is itself also regulated by several factors and nuclear receptors[45].
The C-24 conjugation of free cholanic acids with glycine or taurine is accomplished by hepatocyte acyltransferases. The reaction proceeds in two steps with the participation of ATP and in the presence of Mg2+. BACS and BAT are involved in amino acid conjugation of bile acids[20]. FXR stimulates bile acid conjugation by inducing expression of genes encoding BACS and BAT, which also are induced by hepatocyte nuclear factor (HNF) 4α[46]. Thus, FXR and HNF4α may coordinately regulate bile acid synthesis and conjugation.
The normal human ratio of the glycine to taurine conjugates of bile acids is 3:1. The ratio of the glycine/taurine conjugates may be altered under the influence of alimentary and hormonal factors, in some liver diseases. The presence of unconjugated bile acids in the bile most frequently is a result of hepatic disease.
In the intestine, glyco- and tauro-conjugated CA and CDCA are deconjugated, and 7α-dehydroxylase activity in bacterial flora removes a 7α-hydroxy group to form secondary bile acids deoxycholic acid (DCA; 3α,12α-dihydroxy) and lithocholic acid (LCA; 3α-monohydroxy), respectively[20].
Bile acids may be also exposed to glucuronidation or sulfation, which results in a reduction of their toxic properties and promotes their urinary and fecal excretion[43,47-49]. A small amount of bile acids circulated to the liver is sulfoconjugation at the 3-hydroxy position by sulfotransferase (SULT2A1) and rapidly secreted into bile. Sulfation is the major pathway for detoxification of extremely hydrophobic bile acids in humans[50]. Details of bile acid chemistry, biology, physiology, and synthesis have been reviewed recently[51,52].
BILE PHOSPHOLIPIDS
Phospholipids are a heterogeneous group of substances. They contain fatty acids with a varying length of carbon chain. Phospholipids are an important component of cell membranes, plasma, and bile. In all mammalian species, including humans, bile phospholipids are represented solely by a mixture of PC (lecithin) molecules. The fatty acids of bile PC molecules greatly differ from those of lecithin of plasma and liver tissue. Depending on fatty acid residues at C1 and C2 of the glycerine molecule that is part of PC, there are a variety of lecithins. Human bile predominantly contains 1-palmitoyl, 2-linoleyl, and 1-palmitoyl, 2-oleoyl PCs. This lecithin subpool represents nearly 5% of the total pool of hepatic PCs. The lecithins destined for bile secretion have been shown to be apparently completely synthesized de novo[53]. Selection of certain types of PC seems to occur during bile formation[7]. Bile salts and primarily PC are the main organic solutes in bile, and play a crucial role in cholesterol and dietary lipid solubilization. Bile salts and phospholipids in concert increase the biliary solubility of cholesterol by > 1 million-fold, thereby permitting entry of hepatocellular cholesterol into bile[54]. Hepatic PC biosynthesis is likely to be regulated by bile salts. Biliary lipid secretion is driven by bile salts, the primary metabolites of cholesterol. There are multiple data showing that the biliary secretion of PC (as cholesterol secretion) depends on that of bile acids[55,56]. It has also been shown that the secretion of bile phospholipids is not only associated with that of bile acids, but is regulated by the amount and type of bile salts passing through the hepatocytes[7,57].
The use of electron microscopy has demonstrated that phospholipids are secreted as vesicles into bile, and that vesicle secretion is almost wholly dependent upon MDR3 P-glycoprotein function[58]. Biliary-specific PC molecules are recruited initially from intracellular sources, predominantly the endoplasmic reticulum[59]. A proposed mechanism for PC delivery to the canalicular plasma membrane involves monomeric transfer via binding to the cytosolic PC transfer protein (PC-TP)[60]; a process that is stimulated by low concentrations of bile salts typical of the hepatocytic cytosol. Alternatively, biliary lipid may arrive at the canalicular membrane via intracellular vesicle transport[1].
Biliary PC molecules are translocated from the endoplasmic to the exoplasmic hemileaflet by the action of a transmembrane translocator[61].
MDR3 P-glycoprotein is thought to translocate PCs from the internal to the external hemileaflet of the hepatocyte canalicular plasma membrane; possibly generating microdomains of the more fluid biliary-type PCs. Luminal bile salts, secreted as monomers through the action of bile salt transporters (cBATs), interact preferentially with these microdomains to promote vesiculation of the membrane external hemileaflet; possibly involving nonlamellar phase transitions. Cholesterol, which can freely flip between the internal and external hemileaflets, may be released into bile either by diffusing laterally into nascent vesicles, or by bile-salt-mediated transfer through the aqueous phase. The model is based on data from the study[59] and published work[1,62,63].
Crawford et al[58] proposed the concept that biliary PC molecules are flipped from the internal to the external hemileaflet of the hepatocyte canalicular membrane by the action of MDR3 P-glycoprotein, followed by release of PC vesicles from the external hemileaflet into bile (Figure 4). Formation and detachment of unilamellar vesicles from the canalicular membrane is presumably mediated by the detergent action of luminal bile salts.
Figure 4.
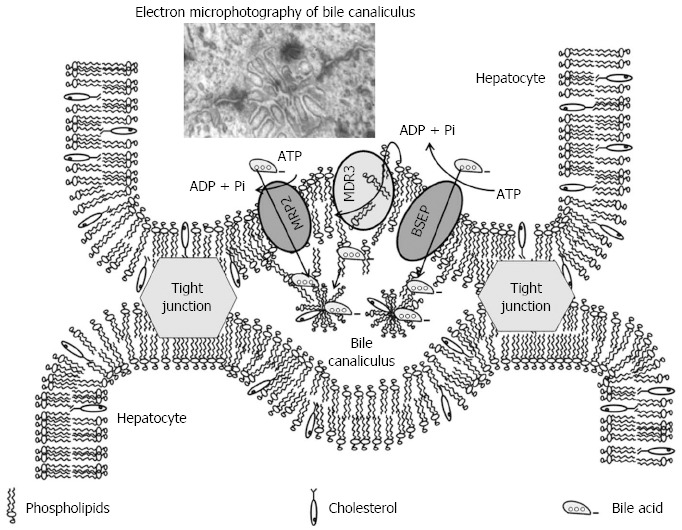
Proposed model for lipid secretion into bile[58]. BSEP: Bile salt export pump; MDR3: Multidrug-resistance protein 3; MRP2: Multidrug-resistance-associated protein 2; ATP: Adenosine triphosphate; ADP: Adenosine diphosphate; Pi: Inorganic phosphate.
Vesiculation of the external hemileaflet provides an explanation of how luminal bile salts can extract large quantities of phospholipid on the basis of their detergent action, without disrupting the integrity of the detergent-resistant canalicular plasma membrane. This remarkable mechanism for selective secretion of membrane phospholipids appears to be uniquely adapted to the detergent environment of the bile canaliculus.
BILE MICELLES
Bile lecithins, bile acid salts, and cholesterol are amphiphiles molecules (Figure 5). In this connection, in the water medium, such as bile, these compounds cannot exist in the monomolecular form and they generate micellar or lamellar structures.
Figure 5.
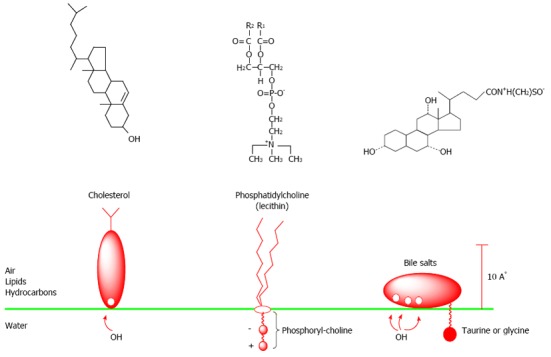
Chemical formulas and simple images of cholesterol, lecithins, and bile salts on a boundary of two phases-water: lipids (air, hydrocarbons).
According to the inclusions into the lipid complex, simple micelles (PC + cholesterol), up to 3 nm in size, mixed (PC + cholesterol + bile acids), 3-6 nm in diameter, and vesicles (PC + cholesterol + bile acids), 25-130 nm in diameter, are identified (Figure 6).
Figure 6.
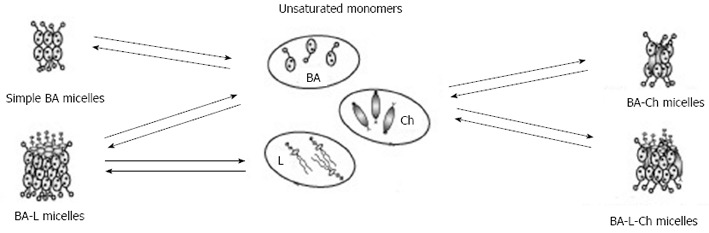
Formation of micelles structures in the system containing bile acids, lecithin, and cholesterol. BA: Bile acids; Ch: Cholesterol; L: Lecithin.
They can all contain amphiphilic proteins on their surface. Lipid molecular inclusions in the micelles of bile acids and the formation of mixed micelles are the main form of interaction of bile acids and lipids in the bile. By forming mixed micelles, bile acids along with lecithin provide cholesterol solubilization. Bile salts as detergents form micelles when the critical concentration of micellar formation (CCM) is achieved. CCM depends on the type of bile salts and their hydrophobicity. Based on their hydrophobic properties, bile acids may be arranged in the following order: deoxycholate > chenodeoxycholate > cholate > ursodeoxycholate[64]. Hydrophobic, but not hydrophilic, bile acids are efficacious endogenous ligands of the nuclear receptors FXR (NR1H4), pregnane X receptor (PXR; NR1I2), and vitamin D receptor (VDR; NR1I1) that play critical roles in the regulation of bile acid synthesis and metabolism[20].
A mixture of bile acids, lecithin, and cholesterol at certain molecular ratios is able to form lamellar liquid-crystalline structures[64]. Vesicles are monolamellar globules that consist of phospholipids and bile acids. At a certain concentration (about 0.1 mmol/L), phospholipids as amphiphiles have been found to produce enclosed globules, in which the polar heads are directed outward, and the nonpolar “tails” are inside. This particle is capable of forming many substances, including protein, and cholesterol, by providing solubilization of the latter by bile acids. Vesiculation is largely determined by the hydrophilic-hydrophobic balance of bile acids and the content of cholesterol[65]. The proportion of bile-mixed micelles and vesicles depends on the concentration and composition of bile acids. The most important feature of phospholipid masses is their ability to change their consistency from crystalline gel to liquid-crystalline state depending on environmental conditions. The liquid-crystalline states are typified by the structural order peculiar to true crystals with the preserved mobility of the molecules observed in solutions. The fusion of monolamellar vesicles may give rise to multilamellar vesicles. The vesicles are a rather stable formation and together with micelles play a pivotal role in the solubilization and transport of cholesterol into the bile[24].
Lipid vesicles 60-80 nm in diameter have been documented repeatedly by electron microscopy and quasielastic light scattering of freshly secreted hepatic bile[1,58]. Microscope laser light scattering of bile canaliculi in isolated rat hepatocyte couplets also detects intraluminal vesicles of similar size; the numbers of which increase upon cellular incubation with bile salts[66].
Acid-dependent and acid-independent bile fractions are identified[67]. The former is associated with the synthesis and active transport of bile acids in the hepatocytes. The volume of the resultant bile is linear with the concentration of bile acids, caused by the osmotic effect of bile acid anions, and Sperbet[68] first argued it in 1959. The bile composition greatly varies in the bile canaliculi due to the increased constant volume of water and bicarbonates irrespective of the secretion of bile acids. This acid-independent bile fraction is regulated by an active Na+ transport. Its physiological role is to dilute and excrete an acid-dependent fraction[69]. Further bile formation occurs in the bile ducts because a fluid enriched with bicarbonates and chlorides is produced in the latter. This process is under the control of secretin (80%) and other gastrointestinal hormones. The volume of the water secreted in the ducts depends to greater degree on the active transport of bicarbonates[70].
ENTEROHEPATIC CIRCULATION OF BILE ACIDS
The bile acids synthesized in the hepatocyte participate in the human body, in the so-called enterohepatic circulation. Conjugated bile acids are secreted from the hepatocytes into the bile canaliculus by canalicular bile salt export pump (BSEP, ABCB11). Some bile acids secreted in the bile duct are reabsorbed in the cholangiocytes and recycled back to hepatocytes (the cholangiohepatic shunt)[20]. The bulk of bile acids secreted are stored in the gallbladder. After each meal, gallbladder contraction empties bile acids into the intestinal tract. Bile acids are mixed up with food masses (together with other digestive secretions), and take an active part in the processes of fat and fat-soluble vitamins metabolism and absorption.
Fatty acids and monoglycerides formed from neutral fats with participation of bile acids and of lipases in the upper parts of the small intestine are absorbed by enterocytes as lipoid-biliary complexes. These complexes disintegrate in the enterocytes. Released monoglycerides and fatty acids are used by the cells as energy and building materials or are transported to the basolateral membrane of the enterocytes and enter the portal venous system. Bile acids may return to the intestine and continue to participate in metabolism and absorption of fat and fat-soluble vitamins. As bile acids are moving through the intestine they may participate 4-6 times in the transportation of monoglycerides and fatty acids in enterocytes.
In the small intestine, bile acids are absorbed by both passive and active mechanisms[71]. Although passive absorption occurs down the length of the intestine, active absorption of bile acids is restricted to the ileum[72,73]. Passing through the intestinal tract, some bile acids are reabsorbed in the upper intestine by passive diffusion, but most bile acids (95%) are reabsorbed in the distal ileum by apical sodium-dependent bile acid transporter (ASBT) located in the brush border membrane[71]. In humans and all other vertebrates examined to date, the ileal epithelium has developed an efficient transport system for the active reclamation of bile acids[51]. Approximately 95% of the bile acids secreted into bile are derived from the recirculating pool[71]. Bile acids are transdiffused across the enterocytes to the basolateral membrane where the organic solute transporter and heterodimer (OST/OST) effluxes bile acids into the portal blood circulation[74,75], transports them to the sinusoids, where they are taken up by Na+-dependent taurocholate cotransport peptide (NTCP) into hepatocytes[20]. The bulk of bile acids are absorbed by hepatocytes into the liver, and again excreted into the bile. Later, this enterohepatic cycle recurs. Hepatocytes are known to secrete as much as 90% of the bile acids that have returned into the cells during enterohepatic circulation, and about 10% of the newly synthesized bile acids[20]. A bile acid pool of about 3 g is recycled 4-12 times a day[71]. At a fundamental level, the bile acid enterohepatic circulation can be viewed as a series of storage chambers (gallbladder and small intestine), valves (sphincter of Oddi and ileocecal valve), mechanical pumps (canaliculi, biliary tract, and small intestine), and chemical pumps (hepatocyte and enterocyte transporters)[71].
The enterohepatic circulation of bile acids serves as an important physiological route for recycling bile acids and absorption of nutrients, as well as regulation of whole-body lipid metabolism[20]. Enterohepatic circulation ensures a continuous supply of bile acids to be used repeatedly for lipid absorption during the digestion of a single meal or multiple meals throughout the day[76]. Efficient intestinal reabsorption and hepatic extraction of bile acids also enables effective recycling and conservation that largely restricts these potentially cytotoxic detergents to the intestinal and hepatobiliary compartments.
FXR plays a key role in the control of enterohepatic circulation of bile acids. FXR induces the expression of BSEP in the canalicular membrane, which is the driving force for bile formation. FXR inhibits Ntcp transcription by SHP-dependent inhibition of retinoid X receptor/retinoic acid receptor induction of Ntcp[77]. Thus, FXR plays a critical role in the coordination of bile acid synthesis, biliary bile acid secretion, intestinal bile acid reabsorption and secretion, and bile acid uptake into hepatocytes.
Hepatocytes hold a central position in the enterohepatic circulation of bile acids, with which their highest (as compared to other cells) content of cholanic acids is associated.
Molecular mechanisms of hepatocyte bile secretion
Hepatocyte secretion of bile is the passive transhepatocellular filtration of water and electrolytes (Na+, K+, and Cl-) from blood into the bile canalicular cavity, which is caused by the osmotic gradient between the bile and blood, which results from active transport of organic and inorganic anions from blood and hepatocytes to the bile canaliculi. The ionic transport systems that govern ionic and electrical gradients onto the membrane surface (Na+/K+, Na+/H+, Na+/HCO3-, Cl-/HCO3-, and Na+/Ca2+) are the basis for the mechanism of targeted bile secretion[78]. The transport of bile salts, organic anions and cations, bilirubin and other substances from the portal blood into the biliary system is accomplished through the action of an array of transporter proteins in the hepatocytes[79].
The hepatocytes are polar cells in which the transport of osmotically active bile components has a strict direction from the sinusoidal (basal) surface to its canalicular (apical) part. There is evidence on the importance of taurocholic acid in the directed transport of substances in the liver cells[44]. This vectorial trans-hepatocellular movement of bile acids is a remarkably concentrative transport process that is driven by a distinct set of primary (ATP-dependent), secondary (Na+ gradient-dependent), and tertiary (OH- or HCO3--dependent anion exchange) transport systems at the sinusoidal and canalicular plasma membranes[80,81]. Bile secretion is a highly regulated process. To maintain this process, liver cells must transport bile acids efficiently from the portal blood into bile. ATP binding cassette (ABC) transporters[57] mediate transport of bile salts. Expression of these transporters is regulated in a coordinate fashion by a set of nuclear hormone receptors explaining the old observation of coupling between bile salt secretion and biliary lipid secretion. Over the past two decades, there has been significant progress toward identifying the individual membrane transporters and unraveling their complex regulation[74]. The hepatocytes are polarized cells that express differential transport systems in their plasma membrane domains. Molecular cloning has identified most of these transport proteins, and their transport properties characterized by functional studies. The NTCP, the bile salt export pump (BSEP), the ASBT, and the organic solute transporter OSTα-OSTβ, the major bile acid transporters that control the fate of bile acids, through either absorption and enterohepatic cycling or excretion and elimination from the body.
These transporters play a key role in the vectorial transfer of solutes and water from sinusoidal blood into the bile, thus contributing to bile formation and the biliary excretion of various xenobiotics. In the liver and intestine, transporters play a critical role in maintaining the enterohepatic circulation and bile acid homeostasis[71,82].
Bile acids determine the secretion of an acid-dependent bile fraction. The transport of bile acids from the sinusoidal space to the bile canaliculus comprises their penetration across the sinusoidal and canalicular membranes and movement inside the hepatocytes. The molecular and metabolic processes occurring on the hepatocyte sinusoidal membrane involve: (1) hepatocyte absorption of bile acids and proteins from blood; and (2) transmembrane transfer of inorganic ions. The principal intracellular molecular and metabolic processes determining the mechanisms of hepatocyte bile secretion are: (1) biosynthesis and conjugation of bile acids; (2) hydroxylation and conjugation of bile acids entering the hepatocytes from the blood; and (3) transfer of bile acids and proteins from the perisinusoidal to pericanalicular area of the hepatocytes.
The molecular mechanisms of hepatocyte canalicular membrane function ensure the transmembrane transfer of bile acids, proteins, inorganic and organic cations and anions into the bile canalicular cavity.
Molecular physiology of the transporting systems of the hepatocytic sinusoidal membrane: The specific feature of transport of bile acids from blood to the hepatocyte across the sinusoidal membrane is to overcome the high gradient of their concentration and electrical potential. Hepatocytes absorb bile acids from the blood by the bile acid carriers, which are integral hepatocyte sinusoidal membrane proteins[78] (Figure 7).
Figure 7.
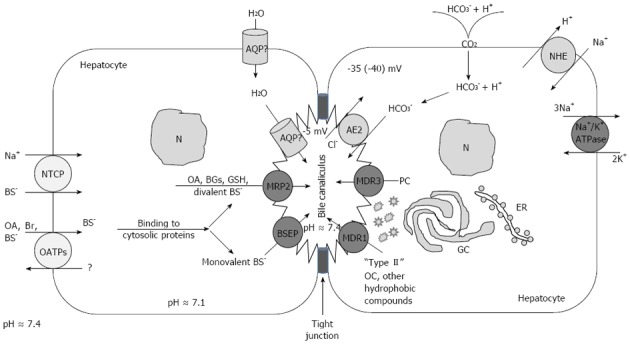
Mechanisms of the transport of bile acids, water, and electrolytes through the hepatocyte (transcellular pathway) and intercellular space (paracellular pathway). Localization and function of sinusoidal and canalicular hepatocellular transporters. The Na+-dependent sinusoidal uptake of bile salts (BS) is mediated by Na+-dependent taurocholate cotransport peptide. The Na+-independent hepatic uptake of organic anions (OA-), BSs and type II organic cations (OC+) is mediated by members of the OATP family. Sinusoidal uptake of type I OC+ is mediated by OCT1. Transport across the canalicular membrane is driven mainly by ATP-dependent export pumps. MDR1 mediates canalicular excretion of amphiphilic type II OC+ and other hydrophobic compounds. MDR3 functions as a PC flippase. BSEP mediates apical excretion of BSs. MRP2 transports non-bile-salt organic anions, such as bilirubin glucuronides (BGs), GSH, and sulfated/glucuronidated bile salts. Canalicular transport of HCO3- is mediated by the Cl-/HCO3- exchanger AE2. AQP9 and AQP8 are involved in the transport of water across the sinusoidal and the canalicular membrane, respectively. The nature of the water channels in human liver has been characterized[84]. GC: Golgi apparatus (complex); ER: Endoplasmic reticulum; N: Nucleus.
Liver sinusoids possess a specific architecture that allows passage of organic compounds bound to albumin through endothelial fenestrae into the space of Disse, from where they can be taken up by the sinusoidal transport systems of the hepatocytes[83,84].
The bulk (about 85%) of the bile acids are present in plasma as a complex of plasma proteins (mainly, albumin)-bile acid, therefore, it has been shown that albumin performs its inherent transport function and contributes to the interaction of bile acids with specific sinusoidal membrane receptors in the mechanisms of hepatocyte absorption of bile acids. The bile acids in sinusoidal blood are efficiently taken up by hepatocytes from Disse’s space despite being highly albumin bound, due to the existence in the basolateral membrane of transporters[85,86]. The powerful techniques of molecular biology have enabled gene cloning of the transporters involved in biliary secretion and the enterohepatic circulation of bile acids. This has permitted elucidation of their function as well as their regulation by nuclear receptors[87]. The enterohepatic circulation results from efficient ileal absorption, and is highly regulated at two sites. In the hepatocytes, biosynthesis of bile acids is regulated by negative feedback by the nuclear receptor FXR as well as by cytokines and by a peptide (FGF-19) liberated by bile acids from the ileal enterocytes. In the ileal enterocytes, bile acid reclamation is regulated by negative feedback by FXR and other nuclear receptors. BSEP mediates uphill canalicular bile acid secretion[87]. The plasma membranes of hepatocytes have been found to contain the proteins that selectively bind bile acids[87]. This uptake is carried out against an electrochemical gradient, is saturable[88], and depends on the structure of the bile acids. It is more efficient for trihydroxy- than for dihydroxy-bile acids and for conjugated more than for unconjugated bile acids[89]. Transporters on the basolateral membrane, which faces the space of Disse, are responsible for the uptake of bile salts and organic anions[79]. The liver cells have an active transport system to transfer bile acids from the blood to the hepatocytes. Basolateral uptake transporters can be divided into Na+-dependent and Na+-independent systems. The NTCP, the main Na+-dependent bile acids transporter[90-92] is only expressed in the basolateral membrane[93]. NTCP, (gene name SLC10A1) is the founding member of the SLC10 family of solute carrier proteins, which includes two bile acid carriers (SLC10A1/NTCP and SLC10A2/ASBT), one steroid sulfate transporter (SLC10A6/SOAT), and four orphan carriers (SLC10A3, SLC10A4, SLC10A5, SLC10A7)[94-96]. Na+-dependent uptake involves co-transport of solutes with Na+. Moreover, the driving force is the energy of ATP generated by Na+,K+-АТPase. This enzyme is located on the sinusoidal and lateral membranes of hepatocytes[97,98] and undetectable on the canalicular (apical) part[99,100]. The active transport of bile acids ensures their continuous hepatocyte pumping from blood to bile, thus causing their low level in the blood flowing from the liver and in plasma as a whole. The properties of NTCP satisfy all the functional criteria for hepatocyte Na+-coupled bile acid uptake[71,94]. The major physiological substrates of NTCP include all the major glycine- and taurine-conjugated bile acids[93,101-104]. Depending on the structure of the bile acid and NTCP, unconjugated bile acids are moderate or weak substrates[102,105,106] and sulfated bile acids appear to be only weakly transported[107]. The high level of NTCP expression at the sinusoidal membrane of hepatocytes and high affinity of NTCP for conjugated bile acids promote efficient extraction of bile acids from portal blood. Thus, NTCP functions to maintain the enterohepatic circulation of bile acids and maintain plasma concentrations at a minimum.
The uptake of bile acids by Na+-independent mechanisms seems to be mediated by less-specific transporters, known as organic anion-transporting polypeptides (OATPs), which exchange these molecules for other anions, such as HCO3-, glutathione (GSH) or even other bile acids[89,90,108].
Four OATPs have been cloned and characterized from human liver: OATP1A2 (SLCO1A2/SLC21A3; formerly, OATP-A), OATP1B1 (SLC21A6; formerly, OATP-C or LST-1), OATP1B3 (SLC21A8; formerly, OATP-8) and OATP2B1 (SLC21A9; formerly, OATP-B). These transporters may take up bile acids (mainly unconjugated forms), endogenous OA- (thyroid hormones, monoconjugated bilirubin) and xenobiotic compounds (e.g., toxins, drugs and food components)[109]. In contrast to NTCP, members of the OATP family expressed on the hepatocyte sinusoidal membrane, such as Oatp1a1 (Slco1a1), efficiently transport unconjugated or sulfated bile acids, suggesting that these carriers participate in the hepatic uptake of those bile acid species in vivo[89,102,110]. The heterodimeric protein OSTα/OSTβ is expressed at the basal membrane of hepatocytes and cholangiocytes[111]. This is a sodium-independent bile acid transporter that may play a role in bile acid efflux from hepatocytes toward blood when these compounds are accumulated under cholestatic conditions. Moreover, in cholangiocytes, in addition to playing a similar role, this transporter may also be involved in the cholehepatic shunting of bile acids.
Hepatocellular uptake of organic cations is mediated by two separate transport systems, which depends on the substrate molecular size[112]. Thus, small (type I) organic cations are taken up by the organic cation transporter, OCT1/Oct1 (SLC22A1/Slc22a1), which is electrogenic in nature. In contrast, human OATP-A mediates the uptake of bulky (type II) organic cations. In addition to conjugated and unconjugated bile salts, Oatps/OATPs accept other cholephilic compounds, including glucuronidated (and maybe unconjugated) bilirubin, exogenous organic anions (e.g., sulfobromophthalein), leukotrienes, estrogen-conjugates (e.g., estrone-3-sulfate or estradiol-17-β-d-glucuronide), thyroid hormones, mycotoxins, and numerous xenobiotics[92,113-115].
Molecular physiology of the intracellular processes underlying the hepatocyte secretion of bile: In order to explain the transit of bile acids from the sinusoidal membrane to the pericanalicular region, two different, not mutually exclusive, mechanisms have been proposed: (1) simple diffusion of bile acids bound to intracellular proteins[116]; and (2) vesicular transport of bile acids driven by cytoskeleton contractile activity[117,118]. Two arguments have been raised against the role of the second mechanism. One is that hepatic transit of labeled bile acids is too fast[118]. The second is that the baseline secretion of bile acids is not modified by microtubular disruption[117]. However, the overload of bile acids intensifies the vesicular trafficking from the Golgi complex to the pericanalicular zone[119], and under these circumstances the alteration in the functional integrity of the cytoskeleton results in impaired bile acid secretion[120] and subsequent cholestasis[121].
The quantity of ABC transporters in the apical membrane is regulated by the amount of biliary components available for secretion[122,123]. The regulated intracellular vesicular traffic of canalicular ABC transporters[123,124] is crucial for normal bile secretion. BSEP (formerly SPGE, a sister of P-glycoprotein) is the main, if not the only, canalicular bile acid transporter[125], and it is also located in subcanalicular vesicles that may act as an intracellular pool. It is therefore probable that the impaired secretion of bile acids observed in overloaded conditions is an indirect result of the distortion of the increased vesicular traffic of transporters to the canalicular membrane[120]. These and other studies[126,127] have established the actual role of vesicular trafficking in hepatocytes, and have demonstrated that specific vesicle trafficking machinery is required for membrane polarity. The overall functions based on hepatocyte polarity are not attributable to the mere presence of transporters in both poles of these cells[128] but also to their intracellular trafficking and temporary anchorage to the different hepatocyte membranes (Figure 7).
Molecular physiology of the transporting systems of the hepatocyte canalicular membrane: After traversing the cell by Fick’s diffusion, cholephilic compounds mostly bound to high-affinity cytosolic proteins are excreted into the bile mainly by ATP-dependent pumps of the superfamily of ABC transporters; in particular those belonging to the family of multidrug-resistance proteins, MDR/Mdr, or to the family of multidrug-resistance-associated proteins, MRP/Mrp[57,71].
MDRs/Mdrs are members of the ABC superfamily that were originally described in cancer cell lines, where they confer resistance to therapeutic agents. Three gene products were identified in rodents, Mdr1a (Abcb1a), Mdr1b (Abcb1b) and Mdr2 (Abcb4), and two in humans, MDR1 (ABCB1) and MDR 3 (ABCB4). MDR1/Mdr1 functions as an efflux pump for a wide range of amphiphilic, bulky type II cationic drugs, together with other hydrophobic compounds, such as endogenous and exogenous metabolites or toxins, steroid hormones, hydrophobic peptides and even glycolipids[113]. Two closely related but functionally distinct Mdr1 isoforms, mdr1a and mdr1b, are present in the murine but not human phenotype[129]. MDR3/Mdr2 functions as a flippase, which translocates PC from the inner to the outer hemileaflet of the canalicular membrane, followed by release of PC-containing vesicles from the outer hemileaflet into bile; a process facilitated by the detergent properties of luminal bile salts[58].
Monoanionic bile salts are excreted in the canalicular pole by the bile salt export pump (BSEP/Bsep; ABCB11/abcb11); another member of the MDR family[130]. In contrast, canalicular efflux of divalent, bipolar sulfated or glucuronidated bile salts is mediated by the multidrug-resistance-associated protein 2 (MRP2/Mrp2; ABCC2/Abcc2)[131,132]. This carrier is also engaged in the biliary excretion of many other organic anions, including glutathione-S-conjugates (e.g., leukotriene C4 or sulfobromophthalein, among others), glucuronides (e.g., bilirubin and estrogens), and reduced (GSH) and oxidized glutathione (GSSG) - the former with low affinity[133,134]. Both GSSG and GSH are major determinants of the so-called “canalicular bile-salt-independent bile flow”[135].
The canalicular membrane domain also contains the electroneutral anion exchanger 2 (AE2/Ae2; SLC4A2/slc4a2), which extrudes HCO3- by exchanging the anion for biliary Cl-[136]. It functions to regulate intracellular pH when hepatocytes are exposed to an alkaline load[136]. In addition, AE2/Ae2 plays a role in bile flow generation, because HCO3- excretion is thought to be an additional primary driving force of the canalicular bile-salt-independent bile flow[136,137]. Both in humans and rats, three transcript variants of AE2/Ae2 have been described: the full-length transcript AE2a/Ae2a, expressed from the upstream promoter in most tissues, and the alternative transcripts AE2b1/Ae2b1 and AE2b2/Ae2b2, expressed in a more tissue-restricted fashion (mainly in liver and kidney). AE2b1/2/Ae2b1/2 transcription is driven from overlapping promoter sequences within intron 2, which results in AE2/Ae2 protein isoforms with short N-terminal differences[138,139].
In water transporters, for a solute to drive blood-to-bile vectorial water transport primarily, resultant osmotic forces need to be associated with aquaporin (AQP)-mediated transcellular movement of water molecules from plasma to the bile canaliculus[140]. Both immunochemical and functional studies have demonstrated the constitutive expression of the water channel AQP9 at the basolateral membrane of rat hepatocytes, and the regulated expression of the water channel AQP8 at the hepatocellular canalicular membrane domain[140-142]. AQP8 is suggested to play a role in bile formation, facilitating the osmotic movement of water under choleretic stimulus[140,142]. AQP isoforms that mediate polarized water transport in human hepatocytes, if any, remain to be identified.
Gallbladder
The bile formed outside the digestive periods enters the gallbladder that performs two important functions: concentration of bile and its storage up to the evacuation into the duodenum. Minor quantities of bile acids (about 1.3%) are absorbed in the gallbladder walls. Micelles and vesicles enter the gallbladder where the concentration of lipids is increased by water absorption, which causes some physicochemical changes in the bile (Figure 8).
Figure 8.
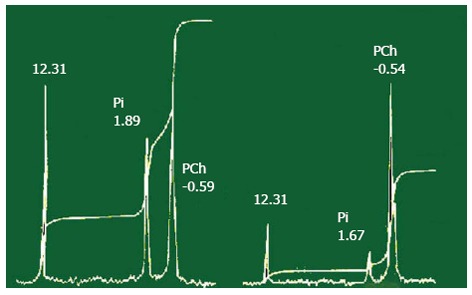
31P-NMR spectroscopy hepatic (left part of figure) and gallbladder (right part of figure) bile. Signals: 12,31 (standard); inorganic phosphate (Pi); phosphatidilcholine (PCh). PCh signal of gallbladder bile is increased with respect to such signals from hepatic bile. Signal of standard and inorganic phosphate of gallbladder bile are decreased with respect to such signals of hepatic bile. The concentration of lipids is increased by water absorption by gallbladder mucosa.
If the gallbladder functions well due to its contraction, all agglomerated vesicles and micelles with bile flow reach the duodenum. The gallbladder mucosa actively absorbs amino acids (this has been established by 35S-labelled methionine) and an albumin bile protein fraction[143].
Thus, the gallbladder takes an active part in changing the composition of bile via reabsorption of its components into the blood and making the bile components circulate along the small circuit: liver-gallbladder-blood-liver.
The flow of bile is lowest during fasting, and a majority of that is diverted into the gallbladder for concentration. When chyme from an ingested meal enters the small intestine, acid and partially digested fats and proteins stimulate secretion of cholecystokinin and secretin. These enteric hormones have important effects on pancreatic exocrine secretion. They both are also important for secretion and flow of bile.
Cholecystokinin: The name of this hormone describes its effect on the biliary system-cholecysto = gallbladder and kinin = movement. The most potent stimulus for release of cholecystokinin is the presence of fat in the duodenum. Once released, it stimulates contractions of the gallbladder and common bile duct, resulting in delivery of bile into the gut.
Secretin: This hormone is secreted in response to acid in the duodenum. Its effect on the biliary system is similar to that seen in the pancreas: it simulates biliary duct cells to secrete HCO3- and water, which expands the volume of bile and increases its flow into the intestine.
Sphincter of Oddi
The sphincter of Oddi is located at the interface of the common bile duct and the main pancreatic duct at their confluence into the duodenum. Anatomical and immunohistochemical studies have indicated that the sphincter of Oddi is richly innervated with cholinergic, adrenergic, and peptidergic neurons.
The location of the sphincter of Oddi determines its function: to regulate the entry of bile and pancreatic juice into the duodenum and to prevent reflux of duodenal contents into the common bile duct and the main pancreatic duct. The sphincter coordinates the time and rate of secretion of about 3 L/d of bile and pancreatic juice into the duodenum[144]. Normally, the sphincter is characterized by considerable phase contractions throughout the interdigestive period.
Transport of bile acids along the portal venous system
Bile acid salts come from the intestine into the portal venous system. Bile acids with venous blood enter the liver where they are apparently completely (99%) absorbed by hepatocytes. And only a small quantity (about 1%) of bile acids enters the peripheral blood. In this connection, in healthy individuals the blood concentration of circulating bile acid salts is small.
In the hepatocytes, the secondary bile acids, deoxycholic and lithocholic, are subject to hydroxylation and they conjugate with glycine or taurine. Bile acids come as conjugates from the liver into the bile again. The normal enterohepatic circulation of bile acids occurs 2-6 times daily depending upon the dietary regimen.
It has been shown that the normal kidneys are not involved in the excretion of bile acids from the body. Portal venous blood bile acids are bound by plasma proteins, mainly albumins[145] and, to a lesser extent, by α-and β-globulins. The data available suggest binding of blood bile salts to lipoproteins[146,147]. The bile acids are loosely bound to lipoproteins. In healthy individuals, bile acids are detectable in all classes of lipoproteins[147].
METABOLISM OF BILE PIGMENTS
Bilirubin is a principal bile pigment. Bilirubin is formed in the cells of the reticuloendothelial system. Unconjugated bilirubin is a product of heme catabolism (Figure 9). Degradation of heme involves its conversion to biliverdin followed by reduction of biliverdin to bilirubin[148,149]. Two different enzyme systems are involved in the production of bilirubin from heme: (1) microsomal heme oxygenase[150-152]; and (2) cytosolic biliverdin reductase[153,154].
Figure 9.
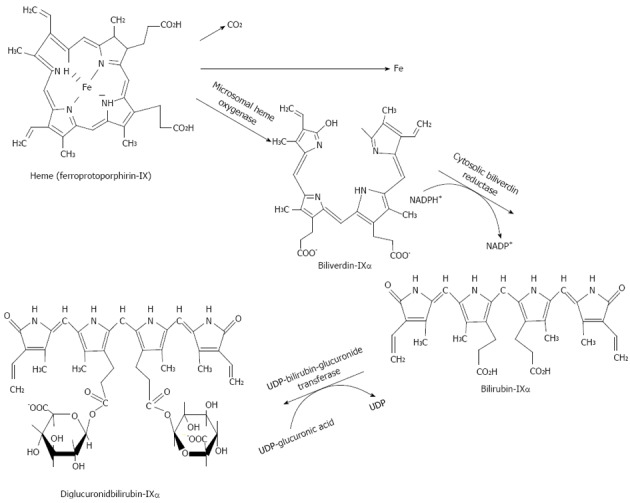
Formation of IXα bilirubin from heme.
The heme oxygenase activity is detectable in all body tissues. However, the highest activity of this enzyme is found in the spleen[150]. Heme oxygenase has an evident stereospecificity for the α-meso-bridge of a heme molecule, resulting in the formation of primarily IX-α isomers of biliverdin in man.
Cytosolic biliverdin reductase is also present in all body tissues; its activity is most pronounced in the spleen, liver, and kidney.
A gram of hemoglobin produces 36.2 mg of bilirubin[151]. In an adult, the daily bilirubin production determined by different methods is 3.9 ± 0.7 mg/kg (or 250-350 mg/d) - when determined with labeled bilirubin; and 4.4 ± 0.7 mg/kg established from СО2 formation when heme is converted to bilirubin[149,154]. Normally, a human being generates bilirubin IX-α and a minor quantity of its structural isomers: bilirubins IX-β, IX-γ or IX-δ.
Bilirubin is liberated from the endothelial tissue cells to plasma, binds to albumin, and is delivered to the hepatocytes and taken up by liver cells in a form dissociated from albumin. Bilirubin transport across the basolateral part of a membrane within the hepatocyte is accomplished by transport systems such as organic anions transport protein[155], via the flip-flop mechanism[156], or by simple diffusion due to chemical concentration gradient. The uptake of bilirubin is highly effective because of its rapid hepatic metabolism and excretion into the bile and of the presence of cytosolic binding proteins, such as glutathione-S-transferase. Unconjugated bilirubin is a nonpolar (fat-soluble) substance. In the endoplasmic reticulum of hepatocytes, bilirubin is converted by microsomal UDP-glucuronyl transferase to a polar conjugate with glucuronic acid. Mono- and diglucuronide bilirubin is formed. Water-soluble conjugate of bilirubin is easily excreted into bile. Much smaller fractions of bilirubin are conjugated to sulfates, glucose, or xylose[157].
The physiological significance of conjugation of bilirubin to sugars or glucuronic acid is diminishing its toxic properties through increasing molecular hydrophilicity, which improves bilirubin transport across the membrane structures and ensures secretion into the bile. However, IXβ, IXγ, and IXδ isomers of bilirubin require no conjugation for bile secretion because they have no rigid structure as compared to the molecule of bilirubin IXα. The latter has a rigid structure due to intramolecular hydrogen bonds. Interestingly, the destruction of the bonds can improve the biliary secretion of bilirubin IXα. It has been demonstrated that UV blood irradiation results in destruction of intramolecular hydrogen bonds in bilirubin. This facilitates the diffusion of bilirubin IXα molecules across the membrane structures without conjugation with glucuronic acid or sugars.
A greater proportion of conjugated bilirubin in the bile is present in the mixed micelles containing cholesterol, phospholipids, and bile acids. Conjugated bilirubin is hydrolyzed by bacterial glucuronidases under urobilinogens. Having a nonpolar molecule, urobilinogen is well absorbed in the small intestine, and in minimal quantities in the colon. The liver and kidneys re-excrete small quantities of absorbed urobilinogen. In hepatocyte dysfunction, hepatic urobilinogen re-excretion is impaired and renal excretion is increased[151,153,154].
SECRETION OF WATER AND ELECTROLYTES INTO BILE
Formation of bile and generation of bile flow are driven by the active secretion of bile salts, lipids and electrolytes into the canalicular and bile duct lumens followed by the osmotic movement of water[158]. Thus, water has to cross rapidly into and out of the cell interior driven by osmotic forces. Bile as a fluid, results from complicated interplay of hepatocyte and cholangiocyte uptake, secretion and concentration that involves various transporters of lipids, anions, cations, and water. Considering bile is composed of > 95% water, the molecular basis and regulatory mechanisms of water transport in hepatocytes during bile formation are still under evaluation[159].
Theoretically, water can flow through the hepatocyte epithelial barrier either across tight junctions between adjacent hepatocytes (paracellular route) or across hepatocyte plasma membranes (transcellular route). The paracellular route was traditionally proposed as the major pathway for water movement.
The tight junctions play an important role in the paracellular secretion of water and the dissolved low-molecular-weight compounds of the latter. Water and electrolytes are assumed to pass from the intercellular space through the tight junctions into the bile canalicular lumen[99]. Moreover, the selectivity of electrolyte excretion is considered to be cause by the presence of a negative charge at the site of a tight junction. This charge serves also as a barrier to regurgitation of substances from the bile canaliculus into the sinusoidal space. The molecular mechanisms of paracellular permeability are currently associated with the functioning of the specific tight junction protein ZO-1. ZO-1 is phosphorylated with protein kinases, which is of importance in the molecular mechanisms responsible for the regulation of paracellular permeability[160]. Tight junctions exhibit low water permeability but allow electrolyte permeation that enables canalicular spaces to shrink below the van’t Hoff equilibrium during the osmotic maneuver[161]. Nonetheless, the experimental data supporting this view remain limited and largely indirect[162].
The cloning and functional characterization of a family of proteins that works as membrane water channels, named AQPs[163], challenged the former concepts of water transport and contributed to the better understanding of bile physiology. The discovery of the AQP water channels has clarified the mechanisms by which water, the major component of bile, moves across the hepatobiliary epithelia[164].
Direct osmotic water permeability assessment by stopped-flow spectrophotometry in canalicular and sinusoidal plasma membrane vesicles revealed the presence of both lipid (non-channel) and AQP-mediated pathways for sinusoidal and canalicular water movement[165]. The study demonstrated that the canalicular plasma membrane domain has lower water permeability than the sinusoidal membrane, and thus it is rate limiting for transcellular water transport in hepatocytes[159]. However, upon cAMP stimulus the intracellular AQP8 inserts to the canalicular domain and so this membrane becomes highly water permeable. Therefore, the transcellular pathway via water channels seems to account for most of the water entering the bile canaliculus. Hepatocytes express AQPs, a family of membrane channel proteins that facilitate the osmotically driven movement of water molecules. AQP8 is localized to canalicular membranes and modulates membrane water permeability, providing a molecular mechanism for the osmotically coupled transport of solute and water during bile formation.
There is experimental evidence suggesting that defective hepatocyte AQP8 expression leads to alterations in normal bile physiology. Thus, AQP8 protein is downregulated (and canalicular water permeability decreased), in established rat models of cholestasis, such as sepsis-associated cholestasis, estrogen-induced cholestasis, and extrahepatic obstructive cholestasis[166].
The entry of inorganic cations and anions into the hepatocytes is effected with the participation of specific ion channels and carriers, which include Na+,K+-АТPase, Na+/H+-exchanger, Na+/HCO3- cotransporter, and Na+-independent carrier of SO42-[153]. It is suggested[167] that by analogy with the mechanisms of water reabsorption from the bile in the gallbladder, water is reabsorbed by the bile duct epithelial cells as a result of Na+-coupled cotransport of Cl-. Enhanced reabsorption after removal of the gallbladder is regarded as an adaptive mechanism that provides bile concentration.
The primary mechanism of biliary copper excretion involves ATP7B-mediated vesicular sequestration of copper rather than direct copper translocation across the canalicular membrane[168].
The microfilament system is of no small importance in the canalicular secretion of bile. Bile is transported to the ducts via contraction of a canaliculus, which is associated with the activity of microfilaments. The latter play a great role in the regulation of permeability of a tight junction for water and electrolytes.
SECRETION OF PROTEINS INTO BILE
Proteins, as bile acids, are referred to as the osmotically active components of bile, which determine the rate of its secretion[99]. Albumin and the other proteins that are close to its molecular weight make a major contribution to the creation of an osmotic gradient.
Tens of different proteins with a molecular weight of 6-220 kDa are detectable in the bile[99]. The greater part of them are blood proteins; the lesser are the proteins entering the bile directly from the hepatocytes and epithelial cells of bile ducts. The experimental data[99] suggest that many plasma proteins interact with the specific receptors available on the sinusoidal membrane of hepatocytes and form a plasma protein-receptor complex that enters the liver cells via endocytosis.
The intracellular transfer of proteins from the perisinusoidal area of a hepatocyte to its pericanalicular one is mainly accomplished by targeted vesicular transport. The large proportion of proteins entering the hepatocyte from blood as a receptor-plasma protein complex generate a receptosome (endosome) and are transported in such a form to the Golgi apparatus, then to the canalicular membrane[99]. The newly synthesized proteins entering the bile canaliculi are also transported just from the hepatocytes by targeted vesicular transport. It is presumed that apolipoproteins perform in the bile the same function as lipoproteins in the serum, that is, ensure lipid transport[169].
Apoproteins are taken up from the sinusoidal space by endocytosis, transported in the hepatocytes as vesicles and released into the bile across the canalicular membrane by exocytosis[170].
In conclusion, the cholesterol that is newly synthesized in the hepatocytes serves as a substrate for the synthesis of bile acids. The synthesis of bile acids is a key point in the formation of bile. Acid-dependent and acid-independent bile fractions are identified. The structure of a hepatocyte provides a targeted transport of bile constituents from the basolateral to apical membrane of a liver cell. The transport of bile acids across the sinusoidal membrane is associated with the overcoming of a high concentration gradient and an electrical potential and it is accomplished by an active transport with the participation of carrier proteins. The vesicles are the basic form of the transport of lipids in and out of the cell itself. Bile lipids are secreted into the bile canaliculi as monolamellar vesicles. Bile acids pass across the apical part of a hepatocytic membrane independently, by ABC transporters, solubilize the phospholipids and cholesterol from the membrane surface in the bile canalicular lumen.
There is a constant transformation of vesicles to and from micelles in the bile canaliculi and ducts as a result of absorptive and secretory processes with the participation of bile acids. All the aforesaid suggests considerable progress made in the understanding of the mechanisms of bile formation and excretion, which has made possible due to the advances of fundamental investigations in cell biology, molecular biology and biochemistry.
ACKNOWLEDGMENTS
The author to express thanks to professor Gennadiy Vasilievich Saparin and Peter Valentinovich Ivannikov for receiving bile image by CCL SEM. The author expresses his gratitude to professor Vladimir Mitrofanovich Pisarev for assistance and advice in preparing the article.
Footnotes
P- Reviewer: Vasiliy IR S- Editor: Cui XM L- Editor: Kerr C E- Editor: Zhang DN
References
- 1.Crawford JM, Möckel GM, Crawford AR, Hagen SJ, Hatch VC, Barnes S, Godleski JJ, Carey MC. Imaging biliary lipid secretion in the rat: ultrastructural evidence for vesiculation of the hepatocyte canalicular membrane. J Lipid Res. 1995;36:2147–2163. [PubMed] [Google Scholar]
- 2.Esteller A. Physiology of bile secretion. World J Gastroenterol. 2008;14:5641–5649. doi: 10.3748/wjg.14.5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poupon R. [Molecular mechanisms of bile formation and cholestatic diseases] Bull Acad Natl Med. 2003;187:1261–1274; discussion 1261-1724. [PubMed] [Google Scholar]
- 4.Pavlov IP. Lectures on Physiology 1912-1913. I. Physiology of digestion. In: Kupalov PS, editor. Lecture 23: Procedure for obtaining bile. Value of bile in a digestive process. Razenkov IP, editor. Moscow: Izdatelstvo Akademii Meditsinskikh Nauk USSR; 1949. [Google Scholar]
- 5.Arrese M, Trauner M. Molecular aspects of bile formation and cholestasis. Trends Mol Med. 2003;9:558–564. doi: 10.1016/j.molmed.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Kubitz R, Häussinger D. Osmoregulation of bile formation. Methods Enzymol. 2007;428:313–324. doi: 10.1016/S0076-6879(07)28018-8. [DOI] [PubMed] [Google Scholar]
- 7.Alvaro D, Cantafora A, Attili AF, Ginanni Corradini S, De Luca C, Minervini G, Di Biase A, Angelico M. Relationships between bile salts hydrophilicity and phospholipid composition in bile of various animal species. Comp Biochem Physiol B. 1986;83:551–554. doi: 10.1016/0305-0491(86)90295-6. [DOI] [PubMed] [Google Scholar]
- 8.Loginov AS, Chebanov SM, Saparin GV, Obyden SK. The morphology and composition of cholesterol, protein, and bilirubin deposits in dried human bile: cathodoluminescence and backscattered electron imaging. Scanning. 1998;20:442–446. doi: 10.1002/sca.1998.4950200604. [DOI] [PubMed] [Google Scholar]
- 9.Fu D, Lippincott-Schwartz J, Arias IM. Cellular mechanism of bile acid-accelerated hepatocyte polarity. Small GTPases. 2011;2:314–317. doi: 10.4161/sgtp.18087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bryant DM, Mostov KE. From cells to organs: building polarized tissue. Nat Rev Mol Cell Biol. 2008;9:887–901. doi: 10.1038/nrm2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kojima T, Yamamoto T, Murata M, Chiba H, Kokai Y, Sawada N. Regulation of the blood-biliary barrier: interaction between gap and tight junctions in hepatocytes. Med Electron Microsc. 2003;36:157–164. doi: 10.1007/s00795-003-0220-5. [DOI] [PubMed] [Google Scholar]
- 12.Meier PJ. Transport polarity of hepatocytes. Semin Liver Dis. 1988;8:293–307. doi: 10.1055/s-2008-1040551. [DOI] [PubMed] [Google Scholar]
- 13.Ballatori N, Clarkson TW. Biliary secretion of glutathione and of glutathione-metal complexes. Fundam Appl Toxicol. 1985;5:816–831. doi: 10.1016/0272-0590(85)90165-4. [DOI] [PubMed] [Google Scholar]
- 14.Keppler D. Export pumps for glutathione S-conjugates. Free Radiac Biol Med. 1999;27:985–991. doi: 10.1016/s0891-5849(99)00171-9. [DOI] [PubMed] [Google Scholar]
- 15.Tsukada N, Ackerley CA, Phillips MJ. The structure and organization of the bile canalicular cytoskeleton with special reference to actin and actin-binding proteins. Hepatology. 1995;21:1106–1113. [PubMed] [Google Scholar]
- 16.Vasudevan DM, Sreekumari S, Vaidyanathan K. Textbook of Biochemistry for Medical Students. Chapter 13. Cholesterol and lipoproteins. 7th ed. New Delhi: Jaypee brothers medical publishers (P) Ltd; 2013. pp. 169–183. [Google Scholar]
- 17.Partington JR. A History of Chemistry, Vol. IV. London: Macmillan and Co.,Ltd; 1964. p. 335. [Google Scholar]
- 18.Marry R, Grenner D, Mayes P, Roduell V. Human biochemistry. Moscow: Mir Publishers; 1993. pp. 281–284. [Google Scholar]
- 19.Nilsson LM, Sjövall J, Strom S, Bodin K, Nowak G, Einarsson C, Ellis E. Ethanol stimulates bile acid formation in primary human hepatocytes. Biochem Biophys Res Commun. 2007;364:743–747. doi: 10.1016/j.bbrc.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 20.Chiang JY. Bile acids: regulation of synthesis. J Lipid Res. 2009;50:1955–1966. doi: 10.1194/jlr.R900010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith LP, Nierstenhoefer M, Yoo SW, Penzias AS, Tobiasch E, Usheva A. The Bile Acid Synthesis Pathway Is Present and Functional in the Human Ovary. PLoS One. 2009;4:e7333. doi: 10.1371/journal.pone.0007333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferdinandusse S, Houten SM. Peroxisomes and bile acid biosynthesis. Biochim Biophys Acta. 2006;1763:1427–1440. doi: 10.1016/j.bbamcr.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137–174. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- 24.Carey MC. Pathogenesis of gallstones. Am J Surg. 1993;165:410–419. doi: 10.1016/s0002-9610(05)80932-8. [DOI] [PubMed] [Google Scholar]
- 25.Li T, Jahan A, Chiang JY. Bile acids and cytokines inhibit the human cholesterol 7 alpha-hydroxylase gene via the JNK/c-jun pathway in human liver cells. Hepatology. 2006;43:1202–1210. doi: 10.1002/hep.21183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li T, Kong X, Owsley E, Ellis E, Strom S, Chiang JY. Insulin regulation of cholesterol 7alpha-hydroxylase expression in human hepatocytes: roles of forkhead box O1 and sterol regulatory element-binding protein 1c. J Biol Chem. 2006;281:28745–28754. doi: 10.1074/jbc.M605815200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song KH, Chiang JY. Glucagon and cAMP inhibit cholesterol 7alpha-hydroxylase (CYP7A1) gene expression in human hepatocytes: discordant regulation of bile acid synthesis and gluconeogenesis. Hepatology. 2006;43:117–125. doi: 10.1002/hep.20919. [DOI] [PubMed] [Google Scholar]
- 28.Song KH, Ellis E, Strom S, Chiang JY. Hepatocyte growth factor signaling pathway inhibits cholesterol 7alpha-hydroxylase and bile acid synthesis in human hepatocytes. Hepatology. 2007;46:1993–2002. doi: 10.1002/hep.21878. [DOI] [PubMed] [Google Scholar]
- 29.Song KH, Li T, Owsley E, Strom S, Chiang JY. Bile acids activate fibroblast growth factor 19 signaling in human hepatocytes to inhibit cholesterol 7alpha-hydroxylase gene expression. Hepatology. 2009;49:297–305. doi: 10.1002/hep.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bove KE, Heubi JE, Balistreri WF, Setchell KD. Bile acid synthetic defects and liver disease: a comprehensive review. Pediatr Dev Pathol. 2004;7:315–334. doi: 10.1007/s10024-002-1201-8. [DOI] [PubMed] [Google Scholar]
- 31.Chiang JY. Bile acid regulation of gene expression: roles of nuclear hormone receptors. Endocr Rev. 2002;23:443–463. doi: 10.1210/er.2000-0035. [DOI] [PubMed] [Google Scholar]
- 32.Chiang JY. Regulation of bile acid synthesis: pathways, nuclear receptors, and mechanisms. J Hepatol. 2004;40:539–551. doi: 10.1016/j.jhep.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Chiang JY, Kimmel R, Weinberger C, Stroup D. Farnesoid X receptor responds to bile acids and represses cholesterol 7alpha-hydroxylase gene (CYP7A1) transcription. J Biol Chem. 2000;275:10918–10924. doi: 10.1074/jbc.275.15.10918. [DOI] [PubMed] [Google Scholar]
- 34.Yang Y, Zhang M, Eggertsen G, Chiang JY. On the mechanism of bile acid inhibition of rat sterol 12alpha-hydroxylase gene (CYP8B1) transcription: roles of alpha-fetoprotein transcription factor and hepatocyte nuclear factor 4alpha. Biochim Biophys Acta. 2002;1583:63–73. doi: 10.1016/s1388-1981(02)00186-5. [DOI] [PubMed] [Google Scholar]
- 35.Chen W, Chiang JY. Regulation of human sterol 27-hydroxylase gene (CYP27A1) by bile acids and hepatocyte nuclear factor 4alpha (HNF4alpha) Gene. 2003;313:71–82. doi: 10.1016/s0378-1119(03)00631-0. [DOI] [PubMed] [Google Scholar]
- 36.Hunt MC, Yang YZ, Eggertsen G, Carneheim CM, Gåfvels M, Einarsson C, Alexson SE. The peroxisome proliferator-activated receptor alpha (PPARalpha) regulates bile acid biosynthesis. J Biol Chem. 2000;275:28947–28953. doi: 10.1074/jbc.M002782200. [DOI] [PubMed] [Google Scholar]
- 37.Pineda Torra I, Claudel T, Duval C, Kosykh V, Fruchart JC, Staels B. Bile acids induce the expression of the human peroxisome proliferator-activated receptor alpha gene via activation of the farnesoid X receptor. Mol Endocrinol. 2003;17:259–272. doi: 10.1210/me.2002-0120. [DOI] [PubMed] [Google Scholar]
- 38.Shefer S, Salen G, Nguyen L, Batta AK, Packin V, Tint GS, Hauser S. Competitive inhibition of bile acid synthesis by endogenous cholestanol and sitosterol in sitosterolemia with xanthomatosis. Effect on cholesterol 7 alpha-hydroxylase. J Clin Invest. 1988;82:1833–1839. doi: 10.1172/JCI113799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gälman C, Angelin B, Rudling M. Bile acid synthesis in humans has a rapid diurnal variation that is asynchronous with cholesterol synthesis. Gastroenterology. 2005;129:1445–1453. doi: 10.1053/j.gastro.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 40.Dietschy JM, Turley SD, Spady DK. Role of liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. J Lipid Res. 1993;34:1637–1659. [PubMed] [Google Scholar]
- 41.Dietschy JM, Turley SD. Control of cholesterol turnover in the mouse. J Biol Chem. 2002;277:3801–3804. doi: 10.1074/jbc.R100057200. [DOI] [PubMed] [Google Scholar]
- 42.Small DM, Rapo S. Source of abnormal bile in patients with cholesterol gallstones. N Engl J Med. 1970;283:53–57. doi: 10.1056/NEJM197007092830201. [DOI] [PubMed] [Google Scholar]
- 43.Gu JJ, Hofmann AF, Ton-Nu HT, Schteingart CD, Mysels KJ. Solubility of calcium salts of unconjugated and conjugated natural bile acids. J Lipid Res. 1992;33:635–646. [PubMed] [Google Scholar]
- 44.Marcinkiewicz J, Kontny E. Taurine and inflammatory diseases. Amino Acids. 2012:Epub ahead of print. doi: 10.1007/s00726-012-1361-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen W, Guo JX, Chang P. The effect of taurine on cholesterol metabolism. Mol Nutr Food Res. 2012;56:681–690. doi: 10.1002/mnfr.201100799. [DOI] [PubMed] [Google Scholar]
- 46.Inoue Y, Yu AM, Inoue J, Gonzalez FJ. Hepatocyte nuclear factor 4alpha is a central regulator of bile acid conjugation. J Biol Chem. 2004;279:2480–2489. doi: 10.1074/jbc.M311015200. [DOI] [PubMed] [Google Scholar]
- 47.Jansen PL, Mulder GJ, Burchell B, Bock KW. New developments in glucuronidation research: report of a workshop on “glucuronidation, its role in health and disease”. Hepatology. 1992;15:532–544. doi: 10.1002/hep.1840150328. [DOI] [PubMed] [Google Scholar]
- 48.Stiehl A. Disturbances of bile acid metabolism in cholestasis. Clin Gastroenterol. 1977;6:45–67. [PubMed] [Google Scholar]
- 49.Yousef I, Mignault D, Tuchweber B. Effect of complete sulfation of bile acids on bile formation: role of conjugation and number of sulfate groups. Hepatology. 1992;15:438–445. doi: 10.1002/hep.1840150314. [DOI] [PubMed] [Google Scholar]
- 50.Hofmann AF. Detoxification of lithocholic acid, a toxic bile acid: relevance to drug hepatotoxicity. Drug Metab Rev. 2004;36:703–722. doi: 10.1081/dmr-200033475. [DOI] [PubMed] [Google Scholar]
- 51.Hofmann AF, Hagey LR. Bile acids: chemistry, pathochemistry, biology, pathobiology, and therapeutics. Cell Mol Life Sci. 2008;65:2461–2483. doi: 10.1007/s00018-008-7568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Monte MJ, Marin JJ, Antelo A, Vazquez-Tato J. Bile acids: chemistry, physiology, and pathophysiology. World J Gastroenterol. 2009;15:804–816. doi: 10.3748/wjg.15.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kawamoto T, Okano G, Akino T. Biosynthesis and turnover of individual molecular species of phosphatidylcholine in liver and bile. Biochim Biophys Acta. 1980;619:20–34. [PubMed] [Google Scholar]
- 54.Carey MC, Lamont JT. Cholesterol gallstone formation. 1. Physical-chemistry of bile and biliary lipid secretion. Prog Liver Dis. 1992;10:139–163. [PubMed] [Google Scholar]
- 55.Ivanchenkova RA. Neurohumoral regulation of bile production and excretion processes. Klinicheskaya Meditsina. 1986;64:24–32. [PubMed] [Google Scholar]
- 56.Nilsson S, Scherstén T. Importance of bile acids for phospholipid secretion into human hepatic bile. Gastroenterology. 1969;57:525–532. [PubMed] [Google Scholar]
- 57.Groen AK, Oude Elferink RP. Lipid transport into bile and role in bile formation. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:131–135. doi: 10.2174/1568008054064887. [DOI] [PubMed] [Google Scholar]
- 58.Crawford AR, Smith AJ, Hatch VC, Oude Elferink RP, Borst P, Crawford JM. Hepatic secretion of phospholipid vesicles in the mouse critically depends on mdr2 or MDR3 P-glycoprotein expression. Visualization by electron microscopy. J Clin Invest. 1997;100:2562–2567. doi: 10.1172/JCI119799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patton GM, Fasulo JM, Robins SJ. Hepatic phosphatidylcholines: evidence for synthesis in the rat by extensive reutilization of endogenous acylglycerides. J Lipid Res. 1994;35:1211–1221. [PubMed] [Google Scholar]
- 60.Cohen DE, Leonard MR, Carey MC. In vitro evidence that phospholipid secretion into bile may be coordinated intracellularly by the combined actions of bile salts and the specific phosphatidylcholine transfer protein of liver. Biochemistry. 1994;33:9975–9980. doi: 10.1021/bi00199a021. [DOI] [PubMed] [Google Scholar]
- 61.Berr F, Meier PJ, Stieger B. Evidence for the presence of a phosphatidylcholine translocator in isolated rat liver canalicular plasma membrane vesicles. J Biol Chem. 1993;268:3976–3979. [PubMed] [Google Scholar]
- 62.Crawford JM. Role of vesicle-mediated transport pathways in hepatocellular bile secretion. Semin Liver Dis. 1996;16:169–189. doi: 10.1055/s-2007-1007230. [DOI] [PubMed] [Google Scholar]
- 63.Elferink RP, Tytgat GN, Groen AK. Hepatic canalicular membrane 1: The role of mdr2 P-glycoprotein in hepatobiliary lipid transport. FASEB J. 1997;11:19–28. doi: 10.1096/fasebj.11.1.9034162. [DOI] [PubMed] [Google Scholar]
- 64.van de Heijning BJ, Stolk MF, van Erpecum KJ, Renooij W, Groen AK, vanBerge-Henegouwen GP. Bile salt-induced cholesterol crystal formation from model bile vesicles: a time course study. J Lipid Res. 1994;35:1002–1011. [PubMed] [Google Scholar]
- 65.Carey MC, Cahalane MJ. Whither biliary sludge? Gastroenterology. 1988;95:508–523. doi: 10.1016/0016-5085(88)90513-6. [DOI] [PubMed] [Google Scholar]
- 66.Möckel GM, Gorti S, Tandon RK, Tanaka T, Carey MC. Microscope laser light-scattering spectroscopy of vesicles within canaliculi of rat hepatocyte couplets. Am J Physiol. 1995;269:G73–G84. doi: 10.1152/ajpgi.1995.269.1.G73. [DOI] [PubMed] [Google Scholar]
- 67.Paumgartner G, Sauerbruch T. Secretion, composition and flow of bile. Clin Gastroenterol. 1983;12:3–23. [PubMed] [Google Scholar]
- 68.Sperber I. Secretion of organic anions in the formation of urine and bile. Pharmacol Rev. 1959;11:109–134. [PubMed] [Google Scholar]
- 69.Gumucio JJ, Miller DL. Functional implications of liver cell heterogeneity. Gastroenterology. 1981;80:393–403. [PubMed] [Google Scholar]
- 70.Anwer MS, Hegner D. Role of inorganic electrolytes in bile acid-independent canalicular bile formation. Am J Physiol. 1983;244:G116–G124. doi: 10.1152/ajpgi.1983.244.2.G116. [DOI] [PubMed] [Google Scholar]
- 71.Dawson PA, Lan T, Rao A. Bile acid transporters. J Lipid Res. 2009;50:2340–2357. doi: 10.1194/jlr.R900012-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Krag E, Phillips SF. Active and passive bile acid absorption in man. Perfusion studies of the ileum and jejunum. J Clin Invest. 1974;53:1686–1694. doi: 10.1172/JCI107720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schiff ER, Small NC, Dietschy JM. Characterization of the kinetics of the passive and active transport mechanisms for bile acid absorption in the small intestine and colon of the rat. J Clin Invest. 1972;51:1351–1362. doi: 10.1172/JCI106931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dawson PA, Hubbert M, Haywood J, Craddock AL, Zerangue N, Christian WV, Ballatori N. The heteromeric organic solute transporter alpha-beta, Ostalpha-Ostbeta, is an ileal basolateral bile acid transporter. J Biol Chem. 2005;280:6960–6968. doi: 10.1074/jbc.M412752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ballatori N, Christian WV, Lee JY, Dawson PA, Soroka CJ, Boyer JL, Madejczyk MS, Li N. OSTalpha-OSTbeta: a major basolateral bile acid and steroid transporter in human intestinal, renal, and biliary epithelia. Hepatology. 2005;42:1270–1279. doi: 10.1002/hep.20961. [DOI] [PubMed] [Google Scholar]
- 76.Hofmann AF, Schteingart CD, Lillienau J. Biological and medical aspects of active ileal transport of bile acids. Ann Med. 1991;23:169–175. doi: 10.3109/07853899109148043. [DOI] [PubMed] [Google Scholar]
- 77.Denson LA, Sturm E, Echevarria W, Zimmerman TL, Makishima M, Mangelsdorf DJ, Karpen SJ. The orphan nuclear receptor, shp, mediates bile acid-induced inhibition of the rat bile acid transporter, ntcp. Gastroenterology. 2001;121:140–147. doi: 10.1053/gast.2001.25503. [DOI] [PubMed] [Google Scholar]
- 78.Meier PJ. The bile salt secretory polarity of hepatocytes. J Hepatol. 1989;9:124–129. doi: 10.1016/0168-8278(89)90085-8. [DOI] [PubMed] [Google Scholar]
- 79.Shaffer EA. Cholestasis: the ABCs of cellular mechanisms for impaired bile secretion--transporters and genes. Can J Gastroenterol. 2002;16:380–389. doi: 10.1155/2002/842151. [DOI] [PubMed] [Google Scholar]
- 80.Alrefai WA, Gill RK. Bile acid transporters: structure, function, regulation and pathophysiological implications. Pharm Res. 2007;24:1803–1823. doi: 10.1007/s11095-007-9289-1. [DOI] [PubMed] [Google Scholar]
- 81.Trauner M, Boyer JL. Bile salt transporters: molecular characterization, function, and regulation. Physiol Rev. 2003;83:633–671. doi: 10.1152/physrev.00027.2002. [DOI] [PubMed] [Google Scholar]
- 82.Lam P, Soroka CJ, Boyer JL. The bile salt export pump: clinical and experimental aspects of genetic and acquired cholestatic liver disease. Semin Liver Dis. 2010;30:125–133. doi: 10.1055/s-0030-1253222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roma MG, Crocenzi FA, Mottino AD. Dynamic localization of hepatocellular transporters in health and disease. World J Gastroenterol. 2008;14:6786–6801. doi: 10.3748/wjg.14.6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reichen J. The Role of the Sinusoidal Endothelium in Liver Function. News Physiol Sci. 1999;14:117–121. doi: 10.1152/physiologyonline.1999.14.3.117. [DOI] [PubMed] [Google Scholar]
- 85.Frimmer M, Ziegler K. The transport of bile acids in liver cells. Biochim Biophys Acta. 1988;947:75–99. doi: 10.1016/0304-4157(88)90020-2. [DOI] [PubMed] [Google Scholar]
- 86.Hagenbuch B, Meier PJ. Sinusoidal (basolateral) bile salt uptake systems of hepatocytes. Semin Liver Dis. 1996;16:129–136. doi: 10.1055/s-2007-1007226. [DOI] [PubMed] [Google Scholar]
- 87.Hofmann AF. Biliary secretion and excretion in health and disease: current concepts. Ann Hepatol. 2007;6:15–27. [PubMed] [Google Scholar]
- 88.Erlinger S. Bile flow. In: Arias IM, Popper H, Jacoby WB, Schachter D, Shafritz DA, et al., editors. The Liver: Biology and Pathobiology. New York: Raven Press; 1988. pp. 643–664. [Google Scholar]
- 89.Meier PJ, Eckhardt U, Schroeder A, Hagenbuch B, Stieger B. Substrate specificity of sinusoidal bile acid and organic anion uptake systems in rat and human liver. Hepatology. 1997;26:1667–1677. doi: 10.1002/hep.510260641. [DOI] [PubMed] [Google Scholar]
- 90.Meier PJ, Stieger B. Bile salt transporters. Annu Rev Physiol. 2002;64:635–661. doi: 10.1146/annurev.physiol.64.082201.100300. [DOI] [PubMed] [Google Scholar]
- 91.Arrese M, Ananthananarayanan M, Suchy FJ. Hepatobiliary transport: molecular mechanisms of development and cholestasis. Pediatr Res. 1998;44:141–147. doi: 10.1203/00006450-199808000-00001. [DOI] [PubMed] [Google Scholar]
- 92.Bohan A, Boyer JL. Mechanisms of hepatic transport of drugs: implications for cholestatic drug reactions. Semin Liver Dis. 2002;22:123–136. doi: 10.1055/s-2002-30099. [DOI] [PubMed] [Google Scholar]
- 93.Hagenbuch B, Meier PJ. Molecular cloning, chromosomal localization, and functional characterization of a human liver Na+/bile acid cotransporter. J Clin Invest. 1994;93:1326–1331. doi: 10.1172/JCI117091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hagenbuch B, Dawson P. The sodium bile salt cotransport family SLC10. Pflugers Arch. 2004;447:566–570. doi: 10.1007/s00424-003-1130-z. [DOI] [PubMed] [Google Scholar]
- 95.Geyer J, Wilke T, Petzinger E. The solute carrier family SLC10: more than a family of bile acid transporters regarding function and phylogenetic relationships. Naunyn Schmiedebergs Arch Pharmacol. 2006;372:413–431. doi: 10.1007/s00210-006-0043-8. [DOI] [PubMed] [Google Scholar]
- 96.Godoy JR, Fernandes C, Döring B, Beuerlein K, Petzinger E, Geyer J. Molecular and phylogenetic characterization of a novel putative membrane transporter (SLC10A7), conserved in vertebrates and bacteria. Eur J Cell Biol. 2007;86:445–460. doi: 10.1016/j.ejcb.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 97.Blitzer BL, Boyer JL. Cytochemical localization of Na+, K+-ATPase in the rat hepatocyte. J Clin Invest. 1978;62:1104–1108. doi: 10.1172/JCI109216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Latham PS, Kashgarian M. The ultrastructural localization of transport ATPase in the rat liver at non-bile canalicular plasma membranes. Gastroenterology. 1979;76:988–996. [PubMed] [Google Scholar]
- 99.Blitzer BL, Boyer JL. Cellular mechanisms of bile formation. Gastroenterology. 1982;82:346–357. [PubMed] [Google Scholar]
- 100.Sztul ES, Biemesderfer D, Caplan MJ, Kashgarian M, Boyer JL. Localization of Na+,K+-ATPase alpha-subunit to the sinusoidal and lateral but not canalicular membranes of rat hepatocytes. J Cell Biol. 1987;104:1239–1248. doi: 10.1083/jcb.104.5.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Boyer JL, Ng OC, Ananthanarayanan M, Hofmann AF, Schteingart CD, Hagenbuch B, Stieger B, Meier PJ. Expression and characterization of a functional rat liver Na+ bile acid cotransport system in COS-7 cells. Am J Physiol. 1994;266:G382–G387. doi: 10.1152/ajpgi.1994.266.3.G382. [DOI] [PubMed] [Google Scholar]
- 102.Hata S, Wang P, Eftychiou N, Ananthanarayanan M, Batta A, Salen G, Pang KS, Wolkoff AW. Substrate specificities of rat oatp1 and ntcp: implications for hepatic organic anion uptake. Am J Physiol Gastrointest Liver Physiol. 2003;285:G829–G839. doi: 10.1152/ajpgi.00352.2002. [DOI] [PubMed] [Google Scholar]
- 103.Kramer W, Stengelin S, Baringhaus KH, Enhsen A, Heuer H, Becker W, Corsiero D, Girbig F, Noll R, Weyland C. Substrate specificity of the ileal and the hepatic Na(+)/bile acid cotransporters of the rabbit. I. Transport studies with membrane vesicles and cell lines expressing the cloned transporters. J Lipid Res. 1999;40:1604–1617. [PubMed] [Google Scholar]
- 104.Platte HD, Honscha W, Schuh K, Petzinger E. Functional characterization of the hepatic sodium-dependent taurocholate transporter stably transfected into an immortalized liver-derived cell line and V79 fibroblasts. Eur J Cell Biol. 1996;70:54–60. [PubMed] [Google Scholar]
- 105.Mita S, Suzuki H, Akita H, Stieger B, Meier PJ, Hofmann AF, Sugiyama Y. Vectorial transport of bile salts across MDCK cells expressing both rat Na+-taurocholate cotransporting polypeptide and rat bile salt export pump. Am J Physiol Gastrointest Liver Physiol. 2005;288:G159–G167. doi: 10.1152/ajpgi.00360.2003. [DOI] [PubMed] [Google Scholar]
- 106.Mita S, Suzuki H, Akita H, Hayashi H, Onuki R, Hofmann AF, Sugiyama Y. Vectorial transport of unconjugated and conjugated bile salts by monolayers of LLC-PK1 cells doubly transfected with human NTCP and BSEP or with rat Ntcp and Bsep. Am J Physiol Gastrointest Liver Physiol. 2006;290:G550–G556. doi: 10.1152/ajpgi.00364.2005. [DOI] [PubMed] [Google Scholar]
- 107.Craddock AL, Love MW, Daniel RW, Kirby LC, Walters HC, Wong MH, Dawson PA. Expression and transport properties of the human ileal and renal sodium-dependent bile acid transporter. Am J Physiol. 1998;274:G157–G169. doi: 10.1152/ajpgi.1998.274.1.G157. [DOI] [PubMed] [Google Scholar]
- 108.Pellicoro A, Faber KN. Review article: The function and regulation of proteins involved in bile salt biosynthesis and transport. Aliment Pharmacol Ther. 2007;26 Suppl 2:149–160. doi: 10.1111/j.1365-2036.2007.03522.x. [DOI] [PubMed] [Google Scholar]
- 109.Kullak-Ublick GA, Hagenbuch B, Stieger B, Schteingart CD, Hofmann AF, Wolkoff AW, Meier PJ. Molecular and functional characterization of an organic anion transporting polypeptide cloned from human liver. Gastroenterology. 1995;109:1274–1282. doi: 10.1016/0016-5085(95)90588-x. [DOI] [PubMed] [Google Scholar]
- 110.Meng LJ, Wang P, Wolkoff AW, Kim RB, Tirona RG, Hofmann AF, Pang KS. Transport of the sulfated, amidated bile acid, sulfolithocholyltaurine, into rat hepatocytes is mediated by Oatp1 and Oatp2. Hepatology. 2002;35:1031–1040. doi: 10.1053/jhep.2002.32667. [DOI] [PubMed] [Google Scholar]
- 111.Ballatori N. Biology of a novel organic solute and steroid transporter, OSTalpha-OSTbeta. Exp Biol Med (Maywood) 2005;230:689–698. doi: 10.1177/153537020523001001. [DOI] [PubMed] [Google Scholar]
- 112.van Montfoort JE, Müller M, Groothuis GM, Meijer DK, Koepsell H, Meier PJ. Comparison of “type I” and “type II” organic cation transport by organic cation transporters and organic anion-transporting polypeptides. J Pharmacol Exp Ther. 2001;298:110–115. [PubMed] [Google Scholar]
- 113.Müller M, Jansen PL. Molecular aspects of hepatobiliary transport. Am J Physiol. 1997;272:G1285–G1303. doi: 10.1152/ajpgi.1997.272.6.G1285. [DOI] [PubMed] [Google Scholar]
- 114.Ito K, Suzuki H, Horie T, Sugiyama Y. Apical/basolateral surface expression of drug transporters and its role in vectorial drug transport. Pharm Res. 2005;22:1559–1577. doi: 10.1007/s11095-005-6810-2. [DOI] [PubMed] [Google Scholar]
- 115.Niemi M. Role of OATP transporters in the disposition of drugs. Pharmacogenomics. 2007;8:787–802. doi: 10.2217/14622416.8.7.787. [DOI] [PubMed] [Google Scholar]
- 116.Kaplowitz N. Physiological significance of glutathione S-transferases. Am J Physiol. 1980;239:G439–G444. doi: 10.1152/ajpgi.1980.239.6.G439. [DOI] [PubMed] [Google Scholar]
- 117.Crawford JM, Berken CA, Gollan JL. Role of the hepatocyte microtubular system in the excretion of bile salts and biliary lipid: implications for intracellular vesicular transport. J Lipid Res. 1988;29:144–156. [PubMed] [Google Scholar]
- 118.Lamri Y, Roda A, Dumont M, Feldmann G, Erlinger S. Immunoperoxidase localization of bile salts in rat liver cells. Evidence for a role of the Golgi apparatus in bile salt transport. J Clin Invest. 1988;82:1173–1182. doi: 10.1172/JCI113714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Crawford JM, Vinter DW, Gollan JL. Taurocholate induces pericanalicular localization of C6-NBD-ceramide in isolated hepatocyte couplets. Am J Physiol. 1991;260:G119–G132. doi: 10.1152/ajpgi.1991.260.1.G119. [DOI] [PubMed] [Google Scholar]
- 120.Dubin M, Maurice M, Feldmann G, Erlinger S. Influence of colchicine and phalloidin on bile secretion and hepatic ultrastructure in the rat. Possible interaction between microtubules and microfilaments. Gastroenterology. 1980;79:646–654. [PubMed] [Google Scholar]
- 121.Trauner M, Meier PJ, Boyer JL. Molecular pathogenesis of cholestasis. N Engl J Med. 1998;339:1217–1227. doi: 10.1056/NEJM199810223391707. [DOI] [PubMed] [Google Scholar]
- 122.Gerloff T, Stieger B, Hagenbuch B, Madon J, Landmann L, Roth J, Hofmann AF, Meier PJ. The sister of P-glycoprotein represents the canalicular bile salt export pump of mammalian liver. J Biol Chem. 1998;273:10046–10050. doi: 10.1074/jbc.273.16.10046. [DOI] [PubMed] [Google Scholar]
- 123.Kipp H, Pichetshote N, Arias IM. Transporters on demand: intrahepatic pools of canalicular ATP binding cassette transporters in rat liver. J Biol Chem. 2001;276:7218–7224. doi: 10.1074/jbc.M007794200. [DOI] [PubMed] [Google Scholar]
- 124.Gatmaitan ZC, Nies AT, Arias IM. Regulation and translocation of ATP-dependent apical membrane proteins in rat liver. Am J Physiol. 1997;272:G1041–G1049. doi: 10.1152/ajpgi.1997.272.5.G1041. [DOI] [PubMed] [Google Scholar]
- 125.Strautnieks SS, Bull LN, Knisely AS, Kocoshis SA, Dahl N, Arnell H, Sokal E, Dahan K, Childs S, Ling V, et al. A gene encoding a liver-specific ABC transporter is mutated in progressive familial intrahepatic cholestasis. Nat Genet. 1998;20:233–238. doi: 10.1038/3034. [DOI] [PubMed] [Google Scholar]
- 126.Wakabayashi Y, Lippincott-Schwartz J, Arias IM. Intracellular trafficking of bile salt export pump (ABCB11) in polarized hepatic cells: constitutive cycling between the canalicular membrane and rab11-positive endosomes. Mol Biol Cell. 2004;15:3485–3496. doi: 10.1091/mbc.E03-10-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lapierre LA, Kumar R, Hales CM, Navarre J, Bhartur SG, Burnette JO, Provance DW, Mercer JA, Bähler M, Goldenring JR. Myosin vb is associated with plasma membrane recycling systems. Mol Biol Cell. 2001;12:1843–1857. doi: 10.1091/mbc.12.6.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Paulusma CC, Bosma PJ, Zaman GJ, Bakker CT, Otter M, Scheffer GL, Scheper RJ, Borst P, Oude Elferink RP. Congenital jaundice in rats with a mutation in a multidrug resistance-associated protein gene. Science. 1996;271:1126–1128. doi: 10.1126/science.271.5252.1126. [DOI] [PubMed] [Google Scholar]
- 129.Silverman JA, Raunio H, Gant TW, Thorgeirsson SS. Cloning and characterization of a member of the rat multidrug resistance (mdr) gene family. Gene. 1991;106:229–236. doi: 10.1016/0378-1119(91)90203-n. [DOI] [PubMed] [Google Scholar]
- 130.Suchy FJ, Ananthanarayanan M. Bile salt excretory pump: biology and pathobiology. J Pediatr Gastroenterol Nutr. 2006;43 Suppl 1:S10–S16. doi: 10.1097/01.mpg.0000226385.71859.5f. [DOI] [PubMed] [Google Scholar]
- 131.Kullak-Ublick GA, Stieger B, Hagenbuch B, Meier PJ. Hepatic transport of bile salts. Semin Liver Dis. 2000;20:273–292. doi: 10.1055/s-2000-9426. [DOI] [PubMed] [Google Scholar]
- 132.Akita H, Suzuki H, Ito K, Kinoshita S, Sato N, Takikawa H, Sugiyama Y. Characterization of bile acid transport mediated by multidrug resistance associated protein 2 and bile salt export pump. Biochim Biophys Acta. 2001;1511:7–16. doi: 10.1016/s0005-2736(00)00355-2. [DOI] [PubMed] [Google Scholar]
- 133.Paulusma CC, van Geer MA, Evers R, Heijn M, Ottenhoff R, Borst P, Oude Elferink RP. Canalicular multispecific organic anion transporter/multidrug resistance protein 2 mediates low-affinity transport of reduced glutathione. Biochem J. 1999;338(Pt 2):393–401. [PMC free article] [PubMed] [Google Scholar]
- 134.Yang B, Hill CE. Nifedipine modulation of biliary GSH and GSSG/ conjugate efflux in normal and regenerating rat liver. Am J Physiol Gastrointest Liver Physiol. 2001;281:G85–G94. doi: 10.1152/ajpgi.2001.281.1.G85. [DOI] [PubMed] [Google Scholar]
- 135.Ballatori N, Truong AT. Glutathione as a primary osmotic driving force in hepatic bile formation. Am J Physiol. 1992;263:G617–G624. doi: 10.1152/ajpgi.1992.263.5.G617. [DOI] [PubMed] [Google Scholar]
- 136.Banales JM, Prieto J, Medina JF. Cholangiocyte anion exchange and biliary bicarbonate excretion. World J Gastroenterol. 2006;12:3496–3511. doi: 10.3748/wjg.v12.i22.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hardison WG, Wood CA. Importance of bicarbonate in bile salt independent fraction of bile flow. Am J Physiol. 1978;235:E158–E164. doi: 10.1152/ajpendo.1978.235.2.E158. [DOI] [PubMed] [Google Scholar]
- 138.Medina JF, Lecanda J, Acín A, Ciesielczyk P, Prieto J. Tissue-specific N-terminal isoforms from overlapping alternate promoters of the human AE2 anion exchanger gene. Biochem Biophys Res Commun. 2000;267:228–235. doi: 10.1006/bbrc.1999.1951. [DOI] [PubMed] [Google Scholar]
- 139.Aranda V, Martínez I, Melero S, Lecanda J, Banales JM, Prieto J, Medina JF. Shared apical sorting of anion exchanger isoforms AE2a, AE2b1, and AE2b2 in primary hepatocytes. Biochem Biophys Res Commun. 2004;319:1040–1046. doi: 10.1016/j.bbrc.2004.05.080. [DOI] [PubMed] [Google Scholar]
- 140.Marinelli RA, Gradilone SA, Carreras FI, Calamita G, Lehmann GL. Liver aquaporins: significance in canalicular and ductal bile formation. Ann Hepatol. 2004;3:130–136. [PubMed] [Google Scholar]
- 141.Huebert RC, Splinter PL, Garcia F, Marinelli RA, LaRusso NF. Expression and localization of aquaporin water channels in rat hepatocytes. Evidence for a role in canalicular bile secretion. J Biol Chem. 2002;277:22710–22717. doi: 10.1074/jbc.M202394200. [DOI] [PubMed] [Google Scholar]
- 142.García F, Kierbel A, Larocca MC, Gradilone SA, Splinter P, LaRusso NF, Marinelli RA. The water channel aquaporin-8 is mainly intracellular in rat hepatocytes, and its plasma membrane insertion is stimulated by cyclic AMP. J Biol Chem. 2001;276:12147–12152. doi: 10.1074/jbc.M009403200. [DOI] [PubMed] [Google Scholar]
- 143.Zamychkina KS, Kriukova LV. Absorption of methionine from the gall-bladder in vivo and in vitro. Biull Eksp Biol Med. 1967;63:28–30. [PubMed] [Google Scholar]
- 144.Rehman A, Affronti J, Rao S. Sphincter of Oddi dysfunction: an evidence-based review. Expert Rev Gastroenterol Hepatol. 2013;7:713–722. doi: 10.1586/17474124.2013.849197. [DOI] [PubMed] [Google Scholar]
- 145.Roda A, Cappelleri G, Aldini R, Roda E, Barbara L. Quantitative aspects of the interaction of bile acids with human serum albumin. J Lipid Res. 1982;23:490–495. [PubMed] [Google Scholar]
- 146.Salvioli G, Lugli R, Pradelli JM, Gigliotti G. Bile acid binding in plasma: the importance of lipoproteins. FEBS Lett. 1985;187:272–276. doi: 10.1016/0014-5793(85)81257-6. [DOI] [PubMed] [Google Scholar]
- 147.Hedenborg G, Norlander A, Norman A, Svensson A. Bile acid transport by serum lipoproteins in patients with extrahepatic cholestasis. Scand J Clin Lab Invest. 1986;46:745–750. doi: 10.3109/00365518609084046. [DOI] [PubMed] [Google Scholar]
- 148.Bissell DM. In: Bile pigments and jaundice. Ostrow JD, editor. New-York: Marcel Dekker Inc; 1986. pp. 133–156. [Google Scholar]
- 149.Kapitulnik J, Maines MD. The role of bile pigments in health and disease: effects on cell signaling, cytotoxicity, and cytoprotection. Front Pharmacol. 2012;3:136. doi: 10.3389/fphar.2012.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988;2:2557–2568. [PubMed] [Google Scholar]
- 151.Lundvig DM, Immenschuh S, Wagener FA. Heme oxygenase, inflammation, and fibrosis: the good, the bad, and the ugly? Front Pharmacol. 2012;3:81. doi: 10.3389/fphar.2012.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fevery J, Vanstapel F, Blanckaert N. Bile pigment metabolism. Baillieres Clin Gastroenterol. 1989;3:283–312. doi: 10.1016/0950-3528(89)90002-x. [DOI] [PubMed] [Google Scholar]
- 153.Gibbs PE, Tudor C, Maines MD. Biliverdin reductase: more than a namesake - the reductase, its Peptide fragments, and biliverdin regulate activity of the three classes of protein kinase C. Front Pharmacol. 2012;3:31. doi: 10.3389/fphar.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Landaw SA, Callahan EW, Schmid R. Catabolism of heme in vivo: comparison of the simultaneous production of bilirubin and carbon monoxide. J Clin Invest. 1970;49:914–925. doi: 10.1172/JCI106311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tiribelli C, Ostrow JD. New concepts in bilirubin chemistry, transport and metabolism: report of the Second International Bilirubin Workshop, April 9-11, 1992, Trieste, Italy. Hepatology. 1993;17:715–736. doi: 10.1002/hep.1840170428. [DOI] [PubMed] [Google Scholar]
- 156.Zucker SD, Goessling W, Gollan JL. Kinetics of bilirubin transfer between serum albumin and membrane vesicles. Insight into the mechanism of organic anion delivery to the hepatocyte plasma membrane. J Biol Chem. 1995;270:1074–1081. doi: 10.1074/jbc.270.3.1074. [DOI] [PubMed] [Google Scholar]
- 157.Chowdhury JR, Chowdhury NR. Conjugation and excretion of bilirubin. Semin Liver Dis. 1983;3:11–23. doi: 10.1055/s-2008-1040667. [DOI] [PubMed] [Google Scholar]
- 158.Calamita G, Ferri D, Gena P, Liquori GE, Marinelli RA, Meyer G, Portincasa P, Svelto M. Water transport into bile and role in bile formation. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:137–142. doi: 10.2174/1568008054064850. [DOI] [PubMed] [Google Scholar]
- 159.Lehmann GL, Larocca MC, Soria LR, Marinelli RA. Aquaporins: their role in cholestatic liver disease. World J Gastroenterol. 2008;14:7059–7067. doi: 10.3748/wjg.14.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fricker G, Hugentobler G, Meier PJ, Kurz G, Boyer JL. Identification of a single sinusoidal bile salt uptake system in skate liver. Am J Physiol. 1987;253:G816–G822. doi: 10.1152/ajpgi.1987.253.6.G816. [DOI] [PubMed] [Google Scholar]
- 161.Jessner W, Zsembery A, Graf J. Transcellular water transport in hepatobiliary secretion and role of aquaporins in liver. Wien Med Wochenschr. 2008;158:565–569. doi: 10.1007/s10354-008-0597-9. [DOI] [PubMed] [Google Scholar]
- 162.Masyuk AI, Marinelli RA, LaRusso NF. Water transport by epithelia of the digestive tract. Gastroenterology. 2002;122:545–562. doi: 10.1053/gast.2002.31035. [DOI] [PubMed] [Google Scholar]
- 163.King LS, Kozono D, Agre P. From structure to disease: the evolving tale of aquaporin biology. Nat Rev Mol Cell Biol. 2004;5:687–698. doi: 10.1038/nrm1469. [DOI] [PubMed] [Google Scholar]
- 164.Portincasa P, Calamita G. Water channel proteins in bile formation and flow in health and disease: when immiscible becomes miscible. Mol Aspects Med. 2012;33:651–664. doi: 10.1016/j.mam.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 165.Marinelli RA, Tietz PS, Caride AJ, Huang BQ, LaRusso NF. Water transporting properties of hepatocyte basolateral and canalicular plasma membrane domains. J Biol Chem. 2003;278:43157–43162. doi: 10.1074/jbc.M305899200. [DOI] [PubMed] [Google Scholar]
- 166.Marinelli RA, Lehmann GL, Soria LR, Marchissio MJ. Hepatocyte aquaporins in bile formation and cholestasis. Front Biosci (Landmark Ed) 2011;16:2642–2652. doi: 10.2741/3877. [DOI] [PubMed] [Google Scholar]
- 167.Graf J, Henderson RM, Krumpholz B, Boyer JL. Cell membrane and transepithelial voltages and resistances in isolated rat hepatocyte couplets. J Membr Biol. 1987;95:241–254. doi: 10.1007/BF01869486. [DOI] [PubMed] [Google Scholar]
- 168.Cater MA, La Fontaine S, Shield K, Deal Y, Mercer JF. ATP7B mediates vesicular sequestration of copper: insight into biliary copper excretion. Gastroenterology. 2006;130:493–506. doi: 10.1053/j.gastro.2005.10.054. [DOI] [PubMed] [Google Scholar]
- 169.Ivanchenkova RA. Some aspects of cholepoiesis. Klinicheskaya Meditsina. 1999;77:18–22. [PubMed] [Google Scholar]
- 170.Kibe A, Holzbach RT, LaRusso NF, Mao SJ. Inhibition of cholesterol crystal formation by apolipoproteins in supersaturated model bile. Science. 1984;225:514–516. doi: 10.1126/science.6429856. [DOI] [PubMed] [Google Scholar]


